Abstract
A cellulosomal scaffoldin gene, termed cipBc, was identified and sequenced from the mesophilic cellulolytic anaerobe Bacteroides cellulosolvens. The gene encodes a 2,292-residue polypeptide (excluding the signal sequence) with a calculated molecular weight of 242,437. CipBc contains an N-terminal signal peptide, 11 type II cohesin domains, an internal family III cellulose-binding domain (CBD), and a C-terminal dockerin domain. Its CBD belongs to family IIIb, like that of CipV from Acetivibrio cellulolyticus but unlike the family IIIa CBDs of other clostridial scaffoldins. In contrast to all other scaffoldins thus far described, CipBc lacks a hydrophilic domain or domain X of unknown function. The singularity of CipBc, however, lies in its numerous type II cohesin domains, all of which are very similar in sequence. One of the latter cohesin domains was expressed, and the expressed protein interacted selectively with cellulosomal enzymes, one of which was identified as a family 48 glycosyl hydrolase on the basis of partial sequence alignment. By definition, the dockerins, carried by the cellulosomal enzymes of this species, would be considered to be type II. This is the first example of authentic type II cohesins that are confirmed components of a cellulosomal scaffoldin subunit rather than a cell surface anchoring component. The results attest to the emerging diversity of cellulosomes and their component sequences in nature.
Bacteroides cellulosolvens is a mesophilic, anaerobic bacterium known to bind tightly and to degrade crystalline forms of cellulose (13, 14, 27). Most of its cellulases appear to be associated with the cell (29). We have shown previously that the bacterium produces cellulosome-like complexes, in both the cell-associated and extracellular fractions (18). The biochemical evidence in favor of a cellulosome in this bacterium includes the production of high-molecular-weight cellulolytic complexes, the cross-reactivity of a high-molecular-weight scaffoldin-like glycopolypeptide with cellulosome-specific antibodies from Clostridium thermocellum, and the presence of cell surface protuberance-like organelles (16, 19). These properties of the cellulase system of B. cellulosolvens indicated the presence of a cellulosome-like entity, similar to the cellulosome of C. thermocellum. Furthermore, extensive structural analysis revealed that B. cellulosolvens cell surface oligosaccharides were strikingly similar to cellulosome-associated oligosugars from C. thermocellum (11, 12).
The cellulosome of C. thermocellum is characterized by a definitive scaffoldin subunit that integrates the enzyme subunits into the complex (10, 17). For this purpose, the scaffoldin subunit contains multiple copies of type I cohesin domains, each of which binds strongly to a complementary dockerin domain, contained as a component part of each enzyme subunit. The scaffoldin of C. thermocellum also contains its own dockerin—a type II dockerin domain—that interacts selectively with complementary type II cohesins, contained on at least three different surface proteins. In C. thermocellum, the type I cohesin-dockerin interaction apparently defines the incorporation of the enzyme subunits into the cellulosome, whereas the type II cohesin-dockerin interaction appears to mediate the anchoring of the cellulosome onto the cell surface (1, 6).
We have recently pursued a genetic program for expanding our earlier biochemical findings regarding the distribution and variety of cellulosomes in various microbial species (3). For this purpose, we have searched for the elusive scaffoldin component—the definitive subunit which integrates the various cellulosomal enzymes into the complex—in bacteria that were previously suspected of harboring cellulosomes. In this framework, we recently reported a unique scaffoldin sequence in a related bacterium, Acetivibrio cellulolyticus, which includes a catalytic domain as an integral part of its primary structure (7). Otherwise, the A. cellulolyticus scaffoldin resembles that of C. thermocellum in that it includes a C-terminal type II dockerin domain and an internal cellulose-binding domain (CBD), as opposed to the other known clostridial cellulosomes from C. cellulolyticum, C. cellulovorans, and C. josui (15, 31, 37).
Both A. cellulolyticus and B. cellulosolvens have been shown to belong to the clostridial assemblage on the basis of 16S rRNA (23). Nevertheless, it is becoming clear that the phylogenetic relationship between cellulolytic bacteria does not necessarily reflect the characteristics of their respective cellulase systems. In this communication, we confirm that B. cellulosolvens produces a bona fide cellulosome. The very large scaffoldin gene, termed cipBc, was sequenced and found to contain type II cohesins rather than the type I cohesins that characterize the other known scaffoldins. Moreover, the number of cohesins is the highest thus far encountered in a single polypeptide. In addition, the scaffoldin contains an internal CBD and a C-terminal dockerin domain but lacks an associated domain X or hydrophilic domain that has been found in all scaffoldins thus far described.
MATERIALS AND METHODS
Organism and growth conditions.
B. cellulosolvens ATCC 35603 (28) was purchased from the German Collection of Microorganisms and Cell Cultures (DSMZ; Braunschweig, Germany). Cells were grown anaerobically at 35°C in serum bottles containing the DSMZ-recommended medium (medium 315 for B. cellulosolvens), which included either 0.3% cellobiose (Sigma Chemical Co., St. Louis, Mo.) or cellulose (Avicel; E. Merck AG, Darmstadt, Germany) as the carbon source. Cells were grown to mid-exponential phase (36 to 48 h), the culture was centrifuged (10,000 × g, 10 min), and both supernatant fluids and cells were stored for further use.
Identification of candidate scaffoldin sequences from B. cellulosolvens.
The procedure followed for identifying candidate scaffoldin bands from cell-free culture fluids of B. cellulosolvens was essentially as described earlier for A. cellulolyticus (7). Briefly, cellulose-adsorbed proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), blotted onto nitrocellulose sheets, and stained using an α-galactose-specific lectin from Griffonia simplicifolia and an antibody preparation against the C. thermocellum cellulosome. Candidate protein bands, recognized by both probes, were extracted from the gel. The extracted proteins were subjected to proteolysis, and the resultant peptides were resolved by reverse-phase high-pressure liquid chromatography and were collected (24). The purified peptide peaks were analyzed and sequenced by Edman degradation (Protein Center, Technion, Haifa, Israel).
DNA preparation.
B. cellulosolvens genomic DNA was isolated as described earlier (26). Plasmid DNA was purified using the High Pure plasmid isolation kit (Boehringer Mannheim Corp., Indianapolis, Ind.). A Spin-X microcentrifuge filter (0.2-μm-pore-size nylon filter; Costar, Corning, N.Y.) was used for DNA purification from agarose gels.
Consensus PCR.
An expand high-fidelity PCR system (Boehringer Mannheim) was used in all PCRs. PCR was performed using a Mastercycler personal instrument (Eppendorf, Hamburg, Germany), programmed as follows: a 3-min predenaturation step at 95°C was followed by 30 cycles comprising a 45-s denaturation step at 94°C, an annealing step of 45 to 60 s at 40 to 60°C (depending on the primer), and an extension step at 72°C for 1 to 3 min (depending on the length of the product). Degenerate oligonucleotide primers were generated from the peptide sequence (see Table 1). PCR was carried out under various annealing temperatures (40 to 60°C) in order to obtain specific amplified products using the genomic DNA as template. Purified PCR products were cloned into the pGEM-T Easy vector system (Promega, Madison, Wis.) and were sequenced. Sequences were compared with GenBank and known cellulosome-related proteins.
