Summary
Background
Little is known about post-acute sequelae of SARS-CoV-2 infection (PASC) after acquiring SARS-CoV-2 infection during pregnancy. We aimed to evaluate the association between acquiring SARS-CoV-2 during pregnancy compared with acquiring SARS-CoV-2 outside of pregnancy and the development of PASC.
Methods
This retrospective cohort study from the Researching COVID to Enhance Recovery (RECOVER) Initiative Patient-Centred Clinical Research Network (PCORnet) used electronic health record (EHR) data from 19 U.S. health systems. Females aged 18–49 years with lab-confirmed SARS-CoV-2 infection from March 2020 through June 2022 were included. Validated algorithms were used to identify pregnancies with a delivery at >20 weeks’ gestation. The primary outcome was PASC, as previously defined by computable phenotype in the adult non-pregnant PCORnet EHR dataset, identified 30–180 days post-SARS-CoV-2 infection. Secondary outcomes were the 24 component diagnoses contributing to the PASC phenotype definition. Univariable comparisons were made for baseline characteristics between individuals with SARS-CoV-2 infection acquired during pregnancy compared with outside of pregnancy. Using inverse probability of treatment weighting to adjust for baseline differences, the association between SARS-CoV-2 infection acquired during pregnancy and the selected outcomes was modelled. The incident risk is reported as the adjusted hazard ratio (aHR) with 95% confidence intervals.
Findings
In total, 83,915 females with SARS-CoV-2 infection acquired outside of pregnancy and 5397 females with SARS-CoV-2 infection acquired during pregnancy were included in analysis. Non-pregnant females with SARS-CoV-2 infection were more likely to be older and have comorbid health conditions. SARS-CoV-2 infection acquired in pregnancy as compared with acquired outside of pregnancy was associated with a lower incidence of PASC (25.5% vs 33.9%; aHR 0.85, 95% CI 0.80–0.91). SARS-CoV-2 infection acquired in pregnant females was associated with increased risk for some PASC component diagnoses including abnormal heartbeat (aHR 1.67, 95% CI 1.43–1.94), abdominal pain (aHR 1.34, 95% CI 1.16–1.55), and thromboembolism (aHR 1.88, 95% CI 1.17–3.04), but decreased risk for other diagnoses including malaise (aHR 0.35, 95% CI 0.27–0.47), pharyngitis (aHR 0.36, 95% CI 0.26–0.48) and cognitive problems (aHR 0.39, 95% CI 0.27–0.56).
Interpretation
SARS-CoV-2 infection acquired during pregnancy was associated with lower risk of development of PASC at 30–180 days after incident SARS-CoV-2 infection in this nationally representative sample. These findings may be used to counsel pregnant and pregnant capable individuals, and direct future prospective study.
Funding
National Institutes of Health (NIH) Other Transaction Agreement (OTA) OT2HL16184.
Keywords: PASC, Pregnancy, SARS-CoV-2 infection
Research in context.
Evidence before this study
The COVID-19 pandemic has revealed extensive knowledge about the short-term effects and complications of Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) infection. Attention is now increasingly turning to long-term health after infection, termed post-acute sequelae of SARS-CoV-2 infection (PASC) or Long COVID. There is limited knowledge about PASC after SARS-CoV-2 infection during pregnancy, which represents a time of significant physiologic changes and altered immune adaptations. We searched PubMed with terms “PASC”, “COVID”, “long COVID”, “pregnancy”, and “pregnant” from database inception through September 26, 2023. We did not apply language restrictions. The PRIORITY study was identified, which identified 26% of individuals with persistent symptoms eight weeks post-SARS-CoV-2 infection among a cohort with COVID-19 during pregnancy or up to 6 weeks postpartum. No other studies were identified that described PASC following SARS-CoV-2 infection in pregnancy. The RECOVER Pregnancy Cohort is enrolling and plans to characterize the incidence and phenotype of PASC after SARS-CoV-2 infection in pregnancy but these results are not yet available.
Added value of this study
To our knowledge, this is the first study to describe the incidence of PASC following acquisition of SARS-CoV-2 infection during pregnancy, and to assess the association between acquisition of SARS-CoV-2 infection during pregnancy compared with acquired outside of pregnancy. In those with SARS-CoV-2 infection acquired during pregnancy, the incidence of PASC in the subsequent 30–180 days was 25.5%. The incidence of PASC was lower in those acquiring SARS-CoV-2 infection during pregnancy compared with acquiring SARS-CoV-2 infection outside of pregnancy (25.5% vs 33.9%; aHR 0.85, 95% CI 0.80–0.91).
Implications of all the available evidence
The results of this retrospective cohort demonstrate acquiring SARS-CoV-2 infection in pregnancy as compared with acquiring SARS-CoV-2 infection outside of pregnancy was associated with a lower incidence of PASC in the interval 30–180 days. These findings may be used to inform clinic counseling and care. Future prospective study is necessary to confirm and expand these findings.
Introduction
As the coronavirus disease 2019 (COVID-19) pandemic has evolved, the short-term course and complications from Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) infection are now well described.1 Increasingly, attention has turned to understanding the lingering and long-term health implications, known as post-acute sequelae of SARS-CoV-2 infection (PASC), or Long COVID. The World Health Organisation recognises PASC as symptoms occurring by three months post-SARS-CoV-2 infection, lasting for at least two months, and not explained by an alternative diagnosis.2 PASC has widespread effects with symptoms described in nearly every organ system, and studies suggest one-third of those with COVID-19 will go on to develop PASC.3 Ongoing research efforts are focused on describing the natural course of PASC, identifying risk factors for its development and understanding its incidence in varying populations.4
Little is known about PASC after SARS-CoV-2 infection acquired during pregnancy.5 Pregnancy-related COVID-19 is associated with worse perinatal outcomes and maternal morbidity, such as higher rates of preterm birth, hypertensive disorders of pregnancy, and thromboembolic events.6,7 It is unclear whether acquiring SARS-CoV-2 infection during pregnancy influences the likelihood of developing PASC. As pregnancy results in significant physiologic changes and differences in immune response, the risk for development of PASC may differ from the general population.
