Abstract
Objective
The second iteration of the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE-II) initiative recommends use of the Simple Endoscopic Score for Crohn’s disease (SES-CD) as a treatment target for patients with CD. We aimed to assess whether the STRIDE-II endoscopic endpoints are achievable and whether the degree of mucosal healing (MH) affects long-term outcomes.
Design/method
We performed a retrospective observational study between 2015 and 2022. Patients with CD who had baseline and follow-up SES-CD scores after biological therapy initiation were included. The primary outcome was treatment failure, defined as the need for: (1) change of biological therapy for active disease (2) corticosteroid use (3) CD-related hospitalisation or (4) surgery. We compared rates of treatment failure with the degree of MH achieved. Patients were followed up until treatment failure or study end (August 2022).
Results
50 patients were included and followed up for median 39.9 (34.6–48.6) months. Baseline characteristics: 62% male, median age 36.4 (27.8–43.9) years, disease distribution (L1: 4, L2: 11, L3: 35, perianal: 18). The proportion of patients achieving STRIDE-II end-points were: SES-CD 2–25 (50%) and >50% reduction in SES-CD—35 (70%). Failure to achieve SES-CD 2 (HR 11.62; 95% CI 3.33 to 40.56, p=0.003) or >50% improvement in SES-CD (HR 30.30; 95% CI 6.93 to 132.40, p<0.0001) predicted treatment failure.
Conclusion
Use of SES-CD is feasible in real-world clinical practice. Achieving an SES-CD 2 or a greater than 50% reduction, as set out by STRIDE-II, is associated with reduced rates of overall treatment failure including CD-related surgery.
Keywords: Crohn's disease, endoscopic procedures
WHAT IS ALREADY KNOWN ON THIS TOPIC
Validated endoscopic indices for Crohn’s disease (CD), such as the Simple Endoscopic Score (SES-CD), are used routinely in clinical trials to provide objective evaluations of disease activity. Although endoscopic CD scoring is performed less frequently in clinical practice, Selecting Therapeutic Targets in Inflammatory Bowel Disease-II (STRIDE-II) has recommended that routine use of SES-CD should be considered to assess the achievement of treatment targets. However, the real-world feasibility and long-term impact of using SES-CD in routine clinical practice remains unclear.
WHAT THIS STUDY ADDS
SES-CD endoscopic targets, as set out by STRIDE-II, appear feasible and achievable in real world practice. Achieving an SES-CD 2 or a reduction of greater than 50% is associated with a reduction in rates of overall treatment failure including CD-related surgery.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
IBD services should consider incorporating follow-up endoscopy, including objective scoring, into management pathways for patients with CD commencing new treatments. Failure to achieve specified endoscopic targets appears associated with less favourable outcomes and should lead to consideration of optimisation or a switch in therapy. Collection of objective endoscopic and clinical data routinely will also enable evaluation of real-world outcomes and help further define optimum targets for treatment in this setting.
