Abstract
The neuromuscular junction (NMJ) is a specialized chemical synapse that converts neural impulses into muscle action. Age-associated NMJ degeneration, which involves nerve terminal and postsynaptic decline, denervation, and loss of motor units, significantly contributes to muscle weakness and dysfunction. Although physical training has been shown to make substantial modifications in NMJ of both young and aged animals, the results are often influenced by methodological variables in existing studies. Moreover, there is still lack of strong consensus on the specific effects of exercise on improving the morphology and function of the ageing NMJ. Consequently, the purpose of this study was to conduct a systematic review to elucidate the effects of exercise training on NMJ compartments in the elderly.
We conducted a systematic review using PubMed, Embase, and Web of Science databases, employing relevant keywords. Two independent reviewers selected studies that detailed NMJ changes during exercise in ageing, written in English, and available in full text.
In total, 20 papers were included. We examined the altered adaptation of the NMJ to exercise, focusing on presynaptic and postsynaptic structures and myofibers in older animals or humans. Our findings indicated that aged NMJs exhibited different adaptive responses to physical exercise compared to younger counterparts. Endurance training, compared with resistance and voluntary exercise regimens, was found to have a more pronounced effect on NMJ structural remodeling, particularly in fast twitch muscle fibers. Physical exercise was observed to promote the formation and maintenance of acetylcholine receptor (AChR) clusters by increasing the recombinant docking protein 7 (Dok7) expression and stabilizing Agrin and lipoprotein receptor-related protein 4 (LRP4). These insights suggest that research on exercise-related therapies could potentially attenuate the progression of neuromuscular degeneration.
Translational potential of this article: This systematic review provides a detailed overview of the effects of different types of physical exercise on improving NMJ in the elderly, providing scientific support for the timely intervention of muscle degeneration in the elderly by physical exercise, and providing help for the development of new therapeutic interventions in the future.
Keywords: Ageing, Exercise, Neuromuscular junction, Physical training, Systematic review
Graphical abstract
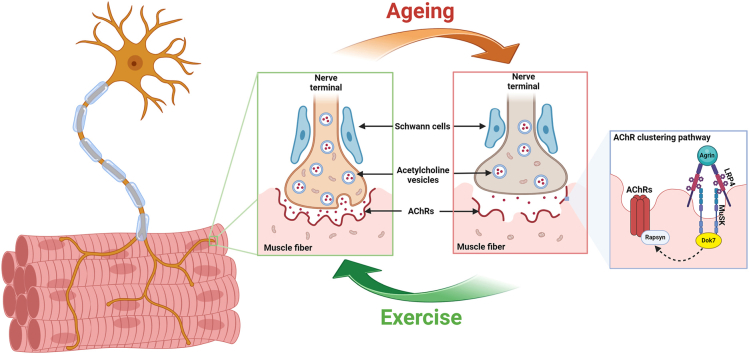
During ageing, the neuromuscular junction undergoes a continuous process of degeneration and remodeling. This process includes the atrophy of nerve terminals, widening of the synaptic cleft, fewer postsynaptic membrane folds and increased fragmentation. These changes occur alongside denervation and mitochondrial dysfunction. The pathway responsible for acetylcholine receptor clustering (Agrin-LRP4-MuSK-Rapsyn-Dok7) was downregulated. However, exercise training can decelerate these degenerative alternations.
Translational potential of this article: This systematic review provides a detailed overview of the effects of different types of physical exercise on improving NMJ in the elderly, providing scientific support for the timely intervention of muscle degeneration in the elderly by physical exercise, and providing help for the development of new therapeutic interventions in the future.
1. Introduction
The neuromuscular junction (NMJ) is a highly specialized subcellular structure composed of pre- and postsynaptic compartments and surrounded by Schwann cells. It is located between motor neuron terminals and muscle fibers, effectively converting neural electrical impulses into muscular contractile responses [[1], [2], [3]]. When the action potential reaches the NMJ, the voltage-gated calcium channel of the nerve terminal is activated and calcium enters the nerve, triggering the release of acetylcholine (Ach) from the nerve terminal to the synaptic cleft and then bind to acetylcholine receptors (AChRs) on the postsynaptic membrane. In response to the AChR activation, the myotubes depolarize, causing the release of calcium from the sarcoplasmic reticulum to trigger muscle contraction and movement [4]. The coordinated interplay of presynaptic motor nerve terminals, synaptic cleft, postsynaptic receptors, and the Schwann cells enveloping them forms an efficient and sophisticated electrochemical transmission synapse [5,6]. Agrin, low-density lipoprotein receptor-related protein 4 (LRP4), muscle-specific kinase (MuSK), Dok7 and Rapsyn are essential for the formation and maintenance of the NMJ. Agrin, a neuroterminal secretory protein, is released from nerve terminals into the synaptic cleft where it binds to the LRP4 on the postsynaptic membrane, activating MuSK. With the regulation of Dok7, the activated LRP4/MuSK complex promotes the aggregation and reinforcement of AChR clusters on the postsynaptic membrane. These synapses remain stable in young vertebrates, while ageing introduces a range of functional and morphological alterations in NMJs [7,8], such as nerve terminal sprouting, postsynaptic fragmentation, reduced density of AChRs and synaptic decoupling [9]. These morphological adaptations are associated with a decline in neuromuscular transmission and muscle weakness [10,11]. Age-related degeneration in NMJ also correlates with chronic cycles of muscle fiber denervation and re-innervation, leading to an increase in the proportion of type I fibers and fiber grouping [12,13]. Typically, type I fibers contain a higher proportion of mitochondria than type II fibers [14]. Consequently, a decrease in oxidative capacity can be compensated by a phenotypic shift from oxidative to more glycolytic type I fibers, which can partially restore muscle strength and energy production, albeit at the cost of increased lactic acid production [15,16]. This age-related shift towards type I fiber dominance triggers morphological and functional changes in NMJ [17].
Recent advancements in research have led to the exploration of various strategies, including physical therapy [[18], [19], [20]], medication [21], and exercise [22,23], aimed at decelerating or even reversing NMJ degeneration. Of these, exercise training has gathered significant interest, particularly regarding its application in the elderly [[23], [24], [25]]. A number of studies have established that NMJ morphology in older individuals is more susceptible to changes associated with reduced physical activity [2,26]. Physical exercise is crucial for enhancing physical fitness by increasing muscle strength, fortifying cardiopulmonary function and stimulating metabolic activity [27]. However, the body's response to exercise alters with age. While a consensus has been reached that physical exercise can enhance NMJ in various ways in young rodents, its efficacy in older animals remains to be elucidated. Some studies have reported that exercise induces muscle hypertrophy in young mice but causes weight loss in older ones [28].
To comprehensively understand the effects of exercise on age-related NMJ degeneration, a systematic review was conducted on the morphological, functional and gene expression changes in NMJs following exercise intervention, including those related to nerve terminals, postsynaptic receptors and muscle fibers.
2. Methods
2.1. Search strategy
A systematic literature search was carried out on 30 October 2023 across PubMed, Embase and Web of science databases to identify relevant publications and extract corresponding results. The search strategy employed the following keywords: (exercise OR training) AND (neuromuscular junction OR myoneural junction OR NMJ*) AND (ageing OR aging OR aged OR age). Additionally, the reference lists of included studies and reviews were manually scrutinized for further relevant studies. The study adhered to the PRISMA guidelines.
2.2. Selection criteria
Inclusion criteria included the studies: (1) focused on morphology of NMJ, (2) focused on functional changes of NMJ or mechanism of NMJ following exercise, (3) with full-text articles published in English.
Exclusion criteria were as follows: (1) non-English papers, (2) unavailability of full-text access (including papers that have been newly accepted but not yet available online or some papers that are too dated to retrieve the full text), (3) irrelevance to NMJ, (4) review articles, (5) reports published solely as conference abstracts.
2.3. Study selection
The study selection and data extraction were independently conducted by two reviewers. The initial screening involved the exclusion of clearly irrelevant papers based on their titles and abstracts. Duplicate citations were identified and eliminated using the Endnote reference manager. The remaining potentially relevant articles were assessed in accordance with the inclusion and exclusion criteria. Any disagreements were resolved through discussion with a third reviewer.
2.4. Data extraction
The following information was extracted by two independent reviewers: author, year of publication, animal model, age range, methodology, muscle types, morphological results, functional results, gene/protein expression data and other related data concerning NMJ alternations post-exercise. All studies that met the pre-defined criteria were included in the analysis.
2.5. Data analysis
Given that this study included 3 clinical studies and the animal studies incorporated diverse animal models and methodologies, the data exhibited high heterogeneity. As such, a meta-analysis was deemed unsuitable. Instead, we opted for a qualitative review.
