Abstract
In contrast to the CDC42 homologues of Saccharomyces cerevisiae and Schizosaccharomyces pombe, the WdCDC42 gene in the human pathogenic fungus Wangiella (Exophiala) dermatitidis was found to be nonessential for cell viability. Expression of the constitutively active allele wdcdc42G14V at 37°C induced nonpolarized growth that led to cell enlargement and multiple nucleation. The swollen cells subsequently converted into planate divided bicellular forms or multiply septated sclerotic bodies in post-log phase, when the G14V-altered protein was diminished. The wdcdc42G14V mutation also strongly repressed filamentous growth both in the wild-type strain and in the temperature-sensitive hyphal-form mutant Hf1. In contrast, overexpression of the dominant negative alleles wdcdc42T19N and wdcdc42D120A had no obvious effect on fungal-cell polarization. These results suggested that WdCdc42p plays a unique regulatory role in cellular morphogenesis in W. dermatitidis. Activation of this protein in response to extracellular or intracellular signals seems to commit its yeast-like cells to a phenotype transition that produces sclerotic bodies while repressing hyphal development.
In Saccharomyces cerevisiae and Schizosaccharomyces pombe, the CDC42 gene is essential for cell viability (15, 27) because it plays crucial roles in the regulation of cell polarity via actin cytoskeleton organization and signal transduction (14). The biological functions mediated by Cdc42 GTPase have attracted broad interest, although its mechanism of regulating cellular morphogenesis is still largely obscure. Many Cdc42p target proteins have been identified in S. cerevisiae, such as Bem4 (23), Boi1 (1), Zds1 and Zds2 (2), Bee1 (19), Bni1 (12), Gic1 and Gic2 (3, 4), and Iqg1 (11, 32), all of which are involved in the regulation of actin cytoskeleton organization. Also, the p21-activated serine/threonine kinase family members Ste20p (24, 35) and Cla4p (8) interact with Cdc42p to regulate gene expression and septin organization in mating, filamentous growth, and yeast cytokinesis. Moreover, Cdc42 homologues and regulators have been studied for several other organisms, including humans. The biological functions for human Cdc42p are similar to those in yeast and involve actin cytoskeleton reorganization (30), transcriptional activation through the JNK/SAPK signaling pathway (7, 28), and the induction of cell cycle progression through the G1 phase (31). Some of these effects have been implicated in cell transformation (33), host cell pathogenesis with bacterial cytotoxicity (18), and human immunodeficiency virus replication (21).
The dematiaceous (melanized) fungus Wangiella (Exophiala) dermatitidis is one of many causative agents of human phaeohyphomycosis (17). It is considered a paradigm for studies of this emerging dermatomycosis afflicting humans because of its wide range of clinical manifestations and the increasingly frequent detection of it as a systemic pathogen (25, 26). It also serves as a model for the study of black fungi because of its well-defined polymorphism (36, 37) and cell wall chemistry (38). Of particular interest has been the unique transition from blastic to isotropic growth, whereby yeast-like cells convert to enlarged and transversely septated multicellular forms. This mimics the pathogenic process leading to the production of sclerotic bodies in the tissues of patients infected by the dematiaceous fungi that cause chromoblastomycosis (37). In addition, invasive hyphal growth in W. dermatitidis is also of interest because the CDC42 gene products of S. cerevisiae and Candida albicans have been implicated as a regulator for their filamentous growth (20, 29). Thus, understanding the mechanism regulating phenotypic conversions in W. dermatitidis provides insights into the pathogenesis of diseases caused not only by this species but also by the many other related dematiaceous fungal pathogens of humans.
In this study, we cloned a W. dermatitidis CDC42 homologue, WdCDC42, and confirmed its conserved GTPase function by complementation of the S. cerevisiae cdc42-1ts mutation. However, disruption of WdCDC42 did not result in a lethal phenotype in W. dermatitidis and affected cellular morphologies only under certain stress conditions. Therefore, a series of site-specific mutant alleles of WdCDC42 were generated, which induced dominant lethal phenotypes in S. cerevisiae similar to those reported previously (42). By a newly established integrative transformation system for gene overexpression in W. dermatitidis (41), the constitutively active allele but not dominant negative alleles was found to induce isotropic cell growth leading to the formation of sclerotic bodies and also to strongly repress hyphal development. The results suggested a new biological function of the Cdc42 homologue in this polymorphic fungal model, in which WdCdc42p negatively regulates cell polarization and coordinates with other factors to differentially control cellular phenotypic transitions.
MATERIALS AND METHODS
Strains and media.
Wild-type W. dermatitidis strain 8658 (ATCC 34100; E. dermatitidis CBS 525.76), its temperature-sensitive hyphal mutant Hf1, a parasexually derived diploid (3u2m-428), and an albino strain (ALB303) were routinely cultured in the minimal medium CDN or the complete medium CDY, as described previously (5, 34, 41). For preparation of transformation-competent cells, W. dermatitidis was grown in the rich medium YPD (22). Liquid media were used for the growth of yeast-like cells and multicellular forms of W. dermatitidis, whereas the solid CDY agar containing soluble starch instead of glucose as the sole carbon source was used for the stimulation of hyphal growth. For studies of gene expression under the control of the glaA promoter in transformants, glucose in the media was replaced by an equal amount of xylose for maintenance of the transformants or by 1% soluble starch for the phenotypic characterizations. S. cerevisiae strains DJTD2-16A (MATa cdc42-1 ura3 leu2 trp1 his4 gal2), kindly provided by D. Johnson (University of Vermont, Burlington), and INVSc1 (Invitrogen, Carlsbad, Calif.) were grown in YPD or in SD medium, both with standard compositions (22). The permissive and restrictive temperatures for growth of the S. cerevisiae DJTD2-16A strain and W. dermatitidis Hf1 strain were 25 and 37°C, respectively.
Plasmids and nucleic acid manipulations.
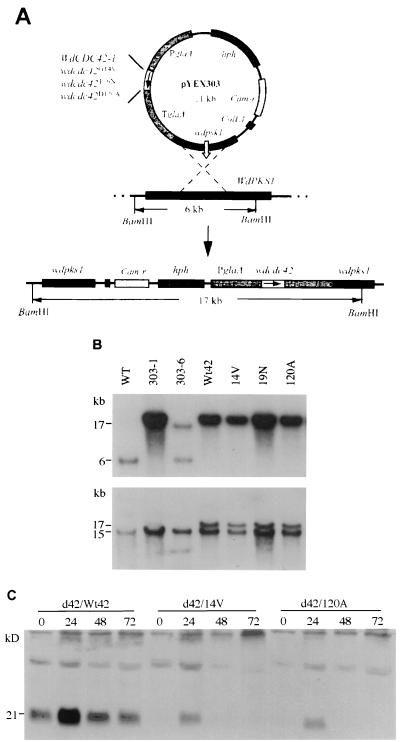
Plasmid pRS315 (42) containing an S. cerevisiae CDC42 gene subclone was provided by D. Johnson (University of Vermont). pCB1551 containing a sulfonylurea resistance allele (SUR) of the Magnaporthe grisea ILV1 gene was obtained from the Fungal Genetics Stock Center (University of Kansas Medical Center, Kansas City). For disruption of WdCDC42, two nonoverlapped partial genomic sequences of WdCDC42 flanking SUR were used to produce pYED42-827 (see Fig. 3A). For overexpression studies, the integrative vector pYEX303 was used, which contains a hygromycin resistance marker, a WdPKS1 fragment of the W. dermatitidis polyketide synthase gene for homologous targeting, and the starch-maltose-inducible glaA promoter, which is also temperature dependent in W. dermatitidis (41).
