Abstract
Background
Acute respiratory infections (ARIs) represent a significant public health concern in the U.S. This study aimed to describe the disease burden of ARIs and identify U.S. populations at high risk of developing complications.
Methods
This scoping review searched PubMed and EBSCO databases to analyze U.S. studies from 2013 to 2022, focusing on disease burden, complications, and high-risk populations associated with ARIs.
Results
The study included 60 studies and showed that ARI is associated with a significant disease burden and healthcare resource utilization (HRU). In 2019, respiratory infection and tuberculosis caused 339,703 cases per 100,000 people, with most cases being upper respiratory infections and most deaths being lower respiratory infections. ARI is responsible for millions of outpatient visits, especially for influenza and pneumococcal pneumonia, and indirect costs of billions of dollars. ARI is caused by multiple pathogens and poses a significant burden on hospitalizations and outpatient visits. Risk factors for HRU associated with ARI include age, chronic conditions, and socioeconomic factors.
Conclusion
The review underscores the substantial disease burden of ARIs and the influence of age, chronic conditions, and socioeconomic status on developing complications. It highlights the necessity for targeted strategies for high-risk populations and effective pathogen detection to prevent severe complications and reduce HRU.
Keywords: acute respiratory infection, disease burden, high risk, risk factors, epidemiology
1. Introduction
Acute respiratory infections (ARIs) are categorized as upper respiratory tract infections or lower respiratory tract infections. They can be caused by a wide range of viral and bacterial pathogens, leading to significant morbidity and mortality worldwide (1–3). They account for 4.25 million deaths annually and are a leading cause of death globally (4). Moreover, the burden of ARIs on healthcare systems has been increasingly recognized, with heightened healthcare resource utilization (HRU), hospitalizations, and outpatient visits attributed to these infections (5–7).
In the U.S., ARIs continue to be a major public health concern. The disease burden during the 2021–2022 influenza season was lower than in the pre-pandemic era, potentially due to quarantine measures and other strategies implemented during the COVID-19 pandemic, as well as the observed interruption of care (8). However, the disease burden increased and even surpassed pre-pandemic levels in the 2022–2023 season (8). During the 2021–2022 influenza season, there were an estimated 9 million influenza illnesses, 4 million influenza-related medical visits, 100,000 influenza-related hospitalizations, and 5,000 influenza deaths (9). In contrast, during the 2022–2023 season, there were an estimated 26–50 million influenza illnesses, 12–24 million influenza-related medical visits, 290,000–630,000 influenza-related hospitalizations, and 18,000–55,000 influenza deaths (10). The disease burden also varies by age group, with the highest illness rate among individuals aged 5–17 years, the highest medical visit and hospitalization rate among those aged 0–4 years, and the highest mortality rate in seniors aged 65 or older (9).
While most ARIs resolve within a few weeks with symptomatic relief such as over-the-counter medications, they can also lead to various complications that significantly contribute to their overall disease burden. For instance, complications of influenza include viral pneumonia, bacterial pneumonia, sinusitis, and otitis media (11). The risk of developing complications may vary among different populations (12).
Understanding the disease burden and risk factors associated with an increased risk of developing complications is crucial for clinical practice, public health initiatives, and future research toward targeted interventions. The majority of previous reviews on this topic were published before the COVID-19 pandemic. To gain insights into the epidemiology of ARIs and to identify populations at increased risk of complications in the U.S., this review examines recent literature on the disease burden of ARIs and populations with a high risk of developing complications from these infections.
2. Methods
We conducted a scoping literature review and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (Supplementary Table S1) (13, 14). The PubMed and EBSCO databases were searched in December 2022 to identify peer-reviewed studies published between January 2013 and December 2022 reporting on disease burden or complications in patients with ARIs. Original research articles, systematic reviews, and meta-analyses were included. Duplicate articles and certain article types (case studies, case reports, clinical trials, studies with sample size less than 100 patients, or studies that demonstrated systematic errors that may affect the validity of results) were excluded. Titles and abstracts of the remaining articles were reviewed, and full-text articles were obtained for further screening. Articles not meeting the inclusion criteria were excluded, as appropriate. Results from the final articles included in this review were categorized into two topics: disease burden of ARI, and populations that have higher risk of developing complications.
2.1. Literature search strategy
Studies were searched using key terms related to respiratory tract infections and disease burden or complications (Supplementary Tables S2, S3). Inclusion criteria comprised studies published in the last 10 years, which were either peer-reviewed publications or publications on websites of professional organizations or federal or global agencies. Furthermore, the study populations included those in the U.S., and all selected publications were required to be written in English.
2.2. Data extraction
Information extracted from articles included, when available: study type, time period or dates study was conducted; data sources, population size, age range, genders, and race/ethnicity; follow-up period; outcome measurements; key findings and statistics; and (when available) if studies were modeling studies and if population vaccination rates were captured or considered. Following data extraction, two reviewers (C.P., C.C.) discussed analysis of queries on findings. All data extraction was conducted using Microsoft Excel 365 (Microsoft Corporation).
3. Results
3.1. Included studies
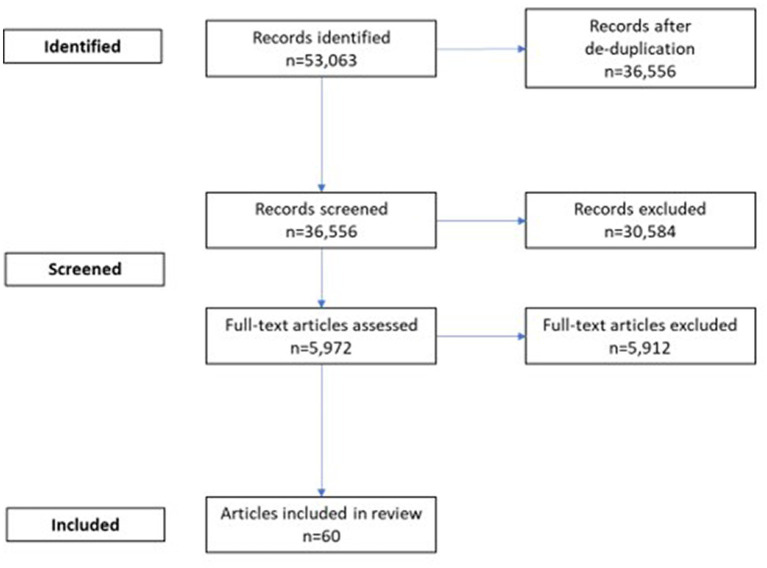
The literature search yielded 5,972 unique records after removing duplicates. After applying exclusion criteria, 60 papers were selected for inclusion in the review, with 39 papers focusing on disease burden (15–54) and 21 papers reporting risk factors associated with the development of ARI complications (Figure 1) (55–74). The findings were summarized in Table 1. Detailed results from each study were included in Supplementary Table S4.
