Abstract
Few metabolites can claim a more central and versatile role in cell metabolism than acetyl-coenzyme A (acetyl-CoA). Acetyl-CoA is produced during nutrient catabolism to fuel the TCA cycle, and it is the essential building block for fatty acid and isoprenoid biosynthesis. It also functions as a signaling metabolite, since it is the substrate for lysine acetylation reactions, enabling modulation of protein functions in response to acetyl-CoA availability. Recent years have seen exciting advances in the understanding of acetyl-CoA metabolism in normal physiology and in cancer, buoyed by new mouse models, in vivo stable isotope tracing approaches, and improved methods for measuring acetyl-CoA, including in subcellular compartments. Efforts to target acetyl-CoA metabolic enzymes are also advancing, including one therapeutic targeting acetyl-CoA synthesis achieving FDA approval. In this article, we will overview the regulation and cancer relevance of major metabolic pathways in which acetyl-CoA participates. We further discuss recent advances in understanding acetyl-CoA metabolism in normal tissues and tumors and the potential for therapeutic targeting of these pathways. The article concludes with commentary on emerging nodes of cancer biology impacted by acetyl-CoA metabolism.
Introduction
Acetyl-CoA is both a central metabolic intermediate and key signaling molecule. Because of its high energy thioester bond linking the acetyl group to Coenzyme A, acetyl-CoA is a thermodynamically activated metabolite that is used in many biochemical pathways1. In mitochondria, acetyl-CoA is produced from glucose, lipid, and amino acid catabolism to power the TCA cycle and electron transport chain, and in the cytosol, it is used for anabolism, as the precursor for fatty acid and isoprenoid biosynthesis (Fig. 1). It is also the cellular substrate for protein lysine acetylation reactions, best known for regulating gene expression through nuclear histone acetylation, and it is an allosteric activator (e.g. of pyruvate carboxylase and pyruvate dehydrogenase kinase)2, 3. Acetyl-CoA’s central role in cell metabolism is so ancient that it is speculated to predate ATP as the cell’s main energy currency4.
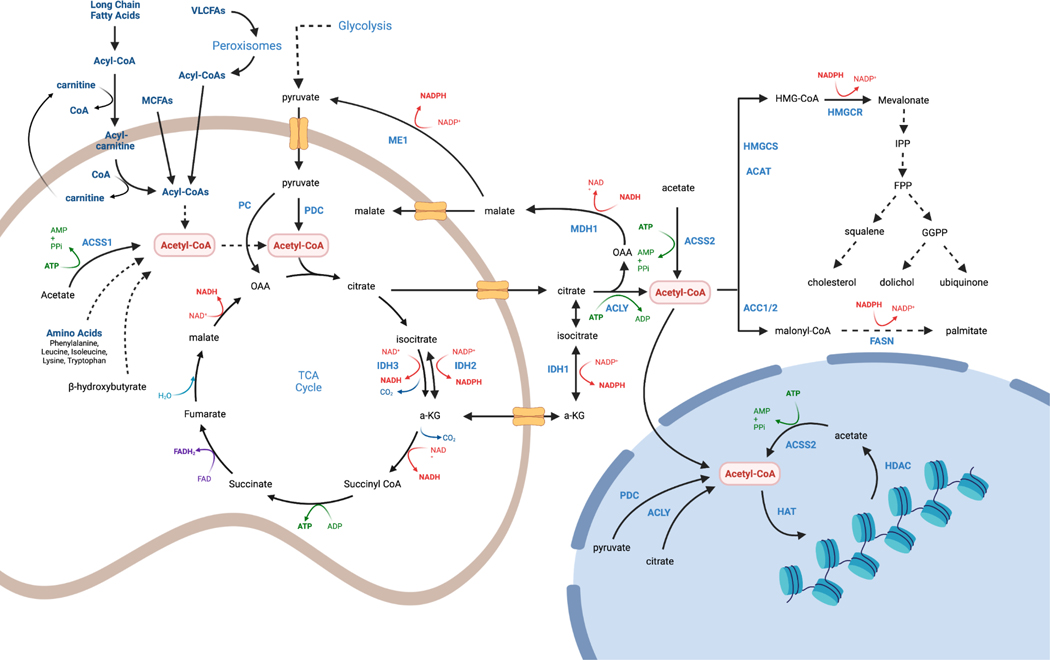
Figure 1. Compartmentalized acetyl-CoA production pathways.
Mitochondrial acetyl-CoA is generated in many cell types from glucose during anabolic conditions, and from other nutrients, such as long and medium chain fatty acids, acetate, amino acids and beta-hydroxybutyrate, during catabolic conditions, though different cell and tissue types have different nutrient preferences. Pyruvate enters mitochondria through the mitochondrial pyruvate carrier (MPC) and undergoes oxidative decarboxylation by pyruvate dehydrogenase complex (PDC) yielding acetyl-CoA, NADH and CO2. Fatty acids are transported into mitochondria as acyl-carnitines via the Cpt1/Cpt2 shuttling system, which operates between the mitochondrial outer and inner membranes (not shown). Peroxisomes can also generate short chain fatty acids that are delivered to mitochondria; medium chain fatty acids do not require the acyl-carnitine shuttle. Acetyl group carbons enter the TCA cycle following a condensation reaction with oxaloacetate catalyzed by citrate synthase (CS). TCA cycle flux generates CO2 and the reducing equivalents that drive the electron transport chain (ETC) and ATP synthesis. Cytosolic acetyl-CoA is used for fatty acid synthesis and in the mevalonate pathway. Cytosolic acetyl-CoA carbons are transferred from mitochondrial citrate via the mitochondrial citrate carrier (Slc25a1). Citrate is cleaved by ATP Citrate Lyase (ACLY) to make cytosolic acetyl-CoA. Citrate may also be generated through reductive carboxylation by the isocitrate dehydrogenases (IDH1/IDH2). Alternatively, cytosolic acetyl-CoA can be derived from acetate via acyl-CoA short-chain synthetase-2 (ACSS2). Acetyl-CoA generated in the cytosol may diffuse into the nucleus for histone acetylation reactions. However, ACLY, ACSS2, and PDC have all been reported in the nucleus where they may generate local acetyl-CoA.
Given the multifaceted roles of acetyl-CoA in the cell, it is perhaps not surprising that it plays important roles in tumor metabolism. For example, it is well established that lipid biosynthesis contributes to tumor growth, enabling membrane production and expansion, as well as lipid-dependent signaling5. Accordingly, the fatty acid synthesis and mevalonate pathways are of substantial interest as therapeutic targets6, 7. Histone and other protein acetylation has also emerged in key context-dependent roles in various aspects of tumorigenesis and may present additional opportunities for therapeutic intervention. Acetyl-CoA metabolic enzymes are frequently overexpressed in cancers, and post-translational modification of these enzymes allows their dynamic regulation in response to various signaling cues. The goal of this review is to discuss the roles of acetyl-CoA in cancer, primarily focusing on its functions and therapeutic potential in the cytosol and nucleus. Targeting mitochondrial acetyl-CoA metabolism has been reviewed in depth elsewhere8, 9. To this end, we first review major metabolic pathways involving acetyl-CoA. We then discuss how changes in acetyl-CoA production and use as both a substrate and a metabolite signal support tumorigenesis, pointing toward promising therapeutic strategies targeting nuclear-cytosolic acetyl-CoA metabolic enzymes. We conclude with a future outlook highlighting the major gaps in our understanding of acetyl-CoA regulation that require more investigation in terms of both basic biology and cancer relevance.
Cellular Roles of Acetyl-CoA
Membranes are impermeable to acetyl-CoA, and thus it must be generated within or transported into each cellular compartment in which it functions. The major pools of acetyl-CoA are divided between the mitochondrial and nuclear-cytosolic compartments. As we will discuss, the nucleus is emerging as an active metabolic compartment in which acetyl-CoA can be produced, but metabolites can also diffuse between cytosol and nucleus. Acetyl-CoA can additionally be generated within peroxisomes, and it can be transported into the endoplasmic reticulum10. To provide a backdrop for discussing acetyl-CoA metabolism in cancer, we begin here with a brief overview of acetyl-CoA functions within the mitochondria, cytosol, and nucleus.
Mitochondrial acetyl CoA
In mitochondria, acetyl CoA enters the TCA cycle upon condensation with oxaloacetate to produce citrate, which is oxidized to yield reducing equivalents and ultimately ATP via the electron transport chain and oxidative phosphorylation. Acetyl-CoA is produced in mitochondria from several nutrient sources (Fig. 1). Pyruvate generated by glycolysis is transported into the mitochondria matrix by the mitochondrial pyruvate carrier (MPC), where it undergoes irreversible oxidative decarboxylation by the multi-enzyme pyruvate dehydrogenase complex (PDC) to produce acetyl-CoA, NADH and CO2. Mitochondrial acetyl-CoA may also be generated by fatty acid β-oxidation, by the catabolism of certain amino acids including the branched chain amino acids (BCAAs) leucine and isoleucine, by ketone body catabolism, and through the ATP-dependent synthesis from acetate. The relative contribution of each of these pathways to generating mitochondrial acetyl-CoA varies with the nutritional state and tissue type.
Cytosolic acetyl CoA
Cytosolic acetyl-CoA is used for de novo lipid and cholesterol biosynthesis, which is critical for building cellular membranes, storing energy, and generating signaling metabolites such as diacylglycerols (DAG) and phosphatidylinositol-3,4,5-trisphosphate (PtdIns(3,4,5)P3). These processes are often upregulated in tumour cells11. It is also necessary for cytosolic protein acetylation, as well as generation of acetylated metabolites including UDP-N-acetylglucosamine, which is crucial for glycosylation12. Cytosolic acetyl-CoA is irreversibly converted to malonyl-CoA by acetyl-CoA carboxylase 1 and 2 (ACC1, ACC2; gene names: ACACA, ACACB) for use in de novo lipogenesis (DNL) and as an inhibitor of fatty acid oxidation (Fig. 1). Cytosolic acetyl-CoA can also be diverted to form mevalonate, the precursor for sterols and isoprenoids, by the consecutive actions of acetyl-CoA acetyl transferase (ACAT), HMG-CoA synthase (HMGCS), and HMG-CoA reductase (HMGCR), the latter step also consuming NADPH (Fig. 1).
When cytosolic acetyl-CoA is needed, mitochondrial citrate is exported to the cytosol where it is cleaved by ATP-citrate lyase (ACLY) in an ATP-consuming reaction that generates oxaloacetate and acetyl-CoA (Fig. 1)13, 14. Citrate can also be produced through α-ketoglutarate reductive carboxylation by the mitochondrial or cytosolic forms of isocitrate dehydrogenase (IDH2 and IDH1, respectively), particularly in cells with mitochondrial defects or in hypoxia15–17. Finally, citrate may be obtained from circulation through the plasma membrane citrate carrier Slc13A5, which is highly expressed in the liver and structurally distinct from the mitochondrial citrate transporter. This could be a particularly significant pathway in liver cancer cells where citrate uptake was shown to protect against nutrient and oxygen stress by feeding the TCA cycle and lipid synthesis 18. Another major route to cytosolic acetyl-CoA is via acyl-CoA short-chain synthetase-2 (ACSS2), which converts acetate to acetyl-CoA at the cost of one ATP (Fig. 1). Acetate can be imported from circulation, with one of its main sources being the gut microbiota, or it can be generated within cells by histone deacetylation reactions (see below), hydrolysis of acetylated metabolites, or in some contexts, directly from pyruvate19, 20. Notably, there are two other acetyl-CoA synthetase isoforms that localize to the mitochondria matrix, ACSS1 and ACSS3, the main difference being that ACSS3 prefers propionate as its substrate21. ACSS2 expression increases upon genetic deletion or inhibition of Acly in certain contexts both in vitro and in vivo, which switches the major source of acetyl-CoA from citrate to acetate to maintain lipogenesis and histone acetylation22, 23. Whether such compensatory mechanisms are important in tumor cells is still under investigation but has clear therapeutic implications.