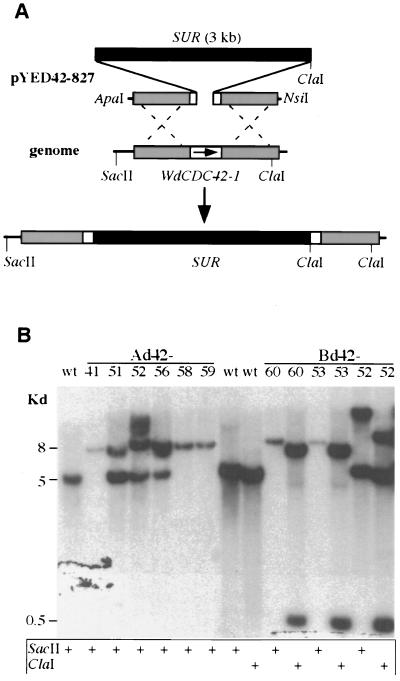
FIG. 3.
Disruption of WdCDC42 by replacement with a SUR selection marker. (A) Strategy for disruption of WdCDC42 with a 5-kb linear DNA fragment containing SUR and partial WdCDC42 sequences. Homologous recombination resulted in the replacement of a 21-bp portion of the WdCDC42 coding sequence (codons 76 to 83) with the 3-kb SUR marker. (B) Southern analysis of wdcdc42 disruption transformants. DNA samples were digested by SacII or ClaI, and the blot was hybridized with a WdCDC42 probe. The fragments of wild-type (wt) WdCDC42 were expected to be 5 kb when cut by SacII and 4.5 kb when cut by ClaI, whereas a fragment containing a SUR insertion would be 8 kb when cut by SacII and would produce two bands of 7 and 0.5 kb when cut by ClaI because of an introduced ClaI restriction site at SUR. Note that Ad42-41, -58, and -59 and Bd42-60 and -53 showed the expected patterns of band shifts and therefore were specific disruption transformants.
The ZAPII cDNA library of W. dermatitidis and its construction by our laboratory were described previously (40). The mRNA used for construction of the library was isolated from W. dermatitidis wild-type yeast-like cells that were first grown at 25°C for 36 h and then shifted to 37°C for an additional 12 h of incubation. cDNA synthesis was carried out with a ZAPII kit (Stratagene, La Jolla, Calif.).
Site-directed mutagenesis was performed using the Morph plasmid DNA mutagenesis kit (5 Prime→3 Prime, Boulder, Colo.) and the WdCDC42 cDNA clone 94AB1 (see Results) as the starting template. The mutagenic oligonucleotides for generating each allele are listed in Table 1. The derived mutant alleles were amplified by using the Expend high-fidelity PCR system (Boehringer Mannheim) and primers 42Bgl and 42Xba (Table 1). The 680-bp PCR fragments were then digested with BglII and XbaI for subcloning into vector pYES2 (Invitrogen) or pYEX303 to produce the respective plasmids (see Results). Finally, the entire coding region sequence of each WdCDC42 mutant allele (e.g. 14V, 19N, or 120A) was confirmed by DNA sequencing in two directions.
TABLE 1.
DNA oligonucleotides used in this study
| Primer | Sequence (5′→3′)a |
|---|---|
| YC42F | AACSTGCCTKCTTATYTCCTA |
| YC42R | CGGAACACTCAACATACTTKA |
| 5′42WD | CAGTCGACACCGCTACCCATCT |
| 3′42WD | ACGATATCCCTGACACGATCCATT |
| 5′42 | ACGTCCGCTATTCATCTTCTAT |
| 5′42Kpn | ATGGTACCTGCGGGTATGACAAAGGTC |
| 3′42Hind | TAAAGCTTACTTCGCCAGCATCGTTTGA |
| 3′42 | CTCGTTTGGGTCAGACTTTCTTG |
| 42-14V | GCCGACCGCGACGTCACCG |
| N-19N | CGGTCGGCAAGAACTGCTTGCTGATATCGTACACCACC |
| 42-120 | CTCAGACCGCTCTACGTGAC |
| 42-186I7N | CCACCAAAGAAGAATATTAAGAAATGCACG |
| 42Bgl | AAAGATCTACGGAATGGTTGTCGCAACG |
| 42Xba | AATCTAGACCGAGTCTCATCAAAGAATCGT |
Sequences for restriction endonuclease recognition sites are in italics. For mutagenic primers, sequences changed from the original DNA sequence of W. dermatitidis are underlined.
DNA blots were prepared and hybridized with a 32P-labeled probe of WdCDC42 derived by PCR with primers 5′42WD and 3′42WD (Table 1) or a probe of WdPKS1 derived from its 2-kb BglII fragment (41). The hybridizations were carried out at 42°C in a solution consisting of 50% formamide, 6× SSPE (1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.7]), 7.5× Denhardt's solution, 0.75% sodium dodecyl sulfate, and 200 μg of denatured DNA/ml, which followed washes with 1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and finally with 0.1× SSC at 25°C. For Northern blotting, total RNA was isolated from the cells by hot acidic phenol extraction (22). Hybridization of the RNA blots was carried out under the same conditions as used for Southern blotting.
Immunoblot analysis.
Proteins from log-phase yeast cells of W. dermatitidis were obtained by glass bead disruption (22). After denaturation, the protein samples (∼60 μg) were separated by sodium dodecyl sulfate–12% polyacrylamide gel electrophoresis and were transferred to nitrocellulose membranes. Total protein loading was estimated by staining with 0.2% Ponceau-S (Sigma, St. Louis, Mo.). WdCdc42p was then detected by using the rabbit anti-yeast CDC42 polyclonal antibody sc-7172 (Santa Cruz Biotechnology, Santa Cruz, Calif.) diluted to 1:500, followed by incubation with a goat anti-rabbit immunoglobulin G-horseradish peroxidase conjugate (Bio-Rad, Richmond, Calif.) diluted to 1:5,000 and finally by reaction with the ECL system (Amersham, Piscataway, N.J.).
Expression in S. cerevisiae.
For expression of WdCDC42 and its mutant alleles in S. cerevisiae, the pYES2-derived plasmids were used to transform yeast strains DJTD2-16A and INVSc1 by the alkali cation method (22). Ura+ transformants were recovered from SD medium (lacking uracil) at 25°C. For temperature sensitivity testing, the transformant cells were replica plated onto media containing either 2% glucose or 2% galactose as the sole carbon source and then incubated for 4 days at 25 or 37°C.
Transformation of W. dermatitidis.
