Abstract
The intestinal anaerobic bacterium Akkermansia muciniphila is specialized in the degradation of mucins, which are heavily O-glycosylated proteins that constitute the major components of the mucus lining the intestine. Despite that adhesion to mucins is considered critical for the persistence of A. muciniphila in the human intestinal tract, our knowledge of how this intestinal symbiont recognizes and binds to mucins is still limited. Here, we first show that the mucin-binding properties of A. muciniphila are independent of environmental oxygen concentrations and not abolished by pasteurization. We then dissected the mucin-binding properties of pasteurized A. muciniphila by use of a recently developed cell-based mucin array that enables display of the tandem repeats of human mucins with distinct O-glycan patterns and structures. We found that A. muciniphila recognizes the unsialylated LacNAc (Galβ1-4GlcNAcβ1-R) disaccharide selectively on core2 and core3 O-glycans. This disaccharide epitope is abundantly found on human colonic mucins capped by sialic acids, and we demonstrated that endogenous A. muciniphila neuraminidase activity can uncover the epitope and promote binding. In summary, our study provides insights into the mucin-binding properties important for colonization of a key mucin-foraging bacterium.
Subject terms: Bacterial adhesion, Bacterial host response, Glycobiology
Intestinal mucus consists of densely O-glycosylated mucins, serving as a nutrient source for bacteria. Elzinga et al. show that mucin-degrading Akkermansia muciniphila selectively binds to O-glycan structures found on human colonic mucins.
Introduction
The human gastrointestinal tract (GIT) is lined by mucus, which plays a key role in the maintenance of intestinal health1. The mucus barrier is mainly composed of mucins, a family of large, heavily O-glycosylated (GalNAc-type, mucin-type) proteins that include secreted mucins (MUC2, 5AC, 5B and 6) and cell membrane attached mucins (MUC1, 3, 4, 12, 13, 17)2. In the intestine, the secreted gel-forming MUC2 mucin binds water and forms a protective, mesh-like network divided into two layers3. A dense mucus layer serves as a barrier for microorganisms and an outer loose mucus layer provides a niche for commensal bacteria that adhere to mucins and their O-glycans4. The O-glycans serve not only as an attachment point but also as a nutrient source for the commensal microbiota, thus having a critical impact on colonization as well as metabolism5–7. Mutualistic bacteria that bind and utilize mucins represent so-called mucolytic bacteria, such as Bifidobacterium spp., Ruminococcus gnavus, Bacteroides thetaiotaomicron and Akkermansia muciniphila6,8.
A. muciniphila is an intestinal symbiont known to bind mucins and also recognized as a mucin-degrader that can utilize individual monosaccharides by expressing a large set of glycoside hydrolases (GHs) and can digest O-glycan trimmed mucins by mucinases9,10 and proteases6,8. A. muciniphila plays a key role in the intestinal host-microbiome ecosystem at the mucosal interface by its abilities to release monosaccharides, e.g., for cross-feeding of other bacteria. Feeding on mucins results in the production of short-chain fatty acids, and in this way, A. muciniphila provides the start of many trophic chains in the human GIT11.
The abundance of A. muciniphila in the healthy human GIT has been negatively correlated with a wide range of disorders, including obesity, diabetes, cardiometabolic diseases, and low-grade inflammation12. This Gram-negative bacterium has also been shown to reinforce the mucosal barrier in mice and humans by increasing the mucus layer thickness13 and administration of both live and pasteurized A. muciniphila reversed high-fat diet-induced metabolic disorders14–16. Thus, A. muciniphila clearly has promising probiotic potential.
The mucin-binding properties of A. muciniphila have so far been studied using human colonic mucins obtained from healthy and diseased donors17,18 or mucin-producing cell lines17,19, but knowledge of A. muciniphila interaction with mucins and recognition of glycans is limited. A recent study identified the role of a mucin-regulated protein (Amuc_1620) in mucin aggregation in the zebrafish gut20, but detailed studies on (additional) ligand-receptor interactions are lacking. The use of isolated mucins for binding studies generally limits the ability to define binding ligands in any structural detail due to their large and heterogenous nature. For example, commercial Porcine Gastric Mucin (PGM) preparations are commonly used, albeit that PGM is not representative of human colonic mucins as both the protein backbone and O-glycans differ considerably21–24.
The recent development of a cell-based mucin platform for the display and production of human mucin reporters containing representative parts of the O-glycodomains with tandem repeated sequences (TRs) and defined O-glycans now provides opportunities for more detailed studies of mucin-binding properties of microbiota members25. These mucin TR reporters containing 150–200 amino acids derived from different human mucins are expressed in human embryonic kidney (HEK293) cells with genetically engineered O-glycosylation capacities, and they are predicted to convey most of the informational content of the native secreted and transmembrane mucins25. Previous work has showcased the use of these O-glycodomains to investigate the binding specificities of microbial and viral adhesins as well as substrate preferences of microbial glycopeptidases9,25,26. Here, we investigated the characteristic mucin-binding requirements of A. muciniphila and demonstrated that A. muciniphila has a strong bias towards core3 MUC2 TRs, representing the most abundant mucin and O-glycoform found in the human colon23. Next, we uncovered that A. muciniphila primarily recognizes the unsubstituted LacNAc disaccharide epitope, which is found widely on complex type O-glycans (core2-4) on mucins following removal of neuraminic acids (and/or fucose if attached). In agreement with this we demonstrate that endogenous neuraminidase activity of A. muciniphila can enhance binding to sialylated mucins.
Results
Pasteurized A. muciniphila binds to PGM in a concentration-dependent manner
We used PGM to establish and optimize an ELISA-based assay for the mucin-binding properties of A. muciniphila. A. muciniphila was grown on a minimal medium supplemented with Glc and GlcNAc, which was previously shown to support rapid growth27. Binding was tested in oxic conditions at different temperatures (4 °C, RT and 37 °C) as well as anoxic conditions at 37 °C to mimic the environment of the human colon. A. muciniphila was found to bind to PGM in a concentration-dependent matter, plateauing between 1 and 10 µg/mL PGM (Fig. 1a). It has been shown that A. muciniphila can survive in certain oxic conditions for up to 10 h28, and we demonstrate that the presence of oxygen did not affect mucin-binding of A. muciniphila. Interestingly, binding was stronger at 4 °C and RT compared to 37 °C (Fig. 1a), which may indicate that some enzymatic degradation of mucins occurs at 37 °C, although such potential degradation clearly did not fully destroy the binding-ligands on PGM. To develop a more robust and transferable assay, we tested mucin-binding of pasteurized A. muciniphila. In preclinical models and human patients, live and pasteurized A. muciniphila were shown to be equivalently effective in alleviating metabolic disorders14,15 indicating that specific beneficial bacterial proteins, including membrane protein Amuc_1100, were still effective after pasteurization15. Moreover, pasteurized A. muciniphila showed comparable binding, albeit with a slightly lower concentration-response compared to that of live cells, which enabled us to proceed in dissecting the binding properties with pasteurized bacteria (Fig. 1b and Supplementary Fig. S1a).
Fig. 1. ELISA binding assays of A. muciniphila with porcine gastric mucin (PGM).
