Hospitals, and particularly intensive care units, are an important breeding ground for the development and spread of antibiotic resistant bacteria. This is the consequence of exposing to heavy antibiotic use a high density patient population in frequent contact with healthcare staff and the attendant risk of cross infection.1,2 Antibiotic resistance increases the morbidity and mortality associated with infections and contributes substantially to rising costs of care resulting from prolonged hospital stays and the need for more expensive drugs.1–3 In this review I will outline the current problems caused by major drug resistant nosocomial pathogens, examine factors that promote antibiotic resistance in hospitals, and discuss strategies for control.
Summary points
The increasing incidence of hospital acquired infections caused by antibiotic resistant pathogens has led to an increase in morbidity and mortality
Resistance results from the interplay of micro-organisms, patients, and the hospital environment, including antibiotic use and infection control practices
An important cause of increasing antibiotic resistance is the selection of resistant bacterial strains by mutation and transfer of mobile resistance genes as a result of excessive antibiotic prescribing by hospital doctors
Increasing antibiotic resistance is also caused by transmission of resistant bacteria within hospitals by cross colonisation of patients via the hands of healthcare staff and subsequent spread between hospitals by transfer of colonised patients
Strategies to control antibiotic resistance in hospitals include multidisciplinary cooperation in implementing local policies on use of antibiotics and infection control measures, timely detection and reporting of the antibiotic resistant strains, improved surveillance, and aggressive control of transmission of epidemic resistant bacteria
The rise in antimicrobial resistance
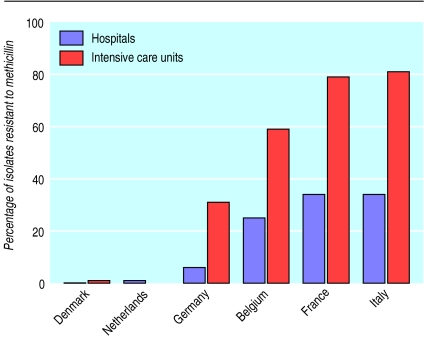
Among pathogens causing hospital infections, Gram positive cocci have become predominant over the past two decades. This trend is related to these pathogens’ capacity for accumulating antibiotic resistance determinants.2 A notable example is that of methicillin resistant strains of Staphylococccus aureus (MRSA), which emerged in the 1970s and increased in frequency as hospital pathogens during the 1980s and ’90s in many countries—with the notable exception of the Scandinavian countries and the Netherlands (figure).4–7 Countries with lower incidence of MRSA infections tend to be more restrictive in antibiotic use, to apply strict infection control measures, and to have better ratios of nurses to patients in their healthcare institutions.6 The rise in MRSA infections was initially associated with epidemics in large teaching hospitals, later spreading to the general hospitals and nursing homes.7 Control stategies, such as contact isolation precautions (for example, systematic use of gloves, gowns, and hand antisepsis for care procedures) and carrier decolonisation with topical antimicrobials, met with varying degrees of success but seemed at least to slow down transmission.6–8 Most MRSA strains are resistant to most other antibiotics, thereby necessitating the use of glycopeptide antibiotics, such as vancomycin. Recently, treatment failures caused by some strains with decreased susceptibility to vancomycin (vancomycin intermediate S aureus (VISA)) were reported in Japan and the United States.9,10 Infections caused by these strains leave very few therapeutic options, and their emergence therefore adds to the rationale for containing MRSA transmission and restricting vancomycin use in hospitals.10
Enterococci, commensal inhabitants of the intestinal and genital tracts, are rising in prominence as hospital pathogens.2 This rise is related to their natural resistance to most commonly used antibiotics and their capacity to acquire resistance to other antibiotics either by mutation (penicillins) or by transfer of resistance genes on plasmids and transposons (aminoglycosides and glycopeptides).2,3 In the United States acquired vancomycin resistance has increased more than 20-fold among nosocomial isolates of enterococci, from 0.3% in 1989 to 10% in 1995.2 This rise paralleled a massive increase in the use of vancomycin in US hospitals and was associated with spread of resistance plasmids and transposons among multiple strains of Enterococcus faecium and E faecalis.2,11 In addition, epidemics of infection with vancomycin resistant enterococci broke out, initially in intensive care units and later in whole hospitals.11–13 Most of these vancomycin resistant strains are resistant to all other effective antimicrobials. In patients with bacteraemia due to these strains, the mortality attributable to the infection is substantial.14 In Europe the incidence of infection caused by multiple resistant enterococci remains lower, although several outbreaks have been reported in transplant and intensive care units.15 About 2-5% of the population in Europe are intestinal carriers of vancomycin resistant E faecium, presumably acquired from the food chain.16
