RESUME
Objectif : Identifier les facteurs prédictifs de la survenue d’infections urinaires communautaires à Escherichia coli productrices de β-Lac-tamase à spectre étendu en pédiatrie.Méthodes : Il s’agissait d’une étude analytique prospective monocentrique ayant inclus des enfants et des adultes jeunes pris en charge pour une infection urinaire communautaire à Escherichia coli productrices de β-Lactamase à spectre étendu. L’étude a été menée au service de néphrologie pédiatrique de l’hôpital Charles Nicolle, Tunis, Tunisie du 1er janvier 2019 au 31 décembre 2020. Les patients ≤20 ans ayant présenté une infection urinaire communautaire à Escherichia coli ont été inclus prospectivement dans notre étude.Résultats : Nous avons colligé 290 infections urinaires chez 218 patients dont 92 infection urinaire à Escherichia coli productrices de β-Lactamase à spectre étendu. L’âge moyen des enfants était de 50,10±54,28 mois, avec une prédominance féminine dans 65,2% des cas. Les facteurs de risque relatifs à l’acquisition de bactéries multirésistantes étaient : une prise d’antibiothérapie dans les trois mois précédents, une antibioprophylaxie, une hospitalisation dans l’année qui a précédé l’infection urinaire, et des soins en ambulatoire dans les six mois précédents (p<0.05). Une résistance à la Ceftazidime, au Céfotaxime, au céfixime, à la Gentamicine et à l’Ofloxacine étaient significativement associée à la présence d’une souche productrice de β-Lactamase à spectre étendu. La résistance aux antibiotiques était significativement plus observée dans la tranche d’âge supérieure à 6 ans. Une cohabitation avec un personnel de la santé était un facteur de risque de résistance à l’Amoxicilline-acide clavulanique. L’étude du profil épidémiologique et des facteurs de risque des infec-tions urinaires à germes multirésistants, notamment à Escherichia coli productrices de β-Lactamase à spectre étendu dans la population pédiatrique, pourrait améliorer l’approche thérapeutique et conduire à une prescription plus rationnelle des antibiotiques.
ABSTRACT
Aim: To identify the predictive factors for the occurrence of community-acquired urinary tract infections caused by Extended-Spectrum β-Lactamase-Producing Escherichia coli in children. Methods: This was a single-center prospective observational study of children and young adults with community-acquired urinary tract infections caused by Extended-Spectrum β-Lactamase-Producing Escherichia coli. The study was conducted in the pediatric nephrology department in Charles Nicolle Hospital, Tunis, Tunisia from January 1st, 2019, to December 31, 2020. Patients ≤20 years with community-acquired urinary tract infection caused by Escherichia coli were included prospectively in our study. Results: We collected 290 urinary tract infections in 218 patients, including 92 urinary tract infections due to Extended-Spectrum β-Lactamase-Producing Escherichia coli. The mean age of children was 50.10±54.28 months, with a female predominance in 65.2% of cases. Risk factors for the acquisition of multidrug-resistant bacteria were antibiotic therapy in the previous three months, antibiotic prophylaxis, hospitalization in the year preceding the urinary tract infections, and outpatient care in the previous six months (p < 0.05). Resistance to Ceftazidime, Cefotaxime, Cefixime, Gentamicin and Ofloxacin was significantly associated with the presence of an Extended-Spectrum β-Lactamase strain. Antibiotic resistance was significantly more observed in the age group above 6 years. Co-habitation with health care worker was a risk factor for resistance to Amoxicillin-Clavulanic Acid. Conclusion: Understanding the epidemiological profile and risk factors for ESBL-producing UTIs, including Extended-Spectrum β-Lactamase-producing Escherichia coli in the pediatric population, could improve the therapeutic approach and lead to more rational prescription of antibiotics.
INTRODUCTION
Urinary tract infections (UTIs) are among the most common bacterial infections. During the first year of life, their incidence is approximately 0.7% in girls and 2.7% in uncircumcised boys (1-5).
Bacteria involved are mainly enterobacteria, with a predominance of Escherichia coli (E. coli) in 60-90% of cases, followed by Proteus mirabilis, then Klebsiella spp. [6,7].
Since the late 1990s, the epidemiology of UTIs has evolved with the emergence of extended-spectrum betalactamase (ESBL)-producing enterobacteria.
ESBLs are a large and very heterogeneous family of bacterial enzymes discovered in 1980 in Europe ( 7 ).
They are induced either by plasmids, or by mutation of the natural genome in Klebsiella spp, coding for a beta-lactamase SHV ( 7 ).