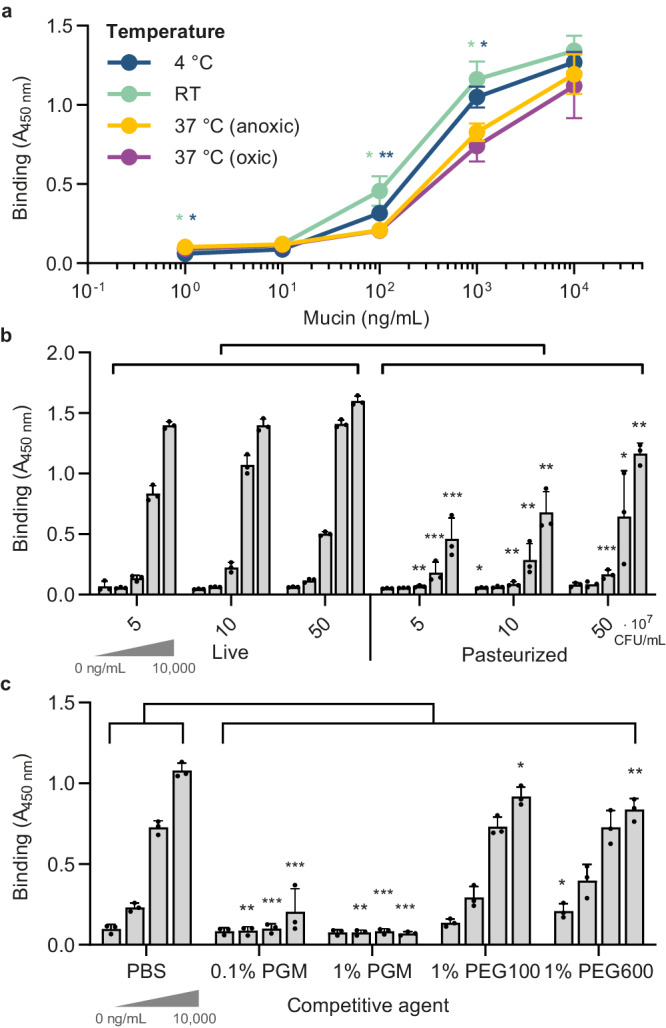
a Binding of live A. muciniphila (5 × 108 CFU/mL in PBS) under oxic conditions at different temperatures (4 °C, RT or 37 °C) and under anoxic conditions at 37 °C to different concentrations of PGM as indicated. The temperature indicates that all incubation steps for this condition were performed at the same respective temperature. Binding to PGM at 37 °C under anoxic conditions serves as a reference for statistical analysis. b Binding of live and pasteurized A. muciniphila (5 × 107−8 CFU/mL) at 4 °C under oxic conditions. PGM was coated at 1, 10, 100, 1000, and 10,000 ng/mL. Binding of live cells serves as a reference for statistical analysis. Note that the concentration of pasteurized cells specified indicate the original concentration of the live equivalent. c Binding inhibition of pasteurized A. muciniphila (5 ×108 CFU/mL) with 1% PEG polymers (100 and 600 kDa) or PGM on binding at 4 °C under oxic conditions. PGM was coated at 0, 100, 1000, and 10,000 ng/mL. Bars and data points represent the mean ± SD of three biological replicates. A student’s two-sided t-test was performed to assess differences in means between conditions. *p < 0.05, **p < 0.01, ***p < 0.001. Source data are provided as a Source Data file.
We tested if PGM in solution could interfere with binding of pasteurized A. muciniphila to mucin. Indeed, already at 0.1% (w/v) PGM could inhibit binding of A. muciniphila cells to coated PGM. This effect was not observed for other polymers such as PEG 100 and 600 kDa, with lower (PEG 100 kDa) or similar viscosity (PEG 600 kDa)29 (Fig. 1c) and was replicated for live bacteria (Supplementary Fig. S1b). Consequently, as a viscous mucin-based medium could potentially interfere with the assay, all subsequent ELISAs were performed with pasteurized bacteria grown on a minimal medium supplemented with Glc and GlcNAc, and carried out under an oxic atmosphere at 4 °C.
Binding of A. muciniphila to mucin depends on the presence of LacNAc
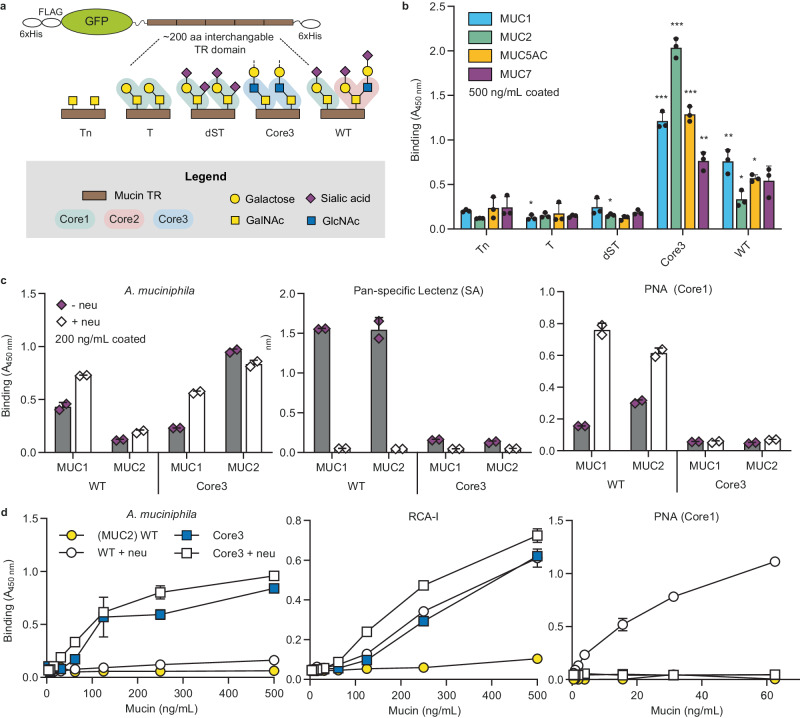
To rationally dissect the binding of A. muciniphila to human mucins, we used the cell-based mucin platform to display and produce the most common O-glycan cores (cores 1-3) and structures (Tn and sialylated core structures) on human mucin TRs (MUC1, 2, 5AC, and 7; Fig. 2a)25. Probing purified mucin TR reporters by ELISA with pasteurized A. muciniphila revealed low/no binding to mucin TRs with core1 (core1/dST) and truncated (Tn/STn) O-glycans, but significant binding to all the mucins with core3 and WT (core1/2) O-glycans (Fig. 2b and Supplementary Fig. S2a, b). The core2 and core3 O-glycans represent the most abundant O-glycan structures in the human intestine23,30,31. Binding to mucin TRs was enhanced by pretreatment with neuraminidase to remove sialic acids; however, interestingly, this enhancement was predominantly observed with the MUC1 reporter and the WT glycoform, while the MUC2 reporter and in particular the core3 glycoform exhibited lower or no significant effect (Fig. 2c, d). We previously demonstrated that the MUC2 reporter in contrast to MUC1 is preferentially glycosylated with core1 O-glycans in WT HEK293 cells despite these having the capacity to produce core232, which resulted in barely detectable levels of core2 O-glycans by our O-glycan profiling of the purified MUC2 reporter expressed in HEK293 WT cells (Supplementary Fig. S3a, b). We therefore had to use higher concentrations (approximately 10 times) of the MUC2 reporter to obtain detectable binding of RCA-1 as well as A. muciniphila in ELISA assays (Fig. 2a). Moreover, HEK293 cells engineered for core3 O-glycosylation capacity were found to produce the trisaccharide core3 O-glycan without substantial sialic acid capping26,33 (Supplementary Fig. S3a, b). Thus, the results suggest that binding of A. muciniphila to the MUC1 and MUC2 reporters were directed to the LacNAc (Galβ1-4GlcNAc) structure common to core2 and core3 O-glycans.
Fig. 2. ELISA binding assays of pasteurized A. muciniphila with purified glycoengineered human mucin TR reporters.
a Graphic depiction of the mucin reporters and the O-glycoforms analyzed. The mucin TR reporters were produced in cells engineered as follows: Tn (KO C1GALT1), T (KO GCNT1, KO ST6GALNAC2/3/4, KO ST3GAL1/2), dST (KO GCNT1, KI ST6GALNAC2/3/4), core3 (KO COSMC, KI B3GNT6). The WT HEK293 cells produce a mixture of core1 (ST) and core2 O-glycans as illustrated. b Binding of pasteurized A. muciniphila (5 × 108 CFU/mL) to isolated mucin TR reporters with five different glycoforms as indicated. Binding to Tn of respective TR domain serves as a reference for statistical analysis. Bars represent the mean ± SD of three biological replicates. A Student’s two-sided t-test was performed to assess differences in means between conditions. *p < 0.05, **p < 0.01, ***p < 0.001. c Binding to mucin reporters (200 ng/mL) with or without pretreatment with Clostridium perfringens neuraminidase (20 mU overnight) (A. muciniphila 1 × 109 CFU/mL) with control binding of pan-specific Lectenz (2 µg/mL) and PNA (0.1 µg/mL). One representative experiment is shown, with bars representing the mean ± SD of 2 technical replicates. d Binding of pasteurized A. muciniphila (5 × 108 CFU/mL) to varying concentrations of WT and Core3 MUC2 reporters pretreated with and without C. perfringens neuraminidase. Binding of RCA-I (0.05 μg/mL) and PNA (0.1 µg/mL) as controls. One representative experiment is shown, representing the mean ± SD of 2 technical replicates. Source data are provided as a Source Data file.
We used lectins as controls to probe for sialic acids (Lectenz) and core1 (Galβ1-3GalNAc) (Peanut agglutinin, PNA34), which confirmed the efficiency of the neuraminidase treatment (Fig. 2c, d). We used Ricinus Communis Agglutinin I (RCA-I35) to detect the exposure level of LacNAc (Galβ1-4GlcNAc), which showed a significant increase for WT MUC2 after neuraminidase treatment as well as a high signal for core3 MUC2 both with/without neuraminidase treatment suggesting the appreciable levels of LacNAc exposed. The RCA-I lectin binding profile revealed a concentration-dependent, sialic acid-sensitive signal for core3 MUC2, similar to A. muciniphila binding patterns (Fig. 2c, d), thus confirming a binding preference of A. muciniphila for non-sialylated LacNAc.