3. Results
3.1. Search results
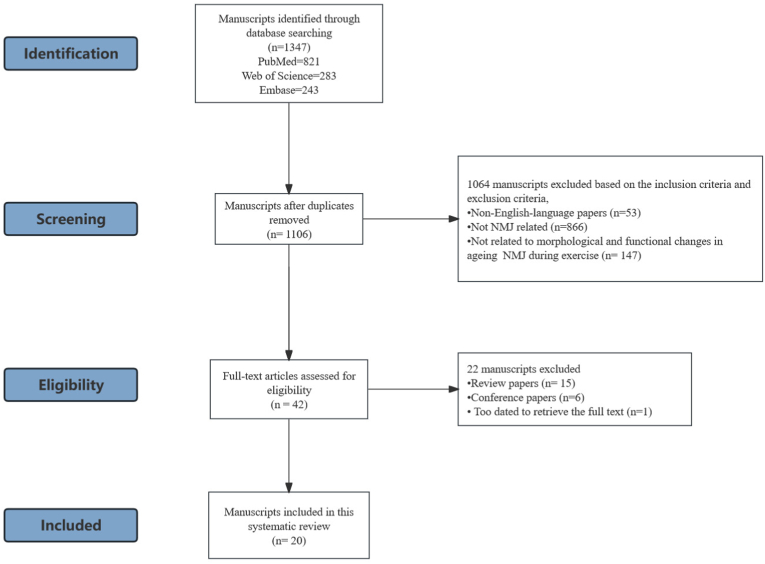
The initial search strategy included 1347 papers from 3 databases (821 from PubMed, 243 from Embase and 283 from Web of Science). After the removal of duplicates, 1106 papers were remained for screening based on selection criteria. Following the evaluation of titles and abstracts, 1064 manuscripts were excluded due to non-compliance with the criteria. Among these, 53 were non-English-language papers, 866 were not related to NMJ and 147 were not associated with morphological and functional changes in ageing NMJ during exercise. After conducting a full-text eligibility assessment, 22 manuscripts were excluded, including 15 review papers, 6 conference reports and 1 paper was too dated to retrieve the full text. Ultimately, 20 studies were selected for systematic analysis [[22], [23], [24], [25],[28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43]]. The flowchart detailing the selection process is shown in Fig. 1.
Figure 1.
Flow diagram for the literature selection process.
3.2. Characteristics of the included studies
The 20 selected studies were conducted between 1984 and 2023. Among these, 11 studies utilized mice [22,25,[28], [29], [30], [31], [32],34,37,41,42], 6 utilized rats [23,24,33,36,38,39] and 3 involved human subjects [35,40,43]. The characteristics of the included studies are shown in Table 1. Furthermore, the exercise protocols varied among the included studies. 5 studies used voluntary running wheel exercise [22,32,34,37,42], 6 studies used endurance training with employed treadmill running [23,29,31,33,36,39] and 6 resistance training with 3 treadmill running [30,38,41], 2 wheel running exercise [25,28] and 1 incorporated ladder climbing for training [24]. The 3 included clinical studies had participants undergoing specifically designed resistance training regimens [35,40,43].
Table 1.
Characteristics of included studies.
| Study | Strain, species | Gender | Age | Training mode | Training protocol | Duration | Muscle types | Methods |
|---|---|---|---|---|---|---|---|---|
| Deschenes et al. [24] (2015) | Fisher 344 rats | Male | 9, 20 months | Resistance exercise | Ladder climbing Animals climbed a 1 m long ladder with an 85° Angle, with additional weights attached to their tails. Each training session featured eight repetitions of ladder climbing, and added resistance was initially set at 50 % of body mass with 30 g increments added weekly. |
3 days/week for 7 weeks | Soleus, Plantaris | Cytofluorescence staining Histochemical Staining |
| Cheng et al. [34] (2013) | C57BL/6J mice | Female | 8 months | Voluntary Exercise | Wheel running A low profile wireless running wheel was placed in mouse cage (2–4 mice per cage). The average distance run was recorded by dividing the total distance by the number of mice in the cage. |
from 8 months to sacrifice | TA | Cytofluorescence staining |
| Chugh et al. [22] (2021) | C57BL/6J mice | Female and Male | 22 months | Voluntary exercise | Wheel running Mice were single housed in individual standard cages fitted with voluntary running wheels. The distance run per day for each mouse was calculated by dividing the total distance by the total number of days |
5 months | Gastrocnemius and soleus | Single fiber electromyography Western blot ELISA |
| Valdez et al. [30] (2010) | Transgenic mice C57BL/6J and CD-1 wild-type mice |
— | 22 months | Voluntary Exercise | Wheel running Mice were housed in cages with monitored running wheels. Running distance was monitored electronically |
1 month | TA, gracilis and GM | NMJ time-lapse imaging |
| Yano et al. [31] (2017) | Balb/c mice | Male | 8 months | Voluntary exercise | Wheel running Mice in exercise groups were individually housed in plastic cages equipped with a running wheel. Exercise volume of each mouse in the training groups was recorded. |
6 months | EDL | Cytofluorescence staining (anti-Synapsin 1, α-BTX) QPCR |
| Sugita et al. [32] (2021) | BALB/c mice and C57BL/6J mice | Male | 6.18 months | Voluntary exercise | Wheel running The exercise group was housed in plastic cages with running wheels |
to 23 months old | EDL and GM | Cytofluorescence staining Toluidine blue staining Western blot analysis |
| Fahim et al. [33] (1997) | C57BL/6NNia mice | Male | 10, 28 months | Endurance exercise | Treadmill running Initially, the exercise began with a moderate walk-jog pace of 15–20 m/min for 30 min/day. The velocity and duration of the exercise were gradually increased until they could run between 25 and 28 m/min for 60 min/day. The animals were exercised at this intensity for the final 8 weeks of their training. |
5 days/week for 12 weeks | GM | Electrophysiological recording Safety Margin of Synaptic Transmission |
| Endo et al. [34] (2021) | C57BL/6 mice | Male | 24 months | Resistance exercise | Treadmill running Mice were subjected to a moderate regimented running exercise on a treadmill at an increasing speed of 12 m/min for 40 min |
3 times/week for 8 weeks | GM | RNA sequencing Quantitative RT -PCR Protein quantification with Immunoblotting Histology and immunostaining |
| Herscovich et al. [28] (1987) | C57BL/6J mice | Female | 4, 10, 18 and 24 months | Resistance exercise | Wheel running The program began with 5 min/day exercise and increased by 5 min every 2nd day. At the beginning of week 2, the maximum training time per day was 30 min. |
10 weeks | GM, quadriceps and the diaphragm muscle | [l25I]-a-BGT |
| Pour et al. [23] (2017) | Wistar rats | Male | 23–24 months | Endurance exercise | Treadmill running The program was commenced at 15 min per session at a speed of 7.5 m per minute and at a slope of 0°. The speed of the treadmill and the duration of the exercise sessions were gradually increased until the final week, to 60 min per session at a speed of 15 m per minute; however, the treadmill inclination was not changed. |
5 days/week for 10 weeks | EDL and soleus | Western blot |
| Gillon et al. [25] (2018) | C57Bl/6j mice | Female | 6, 22–24 months | Resistance exercise | Wheel running Mice in exercise group were given access to a monitored running wheel |
4 months | Soleus | Immunohistochemistry |
| Deschenes et al. [35] (2020) | Fischer 344 rats | Male | 23 months | Endurance exercise | Treadmill running The program began with 2 familiarization trials at 5 m/min with 0 % grade for 5 min. Subsequently, the actual program began with a treadmill velocity of 7.5 m/min at 0 % grade for 15 min. Gradually, the duration was increased so by the end of the 8-week protocol, rats were running for 60 min per session at a velocity of 15 m/min while still at a 0 % grade. |
5 days/week for 8 weeks | Soleus | Cytofluorescent staining of NMJs |
| Deschenes et al. [36] (2018) | Fischer 344 rats | Male | 7, 23 months | Resistance exercise | Treadmill running The training regimen began with a running speed of 5 m/min at a 0 % grade for 5 min. Over the 8 week course of the training program, the training duration was gradually increased to 60 min/session at a velocity of 15 m/min with the treadmill remaining at a 0 % grade. |
5 days/week for 8 weeks | Soleus | Immunofluorescent staining, Microscopy |
| Deschenes et al. [37] (2011) | Fischer 344 rats | Male | 8, 24 months | Endurance exercise | Treadmill running The program began with training sessions of 15 min at a speed of 7.5 m/min at a 0 % grade completed 5 days/week. Treadmill speed and duration of exercise sessions were gradually increased such that by the final week, young and aged rats ran for 60 min per session at a speed of 15 m/min while maintaining a 0 % grade. |
5 days/week for 10 weeks | Soleus and plantaris | Cytofluorescent staining, Histochemical staining |
| Deschenes et al. [38] (2016) | Fischer 344 rats | Male | 8, 24 months | Endurance exercise | Treadmill running The program began with training sessions of 15 min at a speed of 7.5 m/min at a 0 % grade completed 5 days/week. Treadmill speed and duration of exercise sessions were gradually increased such that by the final week, young and aged rats ran for 60 min per session at a speed of 15 m/min, while maintaining a 0 % grade, and still for 5 days/week. |
5 day/week for 10 weeks | Plantaris and EDL | Cytofluorescent staining of NMJs |
| Alshuaib et al. [39] (1990) | C57BL/6J mice | Male | 10 months | Resistance exercise | Treadmill running Animals were trained (30 min/day) on a rodent treadmill with a 10 % upgrade. During the initial month, running began with a speed of 15–20 m/min. For the next seven months, running speed was 25 m/min. |
5 day/week for 10 months | Soleus and EDL | MEPP, EPP |
| Andonian et al. [40] (1987) | C57BL/6NNia mice | Male | 12, 18 and 24–25 months | Endurance exercise | Treadmill training Initially, the exercise began with a moderate walk-jog pace of 15–20 m/min for about 30 min. As the mice became increasingly familiar with the treadmill, the velocity and duration were gradually increased until they could run between 25 and 28 m/min for 60 min per day. |
8 weeks | Soleus and EDL | Immunofluorescence staining |
| Fragala et al. [41] (2014) | Human | Male and female | 61–85 years | Resistance exercise | Progressions of the squat, split squat, leg curl, leg extension, push-up, triceps extension, calf raise, lat pull down, seated low row, biceps curl, shoulder press, abdominal plank, and reverse crunch. | 6 weeks | QUA | Skeletal muscle ultrasound Leg extension trials ELISA |
| Soendenbroe et al. [42] (2020) | Human | Male and female | 64–90 years | Resistance exercise | Leg Extension exercise with one leg only, leaving contralateral leg as a control. | 4.5 h and up to 7 days post exercise | Vastus lateralis | Real time-PCR, Immunohistochemistry, Microscopy, Immunocytochemistry |
| Monti et al. [43] (2023) | Human | Male and female | 78.7 ± 5.9 years | / | Mixed aerobic, strength and balance training | 2 years | / | Neurofilament light chain and C-terminal agrin fragment concentrations in serum |
TA, tibialis anterior muscle; GM, gastrocnemius muscle; EDL, extensor digitorum longus muscle; QUA, Quadriceps femoris muscle; MEPP, miniature endplate potentials; EPP, evoked endplate depolarization.