Figure 1.
Flow diagram of study selection.
Table 1.
Summary of findings.
| Category | Findings |
|---|---|
| Pediatric and adult populations at higher risk of developing complications from acute respiratory infections |
Pediatric population
|
| The disease burden of acute respiratory infections |
Pediatric population
|
3.2. Disease burden of ARI
In 2019, the age-standardized incidence, death, and disability-adjusted life years (DALYs) per 100,000 population of respiratory infection and tuberculosis (RIT) were 339,703 (95% CI 303,184–382,354), 13.6 (95% CI 12.2–14.4), and 384.9 (95% CI 330.6–458.6), respectively (2). Upper respiratory infections constituted the majority of RIT age-standardized incidence rates, while lower respiratory infections made up the highest proportion of RIT age-standardized death and DALY (2).
ARIs significantly contributed to HRU. Approximately 14.5 million outpatient visits for influenza occur annually, with around 80% of visits taking place among the 5–49 age group and approximately 0.7 million visits among seniors aged 65 years and older (45). Each year, an estimated 10% of all children under 18 years and 4% of the entire population < 65 years sought outpatient care for respiratory illness related to influenza (45). Indirect costs, including absenteeism, premature death, and overall direct costs, contributed to the economic burden of influenza, accounting for about $8.0 billion annually in the U.S. (31, 46).
It is noteworthy that the COVID-19 pandemic, which brought about unprecedented changes in societal behavior and public health policies, has had a significant impact on the transmission of non-SARS-CoV-2 respiratory viruses. The adoption of measures such as face masks, improved hand hygiene, social distancing, and the screening and isolation of individuals showing symptoms, along with quarantine protocols, have collectively led to a notable reduction in the community circulation of these viruses (75). Consequently, these changes have been instrumental in substantially decreasing the burden of ARIs in the United States and other parts of the world during the pandemic period (75, 76).
Utilization is likely to continue to increase in the future. The growing elderly population is projected to increase by an estimated 45 million people by 2040, which is expected to significantly contribute to a rise in outpatient pneumococcal pneumonia visits and related hospitalizations (53). Assuming age-specific incidence rates of pneumococcal pneumonia remain constant, outpatient visits for pneumococcal pneumonia are likely to increase by 43%, while hospitalizations due to pneumococcal pneumonia may nearly double between 2004 and 2040 (53). Additionally, ARIs may cause complications that increase HRU. For instance, about 2.2 million outpatient visits occur annually for otitis media related to influenza, with 86% occurring in children under 18 years (45).
Previous research showed each individual ARI pathogen causing significant burden. In recent years, the pediatric population in the U.S. has seen an increase in severe respiratory illnesses due to enterovirus D68 infections, which are most prevalent during late summer and fall (21). Human metapneumovirus infections also pose a significant burden on hospitalizations and outpatient visits for children up to 5 years old, particularly during their first year of life (17). There is substantial annual RSV-attributable HRU and costs in the U.S. across age groups. Among children under 2 years of age, during the 2014–2015 season, it was estimated that between 49,509 and 59,867 community-onset RSV-associated hospitalizations occurred in the U.S., with the highest hospitalization rate in infants under 2 months (15). A previous review showed that the annual RSV-associated hospitalization rates ranged from 8.4 to 40.8 per 1,000 for U.S. infants under 1 year old (22). Across all age groups, RSV infection led to an increased length of hospital stay (1.9–3 days), more emergency department and urgent care visits (0.4–0.5), additional ambulatory visits (0.7–2.7), more outpatient visits (12.1–18.6), and higher number of prescriptions (9.5–14.6) compared to those without RSV, with the highest burden in those aged 65 years and older (42). The adjusted mean annual costs associated with RSV were higher in the elderly population (≥65 years; $12,030–$23,194) than in those under 65 years ($2,251–$5,391) (42). Among children, costs attributable to RSV were higher for those aged 5–17 years ($3,192) than for those aged 1–4 years ($2,251–$2,521) (42).
Various factors may be associated with HRU. Age is one important factor, as increased HRU is associated with both infants under 6 months old and the elderly population (Table 2). Among influenza patients, infants younger than 6 months old were 40% more likely to be admitted to the intensive care unit (ICU) than older infants, and a higher hospitalization rate was observed for infants younger than 3 months old compared to those older than 3 months (64). Elderly age is also linked to 3–9 times the odds of hospitalization (46). One study found that individuals under 18 years of age were more likely to experience an influenza A (H1N1)-related ICU stay than those aged 45–64 years, and the risk for influenza-like illness-related ICU stay was greater for individuals aged 5–12 years than for those aged 45–64 years (74). Additionally, chronic conditions are associated with increased HRU. Specific comorbidities like congestive heart failure, chronic obstructive pulmonary disease, coronary artery disease, and late-stage chronic kidney disease were associated with 2–7 times the odds of hospitalization (46). Increased HRU was also linked to comorbidities like hematological malignancies and chronic kidney disease. ICU admission rate among patients with influenza A (H1N1) or seasonal influenza were higher in patients with asthma or pregnancy (74). Furthermore, socioeconomic factors, such as male gender and non-Hispanic ethnicity, have been found to be associated with influenza A (H1N1)/seasonal influenza-related ICU admission rate (74).
Table 2.