Introduction
Crohn’s disease (CD) is a chronic inflammatory gastrointestinal disease in which transmural inflammation can lead to irreversible bowel damage with progression to fibrosis and/or penetrating disease. Clinical symptoms are clearly important when evaluating patients but disease activity scores have their limitations as treatment goals; 40% of the CD Activity Index (CDAI, a score commonly used as an outcome measure in clinicals trials) derives from subjective reporting of symptoms1 and the weak correlation between clinical disease scores and mucosal healing (MH) has been described several times. In the early 1990s, the GETAID group performed a prospective multicentre study demonstrating a weak correlation between CDAI and CD Endoscopic Index of Severity (CDEIS) (r=0.26, p<0.001)2 and clinical response to treatment with corticosteroids was shown to have no correlation with endoscopic response.3 In the ACCENT 1 study, only 18% of patients with moderate to severely active CD (as per CDAI) had evidence of MH.4 Subsequently, a post hoc analysis of the SONIC trial demonstrated that of the patients with ulceration at baseline who achieved clinical remission at week 26, only 72/136 patients (53%) also achieved endoscopic remission. The sensitivity, specificity and accuracy of CDAI predicting MH were 80.0%, 34.7% and 56.4% respectively.5 6
Assessment of mucosal disease severity at endoscopy, therefore, provides a necessary objective measure of disease activity, particularly when validated scoring systems are employed. The Simple Endoscopic Score for CD (SES-CD, online supplemental table 1) was developed and validated in 2002 as a simplified score derived from, and well correlated to, CDEIS.7 A major component of SES-CD is the degree of ulceration which has been shown to be the most likely lesion to change with therapy.8 9 It includes characteristics deemed to contribute to clinical symptoms that are also easily reproducible with improved inter-rater and intrarater variability.8 10
flgastro-2022-102309supp001.pdf (59.3KB, pdf)
Importantly, MH is associated with favourable long-term outcomes thereby providing an attractive treatment target. In a post hoc analysis of the SONIC study, both MH and a reduction in SES-CD at week 26 were predictive of corticosteroid-free clinical remission, clinical remission and clinical response at week 50.11
With the expanding availability of biological agents and small molecules these objective endoscopic targets are increasingly achievable. However, recommended targets for response and remission differ and largely empirical thresholds are used in clinical trials. The Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) initiative of the International Organisation for the Study of Inflammatory Bowel Diseases provided evidence and consensus-based guidance in 2015 on goals for treatment targets recommended for use in routine clinical practice.12 Clinical disease scores such as Harvey Bradshaw Index (HBI) and CDAI were recommended but were deemed insufficient without simultaneous objective measures of disease activity. Absence of ulceration was considered the most appropriate endoscopic target. However, the minimum degree of MH required for long-term clinical benefit remains unclear. Recently, the guidance on treatment targets has been updated with a recommendation to use objective scores (STRIDE-II). Endoscopic response has been defined as >50% reduction in SES-CD while remission has been defined as an SES-CD 2 or the absence of ulceration.13
We aimed to assess how achievable the endoscopic targets, set out in STRIDE-II, are in real-world clinical practice. Further, we aimed to assess long-term outcomes based on the degree of MH achieved.
Materials and methods
We performed a retrospective observational study in a tertiary IBD unit including consecutive adult patients who fulfilled the following criteria: (1) active CD (2) with paired, baseline and follow-up SES-CD scores (3) recorded after biological therapy initiation. All endoscopies were performed as part of routine clinical care and all endoscopists were experienced IBD clinicians who have had training in the use of SES-CD through clinical trial involvement and local departmental training (senior IBD research fellows, who are also independent endoscopists and/or consultants).
The primary outcome of interest was treatment failure, defined as the need for: (1) change of biological therapy or treatment discontinuation due to active disease, (2) initiation of corticosteroids, (3) CD-related hospitalisation or (4) CD-related surgical resection.
The secondary outcome of interest was to compare rates of treatment failure with the degree of MH achieved. Patients were followed up until treatment failure or until study end (August 2022).
Electronic patient records were searched using a custom-built package (EndoMineR) in R V.3.6.1 (R Foundation for Statistical Computing, Vienna, Austria). Patients with CD receiving biological therapy between 2015 and 2020 were cross-referenced with endoscopies reporting an SES-CD score. Subsequently, biological multidisciplinary meeting (MDM) lists were screened from May 2019 onwards until a total of 50 patients were included. Where endoscopies were reported as ‘normal’ and/or ‘quiescent or no inflammatory disease’, we assigned an SES-CD of 0. Endoscopy reports with subjective descriptors suggesting abnormality, without SES-CD scoring, were excluded as they would prohibit objective and comparative SES-CD assessment which was the aim of this study. Among those patients discussed at the MDM (irrespective of inclusion in our study) we also calculated the proportion of endoscopies with a reported SES-CD score. The components of the SES-CD are shown in online supplemental table 1 and thresholds investigated for definitions of endoscopic remission were SES-CD 2 or absence of ulceration (as per STRIDE-II), and also SES-CD 3. Endoscopic response was defined by a 50% decrease in SES-CD.