3.3. Training regimes
The exercise regimes of the selected studies consisted of resistance training [24,25,28,30,35,40,41], endurance training [23,29,31,33,36,38,39] and voluntary exercise [22,32,34,37,42,43]. Resistance training, which uses body weight or weight bearing goods to provide resistance for muscles, aims to enhance muscle strength during exercise. 1 included animal study had 9- and 20-month-old rats climb a 1-m-long ladder inclined at 85°, with additional weights attached to their tails [24]. 4 studies subjected aged mice to regimented running exercises on a treadmill or running wheel at increasing speeds for durations ranging from 7 weeks to 10 months [25,28,30,41]. 3 clinical trials employed resistance training for major whole-body muscle groups [35,43] and leg muscles [40]. Endurance training, which is event-specific, involves maintaining an elevated heart rate over a prolonged period. 2 of the analyzed studies using this modality used rat models, while 4 used mouse models. These studies adopted treadmill running as an intervention, with training durations spanning from 8 to 10 weeks. In addition to resistance and endurance training, voluntary exercise was incorporated in 5 studies [22,32,34,37,42], where mice in the intervention group were given free wheel running exercises.
3.4. Body weight and muscle mass
11 papers reported on changes in body weight [23,24,30,31,33,[36], [37], [38], [39],42] or muscle weight [23,24,[37], [38], [39],41,42] as indices of metabolic responses during ageing or exercise. Of these studies that evaluated body weight, 4 used mice [30,31,37,42] while 6 used rats [23,24,33,36,38,39]. 2 studies reported a slight but nonsignificant increase in body weight [30,42], while 1 paper reported a significant increase in body weight during ageing in old mice [37]. 5 papers reported an increase in body weight during ageing in rats [23,33,36,38,39], while 3 papers indicated a decrease in body weight during ageing [33,36,38]. Concerning muscle weight, 5 studies reported weight changes in specific muscles during ageing, such as the gastrocnemius (GA) [37,42], tibialis anterior (TA) [23,37,42], extensor digitorum longus (EDL) [23,37,42], soleus [24,37,38,42], and plantaris [24]. 3 papers reported that the muscle weight of elderly mice and rats was higher but not significantly different when compared to younger ones [23,37]. Sugita et al. found that the muscle weight of TA, EDL, and soleus showed no difference, although the GA weight of old mice was lighter but not significantly different [42]. To evaluate the effects of exercise on body weight and muscle mass, 10 papers reported on body weight changes [23,24,30,31,33,36,38,39,41,42] and 5 papers reported on muscle weight change after running exercise with treadmill or running wheel [23,24,38,39,42]. 6 endurance [30,31,33,36,38,39], 2 resistance [24,41] and 1 voluntary study [42] reported that 7–12 weeks running program reduced body weight in the elderly but not significantly, while 1 study reported a significant increase in body weight following 10-week treadmill running with 7.5 m per minute endurance exercise [23]. Regarding muscle weight, 3 studies with endurance training [23,41,42], 1 study with resistant training [41] and 2 studies with voluntary exercise [23,42] reported changes during exercise. 1 paper indicated that a 10-week endurance exercise of treadmill running with 60 min per session at a speed of 15 m/min program significantly improved muscle weight [23]. In another study, it was found that resistance exercise resulted in an increase in the muscle weight of plantaris muscles in aged rats [24]. Meanwhile, 2 papers reported a significant increase in plantaris muscle weight after voluntary exercise [24,42], while another paper reported a decrease in gastrocnemius muscle weight after 8 weeks of resistance training [41]. Pour et al. demonstrated that endurance treadmill running increased the muscle weight of soleus and EDL [23], meanwhile Deschenes et al. showed that resistance training decreased body weight but significantly increased plantaris muscle weight in aged rats [24]. Notably, 2 included studies also investigated the combination of exercise and nutritional supplementation, reporting higher body weight and muscle weight [37,42]. These results revealed contradictory findings concerning body weights in rats and mice during ageing, potentially influenced by variations in the age of the experimental animals.
3.5. NMJ ageing
6 animal studies investigated age-related changes in nerve terminal morphology [24,31,32,36,39,42]. Various muscles were studied, including the soleus [39], EDL [29,36,42], plantaris [36] and GM [31]. 4 studies reported that nerve terminals became larger, more complex and highly branched with advancing age [29,31,36,39]. 1 paper reported a significant increase in the percentage of denervated NMJs in old mice from less than 5 % at 6 months to more than 30 % at 23 months [42]. Andonian et al. reported a slight increase in nerve terminal perimeter in the EDL, paralleled by greater changes in branch number and longitudinal extent length, compared to the soleus muscle [29]. Deschenes (2015) et al. found no significant changes in the nerve terminals in the soleus muscle during ageing [24], but observed increased numbers of nerve terminal branches, total branch length, average branch length, and branching complexity in older plantaris muscle [24]. However, some studies reported that the total branch of nerve terminal became shorter and less complex during ageing [33]. To assess changes in postsynaptic morphology during ageing, 5 papers compared the total and stained area or perimeter of endplates to determine synaptic changes during ageing [25,32,34,39]. 4 papers found that aged AChRs often fragmented into small parts with decreased density, while 1 paper reported that older animals displayed longer total and stained perimeters [28]. 1 paper reported an increased proportion of endplates composed of multiple AChR clusters with age [29]. 4 papers reported muscle fiber degeneration during ageing [28,[38], [39], [40]]. The elderly had significantly smaller type II fibres compared to both their own type I fibres and the type II fibres in the young [40]. 1 paper reported that myofiber did not significantly change. Aged soleus displayed a higher percentage of type I fibers, along with a lower percentage of type II fibers than young muscles [38].
4. Exercise on the NMJ
4.1. Presynaptic compartment
To comprehend the effect of exercise on presynaptic compartment of NMJ, 6 papers examined changes in nerve terminal morphology after exercise in aged animals [29,[31], [32], [33],36,39]. In 3 studies, it was found that nerve terminals in young animals become larger, with longer perimeters and extension lengths [31,36,39]. However, 3 other papers reported significantly smaller and more homogeneous nerve terminals in old animals following exercise [29,36,39]. 1 study found a decrease in nerve terminal perimeter with exercise but little change in extension length and branch number [29]. Another study reported that endurance treadmill running exercise resulted in a greater number of nerve terminal branches and total branch length in young animals, but not in old ones [36]. Interestingly, the average length of individual nerve terminal branches remained unaltered by endurance training [36]. 2 papers suggested less noticeable effects on presynaptic features following exercise [24,32]. 2 papers showed no significant effect of exercise on presynaptic structure of the soleus muscle in aged rats [33]. Andonian et al. reported more dramatic nerve terminal changes in the exercised EDL compared to changes in the older soleus [29]. According to Deschenes et al., Bassoon, a protein that anchors active zones and determines docking sites for presynaptic ACh vesicles, was consistently affected by training [39]. 1 paper detected synaptophysin, found in the membranes of ACh-containing vesicles, to thoroughly assess the consequences of ageing and endurance training on presynaptic NMJ morphology [36]. The age-related function parameters of nerve terminals were investigated in 1 animal study [28]. Herscovich et al. reported a decrease in specific activities of the cholinergic enzymes CAT and AChE with advancing age in examined muscles, revealing more endplates in the quadriceps muscle of old mice compared to young mice [28].