For disruption of WdCDC42, the linear DNA construct was prepared by digestion of pYED42-827 with ApaI and NsiI (see Fig. 3A), whereas for expression of WdCDC42 and its mutant alleles, the plasmids were linearized by NarI (41). Transformation-competent yeast-like cells of W. dermatitidis were prepared from mid-log-phase cultures washed with cold 10% glycerol. Purified plasmid DNA was added to the cell suspensions at a ratio of about 1 μg of DNA per 107 cells. Electroporation was carried out with a Gene Pulser electroporation system (Bio-Rad) at a setting of 1.45 kV, 25 μF, and 200 Ω. Transformants were grown in YPD medium containing 30 μg of hygromycin (Sigma)/ml at 25°C, and albino colonies were selected for further confirmation of plasmid integrations by Southern analysis (see Results).
Photomicroscopy.
Photomicroscopy of W. dermatitidis cells was performed as previously described (5). For staining of the cell wall with Calcofluor (Sigma) or staining of nuclei with DAPI (4′,6′-diamidine-2-phenylindole) (Accurate Chemical, Westbury, N.Y.), fungal cells were fixed for 3 h in 5% formaldehyde and then washed twice with 75% ethanol at room temperature. After staining for 2 min, the samples were repeatedly washed with saline and were finally examined and photographed by using a Zeiss ICM 405 photomicroscope. For documentation of hyphal microcolony growth, cell culture plates were photographed directly with the same photomicroscope immediately after removal from incubation.
Nucleotide sequence accession number.
The genomic sequence of the WdCDC42 gene has been given the GenBank accession number AF162788.
RESULTS
Isolation and characterization of WdCDC42.
Using a PCR-derived 428-bp fragment of the S. cerevisiae CDC42 gene to generate a hybridization probe, we identified two putative CDC42-homologous clones, 94AB1 and 94AB2, by screening a W. dermatitidis cDNA library under low stringency. These clones were confirmed by DNA analysis to contain an identical nucleotide sequence and a deduced amino acid sequence that was 78 to 85% identical to Cdc42p of other organisms (Fig. 1). Therefore, this cloned gene was designated WdCDC42. Its genomic sequence was subsequently obtained by analysis of PCR amplicons derived from W. dermatitidis genomic DNA, which allowed two introns to be identified in its coding region.
FIG. 1.
Comparison of the deduced amino acid sequences of Cdc42 homologues. Reference numbering of the sequences is according to the numbering for the W. dermatitidis Cdc42 protein (Cdc42Wd). The other Cdc42 proteins used for comparison are from S. pombe (Cdc42Sp), C. albicans (Cdc42Ca), S. cerevisiae (Cdc42Sc), Caenorhabditis elegans (Cdc42Ce), Drosophila melanogaster (Cdc42Dm), Homo sapiens (human fetal brain isoform [Cdc42Hsb] and human placental isoform [Cdc42Hsp]), and Gallus gallus Cdc42 (Cdc42Gg). The percentages of identical amino acids are listed at bottom right. Symbol: – at the C-terminal sequence (between 180 and 190), artificially introduced space for alignment. The consensus motif sequences for GTP binding and hydrolysis and for C-terminal prenylation are underlined.
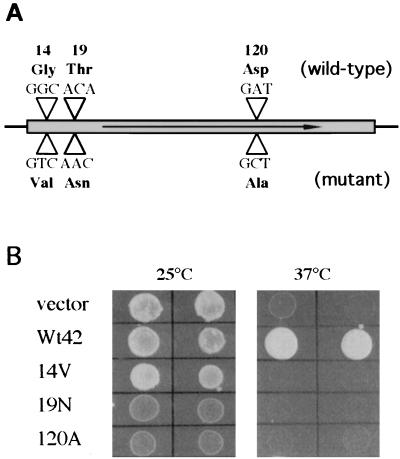
To demonstrate that the cloned WdCDC42 gene is a functional homologue of CDC42, we used the S. cerevisiae cdc42 temperature-sensitive mutant strain DJTD2-16A (15) in cross-species complementation studies. We also generated a series of point mutations in the WdCDC42 cDNA by site-directed mutagenesis. These mutations conferred specific amino acid replacements in the GTP-binding and hydrolysis domains in the Cdc42 GTPase (9, 40), such as Gly-14 to Val (G14V), Thr-19 to Asn (T19N), and Asp-120 to Ala (D120A) (Fig. 2A). Our results showed that the transformants expressing wild-type WdCDC42 (but not expressing mutant wdcdc42G14V, wdcdc42T19N, or wdcdc42D120A) were able to restore the growth of the temperature-sensitive mutant at the restrictive temperature (Fig. 2B). Furthermore, overexpression of the dominant negative alleles wdcdc42T19N and wdcdc42D120A at 25°C in DJTD2-16A resulted in growth retardation and led to terminal phenotypes of enlarged and unbudded yeast cells after prolonged incubation (data not shown). In contrast, the transformant cells overexpressing the constitutively active allele wdcdc42G14V at 25°C produced a morphologically heterogeneous population in which about 30% of cells had elongated buds or were amorphous (data not shown). These dominant mutational phenotypes were virtually identical to those previously produced by overexpression of S. cerevisiae cdc42 mutant alleles (15), suggesting that WdCdc42p has in vivo functions identical to those of S. cerevisiae Cdc42p with respect to control of cell polarity.
FIG. 2.
Generation and functional analysis of wdcdc42 mutant alleles. (A) Diagram of the point mutations introduced into WdCDC42 by site-directed mutagenesis. The three mutant amino acid codons are compared with the wild-type codons, and the codon numbers correspond to their positions in WdCDC42. (B) Expression of WdCDC42 and its mutant alleles with vector pYES2 in the S. cerevisiae strain DJTD2-16A. The transformants are indicated by the allele that each received: Wt42 (wild type), 14V (Gly-14 to Val), 19N (Thr-19 to Asn), and 120A (Asp-120 to Ala). Prior to photography, these transformants were grown for 4 days on SD-galactose agar medium at a temperature of 25 or 37°C.
Disruptions of WdCDC42 in W. dermatitidis.
To reveal the wdcdc42 deletion phenotype in W. dermatitidis, gene disruption experiments were carried out initially in the parasexually derived diploid strain 3u2m-428 (5). One diploid transformant containing a single wdcdc42 disruption was verified by Southern analysis (data not shown) and then subjected to methyl benzimidazole-2-yl-carbamate-induced haploidization. To our surprise, five of the segregants, as determined by Southern analysis, contained only the disrupted allele of wdcdc42 but nonetheless grew well at 25°C in the manner of the wild-type haploid, although their cellular morphologies varied (data not shown). Therefore, the essentiality of WdCDC42 in W. dermatitidis was investigated further in the wild-type haploid and in the albino haploid ALB303. The albino haploid was derived previously from the wild-type parental strain by the targeted integration of vector pYEX303 into the genomic locus of the polyketide synthase gene, WdPKS1, which is known to be required for melanin biosynthesis but not for viability, cell growth, or cellular morphological development in W. dermatitidis (41; unpublished data). In each case, a DNA fragment containing SUR and partial WdCDC42 flanking sequences was employed for targeted gene disruptions by replacement (Fig. 3A). Each of 20 putative disruptants from those two strains was analyzed first by PCR (data not shown) and then by Southern hybridization (Fig. 3B). The results confirmed that WdCDC42 is a single-copy gene in W. dermatitidis. Two independent transformants in the former group (i.e., Bd42-53 and Bd42-60) and three in the latter group (i.e., Ad42-41, Ad42-58, and Ad42-59) contained only the disrupted allele of wdcdc42.