Both mechanisms give the affected bacteria the ability to hydrolyze a wide variety of penicillin’s and cephalosporins ( 7 ).
They are very active against penicillin’s and moderately active against first generation cephalosporins.
Genetic mutations that cause ESBL broaden the spectrum of these enzymes and affect third generation cephalosporins (ceftazidime and cefotaxime) and monobactams (aztreonam) [7].
ESBL producing bacteria do not hydrolyze cephamycins (cefoxitin) or carbapenems (imipenem) and are inhibited by clavulanic acid, tazobactam and sulbactam, which are the classic beta-lactamase inhibitors. The presence of ESBL is frequently associated with resistance to fluoroquinolones ( 8 ).
The prevalence of ESBL-acquired UTIs in children and adults is increasing, reaching 7.4% in North America, 18.8% in Europe, 27.7% in Southeast Asia, and up to 60% in India [9, 11].
Current recommendations for the treatment of UTIs are based on oral cephalosporins for lower UTIs, and third generation cephalosporins (C3G) combined with aminoglycosides in some cases for acute pyelonephritis (12).
The increasing frequency of ESBLproducing Enterobacteriaceae makes the management of UTIs more difficult. The literature on antibiotic therapy for ESBL-acquired UTIs has not provided recommendations for treatment, and there are no randomized controlled trials on this topic to date.
As a result, treatment approaches seem to be heterogeneous. Tunisia, like all countries in the world, is concerned by this growing diffusion of E. coli ESBL.
This study aimed to explore the prevalence and bacteriological profiles of E. coli ESBL UTIs, as well as to examine the predictive factors for their occurrence. The ultimate goal was to develop effective strategies to combat this issue.
METHODES
The clinical features of children and young adults (age≤20 years) with UTI caused by E. Coli were prospectively included in our study. Patients who undergone treatment in the pediatric department of Charles Nicolle hospital between January 1st 2019 and December,31, 2020.
. In the current study, we counted each episode of UTI separately if the same patient had more than one episode of UTI. Urine specimens were obtained either by bladder catheterization or by the midstream or cleancatch method.
Patients were considered to have UTI if a single pathogen (≥104 colony-forming unit (CFU)/ml for catheter specimens or ≥105 CFU/ml for clean-catch specimens) was identified on urine culture. ESBL was identified according the 2010 Clinical and Laboratory Standards Institute guidelines.
The data collected from the medical records database included: gender, age, history of UTI, results of blood culture, results of abdominal ultrasound and voiding cystourethrogram (VCUG) and recent antimicrobial use, details about initial antibiotic therapy, definitive antibiotic therapy, the antibiogram result.
In addition, duration of fever, physical examination data, data from biological examinations including creatinine level, inflammatory tests were also included in our study. UTIs episodes were included in our study as follows:
UTI episodes among patients aged between 0 and 20 years old, treated at the Pediatrics department of the Charles Nicolle Hospital in Tunis between January 1st 2019 and December,31, 2020 n=370.
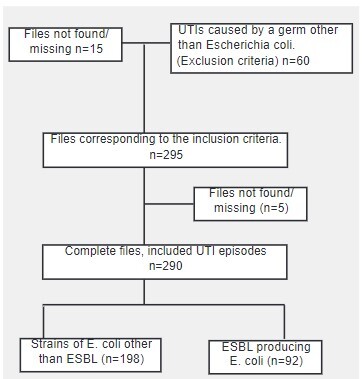
All statistical analyses were performed using the SPSS statistical software package (version 24), Fisher’s exact test and the Mann-Whitney test were used to compare patient characteristics and to determine P values.P-values< 0.05 were statistically significant.
RESULTATS
During the observation period 290 episodes of UTI in 218 patients were enrolled in our study. The baseline characteristics of our population are summarized in Table 1.
The percentage of ESBL-producing pathogens among total E. coli in the study period was 31.72%.
The prevalence of ESBL producing strains tended to decrease with age, it was 43.75% for the age group younger than 3 months and 25.39% for children older than 6 years. These drug resistant strains were more frequent in boys 40% than in girls 28.57%.
Of the 218 patients, 82 were initially treated with cephalosporin antimicrobials, treatment was switched to antimicrobials effective against ESBL in 18 patients after antimicrobial susceptibility was identified.
The other patients continued receiving the same antimicrobials as before. Carbapenem was prescribed initially in 19 cases when risk factors for antibiotic resistance were present.
ESBL isolates were resistant to ceftazidime and cefotaxime in respectively 24.1% and 26.7% of cases. The resistance rates of isolates to the antimicrobials tested are shown in Table 2. Overall, ESBL isolates were resistant to ampicillin.
