Abstract
Background
Dysbiosis of the gut microbiota is pivotal in Crohn’s disease (CD) and modulated by host physiological conditions. Hyperbaric oxygen therapy (HBOT) is a promising treatment for CD that can regulate gut microbiota. The relationship between HBOT and the gut microbiota in CD remains unknown.
Methods
CD patients were divided into an HBOT group (n = 10) and a control group (n = 10) in this open-label prospective interventional study. The fecal samples before and after HBOT were used for 16 S rRNA gene sequencing and fecal microbiota transplantation (FMT). A colitis mouse model was constructed using dextran sulfate sodium, and intestinal and systematic inflammation was evaluated. The safety and long-term effect of HBOT were observed.
Results
HBOT significantly reduced the level of C-reactive protein (CRP) (80.79 ± 42.05 mg/L vs. 33.32 ± 18.31 mg/L, P = 0.004) and the Crohn’s Disease Activity Index (CDAI) (274.87 ± 65.54 vs. 221.54 ± 41.89, P = 0.044). HBOT elevated the declined microbial diversity and ameliorated the altered composition of gut microbiota in patients with CD. The relative abundance of Escherichia decreased, and that of Bifidobacterium and Clostridium XIVa increased after HBOT. Mice receiving FMT from donors after HBOT had significantly less intestinal inflammation and serum CRP than the group before HBOT. HBOT was safe and well-tolerated by patients with CD. Combined with ustekinumab, more patients treated with HBOT achieved clinical response (30%vs.70%, P = 0.089) and remission (20%vs.50%, P = 0.160) at week 4.
Conclusions
HBOT modulates the dysbiosis of gut microbiota in CD and ameliorates intestinal and systematic inflammation. HBOT is a safe option for CD and exhibits a promising auxiliary effect to ustekinumab.
Trial registration
Chinese Clinical Trial Registry, ChiCTR2200061193. Registered 15 June 2022, https://www.chictr.org.cn/showproj.html?proj=171605.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12967-024-05317-1.
Keywords: Hyperbaric oxygen therapy, Crohn’s disease, Gut microbiota, Inflammation, Fecal microbiota transplantation
Introduction
Crohn’s disease (CD) is a chronic and relapsing inflammatory bowel disease (IBD) characterized by an impaired immune response, primarily affecting the gastrointestinal tract and exhibiting a progressive and destructive course [1]. CD is challenging to cure, 30% of patients requiring surgery within 5 years of diagnosis [2]. Therefore, new treatment methods to alleviate the burden of this disease are needed.
The gut microbiota, interacting with both the internal and external environments of the human body, plays a crucial role in the development of CD [3]. Numerous studies have extensively analyzed the characteristics and functional changes of the gut microbiota during CD onset, revealing dysregulation in its composition and functions under CD [4, 5]. The positive effects of fecal microbiota transplantation (FMT) in treating CD further underscore the importance of the gut microbiota [6, 7]. Collectively, the evidence suggests that interventions or modulation targeting the gut microbiota represent a promising direction for future CD treatments.
The composition of the gut microbiota is modulated by the oxygen. Alteration of host oxygenation could change the oxygen content of the luminal environment, suggesting that modulating the oxygenation of the gut could become a new approach for regulating the gut microbiota [8]. Hyperbaric oxygen therapy (HBOT) is a noninvasive treatment used in combination therapies for various diseases and is considered safe, having shown minimal side effects [9]. During HBOT, patients breathe 100% oxygen in a pressurized environment typically at 2.0-2.5 atmosphere absolute [10]. HBOT can improve depressive behavior in mice by reshaping the intestinal microbiota [11]. HBOT can enhance healing in ischemic colonic anastomosis by improving the local anaerobic environment [12]. Similarly, a few case reports have indicated that HBOT can improve clinical manifestations of IBD, although the underlying mechanisms have not been explored [13, 14]. Based on these findings, we hypothesize that HBOT will benefit patients with CD by modulating the composition of the host gut microbiota, thereby influencing the efficacy of biologic agents.
Therefore, we performed an open-label exploratory study to investigate the efficacy and safety of HBOT in CD patients. Omics analysis was employed to comprehensively understand the changes in the gut microbiota from before to after HBOT, followed by validation in an animal model to gain causal insights. Finally, a short-term follow-up was conducted to observe the impact of this therapy on the efficacy of subsequent biologic agents.
Methods
Patients and data collection
This trial was an open-label prospective interventional study and was conducted at the Xiangya Hospital Central South University, Changsha, Hunan Province, China. Patients were diagnosed by a multidisciplinary IBD team consisting of gastroenterologists, radiologists, and pathologists, following the guidelines of the Chinese Medical Association [15]. Patients aged 18 years or older with CD for at least 3 months were eligible for inclusion if they were in an active state with a baseline CD activity index (CDAI) score of 150–450. The treatment options for all patients were decided on after a comprehensive evaluation by the multidisciplinary team. All patients agreed to be treated with biologic agents. Patients with pregnancy, other concomitant diseases, or intolerance to ustekinumab were excluded from this trial. Patients were also not considered for this study if they had any disease contraindicating HBOT.
Grouping and intervention
All patients enrolled in this trial met the indications for ustekinumab and were treated with it. At week 0, patients received a single intravenous infusion of ustekinumab, the dose of which depended on the weight of the patients [16]. The dose of 260 mg was given to patients weighing ≤ 55 kg, 390 mg was given to those weighing > 55 kg and ≤ 85 kg, and 520 mg was given to those weighing > 85 kg. At week 8, patients received maintenance therapy with 90 mg of ustekinumab subcutaneously at a frequency of every 12 weeks. Data were stored and collected in the electronic medical record system.
As this was an open-label exploratory study, all eligible patients were divided into an HBOT group and a control group based on their informed consent. Patients in the HBOT group were treated with 10 sessions of HBOT for 5 days, at two sessions a day, before starting ustekinumab treatment. HBOT was performed under 2.5 atm absolute (ATA) for 100 min (increased pressure for 20 min, ordinary pressure for 60 min and decreased pressure for 20 min), and the patients breathed 100% oxygen with four scheduled breaks to minimize the side effects. This treatment procedure was similar to that in a previous study and remained well within oxygen toxicity limits [17]. Equipment, such as hyperbaric oxygen chambers (YC2410-24, Hoto Oxygen Industrial, Shangdong, China) required for this treatment, was provided by Xiangya Hospital Central South University. Patients in the control group received the ustekinumab treatment routinely during hospitalization at week 0.
Assessment of HBOT safety and short-term outcome
To evaluate the effectiveness of HBOT, the CDAI score, CRP level and fecal calprotectin level of patients before and after hyperbaric oxygen were compared. In terms of safety assessment, the supervising hyperbaric physician also evaluated the common side effects of HBOT, including complaints of trouble equalizing, visual changes and fatigue, in addition to assessing unsolicited adverse events after the treatment. The definition and quantification of side effects were based on Marvin’s review [18] to ensure accuracy and consistency.
DNA extraction, PCR, and 16 S rRNA gene sequencing
Fecal DNA were extracted with the Thermo Scientific KingFisher Apex platform. The V3-V4 region of the bacterial 16 S rRNA gene was amplified by polymerase chain reaction (PCR) using the corresponding fusion primer reaction system (forward primer: 338 F ACTCCTACGGGAGGCAGCAG; reverse primer: 806R GGACTACHVGGGTWTCTAAT). Nucleic acid gel electrophoresis was conducted for quality inspection. Single-stranded cyclized products were produced through denaturation and circularization, while uncyclized linear DNA molecules were digested. Single-stranded circular DNA molecules were replicated via rolling cycle amplification, and a DNA nanoball (DNB) containing multiple copies of DNA was generated. Sufficient-quality DNBs were then loaded into patterned nanoarrays using a high-intensity DNA nanochip technique and sequenced through combinatorial probe-anchor synthesis (cPAS). Raw data were filtered to generate adequate quality clean reads by truncating reads with low-average-quality base pairs and removing reads with adapter contamination, ambiguous bases, or low-complexity. Operating taxonomic units (OTUs) were clustered with a 97% threshold by UPARSE using USEARCH (v7.0.1090) and chimeras were filtered by UCHIME (v4.2.40). Representative OUT sequences were aligned by the RDP classifier (v2.2) against the corresponding database for taxonomic annotations. Alpha diversity was measured by the Shannon index and Simpson index. Beta diversity analysis was conducted by software QIIME software (v1.80).