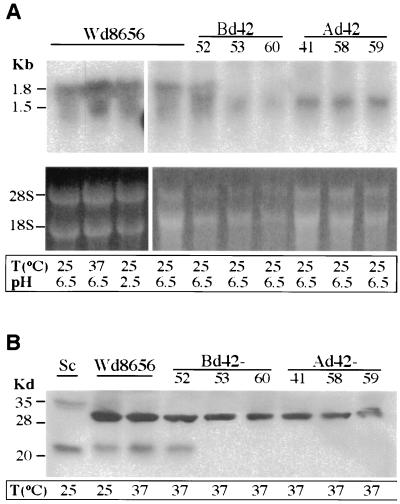
By Northern analysis, both wild-type W. dermatitidis and an ectopic transformant, Bd42-52 (as a control), showed two transcripts of 1.8 and 1.5 kb when hybridized with a WdCDC42 probe. The expression levels of those transcripts were not obviously affected by stress with either high temperature or low pH (Fig. 4A). However, in the wdcdc42 mutants, the 1.8-kb transcript, corresponding in size to the cloned cDNA of WdCDC42, was completely eliminated, whereas the 1.5-kb species continued to be expressed (Fig. 4A). Therefore, the 1.5-kb band was not an alternatively spliced species of the WdCDC42 transcript but possibly encoded another member of the Ras family.
FIG. 4.
Analysis of wdcdc42 disruptants. (A) Northern blot analysis of the wild type (Wd8656) and wdcdc42 disruptants was performed with RNA samples prepared from log-phase cells. The blot was hybridized with a 32P-labeled WdCDC42 probe corresponding to the coding region of the gene. Below the blots, rRNA bands corresponding to each lane in the agarose gels are shown as references for relative RNA amounts. (B) Immunoblotting analysis with an anti-yeast Cdc42 antibody, sc-7172. Approximately 60 μg of total protein of cell lysates was loaded in each lane, and an S. cerevisiae sample (Sc) was used as a positive control. T, temperature.
To rule out the possibility that the wdcdc42 disruptants contained residual WdCdc42p, an immunoblot analysis of the wdcdc42 cell lysates was carried out using anti-yeast Cdc42 antibody. The results showed that wild-type W. dermatitidis, the control strain Bd42-52, and wild-type S. cerevisiae produced positive bands at 21 kDa (Fig. 4B), the expected size of the Cdc42 GTPase homologue. In contrast, none of the wdcdc42 disruptants displayed a 21-kDa band or a truncated form, which again confirmed that the WdCDC42 gene product was completely eliminated in the disruption mutants of W. dermatitidis. Because a polyclonal antibody, sc-7172, was used in the immunoblottings, several other bands, including a 29-kDa species in W. dermatitidis and a 33-kDa species in S. cerevisiae (Fig. 4B), were also sometimes detected, but the signal intensity depended on the wash stringency of blotting. Unlike the 21-kDa band, these bands were all larger than that expected for a Ras-like GTPase, and there were clearly no differences between those from the wild type and those from the disruption mutants of W. dermatitidis in size or intensity. Therefore, these bands were considered to be nonspecific species that cross-reacted with the polyclonal antibody.
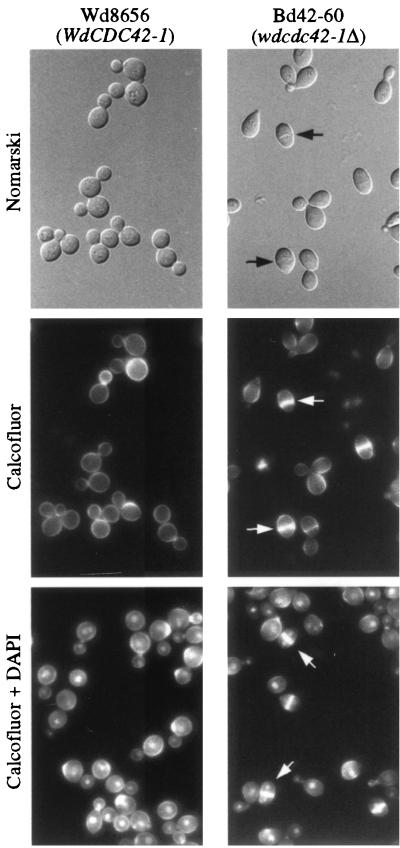
Microscopic observations showed that the cellular phenotypes of the wdcdc42 null mutant were similar to those of a wild-type strain grown in a neutral CDY broth at both 25 and 37°C, in that they retained a yeast form. However, the mutant cells were notably slimmer than the wild-type control cells (Fig. 5). Also, about 6% of the mutant cells formed a transverse septum in mother cells that contained at least two nuclei. Moreover, when the phenotypes of the null mutant and the wild-type strain were compared by culture in acidic (pH 2.5) CDY medium to induce yeast-to-multicellular form (sclerotic body) transition, about 21% of the wild-type cells produced the multicellular forms comparable to that previously reported (16). In contrast, approximately 62% of the null mutant cells converted to less enlarged bicellular forms, suggesting that WdCdc42p might negatively regulate cell polarization.
FIG. 5.
Phenotype of the wdcdc42 null mutant. The cells of the wild-type strain and of a mutant, Bd42-60, were grown in CDY medium at 37°C for 24 h. After fixation with 5% formaldehyde, the samples were stained with Calcofluor or additionally with DAPI. All cells are shown at the same magnification. Note the transverse septum, indicated by arrows, and the presence of a nucleus in each septated cell of the planate forms when also stained with DAPI. Nomarski, Nomarski phase.
A constitutively active allele of WdCDC42 induced isotropic growth and sclerotic-body formation.
The regulatory effects of WdCDC42 in W. dermatitidis were further investigated by overexpression of the wdcdc42 mutant alleles (Fig. 2A) in both the wild-type strain and the wdcdc42 null mutant Bd42-60. Transformations of W. dermatitidis with the wdcdc42 alleles under the control of the glaA promoter were achieved by pYEX303-mediated homologous integration at the WdPKS1 gene locus (Fig. 6A). The transformants with the integrated pYEX303 derivatives at this locus were easily identified by their albino, instead of black, colony phenotypes, as previously described (41). After confirmation of the targeted integrations in the transformants by Southern blotting (Fig. 6B), their proteins were also analyzed by immunoblotting of WdCdc42p at different growth stages. The results indicated that the glaA promoter-controlled expression of WdCdc42p was dramatically increased after 24 h by the shift of cells from 25 to 37°C and that the protein level gradually decreased during post-log phase (Fig. 6C). Although the same patterns of expression were found among all the transformants, the protein levels from the mutant alleles were generally lower than that from the wild-type allele (Fig. 6C). Also, in general the transformants derived from the wdcdc42 null strain seemed phenotypically more sensitive to the mutations (Fig. 7) than did those derived from the wild-type background (data not shown).
