Abstract
The capacity of Escherichia coli to adapt its catabolism to prevailing redox conditions resides mainly in three catabolic branch points involving (i) pyruvate formate-lyase (PFL) and the pyruvate dehydrogenase complex (PDHc), (ii) the exclusively fermentative enzymes and those of the Krebs cycle, and (iii) the alternative terminal cytochrome bd and cytochrome bo oxidases. A quantitative analysis of the relative catabolic fluxes through these pathways is presented for steady-state glucose-limited chemostat cultures with controlled oxygen availability ranging from full aerobiosis to complete anaerobiosis. Remarkably, PFL contributed significantly to the catabolic flux under microaerobic conditions and was found to be active simultaneously with PDHc and cytochrome bd oxidase-dependent respiration. The synthesis of PFL and cytochrome bd oxidase was found to be maximal in the lower microaerobic range but not in a ΔArcA mutant, and we conclude that the Arc system is more active with respect to regulation of these two positively regulated operons during microaerobiosis than during anaerobiosis.
Escherichia coli possesses distinct catabolic routes that enable it to conserve energy efficiently under wide ranges of redox conditions. In environments that provide the cell with external electron acceptors such as oxygen, nitrate, fumarate, and dimethyl sulfoxide, reoxidation of reducing equivalents generated by the oxidation of the energy source occurs in the respiratory chain. This process can be coupled to the formation of a proton motive force (PMF) and thus constitutes an efficient pathway for energy conservation. In the absence of oxygen or other external electron acceptors, ATP synthesis occurs at the level of substrate phosphorylation. Under such fermentative conditions E. coli, when growing on glucose, excretes specific products such as ethanol, acetate, lactate, succinate, and formate (or CO2 and H2). The relative rates of formation of these products are governed by the demand for redox neutrality (5, 21).
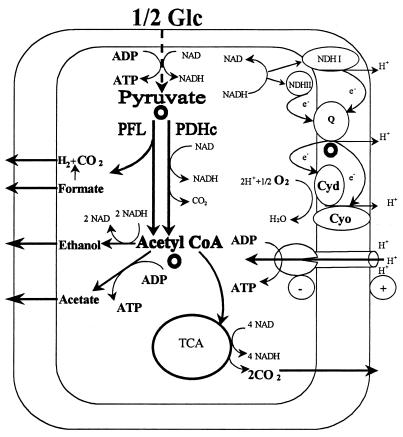
The actual in vivo fluxes of carbon and electrons through the various pathways are determined largely by three major branch points (Fig. 1). The first of these involves the cleavage of pyruvate, which serves as a common substrate for pyruvate formate-lyase (PFL) and the pyruvate dehydrogenase complex (PDHc). Entry into the fermentative pathway depends largely on the activity of PFL, whereas entry into the respiratory pathway is largely governed by the activity of the PDHc. At the second branch point, acetyl-coenzyme A, the product of both of the above reactions, can be converted to either the major fermentation products acetate and ethanol or can subsequently undergo further oxidation in the tricarboxylic acid (TCA) cycle. Finally, E. coli also possesses a branched respiratory chain. The electron flow into respiration can follow alternative routes to oxygen, via a coupled or an uncoupled NADH dehydrogenase (NDH-1 or NDH-2, respectively) to quinone (19, 42). Quinol is then oxidized by either the cytochrome bd or the cytochrome bo terminal oxidase complex, which in turn passes the electrons to oxygen with concomitant reduction of the latter to water. The two terminal oxidases differ in their affinities for oxygen as well as in their H+-to-e− stoichiometries. Cytochrome bd translocates one H+ per e− (44, 48) and has a high affinity for oxygen (10, 34, 49), whereas the low-affinity cytochrome bo oxidase is thought to translocate two H+s per e− (3, 48).
FIG. 1.
Major routes of the anaerobic (left) and aerobic (right) catabolic pathways in E. coli. Open circles, branch points of the respiratory and fermentative catabolism. Glc, glucose.
The regulation of expression of genes encoding catabolic enzymes in enterobacteria has been the subject of many studies. In E. coli, two global regulatory systems which control aerobic respiration and fermentation have been identified. These are the ArcAB two-component regulatory system and the FNR protein (18, 27, 57, 58). FNR serves as an activator of a number of genes whose products are involved in anaerobic respiratory metabolism, whereas the ArcAB system plays an important role in transcriptional regulation under both anaerobic and aerobic conditions. It has been shown to repress a number of genes coding for TCA cycle enzymes, cyoABCDE (coding for the low-affinity terminal oxidase), and, to a lesser extent, transcription of pdhR-aceEF-lpd encoding PDHc and its regulator during fermentative growth (4, 8, 9, 25, 53, 56). Conversely, ArcA acts as an activator of transcription, under appropriate conditions, of two catabolic operons. These are the focApfl operon encoding PFL and a formate transport protein (51, 59) and the cydAB operon coding for cytochrome bd oxidase (61).
Regulation of PFL synthesis and activity in vivo is very complex. Both are controlled by the prevalent oxygen status, which consequently has a major bearing on the control of catabolism (33). First, ArcA and FNR function in combination as anaerobic activators of focApfl gene expression. Second, interconversion of PFL between inactive and active glycyl radical-bearing species occurs at low oxygen tensions and is controlled by the activities of the iron-sulfur protein PFL activase and the product of the adhE gene, PFL deactivase (33, 52). Third, the active glycyl radical form of PFL is irreversibly destroyed by molecular oxygen and hence must be either protected from oxygen damage or converted to the inactive, oxygen-stable species during the transition between anaerobiosis and aerobiosis (63).
Aerobic respiratory activity in vivo is strictly dependent on the presence of terminal oxidases. As the oxygen supply decreases, a concomitant increase in cydAB expression and decrease in cyoABCDE expression occur (61). Cytochrome bd oxidase may therefore provide a means of affording protection to the active PFL enzyme at low oxygen levels. Transcriptional regulation of cyd is both ArcA and FNR dependent, whereby FNR functions as an anaerobic repressor of cyd expression (6, 7, 13).
Although a substantial amount of information regarding the mechanism of signal transduction by the ArcAB system is now available (14, 15, 22, 26, 32, 39–41, 62), no unequivocal proof as to the nature of the signal that stimulates the regulatory cascade in vivo has been presented. Since molecular oxygen has been excluded as the biochemical signal being sensed by ArcB (24, 28, 38), a number of other potential signals have been proposed, including intracellular metabolites (e.g., NADH, d-lactate, and pyruvate), the redox state of the respiratory chain, and the PMF (2, 22, 24, 30). In this study we used steady-state chemostat cultures to investigate in detail the response of E. coli to varying oxygen availability. In particular, we provide evidence to support the dual sensory mechanism of ArcB originally proposed by Matsushika and Mizuno (39), whereby microaerobic activation of the Arc system is mainly determined by a respiration-dependent signal, while deactivation of the system is governed by the redox state of the cell when oxygen levels become less restrictive. Furthermore, we propose that in E. coli respiratory protection of the active species of PFL by cytochrome bd oxidase may occur during microaerobic growth.
