Abstract
The synthesis of new bicyclic lactone derivatives was carried out starting from 2-methyl/phenyl-3a,4,7,7a-tetrahydro-1H-isoindole-1,3(2H)-dione. 6-(Hydroxymethyl)-N-methyl/phenylcyclohex-3-ene-1-carboxamide derivatives were obtained from the reduction of tetrahydro-1H-isoindole-1,3(2H)-diones with NaBH4. Bromination and epoxidation reactions of both compounds were examined, and the structures of the resulting products were determined by spectroscopic methods. Substituted bicyclic lactone compounds, which are interesting rearrangement products in both bromination and epoxidation reactions, were obtained. In particular, hydroxymethyl (−CH2OH) and amide (−CONHR) groups attached to the cyclohexene ring in the bromination and epoxidation reactions were found to be effective in product formation. As a result, a new and applicable method was developed for the synthesis of bicyclic lactone derivatives.
Introduction
Glycosidases, also known as glycoside hydrolases, represent a crucial group of enzymes that facilitate the hydrolytic cleavage of glycosidic bonds.1−4 These enzymes are prevalent across a broad range of organisms, including microorganisms, plants, and animals, and have significant applications in various fields, such as biotechnology, the food industry, and pharmacology. α/β-Glucosidases specialize in breaking down α- or β-linkages at the anomeric center of a glucose fragment. They play essential roles in numerous biochemical pathways that are linked to various metabolic disorders and diseases including diabetes, viral and bacterial infections, lysosomal storage disorders, and cancer.1,2 Consequently, considerable research has been dedicated to developing effective glucosidase inhibitors. These inhibitors are typically designed to mimic a substrate, a transition state, or an enzyme reaction product. As such, various polyhydroxylated cyclic compounds, which are five- or six-membered, are explored as monosaccharide structural analogues and potential inhibitors of glycosidases.5−8
Brazdova et al.9 developed and tested a variety of cyclohexanecarboxylic acids featuring diverse substitution patterns, substituent configurations, and varying lengths of n-alkyl groups R to investigate their impact on inhibitory activity. These molecules, characterized by a six-membered-ring structure adorned with multiple hydrophilic groups, align with the common structural features of many carbohydrate mimetics. The introduction of alkyl groups aimed to bolster the inhibitory effect, a phenomenon similarly noted in prior studies with lipophilic groups on compounds such as 1-deoxynojirimycin derivatives, other iminosugars, alkyl glycosides, and various additional glycosidase inhibitors.9
Cyclitols make up a group of cycloalkanes that are characterized by the presence of at least three hydroxyl groups, with each one attached to a distinct carbon atom in the ring structure. Cyclitol is also used for similar molecules containing one or more double bonds in the ring with various functional groups replacing the hydrogen atoms in such a molecule. Cyclitols are classified as inositols (1), quercitols (2), conduritols (3), and aminoconduritols (4) (Scheme 1).10−12 Cyclitol derivatives are used as sweeteners, glycosidase inhibitors, antidiabetic, antifungal, anticancer, antibiotic, and antiviral reagents.5−8 On the other hand, carbasugars are cyclitol derivatives with significant biological activity. Their synthesis as alternative glycosidase inhibitors has attracted great interest in pharmaceutical and organic chemistry in recent years.4,13−16
Scheme 1. Structures of Commonly Known Cyclitol Derivatives.
On the other hand, syntheses of cyclitol derivatives containing different functional groups from bicyclic or tricyclic systems have been reported in the literature.17−20 Two main reactions, epoxidation and bromination, were used in the synthesis of cyclitol derivatives, and lactone derivatives containing a five-membered ring were synthesized.
Recently, we were interested in the synthesis of y-hydroxycarboxamide 7 from the reduction of bicyclic imide, from which tetrasubstituted cyclohexane derivatives can be prepared by manipulating alkene double bonds. In this context, we were surprised by the formation of rearrangement product 8, which was formed upon bromination of y-hydroxycarboxamide 7. Hydrolysis of iminium 8 gave bicyclic lactone 9 (Scheme 2).21
Scheme 2. Structures of Compounds 6, 7, 8, and 9.
In light of these findings, we decided to investigate the bromination and epoxidation reactions of cyclohexene derivatives containing N-methyl and N-phenyl-carboxamide units. Here, we report the alternative strategy developed for the synthesis of tetrasubstituted cyclohexane derivatives. We also discuss the mechanism of formation of the products in the bromination and epoxidation reactions.
Results and Discussion
For the synthesis of tetrasubstituted cyclohexane derivatives, we used bicyclic imide compounds 6a and 6b obtained in two steps from 3-sulfolene as the key compound. When imide derivatives are reduced with metal hydrides, product formation in these reactions depends on parameters such as the amount of reagent used, type of reagent, and reaction time. Therefore, the conversion of both imide compounds 6a and 6b to the corresponding carboxamide compounds 7a and 7b was carried out under reaction conditions optimized in previous studies.22
In this context, the imide compounds 6a–b were reacted with 2 equiv of NaBH4 in THF/H2O (1:1) and the reaction was quenched after 6 h (Scheme 3). The products were purified, and their structures were elucidated by spectroscopic methods. The carboxamide compounds 7a–b are formed as a result of imide group reduction, with the primary alcohol (−CH2OH) and amide (−CONHR) groups and the peaks belonging to both groups are clearly seen in the 1H and 13C NMR spectra (Figures S1, S2, S4, S5).
