Abstract
Background
Severe yellow fever infection (YFI) may be complicated by a hemorrhagic diathesis. However, the hemostasis profile of YFI has rarely been reported.
Objectives
The aim of this study was to characterize the hemostatic features of YFI by using a rotational thromboelastometry (ROTEM).
Methods
We evaluated clinical, laboratory, and ROTEM parameters in adults with severe YFI and their correlation with hemostatic variables according to bleeding and death.
Results
A total of 35 patients were included (median age, 49 years). ROTEM was performed in 22 patients, of whom 21 (96%) presented bleeding and 4 (18%) died. All patients who died had major bleeding. Patients who died presented prolonged clotting time (CT; median, 2326 seconds; IQR, 1898-2986 seconds) and reduced alpha angle (median, 12°; IQR, 12°-15°) in comparison with patients who had minor (median CT, 644 seconds; IQR, 552-845 seconds and alpha angle, 47°; IQR, 28°-65°) and major (median CT, 719 seconds; IQR, 368-1114 seconds and alpha angle, 43°; IQR, 32°-64°) bleeding who survived. In patients who had bleeding, CT showed a strong negative correlation with factor (F)V (r = −.68), FIX (r = −.84), and FX (r = −.63) as well as alpha angle showed a strong negative correlation with FIX (r = −.92). In patients who died, the correlations were even stronger. A total of 19/21 (90%) patients presented hypocoagulability assessed by ROTEM.
Conclusion
Hypocoagulabitity is the hallmark of the bleeding diathesis of severe YFI. Abnormal CT and alpha angle associated with death and could be used as potential predictors of adverse outcome in severe YFI.
Keywords: bleeding, blood coagulation, blood coagulation factor, thromboelastography, yellow fever
Essentials
-
•
The mechanism of the bleeding diathesis of yellow fever infection is not well understood.
-
•
We assessed clinical, laboratory, and rotational thromboelastometry parameters in adults with severe yellow fever infection.
-
•
Most (90%) patients presented hypocoagulabitity assessed by rotational thromboelastometry.
-
•
Prolonged clotting time and reduced alpha angle associated with death.
1. Introduction
Yellow fever (YF) is a mosquito-borne viral hemorrhagic fever with a high case fatality rate [1]. It is caused by a flavivirus—or YF virus—which is transmitted to humans by mosquitoes Haemagogus and Sabethes [2]. Approximately 15% of individuals infected with YF virus enter the third stage of the disease, which is characterized by variable dysfunction of multiple organs including the liver, kidneys, and cardiovascular system. Multiorgan failure in YF infection (YFI) is associated with high levels of proinflammatory cytokines similar to that seen in systemic immune response syndrome (SIRS) and sepsis [3]. Since there is no specific antiviral therapy available for the treatment of YFI, it mainly consists of supportive care.
A severe bleeding diathesis is the hallmark of YFI and is characterized by coffee-ground hematemesis, melena, hematuria, metrorrhagia, petechiae, ecchymoses, epistaxis, and bleeding from needle puncture sites and gums. However, the biological mechanisms behind this hemorrhagic diathesis are poorly understood. Potential mechanisms are decreased synthesis of coagulation factors by the liver, disseminated intravascular coagulation, and platelet dysfunction. To date, very few studies have investigated the hemostasis in patients with YFI [2,4]. The study by Woodson et al. [2] examined gene expression and protein production of fibrinogen and plasminogen activator-1 after interleukin-6 stimulation of hepatocytes and found that YFI affects the transcriptional and translational expression of fibrinogen and plasminogen activator-1 in human hepatocytes. A study from our group [4] showed that increased international normalized ratio (INR) and activated partial thromboplastin time (aPTT) as well as reduced factor (F)II, FV, FVII, FIX, and protein C were associated with death in severe YFI.
Global tests of hemostasis are increasingly used as tools to assess hemostasis, including the effects of pro- and anticoagulant proteins, fibrinogen, platelets, and red blood cells in coagulation. Furthermore, they have been used to guide therapeutic decisions regarding replacement of blood components in patients with major bleeding, including patients undergoing liver transplant surgery, trauma patients, and patients with obstetrical bleeding [5,6]. Importantly, in patients with acute or chronic liver disease, it has been demonstrated that blood clot formation in patients with cirrhosis [7,8] and acute liver injury/acute liver failure (ALF) is generally preserved [[7], [8], [9], [10]] or mildly hypocoagulable [[8], [9], [10]]. Once patients with YFI develop a major bleeding diathesis and require massive blood transfusion, we hypothesized that blood clot formation assessed by global tests would likely be altered and could potentially assess hemostasis in these patients.
In recent years, Brazil has experienced the most extensive outbreak of YFI, with 1880 confirmed cases and 583 deaths from 2016 to 2018 [11]. The majority of cases (n = 1002; 53.3%) were reported in Minas Gerais State, of whom 340 (33.9%) eventually died [12,13]. This study aimed to characterize the hemostatic features of patients with severe YFI who were admitted to the intensive care unit (ICU) and to assess and correlate global test parameters with routine hemostasis tests and levels of coagulation proteins.
2. Methods
2.1. Patients
Patients with suspected YFI were admitted at Hospital Eduardo de Menezes, Belo Horizonte, Minas Gerais, Brazil, which is a referral center for the treatment of infectious diseases in the State of Minas Gerais. Patients were attended by infectious diseases specialists who were responsible for providing care and collecting clinical data. Inclusion criteria consisted of adult individuals (age, ≥18 years) with severe YFI admitted to the ICU. The criteria for ICU admission were defined according to the Brazilian Ministry of Health [13], which consisted of intense jaundice, hemorrhages, oliguria, intermittent vomiting, decreased level of consciousness, or severe abdominal pain and/or laboratory abnormalities such as transaminase levels 10 times above the reference value (aspartate aminotransferase [AST] generally higher than alanine aminotransferase, unlike acute hepatitis), elevated creatinine (>2 mg/dL), and altered coagulation tests (INR > 1.5 and/or platelet count < 50,000/μL).
The diagnosis of YFI was confirmed by reverse transcriptase polymerase chain reaction according to recommendation of the Ministry of Health [13]. The study was approved by the institutional ethical committees, and all patients/guardians signed a consent form.
2.2. Clinical variables
The following variables of interest were collected at admission: age, sex, race, city of residence, occupation, educational level, weight, height, body mass index, heart and respiratory rate, mean arterial blood pressure, temperature, and SIRS components [3,4]. We also collected data on vaccination status for YFI, comorbidities, bleeding manifestations (type, site, and severity), diagnosis of ALF, infection, hepatic encephalopathy, renal failure, use of amines, dialysis with start and end dates, and transfusion of blood components during hospitalization.