Nuclear acetyl CoA
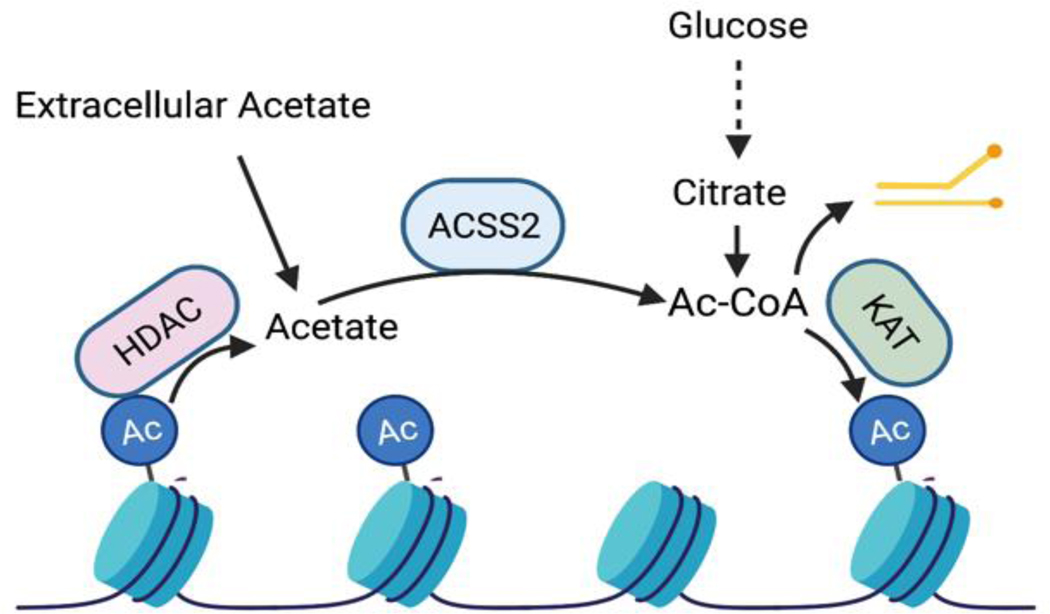
In its role as a signaling metabolite, acetyl-CoA is best known as the substrate for protein lysine acetylation reactions. Although post-translational lysine acetylation occurs throughout the cell on metabolic enzymes, signaling enzymes, transcription factors and histones24, it is currently most appreciated in cancer for its role in nuclear histone acetylation, which is highly sensitive to acetyl-CoA availability25–27 (Fig. 1). Acetyl-CoA may enter the nucleus by diffusion through the nuclear pore, but accumulating evidence also highlights the biological importance of acetyl-CoA produced directly within the nucleus28 (Fig. 1). Indeed, ACLY, which is predominantly cytosolic, is also present in the nucleus25. Nuclear localized ACLY is crucial for its involvement in certain processes, such as DNA damage repair, where it promotes histone acetylation near sites of DNA double strand breaks, enabling BRCA1 recruitment and break repair, a role that is lost if ACLY is confined to the cytosol29. ACSS2 is also both cytosolic and nuclear, and in some contexts, predominantly nuclear30–33. Nuclear ACSS2 regenerates acetyl-CoA from the acetate produced by histone deacetylation (HDAC) reactions, enabling acetyl-CoA recycling within the nucleus31. Such recycling may facilitate gene regulation and enable maintenance of histone and transcription factor acetylation when glucose-derived acetyl-CoA is less available. An emerging concept is that certain high stoichiometry histone acetylation sites (e.g., H3K23) may in fact serve as acetyl-CoA reservoirs, which can be accessed for histone acetylation at key gene regulation sites34, 35 (Box 1). An interesting question is whether these acetyl-CoA reservoirs can also be accessed to feed other metabolic pathways during temporary nutrient deprivation, as is frequently encountered in the tumor microenvironment.
Box 1. Histone acetylation as a participant in metabolism.
Histone acetylation is dynamic. Deacetylation releases acetate that can be recycled back to acetyl-CoA by ACSS2. The concept of histones as an acetate reservoir has been hypothesized, based on evidence that histone acetylation can dramatically fluctuate in response to nutrient availability without corresponding drastic transcriptional alterations, and on calculations that a substantial pool of acetate is deposited on chromatin (histones having the potential to consume up to 3 mM acetyl-CoA)35. Recently, in vitro assays using a histone H3.1 N-terminal peptide with a deuterated acetyl group on acetylated-K23, were used to formally demonstrate that recycling of HDAC-produced acetate could be used by ACSS2 and the KAT CBP to acetylate another lysine residue34. The importance of acetate deposition on histones unrelated to transcription is only beginning to be explored and many questions remain. For example, what is the advantage of maintaining such an acetate reservoir? Since non-enzymatic acetylation can occur in manner dependent on acetyl-CoA concentration, one possible advantage to an acetate reservoir may be to allow on-demand local production of acetyl-CoA for gene regulation, but without allowing acetyl-CoA concentrations to rise to a point at which non-enzymatic histone acetylation- and concomitant dysregulation of gene expression- would become prevalent. Another possibility is that this acetate might also be accessed to feed metabolic processes such as lipid biosynthesis or the TCA cycle under conditions of lipid/nutrient stress. Other unknowns include the mechanisms by which the cell sets and defends its acetate reservoirs, as well as the implications for chromatin regulatory mechanisms if reservoirs dip too low. Research is just beginning to uncover the functional roles and significance of histone acetate reservoirs.
Another potential source of nuclear acetyl-CoA is the pyruvate dehydrogenase complex (PDC). Each subunit of the mitochondrial PDC is also present in the nucleus in response to certain conditions, including mitochondrial stresses, growth factor signaling, and specific developmental cues, where it regulates histone acetylation 36–38 (Fig. 1). While surprising that such a large complex (up to 10 MDa) could translocate to the nucleus, a recent study has delineated a mechanism that involves mitochondrial tethering to the nucleus and entry of PDC independent of nuclear pores39. Finally, carnitine acetyltransferase (CrAT), a mitochondrial enzyme that buffers mitochondrial acetyl-CoA levels through acetylcarnitine synthesis and export, has also been reported to be present in the nucleus to support histone acetylation by regenerating acetyl-CoA from acetylcarnitine40, although the roles and regulation of such a route remain little studied.
Acetyl CoA enzyme regulation in cancer
The production and use of acetyl-CoA in both healthy and cancer cells is dynamically controlled through regulation of metabolic enzymes at both the transcriptional and post-translational levels. The genes encoding ACLY, ACSS2, ACC1, and ACC2 are often co-regulated, along with other lipogenesis genes, by steroid regulatory element-binding proteins (SREBP) transcription factors. SREBPs are classically activated by sterol depletion, and are also activated in cancer by AKT-mTORC1 signaling in response to oncogenic and growth factor signaling41–43 (Fig. 2). SREBP can also cooperate with MYC to activate lipogenic gene expression in MYC-driven cancers, promoting dependence on DNL 44. Moreover, tumor microenvironmental factors such as hypoxia and low pH have each been documented to drive SREBP2-dependent ACSS2 upregulation and acetate-dependent DNL45, 46. Interestingly, in an obesity-associated multiple myeloma model, it was reported that adipocyte-derived angiotensin II may promote SREBP-dependent ACSS2 expression in myeloma cells, which in turn drives acetyl-CoA production and acetylation and activation of the transcription factor IRF4, to which myeloma cells are addicted47. ACLY and ACACA are also co-regulated by the nuclear receptor PPARγ to promote increased DNL in hepatocellular carcinoma48. Finally, NRF2, a transcription factor that regulates antioxidant and other cellular stress response mechanisms and is activated in several cancers was found to regulate ACSS2 in esophageal squamous cell carcinoma49. In terms of clinical correlates with expression, in several cancer types, including cervical cancer, renal cell carcinoma, breast cancer, and grade II and III gliomas, high ACSS2 expression is associated with low survival rates45, 47, 50–52, while conversely, low ACSS2 expression was found to correlate with more aggressive phenotypes and poor survival in hepatocellular carcinoma (HCC) and colorectal cancer53, 54. ACLY is upregulated across numerous cancer types, and high ACLY expression is associated with lower survival rates in ovarian and breast cancers, acute myeloid leukemia, and HCC55–58.
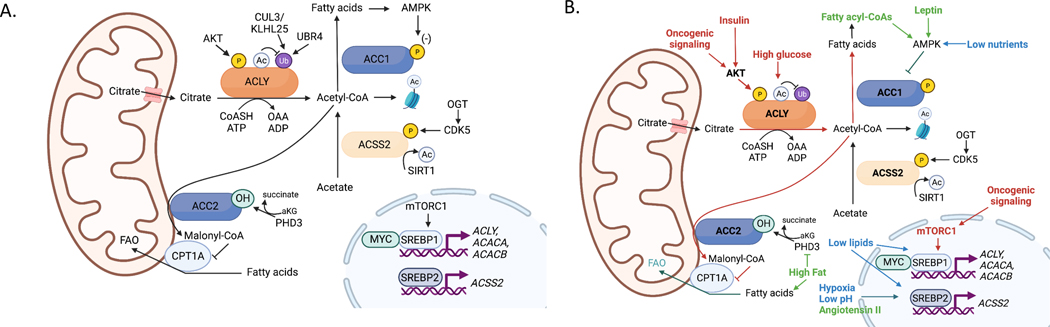
Figure 2. Regulation of acetyl-CoA metabolic enzymes.
(A) Transcriptional and post-translational regulation of acetyl-CoA metabolic enzymes. (B) Impact of oncogenic, microenvironmental and systemic metabolic factors on regulation of acetyl-CoA metabolic enzymes (red, signals associated with oncogenic signaling, high nutrient availability and fatty acid synthesis; green, signals associated with high lipid availability and fatty acid oxidation, and may be linked to obesity-related cancers; blue, signals associated with nutrient stress-inducing adaptation within the tumor microenvironment, which may include fatty acid oxidation or fatty acid synthesis depending on the context). Each of these adaptations may support tumor growth and/or facilitate survival within the tumor microenvironment.
Posttranslational modifications also dynamically modulate acetyl-CoA metabolic enzymes in response to a variety of cues including oncogenic signaling pathways (Fig. 2). ACLY has several described phosphorylation sites, of which serine 455 (S455), located within the enzyme’s disordered loop, is the most well studied. S455 phosphorylation has been reported to enhance enzyme activity59, though it is worth noting that some prior studies had found little change in kinetic parameters60–63. S455 can be phosphorylated by AKT, protein kinase A (PKA), and branched chain ketoacid dehydrogenase kinase (BCKDK)64–66, suggesting that it is a critical node of metabolic regulation. AKT signaling to ACLY occurs downstream of oncogenic and growth factor-mediated signaling and has been shown to promote histone acetylation, consistent with an activating function of this modification26, 29, 67–69. Consistent with an AKT-ACLY-histone acetylation axis, global histone acetylation in human prostate tumors and gliomas correlates positively with pAKT-S473 levels26. Further, exposure of cancer cells to insulin and other growth factors can drive elevated histone acetylation, at least in part through ACLY, with insulin-upregulated gene expression correlating with increased promoter and enhancer histone acetylation67, 70. The regulation of ACLY posttranslational modifications other than pS455 may also have roles in cancer, but are less studied. ACLY tyrosine phosphorylation by the tyrosine-protein kinase Lyn was recently reported in acute myeloid leukemia cells and implicated in promoting lipid synthesis and histone acetylation71. Further, ACLY-S447 and ACLY-S451 are phosphorylated by the glycogen synthase kinase-3 (GSK-3) enzyme, which functions in various cellular processes and is of possible interest as a therapeutic target in cancer72, although a function for these modifications has not yet been described73. The acetylation of ACLY at lysines 540, 546, and 554, which increases under high glucose conditions, has been shown to promote protein stability, lipid synthesis, and tumor growth by blocking UBR4-mediated ubiquitylation 74. ACLY is also ubiquitylated at the same sites by Cullin3 (CUL3)-KLHL25 and levels of CUL3 negatively correlate with ACLY levels in lung cancer75. Collectively, these studies show that ACLY is regulated by extensive post-translational mechanisms, at least some of which exhibit aberrant regulation in cancer.
ACSS2 acetylation and phosphorylation are also regulated by cell growth and survival pathways often dysregulated in cancer. The NAD-dependent deacetylase SIRT1, which increases activity during cell stress, activates ACSS2 via deacetylation of K66176. Tumour-associated stresses such as low nutrients and hypoxia also promote ACSS2 nuclear localization31, 33. Mechanistically, it has been shown that AMPK-dependent phosphorylation of ACSS2 at S659 may expose a nuclear localization sequence33. ACSS2 is also regulated downstream of O-GlcNAc transferase (OGT), an enzyme that mediates the O-GlcNAc post-translational modification, which is elevated in many tumors and implicated in promoting tumor growth77. OGT promotes CDK5-dependent phosphorylation of ACSS2 at S267, resulting in its stabilization and subsequent stimulation of acetate-driven de novo lipogenesis78.
During nutrient deprivation-induced energy stress or excess fatty acid availability, AMPK also phosphorylates ACC1 and ACC2 enzymes to inhibit acetyl-CoA conversion to malonyl-CoA, thereby attenuating lipid synthesis and stimulating fat oxidation79, 80. Mice with alanine knock-in mutations at the sites phosphorylated by AMPK, rendering ACC unable to be inhibited by AMPK, exhibit elevated hepatic DNL and enhanced hepatic tumor formation when challenged with the chemical carcinogen diethylnitrosamine81, 82. ACC2 was also recently found to be hydroxylated and activated by the 2-oxoglutarate-dependent dioxygenase PHD3, suppressing fatty acid oxidation83. Low PHD3 expression in leukemia promoted dependence on fatty acid oxidation83. ACC is also allosterically activated by citrate and inhibited by fatty acyl-CoAs84. Altogether, a wealth of data indicates that the abundance and activity of enzymes that produce and use acetyl-CoA are altered by oncogenic signaling mechanisms, transcriptional regulation, and tumor microenvironmental stresses to promote tumor growth (Fig. 2).
