Abstract
Previous research links risky sexual behavior (RSB) to externalizing problems and to substance use, but little research has been conducted on relationships between internalizing problems (INT) and RSB. The current study addresses that literature gap, using both a twin sample from Colorado (N = 2,567) and a second twin sample from Minnesota (N = 1,131) in attempt to replicate initial results. We explored the hypothesis that the latent variable INT would be more strongly associated with the latent variable RSB for females than for males, examining relationships between INT and RSB via phenotypic confirmatory factor analysis and multivariate twin analyses. We found a small but significant phenotypic association between the latent variables. However, despite using two large twin samples, limited power restricted our ability to identify the genetic and environmental mechanisms underlying this association. Our sex differences hypothesis was not fully supported in either sample and requires further investigation. Our findings illustrate the complexity of the relationship between internalizing problems and risky sexual behavior.
Keywords: risky sexual behavior, internalizing, twin study, CADD, MCTFR
Risky sexual behavior (RSB) is a major public health issue. Examples of RSBs include having unprotected sexual intercourse (vaginal, anal, or oral) having multiple lifetime sexual partners, having multiple concurrent sexual partners, engaging in sexual encounters at an early age, having a high-risk partner (e.g., one who has multiple additional partners), and having sex with a partner who injects/has previously injected substances (Healthwise 2020). Although such sexual behaviors do not always lead to negative consequences, RSBs do increase the risk for unintended pregnancies and contracting sexually transmitted infections (STIs), such as chlamydia, human immunodeficiency virus (HIV) and human papillomavirus (HPV). Certain aspects of RSB, namely lifetime number of sexual partners and age at initial sexual encounter, have previously been shown to be reliably reported and heritable (Huibregtse et al. 2021). In the United States alone, there were 26.2 million new cases of STIs in 2018—the highest annual incidence rate ever—with people aged 15 to 24 years accounting for 45.5% of those cases (Kreisel et al. 2021). Subsequently, an estimated $15.9 billion was spent on direct medical costs for treatment of sexually transmitted infections in 2018 (Chesson et al. 2021). In addition to physical consequences, emotional issues, such as a sense of betrayal and abandonment, altered self-esteem, confusion about romantic feelings, and depression are also associated with sexual activity in people aged 13 to 18 years (Hallfors et al. 2005).
One of the more studied associations is the link between substance use and RSB; drug and alcohol use has been identified as a target health risk behavior and a potential risk factor of RSB (Kann et al. 2018). However, it is possible that RSB facets, such as numerous sexual partners, leads to more frequent sex under the influence of substances, rather than the reverse (Huibregtse et al. 2021).
Risky sexual behavior has also been investigated in conjunction with externalizing disorders (e.g., antisocial personality disorder, conduct disorder, attention problems, etc.), and the association between them is well documented (Timmermans et al. 2008; Ross et al. 2019). For example, previous research has shown that childhood externalizing behaviors predict later RSB (Boislard et al. 2013) and individuals with externalizing disorders engage in greater amounts of RSB compared to those without an externalizing disorder (Ross et al. 2019). Furthermore, twin research suggests a common genetic etiology between externalizing problems and RSB (Barr and Dick 2019).
There are also demonstrated sex differences in RSBs. Men and women differ in both their interest in casual sex, such that men are more willing to engage in casual sex than women are, and in their sexual fantasies, with men having more frequent sexual thoughts and fantasies than women do (Okami and Shackelford 2001). On average, men and women also seem to respond different to perceived sexual risks. In a study on the relationship between perceived HIV risk, HIV knowledge, and RSB, Collado et al. 2015 found that, in contexts where perceived risk of contracting HIV was high, greater knowledge of HIV was associated with reduced risky sexual encounters in women but with increased encounters in men.
While a relationship between externalizing psychopathology and RSB has been robustly identified in the literature, less is known about how internalizing psychopathology may be involved in these behaviors. As a 2008 study by Iacono et al. indicates, behavioral disinhibition, or “lack of constraint, tendency toward impulsivity, or inability to inhibit socially undesirable or otherwise restricted actions” may be one driver of RSBs. Emotional distress and poor self-image potentially play a role in behavioral disinhibition (Niemz et al. 2005), which may also suggest the presence of a unique internalizing pathway to RSBs. Internalizing behaviors are those that correspond to tendencies toward inwardly directed difficulties and overly controlled behavior (Merrell & Gueldner 2010). Such behaviors include symptoms such as emotional distress, frustration, cognitive distortions, withdrawal, and somatization (Achenbach et al. 1987; Bayer et al. 2011). The most commonly observed and diagnosed internalizing disorders are depression and anxiety; the one-year prevalence of major depressive episodes in young adults is nearly 10% (Mojtabai et al. 2016) and prevalence of anxiety in young adults is almost 7% (Goodwin et al. 2020). Social anxiety has been linked to having more sexual partners in some individuals (Rahm-Knigge et al. 2021), and depression has been associated with risky sex and STIs (Pratt et al. 2012). Outside of diagnosed disorders, both depressive symptoms and anxiety symptoms have been associated with risky sexual behaviors (Pozuelo et al. 2022; Wong et al. 2017). There also exist demonstrated sex differences in internalizing behavior, with females showing higher rates of internalizing disorders than males, which could be due to a tendency among women to internalize distress and a tendency among men to externalize their stress (Eaton et al. 2012). Additionally, one study found that females engaging in substance use and sexual activity experience more depressive symptoms than do males engaging in similar behavior (Waller et al. 2006), and another research group found evidence suggesting that the relationship between internalizing problems and RSB may be moderated by sex (Rogers and McKinney 2019). Therefore, we might expect a sex difference in how RSB and internalizing problems relate to one another.
Self-esteem, a measure of global self-worth constructed of both positive and negative feelings about the self (Rosenberg 1985), is also an important component of internalizing disorders. Past research has indicated that poor self-esteem is associated with other internalizing problems (Creemers et al. 2013) and mediates the association between peer relationship issues and internalizing problems (Bosacki et al. 2007). Studies have also linked self-esteem to RSB, finding that low self-esteem in adolescence predicts later risk for engaging in unprotected sex, having a greater number of lifetime sexual partners, and experiencing unplanned pregnancy (Boden and Horwood 2006).
Although a number of studies have investigated links between internalizing problems and RSB, the association remains understudied. Previous research investigating the association between internalizing problems and RSB found that mental health, including internalizing disorders, may mediate increased RSBs in sexual minorities (Oginni et al. 2020); however, the authors did not isolate depression and anxiety from substance use. A second study by the same first author also suggested a link between internalizing problems and RSB, but did not isolate depression and anxiety from other psychosocial adversity (e.g., intimate partner violence) (Oginni et al. 2021). Another group of researchers (Hallfors et al. 2005) suggest that sex and drug use behavior may predict depression, though again, substances were a larger focus, especially in the investigation of sex differences. Additional research suggests that personal factors, such as HIV knowledge and worry about HIV may further mediate an association between internalizing and safer sex practices (Joppa et al. 2014).
There also seem to be genetic influences on risky sexual behavior (Harden 2014; Huibregtse et al. 2021). One study found an association between personality—including neuroticism—and RSB, with evidence to suggest shared genetic influences underlying that association (Zietsch et al. 2010). Previous research also found significant genetic contribution to age at first sexual intercourse specifically, with some evidence of sex differences in the environmental influences on age at first sexual intercourse (Dunne et al. 1997). Another study suggests significant genetic influences on initiation/abstinence of sexual intercourse, number of sexual partners, and age at first sexual intercourse, as well as some differential effects between sexes (Mustanski et al. 2007). Still, to our knowledge, no study has focused on decomposing the genetic and environmental factors underlying the relationship between internalizing problems (specifically and solely) and RSB. Additionally, despite previous evidence of sex differences in both RSB and internalizing problems, potential sex differences in the magnitude of the relationship between RSB and internalizing problems remain understudied.
We present two studies, both of which use genetically-informative twin samples to parse the genetic and environmental influences contributing to the link between internalizing (from here referred to as INT) and RSB. Study 1 uses a Colorado twin sample to examine the association between INT and RSB and the genetic and environmental influences underlying that association to fill an important gap in existing literature. Study 2 uses a Minnesota twin sample in an attempt to replicate our results from Study 1 in an independent sample. Although identical measures were not available across the two studies, inclusion of a constructive replication study—in which two studies are conducted independently in different populations with slightly different measures of the same construct (Lykken 1968)—serves to strengthen and reinforce the impact of the current study. Both studies investigate sex differences in the relationship between INT and RSB. We hypothesize that 1) a positive correlation exists between INT and RSB, 2) sex differences exist in the relationship between INT and RSB, such that the magnitude of the association between INT and RSB will be greater in females than in males, and 3) sex differences exist in the genetic and environmental components underlying the association between INT and RSB. We suspect that sex differences will be in the direction predicted because previous research suggests that females demonstrate more internalizing problems, on average, than males, and because of previous research on sex differences in RSB. Given the public health consequences of RSB, along with prevalence of internalizing disorders, our research has broad implications for both future research and, potentially, public health policy initiatives.
Study 1
Method
Participants
Participants for Study 1 were drawn from the second wave of data collection (occurring in 2002 – 2008) of the Center on Antisocial Drug Dependence (CADD), which was established in 1997 with the primary aims of understanding the genetics and etiology of substance use disorders and related conditions. The CADD is a multiple cohort longitudinal study of adolescent/young adult antisocial behavior and substance use. Two of the CADD data cohorts include the Colorado Longitudinal Twin Study (LTS) and the Colorado Community Twin Study (CTS), which were used in the current analyses. Twins in the analysis were in late adolescence/early adulthood (female mean = 20.09 years, s.d. = 2.64 years; male mean = 19.83 years, s.d. = 2.57 years) and were representative of Colorado demographics (Rhea et al. 2006, 2013a, b, c). The sample was 53.6% female. We chose to study participants of this age as they are likely to be in their peak dating and exploration period, as opposed to an older sample that could be married or in long-term monogamous relationships. There were some cases where either Twin 1 or Twin 2 declined to continue participation, and in those cases, single-responding twins were included. Additionally, 355 participants showed missing data on one or both variables used to define RSB, resulting in a final N = 2,567 individuals (342 female monozygotic twin pairs, 270 male monozygotic twin pairs, 205 female dizygotic twin pairs, 187 male dizygotic twin pairs, 236 opposite sex dizygotic twin pairs, and 87 cotwin missing individuals) who had reported having had sex at least once and reported an age of initiation.