Clinical disease activity was also recorded (HBI) using a threshold of <5 to define clinical remission (as per STRIDE-II recommendations). Combined remission was defined as SES-CD 2 and HBI<5. HBI data were retrieved from endoscopy reports or from the clinical notes within 3 months of the relevant endoscopy. Patient charts were reviewed for demographic, Montreal score, IBD medical and surgical history and treatment failure (as defined above).
Statistics were performed using GraphPad V.9.1.0 Software (San Diego, California, USA). Continuous data are presented as medians with the IQR in brackets. Fisher’s exact test and Mann Whitney-U test were used where applicable. The Kaplan-Meier method was used to assess the cumulative risk of treatment failure reported as logrank HR with 95% CI.
Results
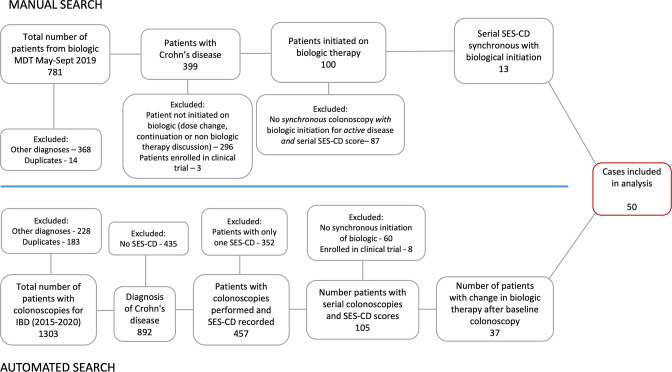
Figure 1 shows the patient selection process. We identified 1303 patients from our electronic patient records and 781 patients from the biological MDM. We included the first 50 consecutive patients who met our inclusion criteria.
Figure 1.
Patient disposition.
To understand the frequency of SES-CD reporting in our institution, we reviewed multiple colonoscopy reports between 2015 and 2022 among the 100 patients from the biological MDM who were initiated on biological therapy. SES-CD was formally scored in 83/178 procedures (46.6%). Of 95, 39 reports with no SES-CD score had alternatively reported a Rutgeerts score (41%) in the postoperative setting.
The baseline characteristics are shown in table 1. The majority of patients were male (31/50, 62%), median age 36.4 (27.8–43.9) years, with ileocolonic distribution (35/50, 70%) and inflammatory phenotype (28/50, 56%). Perianal disease was present in 18/50 (36%). Patients received anti-TNF therapy (31/50, 62%), ustekinumab (17/50, 34%), vedolizumab (1/50, 2%), or risankizumab (1/50, 2%). 27/50 (54%) patients were bio-naïve, 12/50 had undergone prior CD-related surgery (24%) and 23/31 patients on anti-TNF therapy received concomitant immunomodulation. Since this was a real-world study, patients enrolled in clinical trials were excluded and the patient receiving risankizumab did so via a compassionate use scheme.
Table 1.
Baseline characteristics
| Baseline characteristics | n (%) |
| Female sex | 19 (38) |
| Median age (years, IQR) | 36.4 (27.8–43.9) |
| Median age at diagnosis. (IQR) | 23.0 (19.8–35.0) |
| Median disease duration (IQR) | 4.5 (1.0–14.8) |
| Disease location (L1:L2:L3) | 4 (8): 11 (22): 35 (70) |
| +L4 | 4 (8) |
| +p | 18 (36) |
| Disease behaviour (B1: B2: B3) | 28 (56): 11 (22): 11 (22) |
| Prior surgery | 12 (24) |
| No prior biologics (0:1:2: 3) | 27 (54): 14 (28): 5 (10): 4 (8) |
| Biologicaltherapy initiated | |
| Adalimumab | 27 (54) |
| Infliximab | 4 (8) |
| Ustekinumab | 17 (34) |
| Vedolizumab | 1 (2) |
| Risankizumab | 1 (2) |
L1:L2:L3:L4—Montreal score for disease location (ileal: colonic, ileocolonic, upper gastrointestinal; +p—perianal disease; B1: B2: B3—Montreal score for disease behaviour—inflammatory, stricturing, penetrating.
