Abstract
In Enterococcus faecalis, the peptide cCF10 acts as a pheromone, inducing transfer of the conjugative plasmid pCF10 from plasmid-containing donor cells to plasmid-free recipient cells. In these studies, it was found that a substantial amount of cCF10 associates with the envelope of the producing cell. Pheromone activity was detected in both wall and membrane fractions, with the highest activity associated with the wall. Experiments examining the effects of protease inhibitor treatments either prior to or following cell fractionation suggested the presence of a cell envelope-associated pro-cCF10 that can be processed to mature cCF10 by a maturase or protease. A pCF10-encoded membrane protein, PrgY, was shown to prevent self-induction of donor cells by reducing the level of pheromone activity in the cell wall fraction.
In the genus Enterococcus, plasmid-encoded antibiotic resistance genes and virulence genes can be transferred at high frequencies by conjugation (for recent reviews, see references 18, 26, and 43). Transfer of some enterococcal conjugative plasmids from donor to recipient cells is induced by signaling peptides (pheromones). Each pheromone is specific for the induction of transfer of a single plasmid or family of closely related plasmids (43). Five different pheromone plasmids (pAD1, pCF10, pPD1, pOB1, pAM373), each determining a response to a different pheromone, have been characterized to some extent; additional peptide-plasmid groups almost certainly exist (43). All enterococcal sex pheromones identified to date are hydrophobic peptides seven to eight amino acids in length. Two of the best-studied pheromone-inducible plasmids from E. faecalis are pCF10 (23), encoding tetracycline resistance, and pAD1 (42), encoding a cytolysin which is a virulence factor as well as a bacteriocin (29). Pheromone cCF10 (LVTLVFV) specifically induces transfer of pCF10, whereas cAD1 induces pAD1 transfer.
Putative identification of chromosomal gene(s) encoding production of the peptide pheromones has only recently been achieved by computer searches of genomic databases (19, 26). The cPD1, cAM373, cOB1, cAD1, and cCF10 pheromone sequences were found within the signal sequence segments of putative lipoproteins, all of unknown function (19). However, there is no published experimental evidence to indicate that the genes encoding these lipoproteins actually function as the structural genes for the pheromones. If this is the case, the pheromone biosynthetic pathway must entail a type of proteolytic processing that is unique from that of other signaling peptides. An et al. identified chromosomal determinant eep, which is necessary for normal production of several different pheromones (3). The available genetic data for eep (3), as well as the structural features of the deduced eep gene product, are consistent with the notion that Eep could be a membrane protease involved in posttranslational processing of polypeptide precursors into the various mature pheromone peptides. This proposed mechanism of pheromone synthesis is consistent with the proposal of Berg et al. (10) that cAD1 could be produced by the proteolytic processing of lipoprotein signal sequence of staphylococcal plasmid-encoded lipoprotein. Once they have been synthesized, mature pheromones accumulate in the growth medium of producing strains at concentrations in the range of 10−11 M (35, 36). No studies to date have examined whether this excreted pheromone activity comprises the entire pheromone output or if some activity remains cell associated.
The regulatory mechanisms controlling expression of transfer functions conferred by these plasmids have been studied extensively and have been recently reviewed (27). The known regulatory mechanisms for pCF10 are summarized below. Conjugation genes encoded by pCF10 are designated prg (pheromone responsive gene). To induce conjugative transfer, cCF10 is internalized by a plasmid-containing donor cell via the concerted action of the pCF10-encoded pheromone binding protein, PrgZ, and the chromosomal oligopeptide permease (Opp) (32, 33). Pheromone induction involves the direct interaction of cCF10 with one or more intracellular regulatory molecules. Evidence suggests that RNA transcripts from the prgQ and prgS regions of pCF10, as well as the PrgS protein, play a role in transcriptional and translational activation (7, 8, 9, 14, 15, 16, 33) resulting from pheromone internalization. The conjugative transfer genes whose expression is induced by cCF10 include prgB, which encodes a surface adhesin, aggregation substance. This protein mediates cell-cell aggregation and allows for efficient plasmid transfer in liquid medium (6, 39).
The pCF10-containing donor cell has the genetic capability for both cCF10 production (chromosomal) and response (plasmid encoded). Several negative control genes prevent expression of conjugation functions in the absence of exogenous pheromone. Recent results (5) indicate that the PrgX protein and an antisense transcript (Qa) from the prgQ region act together as intracellular negative regulators in uninduced cells. By analogy to the pPD1 and pAD1 systems, cCF10 might bind to PrgX and abolish its activity. High-performance liquid chromatography (HPLC) analysis of culture fluids of pCF10-containing strains demonstrated the presence of levels of cCF10 equal to those produced by plasmid-free recipients (36). Therefore, donor cells must have mechanisms to prevent self-induction of conjugation even if they continue to secrete cCF10. One such mechanism is the secretion of a plasmid-encoded inhibitor peptide. In the pCF10 system, the prgQ gene encodes this peptide, which is called iCF10 (AITLIFI) (36). iCF10 is synthesized as a 22-amino-acid precursor resembling a signal sequence, with the mature iCF10 representing the C-terminal 7 amino acids. Secretion and proteolytic processing of the peptide appear to occur simultaneously via the signal sequence-dependent pathway (36). Donor cells normally secrete cCF10 and iCF10 in a ratio that appears to be sufficient for the peptides to neutralize one another in terms of biological activity (36). The available data suggest that iCF10 competes with cCF10 extracellularly for binding to PrgZ, but that iCF10 is not likely to function intracellularly (9). The other essential negative control gene is prgY (28). Mutations in prgY can be complemented in trans (28). Computer analysis of the PrgY sequence predicted several transmembrane domains (41), and the membrane location of PrgY is suggested by results reported in this study and elsewhere (12). The TraB proteins encoded by the pheromone plasmids pPD1 and pAD1 share significant amino acid sequence similarity to PrgY. These gene products have been implicated in the shutdown of secreted pheromone production by cells carrying the respective plasmids (2, 37, 38). Since culture fluids from pCF10-containing donor cells contain the same amounts of cCF10 as those of plasmid-free recipients (36), it can be concluded that PrgY may block self-induction of donors by a mechanism that is different from that of the TraB proteins.
The present study was undertaken to gain a better understanding of the mechanism of cCF10 biosynthesis and to determine the role of PrgY in preventing self-induction of donor cells. The experiments reported here suggest that there is a significant amount of cCF10 associated with cell membrane and cell wall fractions of E. faecalis cells. Genetic analyses of PrgY suggest that this protein functions to interfere with self-induction of conjugation by cell-envelope-associated cCF10.
