Abstract
Background:
Over 30% of patients with COVID-19 have persistent symptoms that last beyond 30 days and referred to as Long COVID. Long COVID has been associated with a persistent elevation in peripheral cytokines including interleukin-6, interleukin-1β, and tumor necrosis factor-α. This study reports cytokine profiles of patients in our clinic across SARS-COV-2 variant epochs.
Methods:
The clinical cytokine panel was analyzed in patients with Long COVID during periods that were stratified according to variant epoch. The 4 variant epochs were defined as: (1) wild-type through alpha, (2) alpha/beta/gamma, (3) delta, and (4) omicron variants.
Results:
A total of 390 patients had the clinical cytokine panel performed; the median age was 48 years (IQR 38-59) and 62% were female. Distribution by variant was wild-type and alpha, 50% (n = 196); alpha/beta/gamma, 7.9% (n = 31); delta, 18% (n = 72); and omicron, 23% (n = 91). Time to cytokine panel testing was significantly longer for the earlier epochs. Tumor necrosis factor-α (P < .001) and interleukin 1β (P < .001) were significantly more elevated in the earlier epochs (median of 558 days in wild-type through Alpha epoch vs 263 days in omicron epoch, P < .001)). Nucleocapsid antibodies were consistently detected across epochs.
Discussion:
When stratified by variant epoch, patients with early epoch Long COVID had persistently elevated peripheral pro-inflammatory cytokine levels when compared to later epoch Long COVID. Patients with Long COVID have similar clusters of symptoms across epochs, suggesting that the underlying pathology is independent of the peripheral cytokine signature.
Keywords: long COVID, cytokines, SARS-CoV-2, variants, PASC
Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-COV-2), the causative agent of coronavirus disease 2019 (COVID-19), has been confirmed in more than 770 million people and has led to just under 7 million deaths worldwide as of November 2023. 1 The clinical prodrome of COVID-19 is similar to that of other viral syndromes with fever, fatigue, muscle pain, cough being common, occasionally accompanied by diarrhea, altered sense of taste (dysgeusia), and loss of sense of smell (anosmia). 2 The acute symptoms may persist from a few days to several weeks with varied severity, ranging from mild (treated at home with conservative measures) to severe and critical (requiring hospitalization, Intensive Care Unit (ICU) admission, and death). 3
The more severe clinical manifestations of COVID-19 have been thought to be not due to the direct effects of viral infection on host cells, but rather due to the exaggerated immune response with degree of inflammation correlated with disease severity.4,5 A cytokine storm occurs when the degree of cytokines produced in response to an inflammatory driver, which may be infectious or non-infectious in nature, is excessive, and leads to an acute, severe systemic inflammatory response.6,7 An increase in immune tissue damage has been reported, with multiple cytokines being implicated both in acute COVID infections and post COVID inflammatory syndromes, particularly the interleukins [IL] 6, 8, 10, and 1-β, and tumor necrosis factor-alpha [TNF-α].8 -14 This immune system activation is related to cellular components of both the innate (via pathogen-associated molecular pattern (PAMPs)) and adaptive systems.15,16
Greater than 30% of patients who have acute COVID-19 experience persistent or new symptoms temporally related to their infection and that last greater than 30 days.17 -24 These constellation of persistent symptoms has been referred to by a number of names including post COVID syndrome (PoCoS), post-acute sequelae of SARS-CoV2 infection (PASC), and Long COVID (LC). Multiple studies have evaluated the outcomes of LC with a large population-based study from Scotland demonstrating persistent symptoms at 18 months in 48% of patients with PASC and similar findings being reported in a French cohort of 806 patients.25 -28 Initial analysis of the first 108 patients seen in our LC clinic (Jan 19, 2022-April 29, 2022) revealed that IL-6 was elevated in the majority of our patients. 5 Persistent elevation of cytokines (e.g. TNF-α, IL-1β, and IL-6) have been demonstrated in several other studies involving LC patients.5,29 -31 However, most of these studies use research-based cytokine panels which are not readily available in clinics. Based on these studies, our previous IL-6 study, and the need to develop diagnostic tests commonly available in post-COVID clinics, our team used a readily available clinical lab assay for evaluation of our LC patients. The primary aim of the present study was to evaluate and describe the abnormalities on cytokine panels in patients evaluated in our Post-COVID Care Clinic (PCOCC) using this clinical lab assay. In addition, based on clinical observations that cytokines varied based on the SARS-CoV-2 variant epoch the patients were infected in, we sought to stratify and analyze the cytokine profiles by SARS-CoV-2 variant to identify any differences. Here we present our findings using a clinically available cytokine panel in patients with LC across SARS-CoV-2 variants and it’s utility as a diagnostic biomarker.
Methods
This project was approved by the Institutional Review Board (IRB # 21-000558). The medical records of 980 patients seen in the PCOCC seen in a large multispecialty clinic in the midwestern area of the United States who met the definitional criteria for LC (symptoms persisting beyond 30 days without gross evidence of tissue damage on evaluation) were reviewed for whether they had a clinical cytokine panel drawn. At our institution, patients have the option of providing consent for retrospective chart review at any time (i.e., research pre-authorization). Patients who had not provided research pre-authorization were excluded from this analysis. After this exclusion, 309 patients were identified who had been diagnosed with LC and had cytokine panel results available.
The medical records were reviewed, and the following data were abstracted: date of initial SARS-COV-2 infection, age at time of the clinical cytokine laboratory testing, gender, race, ethnicity, clinical cytokine panel lab test results, and SARS-COV-2 nucleocapsid total antibody levels. The clinical cytokine panel comprised the following cytokines: GM-CSF, IFN-α, IFN-β, IFN-γ, IL-1β, IL-10, IL-18, IL-2 Receptor-α soluble, IL-6, MCP-1, MIP-1α, and TNF-α. For individual cytokine results that were less than lower limit of quantitation (LLOQ), the result was converted to a float number based on the LLOQ divided by the square root of 2. 32 Based on date of initial infection and weekly CDC variant data reports, the patients were arbitrarily stratified into SARS-COV-2 variant epochs.33 -35 The wild-type and alpha (B.1.1.7 lineage) variant epoch was from beginning of pandemic until 2021-02-28. The alpha/beta/gamma epoch (B.1.1.7, B.1.351, and P.1 lineages) was defined from 2021-03-01 until 2021-05-31. The delta epoch (B.1.617.2 and AY lineages) was 2021-06-01 until 2021-11-30. The omicron epoch was 2021-12-01 until 2022-06-30.