MATERIALS AND METHODS
Strains and growth conditions.
E. coli strains RM123λRM23 [MC4100 recA Φ(pfl-lacZ)] (50) and RM3133λRM23 [MC4100 ΔarcA::tet Φ(pfl-lacZ)] were used throughout this study. These strains are derivatives of E. coli MC4100 containing a chromosomal pfl-lacZ fusion in the λ attachment site. RM3133 ΔarcA::tet was constructed by transducing the ΔarcA::tet allele from MG1655 arcA::tet (a gift from D. Touati) into MC4100. MC4100 was used in a number of experiments as a control.
Cells were grown in chemostat cultures under glucose-limited conditions (Bioflo 3000 and III; New Brunswick) at a constant dilution rate of 0.15 ± 0.01 h−1 at various oxygen supply rates. Glucose (45 mM) was used as the single carbon and energy source. A simple salts medium as described by Evans et al. (11) was used, but instead of citrate, nitriloacetic acid (2 mM) was used as the chelator. Selenite (30 μg/liter) and thiamine (15 mg/liter) were added to the medium. The pH value was maintained at 7.0 ± 0.1 by titrating with sterile 4 M NaOH, and the temperature was set to 35°C. The cultures were regularly checked for kanamycin resistance by plating on Luria-Bertani (LB) plates containing 20 μg of kanamycin/ml. The ΔarcA genotype was confirmed by Western blot analysis with polyclonal ArcA antiserum each time the ΔarcA strain was used. The strains were maintained in vials in LB medium with 30% (wt/vol) glycerol at −70°C. The oxygen supply was varied by varying the percentage of oxygen in a gas mixture of air and N2 while stirring the culture at a constant speed. In order to prevent long-term adaptive responses the oxygen availability was varied randomly. Residual dissolved oxygen was monitored by an INGOLD polarographic (Ø [diameter of the electrode's tip], 12 mm) O2 sensor with a detection limit of 100 nM and an accuracy of approximately 30 nM as determined by examining O2 input versus O2 output (electrode reading) under conditions similar to those used for cultivation (medium, pH, temperature), but in the absence of cells. The O2 input/output ratio was found to be linear in the range tested (0.05 to 100% of air saturation).
Analytical procedures.
Steady-state bacterial dry weight was measured by the procedure of Herbert et al. (20). Levels of glucose, pyruvate, lactate, formate, acetate, succinate, and ethanol were determined by high-pressure liquid chromatography (LKB) with a REZEX organic acid analysis column (Phenomenex) at a temperature of 65°C with 4 mM H2SO4 as the eluent, using a 2142 refractive index detector (LKB) and an SP 4270 integrator (Spectra Physics). CO2 production and O2 consumption were measured by passing the eluent gas from the fermentor through a Servomex CO2 analyzer and a Servomex O2 analyzer, respectively.
β-Galactosidase activity was measured in permeabilized cells taken from steady-state cultures as originally described by Miller (43) and modified by Giacomini et al. (16). The cytochrome bd content of cells in French press (2.068 × 107 Pa) extracts in 50 mM Tris-HCl, pH 7, was estimated by UV/visual difference spectrophotometry (46). Dithionite-reduced versus oxidized difference spectra (absorption maximum at 625 nm), recorded on an SLM AMINCO DW-2000 UV/visual spectrophotometer, were analyzed to quantitate cytochrome d, using the wavelength pair (628 and 649 nm) and extinction coefficient (18.8 cm−1 mM−1) given by Kita et al. (34).
Concentrations of NADH and NAD were determined in extracts obtained by rapid sampling of chemostat cultures into 5 M KOH and 5 M HCl, respectively, and were assayed after neutralization and filtration as described previously (55).
Calculations of balances and fluxes.
The determination of the steady-state concentrations of biomass and excreted end products and CO2 production and O2 consumption rates allowed the calculation of specific product and substrate consumption rates (q values; millimoles per gram of dry weight per hour). By converting these q values into amounts of carbon atoms produced (it was assumed that 50% of the dry weight consists of carbon [20]) versus amounts consumed per gram of dry weight per hour, the carbon recovery (Crecovery; carbon balance) was determined according to the equation
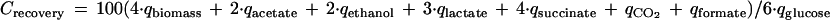 |
Similarly, redox balances were constructed for fully aerobic and fully anaerobic conditions. Here, net NADH and net NAD production rates were calculated on the basis of the stoichiometric values known for fermentation pathways, the TCA cycle, and succinate formation (8). It was assumed that biomass formation from glucose as the carbon source and ammonium ions as the nitrogen source is a redox-neutral process (20):
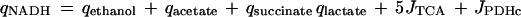 |
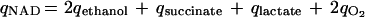 |
where J is the catabolic flux. Redox balances were defined as qNAD/qNADH · 100.
All data present a carbon balance of 95% ± 3%. Redox balances, calculated as indicated, were found to be 95 to 98%. Here, it was assumed that under fully anaerobic conditions PDHc activity was completely absent and that under fully aerobic conditions PFL activity was absent (8).
Typical product formation patterns and carbon and redox balances for a number of steady states are shown in Table 1. The q values were used to calculate the carbon fluxes through PDHc, PFL, and TCA cycles (J values; millimoles per gram of dry weight per hour). For microaerobic conditions, these calculations are based on the scheme depicted in Fig. 1 under the assumption that the prerequisite for a complete redox balance was fulfilled (see also reference 8):
 |
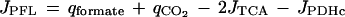 |
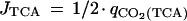 |
where qCO2(TCA) = 2/3 · (qCO2 + qformate − qethanol − qacetate). The calculations for JPFL and JPDHc showed the former flux to approach zero under anaerobic conditions and the latter to approach zero under aerobic conditions, which is consistent with the assumptions made for the calculations of redox balances under these conditions.
TABLE 1.