Acetyl CoA pathways in tumorigenesis
Alterations in acetyl-CoA metabolism have been shown to contribute to tumorigenesis through several pathways, including the mevalonate pathway, DNL, and protein acetylation. Here we discuss the evidence for the importance of each pathway in cancer and how dysregulation of acetyl-CoA metabolism may enforce or support elevated pathway activity.
Lipid, sterol, and isoprenoid synthesis
Substantial preclinical evidence points to the mevalonate pathway as a vulnerability in some cancers7. Specific cancer genetic alterations have been shown to promote mevalonate pathway dependence, including TP53 loss in hepatocellular carcinoma, TP53 mutation in breast cancer, and t(4;14) chromosomal translocation in multiple myeloma85–87. Synthesis of the mevalonate pathway intermediate HMG-CoA is extremely sensitive to cytosolic acetyl-CoA production88, 89, and anti-cancer effects of targeting acetyl-CoA metabolism may be mediated through the mevalonate pathway in some contexts. For example, in a KRASG12D-driven murine model of pancreatic cancer, genetic deletion of Acly in the pancreas impedes tumor formation. Further, acinar-to-ductal metaplasia (ADM), a wound healing response co-opted by mutant KRAS to promote tumor formation90, is inhibited in ex vivo assays by either treatment with statins — which inhibit the conversion of HMG-CoA to mevalonate — or ACLY deficiency, and cholesterol supplementation rescues ADM in statin-treated cells67. Statin treatment is also anti-proliferative in established pancreatic cancer cell lines, in a manner rescuable with mevalonate or GGPP67, 91. While statins are generally insufficient as single agent anti-cancer therapeutics, a number of combination strategies have been proposed to enhance efficacy7. For example, statin treatment can drive activation of a feedback loop involving compensatory activation of SREBP2 and upregulation of the statin target HMGCR; blocking this response enhanced statin-induced apoptosis and suppressed xenograft tumor growth92, 93. Statins have also been used in different combination therapies in clinical trials, with some but not all studies reporting benefits94. The potential for statins as anti-cancer agents is discussed in depth in recent reviews7, 94. Further work is needed to identify optimal contexts for statin use in cancer, as well as to understand the extent to which targeting acetyl-CoA metabolic enzymes could mirror or improve on statin effects.
Fatty acids, either de novo synthesized or imported from circulation, have many potential roles in cancer cells, including as structural components of membranes, signaling molecules, and as fuel for energy production. De novo fatty acid synthesis is upregulated across many cancer types and has been of substantial interest as a therapeutic target6, 11. Inhibiting the conversion of glucose or acetate to lipids via targeting ACLY or ACSS2, respectively, reduces tumor growth in mice45, 50, 95–98. Similarly, targeting FASN exerts anti-cancer effects in some preclinical models6, and the FASN inhibitor TVB-2640 is currently being tested in oncology clinical trials (NCT03808558, NCT02223247, NCT02980029, NCT03032484, NCT03179904, NCT05118776).
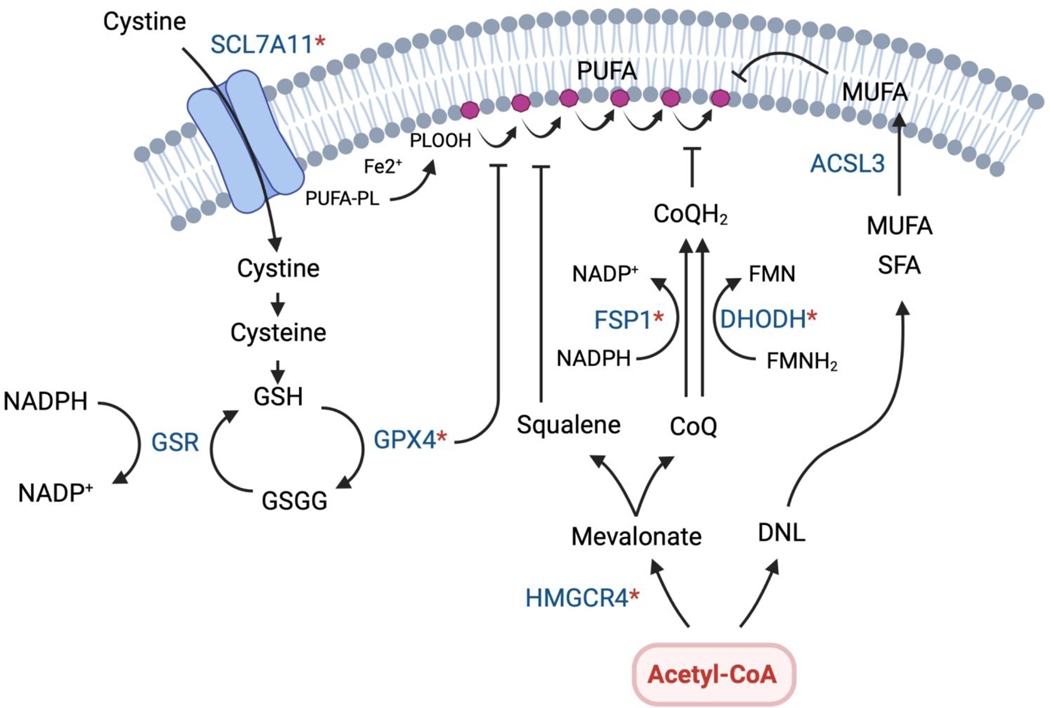
Acetyl-CoA use in the DNL and mevalonate pathways may also defend against oxidative stress and ferroptosis in cancer cells (Fig. 3). Ferroptosis is an iron-mediated mechanism of cell death driven by ROS-dependent peroxidation of polyunsaturated fatty acids (PUFAs) in cell membranes99. Several ferroptosis defense mechanisms are emerging and active in cancer cells100, including the GPX4 glutathione peroxidase pathway and fibroblast-specific protein 1 (FSP1), which is a CoQ oxidoreductase101. In the latter pathway, FSP1 and dihydroorotate dehydrogenase (DHODH) promote NADH-dependent and FMNH2-dependent reduction of CoQ, respectively, which is synthesized from acetyl-CoA to function in this context as an antioxidant and ferroptosis inhibitor101, 102. Notably, statin treatment, which inhibits the mevalonate pathway, was shown to reduce CoQ abundance and trigger compensatory NRF2 upregulation in pancreatic cancer cells; oxidative stress and cell death were induced by targeting this compensatory pathway in conjunction with statins91. Squalene accumulation has also been shown to protect against ferroptosis in lymphomas with loss of squalene monooxygenase103. In a parallel acetyl-CoA driven pathway, de novo synthesized saturated and monounsaturated fatty acids can replace ROS-sensitive PUFAs in membranes thereby reducing overall susceptibility to lipid peroxidation104, 105. Thus, strategies aimed at inhibiting acetyl-CoA use in lipid and isoprenoid synthesis to promote ferroptosis could hold therapeutic potential. For in-depth discussion of ferroptosis and its roles in cancer, the reader is referred to recent reviews100, 106.
Figure 3. Acetyl-CoA pathways have roles in ferroptosis protection.
Ferroptosis is a form of regulated cell death driven by iron-dependent phospholipid peroxidation of membrane PUFAs. If these toxic phospholipid peroxides (PLOOH) are not neutralized by ferroptosis defense mechanisms, they can propagate and damage cell membranes, inducing a unique form of cell death. Many cancer cells, due to their unique metabolic properties and propensity for high ROS production, must engage ferroptosis defense mechanisms to survive. Therefore, ferroptosis may be a targetable vulnerability in many tumor types. Several pathways protect against ferroptosis by reducing lipid peroxides. The major defense pathway is the selenoenzyme glutathione peroxidase 4 (GPX4) pathway, which uses glutathione (GSH) to reduced PLOOH. Additional protection is mediated by squalene and ubiquinol (CoQH), both of which are produced from acetyl-CoA via the mevalonate pathway. FSP1 and DHODH have been show to function in this context by reducing ubiquinone (CoQ) to ubiquinol at different locations in the cell. Ferroptosis sensitivity may also be reduced by actively synthesizing and replacing PUFAs with saturated and monounsaturated fatty acids that are resistant to ferroptosis, which is another mechanism linked to acetyl-CoA production. Ferroptosis regulators for which inhibitors are being considered are indicated with a (*). Currently, the most widely studied inhibitors either target solute carrier family 7 member 11 (SLCA11), which controls cystine uptake and glutathione production (e.g. Erastin and its analogs), or GPX4 (e.g. RSL3, ML162, ML210)100. It will be interesting to see if ACLY or ACSS2 inhibitors synergize with ferroptosis pathway inhibitors against certain cancers.
Protein acetylation
The use of acetyl-CoA as the substrate for the acetylation of histones and other proteins is emerging as an important contributor to tumorigenesis107. Oncogenic metabolic reprogramming and exogenous cues such as growth factors, adipokines, and microenvironmental stimuli can increase acetyl-CoA availability through effects on metabolic enzyme expression or activity. Changes in acetyl-CoA availability have been linked to the regulation of gene expression in several studies. A key question is how specificity in gene regulation is achieved by changes in the availability of a metabolite, with two predominant mechanisms being implicated: 1) nutrient-sensitive regulation of transcription factors (e.g., by acetylation) and 2) compartmentalization of acetyl-CoA production within the nucleus to regulate acetylation at specific loci12. Recent work has demonstrated that the nucleus exhibits distinct acyl-CoA metabolic profiles from the cytosol89, highlighting the potential importance of local acetyl-CoA production.
One process that has been linked transcriptionally to acetyl-CoA availability and metabolically sensitive histone acetylation in cancer cells is DNL. This suggests that an increase in lipogenic acetyl-CoA in the cytosol is coordinated with its ability to act as a gene regulation signal via histone acetylation (Fig. 4). In one study, for example, acetate availability was shown to promote ACACA and FASN expression in hepatocellular carcinoma cells, correlating with increased histone acetylation at the promoters of these genes108. Acetate simultaneously fed fatty acid synthesis directly. In another study in prostate cancer, acetyl-CoA production by a nuclear pyruvate dehydrogenase complex (PDC) promoted histone acetylation at SREBP target genes. Mitochondrial PDC was also needed in this model to supply lipogenic acetyl-CoA via citrate synthesis and cleavage38. Notably, in PDHA1-silenced cells, expression of NLS-tagged and NES-tagged PDHA1 together had a much stronger effect on promoting tumor growth than individually, supporting the notion that compartmentalized functions are coordinated to support tumor growth.
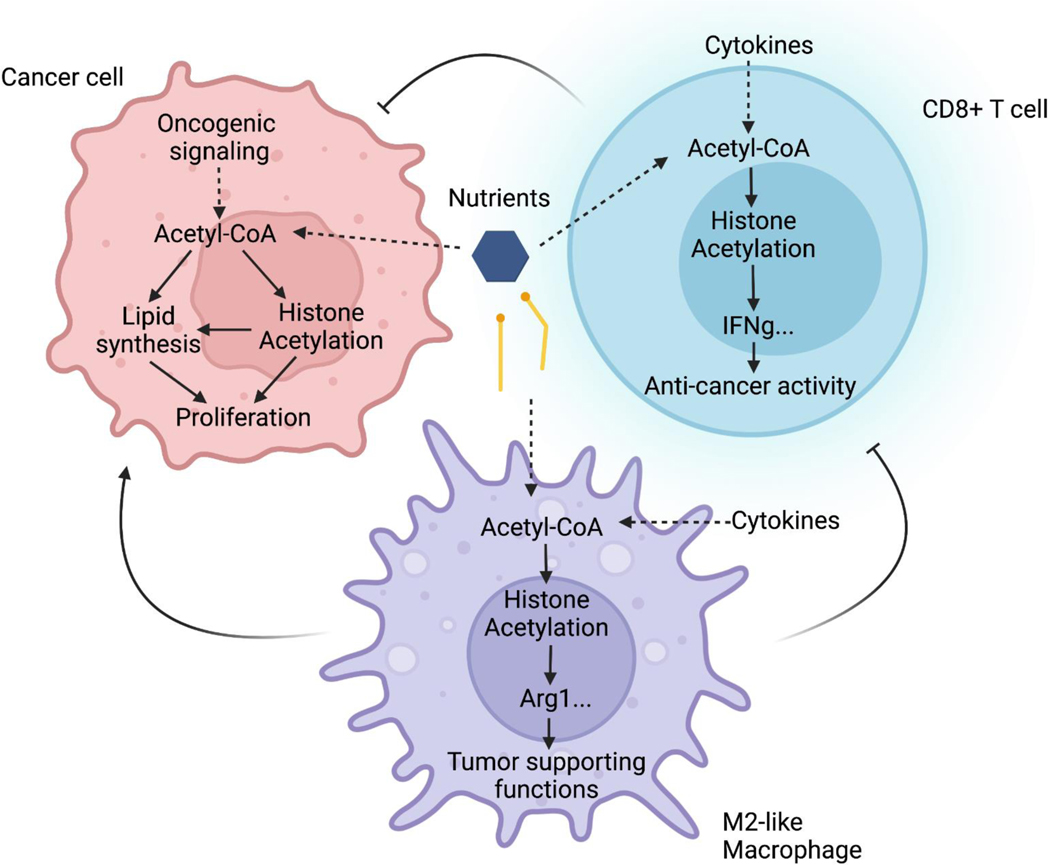
Figure 4. Metabolic regulation of histone acetylation impacts phenotypes of both cancer cells and non-malignant cells in the tumors.