Scheme 3. Synthesis and Their Reactions of γ-Hydroxycarboxamide Derivatives 7.
After synthesizing carboxamide 7 containing three functional groups (−CH2OH, −CONHR, and double bond), we studied the bromination and epoxidation reactions of these compounds. One of the aims of studying these reactions is to synthesize tetrasubstituted cyclohexane derivatives, and the second is the expectation that interesting rearrangement products may arise in both reactions due to the effect of bonded groups in cyclic systems. We very recently studied the bromination of the carboxamide compound 6a. In this reaction, an iminium salt was obtained by the action of both amide (−CONHR) and alcohol (−CH2OH) groups attached to the ring, and the mechanism of product formation was also discussed. Subsequently, hydrolysis of the iminium group yielded the lactone compound. It is very interesting that although only Br2 was used in the reaction, halohydrin (HOBr) was added to the double bond in the final product. Because Br2/H2O or HOBr was not used as a reagent. In this context, when the reaction steps were evaluated, an intramolecular hydroxyl (−OH) group transfer occurred in the reaction. This result makes the applied method even more important (Scheme 3).21
In this context, considering what kind of product can be obtained by protecting the alcohol group that causes lactone formation, the alcohol group was converted to the corresponding silyl ether with tert-butyldimethylsilyl chloride to give compound 10 (Scheme 4).
Scheme 4. Synthesis and Their Reactions of Silyl Ether Compounds.
The bromination reaction of compound 10 containing the protected alcohol group was investigated. The resulting lactone compound obtained in the previous reaction was synthesized. The formation of the same lactone compound can be explained by the fact that the reaction does not proceed similarly to compound 7 as a result of the removal of the silyl group in compound 10 under the reaction conditions. It is predicted that the reaction takes place through the mechanism given in Scheme 5.23−25
Scheme 5. Bromination of Carboxamide Derivative 10 Containing Silyl Ether Group.
When 2 equiv of bromine was used for the bromination reaction, a second bromine atom was found to be attached to the molecule and the exact structure of the molecule was determined by X-ray diffraction (Figure 1). Based on the molecular structure, it is predicted that the second bromine atom is bound to the molecule via the enol form formed by the carbonyl group (Scheme 6).
Figure 1.

(Top) X-ray structure of molecule 12. Thermal ellipsoids are drawn at the 40% probability level. (Bottom) Trapped aqua molecules between layers of molecule 12 and the unit cell are viewed down along the c-axis.
Scheme 6. Bromination of Carboxamide Derivative 7a with Excess Bromine.
The exact conformation and structures of the 5,7a-dibromo-6-hydroxyhexahydro-2-benzofuran-1(3H)-one hydrate (12) was confirmed by X-ray diffraction analysis. Molecule 12 is in racemic form, and only one enantiomer is seen in the asymmetric unit (Figure 1). The molecule 12 was crystallized in the monoclinic P21/c space group. It consists of fused cyclohexane and heterocyclic furan rings with substituted bromine, −OH, and carbonyl oxygen atoms. There is also 1 mol of aqua molecule in this structure. Here, the cyclohexane ring is in the chair conformation and has the most stable conformation. In this cycle, C–C single bonds are in the range of 1.494–1.535(3) Å. Bromine atoms are in cis position and the bond lengths of C4–Br2 and C7–Br1 are 1.963 and 1.982 Å, respectively. Aqua molecules are trapped by hydrogen bonds between strings of molecules 12. These paramount H-bonds are as follows: O3–H···O4 [D···A = 2.651(3)Å], O4–H···O2 [D···A = 2.891(3)Å] and O4–H···O3 [D···A = 2.785(3) Å]. Finally, we can state that the furan ring is in an envelope form. Maximum deviation of C2 atom from meanplane C1/O1/C8/C7 is 0.203 Å.
On the other hand, bromination was carried out in systems containing a phenyl group instead of a methyl group attached to the nitrogen atom in the amide group (Scheme 3). A similar rearrangement product 8b was also observed in the phenyl-containing compound. As a result, lactone compound 9 was obtained through the hydrolysis of compound 8b. However, in the reaction of compound 7b containing phenyl group with 2 equiv. bromine, unlike compound 7a, the second bromine atom was found to be attached to the phenyl ring in the molecule (Scheme 7). This result was confirmed by the 1H NMR spectrum of the crude product (Figures S13 and S14). Since the molecule was hydrolyzed during the purification process, lactone 9 was obtained as a result of hydrolysis. According to the 1H NMR spectrum analysis of compound 13, the bromine atom is not bound to the cyclohexane ring, since there is no change in the number of protons and their signal groups in the cyclohexane ring. On the other hand, the differences in the number of phenyl ring protons and their chemical shift values in molecule 13 indicate that the second bromine atom is bound to the phenyl group. In particular, the fact that phenyl protons change from a multiplet appearance before the reaction to an AB system after the reaction shows that the second bromine atom is bonded to the p-position of the phenyl ring in molecule 13.
Scheme 7. Bromination of Carboxamide Derivative 7b with Excess Bromine.
Interesting rearrangement products were obtained in bromination reactions, and the structures and formation mechanisms of these products were discussed. Next, we decided to examine the epoxidation reactions of carboxamides for which rearrangement products were also expected. First, we carried out the epoxidation reaction of carboxamide 7a with m-CPBA in methylene chloride. As a result of the reaction, epoxy lactone compound 14 was obtained (Scheme 8).