Age-related findings.
| Pathogen/condition | Complications/disease burden | Population | Age-related findings |
|---|---|---|---|
| Community-acquired pneumonia (CAP) | Incidence | Adult | CAP incidence rates were higher for those aged ≥50 years |
| Influenza A (H1N1) | ICU admission | Combined | Individuals younger than 18 years are more likely to be admitted to the ICU due to influenza A (H1N1) compared to those aged 45–64 years |
| Influenza A (H1N1) | Mortality | Adult | Adults over the age of 65 are at an increased risk of mortality from influenza A (H1N1) |
| HMPV | Hospitalization rates | Pediatric | Hospitalization rates for HMPV are highest in infants under 6 months |
| Influenza | 2009 pandemic mortality | Combined | The 2009 influenza pandemic saw a younger age distribution in mortality compared to typical seasonal flu deaths |
| Influenza | Hospital visits | Combined | ~80% of visits occurred in the 5–17 and 18–49 age group |
| Influenza | Hospitalization | Combined | Elderly age was associated with 9 times the odds of hospitalization (≥65 years vs. 5–17 years) and select comorbidities were associated with 2–3 times the odds of hospitalization |
| Influenza | Hospitalization rates | Adult | Hospitalization rates for influenza vary among adults, with those aged 50–64 years experiencing different rates |
| Influenza | Hospitalization rates | Combined | The burden of influenza-associated hospitalizations varies by age, with the highest rates observed in individuals over 65 years |
| Influenza A (H1N1) | ICU admission | Pediatric | Children under the age of 6 months are at an increased risk of requiring ICU admission due to influenza |
| Influenza | ICU admission | Combined | Children aged 5–12 years are at a greater risk of requiring ICU admission for influenza compared to older age groups |
| Influenza | Severe complications | Pediatric | Older children are more likely to experience severe complications from seasonal influenza |
| Influenza and RSV | Hospitalization | Combined | The highest hospitalization rates for influenza are seen in individuals over 75 years old, while for RSV, infants under 1 year are most affected |
| Pneumococcal empyema | Incidence | Pediatric | More complicated cases were observed as age increases |
| Pneumonia | CAP incidence and mortality | Adult | Elderly adults, especially those older than 80 years, experience higher incidence and mortality rates due to CAP |
| Pneumonia | Hospitalizations, costs | Combined | The elderly population experiences an increased incidence and costs of pneumonia episodes, with projected increases in hospitalizations and associated costs |
| Pneumonia | Pneumonia severity | Pediatric | 1-year-olds and 10-year-olds are at a higher risk of developing severe pneumonia compared to two-year-olds |
| Respiratory syncytial virus (RSV) | Hospitalization | Adult | Older adults, particularly those over 75 years, are at an increased risk of hospitalization due to RSV |
| RSV | All-cause mortality | Pediatric | Age was not associated with higher risk |
| RSV | Costs | Combined | Incremental difference in adjusted mean annual costs between RSV and non-RSV controls was higher in elderly (≥65; $12,030–$23,194) than in those aged <65 years ($2251–$5391). Among children, adjusted costs attributable to RSV were higher in children aged 5–17 years ($3192), than in those 1–4 years ($2251–$2521) |
| RSV | Hospitalization | Pediatric | Age 0–2 months had highest age-specific RSV hospitalization rates as compared with 3–23 months |
| RSV | Hospitalization rates, ED visit rates | Pediatric | Hospitalization rates for RSV remain constant over time, with infants aged 0–2 months experiencing higher rates |
| RSV | Hospitalization rates, Healthcare resource use | Pediatric | Infants under 1 year old have higher hospitalization rates for RSV, with the highest healthcare resource use observed in the elderly population |
| RSV | Pulmonary complications | Pediatric | Age was associated with higher risk |
| SARS-Cov-2 | Inpatient death, renal replacement therapy, intubation, vasopressors | Adult | Age does not significantly influence these specific complications in adults |
| SARS-Cov-2 | Intracranial hemorrhage (ICH) | Adult | Age is not a significant factor in the occurrence of ICH in adults with COVID-19 |
| SARS-Cov-2 | Mortality | Combined | Mortality rates due to COVID-19 vary by age and ethnicity, with some groups experiencing higher rates at different ages |
| SARS-Cov-2 | Neurologic complications | Pediatric | Younger children have a higher likelihood of experiencing neurologic complications |
Overall, the results of the studies highlight the significant impact of respiratory infections on healthcare systems, underscoring the need for effective prevention and treatment strategies to mitigate the burden on patients and healthcare resources.
3.3. Populations that have higher risk of developing complications
In the pediatric population, complications assessed included neurologic and pulmonary complications, multisystem inflammatory syndrome, and mortality (55–64). In the adult population, complications studied include intracerebral hemorrhage (ICH), renal complications, respiratory complications, and mortality (65–73). The association between older age and complications in adults is also shown in some studies (72, 73).
Studies suggest that younger age may be a risk factor for complications in children. One study of COVID-19 patients demonstrated that the adjusted odds ratio of developing neurologic complications decreased by 3% for each year older in children under age 18 (56). Another study reported that infants younger than 6 months of age had a 40% higher risk of ICU admission compared to older infants with influenza (64). On the other hand, some studies identified a connection between older age and an increased risk of complications in pediatric populations with high-risk conditions, like transplant and cancer patients. For example, one study found that older age correlated with an increased risk of mortality and pulmonary complications in pediatric solid organ transplant patients with respiratory virus infection (57). Similarly, another study observed that older age was linked to an increased likelihood of experiencing severe complications from seasonal influenza in pediatric cancer patients (63). Moreover, one study identified a potential U-shaped relationship between age and the risk of developing severe pneumonia, with higher risk observed in 1- and 10-year-olds than in 2-and 5-year-olds (58), but other the studies did not show this relationship (67, 68). In adults, it has been reported that those over 65 years of age had a 2.1 times higher risk of mortality in cases of influenza A (H1N1) infection (72). Similarly, another study found that patients older than 75 years had an increased risk of hospitalization due to respiratory syncytial virus (RSV) infections, with odds ratios (95% confidence intervals) of 1.73 (1.15, 2.60) for those aged 75–84 compared to those under 65, and 2.53 (1.67, 3.84) for individuals 85 years and older compared to those under 65 (73). However, studies on SARS-Cov-2 have not established a clear association between age and complications (67, 68). Two single-center studies reported that age was not significantly linked to a composite outcome of inpatient mortality, need for renal replacement therapy (RRT), or hemodialysis (HD), intubation, and vasopressor use in patients with COVID-19 (67). Similarly, another study that investigated risk factors for intracranial hemorrhage (ICH) in COVID-19 patients also found no significant association with age (68).