Zygosity
For approximately 95% of twin participants, DNA samples were collected via cheek swab, and zygosity was determined by analyzing 11 highly polymorphic short tandem repeat polymorphisms (Rhea et al. 2006). Twin pairs had to be concordant for all markers to be considered MZ; those with discordant genotypes were identified as DZ twins. For the remaining 5% of twin participants for whom DNA samples were unavailable, zygosity was determined using parental and self-report zygosity questionnaires and ratings from interviewers (Nichols and Bilbro 1966; see Rhea et al. 2013a, b, c for details). Where discrepancies between zygosity ascertainment occurred, zygosities were reviewed by staff and resolved. Since the time of data collection, zygosities have also been updated based on GWAS testing of the twin pairs.
Measures
Sexual behavior was assessed by self-report using items from the Jessor and Donovan Health Behavior Questionnaire. Number of lifetime sexual partners was scored from a single item: “In your life, how many people have you had sex with?” Responses were free-response format, with the option of refusing to answer. Age at first sexual encounter was also scored from a single item: “How old were you the first time you had sex?” Responses were free-response format. Four participants in Study 1 indicated age at first sexual encounter as 11 or younger. In these at least one of these cases, the participant was already under the care of social services and researchers responsible for data at the time of data collection followed reporting protocols for cases of physical, emotional, or sexual abuse. Because age at first sexual encounter increased (i.e., higher score = older age at sexual initiation), the scale was reversed for multivariate biometrical analyses (i.e., higher score = younger age at sexual initiation). This was done by recoding the oldest age to the smallest score, and so on (e.g., 24 years = 1, 23 years = 2, etc.).
We used measures of depressive symptoms, neuroticism (which encompasses anxiety, alienation, anger, moodiness, and guilt, and demonstrates a strong overlap with general anxiety symptomatology (Uliaszek et al. 2009)), and self-esteem to quantify INT. These three dimensions were chosen primarily because when taken together, they provide a broad measure of negative emotionality (Liu et al. 2011).
Depressive symptom count was measured using a composite of the items on the 20-item Centers for Epidemiological Studies-Depression (CES-D; (Radloff 1977) scale, which has a raw score range of 0 – 60. The instrument asks participants to self-report how often they feel a certain way “during the past week.” Example items from the scale include: “I felt I was just as good as other people (r),” “I felt lonely,” “My sleep was restless,” “People were unfriendly,” and “I felt depressed,” with some items reverse-scored for consistency. All 20 CES-D items were scored on four-point scale (“Rarely (Less than 1 day)” [0], “Some (1–2 days)” [1], “Occasionally (3–4 days)” [2], and “Most (5–7 days)” [3], with higher scores indicating more depressive symptoms. Calculated internal consistency for this scale was high (Alpha = .89).
Neuroticism was measured by nine items of the modified eighteen-item Eysenck Personality Questionnaire (Floderus-Myrhed et al. 1980), in which nine of the items addressed neuroticism and nine addressed extraversion. The nine-item neuroticism scale included items such as: “Do you sometimes feel happy, sometimes sad, without any real reason?”, “Do you often make up your mind too late?”, “Are you touchy about some things?”, and “Do you worry too long after an embarrassing experience?” to which participants either self-reported “Yes” or “No.” The EPQ Neuroticism total score was the mean number of “yes” responses across the nine items, with higher scores indicating greater neuroticism levels. The distribution was approximately normal. Calculated internal consistency for this scale was acceptable (Alpha = .74).
Self-esteem was assessed with the Rosenberg Self-esteem scale, a 10-item scale measuring global self-worth by evaluating both positive and negative feelings about the self (Rosenberg 1985). The scale asks participants to report the extent to which they agree or disagree with each statement. Some sample items from the scale include: “On the whole, I am satisfied with myself,” “At times I think I am no good at all (r),” “I am able to do things as well as most other people,” and “I wish I could have more respect for myself,” with some items reverse-scored according to the scale. All items were scored on a four-point scale (“Strongly disagree” [1], “Disagree” [2], “Agree” [3], and “Strongly agree” [4]) where a higher score indicated higher self-esteem. The scale was reversed for ease of interpretation in multivariate analyses (i.e., higher score = lower self-esteem). Calculated internal consistency for this scale was high (Alpha = .90). Refer to Table 1 for descriptive statistics for all measures.
Table 1.
Descriptive statistics for variables used in Study 1 (CO Sample).
| Entire sample | Males | Females | |||
|---|---|---|---|---|---|
| M (SD) | M (SD) | Min, Max | M (SD) | Min, Max | |
| Num. Part. † | 4.87 (5.74) | 5.24 (6.30) | 1.00, 53.00 | 4.51 (5.14) | 1.00, 60.00 |
| Age at F.S. | 16.97 (2.39) | 16.95 (2.04) | 14.00, 23.00 | 16.82 (2.02) | 9.00, 24.00 |
| Depression * | 10.23 (8.55) | 9.11 (7.41) | 0.00, 57.00 | 11.16 (9.28) | 0.00, 53.00 |
| Neuroticism * | 0.42 (0.24) | 0.39 (0.23) | 0.00, 1.00 | 0.45 (0.25) | 0.00, 1.00 |
| Self-esteem * | 34.35 (5.81) | 34.80 (5.65) | 10.00, 40.00 | 33.97 (5.93) | 10.00, 40.00 |
| Age * | 20.04 (2.72) | 19.83 (2.57) | 16.13, 27.96 | 20.09 (2.64) | 16.52, 29.13 |
Num. Part. = lifetime number of sexual partners; Age at F.S. = age at first sexual encounter; Depression = depressive symptom count; Age = age at assessment;
mean sex difference at p = .05;
mean sex difference is significant, p < .05; analyses were conducted on transformed variables but raw score means are shown here.
Lifetime number of sexual partners, depressive symptoms, and self-esteem all demonstrated strong right skew and were log + 1 transformed to minimize skewness and to meet normality model assumptions.
Statistical procedures
All descriptive statistics, composite variables, transformations, and reverse-scoring were conducted in SPSS version 26 (IBM Corp. 2019). Variable correlations, twin correlations, and t-tests to examine mean sex differences were conducted in R version 4.0.0 (R Core Team 2022). Univariate analysis models were conducted in OpenMx version 2.18.1 (Boker et al. 2011; Neale et al. 2016) and phenotypic CFA models were conducted in Lavaan (Rosseel 2012). Multivariate twin models were conducted in Mplus version 8.5 (Muthén and Muthén 1998–2017). Twin correlations, phenotypic models, and structural equation model parameters were estimated employing maximum likelihood (ML) for analysis of all variables, as all variables were treated as either continuous or quasi-continuous.
Model fit was determined using the following fit indices: the chi-square statistic, the p-value, the comparative fit index (CFI), the root mean square error of approximation (RMSEA) (Hu and Bentler 1999), and the Akaike information criterion (AIC; Akaike 1973). Nested sub-models were also tested, in which nested models were compared to full models, using chi-square difference tests. Chi-square difference tests were used to 1) compare sub-model fit to full model fit, 2) investigate individual parameter significance, 3) test for significant sex differences, and 4) balance fit and number of parameters to choose parsimonious models (Akaike 1987; Williams and Holahan 1994). Individual parameter significance in the biometrical models was tested via one degree of freedom tests by dropping the parameter of interest. A significant p-value (p < .05) resulting from the difference test signifies a significantly worse fit, indicating that the dropped parameter was significant. To test for sex differences in factor loadings, we compared models in which the loadings were freely estimated for males and females to models in which the loadings were constrained to be equal across sex.
Phenotypic analyses
A phenotypic confirmatory factor analysis was run to examine phenotypic correlations and confirm latent variables. Latent variables in the current study were labeled “INT” (for internalizing) and “RSB” (for risky sexual behavior), where INT indicators were depressive symptoms, neuroticism, and self-esteem, and RSB indicators were lifetime number of sexual partners and age at initial sexual encounter. Latent variables were correlated, allowing for identification of the two-indicator latent variable model. Indicator variables were standardized, and the variances of both latent variables were constrained to one for identification. To account for nonindependence of the twin sample, data were clustered by family ID in Lavaan. Missing data were handled using ML options. A multigroup phenotypic confirmatory factor analysis was conducted to examine phenotypic correlations by sex. We conducted measurement invariance testing on the multigroup model and found evidence for metric invariance across male and female latent constructs; configural and scalar invariance were also tested.
Univariate twin analyses
The genetic and environmental etiology of variances and twin covariances of the five variables of interest (e.g., number of lifetime sexual partners, age at first sexual encounter, depressive symptoms, neuroticism, and self-esteem) were first examined using univariate ACE/ADE twin models. Twin models take advantage of differences in genetic similarity between monozygotic (MZ) twins and dizygotic (DZ) twins to parse trait variance into genetic and environmental variance components, where VA represents variance explained by additive genetic influences, VD, non-additive genetic dominance effects, VC, shared environmental influences, and VE, nonshared environmental influences uncorrelated within twin pairs.
Nested sub-models, in which one or more genetic or environmental factors was dropped, resulting in AE, CE, and E models, were tested using standard Chi-square difference tests to determine whether sub-models provide more parsimonious fit alternatives to the full ACE or ADE models. With twin pairs reared together VD and VC are confounded, so non-nested ACE and ADE models were evaluated separately using AIC and other fit indices to determine best fitting models.
Multivariate twin analyses
A bivariate Cholesky decomposition was conducted to investigate genetic and environmental components of the correlation between the latent INT factor and latent RSB factor, and allow for the estimation of genetic (rA) and environmental (rC and rE) correlations across outcomes. The Cholesky decomposes variance into genetic and environmental contributions specific to each variable and shared between the variables. The cross paths, which are often depicted with arrows pointing from variance components on the first latent variable to the second latent variable, do not imply causation as the order of the variables could be exchanged without changing the fit of the model; rather, they are path estimates indicating shared genetic or environmental contributions between variables.