4.2. Postsynaptic compartment
To investigate the morphological changes in the postsynaptic compartment of the NMJ, 5 papers analyzed changes in endplates in aged animals after exercise [[32], [33], [34],37,44]. 2 studies reported that exercise improved the AChR perimeters in aged rats [44] and overlap area in aged mice [34], while 3 studies noted reduced postsynaptic receptor fragmentation following exercise [32,37,42]. 1 paper found a decrease in stained perimeter length in aged rats related to endurance training [33]. Pour et al. observed an increase in AChR expression in the EDL after exercise [23]. Dechenes et al. found a 16 % enlargement of endplates and significant increases in endplate perimeter lengths after resistant training, in addition to a greater dispersion of postsynaptic receptors [44]. Another study reported that the resistance exercise resulted in a 2 times increase in the number of AChRs per field [41].
4.3. Pre- and postsynaptic couple and neuromuscular transmission
To assess NMJ function, 2 papers analyzed pre- and postsynaptic coupling endplates in aged animals [24,42]. 1 study reported significant effects of ageing on presynaptic to postsynaptic coupling [42]. Dechenes et al. reported equal coupling of pre- and postsynaptic morphological features under control conditions and 10 weeks of endurance training [36]. After 5 months of voluntary wheel running, a single-fiber electromyography study on 27-month-old mice demonstrated significant improvements in NMJ transmission [22].
4.4. Muscle fiber characteristics
2 papers investigated muscle fiber performance during ageing and after exercise training [39,44]. 1 clinical study found that elderly individuals had significantly smaller type II fibers compared to younger individuals [40]. Another clinical study reported that the elderly had significantly more d neonatal myosin (MHCn) and neural cell adhesion molecule (NCAM) positive fibres compared to younger individuals [40]. An animal study by Deschenes et al. noted a significant decrease in the expression of type II fibers due to ageing, which was accompanied by a similar increase in the percentage of type I fibers in the plantaris [24]. However, neither ageing nor training significantly changed the fiber type composition in the soleus [24]. In another study, it was reported that the aged soleus displayed a higher percentage of type I fibers and a lower percentage of type II fibers than young muscles. They also found that 7 weeks of resistance training did not significantly affect the fiber type composition in both muscles [24]. No significant differences were found between the previously exercised and control legs in either the young or the elderly for MHCn or NCAM1 [45]. 1 paper noted a decrease in soleus fibers during ageing [39], while another paper showed that control and training animals had similar muscle fiber areas during ageing [44]. For muscle fiber diameter, 2 papers found that exercise had no significant effect on muscle fiber diameter in aged rats [39] and mice [31]. There were no significant changes in the fiber diameters of the soleus or EDL between the control or exercise groups at any of the ages [29], but the diameters of the ageing EDL fibers decreased in both control (−5%) and exercise groups (−10 %) [29]. Regarding muscle fiber typing, Endo et al. showed that exercise improved the proportion of type IIB but decreased type IIA by about 1 % in gastrocnemius muscle of aged mice [41]. Deschenes et al. found type IIA increased from 3.2 % to 4.4 %, while type I had a slighter decrease from 96.8 to 95.6 % after 8 weeks of endurance training [39]. In another resistance training study, they found a 2.9 % decrease in the percentage of type I fibers [44]. Taken together, exercise may increase the muscle fiber size in aged mice and rats and influence the proportion of muscle type IIA and IIB.
4.5. AChRs-related protein/gene expressions
2 papers reported changes in gene expression during exercise [37,42]. Yano et al. performed a correlation analysis between Agrin expression and motor function in each mouse, finding both fragmentation and denervation of the NMJ significantly correlated with Agrin mRNA expression [37]. Sugita et al. found that the expression of Dok7 protein was significantly higher in the exercise group via western blot analysis [42].
Yano et al. confirmed that the incidence of abnormal NMJ was negatively correlated with the expression of Agrin and LRP4 [37]. However, the exact changes in gene expression accompanying exercise remain largely unknown. Immunoblotting results showed that the protein levels of PGC-1α remained unchanged following exercise [41]. Endo et al. reported that there was an absence of anabolic related transcriptional upregulation in pathways in aged rodent models [41]. Notably, genes encoding the transporters and receptor components of glutaminergic transmission were significantly upregulated in exercised muscles [41].
5. Discussion
The neuromuscular junction (NMJ) is a specifically differentiated structure that links presynaptic motor nerve terminals to AChRs, ensuring optimal transmission efficiency. The pre- and postsynaptic membranes display a pretzel-like structure, facilitating the translation of electrical potentials from nerve terminals into muscle contractions [46,47]. Fast- and slow-twitch muscle fibers exhibit distinct NMJ morphologies and transmitter release characteristics [48]. With ageing, the structure and function of NMJs deteriorate, leading to neuromuscular transmission failure and muscle atrophy [48]. This contributes to the decline in muscle mass and muscle power generation, as shown across the studies included in this review [49]. This degradation plays a crucial role in the age-associated decline in neuromuscular function. Moreover, increased fragmentation and reduced overlap of endplates with AChRs and nerve terminals have been observed in both slow and fast-twitch muscles of aged animals, which may explain the reduced transmission amplitude with ageing [50,51]. Miniature endplate potentials (MEPP) and evoked endplate depolarization (EPP) are local depolarizing potentials indicative of NMJ. Reduced MEPP amplitude suggests fewer AChRs or weaker binding between ACh and AChRs. In contrast, prolonged MEPP decay time implies reduced acetylcholinesterase (AChE) activity or increased binding between ACh and AChRs [52]. Fahim et al. demonstrated that young and old muscles respond differently to endurance exercise, corresponding to their morphological responses [31]. Additionally, age-related morphological changes in the alpha motor neuron, partially due to oxidative stress and inflammation, occur during normal ageing. To counteract the degeneration, presynaptic nerve terminals and postsynaptic AChRs undergo structural reorganization. This compensatory mechanism, referred to as NMJ compensation, plays a crucial role in the ongoing process of nerve denervation and reinnervation. Aged animals and humans experience denervation at a faster rate than reinnervation, which causes permanent loss of motor units and future physical dependency [53]. Deschenes et al. found that morphological remodeling of AChRs preceded age-related alterations in skeletal muscles during ageing, including increased AChRs area and perimeter [54]. Therefore, it is essential to intervene and halt the ageing-induced functional denervation process. Investigating morphological and functional alterations of AChRs may help illuminate the detailed mechanisms and the pathogenesis of neuromuscular degeneration and guide new therapeutic targets for this disease.
Exercise is a recommended clinical approach for enhancing muscle quality and physical performance. However, the responses to exercise change with age, with alternations in anabolic response resulting in limited gains in muscle strength and endurance [55]. Exercise promotes various metabolic responses and morphological reconfigurations in elderly NMJs [56]. Morphological reconfiguration of aged NMJ in response to exercise training includes an increase in the perimeter and number of ACh receptors [57]. However, different exercise patterns influence the remodeling of ageing NMJ differently. The adaptations of NMJ to resistance exercise appear to be independent of changes in muscle fiber profile [44], suggesting that exercise-related stimuli directly contribute to NMJ-related changes. Differences in the changes of AChRs morphology and function, in conjunction with various muscle fiber types, species and myofiber compositions in different muscle groups, are summarized in Table 2. Interestingly, ageing caused significant reorganization of cellular aspects of the NMJ, with cellular and subcellular components of NMJs displaying opposite sensitivities to the influence of ageing and exercise training. Remarkable pre- and postsynaptic adaptations of the NMJ occurred as a result of ageing at the gross cellular level, with no evidence of exercise-induced adaptation. Conversely, variables quantified at the active zones of the same synapses exhibited changes in response to exercise training, without displaying any sensitivity to the effects of ageing. To our knowledge, this is the first report indicating different sensitivities of specific components of the NMJ to disparate stimuli such as ageing and exercise training. In older animals, endurance exercise training was found to trend towards an increase in overall muscle mass, as well as a similar tendency for the constituent myofibers, especially those classified as type I, to be larger than those in untrained aged animals. Intriguingly, this suggests that the stimulus of endurance exercise training may activate either catabolic or anabolic pathways to restore homeostatic conditions within myofibers. This highlights the remarkable regulation of various cellular mechanisms that enable myofibers to adapt and function optimally during the tasks they are assigned.