The median time to biological initiation after the baseline colonoscopy was 1.5 (0.7–3.3) months. Follow-up colonoscopy occurred median 15.7 (11.6–21.2) months after biological therapy initiation. Online supplemental table 2 displays HBI and SES-CD results at baseline and follow-up endoscopy with the correlation between the two scores at baseline and follow-up endoscopy shown in online supplemental figure 1a,b, respectively. Thirty-eight per cent of HBI scores were recorded on the day of the procedure. Despite 50% of patients being in clinical remission (HBI <5) at the time of baseline endoscopy, all patients had active endoscopic disease as assessed by SES-CD (76% moderate to severe): baseline HBI versus SES-CD Spearman’s correlation coefficient r=0.14 (−0.15 to 0.41), p=0.33. Only eight (16%) patients underwent follow-up endoscopy within 6–9 months, the time frame specified by STRIDE I.12
flgastro-2022-102309supp002.pdf (141.8KB, pdf)
The proportion of patients achieving the STRIDE-II clinical and endoscopic end-points were: SES-CD - 50% (25/50), absence of ulceration—56% (28/50) and endoscopic response ( 50% reduction in SES-CD)—70% (35/50). SES-CD 3 was achieved in in 64% (32/50) and combined remission was achieved in 38% of patients (19/50).
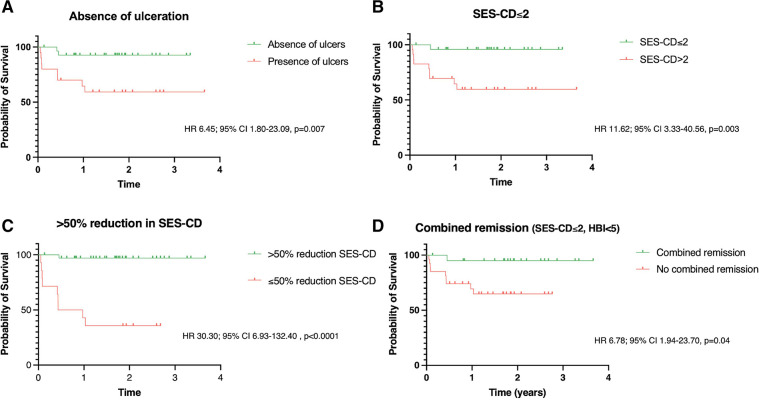
Table 2 shows the rates of MH achieved and the impact on subsequent disease course. Patients were followed up for median 22.5 (16.2–30.9) months from their second endoscopy. The median time to treatment failure was 3.1 (0.7–5.4) months. Overall, 12/50 (24%) patients met our treatment failure criteria including: switch in biological therapy (5/50, 10%), requirement for corticosteroids (5/50, 10%), CD-related acute admission (2/50, 4%), CD-related surgery (4/50, 8%). Bionaive patients, or those who had received just one prior biological therapy, were less likely to experience treatment failure than patients who had previously used two or more biological therapies (p=0.006). Rates of biological switch (n=5) and use of corticosteroids (n=5) were significantly higher in patients failing to achieve SES-CD 2 (both SES-CD 2: 0/25, (0%) vs SES-CD 2: 5/25 (20%), p=0.05). Rates of surgery were significantly higher in patients failing to achieve an SES-CD 3 (SES-CD 3: 1/32 (3%) vs SES-CD>3: 4/18 (22%), p=0.05) or >50% reduction in SES-CD (>50% reduction: 1/35 (3%) vs 50% reduction 4/15 (27%), p=0.02. Failure to achieve >50% reduction in SES-CD at follow-up was the strongest predictor of subsequent treatment failure (HR 30.30; 95% CI 6.93 to 132.40, p<0.0001). Failure to achieve SES-CD (HR 11.62; 95% CI 3.33 to 40.56, p=0.003), absence of ulceration (HR 6.45; 95% CI 1.80 to 23.09, p=0.007) an SES-CD (HR 24.13; 95% CI 5.99 to 97.20, p<0.0001) or combined remission (SES-CD and HBI<5 (HR 6.78; 95% CI 1.94 to 23.70, p=0.04) also predicted subsequent treatment failure. Of note, the median baseline SES-CD in those achieving 50% reduction at follow-up was 11 (8–17), in keeping with moderately active disease. Failure to achieve >50% reduction in SES-CD was also associated with increased rates of requiring corticosteroids, admission or surgery, but not surgery alone (table 2). Figure 2A–D shows Kaplan-Meier curves for the cumulative risk of therapeutic failure according to endoscopic targets as well as combined endoscopic and clinical remission. No significant difference in therapeutic failure was noted when comparing different definitions of MH: SES-CD vs absence of ulcers (p>0.99) and SES-CD vs SES-CD (p=0.36). Only three patients achieved a greater than 25% but 50% reduction in SES-CD, precluding comparative analysis with those patients who achieved >50% reduction in SES-CD.