In addition to its direct roles in metabolism, acetyl-CoA is used for protein modification via acetylation. Nutrient-sensitive histone acetylation has been linked to regulation of gene expression in different cell types, potentially impacting tumor progression. In cancer cells, acetyl-CoA availability for histone acetylation has been linked to gene expression related to lipid metabolism, proliferation, and invasive properties. In macrophages, polarization towards the immune-suppressive M2 phenotype is ACLY-dependent. In T cells, production of IFNγ has been found to be responsive to acetyl-CoA production. The net effect of targeting acetyl-CoA metabolic enzymes may depend on effects in multiple cell types in the tumor microenvironment.
Cell migration, epithelial-mesenchymal transition (EMT), and metastasis have also been linked to nutrient-sensitive acetyl-CoA production. For example, leptin-dependent AMPK activation and ACC phosphorylation was shown to trigger acetyl-CoA accumulation and Smad2 acetylation, thereby promoting EMT in breast cancer109. In HCC, loss of the acetyl-CoA hydrolase ACOT12 boosted acetyl-CoA levels and stimulated expression of the transcription factor Twist2, correlating with increased histone acetylation at this locus110. Finally, high acetyl-CoA abundance was associated with promotion of H3K27ac at genes associated with cell adhesion and migration in glioblastoma cells, and this was linked with Ca2+-dependent activation of the transcription factor NFAT1111. Thus, mechanisms increasing acetyl-CoA availability are associated with regulation of transcription factors in different contexts to promote cancer progression.
Acetyl-CoA metabolism in non-malignant cells is also involved in tumor growth
The roles of acetyl-CoA metabolism in cells within the tumor microenvironment are beginning to be elucidated and will be important to consider in any strategy that targets acetyl-CoA metabolic enzymes. For example, macrophages can take on different phenotypes depending on exogenous stimuli, which can allow them to either promote or oppose tumor growth. While tumor-associated macrophage (TAM) phenotypes are complex and incompletely described by conventional M1 and M2 phenotypes112, M2-like macrophage polarization is generally associated with immune suppression, and ACLY has been shown using both inhibitors and gene knockout to facilitate acquisition of the M2 phenotype 69, 113–115 (Fig. 4). As such, while wild type BMDMs exposed to M2 polarizing stimuli and co-injected with tumor cells promote tumor growth, ACLY-deficient cells do not114. On the other hand, however, growth of tumors implanted into myeloid-specific Acly knockout versus WT mice was not different, even though TAM phenotypes were slightly altered115. Finally, activation of macrophages by CpG DNA, which leads to a macrophage phenotype distinct from either M1 or M2 polarization, stimulates phagocytosis of tumor cells and thereby suppresses tumor growth, in a manner suppressed by inhibitors of fatty acid oxidation and ACLY116. Thus, ACLY may facilitate different functions of macrophages, highlighting that a more complete understanding of ACLY’s role in macrophages in vivo within the tumor microenvironment is needed.
Substantial evidence also points to crucial roles for acetyl-CoA metabolism in T cell biology. CD8-positive T cells (or cytotoxic T lymphocytes) are mediators of adaptive immunity that can kill cancer cells. Activated T cells increase their utilization of glucose, which is required to increase IFN-γ cytokine production through both translational and epigenetic (histone acetylation-dependent [Fig. 4]) mechanisms117–119. This essentially establishes a competition for glucose between T-cells and tumor cells in the microenvironment, which could restrict T-cell function, although this notion has recently been challenged by in vivo studies using PET tracers, which demonstrated preferential uptake of glucose into immune cells over cancer cells120. T-cell impairment caused by glucose restriction is rescued by ex vivo acetate supplementation in an ACSS2-dependent manner121. Additionally, IL-12 stimulation boosts acetyl-CoA production and IFNγ production in T cells exposed to tumor conditioned medium, and either IL-12 or high pyruvate boosted anti-tumor activity of CD8+ T cells in an ACLY-dependent manner upon adoptive transfer into tumor-bearing mice119. CD8+ T cells also require a functional DNL pathway to proliferate, which was determined by conditional Acc1 deletion specifically in CD8+ T cells122. Thus, acetyl-CoA metabolic enzymes are emerging as critical regulators of tumor localized immune cell function. The contribution of acetyl-CoA metabolism in other cells of the TME such as fibroblasts, adipocytes, and B cells, remains understudied.
Tissue-Specific Acetyl-CoA Regulation
A key question of growing importance in cancer biology research is how tumor metabolism is influenced by the metabolism of its cell or tissue of origin123. Thus, there is strong rationale for understanding how acetyl-CoA regulation is normally controlled in cell and tissue lineages from which cancer cells arise. Recent studies are yielding critical information about the tissue-specific requirements for these enzymes in carbohydrate and lipid metabolism, which is additionally important for informing on how pharmacological inhibitors of acetyl-CoA metabolism could impact metabolic homeostasis. Here we focus on liver and pancreas since tumors arise in these tissues and substantial information on acetyl-CoA metabolism is available through studies using genetic models. We also discuss adipose tissue; while adipocytes only rarely become cancerous, adipose tissue activity can impact tumor growth locally and at a distance.
Liver
Non-alcoholic fatty liver disease (NAFLD) has become a leading risk factor for hepatocellular 81carcinoma (HCC), a deadly cancer currently accounting for the 4th highest number of cancer deaths worldwide. Incidence of HCC has been rising in recent decades, and in particular, the percentage of HCC cases linked to NAFLD has risen dramatically (e.g., in the UK, NAFLD-related HCC cases rose from 10% to 35% between 2000 and 2017)124. NAFLD has become a widespread condition, affecting about a quarter of the world’s population and the majority of people with obesity or T2DM124; thus, understanding mechanisms linking obesity and NAFLD to HCC is of high clinical importance (Box 2).
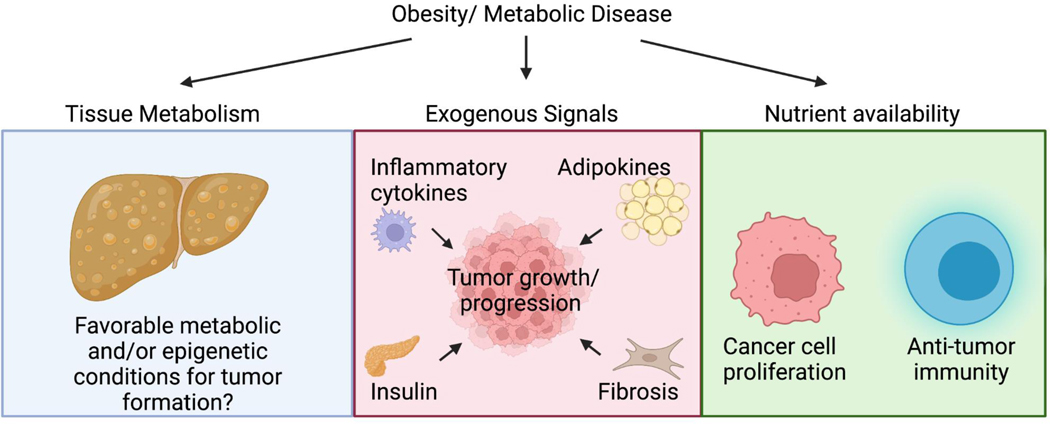
Box 2 |. Obesity, Cancer and Acetyl-CoA regulation:
Obesity is associated with increased risk of death from several types of cancer. While the specific roles of acetyl-CoA metabolism in the link between obesity and cancer is relatively little studied, several reports suggest that it may be an important node. For example, diet-dependent changes in tissue metabolism could contribute to or suppress tumor formation, potentially through changes in acetyl-CoA production or use (blue panel). Along these lines, high fat diet both promotes liver tumorigenesis and has been shown to induce changes in glucose metabolism in the liver in a manner similar to that in hepatocellular carcinoma125. The de novo lipogenesis (DNL) pathway is itself potently regulated by diet in tissues such as liver, with fat suppressing and sugar upregulating lipogenesis genes. Fructose has been implicated in promoting DNL and tumorigenesis in both hepatocellular carcinoma and colorectal cancer130, 194. Notably, ACC activation is sufficient to potentiate carcinogen-induced liver tumorigenesis, while ACC inhibition suppresses tumor growth81. Thus, such changes in tissue metabolism might promote or limit the ability of tumors to form. Changes in tissue metabolism could plausibly also induce epigenetic alterations (e.g., in histone acetylation) to position cells in a favorable context for transformation. A more well established mechanism linking obesity and tumor growth is via altered exogenous signaling cues which may impact tumor growth and progression through effects on tumor cells and non-malignant cells in the tumour microenvironment (red panel). Relevant to acetyl-CoA metabolism, increased insulin/IGF signalling can activate AKT-ACLY signalling, which can influence histone acetylation67, 70, and adipokines such as leptin and angiotensin II have been proposed to drive changes in acetyl-CoA metabolism in cancers, impacting gene expression programs to promote tumor progression33, 109. Finally, changes in nutrient availability could impact nutrient utilization in cancer cells and non-malignant cells in the tumor (green panel). While knowledge is still limited in this area as pertaining to acetyl-CoA metabolism, one study has shown that high fat diet potentiates tumorigenesis in part by suppressing PHD3, resulting in ACC2 inhibition and fatty acid oxidation in cancer cells. Notably, this impacted anti-tumor immune responses since overexpression of PHD3 in cancer cells promoted CD8+ T cell infiltration and reduced tumor growth186.
One metabolic commonality between NAFLD and HCC which supports tumor growth is elevated DNL125–127,48, 128. Moreover, fructose, a potent stimulator of hepatic DNL and major component of the modern diet129, promotes NAFLD and can potentiate HCC in mice130. Thus, defining sources of lipogenic acetyl-CoA and their contributions to DNL could inform strategies to prevent or treat NAFLD and possibly HCC.
Since ACLY links carbohydrate and lipid metabolism through citrate conversion to acetyl-CoA (Fig. 1), it might be anticipated that loss of hepatic Acly would protect against toxic lipid accumulation, particularly driven by diets high in fructose. However, different studies have reached different conclusions about the benefits of targeting hepatic ACLY, which might be explained by the distinct dietary contexts examined. For example, liver specific Acly deficiency surprisingly had no effect on hepatic triglyceride levels or on rates of de novo lipogenesis, as assessed by D2O tracing, either under standard chow-fed conditions or when mice were given sweetened drinking water (50% glucose: 50% fructose) 23. This is because ACSS2 is upregulated by ACLY loss in the context of high fructose consumption, and microbiota-generated acetate, which is converted to acetyl-CoA by ACSS2 in the liver, abundantly supplies lipogenic acetyl-CoA23. Notably, using stable isotope tracing in conjunction with ACSS2 silencing or antibiotics treatment, ACSS2 was found to be required for fructose-dependent DNL if it was consumed rapidly as a bolus; this is because fructose reached and was metabolized directly to acetate by the gut microbiota in this context. If fructose was consumed more gradually on the other hand, this led to flexible use of acetyl-CoA generated either through ACLY or ACSS223. These findings are consistent with recent stable isotope infusion studies in mice, which demonstrated that the liver uses predominantly lactate (which would presumably enter the lipogenic acetyl-CoA pool via ACLY) and acetate to supply acetyl-CoA for fatty acid synthesis131. On the other hand, reduced hepatic lipid accumulation with ACLY deficiency or inhibition with bempedoic acid was observed in a mouse model of NASH that involved high fat, high fructose feeding and thermoneutral conditions132. Interestingly, the effect of bempedoic acid was stronger than that of Acly deletion, which could potentially be due to effects of bempedoic acid in cells other than hepatocytes such as hepatic stellate cells132, or due to effects of bempedoic acid independent of ACLY. Additionally, genetically obese db/db mice have reduced lipid accumulation in the liver upon ACLY silencing133. Intriguingly, on a high fat diet, ACLY deficiency actually resulted in elevated de novo lipogenesis and increased hepatic lipids, which was attributed to upregulation of SREBP1c134. Further work is needed to more completely define the mechanisms through which bempedoic acid reduces NASH, as well as the impact of diet, the microbiota, and other environmental factors such as temperature, on hepatic dependence on ACLY versus ACSS2 for lipogenesis.