Cytokines were measured by a customized bead-based multiplex immunoassay (R&D Systems, Inc). Briefly, threefold serial dilutions of kit standards, which are recombinant human cytokines, were prepared in calibrator diluent. Patient samples and quality control (QC) samples were diluted 1:2 in calibrator diluent; 50 µL of diluted standards, QC, and patient samples were mixed with 50 µL microparticles, which are color-coded magnetic microparticles each conjugated to cytokine-specific antibodies and incubated at room temperature (RT) for 2 h with shaking. After incubation, the immobilized microparticles were washed 3× with a buffered surfactant solution using magnetic platform plate washer. Next, 50 µL of a mixture of biotinylated cytokine-specific antibodies was added to each well, followed by incubation at RT for 1 h with shaking. After 3× with a buffered surfactant solution using magnetic platform plate washer, 50 µL streptavidin-phycoerythrin (PE) conjugate was added to each well and incubated at RT for 30 min with shaking. After washing 3× with a buffered surfactant solution using magnetic platform plate washer, the microparticles were resuspended in 100 µL buffered surfactant solution and analyzed using a FLEXMAP 3D analyzer (Luminex). Data reduction was performed using the XPONENT software. Sensitivity data is not available, but the assays have been tested to show less than 0.5% cross-reactivity or interference.
Nucleocapsid antibody testing was performed on patient sera (obtained from venous blood draw and prepared using standard laboratory technique) using the Roche Elecsys Anti-SARS-CoV-2 electrochemiluminescence assay (Roche Diagnostics, Indianapolis, IN). Briefly, the serum sample is incubated with first a combination of biotinylated and ruthenium labeled SARS-CoV-2 specific nucleocapsid antigen to form a sandwich complex. The sample is washed then incubated with streptavidin-coated microparticles to allow for formation of streptavidin-biotin complexes. The sample is then separated by magnetic capture of the ruthenium labeled antigen-antibody complexes. Residual sample is subject to application of voltage and the electrochemiluminescence signal is analyzed by a Roche Cobas e 801 analytical unit. This assay has received Emergency Use Authorization from the Food and Drug Administration. 36
Descriptive statistics were reported as median (interquartile range) or number of observations (percent of total). For comparison between median values of variant epochs, Kruskal-Wallis rank sum was performed for continuous variables. Post hoc pairwise comparison using the Dunn test with Holm correction was carried out on Kruskal-Wallis test results that reached statistical significance. Statistical analyses were performed using R version 4.2.1.
Results
Demographics
A total of 390 patients were included in the study. The median age of patients when first evaluated in our clinic was 48 years (IQR 38-59) and 62% were female. Race and ethnicity were predominantly white (94%) and non-Hispanic (95%). When divided by epoch of variant predominance, most of our patients (n = 196; 50%) had the wild-type and alpha variant, followed by omicron (91; 23%) and delta (72; 18%) variants. About 289/390 (74%) patients had received at least 1 dose of SAR-CoV-2 vaccine. The patients in our study group had very few comorbidities with hypertension (11%), hyperlipidemia (8%), obstructive sleep apnea (7%), and anxiety (7%) being most common. This data is summarized in Table 1.
Table 1.
Demographic Characteristics.
| Characteristic | N = 390 a |
|---|---|
| Age (years) | 48 [38, 59] |
| Gender | |
| Female | 241 (62%) |
| Male | 149 (38%) |
| Ethnicity | |
| Hispanic or Latino | 11 (3%) |
| Not Hispanic or Latino | 371 (95%) |
| Other/not disclosed | 8 (2%) |
| Comorbidities | |
| Hypertension | 41 (11%) |
| Hyperlipidemia | 30 (8%) |
| Obstructive sleep apnea | 27 (7%) |
| Anxiety | 26 (7%) |
| Vaccination status | |
| Received at least 1 dose of SARS-CoV-2 vaccine | 279 (74%) |
| Race | |
| Black | 6 (1.5%) |
| Native American | 2 (0.5%) |
| Asian | 3 (0.8%) |
| Pacific Islander | 1 (0.3%) |
| White | 366 (94%) |
| Undisclosed/other | 10 (2.6%) |
| SARS-CoV-2 variant by epoch | |
| Wild type through alpha | 196 (50%) |
| Alpha/beta/Ggamma | 31 (8%) |
| Delta | 72 (18%) |
| Omicron | 91 (23%) |
Median (IQR); n (%).