MATERIALS AND METHODS
Strains and medium.
All strains are derivatives of OG1RF (OG1 Rifr Fusr) (22) and, with the exception of OG1RF(pCF389, pMSP5011), were previously constructed. Contents of the strains are shown diagrammatically in the accompanying figures. Strains, appropriate antibiotic resistances, and references are as follows: OG1RF(pCF10), Tetr (23); OG1RF(pMSPS17), Cmr (41); OG1RF(pMSP17H3), Cmr (41); OG1RF(pMSP5011), Cmr (36); OG1RF(pMSP6043), Kanr (28); OG1RF(pCF389), Tetr, Ermr (13). Strains were grown on Todd-Hewitt broth (THB) or TH agar or in M9-YE glucose medium supplemented with antibiotics at the following concentrations: tetracycline, 10 μg/ml; chloramphenicol, 15 μg/ml; kanamycin, 500 μg/ml; erythromycin, 50 μg/ml.
Preparation of membranes.
Various strains were grown at 37°C without shaking in M9-YE medium (24) to an optical density at 600 nm (OD600) of 1.0. Cells were chilled on ice for 30 min and then were harvested by centrifugation at 8,000 rpm (Beckman J2-21 centrifuge; JA20 rotor) for 10 min. The supernatant was collected and was used as the supernatant fraction. The cells were washed twice in an equal volume of phosphate-buffered saline (PBS) buffer, and the pellet was resuspended (1:50 [vol/vol]) in lysis buffer (0.05 M KPO4 buffer [pH 7.0] containing 1 mM MgCl2, 1 mg of lysozyme [Sigma Chemical Co., St. Louis, Mo.]/ml, 20 ng of mutanolysin [Sigma]/ml, 500 μg of DNase [Sigma]/ml, 250 μg of RNase [Sigma]/ml) and incubated for 0.5 h at 37°C. The lysate was centrifuged at 1,100 rpm at 4°C for 20 min to remove unlysed cells (unlysed cell fraction). The supernatant was transferred and centrifuged at 17,000 × g for 20 min at 4°C to collect the cell wall fraction. The supernatant was again transferred to 5 ml of Ultra-clear tubes (Beckman), and membranes were harvested by ultracentrifugation at 50,000 rpm (Beckman Ultracentrifuge Model L-70, SW 50.1 rotor) for 2 h at 4°C. Pelleted cell walls and membranes were resuspended in PBS. In cases in which cells were treated with protease inhibitors, 10 mM EDTA plus the following compounds were added to the lysis solution (final concentrations in parentheses; all from Roche Molecular Biochemicals): aprotinin (2 μg/ml); leupeptin (0.5 μg/ml); phenylmethylsulfonyl fluoride (87 μg/ml). Generation of polyclonal antibody to PrgY and Western blot analysis of PrgY was carried out as described by Bryan et al. (12).
Detection of cCF10 by lipid extraction.
All pellets (unlysed cells, cell walls, and cell membranes) were resuspended at a ratio of 1:20 (1 ml) in PBS. Methanol (2.5 volumes) was mixed with the suspensions. After the pellets were thoroughly resuspended, 1.25 volumes (1.25 ml) of chloroform was added. The suspension was incubated for 0.5 to 3 h at room temperature (no differences were observed with incubation time). The cell debris was removed by centrifugation at 8,000 rpm for 5 min. The supernatant was mixed with 2.5 ml of chloroform and 2.5 ml of H2O. The mixture was centrifuged at 8,000 rpm for 10 min (Beckman J2-21 centrifuge; JA20 rotor), the chloroform layer was harvested and the samples were dried almost to completeness under nitrogen gas. The dried fractions were resuspended in 1 ml of THB and precipitated with 5% trichloroacetic acid (TCA), and the pellets were resuspended in 0.2 ml of PBS. The suspension was then brought to a final pH of 7 by dropwise addition of NaOH, and the final volume was adjusted to 0.5 ml. The sample was then used for either microtiter clumping assays or HPLC fractionation. The culture supernatants from the cells harvested for these fractionation experiments were autoclaved for 15 min at 121°C and 15 lb/in2. They were then subjected to precipitation with 5% TCA, and the pellets were neutralized with NaOH and resuspended in the same final volume as the corresponding cellular fractions so that relative activity levels could be measured directly.
HPLC separation of iCF10 and cCF10.
One hundred microliters of a subcellular fraction (described in the previous paragraph) in PBS was used for HPLC analysis. All separations were carried out using a Waters 486 Absorbance Detector/Gradient Controller, with a Vydac 218 TP C18 reverse-phase column. The solvent system was acetonitrile (AN) in 0.1% trifluoroacetic acid, and elution was carried out according to the following program: 0 to 10 min, 20% AN; 10 to 40 min, linear increase from 20 to 50% AN; 42 to 52 min, column was washed with 80% AN; 52 to 60 min, reequilibration with 20% AN. The flow rate was 1 ml/min, and 1-ml samples were collected every minute for 40 min. These conditions were developed for our chromatography system based on those previously determined to allow for separation of cCF10 from CF10 (36) and on our own empirical testing of elutions of synthetic iCF10 and cCF10 run on our system. The column fractions were lyophilized, resuspended in 200 μl of THB, and assayed for pheromone activity as described in the next section.
Detection of cCF10 activity.
cCF10 extracted from various cell fractions was resuspended in THB, and 200 μl was added to the first well of a round-bottom-well microtiter plate. Twofold serial dilutions were made, and 10 μl of a 15-h OG1RF(pCF10) culture was added to each well (each sample was tested in duplicate). The plates were incubated for 2 h at 37°C with shaking at 600 rpm. A positive clumping reaction was scored when a pellet of cells formed in the bottom of the well and the supernatant cleared significantly. The titer is reported as the reciprocal of the highest dilution which showed a positive clumping reaction.
Determination of location of Tn917 in pCF389.