The National Institutes of Health (NIH) Researching COVID to Enhance Recovery (RECOVER) Initiative is dedicated to studying the long-term effects of COVID-19. Using electronic health record (EHR) datasets through the RECOVER Initiative, a machine learning computable phenotype definition of PASC was previously developed in the non-pregnant adult population.8 The objective of this study was to evaluate the association between acquiring SARS-CoV-2 infection during pregnancy and the development of PASC compared with acquiring SARS-CoV-2 infection outside of pregnancy in the RECOVER Initiative EHR dataset. We hypothesised the physiologic changes of pregnancy would have a protective effect resulting in a lower incidence of PASC in the 30–180 days following SARS-CoV-2 infection acquired in pregnancy.
Methods
Study design and participants
This was a retrospective cohort study using data available through the RECOVER Initiative Patient-Centred Clinical Research Network (PCORnet) EHR dataset. The RECOVER infrastructure leveraged PCORnet to develop a single, unified EHR repository to study PASC across health systems nationwide who continue to refresh their data at least quarterly. The PCORnet EHR dataset is comprised of data from inpatient and outpatient health care encounters for approximately 10 million patients across 19 U.S. health systems.9 Available data include demographics, diagnoses as defined by International Classification of Diseases Tenth Revision (ICD-10) codes, Current Procedural Terminology (CPT) codes, medications, and laboratory results.
For this analysis, females aged 18–49 years with a lab-confirmed SARS-CoV-2 infection between March 1, 2020 and June 1, 2022 were included. A laboratory-confirmed infection was defined by a positive nucleic acid or antigen test for SARS-CoV-2. If there were multiple laboratory-confirmed SARS-CoV-2 infections identified in the study period for an individual, the first positive test was considered as the incident date for SARS-CoV-2 infection in analysis. An acute SARS-CoV-2 infection period was defined from the date of a positive SARS-CoV-2 result through 30 days post-infection (Box 1A).
Box 1. SARS-CoV-2 infection and pregnancy periods.
(A) Baseline, acute SARS-CoV-2 infection, and post-acute SARS-CoV-2 infection (PASC) periods.
The baseline period was defined as the three years (y) prior to the date of laboratory-confirmed positive SARS-CoV-2 result (COVID-19 onset). The acute SARS-CoV-2 infection period was defined from the date of laboratory-confirmed positive SARS-CoV-2 result (COVID-19 onset) plus 30 days. The post-acute SARS-CoV-2 (PASC) period was defined from 30 to 180 days after the acute SARS-CoV-2 infection period.
(B) Pregnancy and postpartum period.
The pregnancy period, in weeks, is defined by the end of pregnancy (delivery date) minus the gestational age at the time of delivery. The postpartum period is defined from the delivery date through 6 weeks postpartum.
The primary exposure was acquiring SARS-CoV-2 during pregnancy compared with outside of pregnancy. Pregnancy was identified using a previously published algorithm of ICD-10 codes.10 In brief, this hierarchical approach excludes abortions (<20 weeks' gestation) and abnormal gestations (e.g., ectopic pregnancy, molar pregnancy), and then identifies pregnancies with an outcome of delivery at >20 weeks’ gestation. Using this approach, individuals with a pregnancy were identified and an end of pregnancy, or delivery, date assigned. The gestational age at delivery was assigned using a validated algorithm developed by Leonard aet al.11 A pregnancy period was defined for each pregnant female in the cohort calculated from the end of pregnancy (delivery) date minus the gestational age (in weeks) at delivery. A 6-week postpartum period was also defined for each pregnant female in the cohort calculated as 42 days from the end of pregnancy date (Box 1B). If multiple pregnancies were identified for an individual during the study period, the pregnancy occurring with a concurrent SARS-CoV-2 infection was included. If multiple pregnancies with a concurrent SARS-CoV-2 infection were identified, only the first pregnancy was included. Individuals with a pregnancy and non-concurrent SARS-CoV-2 infection were excluded from analysis (Box 2).
Box 2. Individuals included with SARS-CoV-2 infection and pregnancy.
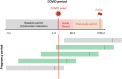 Individuals with SARS-CoV-2 infection during pregnancy (green) were included in analysis. Individuals with a SARS-CoV-2 infection and a pregnancy during the study period but not occurring concurrently (gray) were excluded from analysis.
Individuals with SARS-CoV-2 infection during pregnancy (green) were included in analysis. Individuals with a SARS-CoV-2 infection and a pregnancy during the study period but not occurring concurrently (gray) were excluded from analysis.