Cytokine Panel Results
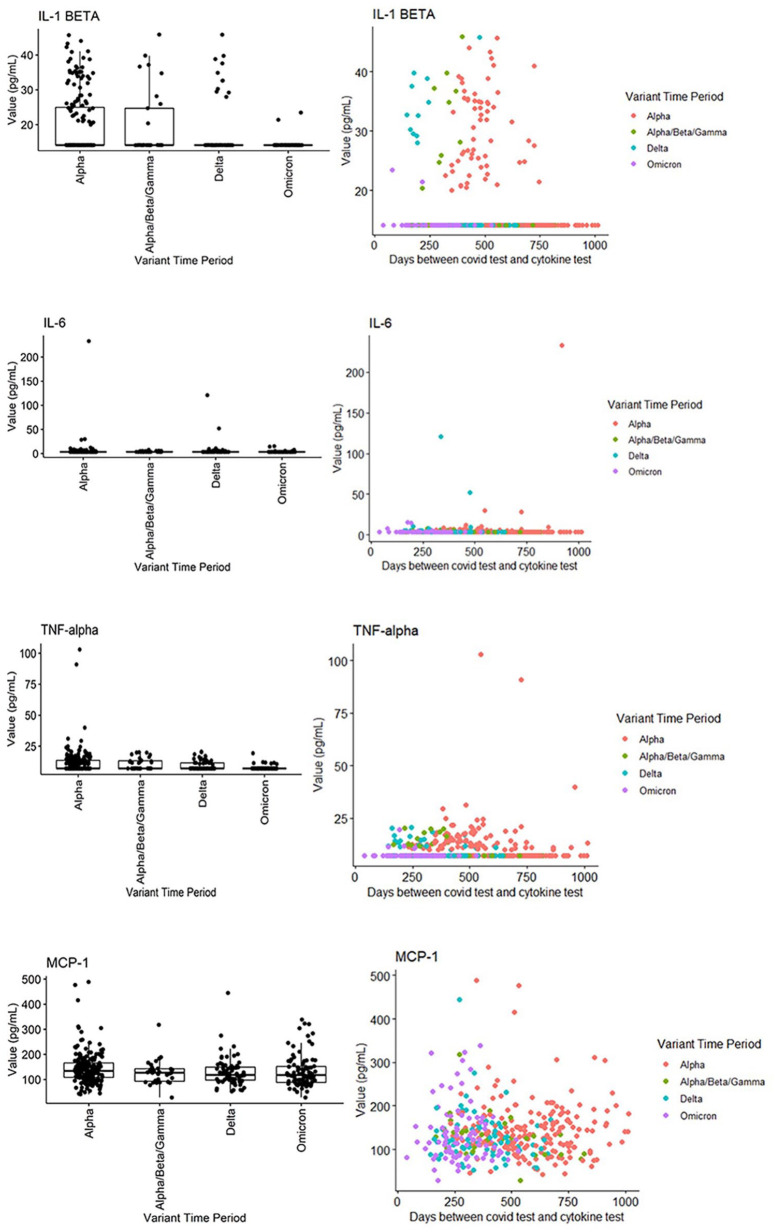
When stratified by SARS-CoV-2 variant epochs, several cytokines were identified as being persistently elevated according to variant of infection. IL-1β was significantly and persistently elevated in the wild-type/alpha, beta, and gamma epochs compared to the delta and omicron epochs (P < .001). TNF-α was significantly and persistently elevated in the wild-type/alpha, beta, gamma, and delta epochs compared to the omicron epoch (P < .001). Interferon-α was also statistically significantly lower in the wild-type/alpha, beta, gamma, and delta epochs, compared to the omicron epoch (P < .001; Figure 1). This data is summarized in Tables 2 and 3.
Figure 1.
Scatter plots of selected cytokine levels by SARS-CoV-2 variant.
Table 2.
Cytokine Levels by SARS-CoV-2 Variant.
| Cytokine | Overall N = 390 a | Alpha N = 196 a | Alpha/beta/gamma N = 31 a | Delta N = 72 a | OmicronN = 91 a | P-value b |
|---|---|---|---|---|---|---|
| GM-CSF | 10.6 (10.6, 10.6) | 10.6 (10.6, 10.6) | 10.6 (10.6, 10.6) | 10.6 (10.6, 10.6) | 10.6 (10.6, 10.6) | .5 |
| IFN-alpha | 14.1 (14.1, 14.1) | 14.1 (14.1, 14.1) | 14.1 (14.1, 14.1) | 14.1 (14.1, 14.1) | 14.1 (14.1, 14.1) | .4 |
| IFN-beta | 14.1 (14.1, 14.1) | 14.1 (14.1, 14.1) | 14.1 (14.1, 14.1) | 14.1 (14.1, 14.1) | 14.1 (14.1, 14.1) | .2 |
| IFN-gamma | 42.4 (42.4, 42.4) | 42.4 (42.4, 42.4) | 42.4 (42.4, 42.4) | 42.4 (42.4, 42.4) | 42.4 (42.4, 42.4) | .3 |
| IL-1 beta | 14 (14, 14) | 14 (14, 25) | 14 (14, 25) | 14 (14, 14) | 14 (14, 14) | <.001 |
| IL-10 | 4.9 (4.9, 4.9) | 4.9 (4.9, 4.9) | 4.9 (4.9, 4.9) | 4.9 (4.9, 4.9) | 4.9 (4.9, 4.9) | .7 |
| IL-18 | 218 (158, 294) | 223 (169, 292) | 259 (174, 351) | 226 (154, 306) | 185 (150, 268) | .058 |
| IL-2 receptor alpha soluble | 488 (385, 634) | 499 (402, 658) | 455 (373, 642) | 502 (403, 610) | 457 (339, 593) | .2 |
| IL-6 | 3.54 (3.54, 3.54) | 3.54 (3.54, 3.54) | 3.54 (3.54, 4.37) | 3.54 (3.54, 3.54) | 3.54 (3.54, 3.54) | .041 |
| MCP-1 | 127 (100, 159) | 134 (109, 166) | 127 (93, 143) | 118 (98, 149) | 118 (88, 152) | .056 |
| MIP-1 alpha | 155.6 (155.6, 155.6) | 155.6 (155.6, 155.6) | 155.6 (155.6, 155.6) | 155.6 (155.6, 155.6) | 155.6 (155.6, 155.6) | .052 |
| TNF-alpha | 7.1 (7.1, 11.7) | 7.1 (7.1, 13.6) | 7.1 (7.1, 13.2) | 7.1 (7.1, 11.5) | 7.1 (7.1, 7.1) | <.001 |
Median (IQR).
Kruskal-Wallis rank sum test.
Table 3.
Nucleocapsid Antibodies by SARS-CoV-2 Variant Epoch.
| Test | Overall N = 211 a | Wild type through Alpha N = 115 a | Alpha/beta/gamma N = 22 a | Delta N = 40 a | Omicron N = 34 a |
|---|---|---|---|---|---|
| Negative | 28 (13%) | 22 (19%) | 2 (9%) | 1 (2.5%) | 3 (8.8%) |
| Positive | 183 (87%) | 93 (81%) | 20 (91%) | 39 (98%) | 31 (91%) |
n (%).