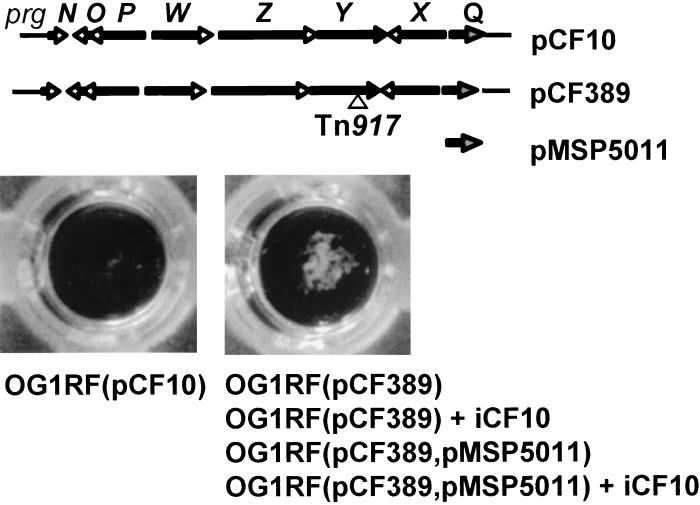
pCF389 DNA was prepared by the method of Anderson and McKay (4). Sequencing was done with a Sequenase Kit (US Biochemistry Corp., Cleveland, Ohio) according to the manufacturer's instructions by using the total plasmid DNA pellet recovered from 100 ml of OG1RF(pCF389) culture (was not completely resuspended, resulting in an extremely viscous reaction mixture) and 100 ng of Tn917 primer CAATAGAGAGATGTCACCGTCAAG (positions 196 to 173 of the published sequence). The junction between Tn917 DNA and pCF389 was at nucleotide 3803 in the published sequence of prgY, corresponding to an insertion in the second codon of amino acid 310 of 383 in PrgY.
Construction of OG1RF(pCF389, pMSP5011).
The plasmid pMSP5011 (previously constructed [36]) was polyethylene glycol purified (31). pMSP5011 contains 600 nucleotides from the intergenic region between prgX and prgR (Fig. 1) encompassing the promoter region for prgQ, ∼500 nucleotides of the prgQ RNA, and the structural gene for iCF10. Electroporation was done by a modification of the method outlined by Dunny et al. (25). Competent OG1RF(pCF389) cells were prepared by growing the cells overnight (13 h) in supplemented M9-YE medium with 0 to 8% glycine in 0.5% increments. The OD660 was measured, and cultures showing a minimum of 60% reduction in growth as compared to that of the 0% glycine culture were pooled. Cells were harvested by centrifugation, washed in a 1:10 (vol/vol) dilution of electroporation buffer (0.625 M sucrose, 1 mM MgCl2, pH 4.0), and resuspended in a 1:30 (vol/vol) dilution of electroporation buffer. Aliquots (40 μl) were made and stored at −70°C. Prior to electroporation, cells were harvested by centrifugation at 13,000 rpm (Heraeus Pico Biofuge) for 2 min at 4°C and washed three times with sterile distilled water (40 μl). The cells were resuspended in 40 μl of sterile distilled water and mixed with 1 μg of pMSP5011. Electroporation was performed in 0.2-cm cuvettes at 2,500 mV and 250 μF, yielding a time constant of approximately 4.0 ms. The cells were immediately resuspended in THB containing 0.25 M sucrose and incubated for 1 h at 37°C prior to plating in TH agar supplemented with 0.25 M sucrose and 15 μg of chloramphenicol/ml. Four colonies were used for further analysis. The presence of pMSP5011 was confirmed by reisolation and restriction enzyme digestion patterns (data not shown).
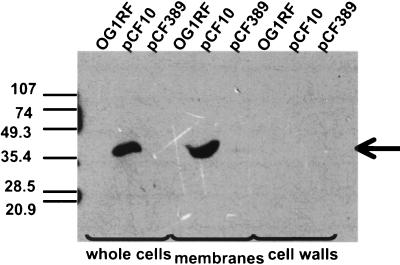
FIG. 1.
Analysis of PrgY in cell fractions of cells used in this study. The presence of PrgY in cellular fractions was analyzed for OG1RF, OG1RF(pCF10) (pCF10 encodes PrgY), and OG1RF(pCF389) (a transposon insert in pCF10 disrupts PrgY). Cellular lysates were prepared by lysozyme-mutanolysin lysis, cell walls were recovered by low-speed centrifugation, and cell membranes were recovered sequentially by high-speed centrifugation. Equal amounts of collected material were electrophoresed through a sodium dodecyl sulfate–7.5% polyacrylamide gel and electroblotted onto a nylon membrane. PrgY was detected by Western blotting with a polyclonal PrgY antibody raised to PrgY peptides. Bands were visualized as described previously (12). The strain used is indicated above each lane, and the fraction analyzed is indicated beneath each group of lanes. The size of the detected band corresponded to the previously predicted size of PrgY (28). The bands detected at the side of the left lane are due to the strong reaction of the premarked molecular weight markers.
RESULTS
cCF10 production is cell envelope associated.
During cell fractionation studies designed to look for internalization of cCF10 into cells, it was observed that coincubation of pCF10-containing cells with cell envelopes isolated from the plasmid-free strain OG1RF induced clumping of the donor cells (data not shown). To further determine what fractions may contain cCF10 activity, supernatant, whole cells, and cell wall and cell membrane fractions were isolated by lysozyme-mutanolysin treatment, centrifugation, and chloroform-methanol extraction of the lipids. The relative purity of the fractions was determined by Western blotting for the pCF10-encoded membrane-associated molecule, PrgY (28). As shown in Fig. 1, essentially all of the detectable PrgY was found in the membrane fraction, suggesting that the wall fraction was not heavily contaminated with membrane material. When the same two fractions were extracted with chloroform-methanol (1) and the extracts were separated by thin-layer chromatography followed by spraying with a reagent to visualize phospholipids (21), the bulk of the reactivity was also in the membrane fraction (data not shown).
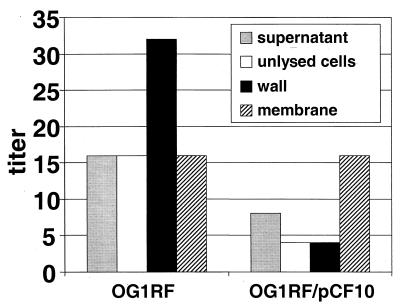
The presence of cCF10 in isolated envelope fractions was determined by a biological assay. Proteins were recovered from the fractions by TCA precipitation, and the titer of active cCF10 present in the fractions was determined by a quantitative microtiter plate assay (see Materials and Methods). Significant levels of cCF10 activity were associated with the cell envelope fractions, including the cell membrane and cell wall fractions of plasmid-free cells (Fig. 2). When fractions from isogenic plasmid-free- or pCF10-containing cells were compared, the membranes were found to contain equivalent amounts of cCF10 activity, while the cell wall, supernatant, and unlysed cell fractions had decreased activity in the plasmid-containing cells.