Males have been found to have a 10–90% higher risk of developing complications in patients with influenza (55, 72). However, some studies in patients with COVID-19 did not find an association between sex and complications (62, 67, 68). Regarding race and ethnicity, Asian (55) and non-Hispanic black (62) populations may have a higher risk of developing complications from influenza and COVID-19, respectively, although some studies did not find an association between race, ethnicity, and complications (58, 67). In the pediatric population, the presence of a neurologic chronic condition was associated with a 3 times higher risk of neurological complications in patients with respiratory virus infection (55, 56, 61). Comorbidities and chronic conditions including asthma, malnutrition, congenital anomalies, and complex chronic medical conditions, were associated with an increased risk of developing complications, e.g., ICH, respiratory failure, pneumonia complications, hospitalization, ICU admission, and mortality in patients with influenza, pneumonia, COVID-19, and RSV (58–60, 64, 70, 71, 73). In adults, additional risk factors, such as alcohol use disorder and increased body-mass index, have been associated with mortality in patients with COVID-19 (65, 69).
Overall, studies suggest that age, sex, and chronic conditions are associated with varying risks of complications in both pediatric and adult populations. In children, younger age, especially infants, may generally be a risk factor for complications, while older age is associated with increased risks in high-risk groups, such as transplant and cancer patients. In adults, older age and male sex are associated with higher risks of complications in some studies, although findings are inconsistent across different infections and complications. Additionally, comorbidities and chronic conditions including asthma, malnutrition, congenital anomalies, and complex chronic medical conditions, were associated with an increased risk of complications. Finally, it should be noted that heterogeneity across studies and unadjusted or unmeasured confounding factors may have contributed to the variation in effect seen.
4. Discussion
In this study, we conducted a literature review from 2013 to 2022 to examine the disease burden of ARIs and identify populations at a higher risk of developing complications. ARIs are associated with a significant disease burden, consistently imposing considerable strain on HRU and individual health. Factors such as age, chronic conditions, and socioeconomic factors influence HRU and risk of complications. Notably, infants under 6 months, elderly populations, and individuals with comorbidities are more likely to experience increased HRU and complications from ARIs.
Given the substantial impact of ARIs on healthcare systems, early intervention is essential (77). This includes prompt testing, prevention strategies, and vaccination programs, especially for vulnerable populations, such as infants, the elderly, and individuals with chronic conditions (78–80). Early testing is crucial for managing ARIs, as it enables timely identification of pathogens, appropriate treatment, and infection control within communities (81–83). In addition, the recent COVID-19 pandemic highlighted the importance of swift and accurate identification of infectious pathogens to facilitate early treatment and prevent serious disease and complications (84, 85). Public health campaigns should emphasize proper hygiene practices, vaccinations, and early testing when experiencing respiratory infection symptoms.
It’s important to note that even among patients with similar symptoms, the predominant pathogens can vary significantly depending on age and other factors. This was highlighted in a previous study that examined the distribution of common pathogens including human rhinovirus/enterovirus (HRV), adenovirus (Adv), influenza, human metapneumovirus (Hmpv), coronavirus, respiratory syncytial virus (RSV), and parainfluenza (PIV) from under 2 years to 65 years and older (86). Notably, this study found statistically significant differences among all age groups for HRV Adv, influenza, RSV, and PIV. These findings underscore the importance of employing diagnostic methods capable of testing for multiple common pathogens to identify the pathogens.
In addition to hygiene and medical interventions, lifestyle factors like a Mediterranean diet, regular exercise, and healthy sleep habits play a crucial role in reducing infection risks by improving the immune system (87). Preventive measures against chronic diseases, tobacco cessation, moderate alcohol consumption, and maintaining a healthy weight are also vital for immune health (87).
The burden of ARI in the U.S. shows significant variation both seasonally and geographically. Typically, the incidence is highest from November to January and lowest from June to August (88). Geographically, southeastern states, including Florida, Georgia, Alabama, South Carolina, and Mississippi, as well as other south states experience a higher ARI burden compared to states in the North (88). Similar findings were reported by a study which found the highest age-standardized RIT incidence and mortality in the East South Central region (2). Furthermore, the ARI burden is influenced by various factors such as the types of circulating viruses, the effectiveness of available vaccines against these viruses, and the vaccination coverage within the population (89).
Healthcare providers need to be aware of the varying risks of complications in pediatric and adult populations based on factors like age, sex, and chronic conditions. This awareness can inform personalized treatment approaches, potentially improving outcomes and reducing complications. Furthermore, HRU and complication risk differ among various pathogens. Early testing contributes to accurate diagnoses, enabling healthcare providers to initiate appropriate treatments promptly, reducing complication risks and HRU. Policymakers and healthcare administrators should consider the significant burden of ARIs when allocating resources and planning services, ensuring sufficient staffing during peak respiratory infection seasons and investing in diagnostic and treatment facilities, including testing infrastructure.
This study has several limitations. First, our literature search may have missed relevant publications due to the nature of a scoping review. Second, eligible studies may include biases due to study design, definitions, and data sources. Finally, as this was a scoping review, there was no critical appraisal of the included studies or the quality of evidence.
Overall, the findings of this study emphasized the significant disease burden associated with ARIs and highlighted populations with a higher risk of developing complications. The disease burden and complication risks vary by pathogens, suggesting that early detection of pathogens could reduce HRU by applying targeted treatment in the initial phase. Further research is needed in terms of strategies to prevent complications, especially in high-risk populations.
Author contributions
CC: Investigation, Writing – original draft, Writing – review & editing. TT: Funding acquisition, Investigation, Writing – original draft, Writing – review & editing. CP: Investigation, Project administration, Supervision, Writing – original draft, Writing – review & editing. ZH: Investigation, Writing – original draft, Writing – review & editing. NR: Investigation, Supervision, Writing – original draft, Writing – review & editing.
Acknowledgments
Trisha Fritz, Senior Multimedia Designer at Premier Inc., provided graphics support.
Funding Statement
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by bioMérieux, Inc.