Table 2.
Impact of achievement of clinical and endoscopic targets on subsequent disease course
| Endoscopic target | Frequency observed, n (%) |
Risk of treatment failure* if target not achieved (logrank HR, 95% CI) | P value | Risk of corticosteroids, admission or surgery if target not achieved (logrank HR, 95% CI) | P value | Risk of surgery if target not achieved (logrank HR, 95% CI) | P value |
| SES-CD 2 | 25 (50) | 11.62 (3.33 to 40.56) | 0.003 | 5.74 (1.16 to 28.56) | 0.07 | 3.44 (0.48 to 24.53) | 0.25 |
| Absence of ulcers | 28 (56) | 6.45 (1.80 to 23.09) | 0.007 | 2.80 (0.55 to 14.18) | 0.23 | 4.21 (0.58 to 30.73) | 0.18 |
| >50% reduction in SES-CD | 35 (70) | 30.30 (6.93 to 132.40) | <0.0001 | 13.74 (2.25 to 83.84) | 0.002 | 8.28 (0.90 to 76.02) | 0.03† |
| SES-CD | 32 (64) | 24.13 (5.99 to 97.20) | <0.0001 | 11.21 (1.98 to 63.40) | 0.005 | 6.68 (0.81 to 55.64) | 0.06 |
| Combined remission (SES-CD 2 and HBI <5) |
19 (38) | 6.78 (1.94 to 23.70) | 0.04 | 2.69 (0.53 to 13.74) | 0.23 | 2.26 (0.38 to 13.57) | 0.37 |
| Combined remission (SES-CD 3 and HBI <5) |
24 (48) | 11.07 (3.18 to 38.49) | 0.004 | 5.43 (1.10 to 26.95) | 0.08 | 3.15 (0.44 to 22.35) | 0.29 |
Bold values signify statistical significance (p value 0.05).
*Treatment failure defined as the need for: (1) change of biological therapy for active disease (2) corticosteroid use (3) CD-related hospitalisation or (4) surgery.
†Non-significant due to 95% CI.
HBI, Harvey Bradshaw Index; SES-CD, Simple Endoscopic Score for Crohn’s Disease.
Figure 2.
Kaplan-Meier curves: survival without therapeutic failure dependent on degree of mucosal healing achieved at follow-up endoscopy. Treatment failure defined as the composite end-point of: biological switch or discontinuation for active disease, corticosteroid use, CD-related admission or surgery (A) endoscopic remission: absence of ulceration (B) endoscopic remission: SES-CD (C) endoscopic response: greater than 50% reduction in SES-CD (D) combined remission: SES-CD and HBI <5. HBI, Harvey Bradshaw Index; SES-CD, Simple Endoscopic Score for Crohn’s Disease.