TABLE 1.
Primers used in this study
| Name | Nucleotide sequencea | Locationb | Comments |
|---|---|---|---|
| SEQ3-F | ATNTTYGGNMGNACNTAYATGAAYYT | Initially unspecified cohesin | Degenerate primer derived from initial protease digest of candidate scaffoldin |
| CBD-R | TGWKYRWARTTWSWCCAGTC | CBD | Degenerate primer designed on the basis of known family III CBD sequences |
| En-F | TTRTCNACRTCRAADATCCA | N terminus | Degenerate primer that aligned with N-terminal portion of signal sequence |
| 24C-R | CATATTCAGGAGCTGATGCAT | Coh-2 | To amplify N terminus of CipBc |
| 24N-R | TTTTCTGCTGCTCCAGAATTC | Coh-2 | To amplify N terminus of CipBc |
| H706N-R | GTCTGTGATGGTGGTAGTGA | Linker between Coh-3 and Coh-4 | Sequencing |
| H706N-F | ATTGGTTCAGGTGTAACAGC | Linker between Coh-3 and Coh-4 | Sequencing |
| COH-Fn | CAGCTCGAGGGTTCAGGAGTAGTATCAACT | Coh-5 | Cohesin expression |
| COH-Rn | GTGGATCCTTACTATCCGTTTATTGAAGAAGCCTG | Coh-5 | Cohesin expression |
| COH-F | AGACCATGGGTTCAGGAGTAGTAGCAAC | Coh-5 | Cohesin expression |
| COH-R | AGTCTCGAGTCCGTTTATTGAAGAAGCCTG | Coh-5 | Cohesin expression, inverse PCR |
| 12F1 | TTAACAGCGACTTGAAGTT | CBD | Inverse PCR, sequencing |
| 12R2 | AGCTTGCTGCTGCACCATT | CBD | Inverse PCR, sequencing |
| 12R1 | GATCATATGTTGATGATCCTG | CBD | Inverse PCR, sequencing |
| 12F2 | CAATTCAGGGTAGAGTTGCA | CBD | Inverse PCR, sequencing |
| SC-F | GGTTCAACATCCATTAAGTTAG | Coh-6 | Genomic-walking PCR |
| 3601-F | ATGGAGTTCAGTTGGACAAT | Coh-7 | Sequencing |
| 4041-F | ACTGAAACTAAAGTATTATTGAA | Coh-8 | Sequencing |
| C-R2 | CAACAACTGGAGCATTAACT | Linker between Coh-10 and Coh-11 | Genomic-walking PCR |
| C-R1 | ATCATTATCAATAGCTGTCAA | Coh-11 | Genomic-walking PCR |
| C-F1 | TCACAAGGTGTATTGAACTT | Coh-11 | Genomic-walking PCR |
| C-F2 | GAACCTTTCAGCATACAGAG | Coh-11 | Genomic-walking PCR |
| M13-R1 | AGCGGATAACAATTTCACACAGGA | pUC19 | Genomic-walking PCR |
| M13-R2 | AACAGCTATGACCATGATTACG | pUC19 | Genomic-walking PCR |
| M13-F1 | GTTTTCCCAGTCACGACGTTG | pUC19 | Genomic-walking PCR |
| M13-F2 | TGTAAAACGACGGCCAGT | pUC19 | Genomic-walking PCR |
Abbreviations for degenerate nucleotides: K, G or T; Y, C or T; W, A or T; R, A or G; S, C or G. N represents A, C, G, or T.
The exact locations of relevant primers are shown in Fig. 3.
Genomic-walking PCR.
Two-step PCRs were applied to amplify the forward and backward sequences of a known region. First, PCR was performed with a combination of a specific primer (Table 1), designed from a previously sequenced region of the target gene, and a pUC19 primer (M13-F1 or M13-R1). Plasmid libraries were used as templates. A 100-fold dilution of the first PCR product served as a template for the second PCR by using a nested primer, designed from an inner sequence of the known region, and a universal pUC19 primer (M13-F2 or M13-R2).
Two-step inverse PCR.
Genomic DNA (5 μg) was digested extensively by PstI and self-ligated by T4 DNA ligase at 4°C overnight. The ligation product was used as a template for the first PCR. Subsequently, the first PCR product (diluted 100-fold) was used as a template for the second PCR employing a nested primer (Table 1), essentially as described above for genomic-walking PCR.
Construction of plasmid genomic libraries.
Four restriction enzymes (EcoRI, HindIII, PstI, and SacI) were applied to construct genomic libraries. Appropriately sized fragments that interacted with a digoxigenin-labeled 1.2-kb scaffoldin-associated DNA probe (see Results) were determined by Southern blotting as described previously (7). B. cellulosolvens genomic DNA (10 μg) was cleaved by restriction enzymes and ligated (4°C overnight) with predigested and alkaline phosphatase-treated pUC19. For genomic-walking PCR, the ligation product was used as the PCR template. For screening a genomic library, the ligation mixture was transferred into Escherichia coli strain XL-1-Blue (Stratagene, La Jolla, Calif.) and was plated onto Luria-Bertani plates containing ampicillin, 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal), and isopropyl-β-d-thio-galactopyranoside (IPTG). The same digoxigenin-labeled probe was used for screening, and positive clones were verified by dot hybridization.
Protein expression and purification.
One of the cohesins from the fully sequenced scaffoldin (cohesin 5) was amplified by PCR using appropriate primers (Table 1), and a His tag was attached to its C terminus by cloning the fragment into the pET28a expression vector (Novagen, Madison, Wis.) using restriction enzymes NcoI and XhoI. In order to prepare the same cohesin with an N-terminal His tag, the fragment, amplified using a different pair of primers, was also cloned into pET14b (Novagen) with restriction enzymes XhoI and BamHI. The respective recombinant construct was transferred into E. coli strain BL21(DE3) (Stratagene), and the protein was expressed at 15°C, essentially according to Gal et al. (9), using an IPTG concentration of 0.1 mM. The supernatant fluids, containing soluble expressed protein, were loaded onto a Ni-nitrilotriacetic acid–agarose column (Qiagen GmbH, Hilden, Germany), washed with imidazole-free wash buffer (50 mM sodium phosphate buffer [pH 6]–10% glycerol–300 mM NaCl), and eluted using elution buffer (wash buffer plus 250 mM imidazole). Protein was purified further by gel filtration fast protein liquid chromatography on Superdex 75 (Pharmacia, Uppsala, Sweden). Both N- and C-terminal His-tagged recombinant proteins proved suitable for subsequent studies.
Western blotting.
Samples of cell-free, cellulose-absorbed B. cellulosolvens proteins (2 μg), purified C. thermocellum cellulosome (2 μg), and His-tagged cohesin 5 (1 μg) were subjected to SDS–6% PAGE and transferred electrophoretically onto nitrocellulose membranes. The blots were blocked with blocking buffer containing 2% bovine serum albumin, 25 mM CaCl2, and 25 μg of His-tagged cohesin 5/ml in Tris-buffered saline (50 mM Tris-HCl [pH 7.5]–150 mM NaCl) and were washed with Tris-buffered saline. Anti-His-horseradish peroxidase antibody (Invitrogen, Carlsbad, Calif.) was used for detection according to the manufacturer's instructions.