Outcomes
The primary outcome was PASC identified from 30 to 180 days after laboratory-confirmed SARS-CoV-2 infection among COVID-19 survivors. PASC was defined based on a previously established computable phenotype among non-pregnant adults in the RECOVER EHR dataset.8 In brief, the PASC phenotype was developed using data-driven computational analysis assessing for PASC signs, symptoms, or medications in a cohort of 35,275 adults (aged ≥20 years) with SARS-CoV-2 infection.8 Through this analysis, 25 conditions were identified as the key contributors to the PASC computable phenotype. For this analysis, PASC was defined as the presence of any of 24 conditions with anaemia excluded a priori based on the known high prevalence of anaemia in pregnant and postpartum individuals.12 Secondary outcomes were the 24 component conditions contributing to the PASC computable phenotype definition, including: diabetes mellitus, pressure ulcers, headache, thromboembolism, joint pain, abdominal pain, sleep disorders, cognitive problems, dyspnoea, oedema, encephalopathy, pulmonary fibrosis, hair loss, constipation, dementia, acute pharyngitis, pulmonary embolism, abnormal heartbeat, malnutrition, malaise and fatigue, chest pain, fever, fluid disorders, or PASC ICD code U099/B948. Each contributing condition was defined using ICD-10 codes (Appendix 1).
Statistics
Univariable comparisons of individual characteristics between females with SARS-CoV-2 infection acquired during pregnancy and females with SARS-CoV-2 infection acquired outside of pregnancy were performed with the standardised mean differences (SMD) reported. Baseline population characteristics assessed included self-reported race and ethnicity, age, body mass index (BMI), area deprivation index (ADI), and tobacco use. Prior studies have identified a higher risk for PASC among individuals with comorbid health conditions and severe COVID-19 disease.4,13 To assess for the presence of pre-existing health conditions (e.g., diabetes mellitus, hypertension), the Elixhauser Comorbidities Index was applied to the cohort for the baseline period of three years prior to the incident SARS-CoV-2 infection date.14 Among the pregnant population, gestational age at SARS-CoV-2 infection and delivery were summarised as median and interquartile range (IQR).
COVID-19 vaccination status was assessed, as prior vaccination has been found to be protective against severe COVID-19 and PASC.15,16 COVID-19 vaccine status was defined as documentation of full vaccination, partial vaccination, or no vaccination prior to the incident SARS-CoV-2 infection date. COVID-19 severity was assessed by location of care as outpatient or inpatient hospitalisation. Inpatient care was defined by hospitalisation in the time interval of one day prior to COVID-19 diagnosis through 16 days post-COVID-19 diagnosis. To reflect severe disease, inpatient care was further classified by intensive care unit (ICU) admission. Finally, the timepoint of SARS-CoV-2 infection within the pandemic timeline was considered. This timepoint functioned as a surrogate for SARS-CoV-2 variant and was included in the models as the Delta variant has been associated with higher COVID-19 complications in, and outside, of pregnancy.17
Using stabilised inverse probability of treatment weighting (IPTW) to adjust for baseline differences, the association between acquiring SARS-CoV-2 infection during pregnancy and PASC was modelled. The incident risk of PASC was reported as adjusted hazard ratio and 95% confidence interval calculated using a Cox proportional hazard model and Walk Chi–Square test. The same approach was used to consider each of the component diagnoses contributing to the PASC computable phenotype. Additionally, the cumulative incidence (CIF) and the difference of cumulative incidence per 100 persons was estimated at 180 days after the infection index date by pregnancy status.
As pregnancy is associated with a higher risk for venous thromboembolism, a sensitivity analysis was performed with the PASC phenotype re-defined excluding thromboembolism and pulmonary embolism as contributory conditions.18 A second sensitivity analysis was performed repeating the primary analyses using a propensity match approach.
Adjustment for multiple comparisons was made using a Bonferroni correction.19 Adjusted p values < 0.05 were considered statistically significant. All analyses were completed using Python 3.9.
Ethics
The RECOVER EHR initiative received Biomedical Research Alliance of New York (BRANY) institutional review board (IRB) approval #21-08-508. This retrospective analysis used deidentified data with a waiver of consent. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were followed for reporting.20
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
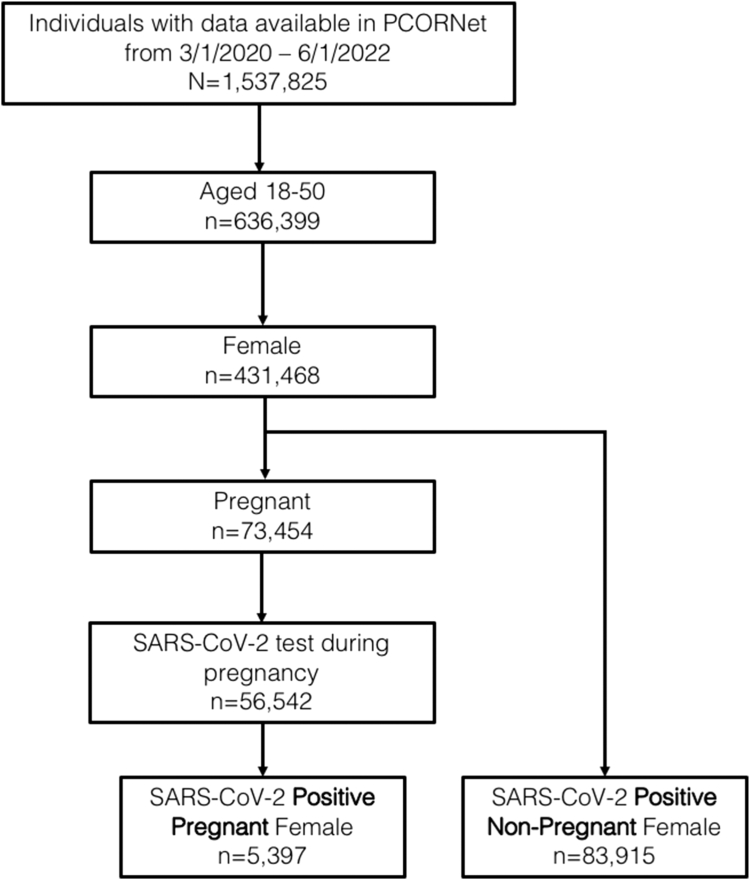
Overall, 83,915 females with SARS-CoV-2 infection acquired outside of pregnancy and 5397 females with SARS-CoV-2 infection acquired during pregnancy were identified within the RECOVER Initiative PCORnet EHR dataset from March 1, 2020 to June 1, 2022 and were included in the analysis (Fig. 1). Females with SARS-CoV-2 infection acquired outside of pregnancy compared with females with SARS-CoV-2 infection acquired during pregnancy were more likely to be older, non-Hispanic White, have documented full COVID-19 vaccination, and have comorbid conditions including chronic hypertension, chronic kidney disease, and class III obesity (Table 1). Females with SARS-CoV-2 infection acquired during pregnancy were more likely to be admitted to the hospital for inpatient management of COVID-19. Among those with SARS-CoV-2 infection acquired during pregnancy, the median gestational age at infection was 34 weeks (IQR 25–38) and the median gestational age at delivery was 39 weeks (IQR 37–39).