ALF was defined as severe acute liver injury with encephalopathy and impaired liver function (INR ≥ 1.5) in a patient with an illness duration of <26 weeks [14]. Infection was defined as a positive blood or urine culture upon exclusion of a contaminant organism and chest X-ray showing a pulmonary infiltrate suggestive of infectious etiology. Renal failure was defined as persistent elevation of urea and creatinine and maintenance of oliguria after hydration demanding continuous dialysis. SIRS was defined according to American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference (1992) [15]: leukocytes, >12,000 or <4000/μL; temperature, <36 °C or >38 °C; respiratory rate; >20 breaths/min; and heart rate, >90 beats/min.
2.3. Blood collection
Peripheral blood was collected in vacutainer tubes containing sodium citrate 3.2% as anticoagulant and 2% EDTA. Fresh whole blood collected in citrate was used to perform rotational thromboelastometry (ROTEM). Platelet-poor plasma was prepared by centrifugation at 1500 rpm for 15 minutes twice. Plasma was separated and stored at −80 °C until processing. At the time of testing, all frozen samples were thawed for 10 minutes at 37 °C. Blood collected in EDTA was used to perform blood cell count.
Coagulation tests were performed at a median time of 3 days (IQR, 2–7 days) after admission to ICU [4].
2.4. Assessment of routine laboratory tests and coagulation factors
We measured prothrombin time (PT)/INR, aPTT, full blood count, AST, alanine aminotransferase, bilirubin, albumin, urea, creatinine, lactate, and phosphate. The PT/INR was determined using recalcified plasma, recombinant human tissue factor, and synthetic thromboplastin (Dade Innovin Reagent; Siemens Healthcare Diagnostics Products GmbH). Thrombin time (TT; STA-Thrombin 2; Stago), PT (STA-Neoplastine CI Plus 10; Stago), aPTT (STA-PTT Kit; Stago), D-dimer (Liatest D-Di Kit; Stago), and protein C (STA-Staclot; Stago) were measured using the STA-R Evolution clot detection system (Stago) by a chromogenic method. Fibrinogen was assessed by coagulometric method modified by Clauss (Fibrinogen-C; Werfen).
The activity levels of FII, FV, FVII, FVIII, FIX, and FX were measured using the Destiny Plus hemostasis analyzer (Alere), with the calibration curves generated from ≥6 dilutions of specific factor-deficient plasmas. Details of blood collection and the methodology for assessing routine clotting tests and coagulation factors were previously described [4].
2.5. ROTEM
ROTEM was assayed in citrated whole fresh blood, ie, within maximum 2 hours after drawing blood. All tests were performed in a single instrument (ROTEM; Pentapharm GmbH) by the same operator (L.L.J.). A total volume of 300 μL of whole blood was pipetted in the cuvette and heated at 37 °C with the addition of 20 μL calcium chloride (STAR-TEM, TEM Innovations GmbH). Clotting was initiated by the addition of 20 μL kaolin. Kinetic changes in clot formation and dissolution were measured for 30 minutes after reaching maximal clot firmness (MCF). The following parameters were analyzed: (i) clotting time (CT), which is the time (in seconds) from the start of the reaction until the initiation of the clot; (ii) clot formation time (CFT), which is the time (in seconds) measured from the initiation of the clot until a clot firmness of 20 mm is detected; (iii) alpha angle (in degree), which reflects the dynamics of clot formation; (iv) MCF (in millimeter), which measures the maximum amplitude or maximum tension/firmness of the clot; and (v) lysis index in 30 minutes, which reflects the activation of the fibrinolytic system. The maximum amplitude (in millimeter) is also demonstrated every 5 minutes after the beginning of reaction by the parameters A5, A10, A15, A20, A25, and A30.
An abnormal ROTEM parameter was defined as a CT or CFT above the upper limit of the normal reference range and an alpha angle or MCF below the lower limit of normal reference range. Hypocoagulability was defined if 2 or more of ROTEM parameters (CT, CFT, alpha angle, and MCF) were outside the limits of the normal reference range [16,17]. The normal ranges in our laboratory were established by assaying 6 adult normal controls (3 women and 3 men) with a median age of 30.0 years (IQR, 27.0-45.5 years). They had no comorbidities, no bleeding disorders, and were free from using any medication. The reference ranges obtained were similar to the ones reported by Scarpelini et al. [18].
2.6. Outcomes
We defined death and bleeding as outcomes. Any bleeding was recorded. If the definition for bleeding severity in patients with acquired bleeding conditions (for instance, hemorrhagic viral fever) was not available, we used the classification proposed by the International Society of Thrombosis and Haemostasis (ISTH) [19].
In brief, according to ISTH, major bleeding was defined as fatal bleeding and/or symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intra-articular or pericardial, or intramuscular with compartment syndrome, and/or bleeding causing a fall in hemoglobin level of 20 g/L or more or leading to transfusion of 2 or more units of whole blood or red cells. The remaining types of bleeding (for instance, epistaxis, petechiae, ecchymoses, oozing of blood from the gums, and bleeding from needle puncture sites) were considered as minor.
2.7. Statistical analysis
For the categorical variables, the number of events and their respective percentages were calculated. For the continuous variables, we calculated median and IQR or means and SDs in case the presentation was nonparametric or parametric, respectively.
Comparison between categorical variables was calculated by using the Fisher’s exact test or Kruskal–Wallis test. Student’s t-test was used for comparison of continuous variables. Mann–Whitney U-test or Kruskal–Wallis test was used for comparing numeric variables with nonparametric distribution. Spearman’s correlation was used to verify the association between numerical variables. P values are reported and considered significant if P was <.05. There was no sample size calculation.
Statistical analyses were performed using R software version 3.5.0 (R Foundation for Statistical Computing) and Statistical Package for Social Sciences software (IBM, SPSS, Inc) version 18.0.
3. Results
3.1. Characteristics of the patients studied
We included 35 adult patients with severe YFI, ie, in the third stage of YFI, admitted to the ICU from December 1, 2017, to April 15, 2018. This period corresponded to the outbreak of YFI in Minas Gerais State. Median age was 49 years (IQR, 38-54 years); most patients (94%) were male, and 77% were not vaccinated against YFI. The median time in the ICU was 10 days (IQR, 7-17 days). Median total bilirubin was 5.7 mg/dL (IQR, 4.0-13.5 mg/dL), and median AST was 4350 units/mL (IQR, 1171-7723 units/mL). A total of 21/35 patients (60%) had bleeding, of whom 14 (66%) had major bleeding. Approximately 60% of patients received blood transfusion with different blood components. A total of 12 patients (34%) died. The characteristics of the study population are detailed in Table 1.