Phylogenetic analysis.
Phylogenetic trees were generated using the ClustalW program (http://www2.ebi.ac.uk/clustalw/). Protein sequences were obtained from the GenBank website (http://www.ncbi.nlm.nih.gov/) or via the Carbohydrate-Active Enzymes server designed by Coutinho and Henrissat (CAZy and CAZyModO websites: http://afmb.cnrs-mrs.fr/∼pedro/CAZY/db.html and http: //afmb.cnrs-mrs.fr/∼pedro/DB/db.html, respectively.) The five-letter abbreviations of bacterial species were selected according to the SwissProt convention (http://www.expasy.ch/sprot/). See also phylogenetic treatment of cellulosomal components in previous publications (2, 7).
Nucleotide sequence accession number.
The DNA sequence for the cipBc gene reported herein was deposited in the GenBank database under accession number AF224509.
RESULTS
Identification of putative B. cellulosolvens scaffoldin subunit.
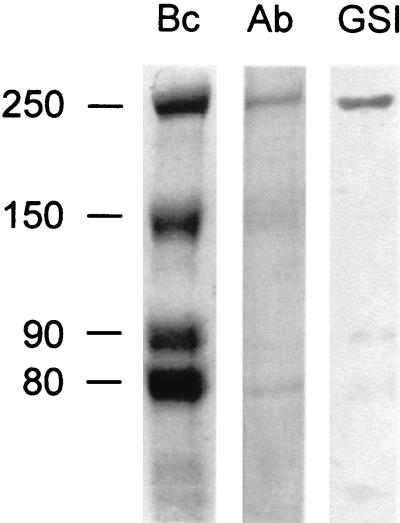
Proteins from the culture fluids of B. cellulosolvens were concentrated and separated by SDS-PAGE. An ∼250-kDa band was identified as a likely candidate for further analysis on the basis of binding to cellulose, immunochemical cross-reactivity with the C. thermocellum scaffoldin, and interaction with G. simplicifolia lectin GS-I (Fig. 1). The designated band was extracted from the gel and subjected to proteolysis, and the amino acid sequence of selected peptides was determined. In one of these peptides, a 16-residue stretch (GTLTFGRTYMNLDSYK) exhibited 50% homology with a cohesin domain from C. thermocellum, thereby corroborating that this peptide was indeed of scaffoldin origin. On the basis of this conserved sequence, a degenerate primer was designed (see “Sequencing strategy” below). Another degenerate primer was designed from a particularly conserved region of the known family IIIa and IIIb CBDs. These two primers provided a handle for identifying the gene encoding the B. cellulosolvens scaffoldin subunit.
FIG. 1.
Identification of scaffoldin-like polypeptides from B. cellulosolvens. Bc, Coomassie brilliant blue-stained SDS-PAGE-separated proteins from concentrated cell-free culture fluids; Ab, Western blot analysis using antibodies specific for the scaffoldin subunit from C. thermocellum; GSI, blotted protein bands cross-reacting with the GS-I lectin from G. simplicifolia. The relative molecular weights (103) of the designated bands are indicated.
Sequencing strategy.
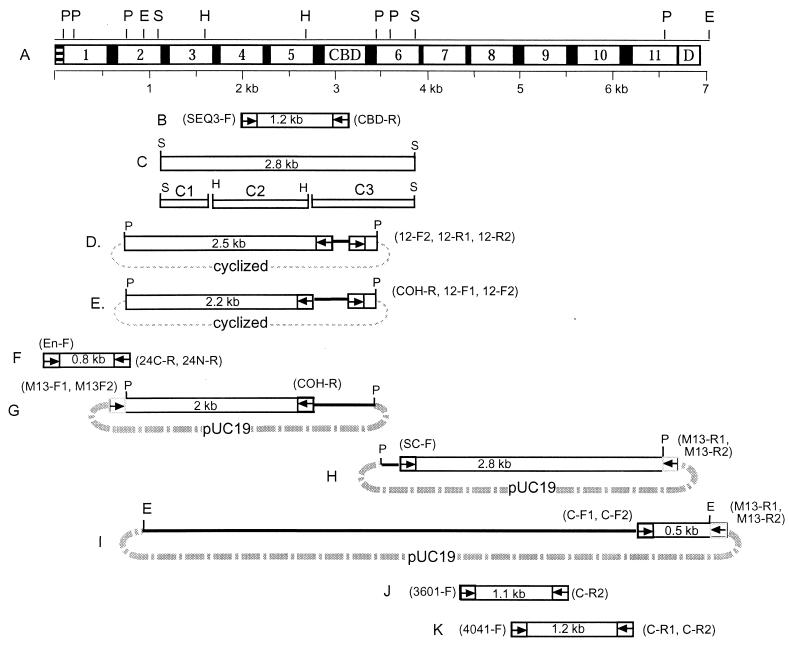
The overall strategy for sequencing the ∼7-kb cipBc gene is presented schematically in Fig. 2, and the primers used in this study are listed in Table 1. Briefly, the following two degenerate primers were initially designed: (i) SEQ3-F, from the above-described peptide sequence (TFGRTYMNL), obtained from the proteolyzed candidate scaffoldin; and (ii) CBD-R (DWSNYTQ), from the conserved region of the family III CBDs. Using these degenerate primers, a 1.2-kb PCR product (Fig. 2B) was amplified from B. cellulosolvens genomic DNA. Sequence analysis showed that this DNA segment indeed contained two type II cohesin domains and a family III CBD. This PCR product was then applied as a probe for Southern blotting and library screening, which resulted in the cloning of a 2.8-kb SacI fragment (Fig. 2D). Two fragments (Fig. 2D and E) were amplified by two-step inverse PCR. In the N terminus, a 0.8-kb PCR fragment (Fig. 2F) was amplified and sequenced by using primers 24C-R and 24N-R and an unspecific degenerate primer (En-F). The remainder of the gene was sequenced by genomic-walking and consensus PCR, as illustrated schematically in Fig. 2G to K. The entire sequence was verified in both directions by overlapping segments.
FIG. 2.
Domain organization of CipBc and overview of sequencing strategy. (A) Domain architecture of CipBc. The polypeptide chain includes 11 cohesins (Coh-1 through Coh-11), an internal CBD, linkers (black), a single C-terminal dockerin domain (D), and an N-terminal signal peptide (stripes). Restriction enzyme sites (E, EcoRI; H, HindIII; P, PstI; S, SacI) and a DNA scale bar are shown. (B) A 1.2-kb PCR fragment obtained with degenerate primers based on peptide sequencing. (C) Top, a 2.8-kb fragment obtained from a SacI genomic library; C1 to C3, subclones of SacI fragment C obtained by using HindIII. (D and E) Respective 2.5- and 2.2-kb fragments, amplified by two-step inverse PCR from PstI-digested and self-ligated genomic DNA. (F) A 0.8-kb PCR product. (G and H) Respective 2- and 2.8-kb fragments, amplified by genomic-walking PCR from the PstI-pUC19 minigenomic library. (I) A 0.5-kb fragment, amplified from the EcoRI-pUC19 minigenomic library. (J and K) Respective 1.1- and 1.2-kb PCR fragments, obtained from genomic DNA. (B to K) An “F” label on a primer indicates forward; “R” indicates reverse direction. Two primers shown on one end indicate two-step PCR. Arrows indicate the location and direction of primers. Dotted lines indicate a cyclic DNA fragment. Broken lines indicate the pUC19 vector. For primer sequences, see Table 1.
