Abstract
Purpose
Post hoc analysis of the phase III HAWK and HARRIER studies to compare the reductions in subretinal hyper-reflective material (SHRM) thickness following brolucizumab 6 mg or aflibercept 2 mg treatment and to assess SHRM thickness and thickness variability as a potential biomarker of visual outcomes in patients with neovascular age-related macular degeneration (nAMD).
Methods
Optical coherence tomography images from the brolucizumab (n=700) and aflibercept (n=696) arms were analysed for the maximum SHRM thickness across the macula over 96 weeks. In a pooled treatment-agnostic analysis, the effect of week 12 SHRM thickness and SHRM thickness variability on best-corrected visual acuity (BCVA) through week 96 were also assessed.
Results
Brolucizumab was associated with numerically higher percentage reductions from baseline in SHRM thickness versus aflibercept in all patients (week 96: 54.4% vs 47.6%, respectively) and also in the matched subgroups with disease activity at week 16 (week 96: 51.6% vs 33.8%, respectively). In eyes with lower SHRM measurements at week 12, mean BCVA gains from baseline were higher at week 96 (<200 µm, +6.47 Early Treatment Diabetic Retinopathy Study letters; ≥200 µm, +3.10 letters). Eyes with the lowest SHRM thickness variability from week 12 to week 96 showed the greatest mean BCVA gains from baseline (week 96: <12 µm, +7.42 letters; >71 µm, −2.95 letters).
Conclusions
In HAWK and HARRIER, greater reductions in maximum SHRM thickness from baseline were observed with brolucizumab compared with aflibercept. Furthermore, the data suggest that SHRM thickness postloading and SHRM thickness variability over time are biomarkers for visual outcomes in patients with nAMD.
Keywords: Retina, Clinical Trial
WHAT IS ALREADY KNOWN ON THIS TOPIC
Subretinal hyper-reflective material (SHRM) often persists following antivascular endothelial growth factor treatment and may have a detrimental effect on visual outcomes in patients with neovascular age-related macular degeneration (nAMD).
Brolucizumab has been shown to provide robust vision gains and superior fluid resolution compared with aflibercept but its impact on SHRM is not known.
WHAT THIS STUDY ADDS
In HAWK and HARRIER, brolucizumab 6 mg resulted in greater reductions in maximum SHRM thickness from baseline than aflibercept 2 mg over the 96-week study period.
A treatment-agnostic analysis also showed that vision outcomes were better in eyes with lower SHRM thickness at 12 weeks postloading and in eyes with less SHRM thickness variability.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study highlights that by more effectively reducing SHRM thickness, brolucizumab may provide greater disease control and that SHRM thickness postloading and thickness variability may be potential biomarkers for 2 year visual outcomes in patients with nAMD.
Introduction
Subretinal hyper-reflective material (SHRM), identified on optical coherence tomography (OCT) as hyper-reflective material located between the neurosensory retina and retinal pigment epithelium (RPE), is common in eyes with neovascular age-related macular degeneration (nAMD) and often persists following antivascular endothelial growth factor (VEGF) treatment.1–4 SHRM components are poorly understood and assumed to vary both temporally and according to neovascular subtype, or location. The composition can alternately represent haemorrhage, fibrin, fibrous scar tissue, type 2 choroidal neovascularisation or vitelliform material.5–7 It is hypothesised that with increased SHRM thickness (or height), a mechanical barrier forms between the retina and RPE, interfering with metabolic exchange and photoreceptor function, and thus the normal visual cycle.1 2 8 9 The presence of SHRM and subsequent development of fibrous scarring is considered a major risk factor associated with sustained visual acuity (VA) loss in nAMD, despite continued treatment.10 11
An early study of SHRM (previously labelled as subretinal tissue) noted that increased thickness at the foveal centre, and increased volume were correlated with decreased VA.8 Subsequently, in the comparison of Age-related Macular Degeneration Treatments Trial (the CATT study), 77% of eyes with nAMD showed SHRM at the time of enrolment, which decreased to 54% 2 years post-treatment with either ranibizumab or bevacizumab.1 A cohort study of CATT found that sustained VA loss in patients was rare; however, at 2 year follow-up in eyes with sustained VA loss, SHRM was typically present or had newly developed.12 SHRM was especially associated with poorer VA outcomes and scar tissue with thicker or wider lesions involving the fovea. It was hypothesised that SHRM thickness rapidly decreases in response to anti-VEGF therapy induction; however, as treatment continues, the pace of SHRM reduction declines due to an increased fibrotic component rendering therapy less effective.1
Brolucizumab is a single-chain variable fragment with a high affinity for VEGF. Its low molecular weight of 26 kDa allows the delivery of more drug per dose compared with other currently available anti-VEGF treatments, and offers the potential for more effective tissue penetration and increased duration of action.13 In the HAWK and HARRIER clinical trials in patients with nAMD, brolucizumab 6 mg demonstrated non-inferiority versus aflibercept in mean change in best-corrected VA (BCVA) at week 48 and week 96 with more than 50% of patients on a q12w dosing interval through year 1.14 15 In addition, brolucizumab 6 mg was associated with greater reductions in intraretinal fluid (IRF), subretinal fluid and sub-RPE fluid through week 96; however, the effect of brolucizumab on SHRM and the association between SHRM and visual outcomes in HAWK and HARRIER have not been evaluated.