Table 1.
Characteristics of included patients with severe yellow fever infection.
| Characteristics | All patients (N = 35) |
|---|---|
| Length of stay in hospital (d), median (IQR) | 10 (7-17) |
| Socio-demographic characteristics | |
| Age (y), median (IQR) | 49 (39-55) |
| Male sex, n (%) | 33 (94) |
| Skin color, n (%) | |
| Mixed | 17 (48.6) |
| White | 11 (31.4) |
| Black | 7 (20) |
| Clinical characteristics | |
| Weight (kg), median (IQR) | 70 (56-85) |
| BMIa (kg/m2), median (IQR) | 25 (22.2-30.4) |
| Heart rate (beats/min), median (IQR) | 81 (69-98) |
| Respiratory rate (breaths/min), median (IQR) | 20 (18-23) |
| Median MABP (IQR) | 94 (75-99) |
| Temperature (°C), median (IQR) | 36 (36.0-37.0) |
| Alcoholisma, n (%) | 17 (48.5) |
| Smokinga, n (%) | 13 (37.1) |
| Vaccination for YF, n (%) | 8 (23) |
| Bleedinga, n (%) | 21 (60) |
| Major bleedinga, n (%) | 14 (66) |
| Hepatic encephalopathy, n (%) | 19 (58) |
| Other infectionb, n (%) | 13 (39) |
| Median No. of SIRS (IQR) | 1.0 (1.0-2.5) |
| Kidney failure, n (%) | 15 (46) |
| Death, n (%) | 12 (34) |
| Laboratory tests | |
| Total bilirubina (mg/dL), median (IQR) | 5.7 (4.0-13.5) |
| AST (units/L), median (IQR) | 4350 (1171-7723) |
| Median INR (IQR) | 1.4 (1.0-1.8) |
| Creatinine at admission (mg/dL), median (IQR) | 1.0 (0.7-2.6) |
| Total leukocytes (μL), median (IQR) | 5400 (3500-8850) |
| Blood transfusion | |
| Fresh frozen plasmaa, n (%) | 19 (68) |
| Cryoprecipitatea, n (%) | 17 (61) |
| Packed red cellsa, n (%) | 15 (54) |
| Plateletsa, n (%) | 17 (61) |
AST, aspartate aminotransferase; BMI, body mass index; INR, international normalized ratio; MABP, mean arterial blood pressure; SIRS, systemic inflammatory response syndrome; YF, yellow fever.
There are some missing data for these variables.
All patients had a bacterial infection.
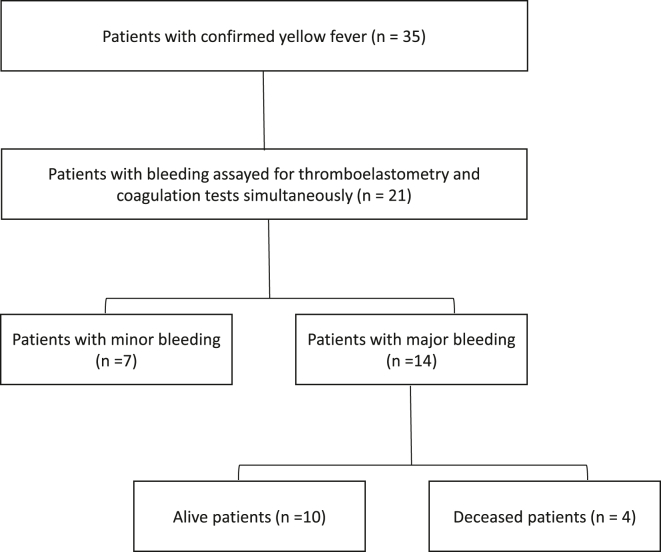
A total of 22/35 (63%) patients with severe YFI admitted to the ICU were assessed for routine laboratory, coagulation factors, and ROTEM on the same day/sample (Figure). Except for a higher death rate in the excluded patients (n = 13) in comparison with the included ones (n = 22; 61.5% vs 17.4%, respectively; P = .01), the remaining characteristics of the 2 groups were similar (Supplementary Table S1).
Figure.
Flow chart of patients enrolled in the study.
3.2. Association of clinical and laboratory variables with death and bleeding
A total of 21/22 patients (96%) had bleeding, of which 14 (67%) were major and 7 (33%) were minor bleeding. A total of 4 (18%) patients died, of whom all (100%) had major bleeding. Therefore, the following analyses refer to the group of 21 patients with severe YFI admitted to the ICU who had bleeding. The outcomes of interest were major/minor bleeding and death.
We compared the characteristics of alive and deceased patients according to minor and major bleeding. Median lactate level (P = .004), creatinine level (P = .007), and leukocyte count (P = .008) were significantly higher in the group that died when compared with the group that survived. As expected, patients who had major bleeding received more units of blood transfusion when compared with the group that had minor bleeding. Although not significant, TT and D-dimer showed a dose-response relationship, with lower levels in patients with minor bleeding/discharged alive than in those with major bleeding/discharged alive, who had lower levels than those with major bleeding/death (Table 2). We found no difference between the minor and major bleeding when death was not accounted for (Table 2).
Table 2.
Characteristics of patients with severe yellow fever infection according to the outcome of bleeding and death.