FIG. 2.
Presence of cCF10 activity in cellular fractions of OG1RF and OG1RF(pCF10) cells. Cellular lysates were prepared by lysozyme-mutanolysin lysis, cell walls were recovered by low-speed centrifugation, and cell membranes were recovered sequentially by high-speed centrifugation. Proteins were recovered from the whole envelope and cell membrane and cell wall fractions by TCA precipitation. For this experiment, as well as those described in subsequent figures, the extracted and concentrated culture supernatant and subcellular fractions were all resuspended in the same final volume so that relative activities in each fraction could be directly compared. The cCF10 activity was detected by microtiter clumping assays; the titers reported represent the reciprocal of the highest dilution that induced clumping (see Materials and Methods). While the absolute amount of cCF10 recovered varied somewhat between experiments, the relative amount of cCF10 in each fraction remained the same. The relative amounts of cCF10 in each fraction shown are representative of at least two independent experiments.
Cell-associated cCF10 activity is a mixture of mature and precursor cCF10.
The genetic data suggest cCF10 is produced by the proteolytic processing of a signal sequence. This process would most likely occur at the cell surface. If a cCF10 precursor existed in the cell envelope and all of the machinery necessary to process the precursor was also present in the cell membrane, this would suggest mature cCF10 could be produced by membranes even after disruption of the cells. In this case, addition of presence of protease inhibitors might decrease the levels of cCF10 in purified subcellular fractions. Conversely, if all the cCF10 in the cell envelope fractions was mature, cCF10 levels should not be affected by the presence of protease inhibitors.
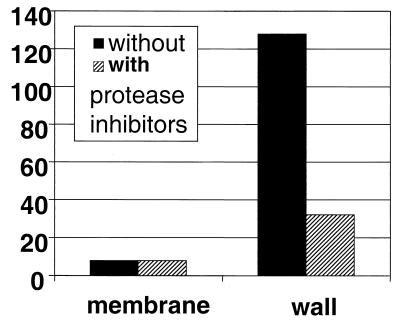
When cell lysis and fractionation of OG1RF cells was carried out in the presence of protease inhibitors, the cell wall fraction showed significantly decreased cCF10 activity (Fig. 3). It should be noted that protease inhibitors reduced the cCF10 titers in fractions only if they were added prior to the 30-min cell lysis step. For fractions isolated in the absence of inhibitors, further incubation at 37°C for 30 min significantly decreased the titer (from 2- to 16-fold in different trials), relative to those of samples incubated in parallel at 0°C. This gradual loss of cCF10 activity at 37°C occurred regardless of whether protease inhibitors were present during preparation of the samples or were added after fractionation. The cCF10 activity associated with isolated membrane fractions was unaffected by the presence of protease inhibitors during lysis and fractionation (Fig. 3) or by incubation of the isolated fraction at 37°C (not shown).
FIG. 3.
Production of cCF10 activity in subcellular fractions of OG1RF cells in the presence and absence of protease inhibitors. Cellular lysates were prepared by lysozyme-mutanolysin lysis. The lysis steps were carried out either in the presence or absence of protease inhibitors (see Materials and Methods). Cell walls were recovered by low-speed centrifugation, and cell membranes were recovered sequentially by high-speed centrifugation. Proteins were recovered from cell membranes or cell wall fractions by chloroform-methanol lipid extraction followed by TCA precipitation. The presence of cCF10 was detected by microtiter clumping assay, as described above. The relative amounts of cCF10 in each fraction shown are representative of at least two independent experiments.
We also carried out HPLC analysis of the cCF10 activity in the isolated subcellular fractions from these experiments. cCF10 was harvested by TCA precipitation and then further purified by HPLC under conditions that separate cCF10 and iCF10. All of the cCF10 activity detected in the HPLC fractions had a retention time identical to that of synthetic cCF10 (27 to 28 min), indicating that the only molecular species in these preparations with biological activity is mature cCF10. Taken together, these data suggest the presence of a precursor that is converted to cCF10 by proteolytic processing at the cell surface. These results do not allow for a conclusive determination of whether this processing actually occurs in the wall or at another location, such as the membrane or the membrane-wall interface.
Reduction of cell wall-associated cCF10 activity by pCF10 is due to the presence of PrgY.
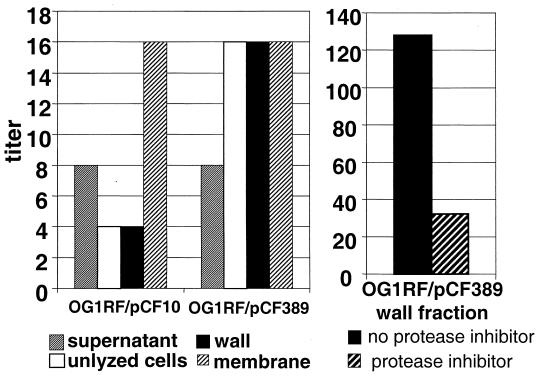
Although pCF10-containing donor cells continue to produce cCF10, this endogenous pheromone does not self-induce conjugation. Nakayama et al. found that iCF10 neutralizes cCF10 activity in the supernatant of donor cultures (38). It was of interest to determine the effects of iCF10 and PrgY on the cell-associated pheromone activity described above. Plasmid pCF389 is a pCF10 derivative containing a prgY::Tn917 insertion at amino acid 310 of 389. This insertion results in the nonpolar mutation of prgY. The amount of cell-associated cCF10 was measured for OG1RF(pCF10) and OG1RF(pCF389) cells (Fig. 4). The cCF10 activity in the wall fraction of the PrgY mutant was significantly higher. The addition of protease inhibitors prior to lysis and fractionation had the same effect on the PrgY mutant, as was observed with plasmid-free cells, suggesting that the cell-associated cCF10 in the PrgY mutant is probably a similar mix of precursor and mature cCF10. These data suggest that PrgY reduces cell wall-associated cCF10 activity.
FIG. 4.
Comparison of cCF10 production by cell fractions of OG1RF(pCF10) and the prgY-negative derivative OG1RF(pCF389). Cellular lysates were fractionated as described above. Pheromone activity recovered from whole-cell lysates, cell membranes, or cell wall fractions by chloroform-methanol extraction followed by TCA precipitation was detected by microtiter clumping assays. In some experiments, protease inhibitors were present during cell lysis and fractionation (see Materials and Methods). The relative amounts of cCF10 in each fraction shown above are representative of at least two independent experiments.