A. muciniphila selectively binds core3 O-glycans on MUC2
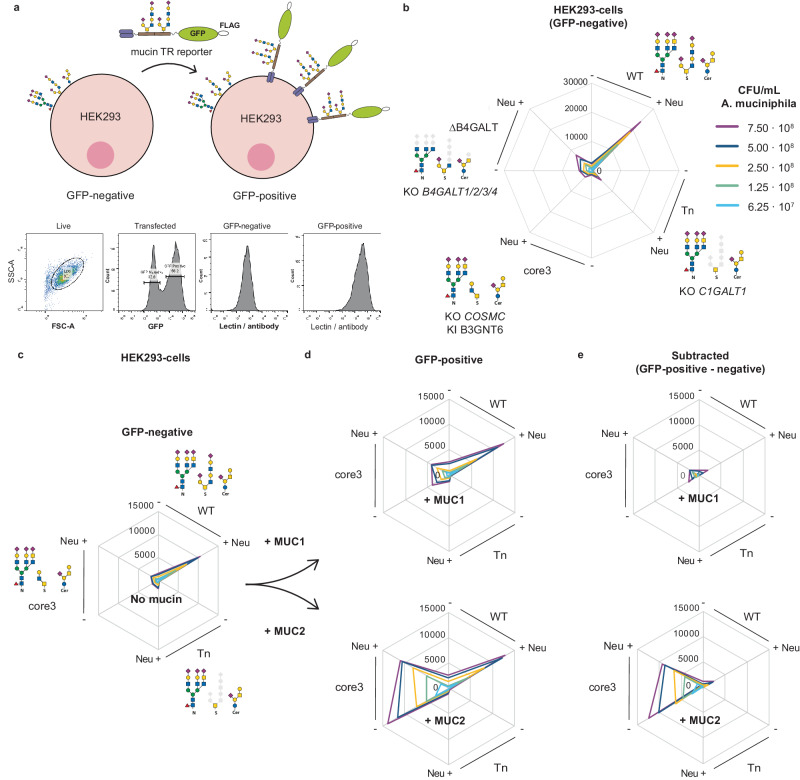
Since the LacNAc disaccharide is a common terminal structure found on most types of glycoconjugates, including glycolipids and N-glycoproteins, and to further evaluate the glycan and glycoconjugate binding properties, we employed the cell-based mucin TR display platform with flow cytometry analysis (Fig. 3a). Pasteurized A. muciniphila bound to HEK293 WT cells without introduction of mucin reporters, but only after neuraminidase treatment to remove sialic acid capping (Fig. 3b). The binding was eliminated by loss of elongated O-glycans (KO C1GALT1, Tn), which suggests that A. muciniphila preferentially binds O-glycans. Moreover, A. muciniphila binding was also lost when tested in cells where LacNAc synthesis (KO B4GALT1/2/3/4, ∆B4GALT) was eliminated, which further supports LacNAc as the binding epitope. Next, we expressed cell membrane bound mucin reporters in the glycoengineered HEK293 cells (Fig. 3c–e). A. muciniphila is known to express mucinases36, and these could potentially interfere with the binding assays performed. To exclude degradation of the mucin reporters during our binding studies with pasteurized A. muciniphila bacteria, we took advantage of the design of the reporters with N-terminal FLAG-tags (Fig. 2a). Incubation of mucin reporters expressed in cells with pasteurized A. muciniphila did not significantly affect binding of anti-FLAG antibodies (Supplementary Fig. S4), suggesting that the endogenous A. muciniphila mucinases are not active under the conditions of our assays. Pasteurized A. muciniphila again only bound to WT HEK293-cells following neuraminidase treatment and the binding was not appreciably affected by the expression of MUC1 or MUC2 TR reporters (Fig. 3d, e and Supplementary Fig. S5a, b). This finding was in agreement with the indiscriminate binding to different mucin TRs by ELISA (Fig. 2a), with the notable exception of binding to core3 engineered cells. While A. muciniphila binding to engineered core3 HEK293 cells with and without expression of MUC1 was very low, the binding to core3 HEK293 cells expressing the MUC2 TR reporter was markedly enhanced, and this strong binding was largely independent of pretreatment with neuraminidase (Fig. 3b). These results recapitulate findings with the secreted mucin reporters by ELISA, including the finding that core3 O-glycans in HEK293 cells are not sialylated (Fig. 2b, c). Moreover, the results suggest that the major intestinal mucin MUC2 may serve as a preferential scaffold for presentation of core3 O-glycans recognized by A. muciniphila. This conclusion is based on the low binding to core3 O-glycan engineered cells with or without expression of MUC1. We previously demonstrated that the MUC1 TR reporters expressed in core3 engineered HEK293 cells do acquire core3 O-glycans, albeit with reduced number of O-glycans per TR26,37, but the MUC2 TR reporters are predicted to present much denser clusters of core3 O-glycans that appear to be favored by A. muciniphila. In line with this, the human intestinal MUC2 was shown to predominantly express core3 O-glycans38,39 but further studies into this are clearly needed.
Fig. 3. Flow cytometry analysis of pasteurized A. muciniphila binding to glycoengineered HEK293 cells.
a Schematic depiction of the gating strategy (GFP-positive/negative) for assessing binding to glycans on HEK293WT cells and on the mucin-GFP reporters expressed on a subpopulation of HEK293 cells using transient transfections. Live cells were gated on the side scatter area (SSC-A) versus forward scatter area (FSC-A) plot followed by gating on GFP positive cells (expressing mucin-GFP reporters) or GFP negative (not expressing mucin-GFP reporters). b Binding of A. muciniphila (0.625–7.5 × 108 CFU/mL) to HEK293 cells glycoengineered as indicated without transfection of GFP-mucin reporters. Cells were analyzed with (+) and without (−) pretreatment with Clostridium perfringens neuraminidase (Neu, 10 mU, 1 h). Data points represent the average median fluorescence intensity (MFI) of two biological replicates. c–e Binding of A. muciniphila to glycoengineered HEK293 cells transiently transfected with GFP-tagged mucin reporters (MUC1, MUC2) using the GFP-gating strategy. c Binding to the GFP-negative cell population not expressing the mucin reporters reproduce binding found in b. Binding the GFP-positive cell population expressing the mucin reporters (cells) is shown in d and in e after subtraction of binding to the GFP-negative cell population. Note, expression of the MUC1 reporter did not significantly contribute to the binding of A. muciniphila, while expression of the MUC2 reporter selectively enhanced binding only in cells glycoengineered for core3 O-glycosylation (also note that neuraminidase pretreatment did not significantly affect this binding). One representative experiment is shown of n = 2 biological replicates. Source data are provided as a Source Data file.
Since the dissection analysis was carried out predominantly with pasteurized A. muciniphila, we confirmed that the selective specificity for LacNAc carried on core2 and core3 O-glycans and MUC2 was also found with live bacteria, as demonstrated by ELISA (Supplementary Fig. S6).
Activity of endogenous A. muciniphila neuraminidases are needed for binding to mucins
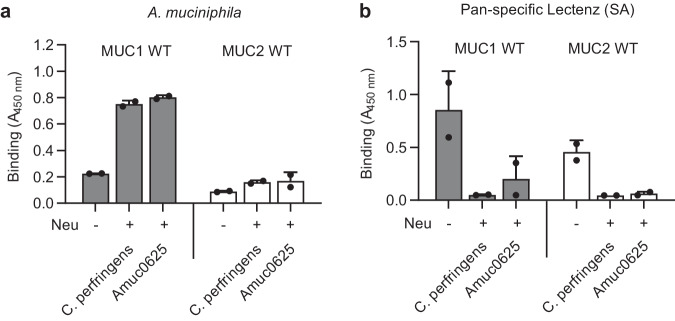
While HEK293 cells, for yet unknown reasons, appear to be incapable of sialylating the engineered core3 O-glycans, it is clear that, e.g., MUC2 isolated from the human colon contains highly sialylated O-glycans including core3 O-glycans40. We, therefore, predict that desialylation is generally needed to expose the A. muciniphila LacNAc epitopes. The genome of A. muciniphila encodes exo-neuraminidases, including Amuc_0625 and 183536,41. Amuc_0625 is expressed higher during growth on mucin41 and has been extensively characterized for its optimal pH, temperature, and substrate specificity42,43. We therefore tested purified Amuc_0625 produced in E. coli42 with isolated secreted MUC1 and MUC2 TR reporters (Figs. 2c, d and 3), and found that pretreatment with Amuc_0625 produced the same enhancement of binding to the two mucin TRs as observed after desialylation with the C. perfringens neuraminidase (Fig. 4a, b). A similar effect was demonstrated for the Amuc_1835 neuraminidase (Supplementary Fig. S7a). The neuraminidase activities detectable with live and pasteurized A. muciniphila bacteria were very low (>10x lower compared to 10 mU C. perfringens neuraminidase) (Supplementary Fig. S7b). Overall, these results show that A. muciniphila has the capacity to remove sialic acids and promote binding to mucins in the mucus.