In this post hoc analysis of the HAWK and HARRIER study populations, we compared reductions in SHRM thickness in the brolucizumab 6 mg and aflibercept 2 mg treatment arms, first in all patients as well as in patients with disease activity identified at week 16 (disease activity was determined by the investigator but guidance provided in the study protocol included a decrease in BCVA≥5 letters compared with baseline; decrease in BCVA≥3 letters and central subfield thickness (CST) increase ≥75 µm compared with week 12; decrease in BCVA≥5 letters due to nAMD disease activity compared with week 12; new or worse IRF compared with week 12). As brolucizumab-treated patients with disease activity were adjusted to a every 8 weeks interval until the end of the study, these patients matched aflibercept-treated patients in terms of number of injections and treatment interval. Through a pooled, treatment-agnostic analysis of these two large study cohorts, we also investigated the impact of SHRM thickness and thickness fluctuations on visual outcomes.
Study design and population
HAWK (NCT02307682) and HARRIER (NCT02434328) were two phase III, 96-week, prospective, randomised, double-masked and multicentre clinical studies. Inclusion and exclusion criteria, detailed trial protocol, statistical analyses, randomisation, and oversight have been previously published.14 15 Patients were randomised 1:1:1 to receive either brolucizumab 3 mg, brolucizumab 6 mg or aflibercept 2 mg (in HAWK), or 1:1 to receive brolucizumab 6 mg or aflibercept 2 mg (in HARRIER). Following 3 monthly loading doses at weeks 0, 4 and 8, brolucizumab study eyes were given an intravitreal injection every 12 weeks and the dose was adjusted to every 8 weeks for the remainder of the study period if disease activity was detected at any of the predefined assessment visits; aflibercept was dosed every 8 weeks following 3 monthly loading doses, as per label at study initiation. The data set for this analysis includes all randomised and treated patients from both HAWK and HARRIER with at least two evaluable postbaseline OCT images, analysed by the Doheny Image Reading and Research Laboratory, Los Angeles, California, USA.
Study assessments
In this retrospective analysis, OCT images from patients treated with brolucizumab 6 mg (n=700) or aflibercept 2 mg (n=696) were assessed in a masked, subcompartment analysis. SHRM was identified in the subretinal space and measured from the inner surface of the hyper-reflective material to the inner surface of the RPE (if present) or to the Bruch’s membrane if RPE was absent. Graders reviewed all B-scans to determine and measure the maximum SHRM thickness across the entire macula (OCT scan area 6 mm×6 mm). The graders were masked to treatment assignments for the patients. Maximum SHRM thickness was measured at baseline, and at weeks 4, 8, 12, 16, 20, 24, 32, 68 and 96.
The reduction in SHRM thickness across the entire macula in the brolucizumab and aflibercept treatment arms from baseline to week 96 was compared in the overall HAWK and HARRIER study population. Additionally, SHRM thickness reduction was also assessed in the subgroup of patients with disease activity at week 16 in both treatment arms (the first assessment visit postloading phase).
In a pooled, treatment-agnostic analysis, the 2-year BCVA outcomes were compared in patients with different SHRM thickness cut-off thresholds (100 µm and 200 µm) at week 12, when the full impact of the loading phase (3 monthly loading doses) is observed. An analysis of the association between SHRM thickness variability and visual outcomes was also performed using the pooled treatment-agnostic data. The SD of a patient’s maximum SHRM thickness (SD (SHRM)) during the maintenance phase (weeks 12–96) was used as a metric of individual SHRM variability/stability. Patients were grouped into quartiles of increasing SD (SHRM thickness), designated as Q1 (lowest variability) to Q4 (highest variability) and the BCVA outcomes in each of these quartiles compared.
VA assessments were conducted at baseline and every 4 weeks thereafter by masked investigators. BCVA was measured using Early Treatment Diabetic Retinopathy Study charts.
Statistical analysis
Maximum SHRM thickness across the macula and BCVA change from baseline were analysed using the analysis of covariance model with treatment and age category (<75, ≥75) as fixed effects and the baseline value of the corresponding endpoint as a covariate. Missing values were imputed using the last observation carried forward method. P values were two sided, non-adjusted for multiplicity and considered statistically significant below 0.05.
Results
In this post hoc analysis of the HAWK and HARRIER studies, the total analysis set included 96% of randomised patients (n=1396/1459) treated with either brolucizumab 6 mg or aflibercept 2 mg and with at least two evaluable OCT images from postbaseline. The baseline characteristics of the analysis population were well balanced across both treatment arms (table 1). Of the overall data set, 79.3% of patients exhibited SHRM at baseline in HAWK and HARRIER (brolucizumab 6 mg: 79.4%; aflibercept 2 mg: 79.3%).