Brief description of the cipBc scaffoldin gene.
The various sequences obtained as described in the previous section were compiled in order, revealing a 7-kb DNA sequence, which contained a single open reading frame (Fig. 3). The deduced peptide (excluding the signal sequence) contained 2,292 amino acids with an estimated molecular mass of 242,437 kDa. The signal sequence was consistent with those of other known scaffoldins (7), and their comparison suggested that one or two of the initial residues of the gene remained unsequenced.
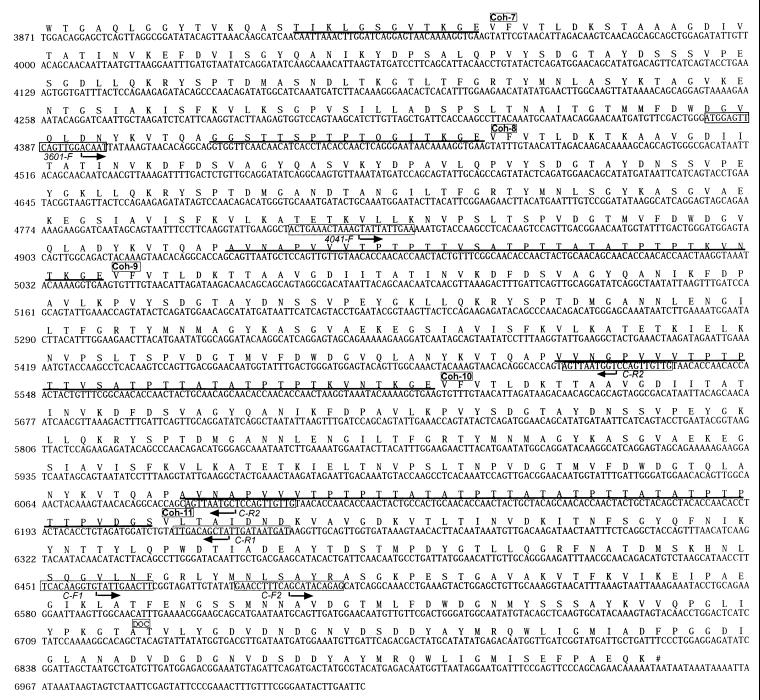
FIG. 3.
Nucleotide and deduced amino acid sequences of the B. cellulosolvens scaffoldin subunit (CipBc). The presumed beginning of each cohesin, CBD, or dockerin domain is labeled. The signal sequence is shown in italics, and the intermodular linker sequences are underlined. Primer sequences are boxed, and their directionality is indicated by an arrow.
CipBc cohesin domains.
The CipBc scaffoldin is organized into 11 type II cohesin domains, an internal family III CBD, and a C-terminal dockerin domain (Fig. 2A). The CBD is preceded by 5 cohesin domains and followed by another 6 cohesins downstream. The domains are separated by distinctive linker sequences, most of which are rich in proline and threonine residues (Fig. 3). In length and composition, the CipBc linkers are quite similar to those of the CipA scaffoldin from C. thermocellum. In contrast to all other scaffoldins thus far described, CipBc lacks a domain X or similar type of hydrophilic domain, suggesting that the latter types of domain may be indicative of scaffoldin components but not definitive.
Unlike all other scaffoldin-based cohesins described to date, the cohesin domains of CipBc can clearly be classified as type II cohesins (Fig. 4). Phylogenetic analysis places all 11 CipBc cohesins in a cluster in close proximity to the type II cohesins from C. thermocellum anchoring proteins (Fig. 4A). Cohesins 8, 9, and 10 form a well-conserved group, as do cohesins 2 through 6. As in many other scaffoldins, the N- and C-terminal cohesins are the most divergent. The sequences of the type II cohesin domains thus far described all contain a characteristic insert and distinctive set of conserved residues, which are largely different than those of the type I cohesins.
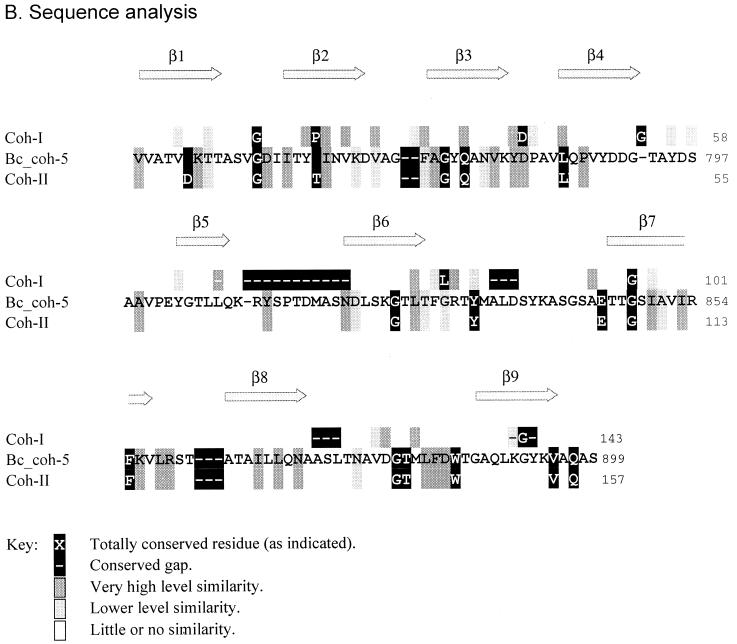
FIG. 4.
Assignment of the B. cellulosolvens cohesins as type II cohesins. (A) Phylogenetic analysis of CipBc cohesin sequences. The type I cohesins include those from the other known scaffoldins and two other cellulase-binding surface proteins (OlpA from C. thermocellum and OrfX from C. cellulolyticum). In addition to the CipBc cohesins, type II cohesins include the anchoring proteins from C. thermocellum and a putative anchoring protein from A. cellulolyticus. See Materials and Methods for sources of the sequences and abbreviations used in this and subsequent figures. The scale bar in this and subsequent figures indicates percentage (0.1) of amino acid substitutions. (B) Alignment of CipBc cohesin sequences versus types I and II cohesins (Coh-I and Coh-II, respectively). The positions of the β strands, known from the crystal structure of type I cohesins from C. thermocellum, are also shown. The sequence for cohesin 5 (Bc_coh-5) is shown as representative of the 11 B. cellulosolvens cohesins. The conserved sequence identities, similarities, and gaps of the B. cellulosolvens cohesins coincide with those of the type II cohesins.