| Characteristics | All patients |
Alive patients |
Deceased patients |
P valuec | |||
|---|---|---|---|---|---|---|---|
| (N = 21) |
(n = 17) |
(n = 4) |
|||||
| Minor bleeding |
Major bleeding |
P valueb | Minor bleeding |
Major bleeding |
Major bleeding |
||
| (n = 7) | (n = 14) | (n = 7) | (n = 10) | (n = 4) | |||
| Characteristics | |||||||
| Time in ICU (d), median (IQR) | 4 (2-16) | 3 (1.8-4.2) | .15 | 4 (2-16) | 2 (1-3) | 4 (3-7) | .07 |
| Socio-demographic characteristics | |||||||
| Age (y), median (IQR) | 41 (37-51) | 51 (44-59) | .26 | 41 (37-51) | 51 (36-59) | 50 (45-57) | .52 |
| Male sex, n (%) | 7 (100) | 13 (92.8) | .46 | 7 (100) | 9 (90) | 4 (100) | .71 |
| Weight (kg), median (IQR) | 75 (65-84) | 70 (54-76) | .25 | 75 (65-84) | 70 (52-72) | 73 (57-86) | .32 |
| BMIa (kg/m2), median (IQR) | 22 (21-29) | 23 (21-29) | .85 | 22 (21-29) | 23 (20-24) | 30 (24-32) | .48 |
| Clinical characteristics | |||||||
| Median MABP (IQR) | 83 (64-98) | 89 (77-99) | .57 | 83 (64-98) | 87 (72-104) | 93 (83-98) | .76 |
| Heart rate (beats/min), median (IQR) | 70 (56-97) | 88 (68-98) | .48 | 70 (56-97) | 84 (58-98) | 94 (78-104) | .49 |
| Respiratory rate (breaths/min), median (IQR) | 19 (16-21) | 19 (17-21) | .86 | 19 (16-21) | 19 (17-20) | 23 (17-30) | .5 |
| Temperature (°C), median (IQR) | 36 (35-37) | 36 (35-38) | .77 | 36 (35-37) | 37 (36-38) | 36 (35-37) | .72 |
| Vaccination for YF, n (%) | 1 (14) | 2 (14) | .81 | 1 (14) | 2 (20) | 0 (0) | .81 |
| Hepatic encephalopathy, n (%) | 2 (29) | 9 (64) | .38 | 2 (29) | 7 (70) | 2 (50) | .07 |
| Renal failure, n (%) | 2 (29) | 7 (50) | .42 | 2 (29) | 4 (40) | 3 (75) | .08 |
| Infectiona, n (%) | 2 (29) | 7 (50) | .42 | 2 (29) | 5 (50) | 2 (50) | .13 |
| Median No. of SIRS (IQR) | 2 (0.5-2) | 2 (1-3) | .17 | 2 (0.5-2) | 2 (1-3) | 3 (1-3) | .05 |
| Laboratory tests | |||||||
| AST (units/L), median (IQR) | 1179 (729-9901) | 1334 (525-3839) | .70 | 1179 (729-9901) | 2785 (460-5453) | 773 (437-2323) | .57 |
| ALT (units/L), median (IQR) | 722 (121-5166) | 1085 (197-2781) | .90 | 722 (121-5166) | 1922 (383-3139) | 134 (96-859) | .10 |
| Total bilirubin (mg/dL), median (IQR) | 8.4 (3.4-20.4) | 4 (2.9-9.8) | .50 | 8.4 (3.4-20.4) | 3.9 (1.6-7.1) | 9.8 (4.7-16.5) | .26 |
| Albumin (g/dL), median (IQR) | 2.9 (2.8-3.1) | 2.9 (2.6-3.1) | .77 | 2.9 (2.8-3.1) | 2.8 (2.6-3.1) | 2.9 (2.5-3.6) | .93 |
| Lactate (mmol/L), median (IQR) | 1.4 (1.1-1.8) | 1.8 (1.2-5.6) | .45 | 1.4 (1.1-1.8) | 1.2 (1.1-2.0) | 9.2 (4.3-12.8) | .004 |
| Creatinine (mg/dL), median (IQR) | 0.9 (0.7-0.9) | 1.3 (0.9-2.9) | .17 | 0.9 (0.7-0.9) | 0.9 (0.8-1.9) | 6.5 (3.0-7.4) | .007 |
| Median leukocyte count/μL (IQR) | 5300 (2300-7200) | 5400 (2950-11,150) | .70 | 5300 (2300-7200) | 3400 (2300-6150) | 15,750 (8975-33,325) | .008 |
| Median platelet count/μL (IQR) | 55,000 (37,000-236,000) | 51,000 (38,000-113,000) | .68 | 55,000 (37,000-236,000) | 51,000 (38,000-113,000) | 58,000 (34,000-157,000) | .91 |
| aPTT (s), median (IQR) | 48 (22-80) | 36 (23-68) | .63 | 48 (22-80) | 30 (21-40) | 76 (47-93) | .09 |
| Median INR (IQR) | 1.2 (1.0-1.6) | 1.0 (1.0-1.5) | .49 | 1.2 (1.0-1.6) | 1.0 (1.0-1.2) | 1.6 (1.4-1.7) | .09 |
| TT (s), median (IQR) | 22 (16-49) | 37 (26-93) | .20 | 22 (16-49) | 35 (24-63) | 82 (26-300) | .26 |
| D-dimer (μg/mL), (IQR) | 0.45 (0.42-11.29) | 8 (2-9) | .43 | 0.45 (0.42-11.29) | 2.3 (1.5-13.3) | 9.6 (9.3-9.9) | .63 |
| Blood transfusion | |||||||
| Transfusion of fresh frozen plasma, n (%) | 3 (43) | 10 (71) | .20 | 3 (43) | 7 (70) | 3 (75) | .0001 |
| Transfusion of cryoprecipitate, n (%) | 2 (29) | 9 (64) | .12 | 2 (29) | 6 (60) | 3 (75) | .001 |
| Transfusion of packed red cells, n (%) | 2 (29) | 7 (50) | .34 | 2 (29) | 4 (40) | 3 (75) | .001 |
| Transfusion of platelets, n (%) | 3 (43) | 10 (71) | .20 | 3 (43) | 8 (80) | 2 (50) | .001 |
ALT, alanine aminotransferase; aPTT, activated partial thromboplastin time; AST, aspartate aminotransferase; BMI, body mass index; ICU, intensive care unit; INR, international normalized ratio; MABP, mean arterial pressure; SIRS, systemic inflammatory response syndrome; TT, thrombin time; YF, yellow fever.
All patients had a bacterial infection.
Comparison between the 2 groups was performed using the Mann–Whitney U-test, considering alpha at 5%.
Comparison between the 3 groups was performed using the Kruskal–Wallis statistic test, considering alpha at 5%.
Median FV and FIX were significantly lower in patients who had major bleeding/death compared with the group that had bleeding/discharged alive (P = .04 and P = .03, respectively). Patients who died presented significantly more prolonged CT (median CT, 2326 seconds; IQR, 1898-2986 seconds) and reduced alpha angle (median, 12°; IQR, 12°-15°) in comparison with patients who had minor bleeding/discharged alive (median CT, 644 seconds; IQR, 552-845 seconds and alpha angle, 47°; IQR, 28°-65°) and major bleeding/discharged alive (median CT, 719 seconds; IQR, 368-1114 seconds and alpha angle, 43°; IQR, 32°-64°; Table 3).
Table 3.