Fig. 1.
Study cohort.
Table 1.
Baseline characteristics of pregnant and non-pregnant females with SARS-CoV-2 infection.
| Baseline characteristic | Pregnant females N = 5397 | Non-pregnant females N = 83,915 | SMDa | SMD (weighted) |
|---|---|---|---|---|
| Race | ||||
| Asian | 225 (4.2) | 3368 (4.0) | 0.01 | 0.01 |
| Black | 1187 (22.0) | 17,834 (21.3) | 0.02 | 0.03 |
| Otherb | 969 (18.0) | 8091 (9.6) | 0.24 | 0.01 |
| White | 2377 (44.0) | 41,813 (49.8) | −0.12 | 0.03 |
| Ethnicity | ||||
| Hispanic | 1500 (27.8) | 12,005 (14.3) | 0.34 | 0.04 |
| Missing | 180 (3.3) | 8507 (10.1) | −0.27 | −0.14 |
| Non-Hispanic | 3717 (68.9) | 64,403 (75.6) | −0.15 | 0.05 |
| Age | 30 (26–34) | 35 (28–42) | −0.62 | 0.03 |
| Body mass index (kg/m2) | 31 (27–36) | 29 (24–36) | −0.07 | 0.03 |
| Area deprivation index | 38 (16–71) | 38 (15–67) | 0.05 | 0.09 |
| Tobacco use (current) | 157 (2.9) | 4866 (5.8) | −0.14 | 0.02 |
| Comorbid health conditions | ||||
| Asthma | 535 (9.9) | 8913 (10.6) | −0.02 | 0.04 |
| Chronic hypertension | 222 (4.1) | 10,815 (12.9) | −0.32 | −0.02 |
| Chronic kidney disease | 34 (0.6) | 2214 (2.6) | −0.16 | −0.05 |
| Chronic pulmonary disorders | 560 (10.4) | 10,347 (12.3) | −0.06 | 0.04 |
| Diabetes mellitus, type 1 or 2 | 148 (2.7) | 5425 (6.5) | −0.18 | −0.02 |
| Class III obesity | 360 (6.7) | 8255 (9.8) | −0.12 | −0.01 |
| Inflammatory bowel disorder | 27 (0.5) | 1080 (1.3) | −0.08 | −0.03 |
| Seizure disorder | 45 (0.8) | 1301 (1.6) | −0.07 | 0.02 |
| Mental health condition | 529 (9.8) | 13,389 (16.0) | −0.18 | 0.0 |
| Substance use | 273 (5.1) | 5527 (6.6) | −0.07 | 0.06 |
| Anemia | 542 (10.0) | 7574 (9.0) | 0.03 | 0.03 |
| Systemic lupus erythematosus | 23 (0.4) | 997 (1.2) | −0.09 | 0.01 |
| ICU admission for COVID-19 | 80 (1.5) | 911 (1.1) | 1.12 | 0.02 |
| COVID-19 vaccine status | ||||
| Fully vaccinated | 188 (3.5) | 10,270 (12.2) | −0.33 | −0.08 |
| Partially vaccinated | 130 (2.4) | 4681 (5.6) | −0.16 | 0.0 |
| Not vaccinated | 5082 (94.2) | 69,031 (82.3) | 0.38 | 0.07 |
| SARS-CoV-2 infection period | ||||
| 3/2020–6/2020 | 668 (12.4) | 5399 (6.4) | 0.20 | −0.02 |
| 7/2020–10/2020 | 721 (13.4) | 8777 (10.5) | 0.09 | 0.01 |
| 11/2020–2/2021 | 1321 (24.5) | 18,501 (22.0) | 0.06 | 0.01 |
| 3/2021–6/2021 | 554 (10.3) | 4873 (5.8) | 0.16 | 0.02 |
| 7/2021–10/2021 | 799 (14.8) | 9872 (11.8) | 0.09 | 0.02 |
| 11/2021–2/2022 | 1333 (24.7) | 31,160 (37.1) | −0.27 | −0.04 |
| 3/2022–6/2022 | 1 (0) | 5333 (6.4) | −0.37 | 0.0 |
Data are n (%) or median (interquartile range).
SMD, standardized mean difference; ICU, intensive care unit.
A standardized mean difference of >0.10 or <–0.10 indicates a meaningful effect size difference between the two samples.
Other includes American Indian or Alaska Native, Native Hawaiian or Other Pacific Islander, or identify as multiple races.