Time to Testing
The time from infection to laboratory evaluation with cytokine panel and nucleocapsid antibody was also significantly different between the SARS-CoV-2 variant epochs with the time to testing longest for the wild-type to Alpha variant and shortest for the omicron BA.2 and subvariants epoch (median of 558 days vs 263 days, P < .001)). This data is summarized in Table 2.
Nucleocapsid Antibody Testing
The nucleocapsid antibody test was performed on over half of the PCOCC patients (54%; n = 211). Overall, 87% (n = 183) of those patients tested had a positive nucleocapsid antibody test (Table 2). Patients infected during the earlier wild-type or alpha variant epoch (81%; 93/115) had lower positivity rates compared to later periods such as delta (98%; 39/40) and omicron (91%; 31/34) but time to testing was longer as mentioned above.
Discussion
To our knowledge, this is the first study to describe the differences in the cytokine profiles of patients with LC in relation to the SARS-CoV2 variant of infection. There was attenuation in the level of peripheral cytokines among patients with LC associated with the Omicron variant family even though time from infection to testing was shorter in this group. In addition, the current study highlights the successful implementation of a clinical cytokine assay outside of the research context. Earlier studies had proposed the use of peripheral cytokines—in particular TNF-α, IL-1β, and IL-6 as a potential biomarker panel for patients with LC based on data gathered during the period dominated by wild type, alpha and delta variants.29,30 While our data correlates positively with those studies during that period, we also demonstrate that this signal is lost in patients with LC during the Omicron epoch. Hence, peripheral cytokine panels do not currently have demonstrable utility in diagnosis of LC after infection with Omicron subvariants.
These observations have potential implications on the putative pathophysiology of LC since patients with LC after Omicron infection still present with the classic features of fatigue, post exertional malaise, myalgia, dyspnea, paresthesias, chest pain, and orthostatic intolerance, despite the absence of persistence of peripheral cytokine elevation.5,22,37,38 It is possible that these persistent symptoms could be related to central nervous system inflammation (neuroinflammation) as a potential pathophysiology of LC and indeed discordance between serum and cerebrospinal fluid (CSF) cytokine levels in neuroinflammatory disorders is well reported.39,40 Previous studies have supported the presence of neuroinflammation in patients with LC by demonstrating both hypometabolic changes on 14FDG PET scans and inflammatory changes on [18F]DPA-714 PET scans which specifically target inflammation. Additionally, these studies demonstrate discordance between neuroinflammation on PET scan and peripheral cytokine levels.41 -45 Based on this data, we surmise that the pathophysiology of LC may approximate that of these other neuroinflammatory disorders with central neuroinflammation despite normal peripheral cytokine panels, which raises the need for alternative diagnostic methods.
When the cytokine panels were compared across epochs, the time from initial infection to obtaining the cytokine panel had decreased significantly with the Omicron SARS-CoV-2 variant and its subvariants when compared to the earlier variants (wild type through delta), which implied that the peripheral cytokine elevations if present attenuated more rapidly with the Omicron variants. The lack of persistent peripheral cytokine elevation in patients with LC during Omicron suggests the role of neuroinflammation and dictates careful consideration of other putative mechanisms of LC including spike protein persistence, endothelial dysfunction, and microthrombosis.46 -49 Notably a very high percentage (87%) of our patients had persistently positive nucleocapsid antibodies after infection with SARS-CoV-2 at a median duration of 406 days. This is a much higher rate than has been reported in the literature wherein 30% to 60% of patients infected with SARS-CoV-2 experienced seroreversion of the nucleocapsid antibody levels with the majority occurring within the first 270 days.50,51 This may represent a facet of the dysfunctional and enhanced immune response seen in patients with LC.
Based on the data presented in this study, we hypothesize that LC is primarily driven via a central neuroinflammatory pathology—which may be related to persistence of viral proteins including spike protein and local inflammatory effects including endothelial dysfunction and alterations in cerebral blood flow It would be instructive to compare the patterns of neuroinflammation on PET scan in LC patients from different variant epochs, as if these patterns are similar, it would lend credence to this central neuroinflammation theory. While the roles of endothelial dysfunction and microthrombi remain debated, their effects would need to be predominantly located within the central nervous system to drive this pathology.46,47,49 Endothelial dysfunction as a pathogenic mechanism involving the central nervous system has been identified in previous research.52 -54 11 brains evaluated in a recent autopsy studies of 44 unvaccinated individuals who died with COVID-19 demonstrated vascular congestion and few other histopathologic changes in the brain. 55 Another autopsy series of 9 brains of people who were unvaccinated and died from COVID-19 in the first 3 months of the US pandemic demonstrated perivascular fibrinogen leakage, immunostaining for cell adhesion molecules including Integrilin β3, CD61, and PECAM-1 (platelet/endothelial cell adhesion molecule 1), and platelet coagulation.56,57 These studies additionally showed the presence of immune complex deposition with complement, IgM, and IgG complexes being detected in the walls of blood vessels in the brain. The role of endothelial dysfunction in LC has been further supported by the recent clinical trial demonstrating improvement in fatigue and cognitive dysfunction in patients with LC when treated with L-arginine and Vitamin C.58,59 L-arginine is a precursor to nitric oxide which may exert multiple effects on endothelial cells including mediating vasodilation and endothelial reactivity, thereby increasing maximal oxygen uptake.58,60,61 The role of the spike protein and its mRNA in driving inflammation continues to be evaluated with early reports suggesting persistence of the spike protein in the serum and brains of patients with LC, and recent work suggests that nuclear translocation of these molecules may be a novel inflammatory mechanism.48,62 -64 The role of the spike protein in neuroinflammation and endothelial dysfunction would also explain post-vaccine LC, which has been well reported.65,66 Based on this information, it is likely that LC is driven by multiple factors including CNS endothelial cell dysfunction, cerebral blood flow, and neuroinflammation—all of which may be driven by persistent spike protein. Further pathophysiologic studies including evaluating the CNS cytokine profile in LC, neuroinflammatory change across variants using functional imaging, and endothelial function and spike protein assays are warranted to enhance the roles of these pathways and our understanding of LC.