Table 1.
Demographic and baseline ocular characteristics of patients included in this post hoc analysis
| Brolucizumab 6 mg (n=700) | Aflibercept 2 mg (n=696) | |
| Age, mean (SD), years | 75.7 (8.8) | 75.7 (8.3) |
| Females, n (%) | 393 (56.1) | 387 (55.6) |
| Ethnicity, n (%) | ||
| White | 597 (85.3) | 595 (85.5) |
| Asian | 81 (11.6) | 76 (10.9) |
| BCVA, mean (SD), letters | 61.2 (13.1) | 60.4 (13.3) |
| CST, mean (SD), μm | 468.2 (168.6) | 461.8 (149.4) |
| Type of CNV, n (%) | ||
| Predominantly classic | 252 (36.0) | 249 (36.0) |
| Minimally classic | 71 (10.1) | 67 (9.7) |
| Occult | 377 (53.9) | 375 (54.3) |
| Lesion area associated with CNV, mean (SD), mm2 | 3.6 (3.6) | 3.7 (3.9) |
| Presence of fluid, n (%) | ||
| SRF | 485 (69.3) | 492 (70.7) |
| IRF | 329 (47.0) | 316 (45.4) |
| Sub-RPE fluid | 282 (40.3) | 274 (39.4) |
BCVA, best-corrected visual acuity; CNV, choroidal neovascularisation; CST, central subfield thickness; IRF, intraretinal fluid; RPE, retinal pigment epithelium; SRF, subretinal fluid.
Absolute reduction in SHRM thickness through week 96
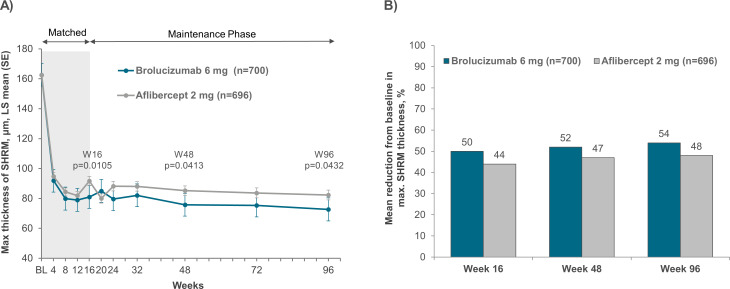
In the pooled dataset from HAWK and HARRIER studies, the least square mean (LSM)±SE SHRM thickness (adjusted for baseline) for the brolucizumab 6 mg group was 162.6 (0.00) µm, 81.0 (2.95) µm, 75.8 (3.22) µm and 72.7 (3.32) µm at baseline, weeks 16, 48 and 96, respectively. The corresponding SHRM thickness in the aflibercept 2 mg group was 162.6 (0.00) µm, 91.7 (2.97) µm, 85.2 (3.24) µm and 82.3 (3.38) µm at baseline, weeks 16, 48 and 96, respectively (figure 1A). Similar trends were observed when each study was analysed separately (online supplemental figure 1). The LSM differences (95% CI) in SHRM thickness between brolucizumab 6 mg and aflibercept 2 mg group at weeks 16, 48 and 96 were: −10.64 (−22.1, 0.8) µm, −9.43 (−22.2, 3.4) µm and −13.19 (−27.2, 0.8) µm for the HAWK study; and −10.89 (−22.7, 0.9) µm, −9.76 (−22.4, 2.8) µm and −7.08 (−19.6, 5.4) µm for the HARRIER study, respectively. Overall, brolucizumab-treated patients had greater reductions in SHRM thickness versus aflibercept-treated patients. Furthermore, SHRM thickness decreased rapidly from baseline following initiation of anti-VEGF treatment and this decrease was maintained up to week 96 in both treatment groups.
Figure 1.
(A) Maximum SHRM thickness and (B) percentage reduction in maximum SHRM thickness in the macula up to week 96 among patients in the brolucizumab and aflibercept arms of the pooled HAWK and HARRIER studies. SHRM, subretinal hyper-reflective material.
bjo-2023-323577supp001.pdf (608.6KB, pdf)
Percentage reduction in SHRM thickness through week 96
The percentage reduction in SHRM thickness also decreased to week 96 in both treatment arms. In the pooled dataset from HAWK and HARRIER (figure 1B), brolucizumab was associated with a numerically greater percentage reduction in SHRM thickness across the macula compared with aflibercept. A similar trend in percentage reduction was also observed when each study population was analysed separately (online supplemental figure 2). The mean percentage reduction in SHRM thickness in the pooled studies was: 50.1%, 52.3% and 54.4% at weeks 16, 48 and 96, respectively, for brolucizumab; and 43.9%, 47.0% and 47.6%, respectively, for aflibercept.