Fig. 4. ELISA analysis of endogenous A. muciniphila neuraminidases with human mucin TR reporters.
a Immobilized recombinant purified mucin reporters (250 ng/mL) were incubated with recombinant A. muciniphila Amuc_0625 neuraminidase (1.4 mU) or control C. perfringens neuraminidase (2.5 mU) and binding of pasteurized A. muciniphila (5 ×108 CFU/mL) tested. b Analysis as in a) with the sialic acid binding pan-specific Lectenz (2.0 μg/mL). Plates were read at 450 nm. Bars represent the mean ± SD of 2 biological replicates. Source data are provided as a Source Data file.
Discussion
A. muciniphila is a well-known mucus-adapted intestinal symbiont with specific binding to O-glycans. Here, we dissected mucin-binding properties of A. muciniphila using a novel cell-based platform for production and display of human mucin reporters with defined O-glycans. We showed that mucin binding of the anaerobic A. muciniphila is independent of environmental oxygen concentrations and that both live and pasteurized cells bind similarly to mucins. The use of pasteurized A. muciniphila cells allowed us to uncouple the metabolic and enzymatic activity from the major mucin-binding properties. We demonstrated that mucin binding of A. muciniphila is dependent on LacNAc epitopes preferentially carried on O-glycans, which may be exposed after desialylation by endogenous neuraminidases, like Amuc_0625 and Amuc_1835. While A. muciniphila was found to bind several different mucin TRs indiscriminately with core2 O-glycans, we demonstrated select binding to MUC2 with core3 O-glycans displayed on cells providing the compelling scenario that the mucin-binding properties of A. muciniphila is aligned with the intestinal mucus and the characteristic composition of MUC2 with core3 based O-glycans38–40,44.
Our study investigated the binding of A. muciniphila under different assay conditions (temperature, oxygen) and molecular cues (glycosylation, mucin TR sequence). We used the commonly used mucin PGM for optimization of assays to ensure we were tracing the previously reported mucin-binding properties21. PGM preparations consist of different mucins (MUC5AC and MUC6) with heterogenous O-glycans that are quite different from human colonic MUC223,24, but PGM O-glycans also have a low degree of sialylation and include core2 O-glycan structures with LacNAc termini21,45.
We demonstrate that the binding of A. muciniphila to human mucins is at least in part directed by complex type (core2/3) O-glycans carrying LacNAc epitopes. Our dissection of the A. muciniphila binding specificity indicated that LacNAc on O-glycans is the preferred ligand compared to LacNAc found on glycolipids and N-glycoproteins (Fig. 3a). However, how apparent selectivity for mucin TRs depended on the structures of O-glycans is currently poorly understood. Thus, we found that A. muciniphila binding to complex O-glycans appeared to be largely independent of the type of mucins (Fig. 2b), except when mucins were displayed on the cell surface with core3 O-glycans where binding to MUC2 was substantially better than to MUC1 (Fig. 3c–e). While MUC2 with core3 O-glycans was found to be the best ligand in both ELISA and flow cytometry assays, we only observed this clear selectivity for MUC2 with core3 O-glycans when presented on cells. Our results are in line with previous studies showing A. mucinipihila binding to colon carcinoma cells Caco-2 and HT29 cells17,19 which have relatively low expression of mucins46,47, but express core2 glycans as well as I-branched (containing additional poly-LacNAc) glycans on their cell surface48. Interestingly, previous studies have shown contradicting results regarding binding of A. mucinipihila to human colonic mucins17,18, which may be explained by variations in O-glycosylation although the O-glycan structures were not always quantified. Regardless, the observed preference of A. mucinipihila for core3 O-glycans on MUC2 in our study clearly correlates with the human intestinal mucus being the colonization niches of this bacterium23,24.
Our study implicates a lectin-like adhesin in A. muciniphila, but little is known about microbial adhesins from intestinal bacteria49,50. A recent metagenomic screening of human intestinal microbiota revealed thousands of sequences predicted to encode lectins50. The most common carbohydrate-binding domain in human intestinal microbiota is a domain previously described as Bacteroidetes-Associated Carbohydrate-binding Often N-terminal domain (BACON). Of interest, this BACON-like domain is also present in some proteins of A. muciniphila36,50,51. This domain has not been extensively characterized but is suggested to be involved in the binding of mucin glycans51–53. Additionally, as annotated in the CAZy-database, genomes of A. muciniphila strains contain several types of other carbohydrate-binding modules (CBMs) found in e.g. glycoside hydrolases54. The type IV pili of A. muciniphila55 may also serve roles in binding to mucins, and in general it may be predicted that multiple adhesins and glycan-binding proteins are involved in binding to mucins. Regardless, the mucin-binding properties directed by LacNAc epitopes identified here with the mucin display dissection strategy correlated with previous mucin-binding studies using PGM.
In the mucus layer the abundant O-glycans on mucins are utilized by the microbiota through use of a diverse group of glycosidases that sequentially trim the mucin glycans6,8,56. A recent study demonstrated that neuraminidases (and fucosidases) are crucial for growth of A. muciniphila on mucin57. Here, we showed that removal of terminal sialic acids on O-glycans is prerequisite for uncovering the A. muciniphila LacNAc O-glycan epitope on human mucins and demonstrate that A. muciniphila is equipped with several neuraminidases for this purpose (Fig. 4b)57,58. Similarly, the A. muciniphila O-glycanases (Amuc_0724, 0875 and 2108) require removal of sialic acids to release core1 O-glycans from mucins58. Further the two M60-like peptidases (Amuc_1514 and Amuc_0627)59 involved in A. muciniphila directed mucin degradation were shown to be inhibited by O-glycans capped with sialic acids9,59, and recent studies found that the Amuc_06279 and Amuc_143810 metallopeptidases selectively cleaved mucins with truncated T and Tn O-glycans without sialic acid capping. Our study suggests that the LacNAc-mediated mucin-binding properties of A. muciniphila enable it to adhere to mucins in their early stages of degradation in the mucus, i.e., after removal of sialic acids and before removal of galactose on LacNAc-based O-glycans. Human mucins mainly carry LacNAc-based core2-4 O-glycans that upon desialylation in the microbe-rich outer mucus layer will display abundant LacNAc terminated O-glycans. Subsequent removal of galactose residues of LacNAc O-glycans would then liberate and retain A. muciniphila in the mucus before the mucins are fully degraded and/or released into the lumen. Pathogenic bacteria, in contrast, are known to express mucinases, such as StcE from EHEC60, that efficiently cleave mucins with complex and sialylated O-glycans25,26,60, but interestingly not core3 O-glycans25. Taken together, we could speculate about a scenario where commensal bacteria like A. muciniphila degrade mucins after trimming of O-glycans, thus limiting the processing to the outer mucus layer and preventing destruction of the mucus barrier function, while pathogenic species with more potent mucinases can degrade nascent mucins and penetrate the mucus barrier.
Our study showcases the value of the cell-based mucin platform to discover and dissect mucin binding properties of microbes. The next step will be to identify the microbial adhesins underlying mucin binding, and our study here illustrates that the cell-based platform can be used to produce secreted mucin reporters that potentially can be used to label and identify such adhesins. Different human isolates of A. muciniphila19 were previously suggested to exhibit different mucin binding properties, and it would be interesting to extend studies to representatives of all four A. muciniphila phylogroups identified in human beings and other mammalian hosts61–64. Furthermore, to better represent the human intestinal tract in vivo, binding experiments could be extended to a more dynamic environment, e.g., using microfluidics29. Our study identified one mucin-binding property of A. muciniphila that was originally identified with PGM, retained in pasteurized cells, and characterized here. However, it is conceivable that A. muciniphila contain other mucin-binding properties, but further studies are needed to address this. Our study did not explore O-glycan modifications, e.g., sulfation65, and more elaborated O-glycans such as fucosylated and blood group related O-glycans. The binding of A. muciniphila to mucins also remains to be studied in the context of a microbial community with microbes competing for ligands.