Scheme 8. Synthesis of Hexahydrooxireno[2,3-f]isobenzofuran-3(1aH)-one (14) and 6-Hydroxy-1-oxooctahydro-2-benzofuran-5-yl-3-chlorobenzoate (15).
After separation, 1H and 13C NMR spectroscopic data showed that a product different from expected was formed (Figures S18 and S19). In the 1H NMR spectrum of the product, CH2 protons in the lactone ring give the AB system at δ = 4.40 and 4.03 ppm. In addition, the epoxide ring protons resonate in the form of the multiplet at δ = 3.23–3.18 ppm due to the asymmetry in the molecule. These protons are split into neighboring CH2 protons. In the 13C NMR spectrum of compound 14, the carbonyl carbon and –OCH2– carbon in the lactone ring resonate at δ = 170 and 60 ppm, respectively. Additionally, 8 lines in the 13C NMR spectrum are in complete agreement with the structure of the molecule. The exact stereochemistry of the epoxide ring in 14 was later confirmed by single-crystal X-ray analysis of the epoxide ring opening product 15.
When the reaction mechanism is examined, depending on the structure of the lactone molecule 14, three different reactions occur. These are epoxide, amide hydrolysis, and lactone formation. Amide hydrolysis and lactonization can also occur in a single step. Phenyl isomer 7b also gave the same syn-epoxide 14.
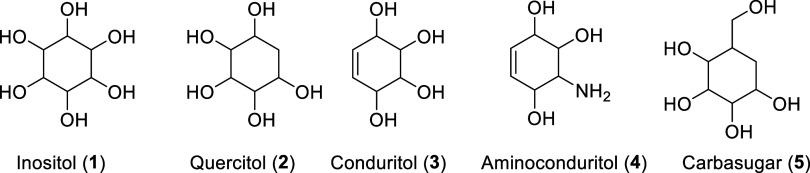
When the reaction time was prolonged or the reaction temperature was increased to 35–40 °C, the epoxide opening product 15 was obtained (Scheme 8). Compound 15 was crystallized from CH2Cl2–hexane solvent mixture and X-ray analysis was performed (Figure 2).
Figure 2.
(Top) X-ray structure of dimeric molecule 15. Thermal ellipsoids are drawn at the 40% probability level. (Bottom) Stacking motif and the unit cell viewed down along the c-axis.
The exact conformation and structures of 6-hydroxy-1-oxooctahydro-2-benzofuran-5-yl-3-chlorobenzoate (15) were confirmed by X-ray diffraction analysis. Molecule 15 is in racemic form, and only one enantiomer is seen in the asymmetric unit (Figure 2). The molecule 15 crystallizes in monoclinic space group C2/c with eight molecules in the unit cell. The core structure is again a fused cyclohexane and lactone ring, and −OH, carbonyl, and m-CPBA groups are attached to the core unit. Two enantiomers form a dimeric structure with O3–H–O5 [D···A = 2.849(3)Å] hydrogen bonding (Figure 2). The cyclohexane ring in the chair conformer and for this cycle C–C single bonds are in the 1.508–1.528(3) Å range. Here again, we can state that the furan ring is in the envelope form. Maximum deviation of C14 atom from meanplane C12/O4/C13/C11 is 0.224 Å. The racemic nature of the molecules significantly affects the supramolecular structures and crystal lattice motifs (Figure 2).
We expected the formation of the anti-epoxy isomer due to the asymmetry in molecule 7. However, X-ray analysis of epoxy ring opening product 15 showed that the syn-epoxy isomer was formed in the epoxidation reaction. In our previous studies, we expected the formation of the anti-epoxide isomer due to the steric effect but found that the syn-isomer was formed in much larger amounts.26,27 This result, the further formation of the syn-isomer, was explained by the dipole–dipole interaction between the imide ring and the per acid in the compound. Similar to these examples, an important factor in the formation of the syn-epoxy isomer from the epoxidation of compound 7 is the intermolecular interactions (hydrogen bonding, etc.) of the per acid with the alcohol and amide groups in the molecule, resulting in the per acid approaching the molecule from the same side as the alcohol and amide group to form the syn-isomer.
In order to investigate the effect of the alcohol group on isomer formation in epoxidation reactions, the OH group was protected with TBSCl as in the bromination reaction, and the epoxidation reaction was carried out. After purification, it was determined by spectroscopic methods that a new rearrangement product was formed from the epoxidation of the carboxamide molecule containing the protected alcohol group (Scheme 9). In the 1H NMR spectrum of compound 16, signal groups belonging to the epoxide and amide group are not present (Figures S24 and S25). This indicates that the epoxide ring is opened, and the amide group is hydrolyzed. In addition, −CH2OTBS protons give the AB system at δ = 3.43 and 3.33 ppm. H4 and H5 protons in the six-membered ring resonate at δ = 4.58 and 4.15 ppm, respectively. The presence of 14 lines in 13C NMR is in agreement with the structure.
Scheme 9. Epoxidation of the Carboxamide Derivative 10.
When the products formed in the epoxidation reaction of compounds 7 and 10 are compared, syn-isomer is formed in compound 7 (free alcohol group) while anti-isomer is formed in compound 10 (protected alcohol group) and the carboxylic acid group formed by hydrolysis of the amide group attacks from behind and causes the epoxide ring to open. This result supports our suggestion of explaining the syn-isomer. Thus, two different lactones were obtained from the epoxidation of compound 7 and compound 10. However, since the amide group is hydrolyzed in the reaction medium, the same lactone 16 is formed from both compounds 10a and 10b.