FIG. 6.
Transformation of the W. dermatitidis cells with WdCDC42 and its mutant alleles. (A) The integrative vector pYEX303-derived plasmids contain WdCDC42 or a mutant allele under the control of the promoter glaA. Prior to electroporation, the plasmids were linearized by NarI at the sequence of wdpks1 for targeting to the locus of WdPKS1, a gene for the melanin biosynthetic pathway in W. dermatitidis. (B) Southern analysis of the resulting albino transformants. When hybridized with a WdPKS1 probe (upper panel), the DNA digested by BamHI showed a 6-kb WdPKS1 fragment in the wild-type strain (WT), which as expected was replaced by a 17-kb BamHI-digested fragment after integration with a pYEX303-derived plasmid. Also as expected, the melanized ectopic transformant 303-6 retained the 6-kb fragment. The same blot was also hybridized with a WdCDC42 probe (lower panel), which confirmed that the integrated WdCDC42 alleles overlapped the 17-kb hybridization bands, whereas the endogenous WdCDC42 gene corresponded with the 15-kb fragments in BamHI digestions. (C) Immunoblot with antibody sc-7172 of WdCdc42p from transformant cells. Cell were grown in a soluble-starch-containing medium at 25°C and were then shifted to 37°C. The number under each strain is the incubation time in hours.
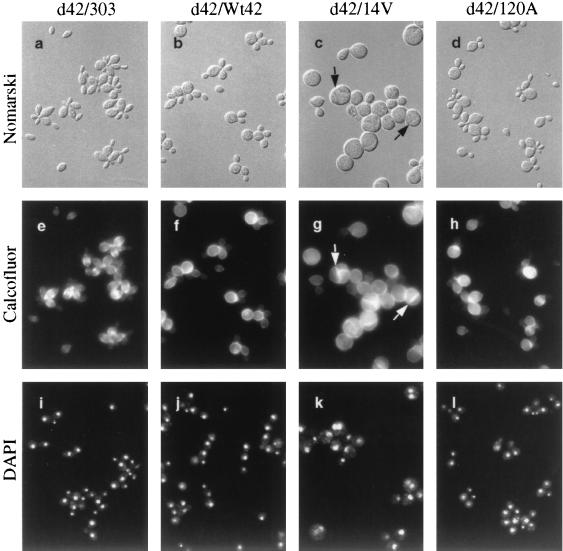
FIG. 7.
Cellular morphologies of W. dermatitidis overexpressing WdCDC42 or its mutant alleles. The albino transformants were derived from a wdcdc42 null strain (d42) and contained pYEX303 (d42/303), pYEX303-Wt42 (d42/Wt42), pYEX303-14V (d42/14V), or pYEX303-120A (d42/120A). The transformant carrying pYEX303-19N had a phenotype identical to that of the transformants containing pYEX303-120A (data not shown). Before photography, cells were grown in a starch-containing CDY liquid medium for 3 days at 37°C. Cell samples were then fixed with 5% formaldehyde and stained with Calcofluor or DAPI. All cells are shown at the same magnification. Note the transverse septa in the wdcdc42G14V planate cell and sclerotic body, indicated by arrows. Nomarski, Nomarski phase.
In CDY-starch liquid medium at 25°C, all transformants in the wdcdc42 null background grew in a yeast form like the wild-type strain, but those carrying the constitutively active allele wdcdc42G14V often had an unusual, peanut-like shape (data not shown). However, after a shift to 37°C, the wdcdc42G14V transformants displayed unexpected morphological changes: the yeast-like cells showed decreased bud formation and increased isotropic growth (phase I) in the first day of culture, suggesting that the G14V-altered WdCdc42 protein had induced depolarization of cell wall expansion in this fungus. By early stationary phase (∼48 h), approximately 30% of the isotropically enlarged cells had developed a transverse septum to become planate divided forms; by middle or late stationary phase (∼72 h), some of the swollen cells had also produced the multiple septa characteristic of sclerotic bodies (phase II) (Fig. 7c). Cell wall and nuclear staining (Fig. 7g and k) showed that the transverse septations were only in forms containing multiple nuclei. Notably, the septa were formed when the expression of the G14V-altered protein had diminished (Fig. 6C). Under the same growth conditions, the transformant cells containing the highly expressed plasmid-borne wild-type WdCDC42 allele (Fig. 6C) induced only a low percentage of phase I phenotypes (Table 2). In contrast, expression of the wdcdc42T19N and wdcdc42D120A alleles resulted neither in any obvious cell polarity change (Fig. 7d, h, and l; Table 2; data not shown) nor in a lethal effect in W. dermatitidis.
TABLE 2.
Change in the percentage of cell types induced by transformation and expression of wdcdc42 mutant allelesa
| Strainb | % of cells in form of:
|
||
|---|---|---|---|
| Yeast | Sclerotic bodiesc
|
||
| Phase I | Phase II | ||
| D42-60/303 | 99.3 | 0 | 0.6 |
| D42-60/Wt42 | 97.7 | 1.3 | 0.6 |
| D42-60/14V | 33.3 | 34.0 | 32.6 |
| D42-60/120A | 99.7 | 0 | 0.3 |
Values are the averages for two independent transformants. Three hundred yeast and sclerotic bodies were counted for each sample.
All transformants were derived from the wdcdc42 null strain Bd42-60. CDY was used as the basic growth medium, in which dextrose was replaced with soluble starch. Mid-log-phase cells harvested from a 25°C culture were diluted in a medium prewarmed at 37°C and incubated at this temperature for 3 days.
The term “sclerotic bodies” refers to an isotropically enlarged cell phenotype that contains multiple nuclei (phase I) and at least one transverse septum (phase II).
The constitutively active allele of WdCDC42 repressed hyphal development.
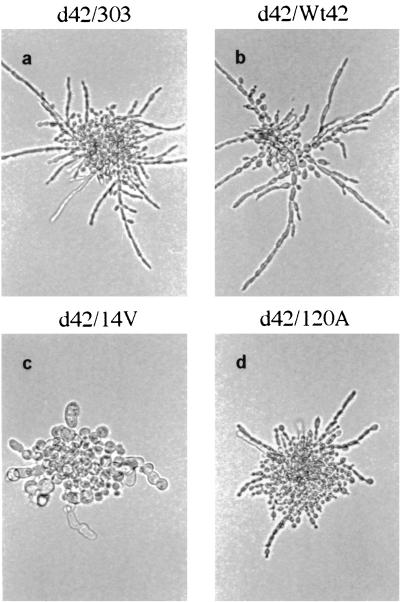
The effect of WdCdc42p activity on hyphal growth in W. dermatitidis was determined on CDY-starch agar at 37°C, because this fungus exhibits the hyphal phenotype more homogeneously on solid media with less-available carbon sources or limited nitrogen (unpublished data). Microscopic observations showed that wdcdc42 null cells initiated apical growth and elongation after 3 h of incubation (data not shown) and then formed hyphal microcolonies within 24 h (Fig. 8a), suggesting that WdCDC42 was not required for hyphal growth in W. dermatitidis. In contrast, the transformant carrying the constitutively active allele wdcdc42G14V displayed the nonpolarized cell expansion that resulted in large ovoid or spherical morphologies (Fig. 8c). However, the cells overexpressing a dominant negative allele (wdcdc42T19N or wdcdc42D120A) produced apically attached buds initially (Fig. 8d) but still formed normal hyphae after prolonged incubation.