The demographics of our study group were typical of patient populations with LC, with women in their third to fifth decade of life being most represented.22,38 Given our geographical location in the Midwest United States, our population is largely non-Hispanic white (94%) in ethnicity and race (respectively), which does limit generalizability of our findings, thereby inviting further study in more diverse populations. In addition, the analysis of patients by variant epoch is inexact as SARS-CoV-2 genotyping was not widely performed on individual SARS-CoV-2 tests in the USA; thus, patients close to the time bounds of the defined variant epoch may have been inadvertently misclassified. We have tried to minimize this potential for error by allowing a period of time between cutoffs for crossover of variant prevalence. This approach has been previously validated in real world analyses of efficacy of anti-spike neutralizing monoclonal antibodies against SARS-CoV-2 variants.33 -35 However, the explosion of multiple subvariants in the Omicron lineage has made an epoch-based stratification mechanism obsolete as multiple Omicron sub-variants are circulating at any given time. 67 A potential further confounder is our inability to account for multiple COVID infections especially if they were asymptomatic or untested. Potential co-infection with other viruses was also not accounted for, but patients are generally afebrile without a viral prodrome in order to be seen in clinic.
The data gleaned from our study has implications for future research. Correlation of neuroinflammatory changes with the presence of peripheral cytokine elevation will be instructive as to whether these 2 processes can occur independently in patients with LC. By comparing imaging studies for neuroinflammation across variants, it can be ascertained whether the neuroinflammatory changes seen in LC are preserved across variants, and if there are subtle variations that may correlate with clinical phenotype of LC. Interestingly, there is some overlap with the cytokine profile developed shortly post vaccination in patients with mRNA vaccines who have elevated IL-6 and TNF-α levels, but this similarity is not present with an adenovirus vector.68,69 Other areas that demand future research include the interplay at the molecular level between persistent viral protein and RNA fragments and the immune system, as this interplay may drive the endothelial dysfunction and other physiologic changes seen in LC. There is no data on the cytokine profile seen in patients with post-vaccine LC or whether this cytokine profile is pathologically prolonged in these patients, and further studies on long term vaccine immune outcomes are needed. Further work is also needed as to whether these immune perturbations are indeed correlated with changes in patient symptoms as they recover or worsen.
Limitations of this study include the above-mentioned generalizability concerns due to the homogeneity of the patient population and the use of a clinical cytokine panel which reported normal values in many cases at the limit of detection of the test (i.e., <20). While this approach does have the benefit of ready translation into clinical practice, the lack of exact data for patients in the normal range did provide challenges for statistical analysis. We opted to treat these values by taking limit of detection divided by the square root of 2 (e.g.,<20 was treated as 14.1), thereby maximizing rigor. 32 Additionally, literature demonstrates differential T cell profiles in patients with LC compared to healthy controls, in particular decreased activity and number of regulatory T cells (Treg) which corresponds with decreased production of immune suppressive cytokines (IL-10 and TNF-β), which may be related to the immune dysfunction noted in LC. 70
Conclusions
While peripheral cytokines were previously noted to be persistently elevated in patients with LC in earlier variants of SARS-CoV-2, this study demonstrates that both the amplitude and duration of peripheral cytokine elevations in patients with LC during the Omicron epoch are attenuated compared to these previous variants. As such, diagnosis of LC using a peripheral cytokine panel is no longer feasible with the current circulating variants. This observation also has significant implications on the putative role of peripheral inflammation in patients with LC, implying that central neuroinflammation rather than peripheral inflammation may be the core pathophysiologic change driving this poorly understood disease process. Further work is needed to evaluate which molecular mechanisms are at play in this central neuroinflammation with spike protein and mRNA persistence and endothelial dysfunction being the most likely contributors.
Acknowledgments
We would like to acknowledge our research coordinator team Shawn Fokken, Jennifer Hanson, Obediah Bauer, and Megan Erickson for their significant investment of the time and effort into our LC Research.
Footnotes
Abbreviations: COVID-19: Coronavirus disease 2019
GM-CSF: Granulocyte Macrophage Colony Stimulating Factor
CSF: Cerebrospinal Fluid
ICU: Intensive Care Unit
IFN: Interferon
IL: Interleukin
IRB: Institutional Review Board
LC: Long COVID
LLOQ: Lower Limit of Quantitation
MCP-1: Monocyte Chemoattractant Protein-1
MIP-1α: Macrophage Inflammatory Protein 1-α
PASC: Post Acute Sequelae of SARS-CoV-2 Infection
PCOCC: Post COVID Care Clinic
PoCoS: Post COVID Syndrome
QC: Quality Control
RT: Room Temperature
SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus-2
TNF: Tumor Necrosis Factor
Author Contributions: Conceptualization and funding acquisition were performed by RG and RTH; Data curation and formal analysis were performed by BRS and SY; Methodology was developed by RG, RTH, MRS, ADB, and RRR; Project coordination was performed by RG, SY, RTH, and ITC; Supervision was performed by RG, RTH, and ITC; Visualization performed by RG; Writing (original draft) was performed by RG, SY, RTH, and BRS; All authors participated in writing (review and editing) and investigation.