Fecal microbiota transplantation
Before fecal microbiota transplantation (FMT), mice were fed with a cocktail of antibiotics (ampicillin 1 g/L, metronidazole 1 g/L, neomycin 1 g/L, and vancomycin 0.5 g/L) for 1 week to deplete microbiota, followed by wash-out period of 4 days to metabolize antibiotics before FMT, as reported in the previous literature [19]. Two patients’ (donors) feces before and after HBOT as donors were randomly selected for FMT. According to a previous study, 500 mg fresh stools from each donor before or after HBOT were weighed and homogenized by mixing with PBS buffer at a ratio of 100 mg/ml. After that, the mixture was centrifuged at 2500 × g for 5 min, and the supernatant was collected under anaerobic conditions. The prepared fecal samples were pooled and divided into the before HBOT (BH) group and the after HBOT (AH) group based on the different sources of stools. From 3 days before dextran sodium sulfate (DSS) intervention, the mice were colonized by oral gavage with 200 µl of BH or AH fecal slurry until the end of DSS intervention. The mice in each group were treated once daily.
DSS-induced colitis
C57BL/6J male mice were induced with colitis by administering 2.5% DSS (36,000 to 50,000 molecular weight; MP Biomedicals) in their drinking water for 7 days. Each mouse received the same volume of liquid. The body weight and stool consistency were monitored daily [20]. The mice were euthanized on day 8. The tissue and feces were collected for subsequent experiments.
Hematoxylin and eosin staining and histopathological analysis
The distal colon tissue was selected and fixed in 10% formalin solution, followed by embedding in paraffin. The tissue was then cut into 5-µm-thick sections and stained with hematoxylin and eosin (H&E) according to the standard protocols. Neutral balsam was used to cover the slides. According to published standards [20], histological blind scoring was assessed based on the degree of damage and inflammatory infiltration in the mucosal layer, submucosal layer, mucosal muscle layer, and serosal layer of the colon tissue. The total pathological score for each mouse was calculated.
Real-time qPCR
According to the manufacturer’s instructions, RNA from mouse colon tissue was isolated with TRIzol reagent (TransGen), and reverse transcribed into cDNA with the PerfectStart Uni RT-qPCR Kit (TransGen). The DNA from mouse stool was extracted using the EasyPure Stool Genomic DNA Kit (TransGen). Real-time PCR (RT-PCR) was performed by using SYBR Green Master mix with ROX and an ABI QuantStudio 5 Flex instrument. The primers were listed in Table 1S. The gene expression was normalized to Gapdh. The specific genes were quantified to indicate the expression profile of the microbial community and normalized to total bacterial 16 S rRNA.
Table 1.
Baseline characteristics of study patients
| Variable | Control group(n = 10) | HBOT group(n = 10) | P value |
|---|---|---|---|
| Age, mean ± SD, years | 31.92 ± 13.08 | 27.20 ± 7.26 | 0.332 |
| BMI, mean ± SD, kg/m2 | 19.4 ± 2.2 | 18.6 ± 2.5 | 0.457 |
| Male, n (%) | 6(60%) | 8(80%) | 0.628 |
| Location, n (%) | |||
| L1 | 0(0%) | 0(0%) | 1.000 |
| L2 | 4(40%) | 2(20%) | 0.314 |
| L3 | 6(60%) | 8(80%) | 0.314 |
| L4 | 0(0%) | 0(0%) | 1.000 |
| Behavior, n (%) | |||
| B1 | 3(30%) | 3(30%) | 1.000 |
| B2 | 7(70%) | 5(50%) | 0.650 |
| B3 | 0(0%) | 2(20%) | 0.474 |
| Perianal fistulas, n (%) | 6(60%) | 8(80%) | 0.628 |
| Surgical history, n (%) | 4(40%) | 3(30%) | 1.000 |
| CDAI, mean ± SD | 233.47 ± 53.84 | 274.87 ± 65.54 | 0.140 |
| Serological examination | |||
| WBC, mean ± SD, 109/L | 6.49 ± 1.91 | 7.38 ± 2.28 | 0.357 |
| HGB, mean ± SD, g/L | 120.42 ± 22.71 | 118.40 ± 15.78 | 0.819 |
| PLT, mean ± SD, 109/L | 325.83 ± 116.34 | 437.00 ± 167.15 | 0.101 |
| NLR, mean ± SD | 4.42 ± 2.38 | 4.47 ± 1.77 | 0.958 |
| PLR, mean ± SD | 315.95 ± 193.91 | 291.96 ± 129.79 | 0.749 |
| ALB, mean ± SD, g/L | 31.6 ± 4.7 | 29.6 ± 4.3 | 0.334 |
| D-D, mean ± SD, mg/L | 0.17 ± 0.09 | 0.21 ± 0.10 | 0.359 |
| ESR, mean ± SD, mm/h | 61.75 ± 36.29 | 83.80 ± 19.30 | 0.107 |
| CRP, mean ± SD, mg/L | 41.12 ± 40.61 | 80.79 ± 42.05 | 0.046 |
| FC, n (%) | 1.000 | ||
| >1800ug/g | 10 (100%) | 10 (100%) | |
| 20ug/g- 1800ug/g | 0 (0%) | 0 (0%) | |
| ≤20ug/g | 0 (0%) | 0 (0%) | |
| SES-CD, mean ± SD | 10.8 ± 2.5 | 13.2 ± 3.8 | 0.113 |
Abbreviation BMI, body mass index; CDAI, Crohn’s disease activity index; WBC, white blood cell; HGB, hemoglobin; PLT, platelet; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; FC, fecal calprotectin; SES-CD, simple endoscopic score for Crohn’s disease; SD, standard deviation; HBOT, hyperbaric oxygen therapy
Enzyme-linked immunosorbent assay
Serum samples were collected and centrifuged within one hour and stored at − 80 °C. The expression levels of COX2, PGE2 and CRP in serum were measured by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s protocols (Cambridge, MA, U.S.A.).
Observation of hysteresis effects of HBOT
To monitor the effects of HOBT on CD patients treated with ustekinumab, additional follow-up was conducted. As for safety assessment, in addition to the HBOT-related adverse effects mentioned earlier, any adverse reactions experienced by patients during ustekinumab administration following completion of HBOT should also be documented. The primary outcome was the clinical response at week 4, defined as a reduction of 70 points or more from the baseline in CDAI score [21]. The secondary outcomes included clinical response and endoscopic response at week 8, clinical remission and endoscopic remission at week 8, and the normalization of CRP and fecal calprotectin levels at weeks 4 and 8. Clinical remission was defined as a CDAI less than 150, and endoscopic remission was defined as an SES-CD score below 3. Endoscopic remission was considered when the SES-CD score fell by more than half from baseline [22]. The normalization of CRP was defined as less than 6 mg/L.
Statistical analysis
Continuous variables, expressed as median and interquartile range, were analyzed using Student’s t test or nonparametric tests (Mean-Whitney test) for those not following a normal distribution. Categorical variables, such as clinical response and clinical remission, are expressed as n (%). Differences between categorical variables were analyzed using the chi-square test or Fisher’s exact probability test. All reported P values were two-tailed, and a value of P < 0.05 indicated statistical significance. The microbial variations and grouping significance of HBOT were tested using permutational MANOVA. The genera that differed in proportion before vs. after HBOT were identified with absolute fold change > 2 and adjusted P < 0.05 using the R package “DEseq2”. Correlations between the Shannon index, the level of CRP, and the relative abundance of significant genera were analyzed with the Spearman method. Linear regression models were constructed to evaluate the pairwise correlation. Estimates (Es) were extracted from the fitted linear regression models. The R package “mediation” was then used to perform casual mediation analysis. Average direct effect (ADE) was calculated to measure the direct effect of HBOT treatment on CRP level. Average causal mediated effect (ACME) was calculated to observe the mediated effect of Shannon index on CRP level. Analyses were performed with SPSS software (version 26.0) and R platform (4.2.1). For animal experiments, all analyses were performed with GraphPad Prism (version 9.0).