Multiple antibiotic resistance to useful classes of antibiotics, including the penicillins, cephalosporins, aminoglycosides, and fluoroquinolones, has gradually increased among a number of Gram negative hospital pathogens, especially Klebsiella pneumoniae,2,17 Enterobacter spp,18,19 Pseudomonas aeruginosa,20 and Acinetobacter baumannii.21,22 Epidemic and endemic infections caused by these multiple resistant strains followed intense antibiotic use in many hospitals, particularly in intensive care units.2,18,20,22,23 In a recent European hospital survey 23% of isolates of Klebsiellae were resistant to third generation cephalosporins by production of plasmid encoded extended spectrum β lactamases.17 In many cases, epidemic strains of these Gram negative bacilli showed resistance to nearly all available antibacterial drugs and caused serious nosocomial infections—such as pneumonia and bacteraemia—which were associated with increased mortality.18–21,24 The implementation of contact isolation measures for colonised patients or the modification of policies on antibiotics to curtail the massive use of drugs associated with these outbreaks, or in some cases both of these measures, were generally effective control measures.2,20–22
In addition to this increasing resistance among common agents of nosocomial infection, transmission of community acquired pathogens (such as multiple resistant Mycobacterium tuberculosis and M bovis) was observed in recent years in the United States and Europe in institutions caring for patients infected with HIV.25 This underlined the need for appropriate diagnostic and treatment facilities for these patients, including respiratory isolation facilities in hospitals.26
Factors promoting antimicrobial resistance
The first steps that contribute to the increasing incidence of hospital acquired infections caused by antibiotic resistant pathogens are the selection of resistant mutant strains from the patient’s own flora during antibiotic treatment or the transfer between bacteria of mobile genetic determinants of resistance (plasmids and transposons).2,3,11,20 Subsequently, resistant strains spread among patients in hospital.1,2,3,7,11,12,19–21 Selection of resistance in infecting or colonising bacteria is enhanced by factors related to the patient: immune suppression, infection of foreign bodies that impede local host defences, or presence of a large bacterial inoculum as reservoir of resistant mutants.1,2,3,7,20,21 Other predisposing factors depend on the medical management: use of monotherapy rather than combination therapy may favour selection of resistance in certain infections, as will insufficiently high drug doses or an inappropriate route of administration, which may fail to achieve bactericidal drug levels at the site of infection.2,3,20 Alteration of the endogenous microflora during antibiotic treatment also enhances replacement of susceptible organisms by resistant strains from the hospital microflora. Most commonly, transmission occurs as a result of contact between patients via the contaminated hands of healthcare staff.1,2,7,19–21 Factors predisposing to this transmission include the length of stay in hospital, intensity and duration of exposure to broad spectrum antibiotics, severity of underlying illness, use of invasive devices such as intravenous catheters, or surgery.1,7,20,21,24 Outbreaks with a common source of multiple resistant bacteria, often caused by organisms such as Pseudomonas spp and Acinetobacter spp, are another hazard. These are related to direct exposure of patients to contaminated food, equipment, or fluids—for example, during invasive procedures such as mechanical ventilation or endoscopy.1,7,20,21
The driving force of antibiotic resistance is the widespread use of antibacterial drugs. More than half of patients in acute care hospitals receive antibiotics as treatment or prophylaxis. Hospital doctors often prescribe antibiotics excessively and inappropriately, as shown in many studies.27 Insufficient training in infectious diseases and antibiotic treatment, difficulty of selecting the appropriate anti-infective drugs empirically, insufficient use of microbiological information, need for self reassurance, and fear of litigation are prompting the use of broad spectrum drugs.27 Likewise, compliance of healthcare staff with basic infection control practices—such as hand washing or disinfection—is incomplete, and shortage of healthcare staff often makes isolation precautions difficult to implement.28
Strategies for control
Several societies have published guidelines for optimising antibiotic use and curtailing antibiotic resistance in hospitals.27,29,30 Key components of these guidelines include multidisciplinary coordination between hospital administrators, clinicians, infectious diseases specialists, infection control teams, microbiologists, and hospital pharmacists; formulary based local guidelines on anti-infective treatment; education and regulation of prescriptors by consultant specialists; monitoring and auditing of drug use; surveillance and reporting of resistance patterns of the hospital flora; detection of patients colonised with communicable resistant bacteria and notification of these to the infection control team when isolation of the patient or decolonisation, or both, would be useful; promotion and monitoring of basic hospital infection control practices such as hand hygiene. These guidelines are based more on expert opinion and on the results of descriptive and analytical studies than on evidence from controlled trials, which are difficult to design to evaluate these types of population based intervention. Each hospital has its own ecosystem and microsociety, where determinants of antibiotic resistance are quite specific, and therefore effective solutions will need to be tailored to local epidemiological circumstances and resources.
Figure.