Discussion
In a real-world cohort, treatment targets as set out by STRIDE-II were achieved in about 50% of patients. Failure to achieve an SES-CD 2 or ulcer healing were significantly associated with subsequent treatment failure. Results were similar in patients failing to achieve SES-CD 3. Our data demonstrate that the use of serial objective endoscopic assessments, in this case with SES-CD, is feasible in real world practice and that the STRIDE-II endoscopic remission targets are achievable in a reasonable proportion of patients. In addition, patients achieving these targets have more favourable outcomes with fewer patients requiring biological switch, corticosteroid use or surgery over long-term follow-up. Endoscopic response, defined as an improvement in SES-CD >50%, was also significantly associated with decreased likelihood of treatment failure, thus, highlighting the clear importance of serial assessments in identifying an individual patient’s overall trajectory. These findings are in keeping with the post-hoc analysis of the SONIC trial where both endoscopic response and MH predicted corticosteroid-free remission, clinical remission and clinical response at week 50.11
HBI strongly correlates with CDAI14 and similar to other studies, we have demonstrated the well documented mismatch with regard to clinical and endoscopic remission. These findings reinforce the importance of endoscopic evaluation, in addition to symptom evaluation, to enable therapeutic optimisation and reduce the risk of adverse outcomes. A priori we included HBI scores recorded within 3 months of the endoscopy. While the clinical situation can change in IBD care within a 3-month period, 38% of scores were documented on the day of endoscopy and this was a clinically feasible time frame for data collection given the retrospective nature of our study.
The only baseline characteristic that was associated with treatment failure was prior biological use. It is frequently reported that patients who have tried multiple previous biological therapies may respond less well to future therapy. However, this should not deter clinicians from arranging endoscopic assessment in patients on their first or second biologic as this group still accounted for 42% (5/12) of treatment failures.
The retrospective nature of our analysis naturally introduces bias. Our cohort comprised only a small proportion of cases where biological therapy was initiated due to study selection criteria requiring synchronous serial endoscopy. There may be bias in both directions: patients with active disease may be more likely to accept invasive investigation; conversely non-responders (particularly primary non-responders) may have had an early switch of therapy before endoscopic assessment was indicated. Similar to our methodology, patients included in the post hoc analysis of the SONIC trial who did not proceed to a second endoscopy at week 26 were excluded from the analysis (172/508 (34%) of the initial SONIC cohort was included).11 We also do not have data on biochemical parameters which provide a less sensitive/specific, but also less invasive, objective assessment of disease activity. STRIDE-II recommends normalisation of C reactive protein and a reduction in faecal calprotectin to 250 µg/g as an intermediate treatment target. Since endoscopic remission is considered a long-term treatment target, biochemical markers should be seen as an adjunct to aid therapeutic optimisation en route to follow-up endoscopy and not as an alternative.13 In addition, the inter-rater and intrarater agreement between the endoscopists in our study is unknown. However, this is very difficult to assess outside the setting of central reading as performed in clinical trials. The wide CIs, the higher HRs for SES-CD when compared with SES-CD 2 and the significance of only composite end-points on Kaplan-Meier analysis likely reflect the sample size and larger prospective data would be of use to confirm the true effect sizes to enable us to better inform our patients.
In STRIDE-I, the suggested time frame for endoscopic re-assessment was 6–9 months.12 13 It should be noted that the majority of our patients were assessed after a longer duration of treatment (between 1 and 2 years). Comparison of outcomes in patients who had endoscopic response within or beyond this time frame is confounded by disease severity where patients with more severe or active disease are more likely to undergo earlier endoscopic assessment. The longer median time interval to endoscopic assessment may reflect the practicalities and financial impact of performing routine early follow-up endoscopy, particularly in patients with clinical and biochemical response or remission, in whom an invasive procedure may be less acceptable. In addition, transmural healing has been recommended as an adjunct to endoscopic remission (rather than a treatment target per se). Therefore, in real-world clinical practice, if recent imaging has both been performed and sufficiently informs management, endoscopy is likely to be deferred to a clinically appropriate interval. Nonetheless, our findings, as well as STRIDE recommendations, would still support the notion that endoscopic reassessment has the potential to provide important information that meaningfully guides management. Further research is required to determine whether the duration of treatment required to achieve MH has any impact on long-term outcomes.