FIG. 8.
Microcolony morphologies of W. dermatitidis transformants overexpressing WdCDC42 or its mutant alleles. The same transformant strains as shown in Fig. 7 were grown on a starch-containing CDY agar surface for 24 h at 37°C. The colonies were photographed directly by bright-field microscopy and are all shown at the same magnification.
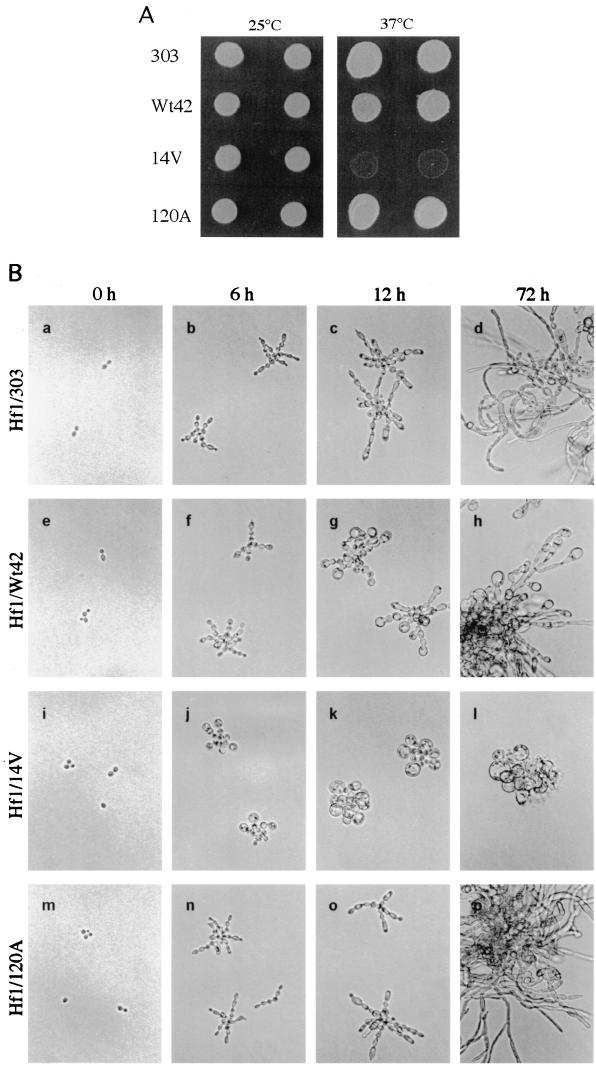
Similar phenomena were observed with overexpression of WdCDC42 and its mutant alleles in Hf1, which contained endogenous wild-type WdCDC42 (data not shown). The Hf1 transformants grew normally as yeasts at 25°C, but at the restrictive temperature of 37°C, the transformants were still viable, except for the transformant containing wdcdc42G14V, which showed growth arrest (Fig. 9A). Microscopic examinations of the Hf1 cells grown on CDY-starch agar at 37°C confirmed that the wdcdc42G14V transformants were inhibited in apical polarization and could undergo only a few cycles of cell division before lysis (Fig. 9B, panels i through l). Although overexpression of wild-type WdCDC42 also induced growth tip enlargement, it did not completely repress apical growth (Fig. 9B, panels e through h). Again, the transformants containing wdcdc42T19N or wdcdc42D120A showed no negative effects on cell polarization or elongation in W. dermatitidis under these conditions (Fig. 9B, panels m through p; data for wdcdc42T19N not shown).
FIG. 9.
Effect of WdCDC42 and its mutant alleles on cell growth and morphological development in strain Hf1. (A) The Hf1 transformants containing pYEX303-derived plasmids were grown on a maltose-containing CDY agar medium for 4 days at 25°C or at 37°C. (B) Cell morphologies of the corresponding Hf1 transformants carrying pYEX303, pYEX303-Wt42, pYEX303-14V, or pYEX303-120A, which were grown on a starch-containing CDY agar surface at 37°C. The inoculum of each strain grown at 25°C is shown in the 0-h photomicrographs. The other photomicrographs were taken at the time indicated at the top. All cells are shown at the same magnification.
DISCUSSION
The zoopathogenic fungus W. dermatitidis exhibits three distinct vegetative growth modes: blastic, apical, and isotropic, which are primarily associated with growth in the yeast, hyphal, and sclerotic-body morphologies, respectively (13). Cellular phenotypic conversions in W. dermatitidis are of great interest because they are potentially relevant to the pathogenicity of this agent of human phaeohyphomycosis. In particular, the unique transition of yeast cells to sclerotic-body forms leads to the dramatic enrichment of cell walls with known or suspected virulence factors, such as melanin (6) and chitin (38, 39, 40). Based on cytological studies of multicellular-body formation, a two-stage process is recognized (5, 13, 36). Stage I is characterized by the production of greatly enlarged, unbudded unicellular forms having multiple nuclei and thickened cell walls. In stage II, isotropic growth continues and the cells produce one or more transverse septa. Moreover, multicellular bodies proliferate slowly through a fission mode, indicating that this unique phenotype not only is a part of the life cycle of W. dermatitidis but also is a stress-resistant form that perhaps contributes its pathogenicity in chronic infection (36). In addition, cell cycle mapping and parasexual genetic analysis of two temperature-sensitive mcm/cdc mutations in this fungus provided clues that at least two different genes normally responsible for bud emergence are also involved in multicellular-body formation under stress conditions (5, 34). We hypothesized that these mutations were possibly in the homologues of CDC24, CDC42, or CDC43 of S. cerevisiae (5, 34).
In this study, we describe the isolation of WdCDC42 and functional characterization of this gene in W. dermatitidis. By means of DNA sequence comparisons and by expression in S. cerevisiae, we confirmed that this gene is a functional counterpart of yeast CDC42. Subsequent sequencing of the WdCDC42 genes of the previously described mcm/cdc strains documented that neither of mutants Mc2 and Mc3 (5, 34) had a defective WdCDC42 allele. However, of greater significance was our finding that the biological functions of WdCDC42 in W. dermatitidis were unexpectedly different from those of CDC42 in S. cerevisiae and other organisms. First, WdCDC42 was not essential for cell viability like the CDC42 homologues in S. cerevisiae and S. pombe (15, 27); our data clearly showed that the WdCDC42 gene disruption resulted in loss of the corresponding gene products in the viable mutants of W. dermatitidis. We suspect that some Ras- or Rho-homologous gene product(s) may take the place of WdCDC42 in the wdcdc42 null mutant, such as a closely related RAC1 homologue that does not exist in S. cerevisiae but has been identified in W. dermatitidis recently (unpublished data). Although several cross-reactive bands that were larger than that of an expected Ras-like GTPase were also detected in the lysates of both the wild-type and the wdcdc42 null mutant cells by immunoblottings with a polyclonal anti-Cdc42 antibody, they were most likely nonspecific signals. However, our data did not exclude the possibility of these unknown factors having a compensatory effect on the wdcdc42 defect in W. dermatitidis.