Results
Demographic characteristics of patients at baseline
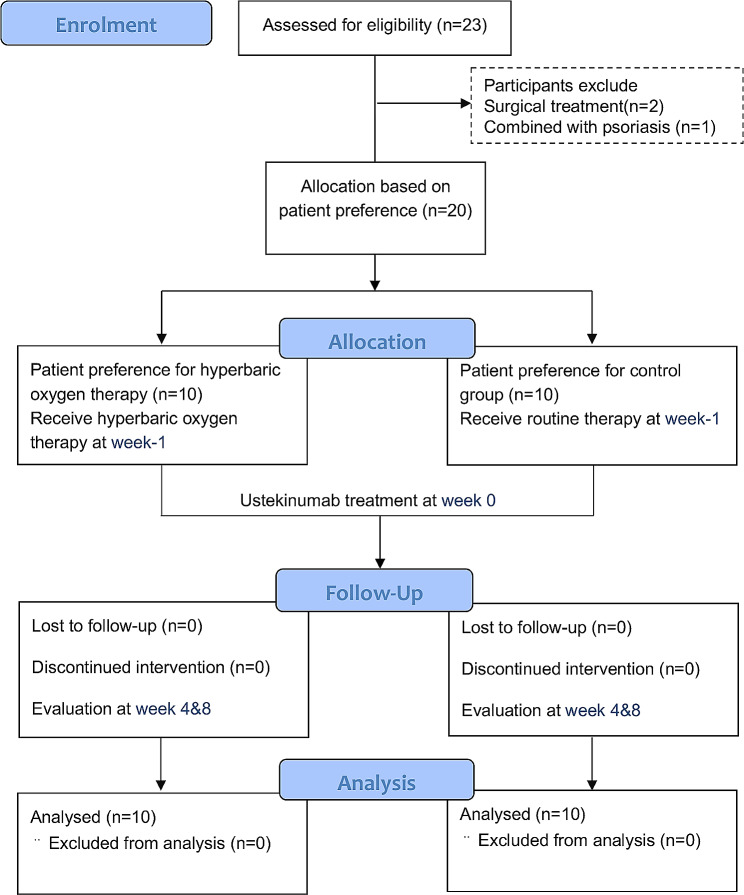
Twenty-three patients were recruited for the study, two of whom withdrew because they chose surgical treatment during the screening period, and one patient withdrew due to concomitant psoriasis. Enrolled patients in this study were divided into two groups according to whether they received HBOT, and a total of 20 patients were finally recruited (Fig. 1). The patients in the two groups were similar in demographic data (P > 0.05). In terms of laboratory tests, all enrolled patients had fecal calprotectin levels > 1800 µg/g. Patients in the HBOT group had higher levels of CRP than those in the control groups (80.79 ± 42.05 mg/L vs. 41.12 ± 40.61 mg/L, P = 0.046), but there were no significant differences in disease activity defined by the CDAI score (P = 0.140) or SES-CD score (P = 0.113). Table 1 displays the baseline characteristics of the study patients.
Fig. 1.
Flow of study recruitment, interventions and outcomes assessments
The levels of CRP and CDAI score decreased after HBOT
After 10 sessions of HBOT, the levels of CRP (80.79 ± 42.05 mg/L vs. 33.32 ± 18.31 mg/L, P = 0.004) and the CDAI (274.87 ± 65.54 vs. 221.54 ± 41.89, P = 0.044) decreased significantly from baseline among the patients in the HBOT group, but the erythrocyte sedimentation rate (ESR) did not decrease (P = 0.251). A decrease in fecal calprotectin levels was observed in three patients after hyperbaric oxygen, in one of whom it returned to the normal level. There was also a mild decrease in the serum levels of hemoglobin, although this decrease was not statistically significant (P > 0.05), as shown in Table 2.
Table 2.
Comparison of disease status before and after 10 sessions of HBOT
| Variable | Before HBOT(n = 10) | After HBOT(n = 10) | P value |
|---|---|---|---|
| CDAI, mean ± SD | 274.87 ± 65.54 | 221.54 ± 41.89 | 0.044 |
| Serological examination | |||
| WBC, mean ± SD, 109/L | 7.38 ± 2.28 | 7.47 ± 2.30 | 0.930 |
| HGB, mean ± SD, g/L | 118.40 ± 15.78 | 108.80 ± 11.71 | 0.139 |
| PLT, mean ± SD, 109/L | 437.00 ± 167.15 | 416.80 ± 149.16 | 0.779 |
| NLR, mean ± SD | 4.47 ± 1.77 | 3.69 ± 1.10 | 0.252 |
| PLR, mean ± SD | 291.96 ± 129.79 | 294.43 ± 80.74 | 0.959 |
| ALB, mean ± SD, g/L | 29.6 ± 4.3 | 31.8 ± 5.3 | 0.322 |
| D-D, mean ± SD, mg/L | 0.21 ± 0.10 | 0.16 ± 0.07 | 0.212 |
| ESR, mean ± SD, mm/h | 83.80 ± 19.30 | 72.40 ± 23.50 | 0.251 |
| CRP, mean ± SD, mg/L | 80.79 ± 42.05 | 33.32 ± 18.31 | 0.004 |
| FC, n (%) | 0.171 | ||
| >1800ug/g | 10 (100%) | 7 (70%) | |
| 200ug/g- 1800ug/g | 0 (0%) | 2 (20%) | |
| ≤200ug/g | 0 (0%) | 1 (10%) |
Abbreviation CDAI, Crohn’s disease activity index; WBC, white blood cell; HGB, hemoglobin; PLT, platelet; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; FC, fecal calprotectin; SES-CD, simple endoscopic score for Crohn’s disease; SD, standard deviation; HBOT, hyperbaric oxygen therapy
Considering the safety of HBOT, the side effects of HBOT include complaints of trouble equalizing, visual changes and fatigue. No patients experienced hyperbaric oxygen-related adverse events, and no patients were unable to tolerate the 10 sessions.
The diversity and composition of the gut microbiota changed after HBOT
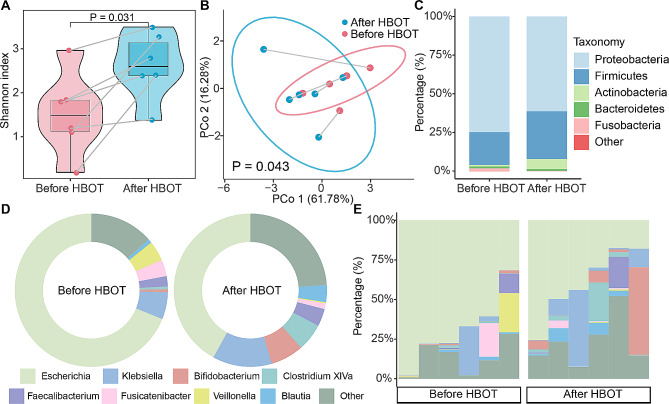
We then explored the alterations in the gut microbiota in patients from before to after HBOT. After quality testing, the fecal samples from six pairs of patients met the requirements. The Shannon index of the gut microbiota in patients after HBOT was significantly higher than that in the same patients before HBOT (P = 0.031, Fig. 2A). Similar notable changes in alpha diversity were also observed with Simpson’s index (Figure S1). The composition of the gut microbiota was significantly altered among patients after HBOT (P = 0.043), and the variations in matched samples before and after HBOT were distinguished by the first principle coordinate axis (Fig. 2B).
Fig. 2.
Diversity and composition of gut microbiota in patient with CD before and after HBOT. (A) The boxplot showed the corresponding change of Shannon index in patients with CD before and after HBOT. (B) Permutational multivariate analysis of variance (Adonis) showed that the composition of the gut microbiota was significantly altered among patients after HBOT (P = 0.043). (C) The stack plot of average relative abundance in the group of before HBOT and after HBOT at phylum level. (D) The circle plot exhibited the compositional differences of genera before and after HBOT. (E) The stack plot of each sample showed the taxonomic composition in individuals with CD. CD, Crohn’s disease; HBOT, hyperbaric oxygen therapy
The averages relative abundance of Proteobacteria decreased from 74.8 to 61.3% after HBOT treatment. The relative abundance of Firmicute and Actinobacteria increased by 9.5% and 5.5%, respectively. Fusobacteria dropped from 1.8% before HBOT to 0.1% after HBOT (Fig. 2C). At the genus level, the average relative abundance of Escherichia decreased from 67.8 to 34.9% after HBOT. The relative abundance of Bifidobacterium, Klebsiella, and Clostridium XIVa rose by 5.2%, 4.8%, and 4.2%, respectively (Fig. 2D). The dominant Escherichia, with over 50% relative abundance in five out of six patients with CD, dropped its abundance to less than 50% in four out of the six after HBOT. (Fig. 2E).