Conclusions
In conclusion, we examined the bromination and epoxidation reactions of cyclohex-3-ene-1-carboxamide derivatives 7 and 10 obtained from the reduction of a bicyclic imide with NaBH4. We obtained the same lactone 9 from the bromination reaction of both compounds and discussed the reaction mechanism. In addition, in compound 7, we examined the reaction of two compounds with methyl and phenyl groups attached to the nitrogen atom with excess bromine. First, we synthesized isobenzofuran derivative 12, which has a second bromine atom attached to the bridgehead, from the reaction of the compound with a methyl group attached to the nitrogen atom with excess bromine. In this reaction, the attachment of the second bromine atom to the ring can be defined as the α-bromination reaction of the carbonyl group. Second, we performed the reaction of the compound in which the phenyl group is attached to the nitrogen atom with excess bromine. As a result of this reaction, we determined that the second bromine atom was bound to the p-position of the phenyl ring, as a result of the electrophilic substitution reaction.
On the other hand, we obtained two different lactone compounds from the epoxidation of compounds 7 and 10. We determined that intermolecular interactions (hydrogen bonds, etc.) play a role in the formation of both lactones. We postulate that the most important reason for reactions to proceed through the proposed mechanism is the canonical forms of the amide group.
As a result, we developed a new lactone synthesis method by bromination and epoxidation reactions depending on the groups in the ring.
Experimental Section
General Information
All reagents used were commercially available unless otherwise specified, and all solvents were distilled before use. Melting points were measured with a Gallenkamp melting point device. 1H NMR and 13C NMR spectra were recorded on a Bruker (400 MHz 1H, 100 MHz 13C) or Varian (400 MHz 1H, 100 MHz 13C). Chemical shift values (δ ppm) are reported using tetramethylsilane (δH 0.00), CDCl3 (δC 77.00), and CD3OD (δC 49.15). The 1H NMR spectra are reported as follows δ (number of protons, multiplicity, coupling constant J Hz). Multiplicities are indicated by s (singlet), d (doublet), t (triplet), q (quartet), dd (doublet of doublet), m (multiplet), and br-s (broad singlet). High-resolution mass spectrometry (HR-MS): Electron spray technique (M+/M−) from the solution. in MeOH (Waters LCT Premier XE UPLC/MS TOF (Manchester, U.K.)).
Synthesis of 6-(Hydroxymethyl)-N-methyl/Phenylcyclohex-3-ene-1-carboxamide (7)
To a stirred solution of isoindole-1,3-dione 6 (2.20 mmol) in THF/H2O (20 mL, 1:1) was added NaBH4 (4.40 mmol) at 0 °C for 5 min. After stirring at room temperature for 6 h, saturated NH4Cl was added to the reaction mixture. The organic layer was separated and the aqueous layer was extracted 3 × 10 mL with EtOAc. The combined organic layer was dried over Na2SO4 and the solvent was removed in vacuo. The residue was purified by silica gel column chromatography (Hexane/EtOAc: 40/60 → 0/100) to afford 7.
6-(Hydroxymethyl)-N-methylcyclohex-3-ene-1-carboxamide (7a)
White solid (95% yield). mp. 88–90 °C. 1H NMR (400 MHz, CDCl3) δ 6.62 (s, 1H), 5.66 (d, J = 2.5 Hz, 2H), 3.97 (brs, 1H), 3.68–3.32 (m, 3H), 2.74 (s, 3H), 2.42–2.04 (m, 4H), 1.84 (dd, J = 16.3, 4.6 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ: 173.42, 127.14, 125.00, 64.09, 41.91, 37.16, 27.22, 26.33, 25.62. HRMS: (ESI), m/z: Calcd. for [M + H]+ C9H15NO2: 170.1103; found: 170.1174.
6-(Hydroxymethyl)-N-phenylcyclohex-3-ene-1-carboxamide (7b)
White solid (96% yield), mp. 155–157 °C. 1H NMR (400 MHz CDCl3): δ 7.96 (brs, 1H, N–H), 7.52–7.47 (m, 2H), 7.36–7.30 (m, 2H), 7.16–7.10 (m, 1H), 5.85 (brs, 2H), 3.75–3.62 (m, 2H), 3.07–3.02 (m, 1H), 2.61–2.26 (m, 3H), 2.24–2.12 (m, 1H), 1.98–1.88 (m, 1H). 13C NMR (100 MHz, CDCl3) δ: 173.41, 137.61, 129.02, 127.35, 124.98, 124.51, 120.13, 64.24, 41.96, 37.23, 26.36. HRMS: (ESI), m/z: [M + H]+ C14H17NO2 calcd. 232.1259; found 232.1313.
(1S,6R)-6-(((tert-Butyldimethylsilyl)oxy)methyl)-N-methyl/Phenylcyclohex-3-ene-1-carboxamide (10)
In a 50 mL single-neck flask, the starting compound 7 (2.16 mmol) and imidazole (4.32 mmol) were dissolved by adding 30 mL of CH2Cl2 and stirred for 5 min at room temperature in N2 atm. Then, tert-butyldimethylsilyl chloride (2.38 mmol) dissolved in 10 mL of CH2Cl2 was added to the mixture and stirred for 16 h at room temperature. When the reaction was completed, 10 mL of a saturated NH4Cl solution was added. The mixture was extracted with CH2Cl2 (3 × 20 mL). The organic phase was washed again with a saturated NH4Cl (20 mL) solution. It was dried over Na2SO4. The crude product was purified on a silica gel column in a 20% EtOAc/n-hexane solvent system.