Typical steady-state product formation patterns and carbon and redox balances under different oxygen availability conditions
| Measurement | % Aerobiosis |
q (mmol · [g of dry weight]−1 · h−1) for:
|
Redox balancea | Carbon balanceb | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| O2 | CO2 | Glucose | Succinate | Lactate | Formate | Acetate | Ethanol | ||||
| 1 | 0 | 0 | 5.56 | 7.18 | 1.31 | 0.07 | 4.68 | 5.22 | 5.06 | 97.2 | 97.2 |
| 2 | 23 | 1.68 | 4.18 | 5.14 | 1.03 | 0.04 | 2.51 | 4.34 | 2.1 | 100 | 96.5 |
| 3 | 53 | 4.69 | 4.88 | 3.23 | 0.22 | 0 | 0 | 3.14 | 0.11 | 100 | 93.6 |
| 4 | 100 | 5.8 | 5.86 | 2.12 | 0.01 | 0 | 0 | 0 | 0 | 99 | 93.9 |
Defined as 100 · qNAD/qNADH (see Materials and Methods).
Ratio of carbon atoms recovered in end products and biomass to carbon atoms consumed, expressed as a percentage.
RESULTS
Experimental setup.
It is important to realize that it is technically rather complicated to obtain data from cells grown under steady-state conditions at very low oxygen tensions. It is not surprising therefore that the bulk of data available deal with cells grown under either fully aerobic or anaerobic conditions. Except for the studies of Wimpenny and Necklen (64) and Rice and Hempfling (49) only a few investigations have been performed under conditions of low oxygen tension (1, 36, 61). From these studies two conclusions can be drawn. First, for different experimental setups (for example, alteration of the geometry of the fermentor) a different oxygen supply may be required to achieve the same physiological response of the cells (49). Second, a certain oxygen supply condition at which further addition of oxygen has no significant effect can be determined (1, 61).
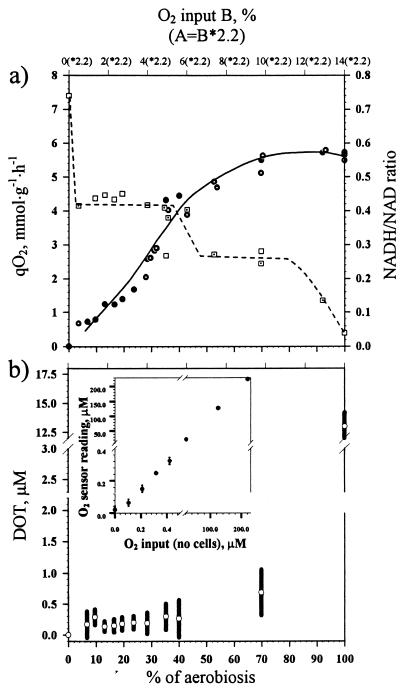
E. coli was grown in chemostat cultures using glucose as the sole carbon and energy source, and the experiments were carried out in two different chemostats, with culture volumes of 1,250 (A) and 1,380 ml (B). Although cells responded qualitatively similarly upon changes in the oxygen supply in the two chemostats, different actual percentages of oxygen input were required to evoke the same response. Consistently, a 2.2-fold-higher percentage of oxygen in the inflowing gas was required for chemostat A than for chemostat B to obtain similar responses of the cells. We assume that this factor reflects the relative differences in Kl,a values (the Kl,a value of the system is the rate of oxygen transfer from the gaseous to the liquid phase [12]) due to a difference in geometry and stirring efficiency between the two vessels. It should be realized that the supply rate of O2 does not define the O2 availability to a cell because the latter is also dependent on the Kl,a values and the biomass. To eliminate the effects of biomass, equal biomass concentrations for the same percentage of aerobiosis in the two chemostats were obtained by using the same glucose concentrations in all the experiments. Increased oxygen contents of the inflowing gas mixture resulted in a new steady state with cells displaying an increased oxygen consumption rate (Fig. 2a). As a consequence, up to an oxygen input of 12 (A) or 5.6% (B), the steady-state dissolved oxygen concentrations in experiments with the wild type did not change significantly (Fig. 2b). Apparently, the organisms responded to these changes in oxygen availability in a “homeostatic” manner, as indeed is exemplified by the observed changes in the composition of the respiratory chain (see below). The cell yield per mole of glucose increased from 20.4 ± 1.5 g under anaerobic conditions to 69.33 ± 0.8 g under fully aerobic conditions. To be able to compare the results obtained from the two fermentors, the data are normalized to the minimal percentage of oxygen in the inflowing gas (30% for chemostat A and 14% for chemostat B) required to reach complete aerobic behavior (i.e., CO2 as the sole end product). Thus, we refer to the minimum oxygen input resulting in complete aerobiosis as 100% aerobiosis. We conclude that dissolved-oxygen tension (DOT) and actual oxygen input are not appropriate parameters to describe the responses of E. coli to variations in oxygen availability. The response of the cells depends on the oxygen transfer rate of a particular setup, and this precludes the possibility of comparing results if actual oxygen input is used. If DOT is used as a variable, it is not possible to discriminate between different responses of the cell within a low-oxygen-supply range. Therefore, we propose to use the percentage of aerobiosis as a variable to study responses of the cell to different oxygen availabilities as described here.
FIG. 2.
(a) Changes in the specific rate of respiration (●, chemostat A;  , chemostat B) and intracellular redox state (NADH/NAD ratio) (□, chemostat A; ⊡, chemostat B) in response to a change in the oxygen supply in wild-type, glucose-limited E. coli. Upper x axis, actual percentage of oxygen in the inflowing gas in chemostat B. It took 2.2-fold more oxygen in the inflowing gas to evoke the same effect in chemostat A. (b) Residual dissolved oxygen concentrations (○) in steady-state glucose-limited chemostat cultures of E. coli (wild type) with increasing oxygen supply. Bars, amplitude of fluctuations in DOT. (Inset) Control of O2 electrode sensitivity in the low (below 1 μM O2) and high ranges. The control was performed in the absence of cells.
, chemostat B) and intracellular redox state (NADH/NAD ratio) (□, chemostat A; ⊡, chemostat B) in response to a change in the oxygen supply in wild-type, glucose-limited E. coli. Upper x axis, actual percentage of oxygen in the inflowing gas in chemostat B. It took 2.2-fold more oxygen in the inflowing gas to evoke the same effect in chemostat A. (b) Residual dissolved oxygen concentrations (○) in steady-state glucose-limited chemostat cultures of E. coli (wild type) with increasing oxygen supply. Bars, amplitude of fluctuations in DOT. (Inset) Control of O2 electrode sensitivity in the low (below 1 μM O2) and high ranges. The control was performed in the absence of cells.
We define the microaerobic range as the range between 0 and 100% aerobiosis.
Glucose catabolism at variable oxygen availability.