Because we hypothesized sex differences in the relationship between INT and RSB, we conducted a five-group bivariate Cholesky decomposition. The five-group bivariate Cholesky decomposition leverages data from five zygosity/sex groupings: monozygotic female twin pairs (MZFF), monozygotic male twin pairs (MZMM), dizygotic female twin pairs (DZFF), dizygotic male twin pairs (DZMM), and opposite-sex dizygotic twin pairs (DZOS). These data allowed us to estimate model parameters and means separately for males and females. As described in more detail above, Chi-square difference tests were used to test whether sex-specific model parameters provided a better fit to the observed data versus models with parameter estimates constrained across sex.
Results
As expected based on previous literature, females reported significantly higher scores on average than males did for all three measures of INT: depressive symptoms, t(2307) = 4.81, p<.001; neuroticism, t(2398) = 6.24, p<.001; and low self-esteem, t(2322) = 3.78, p<.001 (with females reporting lower self-esteem than males). For measures of RSB, there was no significant sex difference for either lifetime number of partners, t(1162) = 1.93, p = .05, or for age at first sexual encounter, t(1349) = 1.21, p = .23. There was a significant sex difference in age at assessment, t(2448) = −2.47, p = .01. T-tests were conducted using transformed data in order to better meet normality assumptions, and thus reflect differences in geometric means, and analyses were adjusted for clustering of twin within families. Raw mean scores are reported in Table 1. There were also some sex differences in the phenotypic correlations among the variables (Table 2; 95% CIs shown in Supplementary Table 1). For females, both number of sex partners and age at first sex show modest but significant correlations with depressive symptoms and neuroticism. Among males, number of sex partners is not significantly correlated with any of the INT measures. The differences observed in the phenotypic correlations hint at a potential sex difference in the relationship between INT and RSB measures.
Table 2.
Phenotypic correlations, by sex, for Study 1 (CO Sample).
| Num. of Partners | Age at F.S. | Depression | Neuroticism | Self-esteem | |
|---|---|---|---|---|---|
| Num. of Partners | 1 | −0.31* | 0.16* | 0.16* | 0.05 |
| Age at F.S. | −0.31* | 1 | −0.12* | −0.15* | −0.07 |
| Depression | 0.04 | −0.11* | 1 | 0.57* | 0.56* |
| Neuroticism | 0.01 | −0.02 | 0.52 * | 1 | 0.46* |
| Self-esteem | 0.03 | −0.12* | 0.56 * | 0.39 * | 1 |
Female estimates shown on the upper diagonal, male estimates shown on the lower diagonal in bold. Depression = depressive symptom count.
significant at alpha = .05. Age at first sex was not reversed when computing the correlations.
Phenotypic relationship between INT and RSB
A phenotypic confirmatory factor analysis was used to assess the relationship between the latent INT and RSB phenotypes. The model fit well, χ2(4) = 5.315, p = .2565, RMSEA = 0.012, CFI = 0.999, AIC = 12392.399, and demonstrated a significant (χ2diff (1) = 11.84, p <.001) phenotypic correlation of .19 between INT and RSB. To test our main hypothesis of sex differences in the magnitude of the correlation between INT and RSB, we conducted a multigroup phenotypic model, as seen in Figure 1 (95% CIs shown in Supplementary Table 2). The multigroup model also fit well, χ2(9) = 13.844, p = .128, RMSEA = 0.031, CFI = 0.995, AIC = 8637.725, and both male (χ2diff (1) = 3.80, p = .049) and female (χ2diff (1) = 21.773, p <.001) correlations between INT and RSB were significant. To investigate potential phenotypic sex differences, we constrained the correlation between the latent variables to be equal for both males and females and compared the model fit model to the original model with freed correlations between the sexes. The phenotypic sex difference in correlations (rmales = 0.13, rfemales = 0.30) was not significant, χ2diff (1) = 3.21, p = .073, but the point estimates of the correlations were in the direction predicted, with females demonstrating a greater correlation between INT and RSB than males.
Figure 1.
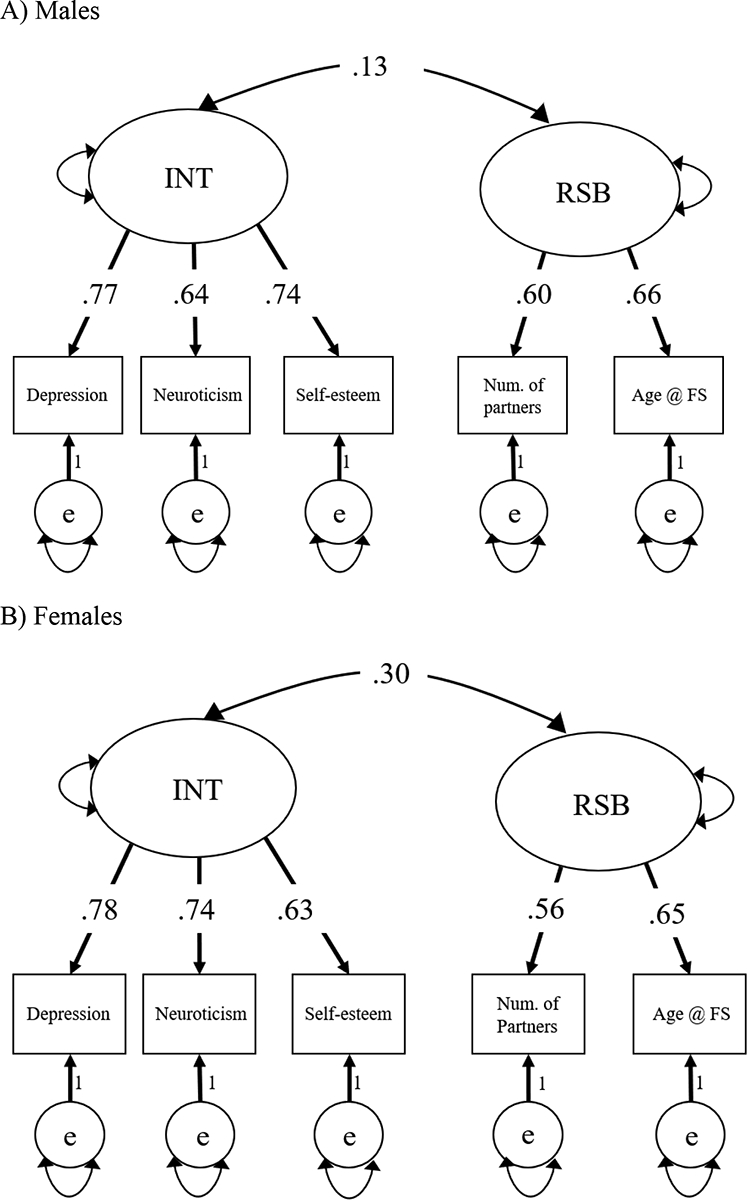
Multigroup phenotypic confirmatory factor analysis for Study 1, separated by sex.
Panel A) Males. Panel B) Females. Standardized estimates shown. In order to estimate the correlations between the latent factors, the measurement models (raw factor loadings) were constrained to be equal across males and females. Latent variable variances fixed to 1.00. Fit: χ2(9) = 13.844, p = .128, RMSEA = 0.031, CFI = 0.995, AIC = 8637.725.
Genetic and environmental influences on indicator variables
Genetic and environmental parameter estimates for each of the five variables were typically in the direction expected based on twin correlations, with few exceptions. ACE models demonstrated the best fit for age at first sexual encounter, depressive symptoms, and neuroticism, while number of lifetime sexual partners and self-esteem were best explained by ADE models, though with some sex differences (twin correlations for all variables are shown in Table 3). All variables demonstrated significant nonshared environmental influences, including measurement error.
Table 3.
Twin correlations for Study 1 (CO Sample).
| MZ vs. DZ | |||||
|---|---|---|---|---|---|
| Num. of Partners | Age at F.S. | Depression | Neuroticism | Self-esteem | |
| MZ | 0.59 | 0.58 | 0.37 | 0.41 | 0.35 |
| DZ | 0.21 | 0.46 | 0.19 | 0.20 | 0.12 |
| MZFF vs. DZFF | |||||
| Num. of Partners | Age at F.S. | Depression | Neuroticism | Self-esteem | |
| MZFF | 0.61 | 0.64 | 0.41 | 0.45 | 0.30 |
| DZFF | 0.51 | 0.50 | 0.22 | 0.21 | 0.09 |
| MZMM vs. DZMM | |||||
| Num. of Partners | Age at F.S. | Depression | Neuroticism | Self-esteem | |
| MZMM | 0.57 | 0.51 | 0.29 | 0.31 | 0.44 |
| DZMM | 0.16 | 0.48 | 0.13 | 0.25 | 0.16 |
| DZOS | |||||
| Num. of Partners | Age at F.S. | Depression | Neuroticism | Self-esteem | |
| DZOS | 0.27 | 0.50 | 0.15 | 0.24 | 0.09 |
MZFF = MZ female twin pairs. DZFF = DZ female twin pairs. MZMM = MZ male twin pairs. DZMM = DZ male twin pairs, DZOS = DZ opposite-sex pairs. Age at F.S. = age at first sex.
Multivariate twin analyses
We used a bivariate Cholesky decomposition to examine the shared genetic and environmental contributions to the relationship between INT and RSB and to calculate genetic and environmental correlations. Model comparisons suggested AE models were the most parsimonious compared to full models, as fitting ACE and ADE models showed that estimates of VC and VD were not significant. The AE model provided a better fit to the data than a CE model based on fit, and the more parsimonious AE model was chosen (refer to Supplementary Table 3 for details).
To investigate sex differences in the relationship between INT and RSB, we computed a five-group sex-limitation Cholesky that allowed for the estimation of separate parameters for males and females (see Figure 2, 95% CIs shown in Supplementary Table 4). The full sex-limitation model fit the data well, though dropping C from the model did not hurt fit, χ2diff(10) = 7.64, p = .66, and the more parsimonious AE model with specific variances on indicators, χ2(273) = 283.589, p = .3171, RMSEA = 0.012, CFI = 0.996, AIC = 11941.006, was selected.
Figure 2.