Figure 4B shows the pattern of similarity along the CipBc cohesin sequence, compared with the pattern in the type I and type II cohesins. The relative level of similarity of the residues at any given position among all 11 CipBc cohesins is shaded according to the key. Totally conserved positions of the given cohesins are shown in the figure with the conserved residue or gaps designated in white letters on a black background. The sequence of CipBc cohesin 5 is illustrated as representative of all 11 cohesins. It is clear from Fig. 4B that the CipBc cohesins can be classified as type II cohesins. All of the residues conserved in the previously described type II cohesins are similarly conserved in the CipBc cohesins. The large gap in the loop region between β-strands 5 and 6 of type I cohesins is not a characteristic of the CipBc cohesins, nor are the smaller gaps that distinguish the type I from the type II cohesins. Moreover, the positions of lower similarity and divergence, observed in the known type II cohesins, are strictly followed among the 11 CipBc cohesins. The combined sequence analysis in Fig. 4B confirms the phylogenetic analysis in Fig. 4A, and it can hence be concluded that the CipBc cohesins are indeed type II cohesins.
CipBc CBD.
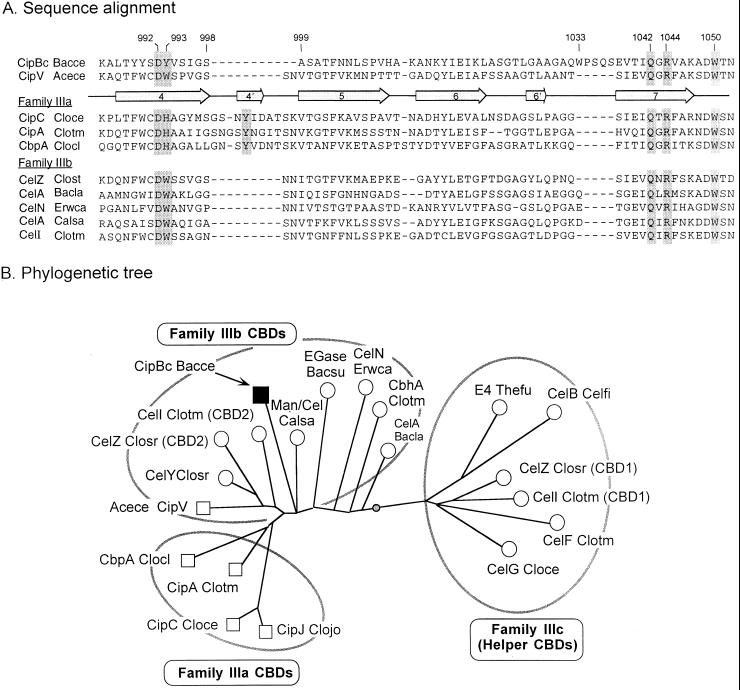
The internal family III CBD of CipBc exhibits several unusual features worth noting. The large 11-residue gap in the sequence (between residues 998 and 999) is consistent with the sequence of a family IIIb CBD as opposed to a family IIIa (Fig. 5A). This conclusion is further supported by phylogenetic analysis of the CipBc sequence together with other family III CBDs (Fig. 5B). The phylogenetic tree shown in the figure places the CipBc CBD in the midst of family IIIb and relatively distant from family IIIa and IIIc CBDs. CipBc is the second scaffoldin CBD, in addition to that of CipV from A. cellulolyticus (7), that is classified as family IIIb instead of IIIa, thus substantiating the notion that the scaffoldin CBDs are more diverse than originally considered. In this context, closer inspection of the CipBc CBD sequence (Fig. 5A) reveals an interesting substitution at one of the postulated cellulose-binding residues (position 993), wherein a tyrosine replaces the histidine of the family IIIa CBDs or the tryptophan of the family IIIb CBDs. Nevertheless, the appearance of tyrosine in this position is consistent with its functioning in cellulose binding as part of a planar aromatic strip as described by Tormo et al. (39). Another interesting feature of the CipBc CBD is a 5-residue insert that includes a tryptophan (W1033). This insert appears in the sequence immediately before β-strand 7. With reference to the structural model for the CipA CBD from C. thermocellum (39), this would imply that the insert is placed near the C-terminal end of β-strand 4 of the putative cellulose-binding face of the molecule. This location implies further that the insert could compensate structurally for the above-mentioned 11-residue deletion. Moreover, W1033 may function as an additional cellulose-binding residue, resembling perhaps the contribution of the deleted tyrosine. Further insight into the implications of these proposals awaits three-dimensional structure analysis.
FIG. 5.
Relationship of the CipBc CBD to other scaffoldin and nonscaffoldin family III CBDs. (A) Sequence alignment of portions of selected family III CBDs, encompassing β-strands 4 through 7 (enumerated arrows). The CipBc CBD and the recently sequenced Acetivibrio CipV CBD (7) are compared to other known scaffoldin CBDs from family IIIa and nonscaffoldin family IIIb CBDs. Shaded residues indicate proposed cellulose-binding residues (39), and numbers refer to presumed positions on the mature CipBc protein. Dashes indicate gaps. (B) Phylogenetic analysis of the family III CBDs. Scaffoldin CBDs are shown as squares. The weighted centroid is shown as a shaded circle on the branch connecting the family IIIb and IIIc CBDs. This analysis is based on a similar analysis of family III CBDs (7).
CipBc dockerin domain.
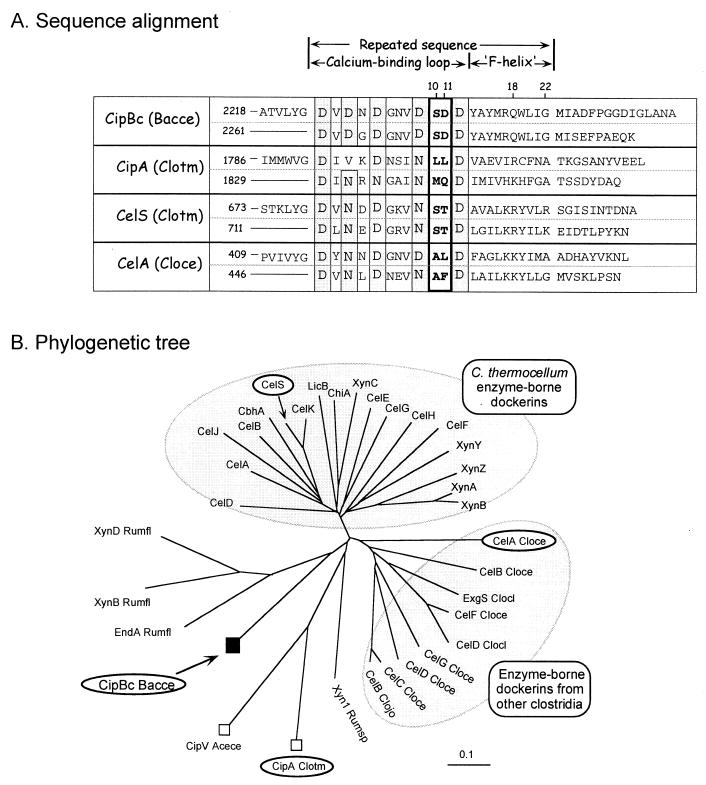
The CipBc scaffoldin carries a dockerin domain at its C terminus. Thus far, only two other scaffoldins, CipA from C. thermocellum and CipV from A. cellulolyticus, have been described which also exhibit a dockerin domain (also at their C termini). The CipBc dockerin sequence shows the normal pattern of a dockerin (Fig. 6A), including a near-perfect repeated sequence. Each repeat displays a typical “F-hand” modification of the EF-hand motif (30), containing a characteristic calcium-binding loop followed by a helix as predicted by the PHD program (32–34). All of the candidate calcium-binding residues are aspartic acids, with no asparagines or other substitutions. The proposed recognition dyads in positions 10 and 11 of the repeated sequence (SD/SD) are similar to those of the C. thermocellum cellulosomal enzymes (25, 30). Also of interest are residues in positions 18 and 22, which we have recently considered as additional determinants that might contribute further to the selectivity of the cohesin-dockerin interaction (A. Mechaly, unpublished results). The latter positions are distinct from those of the C. thermocellum enzymes. Phylogenetic analysis (Fig. 6B) of various dockerin sequences places the CipBc dockerin in a very dispersed branch, which includes ruminococcal enzyme-borne dockerins and the two known scaffoldin dockerins. Distantly separated from this branch are the two large clusters of clostridial enzyme-borne dockerins.