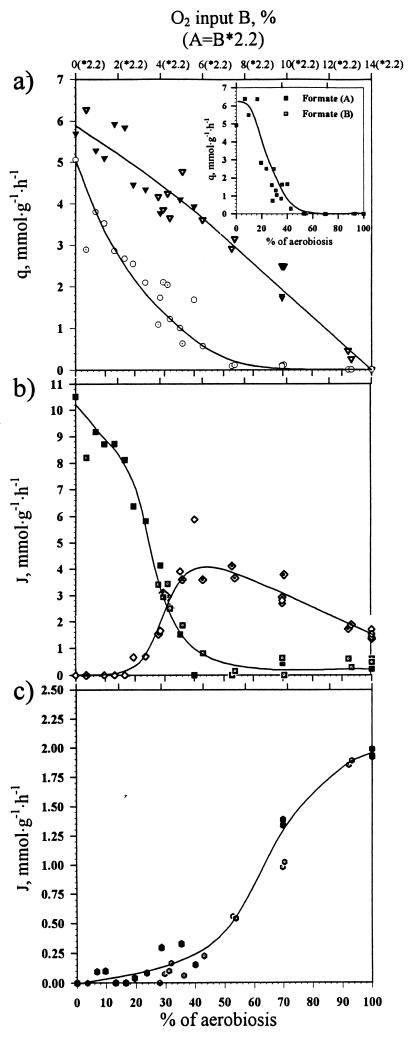
In Fig. 3a the specific production rates of the major fermentation products are presented. For fully aerobic and fully fermentative conditions the data are quantitatively virtually the same as those found earlier (8).
FIG. 3.
(a) Changes in specific rates of ethanol (⊙, chemostat B; ○, chemostat A), acetate (▿, chemostat B; ▾, chemostat A), and formate (inset; ■, chemostat A;  , chemostat B) formation in response to change in oxygen availability in wild-type E. coli. For an explanation of x-axis values, see the Fig. 2 legend. (b) Effect of oxygen availability on distribution of in vivo flux (J) between PFL (■, chemostat A; ◘, chemostat B) and PDHc (◊, chemostat A; ·⃟, chemostat B) in wild-type E. coli. (c) Effect of oxygen availability on in vivo TCA activity (
, chemostat B) formation in response to change in oxygen availability in wild-type E. coli. For an explanation of x-axis values, see the Fig. 2 legend. (b) Effect of oxygen availability on distribution of in vivo flux (J) between PFL (■, chemostat A; ◘, chemostat B) and PDHc (◊, chemostat A; ·⃟, chemostat B) in wild-type E. coli. (c) Effect of oxygen availability on in vivo TCA activity ( , chemostat A;
, chemostat A;  , chemostat B) in wild-type E. coli.
, chemostat B) in wild-type E. coli.
Clearly, the cell switches gradually from completely fermentative to completely respiratory metabolism as more oxygen becomes available, formate being the first product to disappear.
The steady-state specific product formation rates and respiration rates (Fig. 2a) allow for a calculation (based on a closed redox cycle; see Materials and Methods) of the fluxes through TCA, PFL, and PDHc (Fig. 3b and c). From these calculations it follows that under fully fermentative conditions the catabolic flux proceeds solely through PFL. What is of particular interest here is how the flux distribution shifts upon changes in the availability of the electron acceptor. The analysis shows (Fig. 3b) that PFL and PDHc can be active simultaneously under low microaerobic conditions (20 to 40% aerobiosis) and that their in vivo activities change smoothly in response to oxygen availability. It is noteworthy that there is a range where ethanol production is due to PDHc activity. Moreover, as catabolism now proceeds more efficiently with respect to the conservation of energy, a decrease in the qglucose (the specific rate of glucose consumption), and hence a decrease in the absolute value of JPDHc, are observed. TCA cycle activity initiates at 30% aerobiosis and gradually reaches its maximal activity by 100% aerobiosis (Fig. 3c). At levels of aerobiosis greater than 70% acetate is the only product excreted (Fig. 3a).
Respiratory activity at variable oxygen availability.
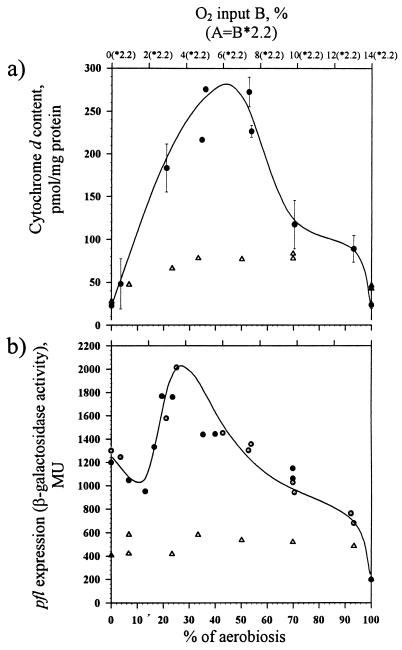
Oxygen consumption was already observed with the lowest oxygen supply rate applied (Fig. 2a). Increasing the oxygen supply resulted in a new steady state, with the cells respiring at a higher specific rate, but it is remarkable that initially (up to an oxygen supply rate allowing approximately 40% of full aerobiosis) no statistically significant changes in dissolved oxygen concentration were detected (Fig. 2b). This suggests that in this range the overall capacity of the respiratory chain is not constant due to either quantitative (affecting the capacity of the respiratory chain) or qualitative (affecting the affinity for oxygen) changes in its composition. This explanation is in accord with the observed changes in the cytochrome bd oxidase content of the cell (Fig. 4a).
FIG. 4.
(a) Effect of oxygen availability on cytochrome d synthesis in wild-type (●) and ΔarcA (Δ) strains of E. coli. For an explanation of x-axis values, see the Fig. 2 legend. (b) Effect of oxygen availability on pfl expression (●, chemostat A;  , chemostat B) in wild-type and ΔarcA (Δ) strains of E. coli.
, chemostat B) in wild-type and ΔarcA (Δ) strains of E. coli.
At very low oxygen availability a flux of electrons through the respiratory chain occurs, although the main carbon flux finds its way to the end products through the fermentative pathway. As NADH is now also reoxidized by respiration, there is no need for a 1:1 ratio of acetate and ethanol production to maintain redox neutrality (Fig. 2a and 3a).
Cellular cytochrome bd oxidase and PFL synthesis at different oxygen availabilities.