Conflict of interest
CC, CP, and NR were employed by Premier Inc. TT and ZH were employed by bioMérieux, Inc.
The authors declare that this study received funding from bioMérieux, Inc. The funder had the following involvement in the study: assistance with study design, collection, analysis, and interpretation of data; co-authoring on this article, and decision to submit for publication.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1325236/full#supplementary-material
References
- 1.Thomas M, Bomar PA. Upper respiratory tract infection. Treasure Island, FL: StatPearls; (2022). [PubMed] [Google Scholar]
- 2.Zhong W, Bragazzi NL, Kong JD, Safiri S, Behzadifar M, Liu J, et al. Burden of respiratory infection and tuberculosis among US states from 1990 to 2019. Clin Epidemiol. (2021) 13:503–14. doi: 10.2147/CLEP.S314802, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rogan M. Respiratory infections, acute. Int Encyclop Public Health. (2017) 2017:332–6. doi: 10.1016/B978-0-12-803678-5.00383-0 [DOI] [Google Scholar]
- 4.Mayor S. Acute respiratory infections are world’s third leading cause of death. BMJ. (2010) 341:c6360. doi: 10.1136/bmj.c6360 [DOI] [Google Scholar]
- 5.Bolek H, Ozisik L, Caliskan Z, Tanriover MD. Clinical outcomes and economic burden of seasonal influenza and other respiratory virus infections in hospitalized adults. J Med Virol. (2023) 95:e28153. doi: 10.1002/jmv.28153, PMID: [DOI] [PubMed] [Google Scholar]
- 6.McLean HQ, Peterson SH, King JP, Meece JK, Belongia EA. School absenteeism among school-aged children with medically attended acute viral respiratory illness during three influenza seasons, 2012-2013 through 2014-2015. Influenza Other Respir Viruses. (2017) 11:220–9. doi: 10.1111/irv.12440, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bureau of Labor Statistics . Employer-reported workplace injuries and illnesses - 2021. Washington, DC: U.S. Department of Labor: (2022). [Google Scholar]
- 8.Centers for Disease Control and Prevention . (2024) Disease burden of flu. Available at: https://www.cdc.gov/flu/about/burden/index.html.
- 9.Centers for Disease Control and Prevention . (2023) Preliminary estimated influenza illnesses, medical visits, hospitalizations, and deaths in the United States – 2021-2022 influenza season. Available at: https://www.cdc.gov/flu/about/burden/2021-2022.htm.
- 10.Centers for Disease Control and Prevention . (2024) 2022-2023 U.S. flu season: preliminary in-season burden estimates. Available at: https://www.cdc.gov/flu/about/burden/preliminary-in-season-estimates.htm.
- 11.Thomas M, Bomar PA. (2023). Upper respiratory tract infection. In: StatPearls [internet]. Treasure Island, FL: StatPearls publishing. Available at: https://www.ncbi.nlm.nih.gov/books/NBK532961/ (Accessed June 27, 2022). [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention . (2022) People at high risk for severe RSV infection. Available at: https://www.cdc.gov/rsv/high-risk/index.html.
- 13.Equator network . (2018) PRISMA extension for scoping reviews (PRISMA-ScR) 2023. Available at: https://www.equator-network.org/reporting-guidelines/prisma-scr/. [DOI] [PubMed]
- 14.Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. (2018) 169:467–73. doi: 10.7326/M18-0850, PMID: [DOI] [PubMed] [Google Scholar]
- 15.Arriola CS, Kim L, Langley G, Anderson EJ, Openo K, Martin AM, et al. Estimated burden of community-onset respiratory syncytial virus-associated hospitalizations among children aged <2 years in the United States, 2014-15. J Pediatr Infect Dis Soc. (2020) 9:587–95. doi: 10.1093/jpids/piz087, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chirikov VV, Simoes EAF, Kuznik A, Kwon Y, Botteman M. Economic-burden trajectories in commercially insured US infants with respiratory syncytial virus infection. J Infect Dis. (2020) 221:1244–55. doi: 10.1093/infdis/jiz160, PMID: [DOI] [PubMed] [Google Scholar]
- 17.Edwards KM, Zhu Y, Griffin MR, Weinberg GA, Hall CB, Szilagyi PG, et al. Burden of human metapneumovirus infection in young children. N Engl J Med. (2013) 368:633–43. doi: 10.1056/NEJMoa1204630, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fell DB, Johnson J, Mor Z, Katz MA, Skidmore B, Neuzil KM, et al. Incidence of laboratory-confirmed influenza disease among infants under 6 months of age: a systematic review. BMJ Open. (2017) 7:e016526. doi: 10.1136/bmjopen-2017-016526, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fletcher MA, Schmitt HJ, Syrochkina M, Sylvester G. Pneumococcal empyema and complicated pneumonias: global trends in incidence, prevalence, and serotype epidemiology. Eur J Clin Microbiol Infect Dis. (2014) 33:879–910. doi: 10.1007/s10096-014-2062-6, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurup S, Burgess R, Tine F, Chahroudi A, Lee DL. SARS-CoV-2 infection and racial disparities in children: protective mechanisms and severe complications related to MIS-C. J Racial Ethn Health Disparities. (2022) 9:1536–42. doi: 10.1007/s40615-021-01092-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma KC, Winn A, Moline HL, Scobie HM, Midgley CM, Kirking HL, et al. Increase in acute respiratory illnesses among children and adolescents associated with rhinoviruses and enteroviruses, including enterovirus D68 - United States, July-September 2022. MMWR Morb Mortal Wkly Rep. (2022) 71:1265–70. doi: 10.15585/mmwr.mm7140e1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLaughlin JM, Khan F, Schmitt HJ, Agosti Y, Jodar L, Simões EAF, et al. Respiratory syncytial virus-associated hospitalization rates among US infants: a systematic review and Meta-analysis. J Infect Dis. (2022) 225:1100–11. doi: 10.1093/infdis/jiaa752, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simoes EAF. The burden of respiratory syncytial virus lower respiratory tract disease in infants in the United States: a synthesis. J Infect Dis. (2022) 226:S143–7. doi: 10.1093/infdis/jiac211, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suh M, Movva N, Bylsma LC, Fryzek JP, Nelson CB. A systematic literature review of the burden of respiratory syncytial virus and