bjo-2023-323577supp002.pdf (353.2KB, pdf)
Absolute and percentage reduction in SHRM thickness through week 96 in patients with disease activity at week 16
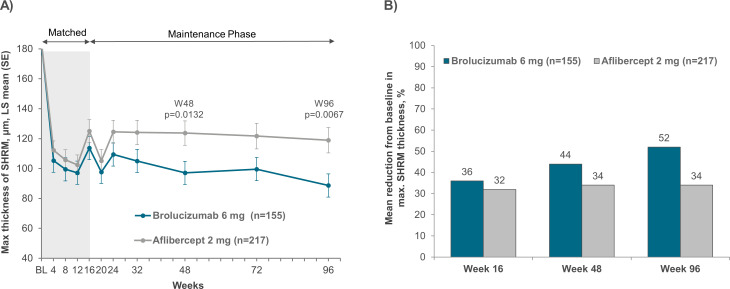
In the matched subgroup of patients with disease activity at week 16, the brolucizumab 6 mg group had LSM (±SE) SHRM thickness (adjusted for baseline) of 183.7 (0.00) µm, 113.7 (7.54) µm, 97.1 (8.15) µm and 88.7 (8.49) µm at baseline, weeks 16, 48 and 96, respectively. The aflibercept group SHRM thickness was 183.7 (0.00) µm, 125.1 (6.34) µm, 123.7 (6.82) µm and 119.0 (7.09) µm at baseline, weeks 16, 48 and 96, respectively. There was a marked difference in SHRM thickness reduction between the two treatment groups and the brolucizumab cohort was associated with statistically significant reductions in SHRM thickness compared with the aflibercept group at week 48 (p=0.0132) and week 96 (p=0.0067) (figure 2A).
Figure 2.
(A) Maximum SHRM thickness and (B) percentage reduction in maximum SHRM thickness in the macula up to week 96 among patients with disease activity at week 16 in the brolucizumab and aflibercept arms of the pooled HAWK and HARRIER studies. SHRM, subretinal hyper-reflective material.
Mean percentage reductions in SHRM thickness were numerically greater for brolucizumab-treated patients with disease activity at week 16, with 35.5%, 43.6% and 51.6% at weeks 16, 48 and 96, respectively, compared with 31.5%, 33.5% and 33.8%, respectively, for aflibercept (figure 2B).
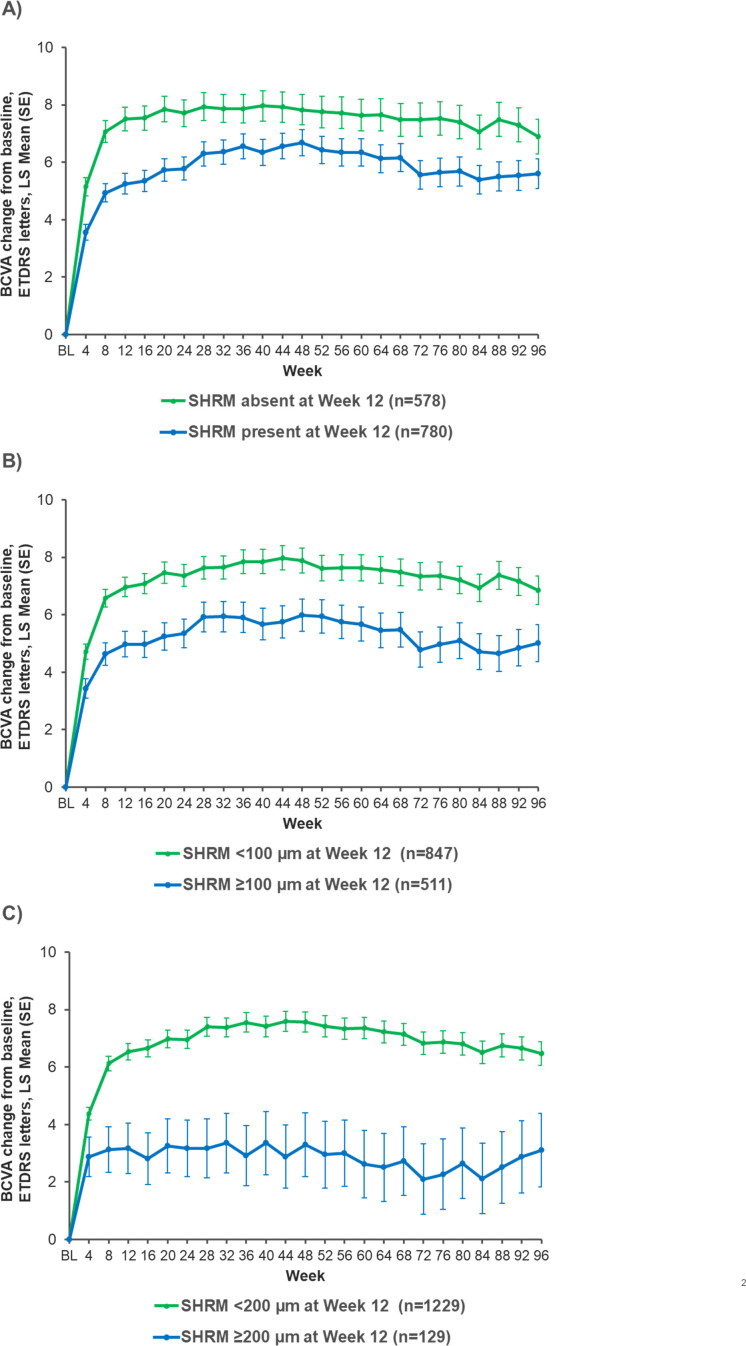
Visual outcomes according to SHRM presence/absence and SHRM thickness cut-offs at week 12
In a pooled, treatment-agnostic analysis of the HAWK and HARRIER data, SHRM was present in 780 (57.4%) eyes postloading at week 12 and absent in 578 (42.6%) eyes. The absence of SHRM at week 12 was associated with better visual outcomes through week 96 (figure 3A). At weeks 16, 48 and 96, those patients without SHRM at week 12 had an LSM (±SE) gain of +7.54 (0.43), +7.83 (0.53) and +6.89 (0.61), respectively, compared with +5.35 (0.37) letters, +6.68 (0.45) letters and +5.60 (0.52) letters, respectively, in those with SHRM present at week 12.