Second, the change of cellular morphology in the wdcdc42 null mutant was very subtle. Even in this background, overexpression of the dominant negative alleles of wdcdc42 did not bring about the cell depolarization and inhibition of yeast-like bud formation (Fig. 7d and 8d) that has been observed in S. cerevisiae (42). In contrast, only expression of the constitutively active allele in W. dermatitidis induced transformant cells to exhibit nonpolarized growth (Fig. 7c) rather than to develop multiple buds or bud elongation (42). These results suggested that WdCdc42p negatively regulated the cell polarization. However, these contradictory effects by the different active states of WdCdc42 GTP-binding protein in W. dermatitidis, compared to those in S. cerevisiae, are not without precedence. In the fission yeast S. pombe, overexpression of the corresponding CDC42 mutant alleles cdc42G12V and cdc42D118A also yielded phenotypes different from those of S. cerevisiae. For example, these two mutant alleles in the fission yeast are not dominant lethal, and both induce enlarged, misshapen cells that contain only a single nucleus (27). Moreover, overexpression of the constitutively active allele cdc42HsG12V in HeLa cells leads to the formation of enlarged multinucleate cells and to cytokinesis arrest (10). These studies suggest that although Cdc42p is a highly conserved component in the molecular machinery involved in cell polarity control, the underlying mechanism of its contribution to cellular morphogenesis is very diverse in different cell types and organisms.
Third, WdCdc42p appeared to play a unique regulatory role in the coordination of cellular morphological transitions in W. dermatitidis. The wild-type protein cycling between the GTP- and GDP-binding states may be required for its normal yeast-like cell division cycle at high temperatures (Fig. 5) and under other stress conditions. When the sustained activation of this GTP protein existed, a signal mimicked by overexpression of the mutant G14V product, the W. dermatitidis cells initiated isotropic growth. This led to the production of sclerotic bodies and simultaneously inhibited hyphal growth (Fig. 7 and 8). Although these phenotypic transitions certainly involve multiple gene regulation phenomena, they are far from fully understood. However, the properties of WdCdc42p elucidated to date seem to provide an important clue to growth mode switching by this Rho-type GTPase in this fungus.
Collectively, our findings revealed that the WdCDC42 gene is a highly conserved member of the CDC42 subfamily. Although it was found to be nonessential for cell viability in W. dermatitidis, the WdCDC42 gene product nonetheless seemed to play an important role in the regulation of stress-induced fungal cellular morphogenesis. As a polymorphic fungus, W. dermatitidis most likely possesses more sophisticated Rho-type GTPase-mediated regulatory pathways than do S. cerevisiae and S. pombe for control of its cell growth and development. We hope that further exploration of these pathways in this pathogen will provide a better understanding of the molecular mechanisms of fungal polymorphism and its contribution to pathogenesis in humans.
ACKNOWLEDGMENTS
We thank B. Harrer for his critical suggestions, D. I. Johnson, M. Ward, and J. Sweigard for providing plasmids and strains, and S. M. Karuppayil, A. L. Mendoza, L. Zheng, Z. Wang, and B. Feng for discussion and technical assistance.
This research was supported by a grant to P. J. Szaniszlo from the National Institute of Allergy and Infectious Diseases (AI 33049).
REFERENCES
- 1.Bender L, Lo H S, Lee H, Kokojan V, Peterson J, Bender A. Associations among PH and SH3 domain-containing proteins and Rho-type GTPases in yeast. J Cell Biol. 1996;133:879–894. doi: 10.1083/jcb.133.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bi E, Pringle J R. ZDS1 and ZDS2, genes whose products may regulate Cdc42p in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:5264–5275. doi: 10.1128/mcb.16.10.5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown J L, Jaquenoud M, Gulli M-P, Chant J, Peter M. Novel Cdc42-binding proteins Gic1 and Gic2 control cell polarity in yeast. Genes Dev. 1997;11:2972–2982. doi: 10.1101/gad.11.22.2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen G C, Kim Y J, Chan C S M. The Cdc42 GTPase-associated proteins Gic1 and Gic2 are required for polarized cell growth in Saccharomyces cerevisiae. Genes Dev. 1997;11:2958–2971. doi: 10.1101/gad.11.22.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper C R, Jr, Szaniszlo P J. Evidence for two cell division cycle (CDC) genes that govern yeast bud emergence in the pathogenic fungus Wangiella dermatitidis. Infect Immun. 1993;61:2069–2081. doi: 10.1128/iai.61.5.2069-2081.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper C R, Jr, Szaniszlo P J. Melanin as a virulence factor in dematiaceous pathogenic fungi. In: Bossche V H, Stevens D A, Odds F C, editors. Host-fungus interplay. Bethesda, Md: National Foundation for Infectious Diseases; 1997. pp. 81–93. [Google Scholar]
- 7.Coso O A, Chiariello M, Yu J-C, Teramoto H, Crespo P, Xu N, Miki T, Gutkind J S. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995;81:1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- 8.Cvrckova F, De Virgilio C, Manser E, Pringle J R, Nasmyth K. Ste20-like protein kinases are required for normal localization of cell growth and for cytokinesis in budding yeast. Genes Dev. 1995;9:1817–1830. doi: 10.1101/gad.9.15.1817. [DOI] [PubMed] [Google Scholar]
- 9.Davis C R, Richman T J, Deliduka S B, Blaisdell J O, Collins C C, Johnson D I. Analysis of the mechanism of the action of the Saccharomyces cerevisiae dominant lethal cdc42G12V and dominant negative cdc42D118A mutations. J Biol Chem. 1998;273:849–858. doi: 10.1074/jbc.273.2.849. [DOI] [PubMed] [Google Scholar]
- 10.Dutartre H, Davoust J, Gorvel J P, Chavrier P. Cytokinesis arrest and redistribution of actin-cytoskeleton regulatory components in cells expressing the Rho GTPase CDC42Hs. J Cell Sci. 1996;109:367–377. doi: 10.1242/jcs.109.2.367. [DOI] [PubMed] [Google Scholar]
- 11.Epp J A, Chant J. An IQGAP-related protein controls actin-ring formation and cytokinesis in yeast. Curr Biol. 1997;7:921–929. doi: 10.1016/s0960-9822(06)00411-8. [DOI] [PubMed] [Google Scholar]
- 12.Evangelista M, Blundell K, Longtine M S, Chow C J, Adames N, Pringle J R, Peter M, Boone C. Bni1p, a yeast formin linking cdc42p and the actin cytoskeleton during polarized morphogenesis. Science. 1997;276:118–122. doi: 10.1126/science.276.5309.118. [DOI] [PubMed] [Google Scholar]
- 13.Geis P A, Jacobs C W. Polymorphism of Wangiella dermatitidis. In: Szaniszlo P J, editor. Fungal dimorphism: with emphasis on fungi pathogenic for humans. New York, N.Y: Plenum Press; 1985. pp. 205–233. [Google Scholar]