Coagulation and global test parameters of patients with severe yellow fever infection according to the outcome of bleeding and death.
| Characteristics | All patients |
Alive patients |
Deceased patients |
P valueb | |||
|---|---|---|---|---|---|---|---|
| (N = 21) |
(n = 17) |
(n = 4) |
|||||
| Minor bleeding |
Major bleeding |
P valuea |
Minor bleeding |
Major bleeding |
Major bleeding |
||
| (n = 7) | (n = 14) | (n = 7) | (n = 10) | (n = 4) | |||
| Coagulation proteins | |||||||
| Fibrinogen (mg/dL), median (IQR) | 315 (177-349) | 187 (128-277) | .08 | 315 (177-349) | 189 (143-322) | 157 (112-189) | .12 |
| FII (%), median (IQR) | 69 (42-115) | 54 (39-71) | .41 | 69 (42-115) | 52 (46-77) | 46 (36-54) | .43 |
| FV (%), median (IQR) | 103 (51-170) | 64 (30-134) | .24 | 103 (51-170) | 129 (76-146) | 31 (30-48) | .04 |
| FVII (%), median (IQR) | 68 (26-91) | 49 (7-76) | .31 | 68 (26-91) | 66 (37-85) | 8 (6-29) | .43 |
| FVIII (%), median (IQR) | 177 (106-278) | 110 (65-210) | .35 | 177 (106-278) | 135 (98-210) | 89 (54-237) | .19 |
| FIX (%), median (IQR) | 63 (28-111) | 30 (11-99) | .29 | 63 (28-111) | 64 (24-117) | 12 (6-13) | .03 |
| FX (%), median (IQR) | 60 (23-90) | 35 (23-81) | .67 | 60 (23-90) | 54 (34-86) | 25 (23-43) | .51 |
| Protein C (%), median (IQR) | 64 (52-82) | 60 (34-70) | .15 | 64 (52-82) | 57 (46-77) | 50 (31-67) | .44 |
| ROTEM parameters | |||||||
| CT (sec), median (IQR) | 644 (552-845) | 1087 (587-2283) | .07 | 644 (552-845) | 719 (368-1114) | 2326 (1898-2986) | .006 |
| CFT (min), median (IQR) | 261 (129-690) | 241 (142-911) | .35 | 261 (129-690) | 390 (136-671) | 543 (2-1085) | .96 |
| α-Angle (°), median (IQR) | 47 (28-65) | 37 (15-60) | .51 | 47 (28-65) | 43 (32-64) | 15 (12-15) | .04 |
| MCF (mm), median (IQR) | 54 (35-58) | 33 (21-61) | .47 | 54 (35-58) | 41 (29-65) | 23 (19-25) | .10 |
| Lysis 30 (%), median (IQR) | 100 (100-100) | 100 (100-100) | .43 | 100 (100-100) | 100 (100-100) | 100 (100-100) | .47 |
| Hypocoagulability | |||||||
| n (%) | 5 (71.4) | 14 (100) | .04 | 5 (71.4) | 10 (100) | 4 (100) | .07 |
CFT, clot formation time; CT, clotting time; F, factor; MCF, maximal clot firmness; ROTEM, rotational thromboelastometry.
P values were considered significant if P < .05.
Comparison between the 2 groups was performed using the Mann–Whitney U-test, considering alpha at 5%.
Comparison between the 3 groups was performed using the Kruskal–Wallis statistic test, considering alpha at 5%.
Except for 2 patients in the group of minor bleeding/discharged alive, all remaining patients (n = 19; 90%) had hypocoagulability, as defined by 2 or more of the ROTEM parameters outside the normal range (Table 3). Except for hypocoagulability, which was more frequent in the group with major bleeding in comparison with minor bleeding (P = .04), the remaining parameters were not different between the groups (Table 3).
The most frequent types of bleeding were hematemesis, oral bleeding, epistaxis, melena, and hematochezia (Supplementary Table S2).
3.3. Correlation between coagulation proteins and ROTEM parameters with bleeding and death
In patients who had bleeding, CT showed a strong negative correlation with FIX (r = −.84; P < .0001) and FX (r = −.63; P < .001) as well as alpha angle showed a strong negative correlation with FIX (r = −.92; P < .0001). There was no correlation between any ROTEM parameters with either INR, aPTT, platelets, FVIII, or protein C in the patients who had bleeding (Table 4).
Table 4.
Correlation of parameters of thromboelastometry and coagulation proteins in patients with any bleeding.
| Characteristic (N = 21) | CT | CFT | α-Angle | MCF | Lysis 30 |
|---|---|---|---|---|---|
| Routine coagulation assays | |||||
| INR | .22 | .17 | −.36 | −.38 | −.40 |
| aPTT | .36 | −.21 | −.19 | .02 | −.27 |
| TT | .83a | −.18 | −.56 | −.41 | −.76 |
| D-dimer | .30 | .93a | −.89a | −.72 | −.90 |
| Platelets | −.17 | −.61 | .07 | −.04 | −.60 |
| Coagulation proteins | |||||
| Fibrinogen | −.47 | −.63a | .91a | .87b | .86 |
| FII | −.55 | −.62 | .86b | .73a | .76 |
| FV | −.68b | −.33 | .76a | .69a | .71 |
| FVII | −.75a | −.10 | .59 | .51 | .39 |
| FVIII | −.32 | −.19 | .22 | .14 | .65 |
| FIX | −.84d | −.43 | .92d | .83b | .85a |
| FX | −.63c | −.69 | .87b | .82b | .81a |
| Protein C | −.52 | −.50 | .39 | .50 | .37 |
aPTT, activated partial thromboplastin time; CFT, clot formation time; CT, clotting time; F, factor; INR, international normalized ratio; MCF, maximal clot firmness; TT, thrombin time.
P < .05.
P < .01.
P < .001.
P ≤ .0001.
In patients who died, the strong negative correlations between CT and FIX (r = −.83; P < .0001) and FX (r = −.77; P < .0001) were maintained, despite the low number of patients in this group. Alpha angle had a strong positive correlation with fibrinogen (r = .82; P < .0001), FV (r = .71; P < .0001), FIX (r = .83; P < .0001), FX (r = .75; P < .0001), and FII (r = .70; P < .001) and a strong negative correlation with D-dimer (r = −.72; P < .001). MCF had a strong positive correlation with fibrinogen (r = .85; P < .0001), FV (r = .69; P < .0001), FIX (r = .85; P < .0001), FX (r = .75; P < .0001), and FII (r = .71; P < .001) and a strong negative correlation with INR (r = −.72; P < .001). There was no correlation of any ROTEM parameters with protein C and FVIII in the patients who died (Table 5). Lysis 30 had no correlation with routine coagulation assays or coagulation proteins, either for bleeding or death outcomes (Tables 4 and 5).
Table 5.