Proportion of S aureus isolates resistant to methicillin recovered from clinical specimens of inpatients in selected European countries. Data for hospitals are derived from Voss et al,4 and data for intensive care units from Vincent et al5
Acknowledgments
Competing interests: None declared.
References
- 1.Flaherty JP, Weinstein RA. Nosocomial infection caused by antibiotic-resistant organisms in the intensive-care unit. Infect Control Hosp Epidemiol. 1996;17:236–248. doi: 10.1086/647287. [DOI] [PubMed] [Google Scholar]
- 2.Gold HS, Moellering RC. Antimicrobial-drug resistance. N Engl J Med. 1996;335:1445–1443. doi: 10.1056/NEJM199611073351907. [DOI] [PubMed] [Google Scholar]
- 3.Cohen ML. Epidemiology of drug resistance: implications for a post-antimicrobial era. Science. 1992;257:1050–1055. doi: 10.1126/science.257.5073.1050. [DOI] [PubMed] [Google Scholar]
- 4.Voss A, Milatovic D, Wallrauch-Schwarz C, Rosdahl VT, Braveny I. Methicillin-resistant Staphylococcus aureus in Europe. Eur J Clin Microbiol Infect Dis. 1994;13:50–55. doi: 10.1007/BF02026127. [DOI] [PubMed] [Google Scholar]
- 5.Vincent JL, Bihari DJ, Suter PM, Bruining HA, White J, Nicolas-Chanoine MH, et al. The prevalence of nosocomial infection in intensive care units in Europe. JAMA. 1995;274:639–644. [PubMed] [Google Scholar]
- 6.Vandenbroucke-Grauls C. Management of methicillin-resistant Staphylococcus aureus in the Netherlands. Rev Med Microbiol. 1998;9:109–116. [Google Scholar]
- 7.Mulligan ME, Murray-Leisure KA, Ribner BS, Standiford HC, John JF, Korvick JA, et al. Methicillin-resistant Staphylococcus aureus: a consensus review of the microbiology, pathogenesis, and epidemiology with implications for prevention and management. Am J Med. 1993;94:313–328. doi: 10.1016/0002-9343(93)90063-u. [DOI] [PubMed] [Google Scholar]
- 8.Struelens MJ, Ronveaux O, Jans B, Mertens R the Groupement pour le Dépistage; l’Etude et la Prévention des Infections Hospitalières. Methicillin-resistant Staphylococcus aureus epidemiology and control in Belgian hospitals, 1991 to 1995. Infect Control Hosp Epidemiol. 1996;17:503–508. doi: 10.1086/647351. [DOI] [PubMed] [Google Scholar]
- 9.Hiramatsu K, Aritaka N, Hanaki H, Kawasaki S, Hosoda Y, Hor S, et al. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet. 1997;350:1670–1673. doi: 10.1016/S0140-6736(97)07324-8. [DOI] [PubMed] [Google Scholar]
- 10.CDC Update. Staphylococcus aureus with reduced susceptibility to vancomycin. United States 1997. MMWR. 1997;46:813. [PubMed] [Google Scholar]
- 11.Frieden TR, Munsiff SS, Low De, Willey BM, Williams G, Faur Y, et al. Emergence of vancomycin-resistant enterococci in New York City. Lancet. 1993;342:76–79. doi: 10.1016/0140-6736(93)91285-t. [DOI] [PubMed] [Google Scholar]
- 12.Karanfil LV, Murphy M, Josephson A, Gaynes R, Mandel L, Hill BC, et al. A cluster of vancomycin-resistant Enterococcus faecium in an intensive care unit. Infect Control Hosp Epidemiol. 1992;13:195–200. doi: 10.1086/646509. [DOI] [PubMed] [Google Scholar]
- 13.Bonten JM, Hayden MK, Nathan C, Van Voorhis J, Matushek M, Slaughter S, et al. Epidemiology of colonisation of patients and environment with vancomycin-resistant enterococci. Lancet. 1996;348:1615–1619. doi: 10.1016/S0140-6736(96)02331-8. [DOI] [PubMed] [Google Scholar]
- 14.Shay DK, Maloney SA, Montecalvo M, Banerjee S, Wormser GP, Arduino MJ, et al. Epidemiology and mortality risk of vancomycin-resistant enterococcal bloodstream infections. J Infect Dis. 1995;172:993–1000. doi: 10.1093/infdis/172.4.993. [DOI] [PubMed] [Google Scholar]
- 15.Jordens JZ, Bates J, Griffiths DT. Faecal carriage and nosocomial spread of vancomycin-resistant Enterococcus faecium. J Antimicrob Chemother. 1994;34:515–528. doi: 10.1093/jac/34.4.515. [DOI] [PubMed] [Google Scholar]