Data Availability: All data related to this study is included within the manuscript at point of publication.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: RG is a consultant for Alpaca Health; RTH is a consultant for Nestle; RG and RTH have received research funding from the GHR Foundation (Minneapolis, MN) and have received research support from electroCore Inc., InteraXon Inc., and Reulay Inc.; ADB is supported by grants from NIAID (grants AI110173 and AI120698), Amfar (#109593), and Mayo Clinic (HH Shieck Khalifa Bib Zayed Al-Nahyan Named Professorship of Infectious Diseases). ADB is a paid consultant for Abbvie, Gilead, Freedom Tunnel, Pinetree therapeutics Primmune, Immunome, MarPam, Rion, Symbiosis, NexImmune and Flambeau Diagnostics, is a paid member of the DSMB for Corvus Pharmaceuticals, Equilium, CSL Behring, and Excision Biotherapeutics. Raymund R. Razonable has received grants from Regeneron, Roche, and Gilead for research not directly related to this study. All authors are employees of the Mayo Clinic. All other authors report no potential conflicts of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: We would like to acknowledge the GHR Foundation (Minneapolis, MN) and the Division of General Internal Medicine at the Mayo Clinic for their generous support of this research.
Ethical Approval: This study was performed as part of a retrospective chart review approved by the Mayo Clinic Institutional Review Board (IRB# 21-000558)
ORCID iDs: Ravindra Ganesh  https://orcid.org/0000-0002-6877-1712
https://orcid.org/0000-0002-6877-1712
Stephanie L. Grach  https://orcid.org/0000-0002-8337-6219
https://orcid.org/0000-0002-8337-6219
Ivana T. Croghan  https://orcid.org/0000-0003-3464-3525
https://orcid.org/0000-0003-3464-3525
References
- 1. World Health Organization. WHO COVID-19 Dashboard. World Health Organization. 2021. Accessed July 27, 2023. https://covid19.who.int/ [Google Scholar]
- 2. Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020;17(5):533-535. doi: 10.1038/s41423-020-0402-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Talukder A, Razu SR, Alif SM, Rahman MA, Islam SMS. Association between symptoms and severity of disease in hospitalised novel coronavirus (COVID-19) patients: a systematic review and meta-analysis. J Multidiscip Healthc. 2022;15:1101-1110. doi: 10.2147/JMDH.S357867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wilson JG, Simpson LJ, Ferreira AM, et al. Cytokine profile in plasma of severe COVID-19 does not differ from ARDS and sepsis. JCI Insight. 2020;5(17):e140289. doi: 10.1172/jci.insight.140289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ganesh R, Grach SL, Ghosh AK, et al. The female-predominant persistent immune dysregulation of the post-COVID syndrome. Mayo Clin Proc. 2022;97(3):454-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Han H, Ma Q, Li C, et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect. 2020;9(1):1123-1130. doi: 10.1080/22221751.2020.1770129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the; Cytokine Storm’ in COVID-19. J Infect. 2020;80(6):607-613. doi: 10.1016/j.jinf.2020.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schultheiss C, Paschold L, Willscher E, et al. Maturation trajectories and transcriptional landscape of plasmablasts and autoreactive B cells in COVID-19. iScience. 2021;24(11):103325. doi: 10.1016/j.isci.2021.103325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Woodruff M, Ramonell R, Cashman K, et al. Dominant extrafollicular B cell responses in severe COVID-19 disease correlate with robust viral-specific antibody production but poor clinical outcomes. Posted online June 22, 2020. medRxiv. doi: 10.1101/2020.04.29.20083717 [DOI] [Google Scholar]
- 10. Borghi MO, Beltagy A, Garrafa E, et al. Anti-phospholipid antibodies in COVID-19 are different from those detectable in the anti-phospholipid syndrome. Front Immunol. 2020;11:584241. doi: 10.3389/fimmu.2020.584241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vlachoyiannopoulos PG, Magira E, Alexopoulos H, et al. Autoantibodies related to systemic autoimmune rheumatic diseases in severely ill patients with COVID-19. Ann Rheum Dis. 2020;79(12):1661-1663. doi: 10.1136/annrheumdis-2020-218009 [DOI] [PubMed] [Google Scholar]
- 12. Wang EY, Mao T, Klein J, et al. Diverse functional autoantibodies in patients with COVID-19. Posted online February 1, 2021. medRxiv. doi: 10.1101/2020.12.10.20247205 [DOI] [PubMed] [Google Scholar]
- 13. Zuo Y, Estes SK, Ali RA, et al. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci Transl Med. 2020;12(570):eabd3876. doi: 10.1126/scitranslmed.abd3876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gu Y, Hsu AC, Pang Z, et al. Role of the innate cytokine storm induced by the influenza A virus. Viral Immunol. 2019;32(6):244-251. doi: 10.1089/vim.2019.0032 [DOI] [PubMed] [Google Scholar]
- 15. Ferrero-Miliani L, Nielsen OH, Andersen PS, Girardin SE. Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1beta generation. Clin Exp Immunol. 2007;147(2):227-235. doi: 10.1111/j.1365-2249.2006.03261.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Takeuchi O, Akira S. Innate immunity to virus infection. Immunol Rev. 2009;227(1):75-86. doi: 10.1111/j.1600-065X.2008.00737.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603-605. doi: 10.1001/jama.2020.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garrigues E, Janvier P, Kherabi Y, et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect. 2020;81(6):e4-e6. doi: 10.1016/j.jinf.2020.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. 2020;370:m3026. doi: 10.1136/bmj.m3026 [DOI] [PubMed] [Google Scholar]