Correlation of parameters of thromboelastometry and coagulation proteins in patients who died and had major bleeding.
| Characteristic (n = 4) | CT | CFT | α-Angle | MCF | Lysis 30 |
|---|---|---|---|---|---|
| Routine coagulation assays | |||||
| INR | .64b | −.21 | −.38 | −.82c | −.17 |
| aPTT | .44a | .3 | −.49a | −.43 | .17 |
| TT | .71b | .08 | −.48 | −.13 | .50 |
| D-dimer | .62b | .74b | −.72c | −.50 | .48 |
| Platelets | −.21 | −.53 | .10 | −.14 | .02 |
| Coagulation proteins | |||||
| Fibrinogen | −.62b | −.7c | .82d | .85d | −.20 |
| FII | −.66 | −.61b | .70c | .71c | −.34 |
| FV | −.62b | −.45 | .71d | .69d | .06 |
| FVII | −.62b | −.12 | .45 | .52a | −.03 |
| FVIII | .22 | .01 | .01 | −.16 | .02 |
| FIX | −.83d | −.57a | .83d | .85d | −.26 |
| FX | −.77d | −.67b | .75d | .75d | −.24 |
| Protein C | −.52a | −.33 | .29 | .45 | −.31 |
aPTT, activated partial thromboplastin time; CFT, clot formation time; CT, clotting time; F, factor; INR, international normalized ratio; MCF, maximal clot firmness; TT, thrombin time.
P < .05.
P < .01.
P < .001.
P ≤ .0001.
Due to small numbers, it was not possible to perform a multivariate analysis to evaluate the association of ROTEM parameters or levels of coagulation factors with the outcomes of death or bleeding.
4. Discussion
We assessed routine coagulation, coagulation factors, and ROTEM parameters in a cohort of patients with severe YFI admitted to ICU. The majority of patients (96%) had bleeding and hypocoagulability (90%). Low FV and FX were associated with death as well as prolonged CT and reduced alpha angle. Alpha angle and CT strongly correlated with liver-produced coagulation factors, mainly FIX and FX. These results suggest that hypocoagulability, due to decreased levels of liver-produced coagulation factors, is the hallmark of the bleeding diathesis of severe YFI. ROTEM seems to be a good tool to assess the bleeding disorder of YFI and may predict adverse outcomes.
An outbreak of YFI occurred in Brazil from 2016 to 2018, totaling about 2000 confirmed cases and 583 deaths until April 2018 [[11], [12], [13]]. The majority of the cases were reported in Minas Gerais State. According to an epidemiologic bulletin from the State Department of Health of Minas Gerais, until June 2018, 1002 cases of YFI were confirmed in the State, of which 340 (33.9%) evolved to death [[11], [12], [13]]. This study enrolled a subset of this population with suspected YFI who were admitted to the ICU of a specialized hospital on infectious diseases in Belo Horizonte, Minas Gerais, during the outbreak occurring from December 2017 to April 2018. Most patients were male, were in their fifth decade of life, worked in rural areas, and were not vaccinated against YFI. Most patients presented with bleeding at admission. As expected, patients who died received more transfusion with fresh frozen plasma and cryoprecipitate.
In this study, patients with severe YFI showed remarkably low levels of liver-produced coagulation factors, of which FV and FIX were the ones that reduced the most. In some patients, FIX levels were as low as 1%. FV was also substantially decreased but was not as low as previously reported in ALF [20]. In spite of an elevated D-dimer in the totality of the patients (which was particularly high in the ones who died), there was no evidence of hyperfibrinolysis in any of the patients, as assessed by ROTEM. Furthermore, plasma FVIII levels were within the normal reference range in all patients evaluated, suggesting against a consumptive coagulopathy. Therefore, elevated D-dimer likely reflects the intense inflammatory state and “cytokine storm,” which is described in the viral hemorrhagic fevers [21]. However, we can not rule out that some level of consumption also takes place. We conclude that the biological mechanism of the bleeding diathesis in YFI mainly involves a severe depletion of liver-produced coagulation factors, likely as a result of ALF in patients with the severe form of YFI. ROTEM parameters, mainly CT and alpha angle, clearly reflected this hemostatic dysfunction and correlated with the levels of several liver-produced coagulation factors.
Viscoelastic tests such as thromboelastography (TEG) and ROTEM are point-of-care assays that rapidly assess the dynamics of clot formation in the whole blood, including clot strength and the stability of the clot using plasmatic and cellular components. TEG and ROTEM have been widely employed as a tool to diagnose the underlying hemostatic defect and guide blood replacement in severe bleeding conditions such as trauma coagulopathy, obstetrical bleeding, and liver transplantation [9,10,22,23]. In inherited bleeding disorders, it has also been used to monitor the response of bypassing agents in patients with hemophilia and inhibitors [24,25] and replacement in FXIII deficiency [26]. To the best of our knowledge, there are no reports of the use of TEG/ROTEM to assess hemostasis in patients with severe YFI with bleeding.
Two studies have investigated the use of TEG/ROTEM in dengue [27,28]. In patients with dengue fever infection and thrombocytopenia, Piza et al. [27] found normal routine coagulation tests but hypocoagulability by ROTEM in about 70% and 55% of patients when tested with intrinsic coagulation pathway assessment (INTEM) and extrinsic coagulation pathway assessment (EXTEM), respectively. Others evaluated the use of TEG or ROTEM to investigate hemostasis in patients with ALF [9], stable cirrhosis [9,28], and patients with acute decompensation of cirrhosis or acute-on-chronic liver failure [8,[29], [30], [31]]. Whereas TEG parameters in these patients are generally within normal limits, ROTEM suggested a hypocoagulable state. The differences in TEG and ROTEM test results are likely explained by the difference in the procoagulant trigger. As the procoagulant trigger is more powerful in ROTEM, the test is less sensitive for anticoagulant factors, which explains a relative hypocoagulability in situations in which pro- and anticoagulant factors are decreased simultaneously. Crochemore et al. [32] reported the use of ROTEM to monitor a severe case of YFI with thrombocytopenia, concluding that ROTEM was an important tool to identify the hypocoagulable state and to guide treatment.
In this study, FV and FIX were significantly lower in patients with YFI who died, compared with those who survived. ROTEM parameters were also associated with mortality. Prolonged CT was the most significant ROTEM parameter associated with death in addition to reduced alpha angle. Plasma levels of various coagulation proteins correlated with ROTEM parameters. The totality of patients who died had major bleeding. Whether the association of hypocoagulable features with bleeding and death indicates a direct link or whether hypocoagulable features are a proxy for increased severity of disease requires further study. Interestingly, routine coagulation tests such as TT, aPTT, INR, and platelets showed weak or no correlation with the ROTEM parameters in the patients who had bleeding. This corroborates with several reports showing that routine coagulation tests do not reflect the hemostatic status of patients with either acute [9,10,33,34] or chronic liver failure [35].