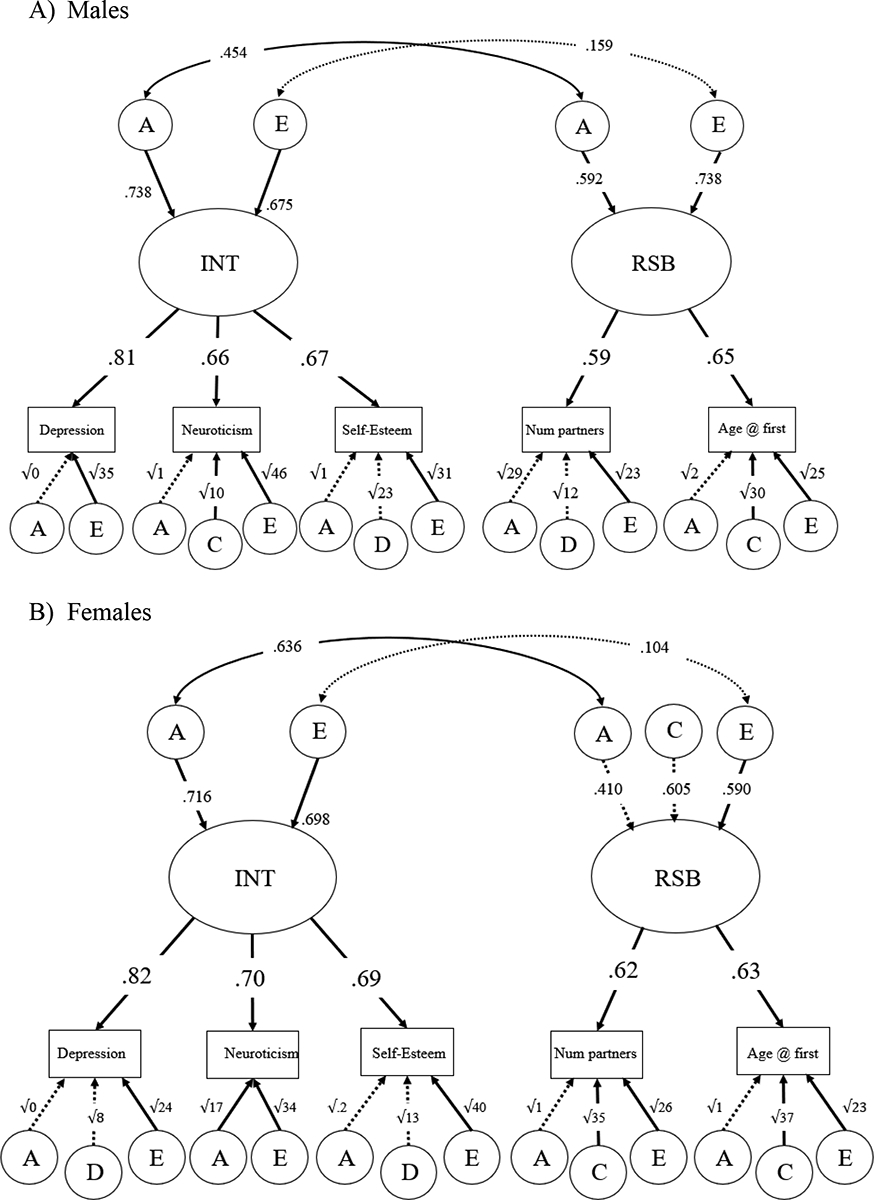
Five-group sex-limitation, with genetic and nonshared environmental correlations.
Panel A) Males. Panel B) Females. Standardized estimates given. Significant paths shown with solid arrows; non-significant paths shown with dotted arrows. Residual A, C, D, and E influences path coefficients are written as the square root of the variances. Fit: χ2(273) = 283.589, p = .3171, RMSEA = 0.012, CFI = 0.996, AIC = 11941.006.
For INT in females, 51% of the variance was accounted for by significant additive genetic factors and 49% of the variance was accounted for by significant nonshared environmental factors. For INT in males, variance was accounted for by significant additive genetic effects (54%) and significant nonshared environmental effects (46%). There was a significant genetic correlation between INT and RSB for both males and females (rfemales = 0.431; rmales = 0.454), as well as a non-significant nonshared environmental correlation (rfemales = 0.101; rmales = 0.159). There was no significant sex difference in shared genetic covariance (i.e., in the genetic cross paths) between INT and RSB, χ2diff(1) = 0.04, p = .834, implying the male and female parameters could be equated without a significant decrease in model fit. As such, a two-group model fit the data well, χ2(102) = 87.766, p = .8413, RMSEA < 0.001, CFI = 1.000, AIC = 11978.011. For path estimates for the cross paths for females and males instead of the reported correlations, see Supplementary Figure 1.
Discussion
The results of Study 1 are consistent with previous research; we found a significant phenotypic correlation between latent factors assessing INT and RSB. We also found that females demonstrated more internalizing symptoms, on average, than males. However, we did not find significant mean sex differences for the RSB indicator variables. Males and females showed similar means for age at initiation and number of sex partners. Furthermore, while we did not find significant sex differences in either phenotypic or genetic correlations, the non-significant phenotypic sex difference (p = .07) was in the expected direction, with the female correlation (r = 0.30) higher than the male (r = 0.13). The lack of significant sex difference in the genetic correlation between INT and RSB suggests that there is no difference between males and females in the genetic and environmental mechanisms underlying the phenotypic correlation. Although the male and female phenotypic correlations could be equated (were not significantly different), the point estimates suggest that the relationship between INT and RSB is potentially greater in females (r = 0.30) than in males (r = 0.13), though statistical evidence suggests this is not meaningful. Furthermore, the genetic correlation between latent variables in females (r = .431) and males (r = .454) were very similar. This finding suggests that the phenotypic correlation for both males and females is based primarily on a significant genetic influence. It is possible that phenotypic sex differences, though not significant, are in the direction observed because females typically display more internalizing symptoms while males tend to display more externalizing symptoms (Eaton et al. 2012).
While Study 1 addressed many aspects of the relationship between INT and RSB, it has certain limitations. First, our findings do not give a clear picture of potential sex differences in the magnitude of the relationship between INT and RSB, as p-values were marginal. Second, it is important to note that the questions measuring RSB (e.g., “In your life, how many people have you had sex with?” and “How old were you the first time you had sex?”) did not formally define “sex,” and therefore, the interpretation of the word “sex” could have varied across participants. Previous research has found that perceived definition of sex can differ widely, even when accounting for generational and gender differences (Sanders et al. 2010). Therefore, it is possible that some participants in the current study only responded to RSB questions in the affirmative based on their individual definition of sex, which could be very broad or very narrow. Additionally, exclusion of participants who did not provide data for one or both of the RSB variables (some of whom may have never had sex) may have limited the contrast between high and low levels of RSB. However, participants who had never had sex did not enter “0” for number of partners; rather, they skipped the item and opted out of all sexual behavior items. Further, all participants could have opted out of all sexual behavior items due to the sensitive and private nature of the items, whether or not they had had sex. Therefore, we cannot confidently include participants who did not disclose having at least one sexual partner, as it is impossible for us to accurately separate truly abstinent participants from those who merely did not wish to answer questions about their sexual behaviors.
Study 2
Given the limitations of Study 1 and its results, we chose to verify our findings in a second genetically-informative twin sample from Minnesota. We aimed to use the same INT indicators used in Study 1 (i.e., depressive symptoms, neuroticism, and self-esteem), but due to differing measures employed across the studies, we were unable to perform an exact replication. Instead, we selected two new indicator variables, alienation and stress reaction, to best approximate the Study 1 INT construct with the available Minnesota data as a constructive replication study (Lykken 1968). Alienation is an established aspect of neuroticism (Mahoney and Quick 2000), and stress reaction also relates to neuroticism, with those with higher neuroticism having more negative stress responses (Miller et al. 2003), and has been previously clustered with internalizing symptoms (Gunthert et al. 1999). Further, both alienation and stress response have been previously clustered under an internalizing subtype and also under broad negative affect (Gjerde et al. 1988; Miller et al. 2003), and negative affect has been shown to be strongly related to internalizing (Crawford et al. 2011; Affrunti and Woodruff-Borden 2016). As such, both indicators were deemed good substitutes for neuroticism and self-esteem for the INT latent variable. Very similar measures of RSB were available in the Minnesota data.
Method
Participants
Participants for Study 2 were drawn from the Minnesota Center for Twin and Family Research (MCTFR), which was established in 1989 with the primary aim of identifying genetic and environmental influences on development and psychological traits (Wilson et al. 2019). The MCTFR includes longitudinal research on twins. The MCTFR twins were identified from Minnesota State birth certificates, which are publicly available. The Younger Cohort of the Minnesota Twin Family Study (MTFS) was included in the current analyses, specifically at follow-up 4 (occurring in 2006 – 2010), as this wave of data contained information on sexual behaviors and participants were similar in age, if slightly older, compared to participants in Study 1. Data for variables of interest existed for 1,527 participants at this wave; similarly to Study 1, there were 393 participants with missing data for one or both RSB variables, and a triplet group was excluded from analyses for a final N = 1,131 individuals (176 female monozygotic twin pairs, 153 male monozygotic twin pairs, 107 female dizygotic twin pairs, 76 male dizygotic twin pairs, and 107 cotwin missing individuals) who provided data on number of partners and age at first sex. Twins included in the analyses were in early adulthood (female mean = 25.23 years, s.d. = 0.75 years; male mean = 25.28 years, s.d. = 0.69 years). The sample was 54.2% female.
Zygosity
Zygosity was determined via several methods. At the time of enrollment, participants and their parents independently completed a zygosity questionnaire that included questions regarding the twins’ degree of physical similarity. Research staff also determined zygosity after rating twins on their degree of physical similarity. An algorithm based on ponderal index (height/weight ratio), cephalic index (head width/length ratio), and fingerprint ridge count was also used to determine zygosity (MCTFS, 2023). Follow-up genotyping was also conducted by either drawing blood or performing a buccal swab (Miller et al. 2012). Subsets of twins have completed subsequent in-person assessments (Wilson et al. 2019).