In contast to ACLY, which is required for embryonic development135, whole body ACSS2 knockout mice are viable and phenotypically normal on a laboratory chow diet32. The fact that they are viable suggests a promising therapeutic window for ACSS2 inhibitors, and importantly, these mice have reduced tumor burden in a liver cancer model50. ACSS2 knockout mice are resistant to obesity and hepatic steatosis when fed a high fat diet, which is associated with lipid metabolic reprogramming in several tissues, including the liver32. Tissue-specific knockout models will be useful in elucidating the direct roles of ACSS2 in cancer cells versus anti-cancer effects that may be exerted via changes in systemic metabolism or in other non-malignant cell types. Cumulatively, the emerging evidence indicates that dietary regimens should be considered when developing and deploying ACLY and ACSS2 therapeutics, though as discussed, more systematic study of the interplay between diet and acetyl-CoA production in liver is needed to inform such potential strategies.
ACC is necessary for committing acetyl-CoA to DNL, and hepatic DNL is suppressed upon its targeting. While whole body ACC1 knockout mice die embryonically136, Acc1 deficiency in the liver does not impair malonyl-CoA levels or DNL due to upregulation of ACC2, suggesting that malonyl-CoA pools can be used flexibly137. Genetic deletion of both ACC1 and ACC2, however, led to reduced liver triglycerides but also elevated plasma triglycerides on chow, high fat, and Western diets, an effect that was also observed in both rodents and humans given a liver targeted ACC inhibitor138. Mechanistically, the elevation in plasma triglyceride was found to be due to reduced synthesis of PUFAs from essential fatty acids, which depends on malonyl-CoA; this PUFA reduction triggered upregulation of SREBP1c and VLDL secretion138. Other studies have similarly reported reduced hepatic lipid levels with ACC inhibition or targeting by antisense oligonucleotides targeting ACC1 and 2 in rodent models81, 139, 140. Reciprocally, as previously noted, loss of the ability to suppress ACC by phosphorylation led to hepatic lipid accumulation82. While the preponderance of evidence supports a model in which inhibiting ACC reduces hepatic lipids, not all studies are in agreement141. Elevated DNL in HCC and the association of HCC with NAFLD and NASH make targeting ACC attractive for this cancer, although different studies investigating this have reached different conclusions. One study using genetic knockout of ACC1 and ACC2 reported elevated tumor formation in mice exposed to the carcinogen DEN, mechanistically implicating the upregulation of antioxidant defenses142. In another study, however, ACC activation enhanced, and an ACC inhibitor reduced liver tumorigenesis81. More work is needed to understand the reasons underlying these different results. Cumulatively, the data point to ACC-dependent DNL as important in both experimental models and humans for hepatic lipid accumulation, making it a target deserving further investigation for its potential in combatting HCC.
Pancreas
A unique feature of the pancreas is that acetyl-CoA pools in acinar cells are derived extensively from the BCAA leucine67, which when catabolized, produces 3 molecules of acetyl-CoA per leucine molecule143 (Fig. 1). Consistently, infusion of 13C-labeled BCAAs in mice revealed that the TCA cycle in the pancreas is heavily fed by BCAAs144. This is interesting in the context of pancreatic cancer because acinar cells are a potential cell type of origin for pancreatic ductal adenocarcinoma (PDA), and moreover, plasma BCAAs are reportedly elevated in individuals who develop pancreatic cancer years preceding their diagnosis145. Kras mutation, which is observed in nearly all cases of human PDA, drives an increase in acinar cell acetyl-CoA abundance in an ACLY-dependent manner, and consistently, Acly deletion reduces tumor formation without impacting normal pancreatic endocrine or exocrine function67. Similarly, pancreatic deletion of Bcat2, which mediates the transamination of BCAAs preceding their mitochondrial catabolism, also protects against PDA 146 further suggesting a role for pancreatic BCAA utilization in early stages of pancreatic tumorigenesis. While ACLY and BCAT2 have emerged as metabolic factors that are needed for efficient tumor formation, the molecular mechanisms through which BCAA and acetyl-CoA metabolism impact pancreatic tumorigenesis largely remain to be defined.
Adipose tissues
In general, adipose tissues come in two varieties, energy storing white adipose tissue (WAT) and thermogenic brown adipose tissue (BAT), both of which additionally secrete endocrine signals that have powerful influences on systemic metabolism147. Adipose tissues have roles in tumor growth through several mechanisms, including providing fatty acids as an energy source to cancer cells, secreting growth-promoting adipokines, modulating systemic insulin sensitivity and glucose homeostasis148, and possibly contributing to the tumor microenvironment following tumor-induced de-differentiation and remodeling into myofibroblasts and macrophage-like cells149. Interestingly, activation of brown adipose tissue was recently shown to suppress tumor growth in mice due to avid glucose uptake, which limited glucose availability to tumors150. Notably, this study also showed that cold exposure in a human patient reduced tumor uptake of 18F-fluorodeoxyglucose150. Therapeutically stimulating brown fat is under intense investigation as an anti-obesity strategy; these new data suggest an unexpected link between non-shivering thermogenesis and tumor growth that may also have therapeutic implications.
In a brown adipocyte differentiation model, ACLY is required for differentiation and lipogenesis in vitro, which correlates with defective histone acetylation and is partially rescued by acetate supplementation68. Interestingly conditional Acly KO in vivo in fully differentiated mature brown adipocytes results in a whitened phenotype with abnormal lipid accumulation68. On the other hand, deleting Acly in all adipocytes results in impairments in handling of dietary carbohydrates, with mice exhibiting reduced white adipose mass, accumulation of hepatic lipids and development of insulin resistance when challenged with high carbohydrate diets151. This is consistent with other evidence that adipose DNL plays important roles in whole body insulin sensitivity152, even though most lipids in adipose tissue are diet-derived or synthesized in the liver. Whether these roles of ACLY in adipose tissue impact tumor progression is unclear, though interestingly, the lipogenic program was suppressed in adipocytes co-cultured with pancreatic cancer cells153, suggesting the possibility that cancer cells might trigger alterations in adipocyte metabolism.
Potential for therapeutic targeting of acetyl-CoA metabolic enzymes in cancer
ACLY
Interest in ACLY as therapeutic target has existed for decades, beginning with the identification of (−)-hydroxycitrate (HC), an ACLY inhibitor which was extracted from the tropical fruit Garcinia cambogia in the 1960s154. HC is available over the counter as a dietary supplement and has attracted interest as a weight loss agent based on favorable metabolic effects in both rodents and humans155, 156. However, a randomized controlled trial failed to find effects of HC in promoting weight loss or fat loss 157. HC has also been investigated as a calorie restriction mimetic (CRM) due to its ability to promote autophagy, which occurs downstream of depletion of acetyl-CoA and acetyl-lysine158, 159. Caloric restriction has been shown to reduce tumor growth160, 161, 162, suggesting the potential for CRMs such as HC for use in oncology. In one study, HC was shown to improve chemotherapy efficacy in a fibrosarcoma subcutaneous allograft model in an autophagy-dependent manner163. This is because immunogenic chemotherapy regimens depend on tumor cell autophagy, which promotes ATP release from the dying tumor cell and immune cell recruitment to the tumor164. It should be noted, however, that context is likely critical for these effects, as autophagy also contributes to immune evasion, and substantial evidence also indicates that inhibiting- rather than promoting- autophagy can exert anti-cancer effects165, 166.
Beyond HC, accumulating evidence suggests that ACLY genetic loss or inhibition can suppress cancer cell proliferation and tumor growth in mice67, 75, 95–97, 111, 167, 168. In terms of in vivo cancer studies using inhibitors, the evidence to date is mainly from xenograft studies, with the inhibitor SB-204990169 shown to suppress growth of both human and murine xenograft tumors75, 96. Another inhibitor, BMS-303141, also suppressed HepG2 xenograft tumor growth when combined with sorafenib167. However, these inhibitors, as well as other inhibitors evaluated in preclinical studies focused on metabolic diseases, have not progressed to clinical trials, at least in part due to poor bioavailability and low target specificity170. The only ACLY inhibitor currently in clinical use is bempedoic acid (NEXLETOL®), which is well tolerated and is FDA approved100 for familial hypercholesterolemia and cardiovascular disease. Bempedoic acid is a pro-drug that is converted to the active molecule bempedoyl-CoA by the hepatic enzyme ACSVL1, and thus it acts specifically in the liver 171. ACSVL1 is expressed in at least a subset of human HCC tumors172, suggesting the possibility that it could be used against HCC. Promisingly, a recent study reported suppression of tumor growth in carcinogen-induced mouse model of HCC using bempedoic acid, and this was enhanced by combining bempedoic acid with anti-PD-L1173. Bempedoic acid or ACLY silencing was also found to suppress metastasis in a colorectal cancer model174. It is important to note that more work is needed to understand if the effects of bempedoic acid and other ACLY inhibitors on tumor growth occur via ACLY-dependent or -independent effects. Bempedoic acid also is known to activate AMPK175, for example, which could well contribute to anti-cancer effects176–178. In sum, however, accumulating preclinical evidence supports ACLY as an attractive target for cancer treatment.
In addition to the currently available ACLY inhibitors, the recent solving of the ACLY homo-tetramer structure by three separate groups opens exciting new potential for inhibiting this enzyme with small molecules179–181. Notably, one study reports developing a novel ACLY inhibitor (NDI-091143) that acts allosterically179, although effects in cells or mice were not reported. In addition to development of improved inhibitors, determining the necessity for ACLY for the normal functioning of cells and tissues of the adult mammal is needed to help identify potential toxicities. Generation of inducible whole-body knockout models could help identify potential side effects associated with systemic ACLY inhibition in adults.
ACSS2:
ACSS2 has also garnered interest as a therapeutic target, especially because ACSS2 KO mice are viable, suggesting a favorable therapeutic window 32. ACSS2 expression is elevated in several cancer types and ACSS2 loss or inhibition shows anti-cancer effects in mouse models of hepatocellular carcinoma (HCC), breast cancer, and multiple myeloma45, 47, 50, 98. These studies have implicated ACSS2’s roles in lipid synthesis and/or regulation of gene expression in promoting tumor growth. An ACSS2 inhibitor called MTB-9655 has recently entered phase I clinical trials in patients with advanced solid tumors (NCT04990739). Overall, ACSS2 is a promising target, underlining the importance of clarifying its mechanistic roles in different cancers, as well as its interactions with diet and other therapeutics.
Acetyl-CoA carboxylase
ACC inhibition suppresses DNL, making it an attractive therapeutic target. Inhibiting ACC has anti-cancer effects in several malignancies, including lung, liver, and lymphoid81, 182, 183, Several ACC inhibitors are currently in clinical trials for non-alcoholic steatohepatitis6, 184. However, in some preclinical models, ACC inhibition could promote tumor progression or impede response to other therapeutics through acetyl-CoA rerouting. For example, ACC inhibition increases acetylation of Smad2 to promote EMT 109. AMPK-dependent ACC inactivation was also shown to reduce the synthesis of polyunsaturated fatty acid (PUFA)-containing lipids, promoting resistance to ferroptosis 185. Moreover, ACC2 inhibition due to PHD3 loss or suppression promotes tumor growth in AML and obesity-linked colon cancer models83, 186. Thus, ACC inhibition holds potential to suppress tumorigenesis, although it may not do so in every context, as noted above. For a comprehensive discussion of lipogenesis inhibitors, we refer the reader to an excellent recent review6.
Conclusions and perspectives
Acetyl-CoA metabolism has been a subject of therapeutic interest for decades; yet its mysteries are still being uncovered. As we have discussed throughout this article, acetyl-CoA production and utilization is dependent on various factors, including nutrient availability, metabolite signals, hormonal and growth factor cues, and cell type. While evidence argues that inhibiting acetyl-CoA metabolic enzymes may be differentially effective depending on these factors, current understanding of how nutrition might be optimally combined with acetyl-CoA pathway inhibitors is limited. Moreover, the contexts in which acetyl-CoA metabolic enzymes can compensate for one another versus serving in non-redundant capacities is poorly defined. Further defining key fates of acetyl-CoA that support tumor growth and potential compensatory mechanisms could identify new targetable vulnerabilities. Genetic or chemical screening approaches aimed at identifying synthetic lethal interactions will be helpful in uncovering such vulnerabilities. Finally, the roles of acetyl-CoA metabolism in non-malignant cell types- including those in the tumor microenvironment and those that regulate systemic metabolism- require continued investigation to better understand how they impact tumor growth.