- 16.Endtz HP, Van den Braak N, Van Belkum A, Kluytmans JA, Koeleman JG, Spanjaard L, et al. Fecal carriage of vancomycin-resistant enterococci in hospitalized patients and those living in the community in the Netherlands. J Clin Microbiol. 1997;35:3026–3031. doi: 10.1128/jcm.35.12.3026-3031.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Livermore DM, Yuan M. Antibiotic resistance and production of extended-spectrum β-lactamases amongst Klebsiella spp from intensive care units in Europe. J Antimicrob Chemother. 1996;38:409–424. doi: 10.1093/jac/38.3.409. [DOI] [PubMed] [Google Scholar]
- 18.Chow JW, Fine MJ, Shlaes DM, Quinn JP, Hooper DC, Johnson MP, et al. Enterobacter bacteremia: clinical features and emergence of antibiotic resistance during therapy. Ann Intern Med. 1991;115:585–590. doi: 10.7326/0003-4819-115-8-585. [DOI] [PubMed] [Google Scholar]
- 19.De Gheldre Y, Maes N, Rost F, De Ryck R, Clevenbergh P, Vincent JL, et al. Molecular epidemiology of an outbreak of multidrug-resistant Enterobacter aerogenes infections and in vivo emergence of imipenem resistance. J Clin Microbiol. 1997;35:152–160. doi: 10.1128/jcm.35.1.152-160.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richard P, Le Floch R, Chamoux C, Pannier M, Espaze E, Richet H. Pseudomonas aeruginosa outbreak in a burn unit: role of antimicrobials in the emergence of multiply resistant strains. J Infect Dis. 1994;170:377–383. doi: 10.1093/infdis/170.2.377. [DOI] [PubMed] [Google Scholar]
- 21.Towner KJ. Clinical importance and antibiotic resistance of Acinetobacter spp. J Med Microbiol. 1997;46:721–746. doi: 10.1099/00222615-46-9-721. [DOI] [PubMed] [Google Scholar]
- 22.Struelens MJ, Carlier E, Maes N, Serruys E, Quint WGV, van Belkum A. Nosocomial colonization and infection with multiresistant Acinetobacter baumannii: outbreak delineation using DNA macrorestriction analysis and PCR-fingerprinting. J Hosp Infect. 1993;25:15–32. doi: 10.1016/0195-6701(93)90005-k. [DOI] [PubMed] [Google Scholar]
- 23.McGowan JE. Antimicrobial resistance in hospital organisms and its relation to antibiotic use. J Infect Dis. 1983;5:1033–1048. doi: 10.1093/clinids/5.6.1033. [DOI] [PubMed] [Google Scholar]
- 24.Lortholary O, Fagon JY, Buu Hoi A, Slama MA, Pierre J, Giral P, et al. Nosocomial acquisition of multiresistant Acinetobacter baumannii: risk factors and prognosis. Clin Infect Dis. 1995;20:790–796. doi: 10.1093/clinids/20.4.790. [DOI] [PubMed] [Google Scholar]
- 25.Guerrero A, Cobo J, Fortún J, Navas E, Quereda C, Asensio A, et al. Nosocomial transmission of Mycobacterium bovis resistant to 11 drugs in people with advanced HIV-1 infection. Lancet. 1997;350:1738–1742. doi: 10.1016/S0140-6736(97)07567-3. [DOI] [PubMed] [Google Scholar]
- 26.Bates JH, Nardell E. American College of Chest Physicians Consensus Statement. Institutional control measures for tuberculosis in the era of multiple drug resistance. Chest. 1995;108:1690–1710. doi: 10.1378/chest.108.6.1690. [DOI] [PubMed] [Google Scholar]
- 27.Working Party of the British Society for Antimicrobial Chemotherapy. Hospital antibiotic control measures in the UK. J Antimicrob Chemother. 1994;34:21–42. [PubMed] [Google Scholar]
- 28.Larson E, Kretzer EK. Compliance with handwashing and barrier precautions. J Hosp Infect. 1995;30:88–106. doi: 10.1016/0195-6701(95)90010-1. [DOI] [PubMed] [Google Scholar]
- 29.Goldmann DA, Weinstein RA, Wenzel RP, Tablan OC, Duma RJ, Gaynes RP, et al. Strategies to prevent and control the emergence and spread of antimicrobial-resistant microorganisms in hospitals. A challenge to hospital leadership. JAMA. 1996;275:234–240. [PubMed] [Google Scholar]
- 30.Schlaes DM, Gerding DN, John JF, Craig WA, Bornstein DL, Duncan RA, et al. Society for Healthcare Epidemiology of America and Infectious Diseases Society of America Joint Committee on the Prevention of Antimicrobial Resistance: guidelines for the prevention of antimicrobial resistance in hospitals. Clin Infect Dis. 1997;25:584–599. doi: 10.1086/513766. [DOI] [PubMed] [Google Scholar]