Significant microbial diversity mediated the causal relationship between HBOT and CRP
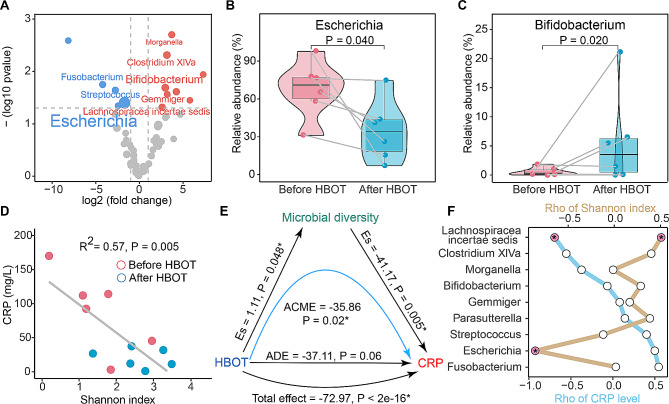
A total of 18 significantly differential genera were identified, with 11 enriched and seven depleted after HBOT. Among genera with relative abundance > 0.5%, Escherichia, Fusobacterium, and Streptococcus markedly reduced after HBOT, and five genera increased after HBOT, including Lachnospiracea incertae sedis, Gemmiger, Bifidobacterium, Clostridium XIVa, and Morganella (Fig. 3A). Escherichia was the leading genus in patients with CD, with a maximum decrease of up to 90.8% after HBOT (Fig. 3B). Bifidobacterium, with the highest relative abundance enriched in after HBOT, notably increased by 21.0% at most (Fig. 3C).
Fig. 3.
Significant genera and the relationship between HBOT, gut microbiota, and CRP. (A) The volcano plot revealed the significantly differential genera after HBOT. The size of genus name represented the average relative abundance in CD. Genera in blue meant depletion and genera in red meant enrichment after HBOT. The boxplot showed the change of Escherichia (B) and Bifidobacterium (C) in corresponding samples before and after HBOT. (D) The scatter plot and linear regression exhibited a significant negative correlation between Shannon index and the level CRP. (E) The plot demonstrated the correlation and casual relationships among HBOT, microbial diversity, and the level of CRP. (F) The plot reveals the coefficients between the relative abundance of significant genera and Shannon index (in brown) / the level of CRP (in blue). Plot in red with a “*” means P value < 0.05 in Spearman test. CRP, C-reactive protein; HBOT, hyperbaric oxygen therapy
We then explored the relationship between gut microbiota and the level of CRP. With the decrease in CRP after HBOT, the Shannon index of the gut microbiota in patients with CD increased. There was a significant negative linear correlation between the CRP level and Shannon index (R2 = 0.57, P = 0.005, Fig. 3D). There were also notable relationships between HBOT, the Shannon index of gut microbiota, and the level of CRP. Through causal mediation analysis, we found that HBOT had a strong total effect on down-regulating the level of CRP. The average direct effect of HBOT on CRP might not be of important (P = 0.06), and the average causal mediation effects of HBOT on CRP mediating the Shannon index were marked (P = 0.02, Fig. 3E). This indicates that ten sessions of HBOT significantly reduce the level of CRP in patients with CD, and gut microbial diversity plays an essential role in mediating this effect.
With an increase in the correlation coefficient between significant differential genera after HBOT and the level of CRP, the rho coefficient between these genera and the Shannon index exhibited a declining trend. Lachnospiracea incertae sedis had a notably positive correlation with the Shannon index and a negative correlation with the level of CRP. The rho between the Escherichia and Shannon index remarkably reached − 0.92, suggesting that the relative abundance of the Escherichia served as a negative indicator for the Shannon index (Fig. 3F).
Fecal microbiota transplantation from gut microbiota after HBOT alleviates colitis in mice
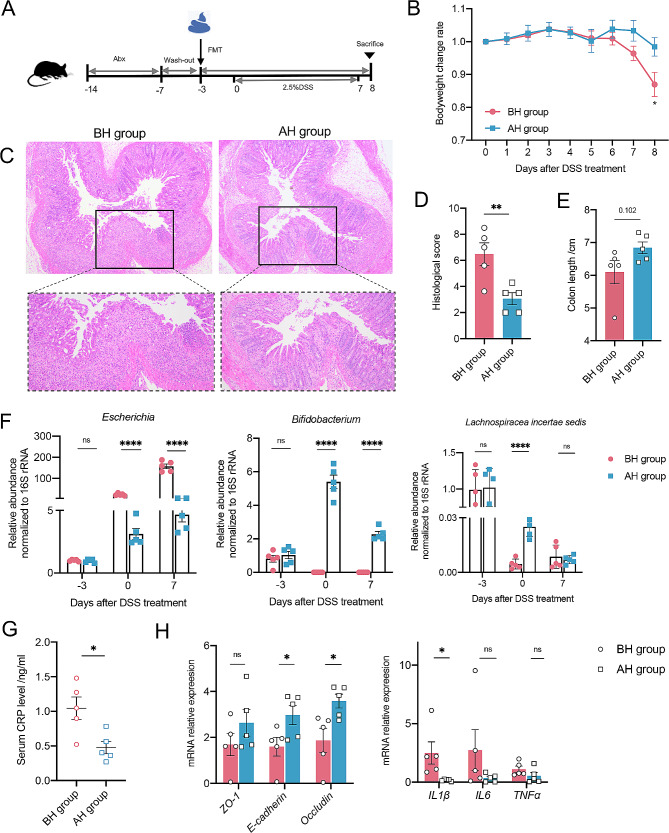
To investigate the potential causal relationship between the widespread gut microbiota alteration induced by HBOT and the reduction of inflammation in patients with CD, a FMT was performed into conventional antibiotic-treated mice fed with DSS (Fig. 4A). The fecal samples used in FMT were from enrolled patients before or after HBOT. Compared with the BH group, FMT from the AH group resulted in significantly attenuated weight loss and increased colon length (Fig. 4B and E). Histological assessments showed much lower inflammatory cell infiltration, mucosal damage, and overall histology score in the colon after FMT form the AH group (Fig. 4C and D). Additionally, the AH group had a lower level of CRP in the serum (Fig. 4G). Further analysis revealed that the fecal samples from mice receiving FMT form AH fecal samples had a lower relative abundance of Escherichia and a higher relative abundance of Bifidobacterium on day 7 of DSS intervention than the BH group, but there were no significant differences in Lachnospiracea incertae sedis (Fig. 4F). At the molecular level, the expression of genes encoding inflammatory proteins was reduced after receiving the FMT from AH samples (Fig. 4H). There were increased expression of genes related to tight junctions in the AH groups, suggesting that the destruction of gut barrier integrity was relatively light (Fig. 4H).
Fig. 4.
Fecal microbiota transplantation from gut microbiota after HBOT alleviates colitis in mice. (A) The experimental design showing an FMT from human donors to mice. (B) Body weights shown as percentage of starting weight (n = 5 per group) and colon length (E). (C) Representative images of distal colon stained with H&E and histological score was calculated (D). (F) Time-course changes in the relative abundance of Escherichia, Bifidobacterium, and Lachnospiracea incertae sedis. (G) Quantification of serum levels of CRP. (H) Expression levels of genes involved in tight junction protein and inflammation. Data points represent individual mice. All data are represented as means ± SEM. p values were calculated by Student’s t test. For (B), (F) Student’s t test was performed independently at each time point; ∗p < 0.05, ∗∗p < 0.01, and∗∗∗p < 0.001. FMT, Fecal microbiota transplantation; H&E, Hematoxylin and eosin staining; CRP, C-reactive protein
HBOT shows a trend toward improving clinical outcomes in patients treated with ustekinumab at week 4
After treatment with ustekinumab at week 4, a higher proportion of patients treated with HBOT achieved clinical response (30% vs. 70%, P = 0.089) and remission (20% vs. 50%, P = 0.160) than patients in the control groups. However, both groups showed similar proportions of clinical response (60% vs. 80%, P = 0.628) and remission (50% vs. 70%, P = 0.325) at week 8. When considering endoscopic remission and endoscopic response, both groups showed similar patient distributions at 8 weeks (P > 0.05).