In summary, our study is the first to demonstrate that A. muciniphila binds to LacNAc present in O-glycans on mucins following removal of sialic acids, which it can perform itself. The results pave the way for future research into colonization, mucin recognition and mucin degradation by an intestinal symbiont that plays a crucial role in the mucosal host-microbe ecosystem.
Methods
Bacterial culture and pasteurization
A. muciniphila MucT (ATCC, BAA-835) was cultured anaerobically in a basal medium66, containing per liter: 0.4 g KH2PO4; 0.53 g Na2HPO4; 0.3 g NH4Cl; 0.3 g NaCl; 0.1 g MgCl2·6H2O; 0.11 g CaCl2; 1 ml alkaline trace element solution; 1 ml acid trace element solution; 1 ml vitamin solution; 0.5 mg resazurin; 4 g NaHCO3; 0.25 g Na2S·7-9H2O. The trace element and vitamin solutions were prepared as described previously67. The acid trace element solution contained the following: 7.5 mM FeCl2, 1 mM H3BO4, 0.5 mM ZnCl2, 0.1 mM CuCl2, 0.5 mM MnCl2, 0.5 mM CoCl2, 0.1 mM NiCl2 and 50 mM HCl. The alkaline trace element solution contained the following: 0.1 mM Na2SeO3, 0.1 mM Na2WO4, 0.1 mM Na2MoO4, 10 mM NaOH. Vitamin solution contained per liter: 0.02 g biotin, 0.2 g niacin, 0.5 g pyridoxine, 0.1 g riboflavin, 0.2 g thiamine, 0.1 g cyanocobalamin, 0.1 g p-aminobenzoic acid and 0.1 g pantothenic acid. All compounds were autoclaved, except the vitamins, which were filter-sterilized. The basal medium was supplemented with 20 g/L Tryptone (Oxoid™, ThermoFisher Scientific™), 4 g/L L-threonine (Sigma-Aldrich), 0.25% (w/v) Glc and 0.275% (w/v) GlcNAc (~25 mM total, Sigma-Aldrich)27. Incubations were done in serum bottles sealed with butyl-rubber stoppers at 37 °C under anoxic conditions provided by a gas phase of 182 kPa (1.8 atm) N2/CO2 (80: 20, v/v). Pilot experiments demonstrated slightly higher binding of bacteria in the stationary phase compared to the exponential phase (OD = 0.6) (Supplementary Fig. S8), so bacteria were harvested at end-exponential or stationary phase (~1 × 109 CFU/mL, optical density at 600 nm (OD600) of 2.8) by pelleting using multiple rounds of centrifugation. The supernatant was discarded and pellets were resuspended in 1 x Phosphate Buffered Saline (PBS) at 1 × 109 CFU/mL (OD600 = 2.8). Bacterial cells were directly used for subsequent experiments (live fraction) or pasteurized for 30 min at 70 °C in a temperature-controlled water bath15. If not directly proceeding to experiments, cells were stored at -20 °C. ELISA data from Fig. 1 were all performed with cells right after pasteurization. Pilot studies for Figs. 1, 2, and 4 were performed with cells right after pasteurization and reproduced with thawed cells to obtain the presented data. All flow cytometry experiments were carried out using thawed cells. In case of thawed cells, batches were not thawed more than three times.
Production and purification of recombinant mucin TR reporters
The original HEK293 was obtained from HEK293 cells were obtained from ATCC (CRL-1573). HEK293 knock-out/knock-in (KO/KI) glycoengineered cells with different O-glycosylation capacities are available as part of the cell-based glycan array resource25,32 and were used to produce secreted mucin TR reporters designed to include representative sequences (150–200 amino acids) from human MUC1, MUC2, MUC5AC and MUC7 TRs25. The following glycoengineered cells were used to produce the different glycoforms of mucin reporters: core2 (WT), T (KO GCNT1, KO ST6GALNAC2/3/4, KO ST3GAL1/2), Tn (KO C1GALT1), dST (KO GCNT1, KI ST6GALNAC2/3/4), and core3 (KO COSMC, KI B3GNT6)25,32. Briefly, cells stably expressing mucin reporters were seeded at a density of 2.5 × 105 cells/mL in serum-free F17 culture media (Invitrogen) supplemented with 0.1% Kolliphor P188 (Sigma) and 4 mM GlutaMax (Gibco) at 37 °C and 5% CO2 under constant agitation (120 rpm) and cultured for 5 days. The culture medium was spun down twice (1000×g, 5 min and 3000×g, 10 min), and mixed 3:1 (v/v) with 4 × binding buffer (100 mM sodium phosphate, pH 7.4, 2 M NaCl), and run through a nickel-nitrilotriacetic acid (Ni-NTA) affinity resin column (Qiagen), pre-equilibrated with washing buffer (25 mM sodium phosphate, pH 7.4, 500 mM NaCl, 20 mM imidazole). After extensive washing with washing buffer mucin TR reporters were eluted with binding buffer containing 200 mM imidazole. Eluted fractions were analyzed by SDS-PAGE and fractions containing the mucin TR reporter were desalted followed by buffer exchange to MilliQ using Zeba spin columns (ThermoFisher Scientific). Purified mucin TR reporters were quantified using a Pierce™ BCA Protein Assay Kit (ThermoFisher Scientific) following the manufacturer’s instructions and evaluated by NuPAGE Novex Bis-Tris (4–12%, ThermoFisher Scientific) Coomassie blue analysis (InstantBlue® Coomassie Protein Stain, Abcam).
Binding of A. muciniphila to mucins by ELISA
Experiments using porcine gastric mucin, PGM
ELISAs were performed as described previously25 and adapted for evaluation of A. muciniphila binding. MaxiSorp 96-well plates (NuncTM, Thermo Scientific) coated with up to 10 µg/mL of dilutions of ethanol-purified and dialyzed commercial PGM type III (Sigma-Aldrich68,) or up to 500 ng/mL purified mucin TR reporters and incubated overnight at 4 °C in 50 μL carbonate-bicarbonate buffer (pH 9.6). Plates were blocked with 100 μL PLI-P buffer (0.5 M NaCl, 3 mM KCl, 1.5 mM KH2PO4, 6.5 mM Na2HPO4 · 2 H2O, 1% Triton-X100, 1% BSA, pH 7.4) for 1 h at RT and incubated with live or pasteurized A. muciniphila resuspended in PBS up to 1 × 109 CFU/mL for 1 h at 4 °C, unless described otherwise. After extensive washing with PBS containing 0.05% Tween-20 (PBS-T), plates were incubated with rabbit anti-A. muciniphila serum (kind gift of Dr. J. Reunanen (University of Helsinki), 1:1000 in PLI-P) for 1 h at 4 °C, followed by extensive washing and incubation with 1 µg/mL HRP-conjugated polyclonal goat anti-rabbit IgG (H + L) (Invitrogen) for 1 h at 4 °C. The ELISA was then developed by addition of TMB substrate (ThermoFisher Scientific™) and stopped with 0.5 M H2SO4 followed by measurement of absorbance at 450 nm (Agilent BioTek, Gen5 software). To test the effect of different temperatures (4 °C, RT and 37 °C), all incubation steps with bacteria and antibodies were carried out at the same temperature. To test the effect of oxygen (at 37 °C), an anoxic environment was created in a box with a Oxoid™ AnaeroGen™ sachet (Thermo Scientific). To test the effect of other compounds in solution during binding, A. muciniphila was resuspended in PBS with 0.1-1% PGM, 1% PEG 100, or 600 kDa (Merck)29.