PrgY mutation does not affect levels of cCF10 in the supernatant.
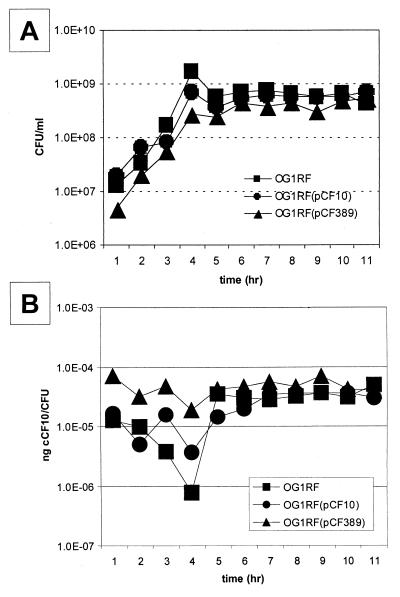
To determine if mutation in prgY affected cCF10 secreted into the growth medium, the number of CFU and amount (in nanograms) of cCF10 produced in the supernatant were measured throughout growth for plasmid-free cells (OG1RF) and for isogenic cells carrying pCF10 or pCF389. The number of CFU were determined by viable count plating (Fig. 5A). All cCF10 activity recovered from the bacterial fractions analyzed in these studies had the same retention time (elution at 27 to 28 min) as synthetic cCF10 run under the same conditions. The amount of cCF10 recovered in the HPLC fraction was determined by the microtiter assay, and the amount (in nanograms) of cCF10 produced per cell was plotted against growth (Fig. 5B). No significant difference in the amount of secreted cCF10 was noted for any of the strains. This suggests that PrgY specifically affects the amount of cell wall-associated cCF10, without reducing the amount released into the growth medium.
FIG. 5.
Production of cCF10 in the growth medium by OG1RF cells containing no plasmid, pCF10, or pCF389. Overnight cultures were diluted 1:10 in fresh medium. (A) Aliquots were removed at various times during growth, and bacterial numbers were determined by viable plate counting. (B) cCF10 was recovered by cell lysis, chloroform-methanol extraction, and TCA precipitation. cCF10 was separated from iCF10 by HPLC, and the amount of cCF10 was determined by performing a microtiter OG1RF(pCF10) clumping. The amount of cCF10 present was calculated following the method of Nakayama et al. (36) and is reported as nanograms of cCF10/CFU.
Reduction of cell wall-associated cCF10 by PrgY is independent of iCF10.
Because a large molar excess of iCF10 is required to block cCF10 activity completely (36), a formal possibility for the constitutively clumpy phenotype of this PrgY mutant is that these cells do not secrete sufficient amounts of iCF10 into the medium to inhibit cCF10. To confirm that the prevention of self-induction by PrgY was independent of iCF10, overproduction of cCF10 was accomplished by adding synthetic iCF10 to the medium and/or overexpressing iCF10 (encoded by prgY) on a multicopy vector. The clumpy phenotype of the PrgY mutant could not be compensated for by addition of a concentration (6 × 10−8 M) of synthetic iCF10 to the growth medium which is greater than 100-fold above the level produced by wild-type donor cells (Fig. 6). Overproduction of iCF10 was accomplished by transforming the plasmid pMSP5011 into the PrgY mutant. pMSP5011 contains the prgQ structural gene encoding iCF10 cloned on a multicopy plasmid. Nakayama et al. (36) showed that pMSP5011 conferred production of over 15 ng of iCF10/ml in plasmid-free cells (OG1RF; 25 times the level conferred by pCF10 in the same host). The PrgY mutant containing the iCF10 overexpression vector remained constitutively clumpy. This phenotype was maintained even in the presence of an additional amount of exogenously added synthetic iCF10 at 6 × 10−8 M (Fig. 6). Because excess iCF10 could not compensate for the lack of PrgY, these two gene products must control self-induction by distinct mechanisms.
FIG. 6.
Inability of iCF10 to suppress the constitutively clumpy phenotype of a prgY mutant. OG1RF(pCF10) or OG1RF(pCF389) derivatives were grown in M9-YE medium or M9-YE medium containing synthetic iCF10 (6 × 10−8 M) and clumping was scored. The wells shown represent the appearance of nonclumpy [OG1RF(pCF10)] or clumpy [OG1RF(pCF389)] strains in liquid culture. The strains exhibiting each phenotype are listed below the appropriate wells. The relevant genes contained in each plasmid are shown above the wells. pCF389 is the pCF10 plasmid containing a transposon insert in prgY at amino acid 310 of 363. pMSP5011 represents the cloned iCF10 structural gene (prgQ).
DISCUSSION
E. faecalis is both a member of the human intestinal tract normal flora and an important opportunistic pathogen causing septicemia, endocarditis, and urinary tract infection when present in normally sterile body sites (30). These organisms frequently carry antibiotic resistance genes (17, 20, 34) that can complicate treatment of diseases as well as serve as a reservoir of antibiotic resistance genes in the intestinal tract. One mechanism for dissemination of these antibiotic resistance genes is high-frequency plasmid transfer accomplished by pheromone-inducible conjugative plasmids, such as pCF10 and pAD1 (17, 20). Except for a few cases (10), pheromone production is unique to enterococcal species, and the available evidence suggests that the host range of the pheromone-responsive plasmids may also be limited to this genus (26, 27, 43). Several aspects of the biology of the pheromone peptides, such as their ability to mediate communication between donor and recipient cells, have been studied at length. In contrast, relatively little information has appeared about the molecular and genetic basis for pheromone production or about the role of these molecules in the producing cell.