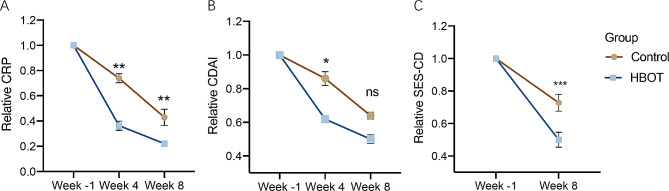
There were no significant differences in the CDAI score or CRP levels between the two groups, either at week 4 or at week 8 (Figure S2). However, when considering the relative change rate of the above parameters, there were greater differences in these two groups (Fig. 5), ignoring the situation where the control group did not receive sham hyperbaric air. At week 4, patients exposed to hyperbaric oxygen exhibited a trend toward higher rates of normalization in CRP (20% vs. 50%, P = 0.160) as well as fecal calprotectin (20% vs. 50%, P = 0.126), but at week 8, the rates in both groups remained similar (Table 3).
Fig. 5.
Time-course changes in the variables assessed for endpoints between control group and HBOT group. Graphs show the relative changes of CRP level (A), CDAI (B) and SES-CD (C) at different time points (n = 10 per group). The relative decline rates at week 4 and/or week 8 compared to baseline were tested by Student’s t test (n = 10 per group), ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. CDAI, Crohn’s disease activity index; CRP, C-reactive protein; SES-CD, simple endoscopic score for Crohn’s disease; HBOT, hyperbaric oxygen therapy
Table 3.
Comparison of clinical outcomes with different treatments
| Variable | Control group(n = 10) | HBOT group(n = 10) | P value |
|---|---|---|---|
| Week 4 | |||
| CDAI, mean ± SD | 197.67 ± 63.71 | 188.87 ± 51.21 | 0.738 |
| CRP, mean ± SD, mg/L | 30.19 ± 16.00 | 25.86 ± 22.40 | 0.875 |
| CRP normalization, n (%) | 2 (20%) | 5 (50%) | 0.160 |
| FC, n (%) | 0.129 | ||
| >1800ug/g | 5 (40%) | 1 (10%) | |
| 200ug/g- 1800ug/g | 3 (40%) | 4 (40%) | |
| ≤200ug/g | 2 (20%) | 5 (50%) | |
| Clinical response, n (%) | 3 (30%) | 7(70%) | 0.089 |
| Clinical remission, n (%) | 2(20%) | 5(50%) | 0.160 |
| Week8 | |||
| CDAI, mean ± SD | 160.52 ± 75.99 | 146.05 ± 64.34 | 0.651 |
| CRP, mean ± SD, mg/L | 16.08 ± 17.91 | 15.9 ± 17.52 | 0.988 |
| CRP normalization, n (%) | 7(70%) | 7(70%) | 1.000 |
| FC, n (%) | 0.476 | ||
| >1800ug/g | 1 (10%) | 0 (0%) | |
| 200ug/g- 1800ug/g | 3(30%) | 2(20%) | |
| ≤200ug/g | 6(60%) | 8 (80%) | |
| SES-CD, mean ± SD | 8.6 ± 5.9 | 7.2 ± 4.8 | 0.568 |
| Clinical response, n (%) | 6(60%) | 8(80%) | 0.628 |
| Clinical remission, n (%) | 5 (50%) | 7 (70%) | 0.325 |
| Endoscopic response, n (%) | 3 (30%) | 3 (30%) | 1.000 |
| Endoscopic remission, n (%) | 1 (10%) | 2(20%) | 0.500 |
Abbreviation CDAI, Crohn’s disease activity index; CRP, C-reactive protein; FC, fecal calprotectin; SES-CD, simple endoscopic score for Crohn’s disease; SD, standard deviation; HBOT, hyperbaric oxygen therapy
Nor did any patient experience treatment-related discomfort during the 8-week follow-up period after taking ustekinumab.
Hyperbaric oxygen therapy ameliorates the inflammatory cascade by regulating COX2/PGE2
Furthermore, we conducted a preliminary investigation of the molecular mechanisms associated with the effects of HBOT in patients with CD. We found that the protein expression of COX2 was significantly decreased by hyperbaric oxygen exposure (0.080 ± 0.003 ng/ml vs. 0.076 ± 0.002 ng/ml, P = 0.014, Figure S3A). Similarly, PGE2, a key inflammatory mediator generated catalytically by COX2, showed the same trend after hyperbaric oxygen treatment (3185.2 ± 389.8 pg/ml vs. 2778.6 ± 364.0 pg/ml P = 0.029, Figure S3B). This result suggested that hyperbaric oxygen may inhibit the occurrence of the inflammatory cascade by regulating the expression of COX2/PGE2.
Discussion
The available therapies for patients with CD are complex due to the various disease characteristics. The reduction in diversity and the altered gut microbial composition plays a pivotal role in the dynamics of CD and emerge as an outstanding potential target for CD treatment [23, 24]. We found that the clinical application of HBOT significantly downregulated CRP and lowered the CDAI in patients with CD. HBOT also mitigated the altered composition of gut microbiota and improved the reduced microbial diversity found in CD. By modulating the gut microbiota, HBOT reduced the serum level of CRP and intestinal inflammation. Prospectively, HBOT combined with ustekinumab improved the efficiency of agents without increasing adverse reactions. An initial exploration of the potential mechanism indicated that HBOT may inhibit the COX2/PGE2 to ameliorate the inflammation, thereby exerting a rapid effect on patients with CD.
Although the pathogenesis of CD remains unclear, many studies have demonstrated that the inflammatory response is involved in the development and progression of the disease [25]. Recent studies have emphasized that early control of inflammation enhances the response to agents and leads to a reduction in complications [26]. Interestingly, our study found that the decrease in CRP was statistically significant after HBOT. This result suggested that HBOT might play a role in reducing the inflammatory load, which has been reported in other studies. However, the ESR, another indicator of reactive inflammation levels, was not significantly decreased. This may be because the ESR was slow to react to positive acute-phase reactants, resulting in slow recognition of the inflammatory response, let alone minor inflammation [27]. Conversely, CRP, with its faster kinetics and a shorter half-life, can decrease rapidly upon inflammation resolution, allowing for monitoring of the treatment response [28]. In addition, CRP has been increasingly used as a secondary endpoint in CD clinical trials, as an increasing number of studies have found that combining biomarker and CDAI-defined endpoints may improve the trial efficiency by increasing the separation of response rates in different intervention groups [29]. Therefore, CRP has been used in over 50 clinical trials as a biomarker of activity in CD [30]. The above results demonstrated that HBOT has good anti-inflammatory effects.
HBOT significantly ameliorated the dysbiosis of the gut microbiota in CD patients. The gut microbiota, as a complex and intensively populated community, is located in an environment determined by host physiological status [8]. HBOT to the host increases oxygenation from intestinal tissue to the lumen, and the composition of the gut microbiota can be altered by directly affecting microbes and by regulating the host immune system [31, 32]. In our study, the relative abundance of predominant Escherichia in patients with CD markably declined after HBOT. As a facultative anaerobic genus, the growth of Escherichia was inhibited under hyperbaric oxygen, according to ex vivo experiments [33]. The relative abundance of Enterobacteriaceae in HBOT treated mice was significantly lower than that in controls [8]. With the decline in Escherichia, more ecological niches became available and the microbial diversity significantly rose. The relative abundance of other genera increased, mainly including Bifidobacterium, Klebsiella, and Clostridium XIVa. However, the anaerobic or aerobic nature of microbes does not represent the trend of the population in their host after HBOT. More attention should be paid to the dynamic pattern of gut microbiota after HBOT in health and diseases.