Figure 3.
BCVA change from baseline by week 12 SHRM (A) presence/absence, (B) thickness cut-off of 100 µm and (C) thickness cut-off of 200 µm (treatment-agnostic pooled data from HAWK and HARRIER). BCVA, best-corrected visual acuity; ETDRS, Early Treatment Diabetic Retinopathy Study; LS, least square; SHRM subretinal hyper-reflective material.
BCVA through week 96 was also compared in patients with SHRM thickness cut-offs of 100 µm and 200 µm at week 12 (figure 3B,C). An association between SHRM thickness and visual outcomes was identified, where greater SHRM thickness at week 12 resulted in worse BCVA outcomes at week 96 (SHRM<100 µm: 6.84 (0.50) letters; SHRM≥100 µm: 5.01 (0.64) letters; SHRM<200 µm: 6.47 (0.41) letters and SHRM≥200 µm: 3.10 (1.28) letters).
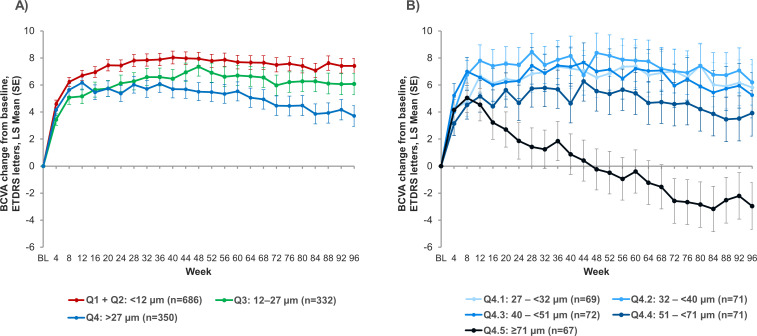
Visual outcomes by quartiles of SHRM thickness variability over time
Finally, the overall patient population was divided into four quartiles based on the SD of their maximum SHRM thickness between week 12 and week 96. A comparison of mean BCVA through week 96 in these various quartiles (figure 4A) showed that higher fluctuations in SHRM thickness led to worse visual outcomes at week 96. In particular, after an initial LSM (±SE) gain of +6.17 (0.53) letters postloading at week 12, mean BCVA change from baseline in the quartile with highest variability (Q4, >27 µm) decreased to +3.71 (0.77) letters at week 96.
Figure 4.
BCVA change from baseline to week 96 for (A) the four SHRM thickness variability quartiles and (B) with Q4 split into further quintiles. (Q1, lowest variability, Q4, highest variability; Q1 and Q2 pooled for clarity). BCVA, best-corrected visual acuity; ETDRS, Early Treatment Diabetic Retinopathy Study; LS, least square; SHRM subretinal hyper-reflective material.
To investigate the impact of even higher SHRM fluctuations on visual outcomes, the quartile with the highest variability was split into further quintiles based on SD of SHRM thickness from week 12 to week 96 (figure 4B). This analysis showed a clear threshold where patients with SHRM fluctuations of >71 µm between week 12 and week 96 lost vision at week 96 (LSM (SE), −2.95 (1.74) letters). A representative case with large fluctuations in SHRM can be seen in online supplemental figure 3.