In order to determine the influence of the oxygen supply on the transcription of Arc-dependent genes for all conditions, the expression of focApfl and cydAB in wild-type and ΔarcA strains was studied. Expression of focApfl was monitored directly as a chromosomal lacZ fusion, while cydAB expression was monitored indirectly by measuring the cytochrome bd content of the cells. Both focApfl and cydAB operons are positively regulated by the phosphorylated form of ArcA (ArcA-P) under anaerobic conditions but are oppositely regulated by the fnr gene product. It was assumed that the cytochrome bd oxidase content correlated with cyd expression (6). From Fig. 4 it can be seen that expression of both operons in the wild type displays a maximum at intermediate oxygen supply rates, whereas this is not the case in the ΔarcA strain, suggesting a strong dependence of expression on ArcA. Increased pfl expression, however, initiates at approximately 15% aerobiosis only. This pattern of expression of both genes is in stark contrast to the gradual changes seen in the overall catabolic fluxes (Fig. 3b and c). Clearly, changes in the synthesis of PFL do not coincide with its in vivo activity. Remarkably, maximal pfl expression coincides with the microaerobic range where PDHc is inactivated (25% aerobiosis). In addition, under fully anaerobic conditions cyd expression is very low and comparable to that under the fully aerated condition.
Changes in NADH/NAD ratio.
Significant differences between the steady-state NADH/NAD ratios of aerobic and anaerobic cultures (0.02 and 0.75, respectively) have been observed previously, as well as a decrease in this ratio whenever an increase in the DOT of a culture is applied (8). We analyzed changes in intracellular concentrations of the pyridine nucleotides over the complete range of oxygen supply rates at small increments to be able to correlate them with changes in Arc-dependent enzyme synthesis and metabolic fluxes in vivo. The total amount of NADH plus NAD was found to be 4 ± 0.3 μmol (g of dry weight)−1 under all conditions. When trace amounts of oxygen were supplied to the culture the NADH/NAD ratio decreased significantly to approximately 60% of the value found under anaerobic conditions (Fig. 2a). Subsequent increases in oxygen supply did not affect the ratio significantly up to the rate (between 30 and 60% aerobiosis) at which a detectable increase in the steady-state DOT occurred (Fig. 2b). This range coincided with the range where major shifts in both pfl and cyd were observed (Fig. 4). At higher oxygen supply rates, the NADH/NAD ratio decreased gradually to the level under fully aerobic conditions.
DISCUSSION
The catabolism of E. coli is to a large extent determined by the availability of an external electron acceptor. Not surprisingly, elaborate sensing and regulatory systems have evolved to monitor the presence and nature of these acceptors and to tune catabolism accordingly. A major role in the regulation of the synthesis of a number of key catabolic enzymes has been assigned to the ArcAB two-component phosphorelay system, which consists of the membrane sensor ArcB and the transcriptional regulator ArcA (22, 23, 25, 29).
To date, the nature of the biochemical signal(s) that triggers phosphorylation of ArcB has not been identified. It is clear that oxygen per se is not the direct signal for ArcB phosphorylation (28, 38). Rather, NADH, d-lactate, acetate, and pyruvate, which can accumulate under microaerobic or anaerobic conditions, have been proposed as potential signals (22), based on the finding that in vitro high concentrations of these intermediates inhibit the intrinsic phosphatase activity of ArcB. Moreover, intracellular concentrations of at least one of the proposed intermediates, NADH, has been found to vary significantly under aerobic, microaerobic, and anaerobic conditions (8, 54).
The PMF (Δp) has been proposed as a signal being sensed by the Arc system (2, 30) as ArcAB-dependent induction of cytochrome bd synthesis has been observed in aerobically growing cells by the addition of protonophores and the inducing potency of the protonophores was found to be proportional to their uncoupling activity.
In this study we have analyzed the expression of two positively regulated, ArcA-P-dependent operons as a function of increasing oxygen availability. Changes in the intracellular redox state (as reflected by the NADH/NAD ratio) and steady-state respiration rates were measured as well. It has been established that cells growing aerobically have a significantly higher value of the PMF than anaerobically grown cells (31), and it seems therefore justified to assume that, with increasing respiration rates, the magnitude of the PMF will increase. In our experiments, the respiration rate reaches its maximum at 60 to 70% aerobiosis, the same condition where the PMF reaches its maximal value (35).
In contrast to the respiration rate, which increased gradually with increasing oxygen availability under stringent microaerobic conditions, the intracellular NADH/NAD ratio was found to remain essentially constant. Over this range, the expression level of the two positively regulated ArcA-P-dependent genes, pfl and cyd, increased. On the other hand, varying oxygen availability under less-stringent microaerobic conditions (above 50% aerobiosis) did not result in significant changes in respiration rate but did affect the NADH/NAD ratio dramatically. Now, derepression of ArcA-P-dependent genes (deduced from the increase of TCA cycle activity) and deactivation of focApfl and cydAB operon expression were observed. So the ArcA-dependent increase in cyd and pfl expression coincides with increasing respiratory activity in the lower range of oxygen availability, whereas the pattern of decreasing NADH concentrations correlates with the decrease in the expression in the higher range of oxygen availability. Whereas in our experiments ArcA-P-dependent pfl and cyd induction coincided with increasing PMF, earlier work (2) suggested an inverse correlation. In both experiments, however, the induction coincided with increasing respiratory activity: it was found earlier that the addition of uncouplers stimulates respiration (45). This discrepancy indicates that the overall electron flow rate per se may operate as a signal. The means by which the electron flow rate can be sensed by ArcB remains to be resolved, but it is tempting to speculate that the recently identified PAS domain of ArcB (60) plays a role in this sensing.
In conclusion, our observations strongly suggest that both the redox state and the rate of respiration rate play a role in ArcAB-dependent regulation, albeit the former does so under conditions of higher oxygen availability than the latter. In this context it is relevant to note that recently Matsushika and Mizuno (39) have shown that dual-signal sensing is possible for ArcB. Their study revealed that ArcB is capable of propagating two types of stimuli through two distinct phosphotransfer pathways (39).
It should be mentioned here that besides the ArcAB system, the regulatory FNR protein is a strong effector for expression of genes involved in the aerobic and anaerobic catabolic pathways. Indeed, the observed differences in expression of pfl and cyd under anaerobic conditions can be explained by interference through FNR regulation of these genes. In contrast to pfl (51) and genes coding for TCA cycle enzymes, for which ArcA-P and FNR exhibit similar effects (17, 25, 37, 38), cyd is subject to competitive ArcA-P induction and FNR repression (7, 12, 61), the latter being dominant under anaerobic conditions.