As one of the leading hallmarks in CD, microbial dysbiosis is characterized by lower reduction and altered composition [34]. Different microbial signatures have been established in various observational studies, and Escherichia is one of the most pivotal genera enriched in CD [35–37]. In our CD cohort, Escherichia occupied over 50% of the relative abundance in five of the six CD patients before HBOT and had a strong linear correlation with microbial diversity. The onset and relapse of CD was suggested to correlate with the systemic immune response to different bacterial events and the level of anti-E. coli was positively correlated with serum inflammatory cytokines including IL6 and IFNα [38]. Mesenteric fat plays a significant role in connecting E. coli with the immunological activity of patients with CD. A high abundance of Escherichia might cause bacterial translocation to mesenteric fat and trigger adipocytes to produce more CRP [39]. The decreases in Bifidobacterium and Clostridium XIVa are a part of the characteristics of dysbiosis in CD as well [34]. Bifidobacterium longum has been demonstrated to have the capacity to relieve colitis and the potential to be an auxiliary treatment [40]. Clostridium XIVa has been strongly correlated with the intestinal metabolism of deoxycholic acid, and the level of bile acid metabolism was also significantly reduced in patients with CD compared with healthy controls [41]. Klebsiella was found elevated after HBOT in our study, but the trend was not significant. The Klebsiella is a potential pathobiont in CD, and a clade of Klebsiella pneumoniae strains could enhance intestinal inflammation [42]. Dysbiosis even contributed to the pathogenesis and activity of CD, and the situation was improved after HBOT.
HBOT ameliorated intestinal inflammation by regulating the gut microbiota. In this study, we performed causal mediation analysis and FMT to identify the causal relationship between HBOT, gut microbiota and inflammation. Consistent with the traits of their human FMT donors, the mice receiving FMT from AH samples exhibited a higher relative abundance of Bifidobacterium and a lower proportion of Escherichia compared with the BH group. In the AH groups, the expression level of tight-junction genes was upregulated, and the gene expression of inflammation was downregulated. Escherichia has the potential to destroy gut barrier integrity, cause bacterial translocation, and induce systematic inflammation [39, 43]. By contrast, the Bifidobacterium produces short-chain fat acids, improves intestinal barrier functions, prevents bacterial translocation, and reduces inflammation [44, 45]. These bacteria play a pivotal role in the mediating the protective function of HBOT, which was verified by the fact that mice receiving FMT from the AH group had significantly lower serum CRP and histology scores in intestinal tissue.
In addition to gut microbiota mediation, there might be other mechanisms underlying the treatment effect of HBOT in CD. There has been little research on the mechanism by which hyperbaric oxygen improves diseases [46]. Based on the observations of our study in the clinical phenomenon, a preliminary exploration was performed. Through the detection of related proteins in the serum of patients before and after hyperbaric oxygen treatment, we found that hyperbaric oxygen could effectively reduce the expression of COX2, which was the key link causing the subsequent inflammatory response. COX2 is regarded as an inducible enzyme which catalyzes the generation of prostaglandins [47]. The expression of COX2 was low in normal tissue cells, but elevated 10 to 80 folds in inflammatory states, resulting in an increase in PGE2 [48]. PGE2, a proinflammatory mediator, is significantly increased during CD. PGE2 plays a positive auxiliary role in TNFα activation in macrophages by regulating intracellular cAMP levels [49]. Our results confirmed that HBOT reduced the expression of COX2 and PGE2, which may decrease the occurrence of the inflammatory cascade in vivo. Early resolution of inflammation may be beneficial to the efficacy of ustekinumab [25]. Therefore, we speculate that hyperbaric oxygen therapy may inhibit the expression of COX2/PGE2, thereby reducing the inflammatory load at the early stage of treatment and effectively promoting the onset of action in ustekinumab treatment.
Although ustekinumab has shown promising therapeutic effects in CD, the onset of action is modest [50]. We found that a higher proportion of patients achieved clinical response and remission at week 4 after hyperbaric oxygen exposure. This may be because hyperbaric oxygen therapy inhibits the continued production of inflammatory factors, allowing more ustekinumab to neutralize interleukins.However, whether the impact of HBOT will affect long-term clinical outcomes still requires time for continuous follow-up. Interestingly, our subsequent follow-up also demonstrated that additional HBOT does not increase the side effects of ustekinumab, but this requires a larger cohort to verify.
Currently, there are still many non-traditional therapies being discovered for patients with CD. Stem cell transplantation has been proven to be effective in treating CD, but it is difficult to operate and expensive [51, 52]. FMT is also an emerging treatment [53, 54], but it depends on stool banks and certain medical technologies [55]. In this study, HBOT was found to have a positive effect on the relief of CD. This therapy is easily accessible and operable, making it a viable option for hospitals with limited resources. While the efficacy of HBOT may be limited, its easy accessibility allows for wider promotion and application, ultimately benefiting more potential patients compared to certain non-traditional methods.
There were some limitations in the study. First, due to the groundbreaking exploratory nature of the research, the sample size was small. There may be some bias in inclusion. Patients with more severe diseases may be more inclined to choose HBOT treatment, which also leads to higher baseline CRP levels in the HBOT group. Therefore, the results should be regarded as evidence that a clinical benefit of HBOT may exist to guide further trial designs. Meanwhile, the patients in control group did not receive sham hyperbaric air and lacked detection of microbial composition. In future prospective studies, we will further balance the baseline characteristics of the two groups and address these shortcomings to further verify our results. Second, patients with CD in our study had a high relative abundance of Escherichia before HBOT and exhibited an increase in Bifidobacterium and Clostridium XIVa after HBOT. The specific functions of these genera should be clarified, and the interactions between the gut microbiota and host during the process of HBOT should be further explored. Finally, this study focused on the mediating effect of the gut microbiota in HBOT treatment. Other mechanisms of HBOT in patients with CD should be further explored, especially the cellular and molecular mechanisms in the host. More in-depth studies need to be performed at the animal and cellular levels.
Conclusion
Hyperbaric oxygen therapy ameliorates intestinal and systematic inflammation by modulating dysbiosis of the gut microbiota in CD. Hyperbaric oxygen has potential benefits for patients with CD undergoing ustekinumab treatment. Nonetheless, high-quality phase 2 clinical studies and basic research are still needed for verification.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank AJE (www.aje.com) for its linguistic assistance during the preparation of this manuscript.
Abbreviations
- CD
Crohn’s disease
- IBD
Inflammatory bowel disease
- ATA
Atm absolute
- PCR
Polymerase chain reaction
- OTUs
Operating taxonomic units
- FMT
Fecal microbiota transplantation
- BMI
Body mass index
- CDAI
Crohn’s disease activity index
- WBC
White blood cell
- HGB
Hemoglobin
- PLT
Platelet
- NLR
Neutrophil-to-lymphocyte ratio
- PLR
Platelet-to-lymphocyte ratio
- ESR
Erythrocyte sedimentation rate
- CRP
C-reactive protein
- FC
Fecal calprotectin
- SES-CD
Simple endoscopic score for Crohn’s disease
- SD
Standard deviation
- HBOT
Hyperbaric oxygen therapy
Author contributions
Study concept and design: YL, HXY, YP, XWL. Acquisition of data: CL, KZL, ZHP, DX, FLH, KKT. Analysis and interpretation of data: YL, RZS, CL, KZL, ZHP, DX, YP. Statistical analysis: YL, RZS, CL, YP. Drafting of the manuscript: YL, RZS, YP, XWL. Critical revision of the manuscript for important intellectual content: YP, XWL. All authors approved the final version of the manuscript, including the authorship list.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No.82230019, Grant No.82170599, and Grant No.82070651).
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Xiangya Hospital Central South University (ID:2022033103). All enrolled patients gave written informed consent for their participation in this study. All animal experiments in this study were carried out with prior approval of Central South University Institutional Animal Care and Use Committee.
Consent for publication
All authors have approved the manuscript and agree with the submission.
Data sharing statement
All data included in this study are available upon request by contact with the corresponding author.
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yong Li, Ruizheng Sun and Chen Lai contributed equally to this work.
Contributor Information
Yu Peng, Email: pengyu918@csu.edu.cn.
Xiaowei Liu, Email: liuxw@csu.edu.cn.