Measures
Sexual behavior was assessed using items from the Sexual Behavior Inventory (SBI; Huibregtse et al. 2011), which was first implemented in the MTFS in 2004. The SBI asks participants to differentiate between casual and committed sex partners, and within those groupings also differentiates between “lifetime” and “the last 12 months,” as well as between oral and penetrative (anal or vaginal) sex. Responses to numbers of sex partners within each grouping and each sexual act were assessed on an ordinal scale and binned (e.g., None, 1–2, 3–5, 6–9, 10–19, 20+). However, the SBI did not ask participants to completely separate either casual and committed partners (i.e., a casual partner who later became a committed partner could be counted twice). Similarly, partners with whom participants have had both oral sex and sexual intercourse could also be counted twice. As such, we chose to focus solely on partners with whom participants have had sexual intercourse in their lifetime, as that value most likely aligns closely with responses given in Study 1. We also chose to create one composite variable across groupings of casual versus committed partners in order to have one variable for lifetime number of sex partners with whom the subject has had sexual intercourse. Finally, because the responses of items were binned, we chose to use the average value for each bin to calculate the number of sex partners a participant has had (e.g., a response of 1–2 partners was coded as 1.5 partners, a response of 3–5 was coded as 4 partners, etc.). We deemed this the best way to approximate the number of partners; while some participants were likely responding on the low end of their bins, others were likely responding on the high end.
Age at first sexual encounter was also assessed by the SBI. The SBI asks participants to differentiate between casual and committed partners, and between oral and penetrative (anal or vaginal) sex. We chose to use the youngest age at sexual intercourse (penetrative sex) across casual or committed partnerships. Responses were free-response format. All participants in Study 2 reported age at first sexual encounter as 12 or older, which was not an outlier age. As in Study 1, the scale was reversed for multivariate biometrical analyses, and participants with valid data for both number of sexual partners and for age at first sexual encounter were included in analysis.
To measure internalizing disorder symptoms, we used measures of alienation and stress reaction. Alienation was measured by the Multidimensional Personality Questionnaire (MPQ; Tellegen and Waller 2008), and was a composite score created from responses to 18 items, which included statements such as: “Some people go out of their way to keep me from getting ahead,”; “Many people try to push me around,”; and “People often try to take advantage of me,” to which participants rated how true they found the statements on a four-point scale (“Definitely true” [1], “Probably true” [2], “Probably false” [3], and “Definitely false” [4]), with most items reverse-oriented and subsequently reverse coded so that higher scores indicated greater feelings of alienation. The distribution was skewed right. Calculated internal consistency for this scale was high (Alpha = .84).
Stress reaction was also measured by the MPQ, and, like alienation, was a composite score created from responses to 18 items. Example items include: “When I want to, I can usually put fears and worries out of my mind,”; “If I have a humiliating experience I get over it very quickly,”; and “I am often nervous for no reason (r),” to which participants rated how true they found the statements on the same four-point scale used for alienation. Most stress reaction items were reverse-oriented and subsequently reverse coded so that higher scores indicated stronger negative stress reaction. The distribution was nearly normal. Calculated internal consistency for this scale was high (Alpha = .83). Refer to Table 4 for descriptive statistics for all measures used in Study 2.
Table 4.
Descriptive statistics for variables used in Study 2 (MN Sample).
| Entire sample | Males | Females | |||
|---|---|---|---|---|---|
| M (SD) | M (SD) | Min, Max | M (SD) | Min, Max | |
| Num. Part. * | 5.87 (5.85) | 6.99 (6.87) | 1.50, 40.00 | 4.93 (4.64) | 1.50, 34.50 |
| Age at F.S. | 19.67 (2.54) | 19.65 (2.62) | 13.00, 27.00 | 19.68 (2.47) | 12.00, 27.00 |
| Alienation † | 30.64 (8.09) | 31.14 (8.13) | 18.00, 58.00 | 30.21 (8.03) | 18.00, 63.00 |
| Stress Reac. * | 40.01 (9.06) | 37.83 (8.37) | 18.00, 65.00 | 41.83 (9.23) | 20.00, 69.00 |
| Age | 25.25 (0.72) | 25.28 (0.69) | 23.80, 27.81 | 25.23 (0.75) | 23.83, 27.96 |
Num. Part. = lifetime number of sexual partners; Age at F.S. = age at first sexual encounter; Stress Reac. = stress reaction; Age = age at assessment.
mean sex difference at p = .05;
mean sex difference is significant, p < .05; analyses were conducted on transformed variables but raw score means are shown here.
Lifetime number of sexual partners and alienation demonstrated right skew and were log + 1 transformed to minimize skewness to meet normality model assumptions.
All descriptive statistics, composite variables, transformations, and reverse-scoring were conducted in SPSS version 27 (IBM Corp. 2020). As in Study 1, variable correlations, twin correlations, and t-tests to examine sex differences were conducted in R version 4.0.0 (R Core Team 2022). Phenotypic analyses were conducted in CFA Lavaan (Rosseel 2012), and multivariate twin analyses were conducted in Mplus version 8.5 (Muthén and Muthén 1998–2017). Twin correlations, phenotypic models, and structural equation models were estimated employing maximum likelihood (ML) for analysis of all variables, as all variables were treated as either continuous or quasi-continuous.
Model fit was determined as it was in Study 1, and model comparisons were again conducted using Chi-squared difference tests. Sex difference models were tested identically as in Study 1.
Phenotypic analyses
As in Study 1, a phenotypic confirmatory factor analysis was run to examine phenotypic correlations and establish latent variables. Latent variables in Study 2 were labeled “INT” and “RSB,” where INT indicators were alienation and stress reaction, and RSB indicators were lifetime number of sexual partners and age at first sexual encounter. Latent variables were correlated and common factor loadings were equalized, allowing for identification of a two-factor, two indicator model. Indicator variables were standardized. Data were clustered by family ID, and missing data were handled using ML options. A multigroup phenotypic confirmatory factor analysis (with separate male and female model parameters) was conducted to examine phenotypic correlations by sex. As in Study 1, we conducted measurement invariance testing on the multigroup model and found evidence for metric invariance across male and female latent constructs; configural and scalar invariance were also tested.
Multivariate twin analyses
A bivariate Cholesky decomposition was conducted to investigate genetic and environmental components of the variance and correlation between latent INT and latent RSB, as in Study 1. We first conducted a two-group bivariate Cholesky decomposition, comparing MZ and DZ twin pairs, and then to investigate potential sex differences in the magnitude of the relationship between INT and RSB, we conducted a four-group bivariate Cholesky decomposition. The Minnesota sample used in Study 2 did not contain opposite-sex dizygotic twin pairs. Model fit for biometrical models was assessed as in Study 1.
Results
Examination of phenotypic sex differences suggested that for Study 2, males trended toward higher scores than females on average for alienation, t(1108) = 1.96, p = .05, whereas females demonstrated a stronger negative stress reaction on average, t(1107) = −7.50, p<.001. Males had significantly more sex partners than did females, t(1095) = 5.42, p<.001, though no significant sex difference in the age at first sex was observed, t(1092) = −0.22, p = .83. As in Study 1, t-tests were conducted using transformed data where appropriate, and reflect differences in geometric means, and analyses were adjusted for clustering of twin within families. Raw mean scores are reported in Table 4. As in Study 1, there were also some potential sex differences in the phenotypic correlations among the variables (Table 5; 95% CIs shown in Supplementary Table 5). For females, both number of sex partners and age at first sex show modest but significant correlations with alienation. Among males, number of sex partners is not significantly correlated with either alienation or stress reaction, though age at first sex is significantly correlated with alienation. The MZ and DZ correlations for all variables are shown in Table 6, and again provide some evidence for sex differences in ACE vs. ADE influences.
Table 5.
Phenotypic correlations, by sex, for Study 2 (MN Sample).
| Num. of Partners | Age at F.S. | Alienation | Stress Reaction | |
|---|---|---|---|---|
| Num. of Partners | 1 | −0.40* | 0.09* | −0.03 |
| Age at F.S. | −0.48* | 1 | −0.14* | −0.05 |
| Alienation | 0.07 | −0.16* | 1 | 0.53* |
| Stress Reaction | 0.01 | −0.06 | 0.58* | 1 |
Female estimates shown on the upper diagonal, male estimates shown on the lower diagonal in bold. Starred estimates are significant at alpha = .05. Age at first sex was not reversed when computing the correlations.
Table 6.
Twin correlations for Study 2 (MN Sample).
| MZ vs. DZ | ||||
|---|---|---|---|---|
| Num. of Partners | Age at F.S. | Alienation | Stress Reaction | |
| MZ | 0.38 | 0.47 | 0.46 | 0.39 |
| DZ | 0.07 | 0.38 | 0.31 | 0.22 |
| MZFF vs. DZFF | ||||
| Num. of Partners | Age at F.S. | Alienation | Stress Reaction | |
| MZFF | 0.32 | 0.47 | 0.53 | 0.43 |
| DZFF | 0.05 | 0.45 | 0.27 | 0.23 |
| MZMM vs. DZMM | ||||
| Num. of Partners | Age at F.S. | Alienation | Stress Reaction | |
| MZMM | 0.36 | 0.48 | 0.38 | 0.25 |
| DZMM | 0.06 | 0.32 | 0.36 | 0.09 |
MZFF = MZ female twin pairs. DZFF = DZ female twin pairs. MZMM = MZ male twin pairs. DZMM = DZ male twin pairs. Age at F.S. = age at first sex.
Phenotypic relationships between INT and RSB
A phenotypic confirmatory factor analysis was used to test the correlation between latent INT and RSB in the combined sample, such that the estimate of the correlation was constrained across males and females. The model fit adequately, χ2(3) = 28.86, p<.001, RMSEA = .09, CFI = 0.959, AIC = 11911.74, and demonstrated a significant (χ2diff (1) = 7.26, p = .01) correlation between INT and RSB of .13. As in Study 1, a multigroup sex-limitation phenotypic model was then used to investigate sex differences (see Figure 3; 95% CIs shown in Supplementary Table 6).
Figure 3.