In stabilised inverse probability of treatment weighting for baseline covariate adjusted analysis, acquiring SARS-CoV-2 infection during pregnancy was associated with a lower incidence of PASC in the subsequent 30–180 days compared with acquiring SARS-CoV-2 infection outside of pregnancy (25.5% vs 33.9%; aHR 0.85, 95% CI 0.80–0.91; p < 0.001). The cumulative incidence of PASC in the 180 days following the incident infection date was 30.8 per 100 persons among those with SARS-CoV-2 infection acquired during pregnancy compared with 35.8 per 100 persons among those with SARS-CoV-2 infection acquired outside of pregnancy (Appendix 2).
When considering the 24 component diagnoses contributing to the overall PASC computable phenotype, SARS-CoV-2 infection acquired during pregnancy was associated with a lower incidence of joint pain, sleep disorders, cognitive problems, dyspnoea, encephalopathy, hair loss, acute pharyngitis, malnutrition, malaise and fatigue, chest pain, and ICD-10 code defined-PASC. SARS-CoV-2 infection acquired during pregnancy compared with acquired outside of pregnancy was associated with a higher incidence of abnormal heartbeat, abdominal pain, and thromboembolism (Table 2).
Table 2.
Association between SARS-CoV-2 infection in pregnant females compared with non-pregnant females and PASC component diagnoses.
| PASC diagnoses | Rate (%) among pregnant females n = 5397 | Rate (%) among non-pregnant females n = 83,915 | aHR (95% CI) | Corrected p value |
|---|---|---|---|---|
| Diabetes mellitus | 0.48 | 0.71 | 0.76 (0.47–1.21) | 0.22 |
| Pressures ulcers | 0 | 0.08 | 0 (0–0) | 0.07 |
| Headache | 4.63 | 5.57 | 1.07 (0.89–1.27) | 0.41 |
| Thromboembolism | 0.50 | 0.35 | 1.88 (1.17–3.04) | 0.003 |
| Joint pain | 5.57 | 8.42 | 0.74 (0.63–0.87) | <0.001 |
| Abdominal pain | 9.41 | 8.96 | 1.34 (1.16–1.55) | <0.001 |
| Sleep disorders | 1.00 | 2.47 | 0.53 (0.39–0.74) | <0.001 |
| Cognitive problems | 0.80 | 2.46 | 0.39 (0.27–0.56) | <0.001 |
| Dyspnea | 4.15 | 8.80 | 0.55 (0.45–0.66) | <0.001 |
| Edema | 3.26 | 5.04 | 0.82 (0.67–1.0) | 0.022 |
| Encephalopathy | 0.21 | 0.71 | 0.41 (0.20–0.85) | 0.002 |
| Pulmonary fibrosis | 0.66 | 0.82 | 0.80 (0.53–1.20) | 0.25 |
| Hair loss | 0.49 | 1.54 | 0.35 (0.22–0.58) | <0.001 |
| Constipation | 2.41 | 2.36 | 1.04 (0.82–1.31) | 0.73 |
| Dementia | 0 | 0.06 | 0 (0–0) | 0.13 |
| Acute pharyngitis | 1.46 | 5.06 | 0.36 (0.26–0.48) | <0.001 |
| Pulmonary embolism | 0.28 | 0.28 | 1.09 (0.57–2.11) | 0.76 |
| Abnormal heartbeat | 6.16 | 4.40 | 1.67 (1.43–1.94) | <0.001 |
| Malnutrition | 0.04 | 0.31 | 0.12 (0.03–0.42) | 0.002 |
| Malaise and fatigue | 1.41 | 4.68 | 0.35 (0.27–0.47) | <0.001 |
| Chest pain | 2.62 | 4.21 | 0.69 (0.56–0.86) | <0.001 |
| Fever | 1.0 | 1.65 | 0.77 (0.54–1.08) | 0.08 |
| Fluid disorders | 1.07 | 1.04 | 1.23 (0.88–1.72) | 0.16 |
| ICD codes (U099/B948) | 0.26 | 0.86 | 0.37 (0.19–0.72) | <0.001 |
PASC, post-acute sequelae of SARS-CoV-2 infection; aHR, adjusted hazard ratio; CI, confidence interval.
In sensitivity analysis with thromboembolism and pulmonary embolism removed as conditions contributing to the PASC computable phenotype definition, SARS-CoV-2 infection acquired during pregnancy was still associated with a lower incidence of PASC (25.2% vs 33.7%; aHR 0.84, 95% CI 0.79–0.90; corrected p < 0.001). In the second sensitivity analysis, SARS-CoV-2 infection acquired during pregnancy compared with propensity score matched females acquiring SARS-CoV-2 infection outside of pregnancy remained associated with a lower incidence of PASC (aHR 0.76, 95% CI 0.71–0.82; Appendix 3).
Discussion
In this population-based retrospective cohort study of COVID-19 survivors, SARS-CoV-2 infection acquired during pregnancy was associated with a lower risk of developing PASC at 30–180 days after infection compared with SARS-CoV-2 infection acquired outside of pregnancy. Similarly, multiple components of the computable PASC phenotype were seen at lower frequencies among those who acquired SARS-CoV-2 infection during pregnancy compared with acquired outside of pregnancy.