health care utilization among United States infants younger than 1 year. J Infect Dis. (2022) 226:S195–s212. doi: 10.1093/infdis/jiac201, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suh M, Movva N, Jiang X, Reichert H, Bylsma LC, Fryzek JP, et al. Respiratory syncytial virus burden and healthcare utilization in United States infants <1 year of age: study of nationally representative databases, 2011-2019. J Infect Dis. (2022) 226:S184–94. doi: 10.1093/infdis/jiac155, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bolge SC, Gutierrez C, Kariburyo F, He D. Burden of pneumonia among hospitalized patients with influenza: real-world evidence from a US managed care population. Pulm Ther. (2021) 7:517–32. doi: 10.1007/s41030-021-00169-2, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown JD, Harnett J, Chambers R, Sato R. The relative burden of community-acquired pneumonia hospitalizations in older adults: a retrospective observational study in the United States. BMC Geriatr. (2018) 18:92. doi: 10.1186/s12877-018-0787-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cavallazzi R, Furmanek S, Arnold FW, Beavin LA, Wunderink RG, Niederman MS, et al. The burden of community-acquired pneumonia requiring admission to ICU in the United States. Chest. (2020) 158:1008–16. doi: 10.1016/j.chest.2020.03.051, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Charu V, Simonsen L, Lustig R, Steiner C, Viboud C. Mortality burden of the 2009-10 influenza pandemic in the United States: improving the timeliness of influenza severity estimates using inpatient mortality records. Influenza Other Respir Viruses. (2013) 7:863–71. doi: 10.1111/irv.12096, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Datta S, Walsh EE, Peterson DR, Falsey AR. Can analysis of routine viral testing provide accurate estimates of respiratory syncytial virus disease burden in adults? J Infect Dis. (2017) 215:1706–10. doi: 10.1093/infdis/jix196, PMID: [DOI] [PubMed] [Google Scholar]
- 31.de Courville C, Cadarette SM, Wissinger E, Alvarez FP. The economic burden of influenza among adults aged 18 to 64: a systematic literature review. Influenza Other Respir Viruses. (2022) 16:376–85. doi: 10.1111/irv.12963, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drijkoningen JJ, Rohde GG. Pneumococcal infection in adults: burden of disease. Clin Microbiol Infect. (2014) 20:45–51. doi: 10.1111/1469-0691.12461 [DOI] [PubMed] [Google Scholar]
- 33.Ehrlich HY, Harizaj A, Campbell L, Colt M, Yuan K, Rabatsky-Ehr T, et al. SARS-CoV-2 in nursing homes after 3 months of serial, Facilitywide point prevalence testing, Connecticut, USA. Emerg Infect Dis. (2021) 27:1288–95. doi: 10.3201/eid2705.204936, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.el Chaer F, Shah DP, Kmeid J, Ariza-Heredia EJ, Hosing CM, Mulanovich VE, et al. Burden of human metapneumovirus infections in patients with cancer: risk factors and outcomes. Cancer. (2017) 123:2329–37. doi: 10.1002/cncr.30599, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fall A, Kenmoe S, Ebogo-Belobo JT, Mbaga DS, Bowo-Ngandji A, Foe-Essomba JR, et al. Global prevalence and case fatality rate of enterovirus D68 infections, a systematic review and meta-analysis. PLoS Negl Trop Dis. (2022) 16:e0010073. doi: 10.1371/journal.pntd.0010073, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferreira-Coimbra J, Sarda C, Rello J. Burden of community-acquired pneumonia and unmet clinical needs. Adv Ther. (2020) 37:1302–18. doi: 10.1007/s12325-020-01248-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim DK, McGeer A, Uleryk E, Coleman BL. Burden of severe illness associated with laboratory confirmed influenza in adults aged 50-64 years: a rapid review. Influenza Other Respir Viruses. (2022) 16:632–42. doi: 10.1111/irv.12955, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lucero-Obusan C, Schirmer PL, Wendelboe A, Oda G, Holodniy M. Epidemiology and burden of influenza in the U.S. Department of Veterans Affairs. Influenza Other Respir Viruses. (2018) 12:293–8. doi: 10.1111/irv.12512, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McLaughlin JM, Johnson MH, Kagan SA, Baer SL. Clinical and economic burden of community-acquired pneumonia in the veterans health administration, 2011: a retrospective cohort study. Infection. (2015) 43:671–80. doi: 10.1007/s15010-015-0789-3, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olasupo O, Xiao H, Brown JD. Relative clinical and cost burden of community-acquired pneumonia hospitalizations in older adults in the United States-a cross-sectional analysis. Vaccines (Basel). (2018) 6:59. doi: 10.3390/vaccines6030059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ortiz JR, Neuzil KM, Cooke CR, Neradilek MB, Goss CH, Shay DK. Influenza pneumonia surveillance among hospitalized adults may underestimate the burden of severe influenza disease. PLoS One. (2014) 9:e113903. doi: 10.1371/journal.pone.0113903, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amand C, Tong S, Kieffer A, Kyaw MH. Healthcare resource use and economic burden attributable to respiratory syncytial virus in the United States: a claims database analysis. BMC Health Serv Res. (2018) 18:294. doi: 10.1186/s12913-018-3066-1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chu JN, Tsoh JY, Ong E, Ponce NA. The hidden colors of coronavirus: the burden of attributable COVID-19 deaths. J Gen Intern Med. (2021) 36:1463–5. doi: 10.1007/s11606-020-06497-4, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldstein E, Greene SK, Olson DR, Hanage WP, Lipsitch M. Estimating the hospitalization burden associated with influenza and respiratory syncytial virus in new York City, 2003-2011. Influenza Other Respir Viruses. (2015) 9:225–33. doi: 10.1111/irv.12325, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matias G, Haguinet F, Lustig RL, Edelman L, Chowell G, Taylor RJ. Model estimates of the burden of outpatient visits attributable to influenza in the United States. BMC Infect Dis. (2016) 16:641. doi: 10.1186/s12879-016-1939-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Near