This study has limitations that need to be addressed. Firstly, we were not able to perform ROTEM in 13/35 (37%) patients with YFI who were admitted to the ICU; 8/13 patients died before drawing blood, and the remaining 5 patients were not tested for different reasons, mostly related to the difficulty of collecting blood due to the patient’s critical condition. Therefore, we cannot exclude the occurrence of a selection bias in the study. Indeed, comparison between the characteristics of the patients included in comparison with the ones excluded showed that the latter were likely to be more severely affected as their death rates were significantly higher. Secondly, due to low numbers, we could not perform a multivariate analysis in order to identify whether ROTEM parameters and other variables were independently associated with the outcomes of bleeding and death. Thirdly, we were unable to assay ROTEM with additional reagents. Fourthly, plasma biomarkers of fibrinolysis, such as plasmin-antiplasmin complex, were not performed. Therefore, we cannot rule out hyperfibrinolysis. Fifthly, no information was captured on patients who were not included due to early death. Therefore, we cannot exclude the occurrence of a selection bias with the noninclusion of more severe patients. Lastly, the study was conducted in 1 center, and therefore, the generability of the results may not apply to other settings. However, this center was the only referral center for the diagnosis and treatment of YFI in our state and, therefore, admitted all patients with severe YFI living in the state.
In conclusion, hypocoagulabitity assessed by ROTEM and low levels of liver-produced coagulation factors are hallmarks of severe YFI. Alpha angle and CT were capable of discriminating an adverse outcome and could potentially be used as predictors of death in severe YFI. ROTEM seems to be a useful tool to assess the hemostatic dysfunction of patients with severe YFI. Further studies should focus on its use to predict outcomes and guide the treatment of the bleeding diathesis of YFI.
Acknowledgments
The authors thank all patients for consenting on the participation on this study and for the staff from Hospital Eduardo de Menezes for their support. The authors also thank Diagnostica Stago Inc. for a kind gift of consumables for performance of D-dimer tests, protein C and thrombin time.
Funding
This work was funded by Governmental grants from Secretarias de Saúde e de Planejamento do Estado de Minas Gerais and Instituto René Rachou, Fiocruz Minas, Belo Horizonte, Minas Gerais, Brazil.
Author contributions
L.L.J. performed the global tests experiments, analyzed the data, and drafted the paper. M.B.F. collected data and contributed to data analysis. N.R.O. and L.S.P. collected data. B.N.C. performed coagulation tests. F.B., D.D.R., and T.L. participated in the study design and wrote the paper. S.M.R. designed the study, got funding, and wrote the paper. All authors revised and approved the final version of the manuscript.
Relationship Disclosure
The authors state that they have no competing conflict of interest.
Footnotes
Handling Editor: Nick van Es
The online version contains supplementary material available at https://doi.org/10.1016/j.rpth.2024.102427
Supplementary material
References
- 1.Barrett A.D., Monath T.P. Epidemiology and ecology of yellow fever virus. Adv Virus Res. 2003;61:291–315. doi: 10.1016/s0065-3527(03)61007-9. [DOI] [PubMed] [Google Scholar]
- 2.Woodson S.E., Freiberg A.N., Holbrook M.R. Coagulation factors, fibrinogen and plasminogen activator inhibitor-1, are differentially regulated by yellow fever virus infection of hepatocytes. Virus Res. 2013;175:155–159. doi: 10.1016/j.virusres.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 3.Balk R.A. Systemic inflammatory response syndrome (SIRS): where did it come from and is it still relevant today? Virulence. 2014;5:20–26. doi: 10.4161/viru.27135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franco M.B., Jardim L.L., de Carvalho B.N., Basques F., Ribeiro D.D., Pereira L.S., et al. Deficiency of coagulation factors is associated with the bleeding diathesis of severe yellow fever. Ann Hematol. 2023;102:1939–1949. doi: 10.1007/s00277-023-05262-x. [DOI] [PubMed] [Google Scholar]
- 5.Kang Y.G., Martin D.J., Marquez J., Lewis J.H., Bontempo F.A., Shaw B.W., Jr., et al. Intraoperative changes in blood coagulation and thrombelastographic monitoring in liver transplantation. Anesth Analg. 1985;64:888–896. [PMC free article] [PubMed] [Google Scholar]
- 6.Kaufmann C.R., Dwyer K.M., Crews J.D., Dols S.J., Trask A.L. Usefulness of thrombelastography in assessment of trauma patient coagulation. J Trauma. 1997;42:716–720. doi: 10.1097/00005373-199704000-00023. [DOI] [PubMed] [Google Scholar]
- 7.Papatheodoridis G.V., Patch D., Webster G.J., Brooker J., Barnes E., Burroughs A.K. Infection and hemostasis in decompensated cirrhosis: a prospective study using thrombelastography. Hepatology. 1999;29:1085–1090. doi: 10.1002/hep.510290437. [DOI] [PubMed] [Google Scholar]
- 8.Kleinegris M.C., Bos M.H., Roest M., Henskens Y., Ten Cate-Hoek A., Van Deursen C., et al. Cirrhosis patients have a coagulopathy that is associated with decreased clot formation capacity. J Thromb Haemost. 2014;12:1647–1657. doi: 10.1111/jth.12706. [DOI] [PubMed] [Google Scholar]
- 9.Stravitz R.T., Fontana R.J., Meinzer C., Durkalski-Mauldin V., Hanje A.J., Olson J., et al. Coagulopathy, bleeding events, and outcome according to rotational thromboelastometry in patients with acute liver injury/failure. Hepatology. 2021;74:937–949. doi: 10.1002/hep.31767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stravitz R.T., Lisman T., Luketic V.A., Sterling R.K., Puri P., Fuchs M., et al. Minimal effects of acute liver injury/acute liver failure on hemostasis as assessed by thromboelastography. J Hepatol. 2012;56:129–136. doi: 10.1016/j.jhep.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ministério da Saúde Febre amarela: Ministério da Saúde atualiza casos no país. 2018. https://www.gov.br/saude/pt-br/assuntos/noticias/2018/marco/febre-amarela-ministerio-da-saude-atualiza-casos-no-pais ; 2018. [accessed October 1, 2023].
- 12.Ministério da Saúde Febre Amarela Silvestre em Minas Gerais. Brasilia, Brasil. Ministério da Saúde 2019. http://www.saude.mg.gov.br/images/noticias_e_eventos/000_2019/jane_fev_mar/Febre_Amarela/Boletim_atualiza%C3%A7%C3%A3o_FA_12-02-2019.pdf ; 2019. [accessed May 14, 2024].