Table 2.
NMJ and myofiber morphology and function during ageing and exercise.
| Study | NMJ measurement | Myofiber measurement | NMJ related results | Muscle fiber |
|---|---|---|---|---|
| Deschenes et al. [45] (2000) |
Presynaptic Branch number; Total branch length; Average branch length; Branching complexity; Presynaptic-to-postsynaptic coupling. Postsynaptic Total endplate perimeter; Stained endplate perimeter; Total endplate area; Stained endplate area; Endplate dispersion |
Immunostaining | Soleus Slow twitch Ageing related Greater endplate area per total nerve terminal branch length; Exercise related Presynaptic structure not impacted; Postsynaptic-to-presynaptic ratio increased in aged rats; Distribution of postsynaptic receptors not affected significantly. Fast twitch Ageing related No significant effects of age or treatment in nerve terminals; Presynaptic-to-postsynaptic coupling ratio not affected. Exercise related No significant effects of exercise in nerve terminals; Significant effect on endplate in both young and aged animals; More dispersed receptor clusters but only among aged endplates; Presynaptic-to-postsynaptic coupling ratio not affected. Plantaris Slow twitch Ageing related Dispersion of ACh receptors not altered; Presynaptic nerve terminal branching patterns increased; Presynaptic to postsynaptic coupling not altered. Exercise related The dispersion of ACh receptors not altered; Presynaptic to postsynaptic coupling not altered. Fast twitch Ageing related Significant enhancement of perimeter lengths and endplate areas; Greater numbers of nerve terminal branches, total branch length, average branch length, and branching complexity; Presynaptic-to-postsynaptic coupling not altered. Exercise related Presynaptic morphology not altered; Presynaptic-to-postsynaptic coupling not altered; Endplate morphology not affected; Dispersion of postsynaptic ACh receptors not affected. |
Soleus Neither age nor treatment affected myofiber size when data from all fiber types were pooled. Neither age nor training significantly changed the fiber type composition of the soleus. Plantaris Total plantaris myofiber size was unaffected by age or by resistance training Ageing resulted in a significant decrease in the expression of type II fibers that was accompanied by a similar increase in the percentage of type I fibers in the plantaris. Resistance training failed to alter fiber-type distribution as ageing did |
| Cheng et al. [34] (2013) | Nerve terminal area; Endplate AChR area; Fragmented endplates | / |
Ageing related More fragmented and complex AChR clusters; Little or no overlying synaptophysin staining of the endplate AChR clusters; Increase in the proportion of endplates comprised of multiple AChR clusters. Exercise related Significantly larger nerve terminal area; The loss of nerve terminal area reduced; A modest increase in endplate AChR area; The area of synaptic overlap increased. |
/ |
| Chugh et al. [22] (2021) | Electrophysiological assessment of neuromuscular transmission | / |
Exercise related No significant effect on motor unit degeneration; A significant improvement in NMJ transmission. |
Muscle contractility demonstrated significant changes with age for tetanic but not for twitch muscle contractility. |
| Valdez et al. [30] (2010) | AChR area changes AChR fragmentation |
/ |
Ageing related The AChR cluster on some muscle fibers were not contacted by an axon; Some AChR site partially denervated; Junctional AChRs were often fragmented into small islands; Misshapen preterminal and terminal axons. Exercise related The frequencies of fragmented, faint, and denervated postsynaptic sites reduced; Less significant effects on presynaptic features. |
The beneficial effects of exercise do not result from prevention of age-related motor neuron loss or muscle fiber degeneration but rather reflect a partial reversal of structural alterations that have already occurred. |
| Yano et al. [31] (2017) | Fragmentation and denervation of NMJ | / |
Ageing related Agrin and LRP4 expression decreased; Both fragmentation and denervation of NMJ showed significant correlation with Agrin mRNA expression. Exercise related NMJ fragmentation and denervation reduced. |
/ |
| Sugita et al. [32] (2021) | 1. NMJ structural features with confocal image 2. Dok7 expression 3. Motor nerve conduction velocity |
EDL, soleus, TA and GM weight |
Exercise related Both fragmentation and denervation of NMJ decreased; Age-related loss of nerve axons attenuated; The number of fragmented AChRs decreased; The proportion of abnormal myelin or motor nerve conduction velocity not affected; Dok7 protein increased. |
No significant differences in body and muscle weight with exercise |
| Fahim et al. [33] (1997) | MEPP and EPP | / |
Ageing related No change in membrane capacitance; MEPP frequencies increased, EPP amplitude decreased. Exercise related No change in membrane capacitance. |
Muscle fiber diameters did not significantly change with either ageing or exercise |
| Endo et al. [34] (2021) | NMJ related gene expression between the sedentary and exercise | / |
Exercise related The expression of genes in the components and regulation of neurotransmission enhanced(Gria1, Gria2, Grin1 and Grin2a); Significant enrichment in positive regulation of excitatory postsynaptic potential, ionotropic glutamate receptor pathway, and neurotransmitter secretion pathway; 2-fold increase in the number of AChRs per field; Significantly higher percentage of complete and partially innervated NMJs; Higher postsynaptic density in the exercise group. |
Notable absence of gene upregulation in the energy metabolism pathways in aged skeletal muscle following exercise; No significant differences in the gastrocnemius fiber type composition after exercise. |
| Fragala et al. [41] (2014) | ELISA of serum CAF | Muscle strength | Circulating CAF increased in response to short-term resistance exercise training in older adults. | Changes in circulating biomarkers did not appear to relate to changes in muscle strength or quality. |
| Herscovich et al. [28] (1987) | The number and distribution of AChR, the specific activities of acetylcholinesterase | / | The AChR number or per endplate in each of the muscle types was not affected by training or ageing. | / |
| Pour et al. [23] (2017) | AChRs expression in EDL and soleus | / | The expression of AChRs increased in the exercise group. | The hypertrophy index of the soleus increased in all the trained groups, |
| Gillon et al. [25] (2018) | Postsynaptic fragmentation; synaptic occupancy; innervation status | / |
Ageing related NMJ fragmentation increased; The proportion of innervated fibers reduced; Endplate occupancy didn't change. Exercise related NMJ fragmentation reduced; The proportion of innervated fibers didn't change; Endplate occupancy increased. |
/ |
| Deschenes et al. [38] (2016) | NMJ morphology | / | Training had no significant effects on NMJs at the cellular level. | Training elicited whole muscle and myofiber trends toward hypertrophy. |
| Deschenes et al. [36] (2018) | Neural and direct muscle stimulation | Percentage of fiber type | Neuromuscular transmission unaffected by ageing and training. | Aged muscle displayed a higher percentage of Type I fibers, along with a lower percentage of Type II fibers than young muscles. |
| Deschenes et al. [37] (2011) | NMJ morphology | / |
Exercise related Non-significant training-induced adaptations in the soleus NMJs; Slow-twitch NMJs of the plantaris displayed morphologic adaptations to exercise in pre- and postsynaptic components. |
/ |
| Soendenbroe et al. [42] (2020) | AChR subunites in mRNA level | MHCe, MHCn in mRNA and protein leveal |
Ageing related The muscle tissue of the elderly women had lower levels of AChR β1 but higher levels of AChR γ mRNA compared to the young women; Exercise related Both the elderly and the young women had a significant upregulation of AChR α1 mRNA in the previously exercised leg compared to the control leg. The exercise response of AChR δ mRNA only reached statistical significance in the elderly. |
Ageing related No difference of type I fibre percentage between young and old; Type II fibres decreased in the elderly; More MHCn- and NCAM-positive fibres in the elderly. Exercise related No significant differences in MHCn or NCAM. |
| Alshuaib et al. [39] (1990) | Resting membrane potential, MEPP amplitude, MEPP frequency, Input resistance, Membranecapacitance, Threshold potential | / |
Exercise related All physiological age-related changes prevented in EDL NMJs but not in soleus NMJs. |
/ |
| Deschenes et al. [35] (2020) | Parameters of branches, vesicles and receptors | Immunohistochemical staining |
Exercise related Vesicles stained area/Total branch length in plantaris increased. |
/ |
| Andonian et al. [40] (1987) | Nerve terminal area | Muscle fibre diameter |
Exercise related The nerve terminal area of soleus not affected while EDL terminals significantly smaller than controls. |
Exercise related No significant change in muscle fiber diameter. |
| Monti et al. [43] (2023) | Neurofilament light chain and C-terminal Agrin fragment | Muscle cross-sectional area |
Exercise related Neurofilament light chain concentration did not change; C-terminal Agrin fragment concentration maintained. |
Exercise related Muscle cross-sectional area reduced. |
NMJ, neuromuscular junction; Ach, acetylcholine; AChR, acetylcholine receptor; LRP4, lipoprotein receptor-related protein 4; Dok7, docking protein 7; CAF, C-terminal Agrin fragment; MHCe, embryonic myosin heavy chain; MHCn, neonatal myosin; NCAM, neural cell adhesion molecule; MEPP, miniature endplate potentials; EPP, evoked endplate depolarization.