Our results suggest that the cytochrome bd content in steady-state cultures of E. coli seems to be adjusted to the oxygen availability in such a manner that the consumption of oxygen is maximized. Up to a certain level, an increased input of oxygen results in an increased respiratory capacity, which as a consequence will maintain a low residual oxygen concentration. This explains why no statistical difference among dissolved oxygen concentrations could be found in wild-type cultures in the microaerobic range, where the most significant microaerobic up-regulation of cyd and pfl occurs. Maximal expression of cyd under microaerobic conditions has been reported earlier (13, 49, 61). Significantly, increased expression of cyd and pfl under microaerobic conditions is coordinated. It is also noteworthy that PFL activity is observed only under conditions where dissolved oxygen concentrations remain low. PFL is, in its active form, a radical-containing enzyme, known to be readily destroyed by oxygen (63). Consequently, it has always been assumed that PFL activity was exclusive to anaerobiosis. We now present proof that this is not the case. Even under microaerobic conditions where respiration reaches its half-maximal rate, as much as half of the total flux from pyruvate is due to PFL activity. We propose that PFL activity can be maintained under these conditions by a mechanism very similar to the so-called respiratory protection known to occur in nitrogen-fixing microorganisms, e.g., Azotobacter vinelandii, where cytochrome bd plays a crucial role in creating an intracellular environment that allows nitrogenase to function by rapidly consuming oxygen via uncoupled respiration (for a review see reference 47). Below 30% aerobiosis, the NADH/NAD ratio is found to remain as high as 0.42, a ratio known to inhibit PDHc activity in vitro (54). Hence, the organism needs an alternative route for pyruvate catabolism. In analogy to the concept of respiratory protection, the physiological role of Arc-dependent up-regulation of both cyd and pfl expression under microaerobic conditions may reside in the rapid consumption of oxygen by cytochrome bd. Thus, the resulting low intracellular oxygen concentration allows for maintaining a high catabolic flux through the oxygen-sensitive PFL.
REFERENCES
- 1.Becker S, Vlad D, Schuster S, Pfeiffer P, Unden G. Regulatory O2 tensions for the synthesis of fermentation products in Escherichia coli and relation to aerobic respiration. Arch Microbiol. 1997;168:290–296. doi: 10.1007/s002030050501. [DOI] [PubMed] [Google Scholar]
- 2.Bogachev A, Murtazina R, Shestopalov A, Skulachev V. Induction of the Escherichia coli cytochrome d by low ΔμH+ and by sodium ions. Eur J Biochem. 1995;232:304–308. doi: 10.1111/j.1432-1033.1995.tb20812.x. [DOI] [PubMed] [Google Scholar]
- 3.Calhoun M W, Oden K L, Gennis R B, de Mattos M J, Neijssel O M. Energetic efficiency of Escherichia coli: effects of mutations in components of the aerobic respiratory chain. J Bacteriol. 1993;175:3020–3025. doi: 10.1128/jb.175.10.3020-3025.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cassey B, Guest J R, Attwood M M. Environmental control of pyruvate dehydrogenase complex expression in Escherichia coli. FEMS Microbiol Lett. 1998;159:325–329. doi: 10.1111/j.1574-6968.1998.tb12878.x. [DOI] [PubMed] [Google Scholar]
- 5.Clark D P. The fermentation pathways of Escherichia coli. FEMS Microbiol Rev. 1989;5:223–234. doi: 10.1016/0168-6445(89)90033-8. [DOI] [PubMed] [Google Scholar]
- 6.Cotter P A, Chepuri V, Gennis R B, Gunsalus R P. Cytochrome o (cyoABCDE) and d (cydAB) oxidase gene expression in Escherichia coli is regulated by oxygen, pH, and the fnr gene product. J Bacteriol. 1990;172:6333–6338. doi: 10.1128/jb.172.11.6333-6338.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cotter P A, Gunsalus R P. Contribution of the fnr and arcA gene products in coordinate regulation of cytochrome o and d oxidase (cyoABCDE and cydAB) genes in Escherichia coli. FEMS Microbiol Lett. 1992;70:31–36. doi: 10.1016/0378-1097(92)90558-6. [DOI] [PubMed] [Google Scholar]
- 8.de Graef M R, Alexeeva S, Snoep J L, Teixeira de Mattos M J. The steady-state internal redox state (NADH/NAD) reflects the external redox state and is correlated with catabolic adaptation in Escherichia coli. J Bacteriol. 1999;181:2351–2357. doi: 10.1128/jb.181.8.2351-2357.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dietrich J, Henning U. Regulation of pyruvate dehydrogenase complex synthesis in Escherichia coli K 12. Identification of the inducing metabolite. Eur J Biochem. 1970;14:258–269. doi: 10.1111/j.1432-1033.1970.tb00285.x. [DOI] [PubMed] [Google Scholar]
- 10.D'Mello R, Hill S, Poole R K. The cytochrome bd quinol oxidase in Escherichia coli has an extremely high oxygen affinity and two oxygen-binding haems: implications for regulation of activity in vivo by oxygen inhibition. Microbiology. 1996;142:755–763. doi: 10.1099/00221287-142-4-755. [DOI] [PubMed] [Google Scholar]
- 11.Evans C G T, Herbert D, Tempest D W. The continuous culture of microorganisms. 2. Construction of a chemostat. In: Norris J R, Ribbons D W, editors. Methods in microbiology. Vol. 2. London, United Kingdom: Academic Press; 1970. pp. 277–327. [Google Scholar]
- 12.Finn R K. Agitation-aeration in the laboratory and industry. Bacteriol Rev. 1954;18:254–274. doi: 10.1128/br.18.4.254-274.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu H A, Iuchi S, Lin E C. The requirement of ArcA and Fnr for peak expression of the cyd operon in Escherichia coli under microaerobic conditions. Mol Gen Genet. 1991;226:209–213. doi: 10.1007/BF00273605. [DOI] [PubMed] [Google Scholar]
- 14.Georgellis D, Kwon O, De Wulf P, Lin E C. Signal decay through a reverse phosphorelay in the Arc two-component signal transduction system. J Biol Chem. 1998;273:32864–32869. doi: 10.1074/jbc.273.49.32864. [DOI] [PubMed] [Google Scholar]