(1S,6R)-6-(((tert-Butyldimethylsilyl)oxy)methyl)-N-methylcyclohex-3-ene-1-carboxamide (10a)
White solid (92% yield), mp. 73–74 °C. 1H NMR (400 MHz, CDCl3) δ 6.03 (s, 2H), 5.66 (s, 4H), 3.52 (t, J = 9.0 Hz, 4H), 2.84–2.63 (m, 8H), 2.36–1.83 (m, 11H), 0.84 (s, 16H), −0.00 (s, 9H). 13C NMR (100 MHz, CDCl3) δ: 174.41, 125.87, 125.43, 77.34, 77.02, 76.70, 64.14, 40.65, 37.64, 26.25, 26.16, 26.01, 25.89, 18.22, −5.41. HRMS: (ESI), m/z: [M + H]+ C15H29NO2Si calcd. 284.1968; found 284.2040.
(1S,6R)-6-(((tert-Butyldimethylsilyl)oxy)methyl)-N-Phenylcyclohex-3-ene-1-carboxamide (10b)
White solid (94% yield), mp. 115–116 °C. 1H NMR (400 MHz, CDCl3) δ 7.94 (s, 1H), 7.40 (d, J = 7.7 Hz, 2H), 7.27–7.09 (m, 2H), 6.96 (t, J = 7.4 Hz, 1H), 5.83–5.55 (m, 2H), 3.63 (dd, J = 10.8, 6.8 Hz, 1H), 3.58–3.46 (m, 1H), 3.01–2.81 (m, 1H), 2.39 (d, J = 16.7 Hz, 1H), 2.24–2.04 (m, 2H), 2.01–1.73 (m, 2H), 0.84 (s, 8H), −0.00 (s, 5H). 13C NMR (100 MHz, CDCl3) δ: 171.90, 138.29, 128.92, 125.64, 125.38, 123.77, 119.52, 64.50, 40.46, 38.00, 26.46, 25.95, 25.52, 18.23, −5.24. HRMS: (ESI), m/z: [M + H]+ C20H31NO2Si calcd. 346.2124; found 346.2196.
Synthesis of 5-Bromo-6-hydroxyhexahydroisobenzofuran-1(3H)-one (9)
To a stirred solution of carboxamide 10 (1.45 mmol) in dichloromethane (20 mL) was added bromine (1.74 mmol) in an ice bath reaction mixture. The reaction was quenched after 5 min. The solvent was removed in vacuo. It was observed that it transformed into a different product on the silica gel column during purification. The lactone compound 9 was obtained in 95% yield mp: 111–113 °C. 1H NMR (400 MHz, CDCl3) δ 4.36–4.23 (m, 2H), 4.12–4.00 (m, 2H), 2.94–2.81 (m, 1H), 2.72–2.62 (m, 1H), 2.52–2.31 (m, 2H), 2.24–2.13 (m, 1H), 2.06–1.96 (m, 1H). 13C NMR (100 MHz, CDCl3) δ: 179.15, 77.58, 77.26, 76.94, 71.23, 67.78, 50.61, 36.22, 31.63, 28.42, 24.98. HRMS: (ESI), m/z: [M + H]+ C8H11BrO3 calcd. 234.9892; found 234.9965.
Synthesis of 5,7a-Dibromo-6-hydroxyhexahydroisobenzofuran-1(3H)-one (12)
To a stirred solution of carboxamide 7 (2.16 mmol) in dichloromethane (20 mL) was added bromine (4.32 mmol) in an ice bath. The reaction was stirred after 10 min. The solvent was removed in vacuo. Lactone compound 12 was obtained in 95% yield by hydrolysis of the product in a silica gel column during purification. White crystal, mp 114–115 °C. 1H NMR (400 MHz, CDCl3) δ 4.63–4.53 (m, 1H), 4.22–4.17 (m, 1H), 4.09 (dd, J = 6.1, 3.0 Hz, 1H), 3.97 (d, J = 9.2 Hz, 1H), 3.09–3.00 (m, 1H), 2.99–2.90 (m, 1H), 2.72 (dd, J = 15.1, 2.6 Hz, 1H), 2.40–2.28 (m, 1H), 2.11–2.01 (m, 1H). 13C NMR (100 MHz, CDCl3) δ: 174.76, 71.16, 69.77, 55.34, 48.03, 41.70, 36.27, 30.06. HRMS: (ESI), m/z: [M + H]+ C8H10Br2O3 calcd. 314.9049; found 314.9051
Synthesis of Hexahydrooxireno[2,3-f]isobenzofuran-3(1aH)-one (14)
Compound 7 (2.16 mmol) and 77% m-CPBA (4.32 mmol) were dissolved in 30 mL of DCM. The reaction was stirred at room temperature for 5 h. The solvent was removed from the evaporator. 10 mL of saturated NaHCO3 solution was added to the residue, and the mixture was extracted with DCM (3 × 20 mL). The organic phase was washed again with saturated NH4Cl (20 mL) solution. It was dried over Na2SO4. The crude product was purified on a silica gel column in a 20% EtOAc/n-Hexane solvent system, 96% yield. White solid, mp. 95–97 °C. 1H NMR (400 MHz, CDCl3) δ 4.36–4.23 (m, 2H), 4.12–4.00 (m, 2H), 2.94–2.81 (m, 1H), 2.72–2.62 (m, 1H), 2.52–2.31 (m, 2H), 2.24–2.13 (m, 1H), 2.06–1.96 (m, 1H). 13C NMR (100 MHz, CDCl3) δ 179.15, 77.58, 77.26, 76.94, 71.23, 67.78, 50.61, 36.22, 31.63, 28.42, 24.98. HRMS: (ESI), m/z: [M + H]+ C8H11BrO3 calcd. 234.9892; found 234.9965.