The Agrin-LRP4-MuSK-Dok7-Rapsyn pathway plays a pivotal role in AChRs clustering and NMJ maturation. Agrin, a heparan sulfate proteoglycan secreted from motor nerve terminals [58,59], binds to the LRP4 in the postsynaptic membrane, The Agrin/LRP4 complex activates MuSK phosphorylation and also recruits Dok7 and induces Dok7 phosphorylation [60]. The Agrin-LRP4-MuSK-Dok7 forms a complex with rapsyn to promote the formation and maintenance of AChRs [61]. Samuel et al. reported a decline in Agrin in AChR areas in old mice [62]. Agrin fragment concentrations have been found to increase in older adults with neuromuscular degeneration compared to aged-matched controls [35]. Studies have reported a decreased expression of Agrin and LRP4 in aged mice, correlating with NMJ fragmentation and denervation progression [37]. Excessive cleavage of Agrin by protease results in functional disintegration at the neuromuscular junction during ageing [59]. Fragala et al. found that an increase in circulating Agrin fragments in response to short-term resistance exercise training in older adults [35]. Previous studies have reported that Wnts could bind to the extracellular carbohydrate recognition domain of MuSK, thereby increasing the phosphorylation levels of MuSK [63,64]. Additionally, there is substantial evidence suggesting that physical exercise could enhance the expression of Wnts [65,66]. This findings suggest that physical exercise may impact the NMJ via the Wnt signaling pathway. Dok7 expression is significantly reduced in old age [67,68], and our included studies show that the expression of Dok7 protein was significantly higher in the exercise group than in sedentary mice [42]. Therapies on increasing Dok7 expression level provide an opportunity to alleviate NMJ degeneration and muscle ageing [[67], [68], [69]].
Mitochondria, critical for ATP production and oxidative phosphorylation [70], can become dysfunctional, leading to an increased production of ROS that may result in neurodenervation and synaptic degeneration. There is growing evidence suggesting that mitochondrial dysfunction, impaired oxidative phosphorylation, and decreased deacetylation contribute to NMJ degeneration [71]. Numerous studies have underscored the importance of synaptic mitochondrial dysfunction to the neuromuscular junction [72,73]. A model of mitochondrial disease revealed that mitochondrial dysfunction hindered locomotive ability and caused aberrant morphology of NMJ [74]. Conversely, physical exercise has been shown to induce mitochondria morphological adaptions and increase the mitochondria's capacity to produce ATP [75]. At the protein level, physical exercise effectively regulated substrate entry and oxidation, the tricarboxylic acid cycle, respiratory chain, and ATP synthesis in mitochondria [76]. Furthermore, physical exercise influenced the expression of sirtuin in skeletal muscle, thereby regulating changes in mitochondrial biogenesis, oxidative metabolism, and cellular antioxidant systems [77]. However, further studies are needed to investigate the relationship and mechanism between mitochondrial acetylation and NMJ degeneration.
There are several limitations in this review. First, the papers included solely addressed changes in the NMJ following exercise in the elderly, which do not offer insights into the differential effects of exercise on the NMJ across various age groups. Second, the variability across the 3 clinical studies was substantial, which posed challenges to deriving consistent conclusions. Third, the absence of standardized analysis methods, resulting from differing training procedures among the studies, could potentially introduce bias in the result interpretation. Lastly, due to the data heterogeneity among the included studies, a meta-analysis could not be conducted.
In conclusion, this systematic review aimed to explore the effect of exercise on ageing NMJ. The NMJ exhibits progressive degeneration in both morphology and function during ageing, accelerating the progression of neuromuscular degeneration. This degeneration involves changes at both the gene and protein levels. As a key pathway to form and stabilize AChRs clusters, the Agrin-LRP4-MuSK-Dok7 pathway undergoes degradation in ageing skeletal muscle. The degradation of Wnt signaling in aged muscle inhibits NMJ remodeling, thereby accelerating NMJ degeneration. Mitochondrial oxidative stress exacerbates the metabolic impairment of NMJs during ageing. Physical training increased the nerve terminal branch, total area, nerve terminal length and branching complexity of the presynaptic compartment, and similar effects have been shown on the postsynaptic compartment. Physical exercise has been shown to promote NMJ hypertrophy and accelerate the remodeling process in the elderly, potentially mitigating NMJ degradation and thus, alleviating the progression of neuromuscular degeneration. The type of exercise affects the NMJ adaptive response, with the effects of endurance training appearing more pronounced than resistance exercise. Different ages used may also have affected the results and the comparison between studies. Therefore, physical exercise could potentially mitigate NMJ degradation in the elderly, thus alleviating the progression of neuromuscular degeneration. However, the mechanisms involved in exercise, such as mitochondrial oxidative stress and the regulation of Wnt-related pathways, have not been clearly elucidated. Moreover, the impact of combining exercise with nutritional supplementation on ageing NMJ warrants further investigation.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgements
This study was supported by the Collaborative Research Fund (Ref: C4032-21GF), General Research Grant (Ref: 14114822), Area of Excellence (Ref: AoE/M-402/20), Research Grant Committee, HKSAR and Group Research Scheme (Ref: C4032-21G), The Chinese University of Hong Kong.
References
- 1.Tabebordbar M., Wang E.T., Wagers A.J. Skeletal muscle degenerative diseases and strategies for therapeutic muscle repair. Annu Rev Pathol. 2013;8:441–475. doi: 10.1146/annurev-pathol-011811-132450. [DOI] [PubMed] [Google Scholar]
- 2.Fralish Z., Lotz E.M., Chavez T., Khodabukus A., Bursac N. Neuromuscular development and disease: Learning from in vitro and in vivo models. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.764732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krause Neto W., Ciena A.P., Anaruma C.A., de Souza R.R., Gama E.F. Effects of exercise on neuromuscular junction components across age: systematic review of animal experimental studies. BMC Res Notes. 2015;8:713. doi: 10.1186/s13104-015-1644-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teh N., Leow L.J. The role of Actin in muscle Spasms in a case series of patients with advanced basal cell Carcinoma treated with a Hedgehog pathway inhibitor. Dermatol Ther. 2021;11(1):293–299. doi: 10.1007/s13555-020-00464-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloch-Gallego E. Mechanisms controlling neuromuscular junction stability. Cell Mol Life Sci. 2015;72(6):1029–1043. doi: 10.1007/s00018-014-1768-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson M.H., Deschenes M.R. The neuromuscular junction: anatomical features and adaptations to various forms of increased, or decreased neuromuscular activity. Int J Neurosci. 2005;115(6):803–828. doi: 10.1080/00207450590882172. [DOI] [PubMed] [Google Scholar]
- 7.Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci. 1995;50(Spec No):11–16. doi: 10.1093/gerona/50a.special_issue.11. [DOI] [PubMed] [Google Scholar]
- 8.Murphy S., Dowling P., Ohlendieck K. Comparative skeletal muscle proteomics using two-dimensional gel electrophoresis. Proteomes. 2016;4(3) doi: 10.3390/proteomes4030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valdez G., Tapia J.C., Lichtman J.W., Fox M.A., Sanes J.R. Shared resistance to aging and ALS in neuromuscular junctions of specific muscles. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0034640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slater C.R. ‘Fragmentation’ of NMJs: a sign of degeneration or regeneration? A long journey with many junctions. Neuroscience. 2020;439:28–40. doi: 10.1016/j.neuroscience.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 11.Willadt S., Nash M., Slater C. Age-related changes in the structure and function of mammalian neuromuscular junctions. Ann N Y Acad Sci. 2018;1412(1):41–53. doi: 10.1111/nyas.13521. [DOI] [PubMed] [Google Scholar]
- 12.Demontis F., Piccirillo R., Goldberg A.L., Perrimon N. Mechanisms of skeletal muscle aging: insights from Drosophila and mammalian models. Dis Model Mech. 2013;6(6):1339–1352. doi: 10.1242/dmm.012559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enns G.M., Hoppel C.L., DeArmond S.J., Schelley S., Bass N., Weisiger K., et al. Relationship of primary mitochondrial respiratory chain dysfunction to fiber type abnormalities in skeletal muscle. Clin Genet. 2005;68(4):337–348. doi: 10.1111/j.1399-0004.2005.00499.x. [DOI] [PubMed] [Google Scholar]