bjo-2023-323577supp003.pdf (171KB, pdf)
Discussion
This post hoc analysis identified reductions in SHRM thickness following treatment with brolucizumab or aflibercept, and assessed its association with VA using pooled data from the HAWK and HARRIER studies. The current study identified the presence of SHRM at baseline in 79.3% of eyes with nAMD and the rapid decline in SHRM thickness from baseline up to week 96 following initiation of anti-VEGF treatment, which is consistent with the CATT study.1 Moreover, we observed that brolucizumab treatment resulted in significant reductions in absolute SHRM thickness and numerically greater reductions in percent thickness across the macula compared with aflibercept. These findings were also replicated in the matched subgroup of patients with disease activity at week 16. By more effectively reducing SHRM thickness, brolucizumab could offer the potential for greater disease control in patients with nAMD. We also observed a transient rise in SHRM thickness in both treatment groups following the matched loading phase. However, in the brolucizumab-treated group, the rise in SHRM thickness was notably smaller and delayed by 4 weeks, reaching a peak at week 20. This prolonged effect is suggestive of a greater durability of the anti-VEGF effect in suppressing disease activity, and reducing SHRM in patients with nAMD.
Greater SHRM thickness postloading at week 12 was associated with poorer visual outcomes after 2 years, thus corroborating results from previous studies, including CATT, about the long-term effects of this morphological feature in patients treated with anti-VEGF therapy.11 16 17 The link between thicker SHRM and poor visual outcomes indicates that SHRM may comprise fibrovascular or fibrocellular tissue that separates the RPE from the photoreceptors, eventually causing photoreceptor dysfunction and loss. Moreover, Casalino et al corroborated the observations from the CATT analysis and revealed that SHRM on SD-OCT may represent fibrotic tissue or mature type 2 neovascular complexes.18 A more in-depth analysis of the HAWK and HARRIER OCT images may identify an association between SHRM morphology and location and the presence of fibrotic tissue, therefore, explaining the poorer visual outcomes of patients with persistent SHRM.19 In addition, identifying eyes with reductions in SHRM thickness specifically at the foveal centre may reveal an even greater impact on visual outcomes.1 Nevertheless, this study clearly demonstrates the impact of SHRM on long-term visual outcomes irrespective of the location within the macula and emphasises the importance of reducing SHRM thickness as much as possible early in the course of anti-VEGF treatment.
Greater fluctuations in SHRM thickness during the maintenance phase in HAWK and HARRIER were also associated with poorer visual outcomes through week 96, suggesting that the damage caused by the thickening SHRM separating (or replacing) photoreceptors from the residual RPE accumulates over time. This finding aligns with previous analyses demonstrating that fluctuations in other nAMD parameters, such as retinal fluid volume and CST,20–22 also contribute to worse visual outcomes in patients.
A strength of the current analysis is the large population size captured from two phase III clinical trials investigating patients with nAMD. In addition, manual grading of the OCT images by masked, certified readers provided a robust analysis of SHRM thickness across the macula. The primary limitation is that this post hoc analysis is based on studies that were not specifically designed for evaluating the impact of SHRM. Therefore, it is not possible to definitively conclude that aggressive anti-VEGF treatment to eliminate SHRM leads to better visual outcomes. In addition, OCT is limited in differentiating the exact nature and composition of SHRM, which can vary from patient to patient and even from visit to visit. OCT angiography can be an important modality to clarify the nature of SHRM material23 24 but was not performed in HAWK and HARRIER. Another limitation is that neither the axial length nor the degree of myopia were accounted for in the treatment-specific analysis and these could have an impact on outcomes. However, with the relatively large sample size, we would expect these characteristics to be reasonably well balanced between the treatment arms.
In summary, brolucizumab achieved greater, clinically meaningful reductions in SHRM thickness over time compared with aflibercept in the HAWK and HARRIER studies. Furthermore, this post hoc analysis indicates that reducing SHRM thickness and variability through anti-VEGF treatment could help to optimise visual outcomes in patients with nAMD.
Acknowledgments
Medical writing support, under the guidance of the authors, was provided by Susan Simpson, PhD (Novartis Ireland), in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3). Medical writing support was funded by Novartis Pharma AG.
Footnotes
Contributors: SS, IS and KG were involved in the conception and design of the study and SS, DS, AMK, RT, AAC and DTW were involved in data collection. All authors made substantial contributions to the analysis and interpretation of data, drafting the article and revising it critically, and final approval of the version to be submitted. SS is the guarantor.
Funding: Financial support was provided by Novartis Pharma AG (Basel, Switzerland).
Disclaimer: The sponsor or funding organisation participated in the design of the study; management, analysis and interpretation of the data; preparation, review and approval of the manuscript.