A significant result of the present study is the finding that in the case of cCF10, the majority of the pheromone produced remains associated with the cell, primarily in the wall fraction. Pheromone activity with an HPLC elution profile identical to that of synthetic cCF10 could be TCA precipitated from unfractionated cell lysates and from cell wall and cell membrane fractions. The addition of a protease inhibitor during disruption of the cells decreased the amount of cCF10 activity associated with the cell wall fraction. This suggested that the cCF10 associated with the cell wall fraction is produced by the proteolytic cleavage of a cCF10 precursor molecule. Based on our results, the precursor could be either cytoplasmic or cell envelope associated. The processing step could take place in the wall, or it might be concurrent with secretion of the peptide across the cytoplasmic membrane. The latter type of secretion mechanism is consistent with the recent identification of the pheromone amino acid sequences within signal sequences of lipoproteins of unknown function (19) and with the dependence of cCF10 production on Eep, a putative membrane protease (3). The eep gene was identified from a screen for chromosomal mutants with reduced capacity for production of pheromone cAD1. An et al. (3) subsequently found that eep mutants showed reduced levels of production of several pheromones. Recently, Brown et al. published a comparative analysis of a family of membrane-associated proteases from several species, ranging from bacteria to humans (11). These enzymes play key roles in proteolytic cleavage of transmembrane protein substrates involved in processes ranging from development to lipid metabolism to neurodegenerative diseases. Although the overall similarity of the proteases is low, they share a conserved active site for proteolysis, known as a membrane-embedded zinc metalloprotease domain. This structure is believed to allow these proteases to cleave their substrates within the membrane; this form of proteolytic maturation has therefore been termed regulated intramembrane proteolysis (RIP). The enterococcal Eep protein is a member of the RIP protease family (11), suggesting that cleavage of the signal peptide pheromone precursor may occur within the membrane, which would be consistent with the results of our analysis of cCF10 production. Since mature cCF10 is significantly more hydrophobic than the putative signal peptide precursor, it seems likely that the RIP process might be coupled to active excretion of the peptide across the membrane.
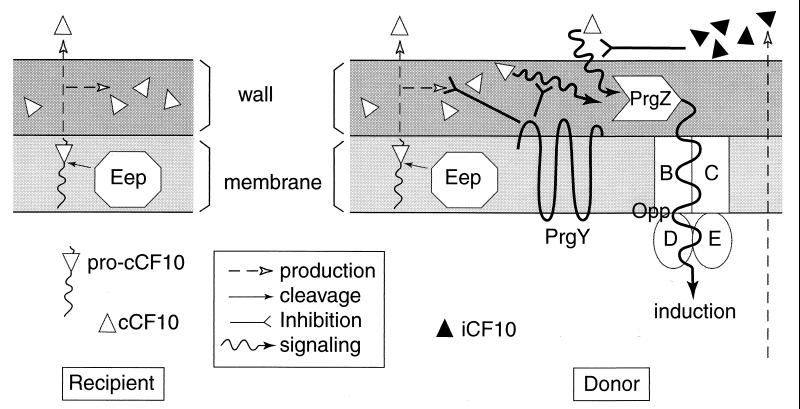
Figure 7 presents a model incorporating the present results with previous studies. In this model, the pheromone precursor is the cleaved signal peptide generated from lipoprotein secretion. The proposed mode of cCF10 synthesis is similar to one proposed by Brown et al. for cAD1 production (11). If gene disruption and cloning studies currently in progress demonstrate that the lipoprotein genes recently identified (19) actually represent the structural genes for the enterococcal pheromones, the mechanism of pheromone biosynthesis depicted in Fig. 7 will be largely confirmed. This mechanism is significantly different from those of any other class of bacterial signaling peptides analyzed to date (27). The data presented here show that both the cell wall and the extracellular medium contain significant cCF10 activity, with most of this activity remaining cell wall-associated. This situation suggests the possibility that the peptide present in the wall could play a role in the physiology of the producing cell. For example, cCF10 might play a role in regulating the expression of activity of the lipoprotein whose signal sequence functions as the propheromone, perhaps via direct interaction between the peptide and the protein. The cell-associated cCF10 could also be important in conjugative signaling between a producing recipient cell and a responding donor cell in close contact with the recipient, e.g., in a surface biofilm. In this regard, it has been shown that extracellular complementation of the Bacillus subtilis peptide signaling factor PhrA may occur as a result of direct cell-cell contact (40). Conceivably, the pheromone-inducible plasmids may have evolved a transfer system designed to function most efficiently between organisms colonizing surfaces and growing in close proximity.
FIG. 7.
Model for pheromone production and control of endogenous pheromone activity by PrgY and iCF10. On the left is the cell envelope of a plasmid-free cell producing cCF10. The synthesis of mature cCF10 is proposed to occur via processing of a propheromone, now believed to represent the cleaved signal peptide from a secreted lipoprotein (19). The Eep protein described by An et al. (3) is proposed to be a membrane protease of the RIP family (11). Some mature pheromone is released into the medium, but a substantial portion remains associated with the cell wall of the organism. In a cell carrying pCF10 (right), both pheromone synthesis and pheromone response functions are present. The plasmid-encoded iCF10 peptide effectively neutralizes the cCF10 released into the medium, while PrgY interferes with a potential autocrine circuit where cell-associated pheromone is immediately reinternalized by the concerted action of the PrgZ binding protein and the chromosomal oligopeptide permease system (33), resulting in self-induction. It is not yet clear whether PrgY acts by sequestering or degrading cell-associated cCF10, by interfering with its interaction with PrgZ, or by some other mechanism.
In cells carrying pCF10, cCF10 released into the growth medium is inhibited by the production of the peptide inhibitor, iCF10. In these studies we found that cell-associated cCF10 was not inhibited by iCF10 but by the action of a transmembrane protein, PrgY. PrgY did not affect the levels of secreted cCF10; rather, PrgY decreased the amount of cell wall-associated cCF10. This suggests that the cCF10 released into the medium is only a fraction of the total pheromone output and that a majority of cell wall-associated cCF10 may have a different fate than secreted cCF10. PrgY shares over 75% amino acid identity with the TraB protein encoded by the pheromone plasmid pPD1 and about 40% identity with the TraB encoded by pAD1. All three of these proteins are predicted to be integral membrane proteins, which has now been confirmed in the case of PrgY (Fig. 1). These proteins are all involved in preventing self-induction of conjugation in plasmid-containing cells. In contrast to our findings for PrgY, the TraB proteins reduce the level of the cognate pheromones secreted into the medium (2, 38). Furthermore, a traB mutation in pPD1 could be suppressed by addition of excess iPD1 inhibitor peptide (36), whereas iCF10 could not suppress the clumpy phenotype associated with a prgY mutant (Fig. 6). These differences may be due to either basic functional differences in between TraB and PrgY or differences in the interaction of the different pheromones with cell wall components during secretion.
In spite of some apparent differences in the various pheromone plasmid systems, it is clear that the prevention of self-induction of conjugation requires two distinct molecules in all cases: an inhibitor peptide which prevents self-induction by secreted pheromone and a membrane protein which acts within the cell envelope. Finally, it should be pointed out that the model (Fig. 7) depicts a direct transit of iCF10 from the cytoplasm to the extracellular medium without much interaction with the cell wall or with plasmid proteins, such as PrgY. This is speculative, since we have not completed a quantitative analysis of iCF10 in subcellular fractions. However, the available evidence does not suggest the presence of a large pool of cell-associated iCF10, suggesting that its primary function may be to complete with cCF10 in the extracellular medium.