Synthesis of 6-Hydroxy-1-oxooctahydroisobenzofuran-5-yl-3-chlorobenzoate (15)
Starting compound 7 (2.16 mmol) and 77% m-CPBA (4.32 mmol) were dissolved in 30 mL of DCM. The reaction was stirred at room temperature for 1 day. The solvent was removed from the evaporator. 10 mL of saturated NaHCO3 solution was added to the residue, and the mixture was extracted with DCM (3 × 20 mL). The organic phase was washed again with saturated NH4Cl (20 mL) solution. It was dried over Na2SO4. The crude product was purified on a silica gel column in a 20% EtOAc/n-hexane solvent system, 92% yield. Colorless crystal, mp. 154–156 °C. 1H NMR (400 MHz, CDCl3) δ 7.96 (t, J = 1.8 Hz, 1H), 7.93–7.88 (m, 1H), 7.59–7.54 (m, 1H), 7.41 (t, J = 7.9 Hz, 1H), 5.21 (dd, J = 7.4, 4.4 Hz, 1H), 4.32 (dd, J = 9.1, 5.2 Hz, 1H), 4.13–4.03 (m, 2H), 2.78 (dtd, J = 17.8, 11.2, 6.3 Hz, 2H), 2.28–2.24 (m, 2H), 2.20–2.11 (m, 1H), 2.07–1.97 (m, 1H). 13C NMR (100 MHz, CDCl3) δ 178.94, 164.36, 134.66, 133.34, 131.65, 129.88, 129.54, 127.77, 71.52, 71.47, 65.20, 36.39, 31.34, 26.00, 25.07. HRMS: (ESI), m/z: [M + H]+ C15H15ClO5 calcd. 311.0608; found 311.0680.
Synthesis 2-(((tert-Butyldimethylsilyl)oxy)methyl)-4-hydroxy-6-oxabicyclo[3.2.1]octan-7-one (16)
After compound 10 (1.45 mmol) was dissolved in DCM, m-CPBA (1.74 mmol) was added. The reaction mixture is monitored by TLC control at room temperature. After 6 h, the reaction was stopped by adding saturated NH4Cl. The crude product was extracted with 3 × 20 mL DCM, and the organic phase was separated. The organic phases were dried over Na2SO4, and the solvent was removed in the evaporator. The crude product was purified on a silica gel column, 84% yield. Yellow viscous. 1H NMR (400 MHz, CDCl3) δ 4.58 (t, J = 5.0 Hz, 1H), 4.10 (t, J = 4.1 Hz, 1H), 3.46–3.38 (m, 1H), 3.32 (dd, J = 10.1, 5.8 Hz, 1H), 2.70 (dt, J = 22.1, 7.4 Hz, 2H), 2.34–2.10 (m, 3H, OH+2H), 1.78 (dd, J = 15.1, 4.9 Hz, 1H), 1.41–1.31 (m, 1H), 0.82 (s, 9H), −0.00 (s, 6H). 13C NMR (100 MHz, CDCl3) δ: 177.82, 79.27, 64.79, 64.46, 39.53, 37.16, 31.36, 30.62, 25.88, 18.25, −5.42. HRMS: (ESI), m/z: [M + H]+ C14H26O4Si calcd. 287.1600; found 287.1672.