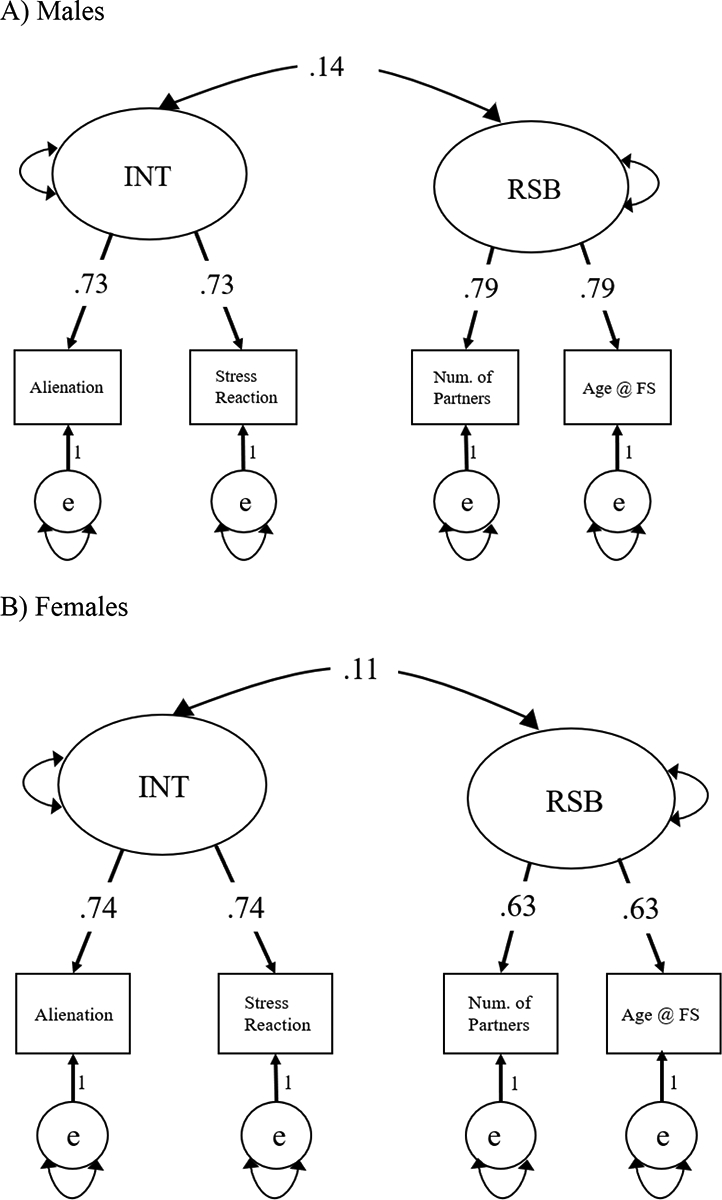
Multigroup phenotypic confirmatory factor analysis for Study 2, separated by sex.
Panel A) Males. Panel B) Females. Standardized estimates shown. In order to estimate the correlations between the latent factors, the measurement models (raw factor loadings) were constrained to be equal across males and females. Fit: χ2(6) = 22.103, p = .001, RMSEA = 0.071, CFI = 0.977, AIC = 11433.825
The factor loadings were constrained to be equal across males and females to ensure the same measurement model. The multigroup sex-limitation phenotypic model fit the data adequately, χ2(6) = 22.10, p = .001, RMSEA = 0.071, CFI = 0.977, AIC =11433.83. Although the male correlation (r = 0.14) between latent INT and RSB was significantly different from zero, χ2diff (1) = 4.74, p = .03, the female correlation (r = 0.11) between latent variables was not, χ2diff (1) = 3.09, p = .08. Additionally, constraining the correlations to be equal did not result in a significant decrement in model fit, χ2diff (1) = 0.34, p = .56, indicating no significant sex difference in the correlation between INT and RSB in the Minnesota sample. In fact, Study 2 point estimates are in the opposite direction to those hypothesized and seen in Study 1.
Multivariate twin analyses
Although females did not demonstrate a significant phenotypic correlation between INT and RSB, we chose to run sex-limitation models as we did in Study 1 because the phenotypic correlation was significant for males, and for the sake of completeness. Multivariate twin analyses were identical to those analyses described and conducted in Study 1. The common effects sex-limitation Cholesky (see Figure 4; 95% CIs shown in Supplementary Table 7) fit the data poorly, (132) = 620.71, p = .000, RMSEA = 0.16, CFI = 0.51, AIC = 11978.97, and therefore should be interpreted with caution. For both sexes, both the genetic correlation (rfemales = 0.149; rmales = 0.151) and non-shared environment correlation (rfemales = 0.150; rmales = 0.172) were not significant. The sex difference in shared genetic covariance (i.e., in the genetic cross paths) between INT and RSB was also not significant, χ2diff(1) = 0.033, p = .86. For estimates of the Cholesky cross paths for females and males instead of the reported correlations, see Supplementary Figure 2.
Figure 4.
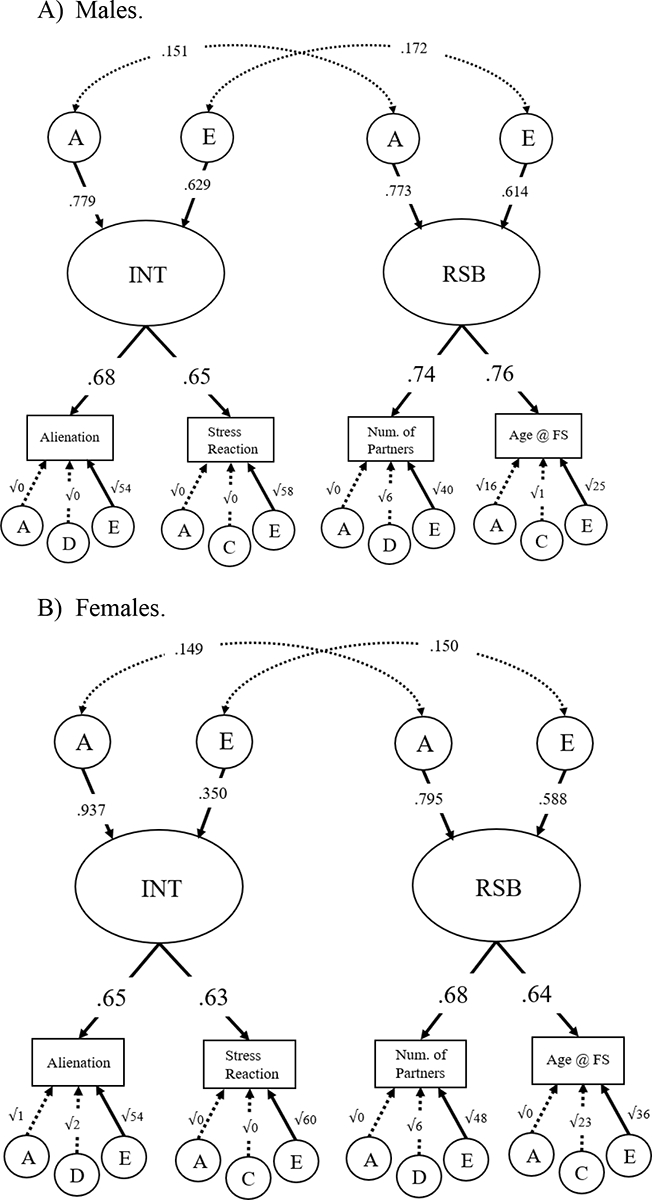
Four-group sex-limitation, with genetic and nonshared environmental correlations.
Panel A) Males. Panel B) Females. Standardized estimates given. Significant paths shown with solid arrows; non-significant paths shown with dotted arrows. Residual A, C, D, and E influences path coefficients are written as the square root of the variances. Fit: χ2(132) = 620.711, p = .000, RMSEA = 0.155, CFI = 0.509, AIC = 11978.97.
Further testing of the cross paths from the Cholesky failed to determine definitively the underlying mechanism (genetic or non-shared environmental) of the significant phenotypic correlation. Testing the significance of individual cross paths followed procedures described previously, and employed Chi-square difference tests and AIC. We failed to reject models in which covariance between latent variables in both males and females was due solely to A or solely to E. We also failed to reject models in which covariance between latent variables was due solely to AE in males (no cross paths in females), and then vice versa (refer to Supplementary Table 8 for details). Essentially, although the phenotypic correlation between INT and RSB was significantly greater than zero (r = 0.13) in the pooled sample of males and females, and in males alone (r = 0.14), we did not find a significant correlation between INT and RSB in females alone (r = 0.11). Given the magnitude of the correlations, we did not have the power to clearly parse apart the genetic and environmental influences underlying the phenotypic relationship between INT and RSB.
Discussion
The results of Study 2 differ from those of Study 1. As in Study 1, we found a significant phenotypic correlation between latent factors INT and RSB in the combined sample of males and females. However, the correlation between INT and RSB was not significant in females in Study 2. Sex differences in both INT and RSB indicator variables followed similar patterns in both studies. We again did not find significant sex differences in the genetic correlation between INT and RSB, and in fact, cannot definitively parse apart the significant phenotypic correlation between latent variables into genetic or environmental components. The lack of significant sex differences in the genetic and non-shared environmental correlations between INT and RSB may have partially been a result of insufficient power, as we found no substantial differences between males and females in the genetic and environmental mechanisms underlying the phenotypic correlation in this sample.
Study 2 has certain limitations. First, we were unable to include a measure of depressive symptoms as an indicator for INT (as was done in Study 1) due to the differing measures employed between studies and a lack of available data. The MCTFR data contained a quasi-continuous measure of major depressive disorder (MDD) symptoms as assessed by DSM-IV symptomatology; however, endorsement of MDD was low and demonstrated an L-shaped distribution, with 85% of participants reporting zero MDD symptoms. Ultimately, we chose to drop MDD from our analyses due to its low endorsement, which limited our replicability analysis to two indicators for INT. The difference in INT measures across the two studies therefore did not allow us to complete an exact replication. For results that retained MDD in the analysis, refer to Supplementary Table 5 and Supplementary Figure 3.