References
- 1.Roda G, Chien Ng S, Kotze PG, Argollo M, Panaccione R, Spinelli A, et al. Crohn’s disease. Nat Rev Dis Primers. 2020;6(1):22. doi: 10.1038/s41572-020-0156-2. [DOI] [PubMed] [Google Scholar]
- 2.Frolkis AD, Dykeman J, Negron ME, Debruyn J, Jette N, Fiest KM, et al. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology. 2013;145(5):996–1006. doi: 10.1053/j.gastro.2013.07.041. [DOI] [PubMed] [Google Scholar]
- 3.Lavelle A, Sokol H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020;17(4):223–37. doi: 10.1038/s41575-019-0258-z. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd-Price J, Arze C, Ananthakrishnan AN, Schirmer M, Avila-Pacheco J, Poon TW, et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 2019;569(7758):655–62. doi: 10.1038/s41586-019-1237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao S, Gao X, Zhu R, Wu D, Feng Z, Jiao N, et al. Microbial genes outperform species and SNVs as diagnostic markers for Crohn’s disease on multicohort fecal metagenomes empowered by artificial intelligence. Gut Microbes. 2023;15(1):2221428. doi: 10.1080/19490976.2023.2221428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao J, Zhao X, Hu S, Huang Z, Hu M, Jin S, et al. Gut microbial DL-endopeptidase alleviates Crohn’s disease via the NOD2 pathway. Cell Host Microbe. 2022;30(10):1435–49. doi: 10.1016/j.chom.2022.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Ma H, Hu T, Tao W, Tong J, Han Z, Herndler-Brandstetter D, et al. A lncRNA from an inflammatory bowel disease risk locus maintains intestinal host-commensal homeostasis. Cell Res. 2023;33(5):372–88. doi: 10.1038/s41422-023-00790-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albenberg L, Esipova TV, Judge CP, Bittinger K, Chen J, Laughlin A, et al. Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology. 2014;147(5):1055–e638. doi: 10.1053/j.gastro.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camporesi EM, Bosco G. Mechanisms of action of hyperbaric oxygen therapy. Undersea Hyperb Med. 2014;41(3):247–52. [PubMed] [Google Scholar]
- 10.Dulai PS, Gleeson MW, Taylor D, Holubar SD, Buckey JC, Siegel CA. Systematic review: the safety and efficacy of hyperbaric oxygen therapy for inflammatory bowel disease. Aliment Pharmacol Ther. 2014;39(11):1266–75. doi: 10.1111/apt.12753. [DOI] [PubMed] [Google Scholar]
- 11.Zhang B, Dong W, Ma Z, Duan S, Han R, Lv Z, et al. Hyperbaric oxygen improves depression-like behaviors in chronic stress model mice by remodeling gut microbiota and regulating host metabolism. CNS Neurosci Ther. 2023;29(1):239–55. doi: 10.1111/cns.13999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Memar MY, Yekani M, Alizadeh N, Baghi HB. Hyperbaric oxygen therapy: antimicrobial mechanisms and clinical application for infections. Biomed Pharmacother. 2019;109:440–7. doi: 10.1016/j.biopha.2018.10.142. [DOI] [PubMed] [Google Scholar]
- 13.Luo L, Qing L, Yao C, Liu D, Li Y, Li T, et al. Efficacy and safety of hyperbaric oxygen therapy for moderate-to-severe ulcerative colitis: a protocol for a systematic review and meta-analysis. BMJ Open. 2021;11(6):e047543. doi: 10.1136/bmjopen-2020-047543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lansdorp CA, Buskens CJ, Gecse KB, Lowenberg M, Stoker J, Bemelman WA, et al. Hyperbaric oxygen therapy for the treatment of perianal fistulas in 20 patients with Crohn’s disease: results of the HOT-TOPIC trial after 1-year follow-up. United Eur Gastroenterol J. 2022;10(2):160–8. doi: 10.1002/ueg2.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inflammatory Bowel Disease Group CSoGCMA. Chinese consensus on diagnosis and treatment in inflammatory bowel disease. (2018, Beijing). J Dig Dis. 2021;22(6):298–317. [DOI] [PubMed]
- 16.Sandborn WJ, Rebuck R, Wang Y, Zou B, Adedokun OJ, Gasink C, et al. Five-year efficacy and Safety of Ustekinumab Treatment in Crohn’s Disease: the IM-UNITI Trial. Clin Gastroenterol Hepatol. 2022;20(3):578–90. doi: 10.1016/j.cgh.2021.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dulai PS, Buckey JC, Jr, Raffals LE, Swoger JM, Claus PL, O’Toole K, et al. Hyperbaric oxygen therapy is well tolerated and effective for ulcerative colitis patients hospitalized for moderate-severe flares: a phase 2A pilot multi-center, randomized, double-blind, sham-controlled trial. Am J Gastroenterol. 2018;113(10):1516–23. doi: 10.1038/s41395-018-0005-z. [DOI] [PubMed] [Google Scholar]
- 18.Heyboer M 3rd, Sharma D, Santiago W, McCulloch N. Hyperbaric oxygen therapy: side effects defined and quantified. Adv Wound Care (New Rochelle). 2017;6(6):210–24. [DOI] [PMC free article] [PubMed]
- 19.Ni Y, Qian L, Siliceo SL, Long X, Nychas E, Liu Y, et al. Resistant starch decreases intrahepatic triglycerides in patients with NAFLD via gut microbiome alterations. Cell Metab. 2023;35(9):1530–47. doi: 10.1016/j.cmet.2023.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Wu J, Wei Z, Cheng P, Qian C, Xu F, Yang Y, et al. Rhein modulates host purine metabolism in intestine through gut microbiota and ameliorates experimental colitis. Theranostics. 2020;10(23):10665–79. doi: 10.7150/thno.43528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Targan SR, Hanauer SB, van Deventer SJ, Mayer L, Present DH, Braakman T, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N Engl J Med. 1997;337(15):1029–35. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- 22.Danese S, Vermeire S, D’Haens G, Panes J, Dignass A, Magro F, et al. Treat to target versus standard of care for patients with Crohn’s disease treated with ustekinumab (STARDUST): an open-label, multicentre, randomised phase 3b trial. Lancet Gastroenterol Hepatol. 2022;7(4):294–306. doi: 10.1016/S2468-1253(21)00474-X. [DOI] [PubMed] [Google Scholar]
- 23.Caparros E, Wiest R, Scharl M, Rogler G, Gutierrez Casbas A, Yilmaz B, et al. Dysbiotic microbiota interactions in Crohn’s disease. Gut Microbes. 2021;13(1):1949096. doi: 10.1080/19490976.2021.1949096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nunez-Sanchez MA, Melgar S, O’Donoghue K, Martinez-Sanchez MA, Fernandez-Ruiz VE, Ferrer-Gomez M et al. Crohn’s Disease, Host-Microbiota Interactions, and Immunonutrition: Dietary Strategies Targeting Gut Microbiome as Novel Therapeutic Approaches. Int J Mol Sci. 2022;23(15). [DOI] [PMC free article] [PubMed]
- 25.Adedokun OJ, Xu Z, Gasink C, Jacobstein D, Szapary P, Johanns J, et al. Pharmacokinetics and exposure Response relationships of Ustekinumab in patients with Crohn’s Disease. Gastroenterology. 2018;154(6):1660–71. doi: 10.1053/j.gastro.2018.01.043. [DOI] [PubMed] [Google Scholar]
- 26.Danese S, Fiorino G, Peyrin-Biroulet L. Early intervention in Crohn’s disease: towards disease modification trials. Gut. 2017;66(12):2179–87. doi: 10.1136/gutjnl-2017-314519. [DOI] [PubMed] [Google Scholar]
- 27.Lapic I, Padoan A, Bozzato D, Plebani M. Erythrocyte sedimentation rate and C-Reactive protein in Acute inflammation. Am J Clin Pathol. 2020;153(1):14–29. doi: 10.1093/ajcp/aqz142. [DOI] [PubMed] [Google Scholar]
- 28.Bray C, Bell LN, Liang H, Haykal R, Kaiksow F, Mazza JJ, et al. Erythrocyte Sedimentation Rate and C-reactive protein measurements and their relevance in Clinical Medicine. WMJ. 2016;115(6):317–21. [PubMed] [Google Scholar]