Experiments using mucin TR reporters
After optimization with PGM, ELISAs were repeated as described above, in which purified mucin TR reporters were coated up to 500 ng/mL and incubated with pasteurized 5 × 108 CFU/mL A. muciniphila. All incubation steps were performed at 4 °C. The following lectins were used as binding references: 0.05 biotinylated μg/mL Ricinus Communis Agglutinin I (RCA-I, #B-1085-1, Vector Laboratories), 0.1 µg/mL biotinylated Peanut Agglutinin (PNA, #B-1075-5, Vector Laboratories) or 2.0 μg/mL pan-specific Lectenz (Lectenz Bio, #SK0501B) as detection probe and 1 μg/mL HRP-conjugated streptavidin (#P0397, Dako) for signal development. For quantification of the coating efficiency, the mucin TR reporters were detected with 0.1 μg/mL anti-FLAG M2-Peroxidase-HRP–conjugated mAb (#A8592, Sigma). The commercial neuraminidase from Clostridium perfringens (C. perfringens, Sigma, #N3001-10UN) and the neuraminidases Amuc_0625 and Amuc_1835 produced with C-terminal His-Tag as described previously42, were used for neuraminidase experiments. Neuraminidase activity assay was performed with 1 mM MU-NANA (2-(4-Methylumbelliferyl)-α-D-N-acetylneuraminic acid) (Sigma-Aldrich) in phosphate buffer (pH 6.0) for 20 min at 37 °C, terminated by addition of Glycine buffer (pH 2.5) with 25% (v/v) ethanol. The signal was quantified using fluorescence (Ex/Em =355/460 nm) with a standard curve of 0.25–0.625 mM MU (4-methylumbelliferone) (Sigma-Aldrich). Two different aliquots of the A. muciniphila neuraminidases were tested to check stability (Supplementary Fig. S9). For the ELISAs, mucin reporters were incubated with 2.5 mU neuraminidase from C. perfringens, 1.4 mU Amuc_0625 or 0.5 mU Amuc_1835 in 20 mM sodium acetate buffer, pH 6.0 for 4 h, or buffer only. Neuraminidase activity of live and pasteurized cells was evaluated using 5 × 108 CFU/mL pasteurized and live cells using the assay described above, for 1 hour under oxic conditions, using phosphate buffer and 10 mU C. perfringens neuraminidase as a reference. Binding to Tn, Core3 and WT mucin TRs (1 µg/mL) was repeated using live A. muciniphila (up to 1 × 109 CFU/mL), with and without neuraminidase treatment (20 mU C. perfringens o/n).
Glycoprofiling by MS
O-glycans on isolated secreted MUC2 reporters (WT and core3) and fetuin (n = 1 for each) were released, derivatized, purified and analyzed by C18 nanoflow liquid chromatography (LC) coupled to mass spectrometry (MS) as described previously33. For each sample, 1 to 2 µg protein was mixed with 25 µL release reagent (20% hydroxylamine and 20% 1,8-diazabicyclo(5.4.0)undec-7-ene (DBU)) and incubated for 1 h at 37 °C. Released O-glycans were enriched by hydrazide beads and labeled with 50 µL 2-aminobenzamide (2-AB) reagent (500 mM 2-AB, 116 mM 2-methylpyridine borane complex (PB) in 45:45:10 methanol:water:acetic acid) for 2.5 h at 50 °C. Labeled glycans were purified by HILIC and porous graphitized carbon (PGC) SPE. Samples were resolved in 20 µL water for MS analysis.
For each sample, 2 µL was injected for nanoLC-MS/MS analysis, using a single analytical column setup. The analytical column was prepared using a PicoFrit Emitter (New Objectives, 75 μm inner diameter), packed with Reprosil-Pure-AQ C18 phase (Dr. Maisch, 1.9 μm particle size, 22–25 cm column length). The emitter was interfaced to an Orbitrap Fusion Lumos mass spectrometer (Thermo Fisher Scientific) via a nanoSpray Flex ion source. Samples were eluted in a 30 min method with a gradient from 3% to 45% of solvent B in 15 min, from 45% to 100% B in the next 5 min and 100% B for the last 10 min at 200 nL/min (solvent A: 0.1% formic acid in water; solvent B: 0.1% formic acid in 80% ACN). A precursor MS scan (m/z 200-1700, positive polarity) was acquired in the Orbitrap at a nominal resolution of 120,000, followed by Orbitrap higher-energy C-trap dissociation (HCD)-MS/MS at a nominal resolution of 50,000 of the 10 most abundant precursors in the MS spectrum (charge states 1–4). A minimum MS signal threshold of 30,000 was used to trigger data-dependent fragmentation events. HCD was performed with an energy of 27% ± 5%, applying a 20 s dynamic exclusion window with a mass tolerance of 25 ppm. Structural annotation and relative quantification of the O-GalNAc glycans was performed as described before using the Minora Feature Detector node in Thermo Proteome Discoverer 2.2.0.388 (Thermo Fisher Scientific lnc.), GlycoWorkbench 2.1 (build 146)69 and the Thermo Xcalibur qual browser 3.0.63. Glycan structure annotation was based on literature33 and MS/MS analysis.
Cell-Binding assays of A. muciniphila to cells transiently expressing mucin TR reporters
Transmembrane GFP-tagged mucin TR reporters were transiently expressed in engineered HEK293 cells and used for flow cytometry study as described previously25. All WT and isogenic HEK293 cells were cultured in DMEM (Sigma-Aldrich) supplemented with 10% heat-inactivated fetal bovine serum (Gibco) and 2 mM GlutaMAX (Gibco) in a humidified incubator at 37 °C and 5% CO2. Cell lines used previously (WT, Tn, Core3) were included in this experiment, as well as ∆B4GALT (KO B4GALT1/2/3/4). Cells were seeded on 24-wells (Nunc) and transfected at ~70% confluency with 0.5 μg of plasmids using Lipofectamine 3000. Cells were harvested 24 h post-transfection and incubated with or without 10 mU neuraminidase of C. perfringens for 1 h at 37 °C and further probed with 0.625–7.5 × 108 CFU/mL pasteurized A. muciniphila on ice or at 4 °C for 1 h, followed by incubation with polyclonal anti-serum to A. muciniphila (1:1000) and cross-absorbed Alexa Fluor™ 647-conjugated goat anti-rabbit IgG (2 μg/mL, Invitrogen) for 1 h. To check the desialylation level, cells were incubated with 2.0 μg/mL biotinylated SiaFindTM pan-specific Lectenz® (Lectenz Bio) pre-incubated with 2 μg/mL Alexa Fluor™ 647-conjugated streptavidin (#S32357, Invitrogen) for 1 h at 4°C. Expression levels of mucin reporters were detected with 0.1 μg/mL APC-conjugated rat IgG2a λ anti-FLAG (#637308, Biolegend). To check for mucin-cleaving activity, WT and Tn cells transiently expressing MUC2 were incubated with up to 7.5 × 108 CFU/mL pasteurized A. muciniphila and next, probed with 0.2 μg/mL APC-conjugated rat IgG2a λ anti-FLAG to check for cleavage of the mucin reporter. All cells were resuspended in PBA for flow cytometry analysis with a spectral analyzer (SA3800 SONY, including software). Median fluorescent intensity (MFI) for all cell populations was quantified using FlowJo software (FlowJo LLC, version 10).
Statistics and reproducibility
Data are represented as the mean of three biological replicates, each representing at least two technical replicates, unless stated otherwise. For binding of A. muciniphila to purified mucins, a biological replicate represents one independent culture of A. muciniphila. For flow cytometry experiments, a biological replicate represents a cell population derived from an independent cell passage. A student’s two-sided t-test was performed to assess differences in means between conditions, unless stated otherwise. Graph and bar chart figures were generated using GraphPad Prism 10 and Excel. No statistical method was used to predetermine the sample size. No data were excluded from the analyses. The experiments were not randomized, and the investigators were not blinded to allocation during experiments and outcome assessment.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Source data
Acknowledgements
This work was supported by a Building Blocks of Life grant from the Dutch Research Council (NWO) (grant no. 737.016.003, J.E.), a FEMS Research and Training Grant (FEMS-GO-2021-069, J.E.), the Spinoza Award and SIAM Gravity Grant 024.002.002 of the Netherlands Organization for Scientific Research (NWO) of W.M.d.V. (J.E. and H.L.P.T.), the Danish National Research Foundation (DNRF107, H.C. and Y.N.), the Novo Nordisk Foundation (0071658, H.C. and Y.N.) and the Lundbeck Foundation (H.C. and Y.N.) and the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (GlycoSkin H2020-ERC; 772735) (to N.H.).
Author contributions
Conceptualization of the study: J.E., Y.N., H.C., W.M.d.V., and H.L.P.T. Performed experiments: J.E., H.L.P.T., and N.H. Analysis and interpretation of data: J.E., Y.N., H.C., N.H., W.M.d.V., and H.L.P.T. Preparation of figures: J.E., H.C., and Y.N. Supervision: H.C., Y.N., W.M.d.V., and H.L.P.T. Wrote first draft: J.E. and H.L.P.T. Contributed to final draft: J.E., N.H., Y.N., H.C., W.M.d.V., and H.L.P.T.; W.M.d.V. provided the Amuc_0625 and Amuc_1835 constructs.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
All elements necessary to allow interpretation and replication of results are provided in the Supplementary Information. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE70 partner repository with the dataset identifier PXD051738. Source data are provided with this paper in the Source Data file. Source data are provided with this paper.