FIG. 6.
Relationship of the CipBc C-terminal dockerin with other dockerins of scaffoldin and nonscaffoldin origin. (A) Sequence alignment of the dockerin domain from CipBc with the type II dockerin from the CipA C. thermocellum scaffoldin and their relationship to selected type I dockerins from various cellulosomal enzyme subunits. Presumed calcium-binding residues are shaded, and proposed recognition residues are indicated in bold. (B) Phylogenetic analysis of selected dockerins. The dockerins included in panel A for sequence alignment are circled. Scaffoldin-borne dockerins are indicated by squares. The scale bar indicates percentage (0.1) of amino acid substitutions.
Interaction of CipBc cohesin domain with B. cellulosolvens components.
In order to identify target proteins that bind the repeating CipBc cohesins, a recombinant form of a representative cohesin domain was prepared and used as a probe for affinity-blotting experiments. For this purpose, cohesin 5 from the cipBc gene was subcloned and fused to a His tag (both N- and C-terminally tagged proteins were designed and prepared). The overexpressed proteins were purified by metal-chelate chromatography on a Ni-nitrilotriacetic acid column. Both of the purified His-tagged cohesin 5 probes proved effective for identification of interacting components derived from spent growth media of cultured B. cellulosolvens. Peroxidase-conjugated antibodies against the His tag were used to detect the cohesin-labeled bands.
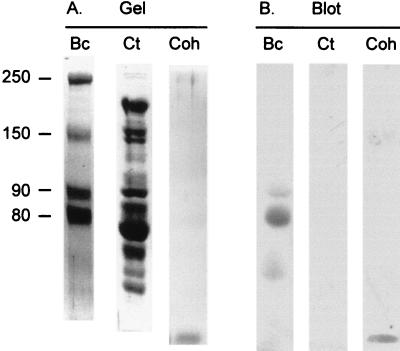
As seen in Fig. 7, the expressed cohesin interacted with at least three different bands, consistent with the notion that the cohesin recognizes several enzymes—presumably subunits of a cellulosome. The major band at approximately 80,000 kDa was extracted from the gel and subjected to proteolysis, and the amino acid sequences of selected peptides were determined. In one of these peptides, a 10-residue stretch exhibited clear homology with a highly conserved region of the family 48 glycosyl hydrolases (Table 2). This type of enzyme is known to be a critical component of every cellulosome thus far described. It therefore appears that the CipBc cohesin 5 (and presumably the other CipBc type II cohesins) binds to enzymes. It would thus follow that these enzymes bear dockerins and that, unlike the enzyme-borne dockerins from other cellulosome species, the CipBc dockerins would be classified as type II. Verification of this premise awaits more extensive sequencing of the cellulosomal enzyme subunits from B. cellulosolvens.
FIG. 7.
Identification of cohesin-binding polypeptides derived from cellobiose-grown cells of B. cellulosolvens. (A) Coomassie brilliant blue-stained SDS-PAGE-separated proteins from cellulose-bound extracellular fraction. (B) Blot of gel in panel A, transferred electrophoretically onto nitrocellulose strips, probed with the His-tagged cohesin 5, and stained immunochemically, using an anti-His-tag antibody. Lanes: Bc, cellulose-adsorbed B. cellulosolvens proteins; Ct, purified cellulosome from C. thermocellum; Coh, purified recombinant type II cohesin 5, containing a His tag. The relative molecular weights (103) of the designated bands are indicated.
TABLE 2.
Sequence of peptide fragment from cellulose-binding cell-derived components of B. cellulosolvens and its similarity to a conserved segment of family 48 glycosyl hydrolases
| Bacterium | Enzyme | Sequence of fragment |
|---|---|---|
| Bacteroides cellulosolvens | “CelS”a | WIFDVDNWYK |
| Clostridium thermocellum | CelSa | WLMDVDNWYG |
| Clostridium cellulolyticum | CelFa | WILDVDNWYG |
| Clostridium josui | CelDa | WLLDVDNWYG |
| Clostridium cellulovorans | ExgSa | WLLDVDNWYG |
| Caldocellum saccharolyticum | CelA | WLMDVDNWYG |
| Anaerocellum thermophilum | CelA | WLMDVDNWYG |
| Clostridium stercorarium | Cbh | WLLDVDNWYG |
| Cellulomonas fimi | CbhB | WLADVDNIYG |
Cellulosomal enzyme.
DISCUSSION
In the initial stages of this project, following the sequencing of the first several domains of CipBc from B. cellulosolvens, it was puzzling to discover, in the same open reading frame, the presence of type II cohesin domains together with a family III CBD (3). Until then, CBDs had been shown to be components of either free glycosyl hydrolase enzymes or scaffoldin subunits, the latter of which contain type I cohesins (38). The type I cohesins were shown to be selective for type I dockerins of the cellulosomal enzymes, thereby serving to mediate cellulosome assembly. Type II cohesin domains have thus far been reported in anchoring proteins from only one bacterium, C. thermocellum (6, 21, 22). Since the type II cohesins were demonstrated to be specific for the type II scaffoldin dockerin (35), the suppositions that this would represent a more general theme in other microbes and that the type II cohesin-dockerin interaction would mediate the attachment of the cellulosome to the cell surface remained.
The compelling question was whether this protein represented a new kind of cellulosomal scaffoldin which contains type II rather than type I cohesins or whether it represented a novel CBD-containing, cell surface anchoring protein. In either case, the mature protein would represent a unique variation of the cellulosome theme. The final sequencing of the complete CipBc and biochemical analysis have resolved the enigma: despite its array of type II cohesins, this protein can be classified as a scaffoldin that binds to various cellulosomal enzymes, in particular a distinctive family 48 glycosyl hydrolase. In this context, it is interesting to note the restricted number of enzymes, which appear to be integrated into the B. cellulosolvens cellulosome, compared to C. thermocellum and C. cellulolyticum. The clear preponderance of the 80-kDa family 48 glycosyl hydrolase may suggest that the B. cellulosolvens cellulosome is an especially large protein complex, comprised mainly of the latter enzyme, reinforced by few additional enzymes. An enzyme of the same family also appears to be a major component of other cellulosomal systems, notably the C. thermocellum cellulosome (20, 40). Presumably the large size and numerous cohesins of CipBc would be of primary importance to the bacterium, although the precise purpose of producing such an unusual and complex protein is currently unclear.