Despite decades of work investigating acetyl-CoA metabolism, gaps still exist in understanding basic acetyl-CoA biology. Armed with a variety of new approaches to study localized metabolite pools187, researchers are just beginning to probe how different perturbations impact acetyl-CoA metabolism in different cellular compartments. In particular, the significance of acetyl-CoA pools in the endoplasmic reticulum and peroxisome remain poorly defined, although recent evidence suggests important biological functions10, 188, 189. Moreover, metabolic regulation has been observed for other acetyl-metabolites such as N-acetylaspartate, N-acetylcysteine, and N-acetylglutamate190, 191, as well as for RNA acetylation (N4-acetylcytidine; ac4C)192, and these topics represent highly interesting areas for further study. How acetyl-CoA-dependent enzymes interact with other acyl-CoAs is also emerging as another potentially important node of metabolic regulation; many acetyl-CoA-dependent enzymes also interact with CoA and other short and/or long chain acyl-CoAs192, 193, which may either result in competitive inhibition of these enzymes or use as alternative substrates (e.g., some KATs can also mediate acylations beyond acetylation21). The field is now poised to make rapid progress in further elucidating these basic biological roles of acetyl-CoA and to hopefully translate these advances towards strategies to help cancer patients.
Acknowledgements
KEW and DAG are supported by R01DK116005. KEW is also supported by R01CA228339, R01CA174761, R01CA248315, and R01CA262055. DAG is also supported by R01DK094004 and R01DK127175.
Glossary
- Thioester bond
Chemical bond using a sulfur instead of oxygen to connect the carboxylate ester, e.g. R-CO-S-R’
- Reductive carboxylation
a reductive pathway of glutamine metabolism in which isocitrate dehydrogenases 1 or 2 operate in reverse to generate isocitrate/citrate from alpha-ketoglutarate and CO2
- De novo lipogenesis
or DNL, is the process of building fatty acids from the non-lipid precursor acetyl-CoA, which can be generated by a variety of pathways but most commonly from carbohydrates
- Ubiquitylation
The post-translational modification process of attaching a ubiquitin to lysine residue, which can function as a regulator signal or form poly-ubiquitin chains targeting a protein for degradation
- Statin
cholesterol lowering drugs that target HMG-CoA reductase
- Mevalonate Pathway
Named after its key intermediate, the five carbon molecule mevalonate, this pathway generates precursors to a large family of isoprenoids that include cholesterol and coenzyme Q10
- Coenzyme Q (CoQ)
Also known as ubiquinone, comprised of a redox active quinone head group and isoprenoid tail synthesized in the mevalonate pathway, that functions as an electron carrier as part of the electron transport chain and as an antioxidant
- Adipokine
An adipocyte derived factor released into the blood that can function in an autocrine or paracrine manner to regulate metabolism
- M2 (and M1) Macrophage
Classification system in which heterogenous macrophages are grouped as either M1, which defines activated proinflammatory macrophages associated with bacteria and virus protection, and M2, which are alternatively activated macrophages that express different markers and are associated with healing and metabolism
- Epigenetic
Refers to non-genetic factors (factors that do not involve changes to DNA sequence) that can influence gene expression, such as histone acetylation and methylation
- PET tracers
Positron emission tomography tracers are chemicals than contain a positron emitting radioisotope that is used to image tumors, such as 18F-fluorodeoxyglucose (18F-FDG), which is a non-metabolizable analog of glucose that can image tumors with high glucose uptake rates
- Stable Isotope Tracing
Technique used to follow the metabolic fate of a tracer molecule delivered to cells or tissues (such as 13C-glucose or 13C-15N-glutamine) in which one or more of the abundant and naturally occurring element (usually C, H, or N) are replaced with less abundant non-radioactive isotopes that can be distinguished in mass by a mass spectrometer
Footnotes
Competing interests
DAG and KEW have no competing interests.
References
- 1.Walsh CT, Tu BP & Tang Y. Eight Kinetically Stable but Thermodynamically Activated Molecules that Power Cell Metabolism. Chem Rev 118, 1460–1494 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sugden MC & Holness MJ Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs. Am J Physiol Endocrinol Metab 284, E855–62 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Adina-Zada A. et al. Allosteric regulation of the biotin-dependent enzyme pyruvate carboxylase by acetyl-CoA. Biochem Soc Trans 40, 567–72 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Martin WF Older Than Genes: The Acetyl CoA Pathway and Origins. Front Microbiol 11, 817 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Currie E, Schulze A, Zechner R, Walther TC & Farese RV Jr. Cellular Fatty Acid Metabolism and Cancer. Cell metabolism (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batchuluun B, Pinkosky SL & Steinberg GR Lipogenesis inhibitors: therapeutic opportunities and challenges. Nat Rev Drug Discov 21, 283–305 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Longo J, van Leeuwen JE, Elbaz M, Branchard E. & Penn LZ Statins as Anticancer Agents in the Era of Precision Medicine. Clin Cancer Res 26, 5791–5800 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Vasan K, Werner M. & Chandel NS Mitochondrial Metabolism as a Target for Cancer Therapy. Cell Metab 32, 341–352 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stacpoole PW Therapeutic Targeting of the Pyruvate Dehydrogenase Complex/Pyruvate Dehydrogenase Kinase (PDC/PDK) Axis in Cancer. J Natl Cancer Inst 109 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Jonas MC, Pehar M. & Puglielli L. AT-1 is the ER membrane acetyl-CoA transporter and is essential for cell viability. J Cell Sci 123, 3378–88 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rohrig F. & Schulze A. The multifaceted roles of fatty acid synthesis in cancer. Nat Rev Cancer 16, 732–749 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Campbell SL & Wellen KE Metabolic Signaling to the Nucleus in Cancer. Mol Cell 71, 398–408 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Srere PA & Bhaduri A. Incorporation of radioactive citrate into fatty acids. Biochim Biophys Acta 59, 487–9 (1962). [DOI] [PubMed] [Google Scholar]
- 14.Bhaduri A. & Srere PA The incorporation of citrate carbon into fatty acids. Biochim Biophys Acta 70, 221–30 (1963). [DOI] [PubMed] [Google Scholar]
- 15.Metallo CM et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature 481, 380–4 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mullen AR et al. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature 481, 385–8 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wise DR et al. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of alpha-ketoglutarate to citrate to support cell growth and viability. Proc Natl Acad Sci U S A 108, 19611–6 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar A. et al. NaCT/SLC13A5 facilitates citrate import and metabolism under nutrient-limited conditions. Cell Rep 36, 109701 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X. et al. Acetate Production from Glucose and Coupling to Mitochondrial Metabolism in Mammals. Cell (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vysochan A, Sengupta A, Weljie AM, Alwine JC & Yu Y. ACSS2-mediated acetyl-CoA synthesis from acetate is necessary for human cytomegalovirus infection. Proc Natl Acad Sci U S A 114, E1528–E1535 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trefely S, Lovell CD, Snyder NW & Wellen KE Compartmentalised acyl-CoA metabolism and roles in chromatin regulation. Mol Metab 38, 100941 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao S. et al. ATP-Citrate Lyase Controls a Glucose-to-Acetate Metabolic Switch. Cell Rep 17, 1037–1052 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao S. et al. Dietary fructose feeds hepatic lipogenesis via microbiota-derived acetate. Nature 579, 586–591 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choudhary C, Weinert BT, Nishida Y, Verdin E. & Mann M. The growing landscape of lysine acetylation links metabolism and cell signalling. Nat Rev Mol Cell Biol 15, 536–50 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Wellen KE et al. ATP-citrate lyase links cellular metabolism to histone acetylation. Science 324, 1076–80 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee JV et al. Akt-dependent metabolic reprogramming regulates tumor cell histone acetylation. Cell Metab 20, 306–19 (2014). This study demonstrated that AKT-dependent phosphorylation of ACLY at serine 455 promotes maintenance of acetyl-CoA production and histone acetylation under glucose limitation and that pAKT-Ser473 correlates with histone acetylation levels in human tumors.
- 27.Takahashi H, McCaffery JM, Irizarry RA & Boeke JD Nucleocytosolic acetyl-coenzyme a synthetase is required for histone acetylation and global transcription. Molecular cell 23, 207–17 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Sivanand S, Viney I. & Wellen KE Spatiotemporal Control of Acetyl-CoA Metabolism in Chromatin Regulation. Trends Biochem Sci 43, 61–74 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sivanand S. et al. Nuclear Acetyl-CoA Production by ACLY Promotes Homologous Recombination. Mol Cell 67, 252–265 e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mews P. et al. Acetyl-CoA synthetase regulates histone acetylation and hippocampal memory. Nature 546, 381–386 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bulusu V. et al. Acetate Recapturing by Nuclear Acetyl-CoA Synthetase 2 Prevents Loss of Histone Acetylation during Oxygen and Serum Limitation. Cell Rep 18, 647–658 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang Z. et al. ACSS2 promotes systemic fat storage and utilization through selective regulation of genes involved in lipid metabolism. Proc Natl Acad Sci U S A 115, E9499–E9506 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li X. et al. Nucleus-Translocated ACSS2 Promotes Gene Transcription for Lysosomal Biogenesis and Autophagy. Mol Cell 66, 684–697 e9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mendoza M. et al. Enzymatic transfer of acetate on histones from lysine reservoir sites to lysine activating sites. Sci Adv 8, eabj5688 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ye C. & Tu BP Sink into the Epigenome: Histones as Repositories That Influence Cellular Metabolism. Trends Endocrinol Metab 29, 626–637 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sutendra G. et al. A Nuclear Pyruvate Dehydrogenase Complex Is Important for the Generation of Acetyl-CoA and Histone Acetylation. Cell 158, 84–97 (2014). [DOI] [PubMed] [Google Scholar]
- 37.Nagaraj R. et al. Nuclear Localization of Mitochondrial TCA Cycle Enzymes as a Critical Step in Mammalian Zygotic Genome Activation. Cell 168, 210–223 e11 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen J. et al. Compartmentalized activities of the pyruvate dehydrogenase complex sustain lipogenesis in prostate cancer. Nat Genet 50, 219–228 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zervopoulos SD et al. MFN2-driven mitochondria-to-nucleus tethering allows a non-canonical nuclear entry pathway of the mitochondrial pyruvate dehydrogenase complex. Mol Cell 82, 1066–1077 e7 (2022). [DOI] [PubMed] [Google Scholar]
- 40.Madiraju P, Pande SV, Prentki M. & Madiraju SR Mitochondrial acetylcarnitine provides acetyl groups for nuclear histone acetylation. Epigenetics 4, 399–403 (2009). [DOI] [PubMed] [Google Scholar]
- 41.Duvel K. et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell 39, 171–83 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Porstmann T. et al. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab 8, 224–36 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeBose-Boyd RA & Ye J. SREBPs in Lipid Metabolism, Insulin Signaling, and Beyond. Trends Biochem Sci 43, 358–368 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gouw AM et al. The MYC Oncogene Cooperates with Sterol-Regulated Element-Binding Protein to Regulate Lipogenesis Essential for Neoplastic Growth. Cell Metab 30, 556–572 e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schug ZT et al. Acetyl-CoA synthetase 2 promotes acetate utilization and maintains cancer cell growth under metabolic stress. Cancer Cell 27, 57–71 (2015). This work demonstrated that ACSS2 is upregulated under lipid- and oxygen-deprived conditions to promote acetate-dependent de novo lipogenesis and support tumor growth.
- 46.Kondo A. et al. Extracellular Acidic pH Activates the Sterol Regulatory Element-Binding Protein 2 to Promote Tumor Progression. Cell Rep 18, 2228–2242 (2017). [DOI] [PubMed] [Google Scholar]
- 47.Li Z. et al. Acetyl-CoA Synthetase 2: A Critical Linkage in Obesity-Induced Tumorigenesis in Myeloma. Cell Metab 33, 78–93 e7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ning Z. et al. USP22 regulates lipidome accumulation by stabilizing PPARgamma in hepatocellular carcinoma. Nat Commun 13, 2187 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Odera JO et al. NRF2/ACSS2 axis mediates the metabolic effect of alcohol drinking on esophageal squamous cell carcinoma. Biochem J 477, 3075–3089 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Comerford SA et al. Acetate dependence of tumors. Cell 159, 1591–602 (2014). This study found that ACSS2 is crucial for acetate capturing for both lipid synthesis and histone acetylation and that mice lacking ACSS2 exhibited reduced liver tumor burden.
- 51. Mashimo T. et al. Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell 159, 1603–14 (2014). Tracing studies in human glioblastoma revealed acetate oxidation in tumors.