Second, we needed to make several inferences when developing the RSB indicators; that the SBI did not clearly separate casual and committed partners, or partners with whom the participant has had oral sex versus sexual intercourse meant that we had to limit evaluation of the number of lifetime partners to responses about lifetime sexual intercourse partners. The use of binning, rather than free response as in Study 1, also required us to infer the average number of sex partners each participant had. This may have restricted our variability on RSB indicators. Next, a high percentage of the sample in Study 2 reported never having had sex, and was subsequently excluded. Relatedly, Study 2 was underpowered compared to Study 1 and did not provide a clear picture of the genetic or environmental components that underly the phenotypic relationship between INT and RSB, which limits our ability to draw conclusions about the nature of this relationship.
Conclusions
The results of both studies demonstrate partial support for our hypotheses. Our first hypothesis, that there exists a positive correlation between INT and RSB, was largely supported. The results of both Study 1 and Study 2 indicate a significant phenotypic correlation between INT and RSB in the combined samples of males and females, although in Study 2 that correlation was only significant for males when the sample was divided by sex. Our second hypothesis, that the magnitude of the correlation between INT and RSB would be greater for females than for males, was not well supported in either study. Study 1 shows a non-significant sex difference trending in the direction predicted, though Study 2 did not find significant sex differences, and, in fact, the relationship was in the opposite direction predicted.
Our third hypothesis, that there are sex differences in the genetic and environmental components underlying the association between INT and RSB, was not supported well in either study. Taking the results of both studies in the aggregate, it is not completely clear whether the phenotypic relationship between INT and RSB is driven primarily by shared genetic factors or by shared environment. In both studies, INT and RSB were assessed by available measures. Although multiple indicator latent phenotypes were analyzed, the correlations between latent INT and RSB were modest, making it difficult to decompose those correlations into genetic and environmental sources. Improved assessment of these constructs may yield increased correlations.
There are several potential reasons for the difference in results between Study 1 and Study 2. Primarily, it was not possible to assess INT or RSB with isomorphic measures across the two studies. The discrepancy is partly driven by the lack of depressive symptoms as an INT indicator in Study 2, which could have resulted in a weak relationship in INT between studies. Phenotypic correlations were also higher in Study 1 and in the expected direction, compared to the lower phenotypic correlations in Study 2. The difference in phenotypic correlations may be indicative of some weakness in the operationalization of INT in Study 2. Additionally, both the age of the overall sample as well as the mean ages at first sex differ between studies. Participants in Study 1 are both younger (m = 19.96 years) than those in Study 2 (m = 25.25 years) and indicate an earlier age at first sex (m = 16.89 years) compared to those in Study 2 (m = 19.67 years). The age difference between samples could partially explain the difference in lifetime number of sexual partners between Study 1 (m = 4.88) and Study 2 (m = 5.87). Alternative explanations for the difference between samples in age at first sex and in number of partners could reflect potentially differing cultural climates between the regions of data collection, Colorado and Minnesota.
In any case, the inclusion of both studies in attempt to replicate initial results from the Colorado sample is impactful, as these results could have important implications to future research and to public health policy. Of interest, our findings suggest a potential unique pathway between INT and RSB, which should be considered in future studies on this or similar topics. Also, the finding that the association between INT and RSB demonstrates shared genetic and environmental variance is of interest as both INT and RSB are prevalent public health issues. Given previous evidence that personal factors such as HIV knowledge may mediate the relationship between INT and RSB (Joppa et al 2014), it is possible that public health policies surrounding sexual education—including STI education—can reduce risky sexual behaviors for individuals with internalizing problems.
Because the relationship between INT and RSB, and specifically the underlying genetic and environmental components of the relationship, are under-studied, future research should continue to explore this topic. Future research might also examine potential causal pathways between INT and RSB, though such models do have certain limitations that must be considered (Heath et al. 1993). Alternatively, researchers could examine longitudinal data to investigate whether changes in RSB over time predict changes in internalizing problems.
Supplementary Material
Funding
Grant support. T32 MH016880, P60 DA011015, R01 DA024417-05.
Footnotes
Conflicts of interest/Competing interests
Not applicable.
Ethics approval
All procedures were approved by institutional review boards at both sites of data collection and comply with the World Medical Association Declaration of Helsinki. Both sites’ institutional review boards have indicated that analyses using the retrospective, anonymized data are not human subjects research and do not require their own approval.
Consent to participate
Consent to participate was provided at the time of original data collection at both sites.
Consent for publication
Consent for publication was provided at the time of original data collection at both sites.
Code availability
Available upon request to the Corresponding Author.
Availability of data and material
The datasets at both sites used and analyzed during the current study are not publicly available due to the fact they are privately managed by the individual sites. However, they may be obtained via authorized access. Please contact the Corresponding Author for details.
References
- Achenbach TM, Edelbrock C, Howell CT (1987) Empirically based assessment of the behavioral/emotional problems of 2- and 3- year-old children. J Abnorm Child Psychol 15:629–650. 10.1007/BF00917246 [DOI] [PubMed] [Google Scholar]
- Affrunti NW, Woodruff-Borden J (2016) Negative affect and child internalizing symptoms: The mediating role of perfection. Child Psychiatry & Human Development 47:358–368. [DOI] [PubMed] [Google Scholar]
- Akaike H (1973) Information theory and an extension of the Maximum Likelihood Principle. In: Petrov BN, Csaki F (eds) International Symposium on Information Theory, pp 267–281 [Google Scholar]
- Akaike H (1987) Factor analysis and AIC. Psychometrika 52:317–332. 10.1007/BF02294359 [DOI] [Google Scholar]
- Barr PB, Dick DM (2019) The genetics of externalizing problems. In: de Wit H, Jentsch JD (eds) Recent Advances in Research on Impulsivity and Impulsive Behaviors. Springer International Publishing, Cham, pp 93–112 [Google Scholar]
- Bayer JK, Rapee RM, Hiscock H, et al. (2011) Translational research to prevent internalizing problems early in childhood. Depress Anxiety 28:50–57. 10.1002/da.20743 [DOI] [PubMed] [Google Scholar]
- Boden JM, Horwood LJ (2006) Self-esteem, risky sexual behavior, and pregnancy in a New Zealand birth cohort. Arch Sex Behav 35:549–560. 10.1007/s10508-006-9060-4 [DOI] [PubMed] [Google Scholar]
- Boislard M-AP, Dussault F, Brendgen M, Vitaro F (2013) Internalizing and externalizing behaviors as predictors of sexual onset in early adolescence. J Early Adolesc 33:920–945. 10.1177/0272431612472982 [DOI] [Google Scholar]
- Boker S, Neale M, Maes H, et al. (2011) OpenMx: An open source extended Structural Equation Modeling framework. Psychometrika 76:306–317. 10.1007/s11336-010-9200-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosacki S, Dane A, Marini Z, Ylc‐Cura (2007) Peer relationships and internalizing problems in adolescents: mediating role of self‐esteem. Emot Behav Difficulties 12:261–282. 10.1080/13632750701664293 [DOI] [Google Scholar]
- Chesson HW, Spicknall IH, Bingham A, et al. (2021) The estimated direct lifetime medical costs of sexually transmitted infections acquired in the United States in 2018. Sex Transm Dis 48:215–221. 10.1097/OLQ.0000000000001380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado A, Loya JM, Yi R (2015) The interaction of HIV knowledge, perceived risk, and sex differences on risky sex. Int J of Sexual Health 27:4, 418–428. [Google Scholar]
- Corp IBM (2019) Released 2019: IBM SPSS statistics for windows, version 26.0. IBM Corp, Armonk [Google Scholar]
- Corp IBM (2020) Released 2020: IBM SPSS statistics for windows, version 27.0. IBM Corp, Armonk [Google Scholar]
- Crawford NA, Schrock M, Woodruff-Borden J (2011) Child internalizing symptoms: Contributions of child temperament, maternal negative affect, and family functioning. Child Psychiatry & Human Development 42:53–64. [DOI] [PubMed] [Google Scholar]
- Creemers DHM, Scholte RHJ, Engels RCME, et al. (2013) Damaged self-esteem is associated with internalizing problems. Front Psychol 4:. 10.3389/fpsyg.2013.00152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duanne MP, Martin NG, Statham DJ et al. (1997) Genetic and environmental contributions to variance in age at first sexual intercourse. Psych Sci 8(3):211–216. [Google Scholar]
- Eaton NR, Keyes KM, Krueger RF, et al. (2012) An invariant dimensional liability model of gender differences in mental disorder prevalence: Evidence from a national sample. J Abnorm Psychol 121:282–288. 10.1037/a0024780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floderus-Myrhed B, Pedersen N, Rasmuson I (1980) Assessment of heritability for personality, based on a Short-Form of the Eysenck Personality Inventory: A study of 12,898 twin pairs. Behavior Genetics 10(2):153–162. [DOI] [PubMed] [Google Scholar]
- Gjerde PF, Block J, Block JH (1988) Depressive symptoms and personality during late adolescence: Gender differences in the externalization-internalization of symptom expression. Journal of Abnormal Psychology 97(4):475–486. [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Weinberger AH, Kim JH, et al. (2020) Trends in anxiety among adults in the United States, 2008–2018: Rapid increases among young adults. J Psychiatr Res 130:441–446. 10.1016/j.jpsychires.2020.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunthert KC, Cohen LH, Armeli S (1999) The role of neuroticism in daily stress and coping. J Pers Soc Psychol 77:1087–1100. 10.1037/0022-3514.77.5.1087 [DOI] [PubMed] [Google Scholar]