- 14.Johnson D I. Cdc42: an essential Rho-type GTPase controlling eukaryotic cell polarity. Microbiol Mol Biol Rev. 1999;63:54–105. doi: 10.1128/mmbr.63.1.54-105.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson D I, Pringle J R. Molecular characterization of CDC42, a Saccharomyces cerevisiae gene involved in the development of cell polarity. J Cell Biol. 1990;111:143–152. doi: 10.1083/jcb.111.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karuppayil S M, Szaniszlo P J. Importance of calcium to the regulation of polymorphism in Wangiella (Exophiala) dermatitidis. J Med Vet Mycol. 1997;35:379–388. doi: 10.1080/02681219780001471. [DOI] [PubMed] [Google Scholar]
- 17.Kwon-Chung K J, Bennett J E. Medical mycology. Philadelphia, Pa: Lea and Febiger; 1992. pp. 337–555. [Google Scholar]
- 18.Lerm M, Selzer J, Hoffmeyer A, Rapp U R, Aktories K, Schmidt G. Deamidation of Cdc42 and Rac by Escherichia coli cytotoxic necrotizing factor 1: activation of c-Jun N-terminal kinase in HeLa cells. Infect Immun. 1999;67:496–503. doi: 10.1128/iai.67.2.496-503.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li R. Bee1, a yeast protein with homology to Wiscott-Aldrich syndrome protein, is critical for the assembly of cortical actin cytoskeleton. J Cell Biol. 1997;136:649–658. doi: 10.1083/jcb.136.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu H, Kohler J, Fink G R. Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science. 1994;262:1741–1744. doi: 10.1126/science.7992058. [DOI] [PubMed] [Google Scholar]
- 21.Lu X, Wu X, Plemenitas A, Yu H, Sawai E T, Abo A, Peterlin B M. Cdc42 and Rac1 are implicated in the activation of the Nef-associated kinase and replication of HIV-1. Curr Biol. 1996;6:1677–1684. doi: 10.1016/s0960-9822(02)70792-6. [DOI] [PubMed] [Google Scholar]
- 22.Lundblad V. Saccharomyces cerevisiae. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. Vol. 2. New York, N.Y: John Wiley & Sons, Inc.; 1994. pp. 13.0.1–13.4.17. [Google Scholar]
- 23.Mack D, Nishimura K, Dennehey B K, Arbogast T, Parkinson J, Toh-e A, Pringle J R, Bender A, Matsui Y. Identification of the bud emergence gene BEM4 and its interactions with Rho-type GTPases in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:4387–4395. doi: 10.1128/mcb.16.8.4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manser E, Leung T, Salihuddin H, Zhao Z-S, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994;367:40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto T, Matsuda T, McGinnis M R, Ajello L. Clinical and mycological spectra of Wangiella dermatitidis infections. Mycoses. 1993;36:145–155. doi: 10.1111/j.1439-0507.1993.tb00743.x. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto T, Ajello L, Matsuda T, Szaniszlo P J, Walsh T J. Developments in hyalohyphomycosis and phaeohyphomycosis. J Med Vet Mycol. 1994;32(Suppl. 1):329–349. doi: 10.1080/02681219480000951. [DOI] [PubMed] [Google Scholar]
- 27.Miller P J, Johnson D I. Cdc42p GTPase is involved in controlling polarized cell growth in Schizosaccharomyces pombe. Mol Cell Biol. 1994;14:1075–1083. doi: 10.1128/mcb.14.2.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minden A, Lin A, Calaret F-X, Abo A, Karin M. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell. 1995;81:1147–1157. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 29.Mösch H-U, Roberts R, Fink G R. Ras2 signals via the Cdc42/Ste20/mitogen-activated protein kinase module to induce filamentous growth in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1996;93:5352–5356. doi: 10.1073/pnas.93.11.5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nobes C D, Hall A. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 31.Olson M F, Ashworth A, Hall A. An essential role for Rho, Rac, and Cdc42 GTPases in cell cycle progression through G1. Science. 1995;269:1270–1272. doi: 10.1126/science.7652575. [DOI] [PubMed] [Google Scholar]
- 32.Osman M A, Cerione R A. Iqg1p, a yeast homologue of the mammalian IQGAPs, mediates Cdc42p effects on the actin cytoskeleton. J Cell Biol. 1998;142:443–455. doi: 10.1083/jcb.142.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiu R-G, Abo A, McCormick F, Symons M. Cdc42 regulates anchorage-independent growth and is necessary for Ras transformation. Mol Cell Biol. 1997;17:3449–3458. doi: 10.1128/mcb.17.6.3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roberts R L, Szaniszlo P J. Yeast-phase cell cycle of the polymorphic fungus Wangiella dermatitidis. J Bacteriol. 1980;144:721–731. doi: 10.1128/jb.144.2.721-731.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simon M-N, De Virgilio C, Souza B, Pringle J R, Abo A, Reed S I. Role for the Rho-family GTPase Cdc42 in yeast mating-pheromone signal pathway. Nature. 1995;376:702–705. doi: 10.1038/376702a0. [DOI] [PubMed] [Google Scholar]
- 36.Szaniszlo P J, Karuppayil S M, Mendoza L, Rennard R J. Cell cycle regulation of polymorphism in Wangiella dermatitidis. Arch Med Res. 1993;24:251–261. [PubMed] [Google Scholar]
- 37.Szaniszlo P J, Mendoza L, Karuppayil S M. Clues about chromoblastomycotic and other dematiaceous fungal pathogens based on Wangiella as a model. In: Bossche H V, Kerridge D, Odds F, editors. Fungal dimorphism. New York, N.Y: Plenum Press; 1993. pp. 241–255. [Google Scholar]
- 38.Szaniszlo P J, Momany M. Chitin, chitin synthase and chitin synthase conserved region homologues in Wangiella dermatitidis. NATO ASI Ser Ser H. 1993;69:229–242. [Google Scholar]
- 39.Wang Z, Zheng L, Hauser M, Becker J M, Szaniszlo P J. WdChs4p, a homolog of chitin synthase 3 in Saccharomyces cerevisiae, alone cannot support growth of Wangiella (Exophiala) dermatitidis at the temperature of infection. Infect Immun. 1999;67:6619–6630. doi: 10.1128/iai.67.12.6619-6630.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Z, Szaniszlo P J. WdCHS3, a gene that encodes a class III chitin synthase in Wangiella (Exophiala) dermatitidis, is expressed differentially under stress conditions. J Bacteriol. 2000;182:874–881. doi: 10.1128/jb.182.4.874-881.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ye X, Feng B, Szaniszlo P J. A color-selectable and site-specific integrative transformation system for gene expression studies in the dematiaceous fungus Wangiella (Exophiala) dermatitidis. Curr Genet. 1999;36:241–247. doi: 10.1007/s002940050496. [DOI] [PubMed] [Google Scholar]
- 42.Ziman M, O'Brien J M, Ouellette L A, Church W R, Johnson D I. Mutational analysis of CDC42Sc, a Saccharomyces cerevisiae gene that encodes a putative GTP-binding protein involved in the control of cell polarity. Mol Cell Biol. 1991;11:3537–3544. doi: 10.1128/mcb.11.7.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]