Early in the pandemic, clinical care and research efforts focused on prevention of the acute morbidity and mortality caused by SARS-CoV-2 infection. Over time, the lasting health implications of COVID-19 have become increasingly pressing with an ongoing need for further evidence to inform clinical care. Studies have consistently identified a host of symptoms described in nearly every organ system in the months following SARS-CoV-2 infection.21 Huang aet al described the 6-month follow-up of 1733 individuals hospitalised in Wuhan, China in 2020 for COVID-19 and surviving to hospital discharge with fatigue and muscle weakness (63%), sleep abnormalities (26%), and anxiety or depression (23%) being common.22
In the RECOVER adult cohort of 9764 adults with and without SARS-CoV-2 infection, 37 symptoms were identified with increased odds ratios among those infected (aOR >1.5) compared with those uninfected at 6-month follow-up. A symptom-based score was developed from these results to define PASC and application of this score identified 23% of SARS-CoV-2 infected individuals developed PASC at 6 months in the same cohort.23 Some symptoms varied by sub-populations in this analysis, which is consistent with other literature describing variable PASC phenotypes, as well as varying incidence of PASC dependant on population risk factors.24
In a population-level study in the United Kingdom, Subramaniam aet al evaluated symptoms of, and risk factors for, PASC among non-hospitalised adults with SARS-CoV-2 infection.25 Among those with at least 12 weeks of follow up after index date, SARS-CoV-2 infection compared with no infection was associated with increased likelihood of experiencing at least one of the WHO long COVID symptoms (5.4% vs 4.3%; aHR 1.26, 95% CI 1.25–1.28). Risk factors most associated with development of PASC symptoms included female sex (aHR 1.52, 95% CI 1.48–1.56), socio-economic deprivation (aHR 1.11, 95% CI 1.07–1.16) and obesity (aHR 1.10, 95% CI 1.07–11.14).25 Consistently identified risk factors for the development of PASC have included severe COVID-19, middle age (40–69 years), female sex, strong antibody response, and the presence of comorbid health conditions including obesity, diabetes, obstructive sleep apnoea, and chronic lung disease.4,26,27 Protective factors have also been identified, including COVID-19 vaccination.15
Prior work has limitedly described differences in PASC following SARS-CoV-2 infection acquired during pregnancy. An interim report from the Pregnancy CoRonavIrus Outcomes RegIsTrY (PRIORITY) study, a prospective cohort study of pregnant and up to 6-week postpartum individuals with SARS-CoV-2 infection, described 26% of SARS-CoV-2 infected participants (n = 155/594) enrolled in 2020 having persistent symptoms eight weeks post-infection.7 Neither data from a non-pregnant comparison group nor follow-up beyond 8 weeks were reported. In a 2022 cross sectional study of pregnant and non-pregnant females in Ecuador, an anonymous 37-item online questionnaire assessed self-reported long COVID symptoms.28 Thirty three of 457 respondents were pregnant; 16 pregnant responders (48.5%) self-reported long COVID symptoms including fatigue (10.6%), hair loss (9.6%), and difficulty concentrating (6.2%). There were no differences in symptoms reported between pregnant and non-pregnant individuals.28 The RECOVER Pregnancy Cohort is currently enrolling pregnant individuals exposed and unexposed to SARS-CoV-2 infection during pregnancy and aims to characterise the incidence and phenotype of PASC following SARS-CoV-2 infection in pregnancy.5 These results will be informative for clinical care, counselling of pregnant individuals with SARS-CoV-2 infection, and public health policy, but are not yet available. To our knowledge, the current study is the first to describe a lower incidence of PASC following acquisition of SARS-CoV-2 infection in pregnancy (PubMed search on 9/26/2023 with search terms PASC, COVID, long COVID, pregnancy, pregnant).
Acquiring SARS-CoV-2 infection during pregnancy as a protective factor against interval PASC differs from the well-described association between COVID-19 in pregnancy and worse perinatal outcomes and maternal morbidity.29,30 In a retrospective cohort study of 14,104 individuals delivering across 17 U.S. hospitals in 2020, those with SARS-CoV-2 infection were more likely to experience maternal mortality or severe morbidity from postpartum hemorrhage, infection, or hypertensive disorders of pregnancy than those without SARS-CoV-2 infection (13.4% vs 9.2%; aRR 1.41, 95% CI 1.23–1.61).6 SARS-CoV-2 infection in pregnancy has also been associated with increased risk for intensive care unit admission, need for mechanical ventilation, and cardiovascular complications among other acute sequelae.31,32 Despite these apparent contradictions of SARS-CoV-2 infection in pregnancy—worse immediate perinatal outcomes but reduced risk for interval PASC—findings likely reflect maternal immune adaptations of pregnancy.
Physiologic changes of pregnancy create a described immune tolerant state. Alterations in cytokine, complement, and T cell regulation, as well as local changes at the uterine–placenta interface, accommodate foetal protection.33 Worse outcomes described with SARS-CoV-2 infection, as well as other viruses such as influenza, during pregnancy may reflect this relatively tolerant immune environment.34 We postulate that these same adaptations down-regulate the robust immune response, thereby causing less inflammatory cellular damage, and may be contributing to a lower incidence of interval PASC.35 These immunoregulatory changes require further elucidation but are beyond the scope of the current study.
Acquiring SARS-CoV-2 infection in pregnancy was associated with an increased likelihood of some PASC phenotype contributing diagnoses, including thromboembolism, abdominal pain, and abnormal heart beat in this study. The risk for thromboembolism is known to be higher during pregnancy and postpartum resulting from a relative increase in prothrombotic factors and venous stasis.18 The association identified between acquiring SARS-CoV-2 infection in pregnancy and thromboembolism may reflect the physiologic effects of pregnancy, rather than PASC itself. The association between acquiring SARS-CoV-2 infection during pregnancy and increased abdominal pain and abnormal heart beat, similarly, may reflect normal pregnancy physiology; however, these differences in physiology (higher prevalence of some PASC symptoms in pregnancy) would move the aHR towards the null and we found a significant association.