- 14.Gouspillou G., Sgarioto N., Norris B., Barbat-Artigas S., Aubertin-Leheudre M., Morais J.A., et al. The relationship between muscle fiber type-specific PGC-1α content and mitochondrial content varies between rodent models and humans. PLoS One. 2014;9(8) doi: 10.1371/journal.pone.0103044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vissing J., Galbo H., Haller R.G. Exercise fuel mobilization in mitochondrial myopathy: a metabolic dilemma. Ann Neurol. 1996;40(4):655–662. doi: 10.1002/ana.410400416. [DOI] [PubMed] [Google Scholar]
- 16.Jeppesen T.D., Schwartz M., Olsen D.B., Vissing J. Oxidative capacity correlates with muscle mutation load in mitochondrial myopathy. Ann Neurol. 2003;54(1):86–92. doi: 10.1002/ana.10594. [DOI] [PubMed] [Google Scholar]
- 17.Xu H., Ranjit R., Richardson A., Van Remmen H. Muscle mitochondrial catalase expression prevents neuromuscular junction disruption, atrophy, and weakness in a mouse model of accelerated sarcopenia. J Cachexia Sarcopenia Muscle. 2021;12(6):1582–1596. doi: 10.1002/jcsm.12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McAvoy M., Tsosie J.K., Vyas K.N., Khan O.F., Sadtler K., Langer R., et al. Flexible multielectrode array for skeletal muscle conditioning, acetylcholine receptor stabilization and epimysial recording after critical peripheral nerve injury. Theranostics. 2019;9(23):7099–7107. doi: 10.7150/thno.35436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castro P., Machanocker D.H., Luna G.F., Barbosa G.M., Cunha J.E., Cunha T.M., et al. Clinical-like cryotherapy in acute knee arthritis protects neuromuscular junctions of quadriceps and reduces joint inflammation in mice. BioMed Res Int. 2022;2022 doi: 10.1155/2022/7442289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee Y.I., Cacciani N., Wen Y., Zhang X., Hedström Y., Thompson W., et al. Direct electrical stimulation impacts on neuromuscular junction morphology on both stimulated and unstimulated contralateral soleus. J Cachexia Sarcopenia Muscle. 2023;14(3):1533–1545. doi: 10.1002/jcsm.13235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozes B., Tong L., Myers M., Moss K., Ridgley A., Sahenk Z. AAV1.NT-3 gene therapy prevents age-related sarcopenia. Aging (Albany NY) 2023;15(5):1306–1329. doi: 10.18632/aging.204577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chugh D., Iyer C.C., Bobbili P., Blatnik A.J., Kaspar B.K., Meyer K., et al. Voluntary wheel running with and without follistatin overexpression improves NMJ transmission but not motor unit loss in late life of C57BL/6J mice. Neurobiol Aging. 2021;101:285–296. doi: 10.1016/j.neurobiolaging.2021.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pour M.B., Joukar S., Hovanloo F., Najafipour H. Long-term low-intensity endurance exercise along with blood-flow restriction improves muscle mass and neuromuscular junction compartments in old rats. Iran J Med Sci. 2017;42(6):569–576. [PMC free article] [PubMed] [Google Scholar]
- 24.Deschenes M.R., Sherman E.G., Roby M.A., Glass E.K., Harris M.B. Effect of resistance training on neuromuscular junctions of young and aged muscles featuring different recruitment patterns. J Neurosci Res. 2015;93(3):504–513. doi: 10.1002/jnr.23495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gillon A., Nielsen K., Steel C., Cornwall J., Sheard P. Exercise attenuates age-associated changes in motoneuron number, nucleocytoplasmic transport proteins and neuromuscular health. GeroScience. 2018;40(2):177–192. doi: 10.1007/s11357-018-0020-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hepple R.T., Rice C.L. Innervation and neuromuscular control in ageing skeletal muscle. J Physiol. 2016;594(8):1965–1978. doi: 10.1113/JP270561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.da Cunha J., Maselli L.M.F., Stern A.C.B., Spada C., Bydlowski S.P. Impact of antiretroviral therapy on lipid metabolism of human immunodeficiency virus-infected patients: old and new drugs. World J Virol. 2015;4(2):56. doi: 10.5501/wjv.v4.i2.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herscovich S., Gershon D. Effects of aging and physical training on the neuromuscular junction of the mouse. Gerontology. 1987;33(1):7–13. doi: 10.1159/000212848. [DOI] [PubMed] [Google Scholar]
- 29.Andonian M.H., Fahim M.A. Effects of endurance exercise on the morphology of mouse neuromuscular junction during ageing. J Neurocytol. 1987;16(5):589–599. doi: 10.1007/BF01637652. [DOI] [PubMed] [Google Scholar]
- 30.Alshuaib W.B., Fahim M.A. Effect of exercise on physiological age-related change at mouse neuromuscular junctions. Neurobiol Aging. 1990;11(5):555–561. doi: 10.1016/0197-4580(90)90117-i. [DOI] [PubMed] [Google Scholar]
- 31.Fahim M.A. Endurance exercise modulates neuromuscular junction of C57BL/6NNia aging mice. J Appl Physiol (1985) 1997;83(1):59–66. doi: 10.1152/jappl.1997.83.1.59. [DOI] [PubMed] [Google Scholar]
- 32.Valdez G., Tapia J.C., Kang H., Clemenson G.D., Gage F.H., Lichtman J.W., et al. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc Natl Acad Sci USA. 2010;107(33):14863–14868. doi: 10.1073/pnas.1002220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deschenes M.R., Roby M.A., Glass E.K. Aging influences adaptations of the neuromuscular junction to endurance training. Neuroscience. 2011;190:56–66. doi: 10.1016/j.neuroscience.2011.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng A., Morsch M., Murata Y., Ghazanfari N., Reddel S.W., Phillips W.D. Sequence of age-associated changes to the mouse neuromuscular junction and the protective effects of voluntary exercise. PLoS One. 2013;8(7) doi: 10.1371/journal.pone.0067970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fragala M.S., Jajtner A.R., Beyer K.S., Townsend J.R., Emerson N.S., Scanlon T.C., et al. Biomarkers of muscle quality: N-terminal propeptide of type III procollagen and C-terminal agrin fragment responses to resistance exercise training in older adults. Journal of Cachexia Sarcopenia and Muscle. 2014;5(2):139–148. doi: 10.1007/s13539-013-0120-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deschenes M.R., Kressin K.A., Garratt R.N., Leathrum C.M., Shaffrey E.C. Effects of exercise training on neuromuscular junction morphology and pre- to post-synaptic coupling in young and aged rats. Neuroscience. 2016;316:167–177. doi: 10.1016/j.neuroscience.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yano M., Minegishi Y., Sugita S., Ota N. Milk fat globule membrane supplementation with voluntary running exercise attenuates age-related motor dysfunction by suppressing neuromuscular junction abnormalities in mice. Exp Gerontol. 2017;97:29–37. doi: 10.1016/j.exger.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 38.Deschenes M.R., Li S., Adan M.A., Oh J.J., Ramsey H.C. Muscle fibers and their synapses differentially adapt to aging and endurance training. Exp Gerontol. 2018;106:183–191. doi: 10.1016/j.exger.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 39.Deschenes M.R., Tufts H.L., Oh J., Li S., Noronha A.L., Adan M.A. Effects of exercise training on neuromuscular junctions and their active zones in young and aged muscles. Neurobiol Aging. 2020;95:1–8. doi: 10.1016/j.neurobiolaging.2020.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Soendenbroe C., Bechshoft C.J.L., Heisterberg M.F., Jensen S.M., Bomme E., Schjerling P., et al. Key components of human myofibre denervation and neuromuscular junction stability are modulated by age and exercise. Cells. 2020;9(4) doi: 10.3390/cells9040893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Endo Y., Zhang Y., Olumi S., Karvar M., Argawal S., Neppl R.L., et al. Exercise-induced gene expression changes in skeletal muscle of old mice. Genomics. 2021;113(5):2965–2976. doi: 10.1016/j.ygeno.2021.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sugita S., Tamura K., Yano M., Minegishi Y., Ota N. The impact of milk fat globule membrane with exercise on age-related degeneration of neuromuscular junctions. Nutrients. 2021;13(7) doi: 10.3390/nu13072310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Monti E., Tagliaferri S., Zampieri S., Sarto F., Sirago G., Franchi M.V., et al. Effects of a 2-year exercise training on neuromuscular system health in older individuals with low muscle function. Journal of Cachexia, Sarcopenia and Muscle. 2023;14(2):794–804. doi: 10.1002/jcsm.13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deschenes M.R., Judelson D.A., Kraemer W.J., Meskaitis V.J., Volek J.S., Nindl B.C., et al. Effects of resistance training on neuromuscular junction morphology. Muscle Nerve. 