The family III CBD of CipBc would be expected to function in the multiplicity of roles performed by a scaffoldin CBD, which include the selective targeting to the substrate of cellulosomal enzymes and probably the intact B. cellulosolvens cell (4). In fact, previous biochemical analyses of the B. cellulosolvens system suggested that the putative cellulosome of this organism is associated very strongly with the cell surface (18). Based on the C. thermocellum system (5, 6, 8, 36), the C-terminal CipBc dockerin would presumably interact with an as-yet unidentified cohesin or set of cohesins of cell surface-based anchoring protein(s).
Another question is whether the CipBc dockerin is a type I or type II dockerin domain. It is very difficult on the basis of differential sequence analysis alone to distinguish between the two types of dockerin, particularly since only two examples of the type II dockerins are known. Thus, the presumed anchoring proteins could bear cohesins of either type I or type II. If they prove to be of type I, then the status of B. cellulosolvens could be the reverse of the C. thermocellum cellulosome, wherein the type I cohesin-dockerin interaction mediates incorporation of enzymes into the complex and the type II interaction fastens the cellulosome to the cell surface. If, on the other hand, the putative anchoring cohesins prove to be of type II, this would suggest that in B. cellulosolvens two kinds of type II cohesins exist in the same cellulosome system, each of which exhibits a different specificity. In the final analysis, such questions will be resolved by identifying and sequencing the interacting components of the B. cellulosolvens system. Future work will include a search for such components.
The results of this work underscore the emerging diversity of the cellulosomes. The scaffoldins appear to have the potential to be extremely diverse in their size, structural organization, and disposition of their modular components. Since we are still at an early stage of discovery, it is difficult to generalize too strictly regarding their classification and anticipated features. To date, the known scaffoldins have been shown to contain a CBD for binding to the substrate and multiple cohesin domains for integrating the dockerin-containing cellulosomal enzymes. Future description of new microbial scaffoldins will contribute further evidence concerning the similarity and diversity among the cellulosome systems in nature.
ACKNOWLEDGMENTS
This work was supported by a contract from the European Commission (Biotechnology Programme, BIO4-97-2303) and by grants from the Israel Science Foundation (administered by the Israel Academy of Sciences and Humanities, Jerusalem). Additional support was provided by the Otto Meyerhof Center for Biotechnology, established by the Minerva Foundation, Germany.
The authors appreciate the expert technical assistance of Rina Kenig. We also thank Adva Mechaly and Shula Michaeli for critical reading of the manuscript.
REFERENCES
- 1.Bayer E A, Chanzy H, Lamed R, Shoham Y. Cellulose, cellulases and cellulosomes. Curr Opin Struct Biol. 1998;8:548–557. doi: 10.1016/s0959-440x(98)80143-7. [DOI] [PubMed] [Google Scholar]
- 2.Bayer E A, Ding S-Y, Mechaly A, Shoham Y, Lamed R. Emerging phylogenetics of cellulosome structure. In: Gilbert H J, Davies G J, Henrissat B, Svensson B, editors. Recent advances in carbohydrate bioengineering. Cambridge, England: The Royal Society of Chemistry; 1999. pp. 189–201. [Google Scholar]
- 3.Bayer E A, Ding S Y, Shoham Y, Lamed R. New perspectives in the structure of cellulosome-related domains from different species. In: Ohmiya K, Hayashi K, Sakka K, Kobayashi Y, Karita S, Kimura T, editors. Genetics, biochemistry and ecology of cellulose degradation. Tokyo, Japan: Uni Publishers Co., Ltd.; 1999. pp. 428–436. [Google Scholar]
- 4.Bayer E A, Morag E, Shoham Y, Tormo J, Lamed R. The cellulosome: a cell-surface organelle for the adhesion to and degradation of cellulose. In: Fletcher M, editor. Bacterial adhesion: molecular and ecological diversity. New York, N.Y: Wiley-Liss, Inc.; 1996. pp. 155–182. [Google Scholar]
- 5.Bayer E A, Shimon L J W, Lamed R, Shoham Y. Cellulosomes: structure and ultrastructure. J Struct Biol. 1998;124:221–234. doi: 10.1006/jsbi.1998.4065. [DOI] [PubMed] [Google Scholar]
- 6.Béguin P, Lemaire M. The cellulosome: an exocellular, multiprotein complex specialized in cellulose degradation. Crit Rev Biochem Mol Biol. 1996;31:201–236. doi: 10.3109/10409239609106584. [DOI] [PubMed] [Google Scholar]
- 7.Ding S-Y, Bayer E A, Steiner D, Shoham Y, Lamed R. A novel cellulosomal scaffoldin from Acetivibrio cellulolyticus that contains a family 9 glycosyl hydrolase. J Bacteriol. 1999;181:6720–6729. doi: 10.1128/jb.181.21.6720-6729.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felix C R, Ljungdahl L G. The cellulosome—the exocellular organelle of Clostridium. Annu Rev Microbiol. 1993;47:791–819. doi: 10.1146/annurev.mi.47.100193.004043. [DOI] [PubMed] [Google Scholar]
- 9.Gal L, Gaudin C, Belaich A, Pagès S, Tardif C, Belaich J-P. CelG from Clostridium cellulolyticum: a multidomain endoglucanase acting efficiently on crystalline cellulose. J Bacteriol. 1997;179:6595–6601. doi: 10.1128/jb.179.21.6595-6601.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerngross U T, Romaniec M P M, Kobayashi T, Huskisson N S, Demain A L. Sequencing of a Clostridium thermocellum gene (cipA) encoding the cellulosomal Sl-protein reveals an unusual degree of internal homology. Mol Microbiol. 1993;8:325–334. doi: 10.1111/j.1365-2958.1993.tb01576.x. [DOI] [PubMed] [Google Scholar]
- 11.Gerwig G, Kamerling J P, Vliegenthart J F G, Morag (Morgenstern) E, Lamed R, Bayer E A. Novel oligosaccharide constituents of the cellulase complex of Bacteroides cellulosolvens. Eur J Biochem. 1992;205:799–808. doi: 10.1111/j.1432-1033.1992.tb16844.x. [DOI] [PubMed] [Google Scholar]
- 12.Gerwig G, Kamerling J P, Vliegenthart J F G, Morag E, Lamed R, Bayer E A. The nature of the carbohydrate-peptide linkage region in glycoproteins from the cellulosomes of Clostridium thermocellum and Bacteroides cellulosolvens. J Biol Chem. 1993;268:26956–26960. [PubMed] [Google Scholar]
- 13.Giuliano C, Khan A W. Cellulase and sugar formation by Bacteroides cellulosolvens, a newly isolated cellulolytic anaerobe. Appl Environ Microbiol. 1984;48:446–448. doi: 10.1128/aem.48.2.446-448.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giuliano C, Khan A W. Conversion of cellulose to sugars by resting cells of a mesophilic anaerobe, Bacteroides cellulosolvens. Biotechnol Bioeng. 1985;27:980–983. doi: 10.1002/bit.260270708. [DOI] [PubMed] [Google Scholar]