AM, Tse J, Young-Xu Y, Hong DK, Reyes CM. Burden of influenza hospitalization among high-risk groups in the United States. BMC Health Serv Res. (2022) 22:1209. doi: 10.1186/s12913-022-08586-y, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nguyen AM, Noymer A. Influenza mortality in the United States, 2009 pandemic: burden, timing and age distribution. PLoS One. (2013) 8:e64198. doi: 10.1371/journal.pone.0064198, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palekar RS, Rolfes MA, Arriola CS, Acosta BO, Guidos PA, Vargas XB, et al. Burden of influenza-associated respiratory hospitalizations in the Americas, 2010-2015. PLoS One. (2019) 14:e0221479. doi: 10.1371/journal.pone.0221479, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reed C, Chaves SS, Daily Kirley P, Emerson R, Aragon D, Hancock EB, et al. Estimating influenza disease burden from population-based surveillance data in the United States. PLoS One. (2015) 10:e0118369. doi: 10.1371/journal.pone.0118369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rolfes MA, Foppa IM, Garg S, Flannery B, Brammer L, Singleton JA, et al. Annual estimates of the burden of seasonal influenza in the United States: a tool for strengthening influenza surveillance and preparedness. Influenza Other Respir Viruses. (2018) 12:132–7. doi: 10.1111/irv.12486, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sibbel S, Sato R, Hunt A, Turenne W, Brunelli SM. The clinical and economic burden of pneumonia in patients enrolled in Medicare receiving dialysis: a retrospective, observational cohort study. BMC Nephrol. (2016) 17:199. doi: 10.1186/s12882-016-0412-6, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsai Y, Zhou F, Kim IK. The burden of influenza-like illness in the US workforce. Occup Med (Lond). (2014) 64:341–7. doi: 10.1093/occmed/kqu022, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wroe PC, Finkelstein JA, Ray GT, Linder JA, Johnson KM, Rifas-Shiman S, et al. Aging population and future burden of pneumococcal pneumonia in the United States. J Infect Dis. (2012) 205:1589–92. doi: 10.1093/infdis/jis240, PMID: [DOI] [PubMed] [Google Scholar]
- 54.Young-Xu Y, van Aalst R, Russo E, Lee JK, Chit A. The annual burden of seasonal influenza in the US veterans affairs population. PLoS One. (2017) 12:e0169344. doi: 10.1371/journal.pone.0169344, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Antoon JW, Hall M, Herndon A, Johnson DP, Brown CM, Browning WL, et al. Prevalence, risk factors, and outcomes of influenza-associated neurologic complications in children. J Pediatr. (2021) 239:32–8.e5. doi: 10.1016/j.jpeds.2021.06.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Antoon JW, Hall M, Howard LM, Herndon A, Freundlich KL, Grijalva CG, et al. COVID-19 and acute neurologic complications in children. Pediatrics. (2022) 150:e2022058167. doi: 10.1542/peds.2022-058167, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Danziger-Isakov L, Steinbach WJ, Paulsen G, Munoz FM, Sweet LR, Green M, et al. A multicenter consortium to define the epidemiology and outcomes of pediatric solid organ transplant recipients with inpatient respiratory virus infection. J Pediatr Infect Dis Soc. (2019) 8:197–204. doi: 10.1093/jpids/piy024, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williams DJ, Zhu Y, Grijalva CG, Self WH, Harrell FE, Jr, Reed C, et al. Predicting severe pneumonia outcomes in children. Pediatrics. (2016) 138:e20161019. doi: 10.1542/peds.2016-1019, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ghimire LV, Chou FS, Moon-Grady AJ. Impact of congenital heart disease on outcomes among pediatric patients hospitalized for influenza infection. BMC Pediatr. (2020) 20:450. doi: 10.1186/s12887-020-02344-x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leyenaar JK, Lagu T, Shieh MS, Pekow PS, Lindenauer PK. Management and outcomes of pneumonia among children with complex chronic conditions. Pediatr Infect Dis J. (2014) 33:907–11. doi: 10.1097/INF.0000000000000317, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilking AN, Elliott E, Garcia MN, Murray KO, Munoz FM. Central nervous system manifestations in pediatric patients with influenza a H1N1 infection during the 2009 pandemic. Pediatr Neurol. (2014) 51:370–6. doi: 10.1016/j.pediatrneurol.2014.04.026, PMID: [DOI] [PubMed] [Google Scholar]
- 62.Zambrano LD, Ly KN, Link-Gelles R, Newhams MM, Akande M, Wu MJ, et al. Investigating health disparities associated with multisystem inflammatory syndrome in children after SARS-CoV-2 infection. Pediatr Infect Dis J. (2022) 41:891–8. doi: 10.1097/INF.0000000000003689, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carr SB, Adderson EE, Hakim H, Xiong X, Yan X, Caniza M. Clinical and demographic characteristics of seasonal influenza in pediatric patients with cancer. Pediatr Infect Dis J. (2012) 31:e202–7. doi: 10.1097/INF.0b013e318267f7d9, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chaves SS, Perez A, Farley MM, Miller L, Schaffner W, Lindegren ML, et al. The burden of influenza hospitalizations in infants from 2003 to 2012, United States. Pediatr Infect Dis J. (2014) 33:912–9. doi: 10.1097/INF.0000000000000321, PMID: [DOI] [PubMed] [Google Scholar]
- 65.Bailey KL, Sayles H, Campbell J, Khalid N, Anglim M, Ponce J, et al. COVID-19 patients with documented alcohol use disorder or alcohol-related complications are more likely to be hospitalized and have higher all-cause mortality. Alcohol Clin Exp Res. (2022) 46:1023–35. doi: 10.1111/acer.14838, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hussein MH, Toraih EA, Attia AS, Burley N, Zhang AD, Roos J, et al. Asthma in COVID-19 patients: an extra chain fitting around the neck? Respir Med. (2020) 175:106205. doi: 10.1016/j.rmed.2020.106205, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fox T, Ruddiman K, Lo KB, Peterson E, DeJoy R, 3rd, Salacup G, et al. The relationship between diabetes and clinical outcomes in COVID-19: a single-center retrospective analysis. Acta Diabetol. (2021) 58:33–8. doi: 10.1007/s00592-020-01592-8, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Melmed KR, Cao M, Dogra S, Zhang R, Yaghi S, Lewis A, et al. Risk factors for intracerebral hemorrhage in patients with COVID-19. J Thromb Thrombolysis. (2021) 51:953–60. doi: 10.1007/s11239-020-02288-0, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Page-Wilson G, Arakawa R, Nemeth S, Bell F, Girvin Z, Tuohy MC, et al. Obesity is independently associated with septic shock, renal complications, and mortality in a multiracial patient cohort hospitalized with COVID-19. PLoS One. (2021) 16:e0255811. doi: 10.1371/journal.pone.0255811, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Panhwar MS, Kalra A, Gupta T, Kolte D, Khera S, Bhatt D, et al. Relation of concomitant heart failure to outcomes in patients hospitalized with influenza. Am J Cardiol. (2019) 123:1478–80. doi: 10.1016/j.amjcard.2019.01.046, PMID: [DOI] [PubMed] [Google Scholar]