- 15.Georgellis D, Lynch A S, Lin E C. In vitro phosphorylation study of the Arc two-component signal transduction system of Escherichia coli. J Bacteriol. 1997;179:5429–5435. doi: 10.1128/jb.179.17.5429-5435.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giacomini A, Corich V, Ollero F J, Squartini A, Nuti M P. Experimental conditions may affect reproducibility of the β-galactosidase assay. FEMS Microbiol Lett. 1992;100:87–90. doi: 10.1111/j.1574-6968.1992.tb14024.x. [DOI] [PubMed] [Google Scholar]
- 17.Guest J R, Green J, Irvine A S, Spiro S. The FNR modulon and FNR-regulated gene expression. In: Lin E C C, Lynch A S, editors. Regulation of gene expression in Escherichia coli. Austin, Tex: Landes Co.; 1996. [Google Scholar]
- 18.Gunsalus R P, Park S-J. Aerobic-anaerobic gene regulation in Escherichia coli: control by the ArcAB and FNR regulons. Res Microbiol. 1994;145:437–450. doi: 10.1016/0923-2508(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi M, Miyoshi T, Takashina S, Unemoto T. Purification of NADH-ferricyanide dehydrogenase and NADH-quinone reductase from Escherichia coli membranes and their roles in the respiratory chain. Biochim Biophys Acta. 1989;977:62–69. doi: 10.1016/s0005-2728(89)80009-x. [DOI] [PubMed] [Google Scholar]
- 20.Herbert D, Phipps P J, Strange R E. Chemical analysis of microbial cells. In: Norris J R, Ribbons D W, editors. Methods in microbiology. 5b. New York, N.Y: Academic Press; 1971. pp. 209–344. [Google Scholar]
- 21.Holms H. Flux analysis and control of the central metabolic pathways in Escherichia coli. FEMS Microbiol Rev. 1996;19:85–116. doi: 10.1111/j.1574-6976.1996.tb00255.x. [DOI] [PubMed] [Google Scholar]
- 22.Iuchi S. Phosphorylation/dephosphorylation of the receiver module at the conserved aspartate residue controls transphosphorylation activity of histidine kinase in sensor protein ArcB of Escherichia coli. J Biol Chem. 1993;268:23972–23980. [PubMed] [Google Scholar]
- 23.Iuchi S, Cameron D C, Lin E C C. A second global regulator gene (arcB) mediating repression of enzymes in aerobic pathways of Escherichia coli. J Bacteriol. 1989;171:868–873. doi: 10.1128/jb.171.2.868-873.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iuchi S, Chepuri V, Fu H A, Gennis R B, Lin E C C. Requirement for terminal cytochromes in generation of the aerobic signal for the arc regulatory system in Escherichia coli: study utilizing deletions and lac fusions of cyo and cyd. J Bacteriol. 1990;172:6020–6025. doi: 10.1128/jb.172.10.6020-6025.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iuchi S, Lin E C C. arcA (dye), a global regulatory gene in Escherichia coli mediating repression of enzymes in aerobic pathways. Proc Natl Acad Sci USA. 1988;85:1888–1892. doi: 10.1073/pnas.85.6.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iuchi S, Lin E C C. Purification and phosphorylation of the Arc regulatory components of Escherichia coli. J Bacteriol. 1992;174:5617–5623. doi: 10.1128/jb.174.17.5617-5623.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iuchi S, Lin E C C. Adaptation of Escherichia coli to redox environments by gene expression. Mol Microbiol. 1993;9:9–15. doi: 10.1111/j.1365-2958.1993.tb01664.x. [DOI] [PubMed] [Google Scholar]
- 28.Iuchi S, Lin E C C. Signal transduction in the Arc system for control of operons encoding aerobic respiratory enzymes. In: Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C.: ASM Press; 1995. pp. 223–231. [Google Scholar]
- 29.Iuchi S, Matsuda Z, Fujiwara T, Lin E C C. The arcB gene of Escherichia coli encodes a sensor-regulator protein for anaerobic repression of the arc modulon. Mol Microbiol. 1990;4:715–727. doi: 10.1111/j.1365-2958.1990.tb00642.x. [DOI] [PubMed] [Google Scholar]
- 30.Iuchi S, Weiner L. Cellular and molecular physiology of Escherichia coli in the adaptation to aerobic environments. J Biochem (Tokyo) 1996;120:1055–1063. doi: 10.1093/oxfordjournals.jbchem.a021519. [DOI] [PubMed] [Google Scholar]
- 31.Kashket E R. Effects of aerobiosis and nitrogen source on the proton motive force in growing Escherichia coli and Klebsiella pneumoniae cells. J Bacteriol. 1981;146:377–384. doi: 10.1128/jb.146.1.377-384.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kato M, Mizuno T, Shimizu T, Hakoshima T. Insights into multistep phosphorelay from the crystal structure of the C-terminal HPt domain of ArcB. Cell. 1997;88:717–723. doi: 10.1016/s0092-8674(00)81914-5. [DOI] [PubMed] [Google Scholar]
- 33.Kessler D, Knappe J. Anaerobic dissimilation of pyruvate. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C.: American Society for Microbiology; 1996. pp. 199–205. [Google Scholar]
- 34.Kita K, Konishi K, Anraku Y. Terminal oxidases of Escherichia coli aerobic respiratory chain. II. Purification and properties of cytochrome b558-d complex from cells grown with limited oxygen and evidence of branched electron-carrying systems. J Biol Chem. 1984;259:3375–3381. [PubMed] [Google Scholar]
- 35.Laszlo D J, Taylor B L. Aerotaxis in Salmonella typhimurium: role of electron transport. J Bacteriol. 1981;145:990–1001. doi: 10.1128/jb.145.2.990-1001.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lawford H G, Rousseau J D. Effect of oxygen on ethanol production by a recombinant ethanologenic E. coli. Appl Biochem Biotechnol. 1994;45–46:349–366. doi: 10.1007/BF02941811. [DOI] [PubMed] [Google Scholar]
- 37.Lynch A S, Lin E C C. Regulation of aerobic and anaerobic metabolism by the Arc system. In: Lin E C C, Lynch A S, editors. Regulation of gene expression in Escherichia coli. Austin, Tex: Landes Co.; 1996. pp. 361–373. [Google Scholar]
- 38.Lynch A S, Lin E C C. Responses to molecular oxygen. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C.: American Society for Microbiology; 1996. pp. 1526–1538. [Google Scholar]
- 39.Matsushika A, Mizuno T. A dual-signaling mechanism mediated by the ArcB hybrid sensor kinase containing the histidine-containing phosphotransfer domain in Escherichia coli. J Bacteriol. 1998;180:3973–3977. doi: 10.1128/jb.180.15.3973-3977.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsushika A, Mizuno T. Mutational analysis of the histidine-containing phosphotransfer (HPt) signaling domain of the ArcB sensor in Escherichia coli. Biosci Biotechnol Biochem. 1998;62:2236–2238. doi: 10.1271/bbb.62.2236. [DOI] [PubMed] [Google Scholar]