Competing interests
W.M.d.V. is a co-founder, inventor of patents, and shareholder of The Akkermansia Company, which commercializes pasteurized A. muciniphila. The University of Copenhagen has filed a patent application on the cell-based display platform. GlycoDisplay Aps, Copenhagen, Denmark, has obtained a license to the patent application field. Y.N. and H.C. are co-founders of GlycoDisplay Aps and hold ownership. The remaining authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors jointly supervised this work: Willem M. de Vos, Hanne L.P. Tytgat.
Contributor Information
Janneke Elzinga, Email: jelzinga@sund.ku.dk.
Hanne L. P. Tytgat, Email: hanne.tytgat@wur.nl
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-48770-8.
References
- 1.Johansson ME, Sjövall H, Hansson GC. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2013;10:352–361. doi: 10.1038/nrgastro.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corfield AP. Mucins: a biologically relevant glycan barrier in mucosal protection. Biochim. Biophys. Acta (BBA) - Gen. Subj. 2015;1850:236–252. doi: 10.1016/j.bbagen.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Nilsson HE, et al. Intestinal MUC2 mucin supramolecular topology by packing and release resting on D3 domain assembly. J. Mol. Biol. 2014;426:2567–2579. doi: 10.1016/j.jmb.2014.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sonnenburg JL, Angenent LT, Gordon JI. Getting a grip on things: how do communities of bacterial symbionts become established in our intestine? Nat. Immunol. 2004;5:569–573. doi: 10.1038/ni1079. [DOI] [PubMed] [Google Scholar]
- 5.McLoughlin K, Schluter J, Rakoff-Nahoum S, Smith AdrianL, Foster KevinR. Host selection of microbiota via differential adhesion. Cell Host Microbe. 2016;19:550–559. doi: 10.1016/j.chom.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 6.Tailford LE, Crost EH, Kavanaugh DW, Juge N. Mucin glycan foraging in the human gut microbiome. Front. Genet. 2015;6:81. doi: 10.3389/fgene.2015.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sonnenburg JL, et al. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science. 2005;307:1955–1959. doi: 10.1126/science.1109051. [DOI] [PubMed] [Google Scholar]
- 8.Derrien M, et al. Mucin-bacterial interactions in the human oral cavity and digestive tract. Gut Microbes. 2010;1:254–268. doi: 10.4161/gmic.1.4.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taleb V, et al. Structural and mechanistic insights into the cleavage of clustered O-glycan patches-containing glycoproteins by mucinases of the human gut. Nat. Commun. 2022;13:4324. doi: 10.1038/s41467-022-32021-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medley BJ, et al. A previously uncharacterized O-glycopeptidase from Akkermansia muciniphila requires the Tn-antigen for cleavage of the peptide bond. J. Biol. Chem. 2022;298:102439. doi: 10.1016/j.jbc.2022.102439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belzer C, et al. Microbial metabolic networks at the mucus layer lead to diet-independent butyrate and vitamin B 12 production by intestinal symbionts. mBio. 2017;8:e00770–00717. doi: 10.1128/mBio.00770-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cani PD, de Vos WM. Next-generation beneficial microbes: the case of Akkermansia muciniphila. Front. Microbiol. 2017;8:1765–1765. doi: 10.3389/fmicb.2017.01765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Everard A, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl Acad. Sci. USA. 2013;110:9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Depommier C, et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat. Med. 2019;25:1096–1103. doi: 10.1038/s41591-019-0495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plovier H, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017;23:107–113. doi: 10.1038/nm.4236. [DOI] [PubMed] [Google Scholar]
- 16.Depommier C, et al. Pasteurized Akkermansia muciniphila increases whole-body energy expenditure and fecal energy excretion in diet-induced obese mice. Gut Microbes. 2020;11:1231–1245. doi: 10.1080/19490976.2020.1737307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reunanen J, et al. Akkermansia muciniphila adheres to enterocytes and strengthens the integrity of the epithelial cell layer. Appl. Environ. Microbiol. 2015;81:3655–3662. doi: 10.1128/AEM.04050-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Earley H, et al. A preliminary study examining the binding capacity of Akkermansia muciniphila and Desulfovibrio spp., to colonic mucin in health and ulcerative colitis. PLoS ONE. 2015;10:e0135280. doi: 10.1371/journal.pone.0135280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Becken, B. et al. Genotypic and phenotypic diversity among human isolates of Akkermansia muciniphila. mBio10.1128/mBio.00478-21 (2021). [DOI] [PMC free article] [PubMed]
- 20.Smith TJ, et al. A mucin-regulated adhesin determines the spatial organization and inflammatory character of a bacterial symbiont in the vertebrate gut. Cell Host Microbe. 2023;31:1371–1385.e1376. doi: 10.1016/j.chom.2023.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karlsson NG, Nordman H, Karlsson H, Carlstedt I, Hansson GC. Glycosylation differences between pig gastric mucin populations: a comparative study of the neutral oligosaccharides using mass spectrometry. Biochem J. 1997;326:911–917. doi: 10.1042/bj3260911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nordman H, et al. Gastric MUC5AC and MUC6 are large oligomeric mucins that differ in size, glycosylation and tissue distribution. Biochem. J. 2002;364:191–200. doi: 10.1042/bj3640191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robbe C, Capon C, Coddeville B, Michalski JC. Structural diversity and specific distribution of O-glycans in normal human mucins along the intestinal tract. Biochem. J. 2004;384:307–316. doi: 10.1042/BJ20040605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robbe C, et al. Evidence of regio-specific glycosylation in human intestinal mucins: presence of an acidic gradient along the intestinal tract *. J. Biol. Chem. 2003;278:46337–46348. doi: 10.1074/jbc.M302529200. [DOI] [PubMed] [Google Scholar]
- 25.Nason R, et al. Display of the human mucinome with defined O-glycans by gene engineered cells. Nat. Commun. 2021;12:4070. doi: 10.1038/s41467-021-24366-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konstantinidi A, et al. Exploring the glycosylation of mucins by use of O-glycodomain reporters recombinantly expressed in glycoengineered HEK293 cells. J. Biol. Chem. 2022;298:101784. doi: 10.1016/j.jbc.2022.101784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Ark KCH, et al. Model-driven design of a minimal medium for Akkermansia muciniphila confirms mucus adaptation. Micro. Biotechnol. 2018;11:476–485. doi: 10.1111/1751-7915.13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ouwerkerk JP, et al. Adaptation of Akkermansia muciniphila to the oxic-anoxic interface of the mucus layer. Appl. Environ. Microbiol. 2016;82:6983–6993. doi: 10.1128/AEM.01641-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Co JY, et al. Mucins trigger dispersal of Pseudomonas aeruginosa biofilms. NPJ Biofilms Microbiomes. 2018;4:23. doi: 10.1038/s41522-018-0067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robbe-Masselot C, et al. Expression of a core 3 disialyl-Le(x) hexasaccharide in human colorectal cancers: a potential marker of malignant transformation in colon. J. Proteome Res. 2009;8:702–711. doi: 10.1021/pr800740j. [DOI] [PubMed] [Google Scholar]
- 31.Xia L. Core 3-derived O-glycans are essential for intestinal mucus barrier function. Methods Enzymol. 2010;479:123–141. doi: 10.1016/S0076-6879(10)79007-8. [DOI] [PubMed] [Google Scholar]
- 32.Narimatsu Y, et al. An atlas of human glycosylation pathways enables display of the human glycome by gene engineered cells. Mol. Cell. 2019;75:394–407.e395. doi: 10.1016/j.molcel.2019.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Haan N, et al. In-Depth profiling of O-glycan isomers in human cells using C18 nanoliquid chromatography-mass spectrometry and glycogenomics. Anal. Chem. 2022;94:4343–4351. doi: 10.1021/acs.analchem.1c05068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lotan R, Skutelsky E, Danon D, Sharon N. The purification, composition, and specificity of the anti-T lectin from peanut (Arachis hypogaea) J. Biol. Chem. 1975;250:8518–8523. doi: 10.1016/S0021-9258(19)40790-4. [DOI] [PubMed] [Google Scholar]
- 35.Chandrasekaran EV, et al. Novel interactions of complex carbohydrates with peanut (PNA), Ricinus communis (RCA-I), Sambucus nigra (SNA-I) and wheat germ (WGA) agglutinins as revealed by the binding specificities of these lectins towards mucin core-2 O-linked and N-linked glycans and related structures. Glycoconj. J. 2016;33:819–836. doi: 10.1007/s10719-016-9678-y. [DOI] [PubMed] [Google Scholar]