Although endoscopic indices (SES-CD and CDEIS) have been incorporated into guidance on efficacy considerations for investigational products in CD,15 16 their use in mainstream clinical practice is limited by their perceived arduousness and complexity. More recently SEMA-CD has been proposed and investigated in a paediatric population.17 This score is derived from SES-CD and aims to simplify scoring even further by assessing the colon as a whole, rather than in four distinct segments, with a multiplication factor for the number of segments involved (regardless of severity). There was a strong correlation between SEMA-CD and SES-CD with good intrarater and inter-rater variability. Central readers also favourably rated SEMA-CD for ease of use. The fact that the SEMA-CD has recently been proposed serves to highlight that ease of use is paramount if we expect endoscopic indices to become standard of care. 46.6% of endoscopy reports from the patients discussed at the biological MDM had SES-CD scores recorded at endoscopy compared with 11%–30% described in other centres.17 Simple measures, applied together as a ‘bundle’, have previously been shown to lead to greater standardisation and quality improvement in terms of IBD endoscopic scoring. This referred specifically to UC but the principle is also likely to be applicable to CD.18 Although absence of ulceration may provide a reasonable alternative where endoscopists or units are unfamiliar with disease specific scores, this method does not enable quantification of endoscopic response. Given that a greater than 50% reduction in SES-CD also has a bearing on outcomes (even in the presence of ongoing ulceration), quantifying the degree of MH with an objective score is preferable as findings may alter patient management.
To conclude, our data demonstrate that endoscopic assessments made using SES-CD provide a useful and objective treatment target that appears feasible for use in real-world clinical practice. Achievement of an SES-CD defined endoscopic response or remission on biological initiation appears associated with a better prognosis. The challenge going forwards is to incorporate routine use of high-quality, objective endoscopic assessments into standard clinical care. In doing so, more outcome data will become available to help us further define optimum real-world targets for treatment.
Footnotes
Twitter: @gastroDS, @SamaanMark
Contributors: SM guarantor; MAS, SM, JM and SZ conception, design; SM, ER, SZ data collection, SM and MAS data analysis and interpretation; MAS, SM and ER manuscript drafting, SM, ER, ES, SH, SZ, SR, SHCA, JS, JM, PMI and MAS review, editing and final approval.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: SM has received an educational grant from Pfizer and speaker fees from Dr Falk Pharma. SH received lecture fees from Pfizer, Janssen, and Takeda. JM has received advisory fees from Janssen, Galapagos, Abbvie and lecture fees from Janssen and Pfizer. PMI has received lecture fees from Abbvie, Warner Chilcott, Ferring, Falk Pharma, Takeda, MSD, Johnson and Johnson, Shire and Pfizer. Financial support for research: MSD, Takeda and Pfizer. Advisory fees: Abbvie Warner Chilcott, Takeda, MSD, Vifor Pharma, Pharmacosmos Topivert, Genentech, Hospira, Samsung Bioepis. MAS has served as a speaker, a consultant and/or advisory board member for Sandoz, Janssen, Takeda, MSD, Falk, Samsung Bioepis. MAS has received advisory fees from Bristol-Myers Squibb, Janssen, Takeda, Sandoz, Samsung Bioepis, Galapagos, AbbVie, and has received lecture fees from Bristol-Myers Squibb, Janssen, Takeda, MSD, Falk, AbbVie, Galapagos.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Data will be shared upon reasonable request to the authors.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1. Best WR, Becktel JM, Singleton JW, et al. Development of a Crohn's disease activity index. National cooperative Crohn's disease study. Gastroenterology 1976;70:439–44. [PubMed] [Google Scholar]