- 20. Ladds E, Rushforth A, Wieringa S, et al. Persistent symptoms after Covid-19: qualitative study of 114 “long Covid” patients and draft quality principles for services. BMC Health Serv Res. 2020;20(1):1144. doi: 10.1186/s12913-020-06001-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ladds E, Rushforth A, Wieringa S, et al. Developing services for long COVID: lessons from a study of wounded healers. Clin Med (Lond). 2021;21(1):59-65. doi: 10.7861/clinmed.2020-0962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Logue JK, Franko NM, McCulloch DJ, et al. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open. 2021;4(2):e210830. doi: 10.1001/jamanetworkopen.2021.0830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Townsend L, Dyer AH, Jones K, et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS One. 2020;15(11):e0240784. doi: 10.1371/journal.pone.0240784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21(3):133-146. doi: 10.1038/s41579-022-00846-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hastie CE, Lowe DJ, McAuley A, et al. Outcomes among confirmed cases and a matched comparison group in the Long-COVID in Scotland study. Nat Commun. 2022;13(1):5663. doi: 10.1038/s41467-022-33415-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Global Burden of Disease Long CC, Wulf Hanson S, Abbafati C, et al. Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA. 2022;328(16):1604-1615. doi: 10.1001/jama.2022.18931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mizrahi B, Sudry T, Flaks-Manov N, et al. Long covid outcomes at one year after mild SARS-CoV-2 infection: nationwide cohort study. BMJ. 2023;380:e072529. doi: 10.1136/bmj-2022-072529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Agergaard J, Gunst JD, Schiøttz-Christensen B, Østergaard L, Wejse C. Long-term prognosis at 1.5 years after infection with wild-type strain of SARS-CoV-2 and Alpha, Delta, as well as Omicron variants. Int J Infect Dis. 2023;137:126-133. doi: 10.1016/j.ijid.2023.10.022 [DOI] [PubMed] [Google Scholar]
- 29. Schultheiß C, Willscher E, Paschold L, et al. From online data collection to identification of disease mechanisms: the IL-1ß, IL-6 and TNF-α cytokine triad is associated with post-acute sequelae of COVID-19 in a digital research cohort. SSRN Electronic Journal. 2021. doi: 10.2139/ssrn.3963839 [DOI] [Google Scholar]
- 30. Schultheiss C, Willscher E, Paschold L, et al. The IL-1beta, IL-6, and TNF cytokine triad is associated with post-acute sequelae of COVID-19. Cell Rep Med. 2022;3(6):100663. doi: 10.1016/j.xcrm.2022.100663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Queiroz MAF, Neves P, Lima SS, et al. Cytokine profiles associated with acute COVID-19 and long COVID-19 syndrome. Front Cell Infect Microbiol. 2022;12:922422. doi: 10.3389/fcimb.2022.922422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Croghan C, Egeghy PP. Methods of dealing with values below the limit of detection using SAS. Southern SAS User Group. 2003;22:24. [Google Scholar]
- 33. O’Horo JC, Challener DW, Speicher L, et al. Effectiveness of monoclonal antibodies in preventing severe COVID-19 with emergence of the delta variant. Mayo Clin Proc. 2022;97(2):327-332. doi: 10.1016/j.mayocp.2021.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Razonable RR, O’Horo JC, Hanson SN, et al. Comparable outcomes for bebtelovimab and ritonavir-boosted nirmatrelvir treatment in high-risk patients with coronavirus disease-2019 during severe acute respiratory syndrome coronavirus 2 BA.2 omicron epoch. J Infect Dis. 2022;226(10):1683-1687. doi: 10.1093/infdis/jiac346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Razonable RR, Tulledge-Scheitel SM, Hanson SN, et al. Real-world clinical outcomes of bebtelovimab and sotrovimab treatment of high-risk persons with coronavirus disease 2019 during the omicron epoch. Open Forum Infect Dis. 2022;9(10):ofac411. doi: 10.1093/ofid/ofac411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Diagnostics R. Elecsys anti-SARS-CoV-2. US FAD-EUA. 2020;5:2. [Google Scholar]
- 37. Ganesh R, Ghosh AK, Nyman MA, et al. PROMIS scales for assessment of persistent post-COVID symptoms: a cross sectional study. J Prim Care Community Health. 2021;12:21501327211030413. doi: 10.1177/21501327211030413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ganesh R, Vanichkachorn GS, Munipalli B, et al. Postacute sequelae of SARS-CoV-2 infection-lessons learned from a coordinated health system response. Mayo Clin Proc Innov Qual Outcomes. 2022;6(4):311-319. doi: 10.1016/j.mayocpiqo.2022.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fertleman M, Pereira C, Dani M, Harris BHL, Di Giovannantonio M, Taylor-Robinson SD. Cytokine changes in cerebrospinal fluid and plasma after emergency orthopaedic surgery. Sci Rep. 2022;12(1):2221. doi: 10.1038/s41598-022-06034-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ellison VJ, Mocatta TJ, Winterbourn CC, Darlow BA, Volpe JJ, Inder TE. The relationship of CSF and plasma cytokine levels to cerebral white matter injury in the premature newborn. Pediatr Res. 2005;57(2):282-286. doi: 10.1203/01.Pdr.0000148286.53572.95 [DOI] [PubMed] [Google Scholar]
- 41. Grach SL, Ganesh R, Messina SA, Hurt RT. Post-COVID-19 syndrome: persistent neuroimaging changes and symptoms 9 months after initial infection. BMJ Case Rep. 2022;15(4):e248448. doi: 10.1136/bcr-2021-248448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Guedj E, Campion JY, Dudouet P, et al. (18)F-FDG brain PET hypometabolism in patients with long COVID. Eur J Nucl Med Mol Imaging. 2021;48(9):2823-2833. doi: 10.1007/s00259-021-05215-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Morand A, Campion JY, Lepine A, et al. Similar patterns of [(18)F]-FDG brain PET hypometabolism in paediatric and adult patients with long COVID: a paediatric case series. Eur J Nucl Med Mol Imaging. 2021;49:913-920. doi: 10.1007/s00259-021-05528-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Visser D, Golla SSV, Verfaillie SCJ, et al. Long COVID is associated with extensive in-vivo neuroinflammation on [18F]DPA-714 PET. Posted online June 4, 2022. medRxiv. 2022. doi: 10.1101/2022.06.02.22275916 [DOI] [Google Scholar]