Crystal Structure Determination
For the determination of the crystal structure, single crystals of molecules 12 and 15 were analyzed using a Rigaku R-AXIS RAPID-S four-circle diffractometer equipped with a two-dimensional area IP detector. Data collection was conducted using graphite-monochromated Mo–Kα radiation (λ = 0.71073 Å) and employed the oscillation scan technique with Δw = 5° increment per image. The lattice parameters were accurately determined via least-squares methods based on all reflections where F2 > 2σ(F2). The integration of intensities, along with corrections for Lorentz and polarization effects and cell refinement, was carried out using the CrystalClear software by Rigaku/MSC, Inc., 2005. The structures were initially solved by direct methods with SHELXS-2013, which facilitated the localization of most of the heaviest atoms. The remaining nonhydrogen atoms were positioned from difference Fourier maps generated through successive cycles of full-matrix least-squares refinement on F2 using SHELXL-2013.28 All nonhydrogen atoms were refined using anisotropic displacement parameters, whereas the hydrogen atoms were modeled with common isotropic displacement factors and positioned using geometric restraints. The refinement process concluded with the final difference Fourier maps, which exhibited no significant residual peaks, indicating a clean and accurate structural model. Crystal data for 12: C8H10O3Br2 H2O, crystal system, space group: monoclinic, P21/c; (no: 14); unit cell dimensions: a = 11.470(4), b = 7.1440(3), c = 13.771(3) Å, α = 90, β = 97.976(59), γ = 90°; volume; 1117.5(2) Å3, Z = 4; calculated density: 1.973 g/cm3; absorption coefficient: 7.241 mm–1; F(000): 648; θ-range for data collection 1.8–25.2°; refinement method: full-matrix least-squares on F2; data/parameters: 1988/130; goodness-of-fit on F2: 1.086; Data completeness; 1.00, final R-indices [I > 2σ(I)]: R1 = 0.055, wR2 = 0.134; largest diff. peak and hole: 0.809 and −0.841 eÅ–3. Crystal data for 15: C15H15O5Cl, crystal system, space group: monoclinic, C2/c; (no: 15); unit cell dimensions: a = 20.195(2), b = 10.1886(3), c = 13.990(2) Å, α = 90, β = 90.083(4), γ = 90°; volume; 2878.5(5) Å3, Z = 8; calculated density: 1.434 g/cm3; absorption coefficient: 0.284 mm–1; F(000): 1296; θ-range for data collection 2.9–25.5°; refinement method: full-matrix least-squares on F2; data/parameters: 2658/191; goodness-of-fit on F2: 1.286; final R-indices [I > 2σ(I)]: R1 = 0.089, wR2 = 0.161; largest diff. peak and hole: 0.286 and −0.380 eÅ–3.
Acknowledgments
The authors thank Tübitak for financial support (grant no. 121Z879). They also thank Atatürk University and Kirşehir Ahi Evran University for the facilities and technical support.
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.4c02183.
1H NMR, 13C NMR, and mass spectrum of 6-(hydroxymethyl)-N-phenylcyclohex-3-ene-1-carboxamide; 1H NMR, 13C NMR, and mass spectrum of 5-bromo-6-hydroxyhexahydroisobenzofuran-1(3H)-one; and 1H NMR, 13C NMR, and mass spectrum of (1R,2S,4S,5S)-2-(((tert-butyldimethylsilyl)oxy)methyl)-4-hydroxy-6-oxabicyclo[3.2.1]octan-7-one (PDF)
Author Contributions
Ö.G.: Investigation, writing—original draft. S.A.: Investigation, methodology. E.Ş.: Software, formal analysis. Y.K.: Conceptualization, methodology, writing—original draft.
The authors declare no competing financial interest.
Supplementary Material
References
- Jacob G. S. Glycosylation inhibitors in biology and medicine. Curr. Opin. Struct. Biol. 1995, 5 (5), 605–611. 10.1016/0959-440X(95)80051-4. [DOI] [PubMed] [Google Scholar]
- Heightman T. D.; Vasella A. T. Recent insights into inhibition, structure, and mechanism of configuration-retaining glycosidases. Angew. Chem., Int. Ed. 1999, 38 (6), 750–770. . [DOI] [PubMed] [Google Scholar]
- Hudlicky T.; Entwistle D. A.; Pitzer K. K.; Thorpe A. J. Modern methods of monosaccharide synthesis from non-carbohydrate sources. Chem. Rev. 1996, 96 (3), 1195–1220. 10.1021/cr9403300. [DOI] [PubMed] [Google Scholar]
- Griffen J. A.; White J. C.; Kociok-Kohn G.; Lloyd M. D.; Wells A.; Arnot T. C.; Lewis S. E. New aminocyclitols with quaternary stereocentres via acylnitroso cycloaddition with an ipso, ortho arene dihydrodiol. Tetrahedron 2013, 69 (29), 5989–5997. 10.1016/j.tet.2013.04.033. [DOI] [Google Scholar]
- de Melo E. B.; da Silveira Gomes A.; Carvalho I. α- and β-Glucosidase inhibitors: chemical structure and biological activity. Tetrahedron 2006, 62, 10277–10302. 10.1016/j.tet.2006.08.055. [DOI] [Google Scholar]
- Legler G. Glycoside Hydrolases: Mechanistic Information from Studies with Reversible and Irreversible Inhibitors. Adv. Carbohydr. Chem. Biochem. 1990, 48, 319–384. 10.1016/S0065-2318(08)60034-7. [DOI] [PubMed] [Google Scholar]