- 2. Cellier C, Sahmoud T, Froguel E, et al. Correlations between clinical activity, endoscopic severity, and biological parameters in colonic or ileocolonic Crohn's disease. A prospective multicentre study of 121 cases. The Groupe d'Etudes Thérapeutiques des affections Inflammatoires Digestives. Gut 1994;35:231–5. 10.1136/gut.35.2.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Modigliani R, Mary JY, Simon JF, et al. Clinical, biological, and endoscopic picture of attacks of Crohn's disease. evolution on prednisolone. Groupe d'Etude Thérapeutique des affections Inflammatoires Digestives. Gastroenterology 1990;98:811–8. 10.1016/0016-5085(90)90002-i [DOI] [PubMed] [Google Scholar]
- 4. Rutgeerts P, Diamond RH, Bala M, et al. Scheduled maintenance treatment with infliximab is superior to episodic treatment for the healing of mucosal ulceration associated with Crohn's disease. Gastrointest Endosc 2006;63:433–42. quiz 64. 10.1016/j.gie.2005.08.011 [DOI] [PubMed] [Google Scholar]
- 5. Colombel J-F, Reinisch W, Mantzaris GJ, et al. Randomised clinical trial: deep remission in biologic and immunomodulator naïve patients with Crohn's disease - a SONIC post hoc analysis. Aliment Pharmacol Ther 2015;41:734–46. 10.1111/apt.13139 [DOI] [PubMed] [Google Scholar]
- 6. Peyrin-Biroulet L, Reinisch W, Colombel J-F, et al. Clinical disease activity, C-reactive protein normalisation and mucosal healing in Crohn's disease in the sonic trial. Gut 2014;63:88–95. 10.1136/gutjnl-2013-304984 [DOI] [PubMed] [Google Scholar]
- 7. Mary JY, Modigliani R. Development and validation of an endoscopic index of the severity for Crohn's disease: a prospective multicentre study. Groupe d'Etudes Thérapeutiques des affections Inflammatoires Du tube Digestif (GETAID). Gut 1989;30:983–9. 10.1136/gut.30.7.983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Daperno M, D'Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn's disease: the SES-CD. Gastrointest Endosc 2004;60:505–12. 10.1016/S0016-5107(04)01878-4 [DOI] [PubMed] [Google Scholar]
- 9. De Cruz P, Kamm MA, Prideaux L, et al. Mucosal healing in Crohn's disease: a systematic review. Inflamm Bowel Dis 2013;19:429–44. 10.1002/ibd.22977 [DOI] [PubMed] [Google Scholar]
- 10. Khanna R, Zou G, D'Haens G, et al. Reliability among central readers in the evaluation of endoscopic findings from patients with Crohn's disease. Gut 2016;65:1119–25. 10.1136/gutjnl-2014-308973 [DOI] [PubMed] [Google Scholar]
- 11. Ferrante M, Colombel J-F, Sandborn WJ, et al. Validation of endoscopic activity scores in patients with Crohn's disease based on a post hoc analysis of data from sonic. Gastroenterology 2013;145:978–86. 10.1053/j.gastro.2013.08.010 [DOI] [PubMed] [Google Scholar]
- 12. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for Treat-to-Target. Am J Gastroenterol 2015;110:1324–38. 10.1038/ajg.2015.233 [DOI] [PubMed] [Google Scholar]
- 13. Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the International organization for the study of IBD (IOIBD): determining therapeutic goals for Treat-to-Target strategies in IBD. Gastroenterology 2021;160:1570–83. 10.1053/j.gastro.2020.12.031 [DOI] [PubMed] [Google Scholar]
- 14. Harvey RF, Bradshaw JM. A simple index of Crohn's-disease activity. Lancet 1980;1:514. 10.1016/s0140-6736(80)92767-1 [DOI] [PubMed] [Google Scholar]
- 15. Agency EM . Guideline on the development of new medicinal products for the treatment of Crohn’s Disease, 2018. [Google Scholar]
- 16. U.S. Department of Health and Human Services FaDA . Crohn’s Disease: Developing Drugs for Treatment Guidance for Industry, 2022. [Google Scholar]
- 17. Adler J, Eder SJ, Gebremariam A, et al. Development and testing of a new simplified endoscopic mucosal assessment for Crohn's disease: the SEMA-CD. Inflamm Bowel Dis 2021;27:1585–92. 10.1093/ibd/izaa307 [DOI] [PubMed] [Google Scholar]
- 18. Kader R, Dart RJ, Sebepos‐Rogers G, et al. Implementation of an intervention bundle leads to quality improvement in ulcerative colitis endoscopy reporting. GastroHep 2020;2:309–17. 10.1002/ygh2.427 [DOI] [Google Scholar]
- 19. Health Research Authority . Defining research, 2017. Available: http://www.hra-decisiontools.org.uk/research/docs/DefiningResearchTable_Oct2017-1.pdf [Accessed 03 Sep 2019].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
flgastro-2022-102309supp001.pdf (59.3KB, pdf)
flgastro-2022-102309supp002.pdf (141.8KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Data will be shared upon reasonable request to the authors.