ACKNOWLEDGMENTS
We thank Dawn Manias, Helmut Hirt, Ed Bryan, and Don Clewell for technical assistance and discussion of unpublished results. We also thank Richard Losick for bringing the work of Brown and colleagues on intramembrane proteolysis to our attention.
This work was supported by PHS grant GM49530 from the NIH. B.A.B. was a recipient of an individual postdoctoral fellowship (IF32-AI08742) from the NIH. M.H.A. was a recipient of the Dennis Watson graduate fellowship from the Dept. of Microbiology, Univ. of Minnesota.
REFERENCES
- 1.Ames G F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968;95:833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.An F Y, Clewell D B. Characterization of the determinant (traB) encoding sex pheromone shutdown by the hemolysin/bacteriocin plasmid pAD1 in Enterococcus faecalis. Plasmid. 1994;31:215–221. doi: 10.1006/plas.1994.1023. [DOI] [PubMed] [Google Scholar]
- 3.An F Y, Sulavik M C, Clewell D B. Identification and characterization of a determinant (eep) on the Enterococcus faecalis chromosome that is involved in production of the peptide sex pheromone cAD1. J Bacteriol. 1999;181:5915–5921. doi: 10.1128/jb.181.19.5915-5921.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson D G, Mckay L L. Simple and rapid method for isolating large plasmid DNA from lactic streptococci. Appl Environ Microbiol. 1983;46:549–552. doi: 10.1128/aem.46.3.549-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bae T, Clerc-Bardin S, Dunny G M. Analysis of expression of prgX, a key negative regulator of the transfer of the Enterococcus faecalis pheromone-inducible plasmid pCF10. J Mol Biol. 2000;297:861–875. doi: 10.1006/jmbi.2000.3628. [DOI] [PubMed] [Google Scholar]
- 6.Bensing B A, Dunny G M. Cloning and molecular analysis of genes affecting expression of binding substance, the recipient-encoded receptor(s) mediating mating aggregate formation in Enterococcus faecalis. J Bacteriol. 1993;175:7421–7429. doi: 10.1128/jb.175.22.7421-7429.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bensing B A, Meyer B J, Dunny G M. Sensitive detection of bacterial transcription initiation sites and differentiation from RNA processing sites in the pheromone-induced plasmid transfer system of Enterococcus faecalis. Proc Natl Acad Sci USA. 1996;93:7794–7799. doi: 10.1073/pnas.93.15.7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bensing B A, Dunny G M. Pheromone-inducible expression of an aggregation protein in Enterococcus faecalis requires interaction of a plasmid-encoded RNA with components of the ribosome. Mol Microbiol. 1997;24:295–308. doi: 10.1046/j.1365-2958.1997.3311709.x. [DOI] [PubMed] [Google Scholar]
- 9.Bensing B A, Manias D M, Dunny G M. Pheromone cCF10 and plasmid pCF10-encoded regulatory molecules act post-transcriptionally to activate expression of downstream conjugation functions. Mol Microbiol. 1997;24:285–294. doi: 10.1046/j.1365-2958.1997.3301710.x. [DOI] [PubMed] [Google Scholar]
- 10.Berg T, Firth N, Skurray R A. Enterococcal pheromone-like activity derived from a lipoprotein signal peptide encoded by a Staphylococcus aureus plasmid. Adv Exp Med Biol. 1997;418:1041–1044. doi: 10.1007/978-1-4899-1825-3_245. [DOI] [PubMed] [Google Scholar]
- 11.Brown M S, Ye J, Rawson R B, Goldstein J L. Regulated intramembrane proteolysis: a control mechanism conserved from bacteria to humans. Cell. 2000;100:391–398. doi: 10.1016/s0092-8674(00)80675-3. [DOI] [PubMed] [Google Scholar]
- 12.Bryan, E. M., T. Bae, M. Kleerebezem, and G. M. Dunny. Improved vectors for nisin controlled gene expression (NICE) in Gram-positive bacteria. Plasmid, in press. [DOI] [PubMed]
- 13.Christie P J, Dunny G M. Identification of regions of the Streptococcus faecalis plasmid pCF10 that encode antibiotic resistance and pheromone response functions. Plasmid. 1986;15:230–241. doi: 10.1016/0147-619x(86)90041-7. [DOI] [PubMed] [Google Scholar]
- 14.Chung J W, Dunny G M. Cis-acting, orientation-dependent, positive control system activates pheromone-inducible conjugation functions at distances greater than 10 kilobases upstream from its target in Enterococcus faecalis. Proc Natl Acad Sci USA. 1992;89:9020–9024. doi: 10.1073/pnas.89.19.9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung J W, Dunny G M. Transcriptional analysis of a region of the Enterococcus faecalis plasmid pCF10 involved in positive regulation of conjugative transfer functions. J Bacteriol. 1995;177:2118–2124. doi: 10.1128/jb.177.8.2118-2124.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung J W, Bensing B A, Dunny G M. Genetic analysis of a region of the Enterococcus faecalis plasmid pCF10 involved in positive regulation of conjugative transfer functions. J Bacteriol. 1995;177:2107–2117. doi: 10.1128/jb.177.8.2107-2117.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clewell D B. Movable genetic elements and antibiotic resistance in enterococci. Eur J Clin Infect Dis. 1990;9:90–102. doi: 10.1007/BF01963632. [DOI] [PubMed] [Google Scholar]
- 18.Clewell D B. Sex pheromones and the plasmid-encoded mating responses in Enterococcus faecalis. Cell. 1993;73:9–12. [Google Scholar]
- 19.Clewell D B, An F Y, Flannagan S E, Antiporta M H, Dunny G M. Enterococcal sex pheromone precursors are part of signal sequences for surface lipoproteins. Mol Microbiol. 2000;35:246–247. doi: 10.1046/j.1365-2958.2000.01687.x. [DOI] [PubMed] [Google Scholar]
- 20.Courvalin P. Resistance of enterococci to glycopeptides. Antimicrob Agents Chemother. 1990;34:2291–2296. doi: 10.1128/aac.34.12.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dittmer J C, Lester R L. A simple specific spray for the detection of phospholipids on thin layer chromatograms. J Lipid Res. 1964;5:126–127. [PubMed] [Google Scholar]