- 41.Matsushika A, Mizuno T. The structure and function of the histidine-containing phosphotransfer (HPt) signaling domain of the Escherichia coli ArcB sensor. J Biochem (Tokyo) 1998;124:440–445. doi: 10.1093/oxfordjournals.jbchem.a022132. [DOI] [PubMed] [Google Scholar]
- 42.Matsushita K, Ohnishi T, Kaback H R. NADH-ubiquinone oxidoreductases of the Escherichia coli aerobic respiratory chain. Biochemistry. 1987;26:7732–7737. doi: 10.1021/bi00398a029. [DOI] [PubMed] [Google Scholar]
- 43.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. pp. 352–355. [Google Scholar]
- 44.Miller M J, Gennis R B. The cytochrome d complex is a coupling site in the aerobic respiratory chain of Escherichia coli. J Biol Chem. 1985;260:14003–14008. [PubMed] [Google Scholar]
- 45.Neijssel O M. The effect of 2,4-dinitrophenol on the growth of Klebsiella aerogenes NCTC 418 in aerobic chemostat cultures. FEMS Lett. 1977;1:47–50. [Google Scholar]
- 46.Olden K, Hempfling W P. The 503-nm pigment of Escherichia coli B: characterization and nutritional conditions affecting its accumulation. J Bacteriol. 1973;113:914–921. doi: 10.1128/jb.113.2.914-921.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poole R K, Hill S. Respiratory protection of nitrogenase activity in Azotobacter vinelandii—roles of the terminal oxidases. Biosci Rep. 1997;17:303–317. doi: 10.1023/a:1027336712748. [DOI] [PubMed] [Google Scholar]
- 48.Puustinen A, Finel M, Haltia T, Gennis R B, Wikstrom M. Properties of the two terminal oxidases of Escherichia coli. Biochemistry. 1991;30:3936–3942. doi: 10.1021/bi00230a019. [DOI] [PubMed] [Google Scholar]
- 49.Rice C W, Hempfling W P. Oxygen-limited continuous culture and respiratory energy conservation in Escherichia coli. J Bacteriol. 1978;134:115–124. doi: 10.1128/jb.134.1.115-124.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sawers G, Bock A. Anaerobic regulation of pyruvate formate-lyase from Escherichia coli K-12. J Bacteriol. 1988;170:5330–5336. doi: 10.1128/jb.170.11.5330-5336.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sawers G, Suppmann B. Anaerobic induction of pyruvate formate-lyase gene expression is mediated by the ArcA and FNR proteins. J Bacteriol. 1992;174:3474–3478. doi: 10.1128/jb.174.11.3474-3478.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sawers G, Watson G. A glycyl radical solution: oxygen-dependent interconversion of pyruvate formate-lyase. Mol Microbiol. 1998;29:945–954. doi: 10.1046/j.1365-2958.1998.00941.x. [DOI] [PubMed] [Google Scholar]
- 53.Smith M W, Neidhardt F C. 2-Oxoacid dehydrogenase complexes of Escherichia coli: cellular amounts and patterns of synthesis. J Bacteriol. 1983;156:81–88. doi: 10.1128/jb.156.1.81-88.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Snoep J L, de Graef M R, Westphal A H, de Kok A, Teixeira de Mattos M J, Neijssel O M. Differences in sensitivity to NADH of purified pyruvate dehydrogenase complexes of Enterococcus faecalis, Lactococcus lactis, Azotobacter vinelandii and Escherichia coli: implications for their activity in vivo. FEMS Microbiol Lett. 1993;114:279–283. doi: 10.1111/j.1574-6968.1993.tb06586.x. [DOI] [PubMed] [Google Scholar]
- 55.Snoep J L, Teixeira de Mattos M J, Postma P, Neijssel O M. Involvement of pyruvate dehydrogenase in product formation in pyruvate-limited anaerobic chemostate cultures of Enterococcus faecalis NCTC 775. Arch Microbiol. 1990;154:50–55. doi: 10.1007/BF00249177. [DOI] [PubMed] [Google Scholar]
- 56.Spencer M E, Guest J R. Transcription analysis of the sucAB, aceEF and lpd genes of Escherichia coli. Mol Gen Genet. 1985;200:145–154. doi: 10.1007/BF00383328. [DOI] [PubMed] [Google Scholar]
- 57.Spiro S, Guest J R. FNR and its role in oxygen-regulated gene expression in Escherichia coli. FEMS Microbiol Rev. 1990;6:399–428. doi: 10.1111/j.1574-6968.1990.tb04109.x. [DOI] [PubMed] [Google Scholar]
- 58.Spiro S, Guest J R. Adaptive responses to oxygen limitation in Escherichia coli. Trends Biochem Sci. 1991;16:310–314. doi: 10.1016/0968-0004(91)90125-f. [DOI] [PubMed] [Google Scholar]
- 59.Suppmann B, Sawers G. Isolation and characterization of hypophosphite-resistant mutants of Escherichia coli: identification of the FocA protein, encoded by the pfl operon, as a putative formate transporter. Mol Microbiol. 1994;11:965–982. doi: 10.1111/j.1365-2958.1994.tb00375.x. [DOI] [PubMed] [Google Scholar]
- 60.Taylor B L, Zhulin I B. PAS domains: internal sensors of oxygen, redox potential, and light. Microbiol Mol Biol Rev. 1999;63:479–506. doi: 10.1128/mmbr.63.2.479-506.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tseng C P, Albrecht J, Gunsalus R P. Effect of microaerophilic cell growth conditions on expression of the aerobic (cyoABCDE and cydAB) and anaerobic (narGHJI, frdABCD, and dmsABC) respiratory pathway genes in Escherichia coli. J Bacteriol. 1996;178:1094–1098. doi: 10.1128/jb.178.4.1094-1098.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsuzuki M, Ishige K, Mizuno T. Phosphotransfer circuitry of the putative multi-signal transducer, ArcB, of Escherichia coli: in vitro studies with mutants. Mol Microbiol. 1995;18:953–962. doi: 10.1111/j.1365-2958.1995.18050953.x. [DOI] [PubMed] [Google Scholar]
- 63.Wagner A F, Frey M, Neugebauer F A, Schafer W, Knappe J. The free radical in pyruvate formate-lyase is located on glycine-734. Proc Natl Acad Sci USA. 1992;89:996–1000. doi: 10.1073/pnas.89.3.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wimpenny J W T, Necklen D K. The redox environment and microbial physiology. I. The transition from anaerobiosis to aerobiosis in continuous culture of facultative anaerobes. Biochim Biophys Acta. 1971;253:352–359. doi: 10.1016/0005-2728(71)90039-9. [DOI] [PubMed] [Google Scholar]