- 36.van Passel MW, et al. The genome of Akkermansia muciniphila, a dedicated intestinal mucin degrader, and its use in exploring intestinal metagenomes. PLoS ONE. 2011;6:e16876. doi: 10.1371/journal.pone.0016876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anggara K, et al. Direct observation of glycans bonded to proteins and lipids at the single-molecule level. Science. 2023;382:219–223. doi: 10.1126/science.adh3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Capon C, Maes E, Michalski JC, Leffler H, Kim YS. Sd(a)-antigen-like structures carried on core 3 are prominent features of glycans from the mucin of normal human descending colon. Biochem. J. 2001;358:657–664. doi: 10.1042/bj3580657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Podolsky DK. Oligosaccharide structures of human colonic mucin. J. Biol. Chem. 1985;260:8262–8271. doi: 10.1016/S0021-9258(17)39465-6. [DOI] [PubMed] [Google Scholar]
- 40.Larsson JM, Karlsson H, Sjövall H, Hansson GC. A complex, but uniform O-glycosylation of the human MUC2 mucin from colonic biopsies analyzed by nanoLC/MSn. Glycobiology. 2009;19:756–766. doi: 10.1093/glycob/cwp048. [DOI] [PubMed] [Google Scholar]
- 41.Ottman, N. et al. Genome-scale model and omics analysis of metabolic capacities of akkermansia muciniphila reveal a preferential mucin-degrading lifestyle. Appl. Environ. Microbiol.10.1128/aem.01014-17 (2017). [DOI] [PMC free article] [PubMed]
- 42.Tailford LE, et al. Discovery of intramolecular trans-sialidases in human gut microbiota suggests novel mechanisms of mucosal adaptation. Nat. Commun. 2015;6:7624. doi: 10.1038/ncomms8624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang K, et al. Biochemical characterisation of the neuraminidase pool of the human gut symbiont Akkermansia muciniphila. Carbohydr. Res. 2015;415:60–65. doi: 10.1016/j.carres.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 44.Thomsson KA, Bäckström M, Holmén Larsson JM, Hansson GC, Karlsson H. Enhanced detection of sialylated and sulfated glycans with negative ion mode nanoliquid chromatography/mass spectrometry at high pH. Anal. Chem. 2010;82:1470–1477. doi: 10.1021/ac902602e. [DOI] [PubMed] [Google Scholar]
- 45.Nordman, H. et al. Mucus glycoproteins from pig gastric mucosa: identification of different mucin populations from the surface epithelium. Biochem. J.15, 903–910 (1997). [DOI] [PMC free article] [PubMed]
- 46.Niv Y, Byrd JC, Ho SB, Dahiya R, Kim YS. Mucin synthesis and secretion in relation to spontaneous differentiation of colon cancer cells in vitro. Int. J. Cancer. 1992;50:147–152. doi: 10.1002/ijc.2910500129. [DOI] [PubMed] [Google Scholar]
- 47.Lesuffleur T, et al. Differential expression of the human mucin genes MUC1 to MUC5 in relation to growth and differentiation of different mucus-secreting HT-29 cell subpopulations. J. Cell Sci. 1993;106:771–783. doi: 10.1242/jcs.106.3.771. [DOI] [PubMed] [Google Scholar]
- 48.Madunic K, et al. Colorectal cancer cell lines show striking diversity of their O-glycome reflecting the cellular differentiation phenotype. Cell. Mol. Life Sci. 2021;78:337–350. doi: 10.1007/s00018-020-03504-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Juge N. Microbial adhesins to gastrointestinal mucus. Trends Microbiol. 2012;20:30–39. doi: 10.1016/j.tim.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 50.Cohen LJ, et al. Unraveling function and diversity of bacterial lectins in the human microbiome. Nat. Commun. 2022;13:3101. doi: 10.1038/s41467-022-29949-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mello LV, Chen X, Rigden DJ. Mining metagenomic data for novel domains: BACON, a new carbohydrate-binding module. FEBS Lett. 2010;584:2421–2426. doi: 10.1016/j.febslet.2010.04.045. [DOI] [PubMed] [Google Scholar]
- 52.Larsbrink J, et al. A discrete genetic locus confers xyloglucan metabolism in select human gut Bacteroidetes. Nature. 2014;506:498–502. doi: 10.1038/nature12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakjang S, Ndeh DA, Wipat A, Bolam DN, Hirt RP. A novel extracellular metallopeptidase domain shared by animal host-associated mutualistic and pathogenic microbes. PLoS ONE. 2012;7:e30287. doi: 10.1371/journal.pone.0030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cantarel BL, et al. The Carbohydrate-Active EnZymes database (CAZy): an expert resource for Glycogenomics. Nucleic Acids Res. 2009;37:D233–D238. doi: 10.1093/nar/gkn663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ottman N, et al. Characterization of outer membrane proteome of Akkermansia muciniphila reveals sets of novel proteins exposed to the human intestine. Front. Microbiol. 2016;7:1157. doi: 10.3389/fmicb.2016.01157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luis AS, Hansson GC. Intestinal mucus and their glycans: a habitat for thriving microbiota. Cell Host Microbe. 2023;31:1087–1100. doi: 10.1016/j.chom.2023.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shuoker B, et al. Sialidases and fucosidases of Akkermansia muciniphila are crucial for growth on mucin and nutrient sharing with mucus-associated gut bacteria. Nat. Commun. 2023;14:1833. doi: 10.1038/s41467-023-37533-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crouch LI, et al. Prominent members of the human gut microbiota express endo-acting O-glycanases to initiate mucin breakdown. Nat. Commun. 2020;11:4017. doi: 10.1038/s41467-020-17847-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shon DJ, et al. An enzymatic toolkit for selective proteolysis, detection, and visualization of mucin-domain glycoproteins. Proc. Natl Acad. Sci. USA. 2020;117:21299–21307. doi: 10.1073/pnas.2012196117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Malaker SA, et al. The mucin-selective protease StcE enables molecular and functional analysis of human cancer-associated mucins. Proc. Natl Acad. Sci. USA. 2019;116:7278–7287. doi: 10.1073/pnas.1813020116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guo X, et al. Genome sequencing of 39 Akkermansia muciniphila isolates reveals its population structure, genomic and functional diverisity, and global distribution in mammalian gut microbiotas. BMC Genomics. 2017;18:800. doi: 10.1186/s12864-017-4195-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ouwerkerk, J. P. et al. Comparative Genomics and Physiology of Akkermansia muciniphila Isolates from Human Intestine Reveal Specialized Mucosal Adaptation. Microorganisms10.3390/microorganisms10081605 (2022). [DOI] [PMC free article] [PubMed]
- 63.Kirmiz, N. et al. Comparative genomics guides elucidation of vitamin B(12) biosynthesis in novel human-associated akkermansia strains. Appl. Environ. Microbiol.10.1128/aem.02117-19 (2020). [DOI] [PMC free article] [PubMed]
- 64.Karcher N, et al. Genomic diversity and ecology of human-associated Akkermansia species in the gut microbiome revealed by extensive metagenomic assembly. Genome Biol. 2021;22:209. doi: 10.1186/s13059-021-02427-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun L, et al. Installation of O-glycan sulfation capacities in human HEK293 cells for display of sulfated mucins. J. Biol. Chem. 2022;298:101382. doi: 10.1016/j.jbc.2021.101382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Derrien M, Vaughan EE, Plugge CM, de Vos WM. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int. J. Syst. Evol. Microbiol. 2004;54:1469–1476. doi: 10.1099/ijs.0.02873-0. [DOI] [PubMed] [Google Scholar]
- 67.Stams AJ, Van Dijk JB, Dijkema C, Plugge CM. Growth of syntrophic propionate-oxidizing bacteria with fumarate in the absence of methanogenic bacteria. Appl. Environ. Microbiol. 1993;59:1114–1119. doi: 10.1128/aem.59.4.1114-1119.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miller RS, Hoskins LC. Mucin degradation in human colon ecosystems: Fecal population densities of mucin-degrading bacteria estimated by a “most probable number” method. Gastroenterology. 1981;81:759–765. doi: 10.1016/0016-5085(81)90503-5. [DOI] [PubMed] [Google Scholar]
- 69.Damerell D, et al. The GlycanBuilder and GlycoWorkbench glycoinformatics tools: updates and new developments. Biol. Chem. 2012;393:1357–1362. doi: 10.1515/hsz-2012-0135. [DOI] [PubMed] [Google Scholar]
- 70.Perez-Riverol Y, et al. The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022;50:D543–d552. doi: 10.1093/nar/gkab1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All elements necessary to allow interpretation and replication of results are provided in the Supplementary Information. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE70 partner repository with the dataset identifier PXD051738. Source data are provided with this paper in the Source Data file. Source data are provided with this paper.