- Lopez O. L.; Bols M. Anomer-Selective Glycosidase Inhibition by 2-N-Alkylated 1-Azafagomines. ChemBioChem 2007, 8, 657–661. 10.1002/cbic.200700012. [DOI] [PubMed] [Google Scholar]
- Mohan S.; Sim L.; Rose D. R.; Pinto B. M. Synthesis of S-alkylated sulfonium-ions and their glucosidase inhibitory activities against recombinant human maltase glucoamylase. Carbohydr. Res. 2007, 342, 901–912. 10.1016/j.carres.2007.01.018. [DOI] [PubMed] [Google Scholar]
- Brazdova B.; Tan N. S.; Samoshina N. M.; Samoshin V. V. Novel easily accessible glucosidase inhibitors: 4-hydroxy-5-alkoxy-1,2-cyclohexanedicarboxylic acids. Carbohydr. Res. 2009, 344, 311–321. 10.1016/j.carres.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Bellomo A.; Camarano S.; Rossini C.; Gonzalez D. Enantiospecific synthesis and insect feeding activity of sulfur-containing cyclitols. Carbohydr. Res. 2009, 344, 44–51. 10.1016/j.carres.2008.09.026. [DOI] [PubMed] [Google Scholar]
- Kuş N. Ş. Biological Properties of Cyclitols and Their Derivatives. Chem. Biodiversity 2024, 21, e202301064 10.1002/cbdv.202301064. [DOI] [PubMed] [Google Scholar]
- Balci M.; Çelik M.; Demir E.; Ertaş M.; Gültekin S. M.; Öztürk N.; Kara Y.; Horasan-Kishalı N. Cyclitols: Conduritols Aminoconduritols and Quersitols. Front. Nat. Prod. Chem. 2005, 1, 169–175. 10.2174/1574089054583696. [DOI] [Google Scholar]
- Mehta G.; Talukdar P.; Mohal N. A general norbornyl based synthetic approach to carbasugars and confused carbasugars. Tetrahedron Lett. 2001, 42, 7663–7666. 10.1016/S0040-4039(01)01454-X. [DOI] [Google Scholar]
- Mehta G.; Lakshminath S.; Talukdar P. A norbornyl route to aminocyclohexitols: syntheses of diverse aminocarbasugars and ‘confused’ aminocarbasugars. Tetrahedron Lett. 2002, 43, 335–338. 10.1016/S0040-4039(01)02125-6. [DOI] [Google Scholar]
- Legler G.The Catalytic Efficiency of Glycoside Hydrolases and Models of the Transition State Based on Substrate Related Inhibitors. In Carbohydrate Mimics: Concepts and Methods; Chapleur Y., Ed.; Wiley-VCH: New York, 1998; pp 463–490. [Google Scholar]
- Rickards R. W.; Duke R. K. Stereospecific total synthesis of the cyclohexane oxide antibiotic eupenoxide. J. Org. Chem. 1984, 49, 1898–1904. 10.1021/jo00185a010. [DOI] [Google Scholar]
- Baran A.; Balci M. Stereoselective Synthesis of Bishomo-inositols as Glycosidase Inhibitors. J. Org. Chem. 2009, 74 (1), 88–95. 10.1021/jo801344f. [DOI] [PubMed] [Google Scholar]
- Sadeghi-Khomami A.; Blake A. J.; Wilson C.; Thomas N. R. Synthesis of a Carbasugar Analogue of a Putative Intermediate in the UDP-Galp-Mutase Catalyzed Isomerization. Org. Lett. 2005, 7 (22), 4891–4894. 10.1021/ol0517877. [DOI] [PubMed] [Google Scholar]
- Brown R. T.; Jameson S. B.; Ouali D.; Tattersall P. I. Synthesis of chiral polyfunctionalised cyclopentanes from the Diels–Alder adduct of furan and maleic anhydride. J. Chem. Res. 2000, 2000, 176–178. 10.3184/030823400103166850. [DOI] [Google Scholar]
- Kasyan L. I.; Krishchik O. V.; Tarabara I. N.; Kas’yan A. O.; Palchikov V. A. Lactonization of epoxyendic anhydride in reactions with amines. Russ. J. Org. Chem. 2006, 42, 501–508. 10.1134/S1070428006040051. [DOI] [Google Scholar]
- Gündoğdu Ö.; Şahin E.; Kara Y. A unique synthesis of N-(Z)-5-bromo-6-hydroxyhexahydro isobenzofuran-1(3H)-ylidene)methanaminium bromide. Chem. Pap. 2024, 78 (2), 1347–1352. 10.1007/s11696-023-03141-3. [DOI] [Google Scholar]
- Gündoğdu Ö.; Atalay A.; Turhan P.; Kishali N. H.; Şahin E.; Bozkaya U.; Kara Y.. Reduction of 2-phenyl-3a,4,7,7a-tetrahydro1H-isoindole-1,3(2H)-dione with NaBH4: Investigation of exoselectivity and reaction mechanism via theoretical computations. 2024.
- Kwon M. S.; Woo S. K.; Na S. W.; Lee E. Total Synthesis of (+)-Exiguolide. Angew. Chem., Int. Ed. 2008, 47 (9), 1733–1735. 10.1002/anie.200705018. [DOI] [PubMed] [Google Scholar]
- Lakouraj M. M.; Mokhtary M. Polyvinylpolypyrrolidone-Bromine Complex, Mild and Efficient Polymeric Reagent for Selective Deprotection and Oxidative Deprotection of Silylethers. Lett. Org. Chem. 2007, 4, 64–67. 10.2174/157017807780037379. [DOI] [Google Scholar]
- Crouch R. D. Selective deprotection of sily ethers. Tetrahedron 2013, 69, 2383–2417. 10.1016/j.tet.2013.01.017. [DOI] [Google Scholar]
- Tan A.; Koc B.; Sahin E.; Kishali N. H.; Kara Y. Synthesis of new cantharimide analogues derived from 3-sulfolene. Synthesis 2011, 7, 1079–1084. 10.1055/s-0030-1258466. [DOI] [Google Scholar]
- Kishikawa K.; Naruse M.; Kohmoto S.; Makoto Yamamoto M.; Kentaro Yamaguch K. Investigation of arene–arene interaction in stereoselective m-CPBA epoxidation. J. Chem. Soc., Perkin Trans. I 2001, 462–468. 10.1039/b004098n. [DOI] [Google Scholar]
- Sheldrick G. M.SHELXS-97 and SHELXL-97, Program for Crystal Structure Solution and Refinement; University of Göttingen: Germany, 1997.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.