- 45. Alshelh Z, Albrecht DS, Bergan C, et al. In-vivo imaging of neuroinflammation in veterans with Gulf War illness. Brain Behav Immun. 2020;87:498-507. doi: 10.1016/j.bbi.2020.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pretorius E, Venter C, Laubscher GJ, et al. Prevalence of symptoms, comorbidities, fibrin amyloid microclots and platelet pathology in individuals with Long COVID/Post-Acute Sequelae of COVID-19 (PASC). Cardiovasc Diabetol. 2022;21(1):148. doi: 10.1186/s12933-022-01579-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pretorius E, Vlok M, Venter C, et al. Persistent clotting protein pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc Diabetol. 2021;20(1):172. doi: 10.1186/s12933-021-01359-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Swank Z, Senussi Y, Manickas-Hill Z, et al. Persistent circulating severe acute respiratory syndrome coronavirus 2 spike is associated with post-acute coronavirus disease 2019 sequelae. Clin Infect Dis. 2023;76(3):e487-e490. doi: 10.1093/cid/ciac722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bonaventura A, Vecchié A, Dagna L, et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol. 2021;21(5):319-329. doi: 10.1038/s41577-021-00536-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Krutikov M, Palmer T, Tut G, et al. Prevalence and duration of detectable SARS-CoV-2 nucleocapsid antibodies in staff and residents of long-term care facilities over the first year of the pandemic (VIVALDI study): prospective cohort study in England. Lancet Healthy Longev. 2022;3(1):e13-e21. doi: 10.1016/S2666-7568(21)00282-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Van Elslande J, Oyaert M, Ailliet S, et al. Longitudinal follow-up of IgG anti-nucleocapsid antibodies in SARS-CoV-2 infected patients up to eight months after infection. J Clin Virol. 2021;136:104765. doi: 10.1016/j.jcv.2021.104765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kim ES, Jeon MT, Kim KS, Lee S, Kim S, Kim DG. Spike proteins of SARS-CoV-2 induce pathological changes in molecular delivery and metabolic function in the brain endothelial cells. Viruses. 2021;13(10):2021. doi: 10.3390/v13102021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. DeOre BJ, Tran KA, Andrews AM, Ramirez SH, Galie PA. SARS-CoV-2 spike protein disrupts blood-brain barrier integrity via RhoA activation. J Neuroimmune Pharmacol. 2021;16(4):722-728. doi: 10.1007/s11481-021-10029-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chen Y, Yang W, Chen F, Cui L. COVID-19 and cognitive impairment: neuroinvasive and blood-brain barrier dysfunction. J Neuroinflammation. 2022;19(1):222. doi: 10.1186/s12974-022-02579-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Stein SR, Ramelli SC, Grazioli A, et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature. 2022;612(7941):758-763. doi: 10.1038/s41586-022-05542-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lee MH, Perl DP, Steiner J, et al. Neurovascular injury with complement activation and inflammation in COVID-19. Brain. 2022;145(7):2555-2568. doi: 10.1093/brain/awac151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lee MH, Perl DP, Nair G, et al. Microvascular injury in the brains of patients with Covid-19. N Engl J Med. 2021;384(5):481-483. doi: 10.1056/NEJMc2033369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Izzo R, Trimarco V, Mone P, et al. Combining L-arginine with vitamin C improves long-COVID symptoms: the LINCOLN Survey. Pharmacol Res. 2022;183:106360. doi: 10.1016/j.phrs.2022.106360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tosato M, Calvani R, Picca A, et al. Effects of l-arginine plus vitamin C supplementation on physical performance, endothelial function, and persistent fatigue in adults with long COVID: a single-blind randomized controlled trial. Nutrients. 2022;14(23):4984. doi: 10.3390/nu14234984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rezaei S, Gholamalizadeh M, Tabrizi R, Nowrouzi-Sohrabi P, Rastgoo S, Doaei S. The effect of L-arginine supplementation on maximal oxygen uptake: a systematic review and meta-analysis. Physiol Rep. 2021;9(3):e14739. doi: 10.14814/phy2.14739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gambardella J, Khondkar W, Morelli MB, Wang X, Santulli G, Trimarco V. Arginine and endothelial function. Biomedicines. 2020;8(8):277. doi: 10.3390/biomedicines8080277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sattar S, Kabat J, Jerome K, Feldmann F, Bailey K, Mehedi M. Nuclear translocation of spike mRNA and protein is a novel pathogenic feature of SARS-CoV-2. Posted online September 27, 2022. bioRxiv. doi: 10.1101/2022.09.27.509633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Crunfli F, Carregari VC, Veras FP, et al. Morphological, cellular, and molecular basis of brain infection in COVID-19 patients. Proc Natl Acad Sci USA. 2022;119(35):e2200960119. doi: 10.1073/pnas.2200960119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rhea EM, Logsdon AF, Hansen KM, et al. The S1 protein of SARS-CoV-2 crosses the blood–brain barrier in mice. Nat Neurosci. 2021;24(3):368-378. doi: 10.1038/s41593-020-00771-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Reddy S, Reddy S, Arora M. A case of postural orthostatic tachycardia syndrome secondary to the messenger RNA COVID-19 vaccine. Cureus. 2021;13(5):e14837. doi: 10.7759/cureus.14837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Potential POTS association with COVID-19 vaccination weaker than with COVID-19 infection. Nat Cardiovasc Res. 2022;1(12):1132-1133. doi: 10.1038/s44161-022-00194-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. CDC. COVID data tracker. CDC. Accessed July 27, 2023. https://covid.cdc.gov/covid-data-tracker/#variant-proportions [Google Scholar]
- 68. Brook B, Fatou B, Checkervarty A, et al. The mRNA vaccine BNT162b2 demonstrates impaired TH1 immunogenicity in human elders in vitro and aged mice in vivo. Res Sq. 2022; rs.3.Rs-2395118. doi: 10.21203/rs.3.rs-2395118/v1 [DOI] [Google Scholar]
- 69. Ewer KJ, Barrett JR, Belij-Rammerstorfer S, et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat Med. 2021;27(2):270-278. doi: 10.1038/s41591-020-01194-5 [DOI] [PubMed] [Google Scholar]
- 70. Dhawan M, Rabaan AA, Alwarthan S, et al. Regulatory T cells (tregs) and COVID-19: unveiling the mechanisms, and therapeutic potentialities with a special focus on long COVID. Vaccines (Basel). 2023;11(3):699. doi: 10.3390/vaccines11030699 [DOI] [PMC free article] [PubMed] [Google Scholar]