- 71.Ponce J, Anzalone AJ, Bailey K, Sayles H, Timmerman M, Jackson M, et al. Impact of malnutrition on clinical outcomes in patients diagnosed with COVID-19. JPEN J Parenter Enteral Nutr. (2022) 46:1797–807. doi: 10.1002/jpen.2418, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shah NS, Greenberg JA, McNulty MC, Gregg KS, Riddell J, Mangino JE, et al. Severe influenza in 33 US hospitals, 2013-2014: complications and risk factors for death in 507 patients. Infect Control Hosp Epidemiol. (2015) 36:1251–60. doi: 10.1017/ice.2015.170, PMID: [DOI] [PubMed] [Google Scholar]
- 73.Wyffels V, Kariburyo F, Gavart S, Fleischhackl R, Yuce H. A real-world analysis of patient characteristics and predictors of hospitalization among US Medicare beneficiaries with respiratory syncytial virus infection. Adv Ther. (2020) 37:1203–17. doi: 10.1007/s12325-020-01230-3, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Placzek HE, Madoff LC. Association of age and comorbidity on 2009 influenza a pandemic H1N1-related intensive care unit stay in Massachusetts. Am J Public Health. (2014) 104:e118–25. doi: 10.2105/AJPH.2014.302197, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chow EJ, Uyeki TM, Chu HY. The effects of the COVID-19 pandemic on community respiratory virus activity. Nat Rev Microbiol. (2023) 21:195–210. doi: 10.1038/s41579-022-00807-9, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maglione M, Pascarella A, Botti C, Ricci G, Morelli F, Camelia F, et al. Changing epidemiology of acute viral respiratory infections in hospitalized children: the post-lockdown effect. Children (Basel). (2022) 9:1242. doi: 10.3390/children9081242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang Y, Eccles R, Bell J, Chua AH, Salvi S, Schellack N, et al. Management of acute upper respiratory tract infection: the role of early intervention. Expert Rev Respir Med. (2021) 15:1517–23. doi: 10.1080/17476348.2021.1988569 [DOI] [PubMed] [Google Scholar]
- 78.Mazagatos C, Delgado-Sanz C, Monge S, Pozo F, Oliva J, Sandonis V, et al. COVID-19 vaccine effectiveness against hospitalization due to SARS-CoV-2: a test-negative design study based on severe acute respiratory infection (SARI) sentinel surveillance in Spain. Influenza Other Respir Viruses. (2022) 16:1014–25. doi: 10.1111/irv.13026, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hanson KE, Azar MM, Banerjee R, Chou A, Colgrove RC, Ginocchio CC, et al. Molecular testing for acute respiratory tract infections: clinical and diagnostic recommendations from the IDSA's diagnostics committee. Clin Infect Dis. (2020) 71:2744–51. doi: 10.1093/cid/ciaa508, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jefferson T, Del Mar CB, Dooley L, Ferroni E, Al-Ansary LA, Bawazeer GA, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database Syst Rev. (2011) 2011:Cd006207. doi: 10.1002/14651858.CD006207.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Das S, Dunbar S, Tang YW. Laboratory diagnosis of respiratory tract infections in children - the state of the art. Front Microbiol. (2018) 9:2478. doi: 10.3389/fmicb.2018.02478, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gradisteanu Pircalabioru G, Iliescu FS, Mihaescu G, Cucu AI, Ionescu ON, Popescu M, et al. Advances in the rapid diagnostic of viral respiratory tract infections. Front Cell Infect Microbiol. (2022) 12:807253. doi: 10.3389/fcimb.2022.807253, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang N, Wang L, Deng X, Liang R, Su M, He C, et al. Recent advances in the detection of respiratory virus infection in humans. J Med Virol. (2020) 92:408–17. doi: 10.1002/jmv.25674, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stojanovic Z, Goncalves-Carvalho F, Marin A, Abad Capa J, Dominguez J, Latorre I, et al. Advances in diagnostic tools for respiratory tract infections: from tuberculosis to COVID-19 - changing paradigms? ERJ Open Res. (2022) 8:00113–2022. doi: 10.1183/23120541.00113-2022, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Muhrer JC. Risk of misdiagnosis and delayed diagnosis with COVID-19: a Syndemic approach. Nurse Pract. (2021) 46:44–9. doi: 10.1097/01.NPR.0000731572.91985.98, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tsagarakis NJ, Sideri A, Makridis P, Triantafyllou A, Stamoulakatou A, Papadogeorgaki E. Age-related prevalence of common upper respiratory pathogens, based on the application of the FilmArray respiratory panel in a tertiary hospital in Greece. Medicine (Baltimore). (2018) 97:e10903. doi: 10.1097/MD.0000000000010903, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Patchett D, Yang J, Northern J, Salinas M, Bauer BA. Viral respiratory infections: an ounce of prevention is worth a pound of cure. Mayo Clin Proc Innov Qual Outcomes. (2021) 5:480–5. doi: 10.1016/j.mayocpiqo.2020.12.008, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.FluView . Weekly US map: Influenza summary update. Atlanta, GA: Centers for Disease Control and Prevention (2023). [Google Scholar]
- 89.Centers for Disease Control and Prevention . Disease burden of flu. (2023). Available at: https://www.cdc.gov/flu/about/burden/index.html (Accessed December 27, 2023).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.