This study has limitations. The use of ICD-10 codes in an EHR dataset risks misclassification and under ascertainment. All individuals included underwent SARS-CoV-2 testing but we cannot address indication for testing in this analysis. Further, individuals with SARS-CoV-2 infection that did not undergo testing or only underwent home testing were not captured in this analysis. While there was sufficient sample size to evaluate the primary outcome of PASC, there was insufficient sample size for sub-analyses by SARS-CoV-2 variant (e.g., Delta, Omicron), gestational age at time of SARS-CoV-2 infection, COVID-19 disease severity, or COVID-19 vaccination status. However, these variables were considered in the weighted modelling approach. We excluded pregnancies at <20 weeks' gestation and therefore the findings are not generalisable to individuals with SARS-CoV-2 infection without an ongoing pregnancy beyond 20 weeks’ gestation. There is also a possibility of residual confounding.
This study also has strengths. We evaluated PASC in individuals acquiring SARS-CoV-2 infection during pregnancy, which has not previously been reported. The selected PCORnet EHR-based study cohort reflects the geographic and racial diversity of the U.S. increasing generalisability of the findings. A data driven analysis approach using inverse probability of treatment weighting allowed for robust modelling and adjustment for confounders.36 Findings were stable in a sensitivity analysis using a propensity score-matched cohort.
In conclusion, we found that acquiring SARS-CoV-2 infection during pregnancy as compared with acquiring SARS-CoV-2 infection outside of pregnancy was associated with a lower incidence of PASC at 30–180 days after infection. The current findings further our understanding of PASC after acquiring SARS-CoV-2 infection in pregnancy to inform patient counselling and direct future research. Further prospective study is necessary to confirm these findings.
Contributors
AMB, CZ, FW, MGW, TWC, and TDM developed the study protocol and analysis plan. CZ and FW accessed, verified, and analysed the data. AMB, CZ, FW, MGW, NG, MF, VJ, TWC, and TDM interpreted the data. AMB and TDM drafted the manuscript. AMB, CZ, FW, MGW, NG, MF, VJ, TWC, and TDM edited the manuscript. All authors had access to the data and accept responsibility for submitting the article for publication.
Data sharing statement
The de-identified data used in this analysis are considered the domain of the contributing health systems. These data were shared with the RECOVER research programme under data sharing agreements. Requests for deidentified data can be considered through the RECOVER research programme but would need the approval of the contributing health systems.
Declaration of interests
Dr. Metz is the site Principal Investigator (PI) for a Pfizer RSV vaccination study that is now complete and a Pfizer pharmacokinetic study of Paxlovid for mild to moderate COVID-19 in pregnancy. She also served on the Medical Advisory Board and is a site PI for a Pfizer SARS-CoV-2 vaccination study in pregnancy. Megan Fitzgerald was compensated for work evaluating COVID re-infections funded through the Patient Lead Research Collaborative. This content is solely the responsibility of the authors and does not necessarily represent the official views of the RECOVER Programme, the National Institutes of Health (NIH), or other funders. This study is part of the NIH Researching COVID to Enhance Recovery (RECOVER) Initiative, which seeks to understand, treat, and prevent the post-acute sequelae of SARS-CoV-2 infection (PASC). For more information on RECOVER, visit https://recovercovid.org/.
Acknowledgements
This research was funded by the National Institutes of Health (NIH) Other Transaction Agreement (OTA) OT2HL161847 (contract number EHR-01-21) as part of the Researching COVID to Enhance Recovery (RECOVER) research programme. Dr. Metz received funding for this work through the National Heart, Lung, and Blood Institute (NHLBI) OT2HL161847. We would like to thank the National Community Engagement Group (NCEG), all patient, caregiver and community Representatives, and all the participants enrolled in the Researching COVID to Enhance Recovery (RECOVER) Initiative. Data contributing sites are included in supplement.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102654.
Appendix A. Supplementary data
References
- 1.National Institutes of Health (NIH) Coronavirus disease 2019 COVID-19 treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/ [PubMed]
- 2.World Health Organization (WHO) A clinical case definition of post COVID-19 condition by a Delphi consensus. https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 [DOI] [PMC free article] [PubMed]
- 3.Akbarialiabad H., Taghrir M.H., Abdollahi A., et al. Long COVID, a comprehensive systematic scoping review. Infection. 2021;49:1163–1186. doi: 10.1007/s15010-021-01666-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hill E., Mehta H., Sharma S., et al. Risk factors associated with post-acute sequelae of SARS-CoV-2 in an EHR cohort: a national COVID cohort collaborative (N3C) analysis as part of the NIH RECOVER program. medRxiv. 2022 doi: 10.1101/2022.08.15.22278603. [DOI] [Google Scholar]
- 5.Metz T.D., Clifton R.G., Gallagher R., et al. Researching COVID to enhance recovery (RECOVER) pregnancy study: rationale, objectives and design. medRxiv. 2023 doi: 10.1101/2023.04.24.23289025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Metz T.D., Clifton R.G., Hughes B.L., et al. Association of SARS-CoV-2 infection with serious maternal morbidity and mortality from obstetric complications. JAMA. 2022;327:748–759. doi: 10.1001/jama.2022.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Afshar Y., Gaw S.L., Flaherman V.J., et al. Clinical presentation of coronavirus disease 2019 (COVID-19) in pregnant and recently pregnant people. Obstet Gynecol. 2020;136:1117–1125. doi: 10.1097/AOG.0000000000004178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zang C., Zhang Y., Xu J., et al. Data-driven analysis to understand long COVID using electronic health records from the RECOVER initiative. Nat Commun. 2023;14:1948. doi: 10.1038/s41467-023-37653-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaushal R., Hripcsak G., Ascheim D.D., et al. Changing the research landscape: the New York City clinical data research Network. J Am Med Inf Assoc. 2014;21:587–590. doi: 10.1136/amiajnl-2014-002764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clapp M.A., James K.E., Friedman A.M. Identification of delivery encounters using international classification of diseases, Tenth revision, diagnosis and procedure codes. Obstet Gynecol. 2020;136:765–767. doi: 10.1097/AOG.0000000000004099. [DOI] [PubMed] [Google Scholar]