- 15.Kakiuchi M, Isui A, Suzuki K, Fujino T, Fujino E, Kimura T, Karita S, Sakka K, Ohmiya K. Cloning and DNA sequencing of the genes encoding Clostridium josui scaffolding protein CipA and cellulase CelD and identification of their gene products as major components of the cellulosome. J Bacteriol. 1998;180:4303–4308. doi: 10.1128/jb.180.16.4303-4308.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamed R, Bayer E A. The cellulosome concept: exocellular/extracellular enzyme reactor centers for efficient binding and cellulolysis. In: Aubert J-P, Beguin P, Millet J, editors. Biochemistry and genetics of cellulose degradation. London, England: Academic Press; 1988. pp. 101–116. [Google Scholar]
- 17.Lamed R, Bayer E A. The cellulosome of Clostridium thermocellum. Adv Appl Microbiol. 1988;33:1–46. [Google Scholar]
- 18.Lamed R, Morag (Morgenstern) E, Mor-Yosef O, Bayer E A. Cellulosome-like entities in Bacteroides cellulosolvens. Curr Microbiol. 1991;22:27–33. [Google Scholar]
- 19.Lamed R, Naimark J, Morgenstern E, Bayer E A. Specialized cell surface structures in cellulolytic bacteria. J Bacteriol. 1987;169:3792–3800. doi: 10.1128/jb.169.8.3792-3800.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamed R, Setter E, Bayer E A. Characterization of a cellulose-binding, cellulase-containing complex in Clostridium thermocellum. J Bacteriol. 1983;156:828–836. doi: 10.1128/jb.156.2.828-836.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leibovitz E, Béguin P. A new type of cohesin domain that specifically binds the dockerin domain of the Clostridium thermocellum cellulosome-integrating protein CipA. J Bacteriol. 1996;178:3077–3084. doi: 10.1128/jb.178.11.3077-3084.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lemaire M, Ohayon H, Gounon P, Fujino T, Béguin P. OlpB, a new outer layer protein of Clostridium thermocellum, and binding of its S-layer-like domains to components of the cell envelope. J Bacteriol. 1995;177:2451–2459. doi: 10.1128/jb.177.9.2451-2459.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin C, Urbance J W, Stahl D A. Acetivibrio cellulolyticus and Bacteroides cellulosolvens are members of the greater clostridial assemblage. FEMS Microbiol Lett. 1994;124:151–155. doi: 10.1111/j.1574-6968.1994.tb07277.x. [DOI] [PubMed] [Google Scholar]
- 24.Matsudaira P, editor. A practical guide to protein and peptide purification for microsequencing. New York, N.Y: Academic Press; 1993. [Google Scholar]
- 25.Mechaly A, Yaron S, Lamed R, Fierobe H-P, Belaich A, Belaich J-P, Shoham Y, Bayer E A. Cohesin-dockerin recognition in cellulosome assembly: experiment versus hypothesis. Proteins. 2000;39:170–177. doi: 10.1002/(sici)1097-0134(20000501)39:2<170::aid-prot7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 26.Murray M G, Thompson W F. Rapid isolation of high-molecular-weight plant DNA. Nucleic Acids Res. 1980;8:4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray W D. Increased cellulose hydrolysis by Bacteroides cellulosolvens in a simplified synthetic medium. J Biotechnol. 1985;3:131–140. [Google Scholar]
- 28.Murray W D, Sowden L C, Colvin J R. Bacteroides cellulosolvens sp. nov., a cellulolytic species from sewage sludge. Int J Syst Bacteriol. 1984;34:185–187. [Google Scholar]
- 29.Murray W D, Sowden L C, Colvin J R. Localization of the cellulase activity of Bacteroides cellulosolvens. Lett Appl Microbiol. 1986;3:69–72. [Google Scholar]
- 30.Pagès S, Belaich A, Belaich J-P, Morag E, Lamed R, Shoham Y, Bayer E A. Species-specificity of the cohesin-dockerin interaction between Clostridium thermocellum and Clostridium cellulolyticum: prediction of specificity determinants of the dockerin domain. Proteins. 1997;29:517–527. [PubMed] [Google Scholar]
- 31.Pagès S, Belaich A, Fierobe H-P, Tardif C, Gaudin C, Belaich J-P. Sequence analysis of scaffolding protein CipC and ORFXp, a new cohesin-containing protein in Clostridium cellulolyticum: comparison of various cohesin domains and subcellular localization of ORFXp. J Bacteriol. 1999;181:1801–1810. doi: 10.1128/jb.181.6.1801-1810.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rost B. PHD: predicting one-dimensional protein structure by profile based neural networks. Methods Enzymol. 1996;266:525–539. doi: 10.1016/s0076-6879(96)66033-9. [DOI] [PubMed] [Google Scholar]
- 33.Rost B, Sander C. Combining evolutionary information and neural networks to predict protein secondary structure. Proteins. 1994;19:55–72. doi: 10.1002/prot.340190108. [DOI] [PubMed] [Google Scholar]
- 34.Rost B, Sander C. Prediction of protein secondary structure at better than 70% accuracy. J Mol Biol. 1993;232:584–599. doi: 10.1006/jmbi.1993.1413. [DOI] [PubMed] [Google Scholar]
- 35.Salamitou S, Raynaud O, Lemaire M, Coughlan M, Béguin P, Aubert J-P. Recognition specificity of the duplicated segments present in Clostridium thermocellum endoglucanase CelD and in the cellulosome-integrating protein CipA. J Bacteriol. 1994;176:2822–2827. doi: 10.1128/jb.176.10.2822-2827.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shoham Y, Lamed R, Bayer E A. The cellulosome concept as an efficient microbial strategy for the degradation of insoluble polysaccharides. Trends Microbiol. 1999;7:275–281. doi: 10.1016/s0966-842x(99)01533-4. [DOI] [PubMed] [Google Scholar]
- 37.Shoseyov O, Takagi M, Goldstein M A, Doi R H. Primary sequence analysis of Clostridium cellulovorans cellulose binding protein A. Proc Natl Acad Sci USA. 1992;89:3483–3487. doi: 10.1073/pnas.89.8.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomme P, Warren R A J, Miller R C, Kilburn D G, Gilkes N R. Cellulose-binding domains—classification and properties. In: Saddler J M, Penner M H, editors. Enzymatic degradation of insoluble polysaccharides. Washington, D.C.: American Chemical Society; 1995. pp. 142–161. [Google Scholar]
- 39.Tormo J, Lamed R, Chirino A J, Morag E, Bayer E A, Shoham Y, Steitz T A. Crystal structure of a bacterial family-III cellulose-binding domain: a general mechanism for attachment to cellulose. EMBO J. 1996;15:5739–5751. [PMC free article] [PubMed] [Google Scholar]
- 40.Wang W K, Kruus K, Wu J H D. Cloning and DNA sequence of the gene coding for Clostridium thermocellum cellulase SS (CelS), a major cellulosome component. J Bacteriol. 1993;175:1293–1302. doi: 10.1128/jb.175.5.1293-1302.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]