- 29.Sands BE, Chen J, Feagan BG, Penney M, Rees WA, Danese S, et al. Efficacy and safety of MEDI2070, an antibody against Interleukin 23, in patients with moderate to severe Crohn’s Disease: a phase 2a study. Gastroenterology. 2017;153(1):77–86. doi: 10.1053/j.gastro.2017.03.049. [DOI] [PubMed] [Google Scholar]
- 30.Porter AC, Aubrecht J, Birch C, Braun J, Cuff C, Dasgupta S, et al. Biomarkers of Crohn’s Disease to support the development of New Therapeutic interventions. Inflamm Bowel Dis. 2020;26(10):1498–508. doi: 10.1093/ibd/izaa215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thom SR. Hyperbaric oxygen: its mechanisms and efficacy. Plast Reconstr Surg. 2011;127(1):S131–41. doi: 10.1097/PRS.0b013e3181fbe2bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez CG, Mills RH, Kordahi MC, Carrillo-Terrazas M, Secaira-Morocho H, Widjaja CE, et al. The host-microbiome response to hyperbaric oxygen therapy in Ulcerative Colitis patients. Cell Mol Gastroenterol Hepatol. 2022;14(1):35–53. doi: 10.1016/j.jcmgh.2022.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zanon V, Rossi L, Castellani E, Camporesi EM, Palu G, Bosco G. Oxybiotest project: microorganisms under pressure. Hyperbaric oxygen (HBO) and simple pressure interaction on selected bacteria. Med Gas Res. 2012;2(1):24. doi: 10.1186/2045-9912-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joossens M, Huys G, Cnockaert M, De Preter V, Verbeke K, Rutgeerts P, et al. Dysbiosis of the faecal microbiota in patients with Crohn’s disease and their unaffected relatives. Gut. 2011;60(5):631–7. doi: 10.1136/gut.2010.223263. [DOI] [PubMed] [Google Scholar]
- 35.Lewis JD, Chen EZ, Baldassano RN, Otley AR, Griffiths AM, Lee D, et al. Inflammation, antibiotics, and Diet as Environmental stressors of the gut Microbiome in Pediatric Crohn’s Disease. Cell Host Microbe. 2015;18(4):489–500. doi: 10.1016/j.chom.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nielsen HB, Almeida M, Juncker AS, Rasmussen S, Li J, Sunagawa S, et al. Identification and assembly of genomes and genetic elements in complex metagenomic samples without using reference genomes. Nat Biotechnol. 2014;32(8):822–8. doi: 10.1038/nbt.2939. [DOI] [PubMed] [Google Scholar]
- 37.Franzosa EA, Sirota-Madi A, Avila-Pacheco J, Fornelos N, Haiser HJ, Reinker S, et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat Microbiol. 2019;4(2):293–305. doi: 10.1038/s41564-018-0306-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Canto E, Zamora C, Garcia-Planella E, Gordillo J, Ortiz MA, Perea L, et al. Bacteria-related events and the immunological response of Onset and Relapse Adult Crohn’s Disease patients. J Crohns Colitis. 2019;13(1):92–9. doi: 10.1093/ecco-jcc/jjy138. [DOI] [PubMed] [Google Scholar]
- 39.Peyrin-Biroulet L, Gonzalez F, Dubuquoy L, Rousseaux C, Dubuquoy C, Decourcelle C, et al. Mesenteric fat as a source of C reactive protein and as a target for bacterial translocation in Crohn’s disease. Gut. 2012;61(1):78–85. doi: 10.1136/gutjnl-2011-300370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yao S, Zhao Z, Wang W, Liu X. Bifidobacterium Longum: Protection against Inflammatory Bowel Disease. J Immunol Res. 2021;2021:8030297. doi: 10.1155/2021/8030297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murakami M, Iwamoto J, Honda A, Tsuji T, Tamamushi M, Ueda H, et al. Detection of gut dysbiosis due to reduced Clostridium Subcluster XIVa using the fecal or serum bile Acid Profile. Inflamm Bowel Dis. 2018;24(5):1035–44. doi: 10.1093/ibd/izy022. [DOI] [PubMed] [Google Scholar]
- 42.Federici S, Kredo-Russo S, Valdes-Mas R, Kviatcovsky D, Weinstock E, Matiuhin Y, et al. Targeted suppression of human IBD-associated gut microbiota commensals by phage consortia for treatment of intestinal inflammation. Cell. 2022;185(16):2879–e9824. doi: 10.1016/j.cell.2022.07.003. [DOI] [PubMed] [Google Scholar]
- 43.Kim N, Gu MJ, Kye YC, Ju YJ, Hong R, Ju DB, et al. Bacteriophage EK99P-1 alleviates enterotoxigenic Escherichia coli K99-induced barrier dysfunction and inflammation. Sci Rep. 2022;12(1):941. doi: 10.1038/s41598-022-04861-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krumbeck JA, Rasmussen HE, Hutkins RW, Clarke J, Shawron K, Keshavarzian A, et al. Probiotic Bifidobacterium strains and galactooligosaccharides improve intestinal barrier function in obese adults but show no synergism when used together as synbiotics. Microbiome. 2018;6(1):121. doi: 10.1186/s40168-018-0494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amabebe E, Robert FO, Agbalalah T, Orubu ESF. Microbial dysbiosis-induced obesity: role of gut microbiota in homoeostasis of energy metabolism. Br J Nutr. 2020;123(10):1127–37. doi: 10.1017/S0007114520000380. [DOI] [PubMed] [Google Scholar]
- 46.Schottlender N, Gottfried I, Ashery U. Hyperbaric oxygen treatment: effects on mitochondrial function and oxidative stress. Biomolecules. 2021;11(12). [DOI] [PMC free article] [PubMed]
- 47.Goncalves S, Yin K, Ito Y, Chan A, Olan I, Gough S, et al. COX2 regulates senescence secretome composition and senescence surveillance through PGE(2) Cell Rep. 2021;34(11):108860. doi: 10.1016/j.celrep.2021.108860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsuge K, Inazumi T, Shimamoto A, Sugimoto Y. Molecular mechanisms underlying prostaglandin E2-exacerbated inflammation and immune diseases. Int Immunol. 2019;31(9):597–606. doi: 10.1093/intimm/dxz021. [DOI] [PubMed] [Google Scholar]
- 49.Fuccelli R, Fabiani R, Sepporta MV, Rosignoli P. The hydroxytyrosol-dependent increase of TNF-alpha in LPS-activated human monocytes is mediated by PGE2 and adenylate cyclase activation. Toxicol Vitro. 2015;29(5):933–7. doi: 10.1016/j.tiv.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 50.Sands BE, Irving PM, Hoops T, Izanec JL, Gao LL, Gasink C, et al. Ustekinumab versus adalimumab for induction and maintenance therapy in biologic-naive patients with moderately to severely active Crohn’s disease: a multicentre, randomised, double-blind, parallel-group, phase 3b trial. Lancet. 2022;399(10342):2200–11. doi: 10.1016/S0140-6736(22)00688-2. [DOI] [PubMed] [Google Scholar]
- 51.Wang R, Yao Q, Chen W, Gao F, Li P, Wu J, et al. Stem cell therapy for Crohn’s disease: systematic review and meta-analysis of preclinical and clinical studies. Stem Cell Res Ther. 2021;12(1):463. doi: 10.1186/s13287-021-02533-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.El-Nakeep S, Shawky A, Abbas SF, Abdel Latif O. Stem cell transplantation for induction of remission in medically refractory Crohn’s disease. Cochrane Database Syst Rev. 2022;5(5):CD013070. doi: 10.1002/14651858.CD013070.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheng F, Huang Z, Wei W, Li Z. Fecal microbiota transplantation for Crohn’s disease: a systematic review and meta-analysis. Tech Coloproctol. 2021;25(5):495–504. doi: 10.1007/s10151-020-02395-3. [DOI] [PubMed] [Google Scholar]
- 54.Imdad A, Nicholson MR, Tanner-Smith EE, Zackular JP, Gomez-Duarte OG, Beaulieu DB, et al. Fecal transplantation for treatment of inflammatory bowel disease. Cochrane Database Syst Rev. 2018;11(11):CD012774. doi: 10.1002/14651858.CD012774.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cammarota G, Ianiro G, Kelly CR, Mullish BH, Allegretti JR, Kassam Z et al. International consensus conference on stool banking for faecal microbiota transplantation in clinical practice. Gut. 2019;68(12):2111-21. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.