- 52.Zhang S, He J, Jia Z, Yan Z. & Yang J. Acetyl-CoA synthetase 2 enhances tumorigenesis and is indicative of a poor prognosis for patients with renal cell carcinoma. Urol Oncol 36, 243 e9–243 e20 (2018). [DOI] [PubMed] [Google Scholar]
- 53.Sun L. et al. Decreased expression of acetyl-CoA synthase 2 promotes metastasis and predicts poor prognosis in hepatocellular carcinoma. Cancer Sci 108, 1338–1346 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bae JM et al. Downregulation of acetyl-CoA synthetase 2 is a metabolic hallmark of tumor progression and aggressiveness in colorectal carcinoma. Mod Pathol 30, 267–277 (2017). [DOI] [PubMed] [Google Scholar]
- 55.Chen Y. et al. ACLY: A biomarker of recurrence in breast cancer. Pathol Res Pract 216, 153076 (2020). [DOI] [PubMed] [Google Scholar]
- 56.Xu Y. et al. Identification and integrative analysis of ACLY and related gene panels associated with immune microenvironment reveal prognostic significance in hepatocellular carcinoma. Cancer Cell Int 21, 409 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wei X. et al. Targeting ACLY Attenuates Tumor Growth and Acquired Cisplatin Resistance in Ovarian Cancer by Inhibiting the PI3K-AKT Pathway and Activating the AMPK-ROS Pathway. Front Oncol 11, 642229 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang J. et al. Low expression of ACLY associates with favorable prognosis in acute myeloid leukemia. J Transl Med 17, 149 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Potapova IA, El-Maghrabi MR, Doronin SV & Benjamin WB Phosphorylation of recombinant human ATP:citrate lyase by cAMP-dependent protein kinase abolishes homotropic allosteric regulation of the enzyme by citrate and increases the enzyme activity. Allosteric activation of ATP:citrate lyase by phosphorylated sugars. Biochemistry 39, 1169–79 (2000). [DOI] [PubMed] [Google Scholar]
- 60.Guy PS, Cohen P. & Hardie DG Purification and physicochemical properties of ATP citrate (pro-3S) lyase from lactating rat mammary gland and studies of its reversible phosphorylation. Eur J Biochem 114, 399–405 (1981). [DOI] [PubMed] [Google Scholar]
- 61.Pentyala SN & Benjamin WB Effect of oxaloacetate and phosphorylation on ATP-citrate lyase activity. Biochemistry 34, 10961–9 (1995). [DOI] [PubMed] [Google Scholar]
- 62.Ranganathan NS, Srere PA & Linn TC Comparison of phospho- and dephospho-ATP citrate lyase. Arch Biochem Biophys 204, 52–8 (1980). [DOI] [PubMed] [Google Scholar]
- 63.Houston B. & Nimmo HG Effects of phosphorylation on the kinetic properties of rat liver ATP-citrate lyase. Biochim Biophys Acta 844, 233–9 (1985). [DOI] [PubMed] [Google Scholar]
- 64.White PJ et al. The BCKDH Kinase and Phosphatase Integrate BCAA and Lipid Metabolism via Regulation of ATP-Citrate Lyase. Cell Metab 27, 1281–1293 e7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Berwick DC, Hers I, Heesom KJ, Moule SK & Tavare JM The identification of ATP-citrate lyase as a protein kinase B (Akt) substrate in primary adipocytes. The Journal of biological chemistry 277, 33895–900 (2002). [DOI] [PubMed] [Google Scholar]
- 66.Guy PS, Cohen P. & Hardie DG Rat mammary gland ATP-citrate lyase is phosphorylated by cyclic amp-dependent protein kinase. FEBS Lett 109, 205–8 (1980). [DOI] [PubMed] [Google Scholar]
- 67. Carrer A. et al. Acetyl-CoA Metabolism Supports Multistep Pancreatic Tumorigenesis. Cancer Discov 9, 416–435 (2019). Mice lacking ACLY in pancreas displayed impaired acinar-to-ductal metaplasia and reduced pancreatic tumor formation in a genetic model of pancreatic cancer.
- 68.Martinez Calejman C. et al. mTORC2-AKT signaling to ATP-citrate lyase drives brown adipogenesis and de novo lipogenesis. Nat Commun 11, 575 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Covarrubias AJ et al. Akt-mTORC1 signaling regulates Acly to integrate metabolic input to control of macrophage activation. Elife 5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Senapati P. et al. Hyperinsulinemia promotes aberrant histone acetylation in triple-negative breast cancer. Epigenetics Chromatin 12, 44 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Basappa J. et al. ACLY is the novel signaling target of PIP2/PIP3 and Lyn in acute myeloid leukemia. Heliyon 6, e03910 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McCubrey JA et al. GSK-3 as potential target for therapeutic intervention in cancer. Oncotarget 5, 2881–911 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hughes K, Ramakrishna S, Benjamin WB & Woodgett JR Identification of multifunctional ATP-citrate lyase kinase as the alpha-isoform of glycogen synthase kinase-3. Biochem J 288 ( Pt 1), 309–14 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin R. et al. Acetylation Stabilizes ATP-Citrate Lyase to Promote Lipid Biosynthesis and Tumor Growth. Molecular cell 51, 506–18 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang C. et al. Cullin3-KLHL25 ubiquitin ligase targets ACLY for degradation to inhibit lipid synthesis and tumor progression. Genes Dev 30, 1956–70 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hallows WC, Lee S. & Denu JM Sirtuins deacetylate and activate mammalian acetyl-CoA synthetases. Proceedings of the National Academy of Sciences of the United States of America 103, 10230–5 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Akella NM, Ciraku L. & Reginato MJ Fueling the fire: emerging role of the hexosamine biosynthetic pathway in cancer. BMC Biol 17, 52 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ciraku L. et al. O-GlcNAc transferase regulates glioblastoma acetate metabolism via regulation of CDK5-dependent ACSS2 phosphorylation. Oncogene 41, 2122–2136 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hardie DG & Pan DA Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochem Soc Trans 30, 1064–70 (2002). [DOI] [PubMed] [Google Scholar]
- 80.Pinkosky SL et al. Long-chain fatty acyl-CoA esters regulate metabolism via allosteric control of AMPK beta1 isoforms. Nat Metab 2, 873–881 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lally JSV et al. Inhibition of Acetyl-CoA Carboxylase by Phosphorylation or the Inhibitor ND-654 Suppresses Lipogenesis and Hepatocellular Carcinoma. Cell Metab 29, 174–182 e5 (2019). This study employed mouse models in which ACC is constitutively active and an ACC inhibitor to reveal that ACC-dependent lipogenesis promotes hepatocellular carcinoma.
- 82.Fullerton MD et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat Med 19, 1649–54 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.German NJ et al. PHD3 Loss in Cancer Enables Metabolic Reliance on Fatty Acid Oxidation via Deactivation of ACC2. Mol Cell 63, 1006–20 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brownsey RW, Boone AN, Elliott JE, Kulpa JE & Lee WM Regulation of acetyl-CoA carboxylase. Biochem Soc Trans 34, 223–7 (2006). [DOI] [PubMed] [Google Scholar]
- 85.Longo J. et al. The mevalonate pathway is an actionable vulnerability of t(4;14)-positive multiple myeloma. Leukemia 35, 796–808 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moon SH et al. p53 Represses the Mevalonate Pathway to Mediate Tumor Suppression. Cell 176, 564–580 e19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Freed-Pastor WA et al. Mutant p53 disrupts mammary tissue architecture via the mevalonate pathway. Cell 148, 244–58 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Trefely S. et al. Subcellular metabolic pathway kinetics are revealed by correcting for artifactual post harvest metabolism. Mol Metab 30, 61–71 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Trefely S. et al. Quantitative subcellular acyl-CoA analysis reveals distinct nuclear metabolism and isoleucine-dependent histone propionylation. Mol Cell 82, 447–462 e6 (2022). This paper reported the development of a rigorous method for quantification of acyl-CoAs in sub-cellular compartments, including mitochondria, cytosol, and nucleus.
- 90.Storz P. Acinar cell plasticity and development of pancreatic ductal adenocarcinoma. Nat Rev Gastroenterol Hepatol 14, 296–304 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McGregor GH et al. Targeting the Metabolic Response to Statin-Mediated Oxidative Stress Produces a Synergistic Antitumor Response. Cancer Res 80, 175–188 (2020). [DOI] [PubMed] [Google Scholar]
- 92.Longo J. et al. An actionable sterol-regulated feedback loop modulates statin sensitivity in prostate cancer. Mol Metab 25, 119–130 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pandyra A. et al. Immediate utility of two approved agents to target both the metabolic mevalonate pathway and its restorative feedback loop. Cancer Res 74, 4772–82 (2014). [DOI] [PubMed] [Google Scholar]
- 94.Jiang W, Hu JW, He XR, Jin WL & He XY Statins: a repurposed drug to fight cancer. J Exp Clin Cancer Res 40, 241 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bauer DE, Hatzivassiliou G, Zhao F, Andreadis C. & Thompson CB ATP citrate lyase is an important component of cell growth and transformation. Oncogene 24, 6314–22 (2005). [DOI] [PubMed] [Google Scholar]
- 96. Hatzivassiliou G. et al. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer cell 8, 311–21 (2005). This study demonstrated that targeting ACLY either using RNAi or an inhibitor could suppress tumor growth.
- 97.Migita T. et al. ATP citrate lyase: activation and therapeutic implications in non-small cell lung cancer. Cancer research 68, 8547–54 (2008). [DOI] [PubMed] [Google Scholar]
- 98.Miller KD et al. Targeting ACSS2 with a Transition-State Mimetic Inhibits Triple-Negative Breast Cancer Growth. Cancer Res 81, 1252–1264 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stockwell BR et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 171, 273–285 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lei G, Zhuang L. & Gan B. Targeting ferroptosis as a vulnerability in cancer. Nat Rev Cancer 22, 381–396 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bersuker K. et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 575, 688–692 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mao C. et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature 593, 586–590 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Garcia-Bermudez J. et al. Squalene accumulation in cholesterol auxotrophic lymphomas prevents oxidative cell death. Nature 567, 118–122 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rysman E. et al. De novo lipogenesis protects cancer cells from free radicals and chemotherapeutics by promoting membrane lipid saturation. Cancer Res 70, 8117–26 (2010). [DOI] [PubMed] [Google Scholar]
- 105.Talebi A. et al. Sustained SREBP-1-dependent lipogenesis as a key mediator of resistance to BRAF-targeted therapy. Nat Commun 9, 2500 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jiang X, Stockwell BR & Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol 22, 266–282 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu JY & Wellen KE Advances into understanding metabolites as signaling molecules in cancer progression. Curr Opin Cell Biol 63, 144–153 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gao X. et al. Acetate functions as an epigenetic metabolite to promote lipid synthesis under hypoxia. Nat Commun 7, 11960 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rios Garcia M. et al. Acetyl-CoA Carboxylase 1-Dependent Protein Acetylation Controls Breast Cancer Metastasis and Recurrence. Cell Metab 26, 842–855 e5 (2017). [DOI] [PubMed] [Google Scholar]
- 110.Lu M. et al. ACOT12-Dependent Alteration of Acetyl-CoA Drives Hepatocellular Carcinoma Metastasis by Epigenetic Induction of Epithelial-Mesenchymal Transition. Cell Metab 29, 886–900 e5 (2019). [DOI] [PubMed] [Google Scholar]
- 111.Lee JV et al. Acetyl-CoA promotes glioblastoma cell adhesion and migration through Ca(2+)-NFAT signaling. Genes Dev 32, 497–511 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Geeraerts X. et al. Macrophages are metabolically heterogeneous within the tumor microenvironment. Cell Rep 37, 110171 (2021). [DOI] [PubMed] [Google Scholar]
- 113.Baardman J. et al. Macrophage ATP citrate lyase deficiency stabilizes atherosclerotic plaques. Nat Commun 11, 6296 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Noe JT et al. Lactate supports a metabolic-epigenetic link in macrophage polarization. Sci Adv 7, eabi8602 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.de Goede KE et al. Myeloid-Specific Acly Deletion Alters Macrophage Phenotype In Vitro and In Vivo without Affecting Tumor Growth. Cancers (Basel) 13 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu M. et al. Metabolic rewiring of macrophages by CpG potentiates clearance of cancer cells and overcomes tumor-expressed CD47-mediated ‘don’t-eat-me’ signal. Nat Immunol 20, 265–275 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chang CH et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 153, 1239–51 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Peng M. et al. Aerobic glycolysis promotes T helper 1 cell differentiation through an epigenetic mechanism. Science 354, 481–484 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chowdhury S. et al. Intracellular Acetyl CoA Potentiates the Therapeutic Efficacy of Antitumor CD8+ T Cells. Cancer Res 82, 2640–2655 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Reinfeld BI et al. Cell-programmed nutrient partitioning in the tumour microenvironment. Nature 593, 282–288 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Qiu J. et al. Acetate Promotes T Cell Effector Function during Glucose Restriction. Cell Rep 27, 2063–2074 e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lee J. et al. Regulator of fatty acid metabolism, acetyl coenzyme a carboxylase 1, controls T cell immunity. J Immunol 192, 3190–9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mayers JR & Vander Heiden MG Nature and Nurture: What Determines Tumor Metabolic Phenotypes? Cancer Res 77, 3131–3134 (2017). [DOI] [PubMed] [Google Scholar]