- 22.Dunny G M, Brown B L, Clewell D B. Induced cell aggregation and mating in Streptococcus faecalis: evidence for a bacterial sex pheromone. Proc Natl Acad Sci USA. 1978;75:3479–3483. doi: 10.1073/pnas.75.7.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunny G M, Funk C, Adsit J. Direct stimulation of the transfer of antibiotic resistance by sex pheromones in Streptococcus faecalis. Plasmid. 1981;6:270–278. doi: 10.1016/0147-619x(81)90035-4. [DOI] [PubMed] [Google Scholar]
- 24.Dunny G M, Zimmerman D L, Tortoello M L. Induction of surface exclusion (entry exclusion) by Streptococcus faecalis pheromones: use of monoclonal antibodies to identify an inducible surface antigen involved in the exclusion process. Proc Natl Acad Sci USA. 1985;82:8582–8586. doi: 10.1073/pnas.82.24.8582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunny G M, Lee L N, LeBlanc D J. Improved electroporation and cloning vector system for gram-positive bacteria. Appl Environ Microbiol. 1991;57:1194–1201. doi: 10.1128/aem.57.4.1194-1201.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunny G M, Leonard B A B, Hedberg P J. Pheromone-inducible conjugation in Enterococcus faecalis: interbacterial and host-parasite chemical communication. J Bacteriol. 1995;177:871–876. doi: 10.1128/jb.177.4.871-876.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunny G M, Leonard B A (Buttaro) Cell-cell communication in Gram-positive bacteria. Annu Rev Microbiol. 1997;51:527–564. doi: 10.1146/annurev.micro.51.1.527. [DOI] [PubMed] [Google Scholar]
- 28.Hedberg P J, Leonard B A (Buttaro), Ruhfel R E, Dunny G M. Identification and characterization of the genes of Enterococcus faecalis plasmid pCF10 involved in replication and in negative control of pheromone-inducible conjugation. Plasmid. 1996;35:46–57. doi: 10.1006/plas.1996.0005. [DOI] [PubMed] [Google Scholar]
- 29.Ike Y, Clewell D B. Evidence that the hemolysin/bacteriocin phenotype of Enterococcus faecalis subsp. zymogenes can be determined by plasmids in different incompatibility groups as well as by the chromosome. J Bacteriol. 1992;174:8172–8177. doi: 10.1128/jb.174.24.8172-8177.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jett B D, Huycke M M, Gilmore M S. Virulence of entercocci. Clin Microbiol Rev. 1994;7:462–478. doi: 10.1128/cmr.7.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krieg P, Milton D. Rapid isolation of plasmid DNA. In: Titus D, editor. Protocols and applications guide. Madison, Wis: Promega Corp.; 1986. p. 106. [Google Scholar]
- 32.Leonard B A (Buttaro), Bensing B A, Hedberg P J, Ruhfel R E, Chung J W, Dunny G M. Pheromone-inducible gene regulation and signalling for the control of aggregation substance expression in the conjugative plasmid pCF10. Dev Biol Stand. 1995;85:27–34. [PubMed] [Google Scholar]
- 33.Leonard B A (Buttaro), Podbielski A, Hedberg P J, Dunny G M. Enterococcus faecalis pheromone binding protein, PrgZ, recruits a chromosomal oligopeptide permease system to import sex pheromone cCF10 for induction of conjugation. Proc Natl Acad Sci USA. 1996;93:260–264. doi: 10.1073/pnas.93.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Macrina F L, Archer G L. Conjugation and broad host range plasmids in streptococci and staphylococci. In: Clewell D B, editor. Bacterial conjugation. New York, N.Y: Plenum; 1993. pp. 313–329. [Google Scholar]
- 35.Mori M, Sakagami Y, Ishii Y, Isogai A, Kitada C, Fujino M, Adsit J C, Dunny G M, Suzuki A. Structure of cCF10, a peptide sex pheromone which induces conjugative transfer of the Streptococcus faecalis tetracycline resistance plasmid, pCF10. J Biol Chem. 1988;263:14574–14578. [PubMed] [Google Scholar]
- 36.Nakayama J, Ruhfel R E, Dunny G M, Isogai A, Suzuki A. The prgQ gene of the Enterococcus faecalis tetracycline resistance plasmid pCF10 encodes a peptide inhibitor, iCF10. J Bacteriol. 1994;176:7405–7408. doi: 10.1128/jb.176.23.7405-7408.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakayama J, Dunny G M, Clewell D B, Suzuki A. Quantitative analysis for pheromone inhibitor and pheromone shutdown in Enterococcus faecalis. Dev Biol Stand. 1995;85:35–38. [PubMed] [Google Scholar]
- 38.Nakayama J, Yoshida K, Kobayashi H, Isogai A, Clewell D B, Suzuki A. Cloning and characterization of a region of Enterococcus faecalis plasmid pPD1 encoding pheromone inhibitor (ipd), pheromone sensitivity (traC), and pheromone shutdown (traB) genes. J Bacteriol. 1995;177:5567–5573. doi: 10.1128/jb.177.19.5567-5573.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olmsted S B, Kao S-M, Vanputte L J, Gallo J C, Dunny G M. Role of pheromone-inducible surface protein Asc10 in mating aggregate formation and conjugal transfer of the Enterococcus faecalis plasmid pCF10. J Bacteriol. 1991;173:7665–7672. doi: 10.1128/jb.173.23.7665-7672.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perego M, Hoch J A. Cell-cell communication regulates the effects of protein aspartate phosphatases on the phosphorelay controlling development in Bacillus subtilis. Proc Natl Acad Sci USA. 1996;93:1549–1553. doi: 10.1073/pnas.93.4.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rufhel R E, Manias D A, Dunny G M. Cloning and characterization of a region of Enterococcus faecalis conjugative plasmid, pCF10, encoding a sex pheromone-binding function. J Bacteriol. 1993;175:5253–5259. doi: 10.1128/jb.175.16.5253-5259.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tomich P K, An F Y, Damle S P, Clewell D B. Plasmid-related transmissibility and multiple drug resistance in Streptococcus faecalis subsp. zymogenes strain DS16. Antimicrob Agents Chemother. 1979;15:828–830. doi: 10.1128/aac.15.6.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wirth R. The sex pheromone system of Enterococcus faecalis: more than just a plasmid-collection mechanism? Eur J Biochem. 1994;222:235–246. doi: 10.1111/j.1432-1033.1994.tb18862.x. [DOI] [PubMed] [Google Scholar]