- 11.Leonard S.A., Panelli D.M., Gould J.B., Gemmill A., Main E.K. Validation of ICD-10-CM diagnosis codes for gestational age at birth. Epidemiology. 2023;34:64–68. doi: 10.1097/EDE.0000000000001557. [DOI] [PubMed] [Google Scholar]
- 12.Anemia in Pregnancy ACOG Practice Bulletin, No. 233. American college of obstetricians and gynecologists. Obstet Gynecol. 2021;138:e55–e64. doi: 10.1097/AOG.0000000000004477. [DOI] [PubMed] [Google Scholar]
- 13.Nalbandian A., Sehgal K., Gupta A., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elixhauser A., Steiner C., Harris D.R., Coffey R.M. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Brannock M.D., Chew R.F., Preiss A.J., et al. Long COVID risk and pre-COVID vaccination in an EHR-based cohort study from the RECOVER program. Nat Commun. 2023;14:2914. doi: 10.1038/s41467-023-38388-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hui L., Marzan M.B., Rolnik D.L., et al. Reductions in stillbirths and preterm birth in COVID-19-vaccinated women: a multicenter cohort study of vaccination uptake and perinatal outcomes. Am J Obstet Gynecol. 2023;228:585.e1–585.e16. doi: 10.1016/j.ajog.2022.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menni C., Valdes A.M., Polidori L., et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study. Lancet. 2022;399:1618–1624. doi: 10.1016/S0140-6736(22)00327-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thromboembolism in Pregnancy ACOG Practice Bulletin, No. 196. American College of Obstetrician and Gynecologists. Obstet Gynecol. 2018;132:e1–e17. doi: 10.1097/AOG.0000000000002706. [DOI] [PubMed] [Google Scholar]
- 19.Bender R., Lange S. Adjusting for multiple testing--when and how? J Clin Epidemiol. 2001;54:343–349. doi: 10.1016/s0895-4356(00)00314-0. [DOI] [PubMed] [Google Scholar]
- 20.von Elm E., Altman D.G., Egger M., et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 21.Groff D., Sun A., Ssentongo A.E., et al. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: a systematic review. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang C., Huang L., Wang Y., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thaweethai T., Jolley S.E., Karlson E.W., et al. Development of a definition of postacute sequelae of SARS-CoV-2 infection. JAMA. 2023;329:1934–1946. doi: 10.1001/jama.2023.8823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang H., Zang C., Xu Z., et al. Data-driven identification of post-acute SARS-CoV-2 infection subphenotypes. Nat Med. 2023;29:226–235. doi: 10.1038/s41591-022-02116-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Subramanian A., Nirantharakumar K., Hughes S., et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat Med. 2022;28:1706–1714. doi: 10.1038/s41591-022-01909-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crook H., Raza S., Nowell J., et al. Long covid-mechanisms, risk factors, and management. BMJ. 2021;374:n1648. doi: 10.1136/bmj.n1648. [DOI] [PubMed] [Google Scholar]
- 27.Hirschtick J.L., Xie Y., Slocum E., et al. A statewide population-based approach to examining Long COVID symptom prevalence and predictors in Michigan. Prev Med. 2023;177 doi: 10.1016/j.ypmed.2023.107752. [DOI] [PubMed] [Google Scholar]
- 28.Vásconez-González J., Fernandez-Naranjo R., Izquierdo-Condoy J.S., et al. Comparative analysis of long-term self-reported COVID-19 symptoms among pregnant women. J Infect Public Health. 2023;16:430–440. doi: 10.1016/j.jiph.2023.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jering K.S., Claggett B.L., Cunningham J.W., et al. Clinical characteristics and outcomes of hospitalized women giving birth with and without COVID-19. JAMA Intern Med. 2021;181:714–717. doi: 10.1001/jamainternmed.2020.9241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Metz T.D., Clifton R.G., Hughes B.L., et al. Disease severity and perinatal outcomes of pregnant patients with coronavirus disease 2019 (COVID-19) Obstet Gynecol. 2021;137:571–580. doi: 10.1097/AOG.0000000000004339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Briller J.E., Aggarwal N.R., Davis M.B., et al. Cardiovascular complications of pregnancy-associated COVID-19 infections. JACC Adv. 2022;1 doi: 10.1016/j.jacadv.2022.100057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith E.R., Oakley E., Grandner G.W., et al. Clinical risk factors of adverse outcomes among women with COVID-19 in the pregnancy and postpartum period: a sequential, prospective meta-analysis. Am J Obstet Gynecol. 2023;228:161–177. doi: 10.1016/j.ajog.2022.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mor G., Cardenas I. The immune system in pregnancy: a unique complexity. Am J Reprod Immunol. 2010;63:425–433. doi: 10.1111/j.1600-0897.2010.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hussain T., Murtaza G., Kalhoro D.H., et al. Understanding the immune system in fetal protection and maternal infections during pregnancy. J Immunol Res. 2022;2022 doi: 10.1155/2022/7567708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saadedine M., El Sabeh M., Borahay M.A., Daoud G. The influence of COVID-19 infection-associated immune response on the female reproductive system. Biol Reprod. 2023;108:172–182. doi: 10.1093/biolre/ioac187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Austin P.C., Stuart E.A. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661–3679. doi: 10.1002/sim.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.