- 13.Brazil. Ministério da Saúde Secretaria de Vigilância em Saúde. Departamento de Imunização e Doenças Transmissíveis. Manual de manejo clínico da febre amarela [recurso eletrônico]/Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Imunização e Doenças Transmissíveis – Brasília. Ministério da Saúde. 2020 ISBN 978-85-334-2818-8. [Google Scholar]
- 14.Lee W.M., Stravitz R.T., Larson A.M. Introduction to the revised American Association for the study of liver diseases position paper on acute liver failure 2011. Hepatology. 2012;55:965–967. doi: 10.1002/hep.25551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. [PubMed] [Google Scholar]
- 16.McMichael M.A., Smith S.A. Viscoelastic coagulation testing: technology, applications, and limitations. Vet Clin Path. 2011;40:140–153. doi: 10.1111/j.1939-165X.2011.00302.x. [DOI] [PubMed] [Google Scholar]
- 17.Whiting D., DiNardo J.A. TEG and ROTEM: technology and clinical applications. Am J Hematol. 2014;89:228–232. doi: 10.1002/ajh.23599. [DOI] [PubMed] [Google Scholar]
- 18.Scarpelini S., Rhind S.G., Nascimento B., Tien H., Shek P.N., Peng H.T., et al. Normal range values for thromboelastography in healthy adult volunteers. Braz J Med Biol Res. 2009;42:1210–1217. doi: 10.1590/s0100-879x2009001200015. [DOI] [PubMed] [Google Scholar]
- 19.Schulman S., Kearon C. Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical in vestigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 20.Pereira L.M., Langley P.G., Hayllar K.M., Tredger J.M., Williams R. Coagulation factor V and VIII/V ratio as predictors of outcome in paracetamol induced fulminant hepatic failure: relation to other prognostic indicators. Gut. 1992;33:98–102. doi: 10.1136/gut.33.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perdomo-Celis F., Salvato M.S., Medina-Moreno S., Zapata J.C. T-cell response to viral hemorrhagic fevers. Vaccines (Basel) 2019;7:11. doi: 10.3390/vaccines7010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Owen C.A. J.r., Rettke S.R., Bowie E.J., Cole T.L., Jensen C.C., Wiesner R.H., et al. Hemostatic evaluation of patients undergoing liver transplantation. Mayo Clin Proc. 1987;62:761–772. doi: 10.1016/s0025-6196(12)62328-3. [DOI] [PubMed] [Google Scholar]
- 23.Holcomb J.B., Minei K.M., Scerbo M.L., Radwan Z.A., Wade C.E., Kozar R.A., et al. Admission rapid thrombelastography can replace conventional coagulation tests in the emergency department: experience with 1974 consecutive trauma patients. Ann Surg. 2012;256:476–486. doi: 10.1097/SLA.0b013e3182658180. [DOI] [PubMed] [Google Scholar]
- 24.Shima M., Matsumoto T., Ogiwara K. New assays for monitoring haemophilia treatment. Haemophilia. 2008;14:83–92. doi: 10.1111/j.1365-2516.2008.01737.x. [DOI] [PubMed] [Google Scholar]
- 25.Tran H.T.T., Sørensen B., Bjørnsen S., Pripp A.H., Tjønnfjord G.E., Andre Holme P. Monitoring bypassing agent therapy - a prospective crossover study comparing thromboelastometry and thrombin generation assay. Haemophilia. 2015;21:275–283. doi: 10.1111/hae.12570. [DOI] [PubMed] [Google Scholar]
- 26.Lovejoy A.E., Reynolds T.C., Visich J.E., Butine M.D., Young G., Belvedere M.A., et al. Safety and pharmacokinetics of recombinant factor XIII-A2 administration in patients with congenital factor XIII deficiency. Blood. 2006;108:57–62. doi: 10.1182/blood-2005-02-0788. [DOI] [PubMed] [Google Scholar]
- 27.Piza F.M., Corrêa T.D., Marra A.R., Guerra J.C., Rodrigues R.D., Villarinho A.A., et al. Thromboelastometry analysis of thrombocytopenic dengue patients: a cross-sectional study. BMC Infect Dis. 2017;17:89. doi: 10.1186/s12879-017-2204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sureshkumar V.K., Vijayan D., Kunhu S., Mohamed Z., Thomas S., Raman M. Thromboelastographic analysis of hemostatic abnormalities in dengue patients admitted in a multidisciplinary intensive care unit: a cross-sectional study. Indian J Crit Care Med. 2018;22:238–242. doi: 10.4103/ijccm.IJCCM_486_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hugenholtz G.C.G., Lisman T., Stravitz R.T. Thromboelastography does not predict outcome in different etiologies of cirrhosis. Res Pract Thromb Haemost. 2017;1:275–285. doi: 10.1002/rth2.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goyal S., Jadaun S., Kedia S., Kumar-Acharya S., Varma S., Nayak B., et al. Thromboelastography parameters in patients with acute on chronic liver failure. Ann Hepatol. 2018;17:1042–1051. doi: 10.5604/01.3001.0012.7205. [DOI] [PubMed] [Google Scholar]
- 31.Blasi A., Calvo A., Prado V., Reverter E., Reverter J.C., Hernández-Tejero M., et al. Coagulation failure in patients with acute-on-chronic liver failure and decompensated cirrhosis: beyond the international normalized ratio. Hepatology. 2018;68:2325–2337. doi: 10.1002/hep.30103. [DOI] [PubMed] [Google Scholar]
- 32.Crochemore T., Savioli F.A., Guerra J.C.C., Kalmar E.M.D.N. Thromboelastometry identifies coagulopathy associated with liver failure and disseminated intravascular coagulation caused by yellow fever, guiding specific hemostatic therapy: a case report. Rev Bras Ter Intensiva. 2020;32:474–478. doi: 10.5935/0103-507X.20200078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lisman T., Bakhtiari K., Adelmeijer J., Meijers J.C., Porte R.J., Stravitz R.T. Intact thrombin generation and decreased fibrinolytic capacity in patients with acute liver injury or acute liver failure. J Thromb Haemost. 2012;10:1312–1319. doi: 10.1111/j.1538-7836.2012.04770.x. [DOI] [PubMed] [Google Scholar]
- 34.Lisman T., Kwaan H.C. Hemostatic dysfunction in liver diseases. Semin Thromb Hemost. 2015;41:445–446. doi: 10.1055/s-0035-1550441. [DOI] [PubMed] [Google Scholar]
- 35.Tripodi A., Primignani M., Chantarangkul V., Viscardi Y., Dell'Era A., Fabris F.M., et al. The coagulopathy of cirrhosis assessed by thromboelastometry and its correlation with conventional coagulation parameters. Thromb Res. 2009;124:132–136. doi: 10.1016/j.thromres.2008.11.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.