Competing interests: SS: Consultant for Novartis, Allergan, Amgen, Apellis, Iveric, Nanoscope, Astellas, Jannsen, Roche/Genentech, Oxurion, Regeneron, 4DMT, Optos, Centervue, Heidelberg, Notal, Biogen, Boehringer Ingelheim. Speaker fees from Optos, Carl Zeiss Meditec, Nidek, Topcon, Novartis. DS: Consultant for Amgen, Bayer, Genentech, Iveric Bio, Novartis, Optovue; Research grants from Amgen, Boehringer, Genentech, Heidelberg, Optovue, Regeneron, Topcon; Speaker fees from Optovue. AMK: Consultant: Abbvie, Adverum, AGTC, Aldebaran Therapeutics, Alimera, Apellis, Arrowhead, Asclepix, Aviceda, Bausch and Lomb, Broadwing Bio, Cholgene, 4DMT, Eyepoint, Frontera Therapeutics, Gemini, Genentech, Graybug, Gyroscope, Iveric Bio, Janssen, Kartos Therapeutics, Kato Pharma, Kodiak, Kriya Therapeutics, Ocular Therapeutix, Oculis, Ocuterra, Opthea, Oxurion, Nanoscope, Novartis, Perfuse, PolyPhotonix, Protagonist, Ray Therapeutics, Recens Medical, Regeneron, Retrotope, Regenxbio, RevOpsis, Roche, Stealth, Thea, Unity Bio, Vanotech, Vial. Research Support: Adverum, Alkahest, Annexon, Apellis, Asclepix, 4DMT, Gemini, Genentech, Inc., Graybug Vision, Gyroscope, Iveric Bio, Kodiak, Neurotech, NGM Bio, Ocular Therapeutix, Oculis, Ocuterra, Opthea, Oxurion, Novartis, Recens Medical, Regenxbio, Roche, Unity Bio. Speaker: Abbvie, Apellis, Bausch and Lomb, Genentech, Novartis. Equity: Aviceda, Recens Medical, Retrotope, RevOpsis, PolyPhotonix. RT: Alcon, Apellis, Bausch and Lomb, FCI, Iveric Bio, Moria, Zeiss, Optovue, Topcon, Alimera, Allergan, Bayer, Novartis, Oculis, Genentech, Roche, Thea. AAC: Consultant: Novartis, Bayer, Roche, Alcon, Apellis; Speaker fees: Novartis, Bayer, Roche, Alcon, Apellis. Nidek; Grant funding: Novartis, Bayer, Roche. IS, KG: Employee of Novartis Pharma AG. DTW: Consultant for Abbvie, Alcon, Apellis, Bayer, Bausch Health, Biogen, Boehringer Ingelheim, Novartis, Ripple Therapeutics, Roche, Topcon, Zeiss. Research grants from Novartis, Roche.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. Novartis is committed to sharing with qualified external researchers, access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided is anonymised to respect the privacy of patients who have participated in the program in line with applicable laws and regulations.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and the studies adhered to the tenets of the Declaration of Helsinki, International Conference on Harmonisation E6 Good Clinical Practice Consolidated Guidelines, and other regulations as applicable and was compliant with the Health Insurance Portability and Accountability Act of 1996. Institutional Review Board (IRB)/Ethics Committee approval was obtained at each study site and all study participants provided written informed consent. Doheny Eye Institute obtained IRB approval to conduct research on ocular images (IRB#15-000083). Participants gave informed consent to participate in the study before taking part.
References
- 1. Willoughby AS, Ying G-S, Toth CA, et al. Subretinal hyperreflective material in the comparison of age-related macular degeneration treatments trials. Ophthalmology 2015;122:1846–53. 10.1016/j.ophtha.2015.05.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kumar JB, Stinnett S, Han JIL, et al. Correlation of subretinal hyperreflective material morphology and visual acuity in neovascular age-related macular degeneration. Retina 2020;40:845–56. 10.1097/IAE.0000000000002552 [DOI] [PubMed] [Google Scholar]
- 3. Pokroy R, Mimouni M, Barayev E, et al. Prognostic value of subretinal hyperreflective material in neovascular age-related macular degeneration treated with bevacizumab. Retina 2018;38:1485–91. 10.1097/IAE.0000000000001748 [DOI] [PubMed] [Google Scholar]
- 4. Yang S, Zhao J, Sun X. Resistance to anti-VEGF therapy in neovascular age-related macular degeneration: a comprehensive review. Drug Des Devel Ther 2016;10:1857–67. 10.2147/DDDT.S97653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee H, Kang KE, Chung H, et al. Automated segmentation of lesions including subretinal hyperreflective material in neovascular age-related macular degeneration. Am J Ophthalmol 2018;191:64–75. 10.1016/j.ajo.2018.04.007 [DOI] [PubMed] [Google Scholar]
- 6. Izumi T, Koizumi H, Maruko I, et al. Optical coherence tomography angiography findings of classic choroidal neovascularization in polypoidal choroidal vasculopathy. Retina 2022;42:123–8. 10.1097/IAE.0000000000003264 [DOI] [PubMed] [Google Scholar]
- 7. Casalino G, Scialdone A, Bandello F, et al. Hyperreflective material as a biomarker in neovascular age-related macular degeneration. Expert Rev Ophthalmol 2020;15:83–91. 10.1080/17469899.2020.1745062 [DOI] [Google Scholar]