- 124.Shah PA, Patil R. & Harrison SA NAFLD-related hepatocellular carcinoma: The growing challenge. Hepatology (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Broadfield LA et al. Fat Induces Glucose Metabolism in Nontransformed Liver Cells and Promotes Liver Tumorigenesis. Cancer Res 81, 1988–2001 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sangineto M. et al. Lipid Metabolism in Development and Progression of Hepatocellular Carcinoma. Cancers (Basel) 12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Calvisi DF et al. Increased lipogenesis, induced by AKT-mTORC1-RPS6 signaling, promotes development of human hepatocellular carcinoma. Gastroenterology 140, 1071–83 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lambert JE, Ramos-Roman MA, Browning JD & Parks EJ Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology 146, 726–35 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Park G, Jung S, Wellen KE & Jang C. The interaction between the gut microbiota and dietary carbohydrates in nonalcoholic fatty liver disease. Exp Mol Med 53, 809–822 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Todoric J. et al. Fructose stimulated de novo lipogenesis is promoted by inflammation. Nat Metab 2, 1034–1045 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang Z. et al. Serine catabolism generates liver NADPH and supports hepatic lipogenesis. Nat Metab 3, 1608–1620 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Morrow MR et al. Inhibition of ATP-citrate lyase improves NASH, liver fibrosis, and dyslipidemia. Cell Metab 34, 919–936 e8 (2022). [DOI] [PubMed] [Google Scholar]
- 133.Wang Q. et al. Abrogation of hepatic ATP-citrate lyase protects against fatty liver and ameliorates hyperglycemia in leptin receptor-deficient mice. Hepatology 49, 1166–75 (2009). [DOI] [PubMed] [Google Scholar]
- 134.Yenilmez B. et al. Paradoxical activation of transcription factor SREBP1c and de novo lipogenesis by hepatocyte-selective ATP-citrate lyase depletion in obese mice. J Biol Chem, 102401 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Beigneux AP et al. ATP-citrate lyase deficiency in the mouse. The Journal of biological chemistry 279, 9557–64 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Abu-Elheiga L. et al. Mutant mice lacking acetyl-CoA carboxylase 1 are embryonically lethal. Proc Natl Acad Sci U S A 102, 12011–6 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Harada N. et al. Hepatic de novo lipogenesis is present in liver-specific ACC1-deficient mice. Mol Cell Biol 27, 1881–8 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kim CW et al. Acetyl CoA Carboxylase Inhibition Reduces Hepatic Steatosis but Elevates Plasma Triglycerides in Mice and Humans: A Bedside to Bench Investigation. Cell Metab 26, 576 (2017). [DOI] [PubMed] [Google Scholar]
- 139.Savage DB et al. Reversal of diet-induced hepatic steatosis and hepatic insulin resistance by antisense oligonucleotide inhibitors of acetyl-CoA carboxylases 1 and 2. J Clin Invest 116, 817–24 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Harriman G. et al. Acetyl-CoA carboxylase inhibition by ND-630 reduces hepatic steatosis, improves insulin sensitivity, and modulates dyslipidemia in rats. Proc Natl Acad Sci U S A 113, E1796–805 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chow JD et al. Genetic inhibition of hepatic acetyl-CoA carboxylase activity increases liver fat and alters global protein acetylation. Mol Metab 3, 419–31 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nelson ME et al. Inhibition of hepatic lipogenesis enhances liver tumorigenesis by increasing antioxidant defence and promoting cell survival. Nat Commun 8, 14689 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Neinast M, Murashige D. & Arany Z. Branched Chain Amino Acids. Annu Rev Physiol 81, 139–164 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Neinast MD et al. Quantitative Analysis of the Whole-Body Metabolic Fate of Branched-Chain Amino Acids . Cell Metab 29, 417–429 e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mayers JR et al. Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nat Med 20, 1193–1198 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li JT et al. BCAT2-mediated BCAA catabolism is critical for development of pancreatic ductal adenocarcinoma. Nat Cell Biol 22, 167–174 (2020). [DOI] [PubMed] [Google Scholar]
- 147.Sakers A, De Siqueira MK, Seale P. & Villanueva CJ Adipose-tissue plasticity in health and disease. Cell 185, 419–446 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lengyel E, Makowski L, DiGiovanni J. & Kolonin MG Cancer as a Matter of Fat: The Crosstalk between Adipose Tissue and Tumors. Trends Cancer 4, 374–384 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhu Q. et al. Adipocyte mesenchymal transition contributes to mammary tumor progression. Cell Rep 40, 111362 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Seki T. et al. Brown-fat-mediated tumour suppression by cold-altered global metabolism. Nature 608, 421–428 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fernandez S. et al. Adipocyte ACLY Facilitates Dietary Carbohydrate Handling to Maintain Metabolic Homeostasis in Females. Cell Rep 27, 2772–2784 e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Smith U. & Kahn BB Adipose tissue regulates insulin sensitivity: role of adipogenesis, de novo lipogenesis and novel lipids. J Intern Med 280, 465–475 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Takehara M. et al. Cancer-associated adipocytes promote pancreatic cancer progression through SAA1 expression. Cancer Sci 111, 2883–2894 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Watson JA, Fang M. & Lowenstein JM Tricarballylate and hydroxycitrate: substrate and inhibitor of ATP: citrate oxaloacetate lyase. Arch Biochem Biophys 135, 209–17 (1969). [DOI] [PubMed] [Google Scholar]
- 155.Onakpoya I, Hung SK, Perry R, Wider B. & Ernst E. The Use of Garcinia Extract (Hydroxycitric Acid) as a Weight loss Supplement: A Systematic Review and Meta-Analysis of Randomised Clinical Trials. J Obes 2011, 509038 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jena BS, Jayaprakasha GK, Singh RP & Sakariah KK Chemistry and biochemistry of (−)-hydroxycitric acid from Garcinia. J Agric Food Chem 50, 10–22 (2002). [DOI] [PubMed] [Google Scholar]
- 157.Heymsfield SB et al. Garcinia cambogia (hydroxycitric acid) as a potential antiobesity agent: a randomized controlled trial. JAMA 280, 1596–600 (1998). [DOI] [PubMed] [Google Scholar]
- 158.Madeo F, Carmona-Gutierrez D, Hofer SJ & Kroemer G. Caloric Restriction Mimetics against Age-Associated Disease: Targets, Mechanisms, and Therapeutic Potential. Cell Metab 29, 592–610 (2019). [DOI] [PubMed] [Google Scholar]
- 159. Marino G. et al. Regulation of autophagy by cytosolic acetyl-coenzyme A. Mol Cell 53, 710–25 (2014). Nutrient deprivation-induced depletion of acetyl-CoA was shown to activate autophagy in a p300-regulated manner.
- 160.Longo VD & Panda S. Fasting, Circadian Rhythms, and Time-Restricted Feeding in Healthy Lifespan. Cell Metab 23, 1048–1059 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lien EC et al. Low glycaemic diets alter lipid metabolism to influence tumour growth. Nature 599, 302–307 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pomatto-Watson LCD et al. Daily caloric restriction limits tumor growth more effectively than caloric cycling regardless of dietary composition. Nat Commun 12, 6201 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pietrocola F. et al. Caloric Restriction Mimetics Enhance Anticancer Immunosurveillance. Cancer Cell 30, 147–160 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Michaud M. et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science 334, 1573–7 (2011). [DOI] [PubMed] [Google Scholar]
- 165.Amaravadi RK, Kimmelman AC & Debnath J. Targeting Autophagy in Cancer: Recent Advances and Future Directions. Cancer Discov 9, 1167–1181 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yamamoto K. et al. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature 581, 100–105 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zheng Y. et al. ATP citrate lyase inhibitor triggers endoplasmic reticulum stress to induce hepatocellular carcinoma cell apoptosis via p-eIF2alpha/ATF4/CHOP axis. J Cell Mol Med 25, 1468–1479 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Shah S. et al. Targeting ACLY sensitizes castration-resistant prostate cancer cells to AR antagonism by impinging on an ACLY-AMPK-AR feedback mechanism. Oncotarget (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pearce NJ et al. The role of ATP citrate-lyase in the metabolic regulation of plasma lipids. Hypolipidaemic effects of SB-204990, a lactone prodrug of the potent ATP citrate-lyase inhibitor SB-201076. Biochem J 334 ( Pt 1), 113–9 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Burke AC & Huff MW ATP-citrate lyase: genetics, molecular biology and therapeutic target for dyslipidemia. Curr Opin Lipidol 28, 193–200 (2017). [DOI] [PubMed] [Google Scholar]
- 171.Pinkosky SL et al. Liver-specific ATP-citrate lyase inhibition by bempedoic acid decreases LDL-C and attenuates atherosclerosis. Nat Commun 7, 13457 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tang Z. et al. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res 45, W98–W102 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gu L. et al. The IKKbeta-USP30-ACLY Axis Controls Lipogenesis and Tumorigenesis. Hepatology 73, 160–174 (2021). [DOI] [PubMed] [Google Scholar]
- 174.Qiao C. et al. IGF1-mediated HOXA13 overexpression promotes colorectal cancer metastasis through upregulating ACLY and IGF1R. Cell Death Dis 12, 564 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Pinkosky SL et al. AMP-activated protein kinase and ATP-citrate lyase are two distinct molecular targets for ETC-1002, a novel small molecule regulator of lipid and carbohydrate metabolism. Journal of lipid research 54, 134–51 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Huang X. et al. Important role of the LKB1-AMPK pathway in suppressing tumorigenesis in PTEN-deficient mice. Biochem J 412, 211–21 (2008). [DOI] [PubMed] [Google Scholar]
- 177.Houde VP et al. AMPK beta1 reduces tumor progression and improves survival in p53 null mice. Mol Oncol 11, 1143–1155 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Dai X. et al. Energy status dictates PD-L1 protein abundance and anti-tumor immunity to enable checkpoint blockade. Mol Cell 81, 2317–2331 e6 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wei J. et al. An allosteric mechanism for potent inhibition of human ATP-citrate lyase. Nature (2019). [DOI] [PubMed] [Google Scholar]
- 180.Wei X, Schultz K, Bazilevsky GA, Vogt A. & Marmorstein R. Molecular basis for acetyl-CoA production by ATP-citrate lyase. Nat Struct Mol Biol 27, 33–41 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Verschueren KHG et al. Structure of ATP citrate lyase and the origin of citrate synthase in the Krebs cycle. Nature 568, 571–575 (2019). These three papers reported for the first time the structure of the ACLY homotetramer.
- 182. Svensson RU et al. Inhibition of acetyl-CoA carboxylase suppresses fatty acid synthesis and tumor growth of non-small-cell lung cancer in preclinical models. Nat Med 22, 1108–1119 (2016). ACC inhibition is demonstrated to suppress tumor growth in genetically engineered lung cancer mouse models.
- 183.Liefwalker DF et al. Metabolic convergence on lipogenesis in RAS, BCR-ABL, and MYC-driven lymphoid malignancies. Cancer Metab 9, 31 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Alkhouri N, Lawitz E, Noureddin M, DeFronzo R. & Shulman GI GS-0976 (Firsocostat): an investigational liver-directed acetyl-CoA carboxylase (ACC) inhibitor for the treatment of non-alcoholic steatohepatitis (NASH). Expert Opin Investig Drugs 29, 135–141 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lee H. et al. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat Cell Biol 22, 225–234 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ringel AE et al. Obesity Shapes Metabolism in the Tumor Microenvironment to Suppress Anti-Tumor Immunity. Cell 183, 1848–1866 e26 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wellen KE & Snyder NW Should we consider subcellular compartmentalization of metabolites, and if so, how do we measure them? Curr Opin Clin Nutr Metab Care 22, 347–354 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Dieterich IA et al. Acetyl-CoA flux regulates the proteome and acetyl-proteome to maintain intracellular metabolic crosstalk. Nat Commun 10, 3929 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.He A. et al. Acetyl-CoA Derived from Hepatic Peroxisomal beta-Oxidation Inhibits Autophagy and Promotes Steatosis via mTORC1 Activation. Mol Cell 79, 30–42 e4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Huber K. et al. N-acetylaspartate pathway is nutrient responsive and coordinates lipid and energy metabolism in brown adipocytes. Biochim Biophys Acta Mol Cell Res 1866, 337–348 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Jung SM et al. In vivo isotope tracing reveals the versatility of glucose as a brown adipose tissue substrate. Cell Rep 36, 109459 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Levy MJ et al. A Systems Chemoproteomic Analysis of Acyl-CoA/Protein Interaction Networks. Cell Chem Biol 27, 322–333 e5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Montgomery DC et al. Global Profiling of Acetyltransferase Feedback Regulation. J Am Chem Soc 138, 6388–91 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Goncalves MD et al. High-fructose corn syrup enhances intestinal tumor growth in mice. Science 363, 1345–1349 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]