- Hallfors DD, Waller MW, Bauer D, et al. (2005) Which Comes first in adolescence—sex and drugs or depression? Am J Prev Med 29:163–170. 10.1016/j.amepre.2005.06.002 [DOI] [PubMed] [Google Scholar]
- Harden KP (2014) Genetic influences on adolescent sexual behavior: Why genes matter for environmentally-oriented researchers. Psychol Bull 140:434–465. 10.1037/a0033564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healthwise Staff (2021. Nov 17) High-risk sexual behaviour. MyHealth.Alberta.ca. https://myhealth.alberta.ca/Health/Pages/conditions.aspx?hwid=tw9064 [Google Scholar]
- Heath AC, Kessler RC, Neale MC, et al. (1993) Testing hypotheses about direction of causation using cross-sectional family data. Behav Genet 23:29–50. 10.1007/BF01067552 [DOI] [PubMed] [Google Scholar]
- Hu L, Bentler PM (1999) Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Model Multidiscip J 6:1–55. 10.1080/10705519909540118 [DOI] [Google Scholar]
- Huibregtse BM, Bornovalova MA, Hicks BM, et al. (2011) Testing the role of adolescent sexual initiation in later-life sexual risk behavior: A longitudinal twin design. Psychol Sci 22:924–933. 10.1177/0956797611410982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huibregtse BM, Hatoum AS, Corley RP, et al. (2021) Etiological overlap between sex under the influence and number of lifetime sexual partners. Behav Genet 51:12–29. 10.1007/s10519-020-10019-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M (2008) Behavioral disinhibition and the development of early-onset addiction: Common and specific influences. Annu Rev Clin Psychol 4:325–348. 10.1146/annurev.clinpsy.4.022007.141157 [DOI] [PubMed] [Google Scholar]
- Joppa MC, Rizzo CJ, Brown LK, et al. (2014) Internalizing symptoms and safe sex intentions among adolescents in mental health treatment: Personal factors as mediators. Children and Youth Services Review 46:177–185. 10.1016/j.childyouth.2014.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kann L, McManus T, Harris WA, et al. (2018) Youth Risk Behavior Surveillance — United States, 2017. MMWR Surveill Summ 67:1–114. 10.15585/mmwr.ss6708a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisel KM, Spicknall IH, Gargano JW, et al. (2021) Sexually transmitted infections among US women and men: Prevalence and incidence estimates, 2018. Sex Transm Dis 48:208–214. 10.1097/OLQ.0000000000001355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Chen X, Lewis G (2011) Childhood internalizing behaviour: Analysis and implications: Childhood internalizing behaviour. J Psychiatr Ment Health Nurs 18:884–894. 10.1111/j.1365-2850.2011.01743.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykken DT (1968) Statistical significance in psychological research. Psychological Bulletin 70(3):151–159. [DOI] [PubMed] [Google Scholar]
- Mahoney JM, Quick BG (2000) Personality correlates of alienation in a university sample. Psychol Rep 87:1094–1100. 10.2466/pr0.2000.87.3f.1094 [DOI] [PubMed] [Google Scholar]
- MCTFS (2023). MTFS Twin Info and Frequently Asked Questions. Minnesota Center for Twin and Family Research. https://mctfr.psych.umn.edu/mtfs-twin-info-and-frequently-asked-questions [Google Scholar]
- Merrell KW, Gueldner (2010) Social and emotional learning in the classroom: Promoting mental health and academic success. New York, NY: Guilford Press. [Google Scholar]
- Miller MB, Basu S, Cunningham J, et al. (2012) The Minnesota Center for Twin and Family Research genome-wide association study. Twin Research and Human Genetics 15(6):767–774. 10.1017/thg.2012.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW, Greif JL, Smith AA (2003) Multidimensional Personality Questionnaire profiles of veterans with traumatic combat exposure: Externalizing and internalizing subtypes. Psychol Assess 15:205–215. 10.1037/1040-3590.15.2.205 [DOI] [PubMed] [Google Scholar]
- Mojtabai R, Olfson M, Han B (2016) National trends in the prevalence and treatment of depression in adolescents and young adults. PEDIATRICS 138:e20161878–e20161878. 10.1542/peds.2016-1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munthén LK, Munthén BO (1998–2017) Mplus User’s Guide. Eighth Edition. Los Angeles, CA: Munthén and Munthén [Google Scholar]
- Mustanski B, Viken RJ, Kaprio J, et al. (2007) Sexual behavior in young adulthood: A population-based twin study. Health Psychol 26(5):610–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale MC, Hunter MD, Pritikin JN, et al. (2016) OpenMx 2.0: Extended Structural Equation and Statistical Modeling. Psychometrika 81:535–549. 10.1007/s11336-014-9435-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols RC, Bilbro WC (1966) The diagnosis of twin zygosity. Acta Genet Stat Med 16:265–275 [DOI] [PubMed] [Google Scholar]
- Niemz K, Griffiths M, Banyard P (2005) Prevalence of pathological internet use among university students and correlations with self-esteem, the General Health Questionnaire (GHQ), and disinhibition. Cyber Psyc Behav 8:562–570. [DOI] [PubMed] [Google Scholar]
- Oginni OA, Jern P, Rahman Q, Rijsdijk FV (2022) Do psychosocial factors mediate sexual minorities’ risky sexual behavior? A twin study. Health Psychology 41(1):76–84. 10.1037/hea0001129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oginni OA, Jern P, Rijsdijk FV (2020) Mental health disparities mediating increased risky sexual behavior in sexual minorities: A twin approach. Arch Sex Behav 49:2497–2510. 10.1007/s10508-020-01696-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okami P, Shackelford TK (2001) Human sex differences in sexual psychology and behavior. Annu Rev Sex Res 12:186–241. [PubMed] [Google Scholar]
- Pozuelo JR, Desborough L, Stein A et al. (2022) Systematic review and meta-analysis: Depressive symptoms and risky behaviors among adolescents in low- and middle-income countries. Journal of the American Academy of Child & Adolescent Psychiatry 61(2):255–276. 10.1016/j.jaac.2021.05.005 [DOI] [PubMed] [Google Scholar]
- Pratt LA, Xu F, McQuillan GM, Robitz R (2012) The association of depression, risky sexual behaviours and Herpes Simplex Virus Type 2 in adults in NHANES, 2005–2008. Sex Transm Infect 88:40–44. 10.1136/sextrans-2011-050138 [DOI] [PubMed] [Google Scholar]
- R Core Team (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/ [Google Scholar]
- Radloff LS (1977) The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Meas 1:385–401. 10.1177/014662167700100306 [DOI] [Google Scholar]
- Rahm-Knigge RL, Prince MA, Conner BT (2021) More likely to have risky sex but less sexually satisfied: A profile of high social interaction anxiety, urgency, and emotion dysregulation. J Psychopathol Behav Assess. 10.1007/s10862-021-09889-w [DOI] [Google Scholar]
- Rhea SA, Bricker JB, Corley RP, et al. (2013a) Design, utility, and history of the Colorado Adoption Project: Examples involving adjustment interactions. Adopt Q 16:17–39. 10.1080/10926755.2012.754810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhea S-A, Bricker JB, Wadsworth SJ, Corley RP (2013b) The Colorado Adoption Project. Twin Res Hum Genet 16:358–365. 10.1017/thg.2012.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhea S-A, Gross AA, Haberstick BC, Corley RP (2013c) Colorado Twin Registry: An update. Twin Res Hum Genet 16:351–357. 10.1017/thg.2012.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhea S-A, Gross AA, Haberstick BC, Corley RP (2006) Colorado Twin Registry. Twin Res Hum Genet 9:941–949. 10.1375/twin.9.6.941 [DOI] [PubMed] [Google Scholar]
- Rogers MM, McKinney C (2019) Parent-child relationship quality and internalizing problems as predictors of risky sexual behavior. J of Family Issues 40(12):1656–1676. 10.1177/0192513X19843156 [DOI] [Google Scholar]
- Rosenberg M (1985) Society and the adolescent self-image. Princeton University Pres, Place of publication not identified [Google Scholar]
- Ross JM, Granja K, Duperrouzel JC, et al. (2019) Risky sexual behavior among adolescents: The role of decision-making, problems from cannabis use and externalizing disorder symptoms. J Clin Exp Neuropsychol 41:300–311. 10.1080/13803395.2018.1550192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosseel Y (2012) “lavaan: An R Package for Structural Equation Modeling.” Journal of Statistical Software, 48(2), 1–36. doi: 10.18637/jss.v048.i02 [DOI] [Google Scholar]
- Sanders SA, Hill BJ, Yarber WL, et al. (2010) Misclassification bias: Diversity in conceptualisations about having ‘had sex.’ Sex Health 7:31–34. 10.1071/SH09068 [DOI] [PubMed] [Google Scholar]
- Tellegen A, Waller NG (2008) Exploring personality through test construction: Development of the multidimensional personality questionnaire. In: Boyle GJ, Matthews G, Saklofske DH (eds) The SAGE Handbook of Personality Theory and Assessment: Personality Measurement and Testing: Personality measurement and assessment, vol 2 (pp. 261–292). 10.4135/9781849200479.n13 [DOI] [Google Scholar]
- Timmermans M, van Lier PAC, Koot HM (2008) Which forms of child/adolescent externalizing behaviors account for late adolescent risky sexual behavior and substance use? J Child Psychol Psychiatry 49:386–394. 10.1111/j.1469-7610.2007.01842.x [DOI] [PubMed] [Google Scholar]
- Uliaszek AA, Hauner KKY, Zinbarg RE, et al. (2009) An examination of content overlap and disorder-specific predictions in the associations of neuroticism with anxiety and depression. J Res Personal 43:785–794. 10.1016/j.jrp.2009.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller MW, Hallfors DD, Halpern CT, et al. (2006) Gender differences in associations between depressive symptoms and patterns of substance use and risky sexual behavior among a nationally representative sample of U.S. adolescents. Arch Womens Ment Health 9:139–150. 10.1007/s00737-006-0121-4 [DOI] [PubMed] [Google Scholar]
- Williams LJ, Holahan PJ (1994) Parsimony‐based fit indices for multiple‐indicator models: Do they work? Struct Equ Model Multidiscip J 1:161–189. 10.1080/10705519409539970 [DOI] [Google Scholar]
- Wilson S, Haroian K, Iacono WG, et al. (2019) Minnesota Center for Twin and Family Research. Twin Res Hum Genet 22:746–752. 10.1017/thg.2019.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JY, Choi EP, Chan CK et al. (2017) Controlling anxiety mediates the influence of childhood adversities on risky sexual behaviors among emerging adults. The Journal of Sex Research 54:1018–1025. 10.1080/00224499.2017.1278569 [DOI] [PubMed] [Google Scholar]
- Zietsch BP, Verweij KJH, Bailey JM et al. (2010) Genetic and environmental influences on risky sexual behavior and its relationship with personality. Behav Genet 40(12):12–21. 10.1007/s10519-009-9300-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets at both sites used and analyzed during the current study are not publicly available due to the fact they are privately managed by the individual sites. However, they may be obtained via authorized access. Please contact the Corresponding Author for details.