2000;23(10):1576–1581. doi: 10.1002/1097-4598(200010)23:10<1576::aid-mus15>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 45.Soendenbroe C., Bechshøft C.J.L., Heisterberg M.F., Jensen S.M., Bomme E., Schjerling P., et al. Key components of human myofibre denervation and neuromuscular junction stability are modulated by age and exercise. Cells. 2020;9(4) doi: 10.3390/cells9040893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Campbell M.J., McComas A.J., Petito F. Physiological changes in ageing muscles. J Neurol Neurosurg Psychiatry. 1973;36(2):174–182. doi: 10.1136/jnnp.36.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bao Z., Cui C., Chow S.K., Qin L., Wong R.M.Y., Cheung W.H. AChRs degeneration at NMJ in aging-associated sarcopenia-A systematic review. Front Aging Neurosci. 2020;12 doi: 10.3389/fnagi.2020.597811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hester G.M., VanDusseldorp T.A., Ha P.L., Kiani K., Olmos A.A., Jabbari M., et al. Microbiopsy sampling for examining age-related differences in skeletal muscle fiber morphology and composition. Front Physiol. 2021;12 doi: 10.3389/fphys.2021.756626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Faulkner J.A., Larkin L.M., Claflin D.R., Brooks S.V. Age-related changes in the structure and function of skeletal muscles. Clin Exp Pharmacol Physiol. 2007;34(11):1091–1096. doi: 10.1111/j.1440-1681.2007.04752.x. [DOI] [PubMed] [Google Scholar]
- 50.Apel P.J., Alton T., Northam C., Ma J., Callahan M., Sonntag W.E., et al. How age impairs the response of the neuromuscular junction to nerve transection and repair: an experimental study in rats. J Orthop Res. 2009;27(3):385–393. doi: 10.1002/jor.20773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kulakowski S.A., Parker S.D., Personius K.E. Reduced TrkB expression results in precocious age-like changes in neuromuscular structure, neurotransmission, and muscle function. J Appl Physiol (1985) 2011;111(3):844–852. doi: 10.1152/japplphysiol.00070.2011. [DOI] [PubMed] [Google Scholar]
- 52.Rocha M.C., Pousinha P.A., Correia A.M., Sebastião A.M., Ribeiro J.A. Early changes of neuromuscular transmission in the SOD1(G93A) mice model of ALS start long before motor symptoms onset. PLoS One. 2013;8(9) doi: 10.1371/journal.pone.0073846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krause Neto W., Ciena A.P., Anaruma C.A., de Souza R.R., Gama E.F. Effects of exercise on neuromuscular junction components across age: systematic review of animal experimental studies. BMC Res Notes. 2015;8:713. doi: 10.1186/s13104-015-1644-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deschenes M.R., Roby M.A., Eason M.K., Harris M.B. Remodeling of the neuromuscular junction precedes sarcopenia related alterations in myofibers. Exp Gerontol. 2010;45(5):389–393. doi: 10.1016/j.exger.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Borst S.E., De Hoyos D.V., Garzarella L., Vincent K., Pollock B.H., Lowenthal D.T., et al. Effects of resistance training on insulin-like growth factor-I and IGF binding proteins. Med Sci Sports Exerc. 2001;33(4):648–653. doi: 10.1097/00005768-200104000-00021. [DOI] [PubMed] [Google Scholar]
- 56.Rogers R.S., Nishimune H. The role of laminins in the organization and function of neuromuscular junctions. Matrix Biol. 2017;57-58:86–105. doi: 10.1016/j.matbio.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu W., Klose A., Forman S., Paris N.D., Wei-LaPierre L., Cortés-Lopéz M., et al. Loss of adult skeletal muscle stem cells drives age-related neuromuscular junction degeneration. Elife. 2017;6 doi: 10.7554/eLife.26464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fallon J.R., Gelfman C.E. Agrin-related molecules are concentrated at acetylcholine receptor clusters in normal and aneural developing muscle. J Cell Biol. 1989;108(4):1527–1535. doi: 10.1083/jcb.108.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bowe M.A., Fallon J.R. The role of agrin in synapse formation. Annu Rev Neurosci. 1995;18:443–462. doi: 10.1146/annurev.ne.18.030195.002303. [DOI] [PubMed] [Google Scholar]
- 60.Zhang S., Ohkawara B., Ito M., Huang Z., Zhao F., Nakata T., et al. A mutation in DOK7 in congenital myasthenic syndrome forms aggresome in cultured cells, and reduces DOK7 expression and MuSK phosphorylation in patient-derived iPS cells. Hum Mol Genet. 2023;32(9):1511–1523. doi: 10.1093/hmg/ddac306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.DeChiara T.M., Bowen D.C., Valenzuela D.M., Simmons M.V., Poueymirou W.T., Thomas S., et al. The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell. 1996;85(4):501–512. doi: 10.1016/s0092-8674(00)81251-9. [DOI] [PubMed] [Google Scholar]
- 62.Samuel M.A., Valdez G., Tapia J.C., Lichtman J.W., Sanes J.R. Agrin and synaptic laminin are required to maintain adult neuromuscular junctions. PLoS One. 2012;7(10) doi: 10.1371/journal.pone.0046663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Strochlic L., Falk J., Goillot E., Sigoillot S., Bourgeois F., Delers P., et al. Wnt4 participates in the formation of vertebrate neuromuscular junction. PLoS One. 2012;7(1) doi: 10.1371/journal.pone.0029976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang B., Liang C., Bates R., Yin Y., Xiong W.C., Mei L. Wnt proteins regulate acetylcholine receptor clustering in muscle cells. Mol Brain. 2012;5:7. doi: 10.1186/1756-6606-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee H.J., Baek S.S. Role of exercise on molecular mechanisms in the regulation of antidepressant effects. J Exerc Rehabil. 2017;13(6):617–620. doi: 10.12965/jer.1735188.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ramakrishna K., Nalla L.V., Naresh D., Venkateswarlu K., Viswanadh M.K., Nalluri B.N., et al. WNT-Β catenin signaling as a potential therapeutic target for neurodegenerative diseases: current status and future perspective. Diseases. 2023;11(3) doi: 10.3390/diseases11030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang Y.T., Crick H.R., Chaytow H., van der Hoorn D., Alhindi A., Jones R.A., et al. Long-term muscle-specific overexpression of DOK7 in mice using AAV9-tMCK-DOK7. Mol Ther Nucleic Acids. 2023;33:617–628. doi: 10.1016/j.omtn.2023.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ueta R., Sugita S., Minegishi Y., Shimotoyodome A., Ota N., Ogiso N., et al. DOK7 gene therapy enhances neuromuscular junction innervation and motor function in aged mice. iScience. 2020;23(8) doi: 10.1016/j.isci.2020.101385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taetzsch T., Valdez G. NMJ maintenance and repair in aging. Curr Opin Physiol. 2018;4:57–64. doi: 10.1016/j.cophys.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu X., Du Y., Trakooljul N., Brand B., Muráni E., Krischek C., et al. Muscle transcriptional profile based on muscle fiber, mitochondrial respiratory activity, and metabolic enzymes. Int J Biol Sci. 2015;11(12):1348–1362. doi: 10.7150/ijbs.13132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Clement J., Wong M., Poljak A., Sachdev P., Braidy N. The plasma NAD(+) metabolome is dysregulated in "normal" aging. Rejuvenation Res. 2019;22(2):121–130. doi: 10.1089/rej.2018.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Du H., Guo L., Yan S., Sosunov A.A., McKhann G.M., Yan S.S. Early deficits in synaptic mitochondria in an Alzheimer's disease mouse model. Proc Natl Acad Sci U S A. 2010;107(43):18670–18675. doi: 10.1073/pnas.1006586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Umbach J.A., Adams K.L., Gundersen C.B., Novitch B.G. Functional neuromuscular junctions formed by embryonic stem cell-derived motor neurons. PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0036049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Suda K., Muraoka Y., Ortega-Yáñez A., Yoshida H., Kizu F., Hochin T., et al. Reduction of Rpd3 suppresses defects in locomotive ability and neuronal morphology induced by the knockdown of Drosophila SLC25A46 via an epigenetic pathway. Exp Cell Res. 2019;385(2) doi: 10.1016/j.yexcr.2019.111673. [DOI] [PubMed] [Google Scholar]
- 75.Stepto N.K., Benziane B., Wadley G.D., Chibalin A.V., Canny B.J., Eynon N., et al. Short-term intensified cycle training alters acute and chronic responses of PGC1α and Cytochrome C oxidase IV to exercise in human skeletal muscle. PLoS One. 2012;7(12) doi: 10.1371/journal.pone.0053080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rocchi A., He C. Regulation of exercise-induced autophagy in skeletal muscle. Curr Pathobiol Rep. 2017;5(2):177–186. doi: 10.1007/s40139-017-0135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vargas-Ortiz K., Pérez-Vázquez V., Macías-Cervantes M.H. Exercise and sirtuins: a way to mitochondrial health in skeletal muscle. Int J Mol Sci. 2019;20(11) doi: 10.3390/ijms20112717. [DOI] [PMC free article] [PubMed] [Google Scholar]