- 8. Keane PA, Liakopoulos S, Chang KT, et al. Relationship between optical coherence tomography retinal parameters and visual acuity in neovascular age-related macular degeneration. Ophthalmology 2008;115:2206–14. 10.1016/j.ophtha.2008.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Keane PA, Patel PJ, Ouyang Y, et al. Effects of retinal morphology on contrast sensitivity and reading ability in neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci 2010;51:5431–7. 10.1167/iovs.09-4846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schmidt-Erfurth U, Waldstein SM. A paradigm shift in imaging biomarkers in neovascular age-related macular degeneration. Prog Retin Eye Res 2016;50:1–24. 10.1016/j.preteyeres.2015.07.007 [DOI] [PubMed] [Google Scholar]
- 11. Fang M, Chanwimol K, Maram J, et al. Morphological characteristics of eyes with neovascular age-related macular degeneration and good long-term visual outcomes after anti-VEGF therapy. Br J Ophthalmol 2023;107:399–405. 10.1136/bjophthalmol-2021-319602 [DOI] [PubMed] [Google Scholar]
- 12. Ying G, Kim BJ, Maguire MG, et al. Sustained visual acuity loss in the comparison of age-related macular degeneration treatments trials. JAMA Ophthalmol 2014;132:915. 10.1001/jamaophthalmol.2014.1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tadayoni R, Sararols L, Weissgerber G, et al. Brolucizumab: a newly developed anti-VEGF molecule for the treatment of neovascular age-related macular degeneration. Ophthalmologica 2021;244:93–101. 10.1159/000513048 [DOI] [PubMed] [Google Scholar]
- 14. Dugel PU, Koh A, Ogura Y, et al. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology 2020;127:72–84. 10.1016/j.ophtha.2019.04.017 [DOI] [PubMed] [Google Scholar]
- 15. Dugel PU, Singh RP, Koh A, et al. HAWK and HARRIER: ninety-six-week outcomes from the phase 3 trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology 2021;128:89–99. 10.1016/j.ophtha.2020.06.028 [DOI] [PubMed] [Google Scholar]
- 16. Alex D, Giridhar A, Gopalakrishnan M, et al. Subretinal hyperreflective material morphology in neovascular age-related macular degeneration: a case control study. Indian J Ophthalmol 2021;69:1862–6. 10.4103/ijo.IJO_3156_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jaffe GJ, Martin DF, Toth CA, et al. Macular morphology and visual acuity in the comparison of age-related macular degeneration treatments trials. Ophthalmology 2013;120:1860–70. 10.1016/j.ophtha.2013.01.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Casalino G, Bandello F, Chakravarthy U. Changes in neovascular lesion hyperreflectivity after anti-VEGF treatment in age-related macular degeneration: an integrated multimodal imaging analysis. Invest Ophthalmol Vis Sci 2016;57:OCT288–98. 10.1167/iovs.15-18753 [DOI] [PubMed] [Google Scholar]
- 19. Casalino G, Stevenson MR, Bandello F, et al. Tomographic biomarkers predicting progression to fibrosis in treated neovascular age-related macular degeneration: a multimodal imaging study. Ophthalmol Retina 2018;2:451–61. 10.1016/j.oret.2017.08.019 [DOI] [PubMed] [Google Scholar]
- 20. Chakravarthy U, Havilio M, Syntosi A, et al. Impact of macular fluid volume fluctuations on visual acuity during anti-VEGF therapy in eyes with nAMD. Eye 2021;35:2983–90. 10.1038/s41433-020-01354-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Evans RN, Reeves BC, Maguire MG, et al. Associations of variation in retinal thickness with visual acuity and anatomic outcomes in eyes with neovascular age-related macular degeneration lesions treated with anti-vascular endothelial growth factor agents. JAMA Ophthalmol 2020;138:1043. 10.1001/jamaophthalmol.2020.3001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dugel PU, Jhaveri CD, Chakravarthy U, et al. Effect of retinal thickness variability on visual outcomes and fluid persistence in neovascular age-related macular degeneration: a post hoc analysis of the HAWK and HARRIER studies. Retina 2022;42:511–8. 10.1097/IAE.0000000000003349 [DOI] [PubMed] [Google Scholar]
- 23. Dansingani KK, Tan ACS, Gilani F, et al. Subretinal hyperreflective material imaged with optical coherence tomography angiography. Am J Ophthalmol 2016;169:235–48. 10.1016/j.ajo.2016.06.031 [DOI] [PubMed] [Google Scholar]
- 24. Kawashima Y, Hata M, Oishi A, et al. Association of vascular versus avascular subretinal hyperreflective material with aflibercept response in age-related macular degeneration. Am J Ophthalmol 2017;181:61–70. 10.1016/j.ajo.2017.06.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bjo-2023-323577supp001.pdf (608.6KB, pdf)
bjo-2023-323577supp002.pdf (353.2KB, pdf)
bjo-2023-323577supp003.pdf (171KB, pdf)
Data Availability Statement
Data are available on reasonable request. Novartis is committed to sharing with qualified external researchers, access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided is anonymised to respect the privacy of patients who have participated in the program in line with applicable laws and regulations.