Regardless of ESBL status, all isolates proved susceptible to colistin (Table 5).
Antibiotic resistance to ceftazidime was statistically associated with CAKUT (P=0.031), and with the use of antibiotics during the last six months (P=0.001) OR 1.61[1.26-2.05], Resistance to Trimethoprim/ sulfamethoxazole (SXT) was associated with recurrence of UTIs P=0.002 OR:1.42[1.14-1.77, with antibiotic prophylaxis (P=0.003) OR:2.5[1.32-4.71], and with clean intermittent catheterization (P=0.02) OR:2.14[1.1-4.14].
Antibiotic resistance to the amoxicillin-clavulanic acid was statistically associated with cohabitation with a member of a health care staff (P=0.006) OR:4.97[1.39-17.6].
Table 1 : Baseline characteristics of the 218 patients with Escherichia coli urinary tract infection follow-up in the pediatric nephrology department at Charles Nicolle hospital, Tunis, Tunisia .
|
Patient profile |
Categorical variables, n(%) |
|
Male |
64 (%) |
|
Female |
154 (%) |
|
Male Female Ratio |
0.41 |
|
Age of diagnosis according to age group (months)
|
24 (1.38) 12 (4.13) 94 (32.4) 54 (18) 126 (43.4) |
|
Previous hospitalization |
89 (30.71) |
|
Recurrent UTI |
152 (52.40) |
|
CAKUT
|
144 (49.65) 68 (23.44) 26 (8.96) 26 (8.96) 6 (2.06) 3 (1.03) 7 (2.41) 6 (2.06) 2 (0.68) |
|
Nephrolithiasis |
3 (1.37) |
|
Kidney transplantation |
1 (0.45) |
|
Upper tract infections |
184 (63.4) |
|
Lower tract infections |
106 (36.55) |
|
ESBL-Producing Escherichia coli |
92 (31.71) |
Table 2 : Resistances among ESBL-producing Escherichia coli isolates .
|
Antibiotic |
Resistance rate (%) |
|
Amoxicillin/clavulanic acid (AMC) |
110 (40,1%) |
|
Cefotaxime (CTX) |
68 (26,7%) |
|
Ceftazidime (CAZ) |
56 (24,1%) |
|
Piperacillin/tazobactam (TZP) |
184 (67,6%) |
|
Ticarcillin (TIC) |
184 (67,6%) |
|
Cefixime (CFM) |
48 (19,6%) |
|
Imipenem (IPM) |
0 |
|
Ertapenem (ETP) |
0 |
|
Gentamicin (GM) |
13 (5,6%) |
|
Amikacin (AN) |
0 |
|
Ofloxacin (OFX) |
23 (9,3%) |
|
Ciprofloxacin (CFX) |
15 (6,2%) |
|
Trimethoprim/sulfamethoxazole (SXT) |
84 (39,3%) |
|
Fosfomycin (FOS) |
2 (1,4%) |
|
Colistin (CT/CL) |
0 |
Table 3 : Comparison of demographic and clinical factors between ESBL and non-ESBL among 290 UTI episodes .
|
Total (N = 290) |
ESBL (n = 92) |
Non-ESBL (n = 198) |
P-value |
|
|
Age, median (IQR), months |
62,18±54,5 |
50,10±54,28 |
67,59±53,95 |
0.011 |
|
Age group months
|
|
|
|
0.35 |
|
Gender
|
|
|
|
0.071 |
|
UTI episode
|
|
|
|
0.229 |
|
Previous hospitalization in the last 3 months |
89 (73.4%) |
49 (53.2%) |
40 (20.2%) |
<0.001 |
|
Bladder dysfunction |
68 (23.44) |
19 (20.6%) |
49 (24.7%) |
0.278 |
|
Vesicoureteric reflux |
26 (8.96) |
18 (9%) |
8 (8.6%) |
0.02 |
|
Megaureter |
26 (8.96) |
15 (7.5%) |
11 (12%) |
0.224 |
|
Ureteropelvic junction obstruction |
6 (2.06) |
6 (3%) |
0 |
0.182 |
|
Renal agenesis |
9 (3.10) |
6 (6.5%) |
3 (1.5%) |
0.05 |
|
Posterior urethral valves |
7 (2.41) |
5 (2.5%) |
2 (2.1%) |
0.035 |
|
Duplex collecting system |
6 (2.06) |
4 (4.3%) |
2 (1%) |
0.068 |
|
Multicystic dysplastic kidney |
2 (0.68) |
2 (2.1%) |
0 |
0.1 |
|
Average time between onset of symptomatology and hospitalization (days) |
3.20 |
7.46 |
0.826 |
|
|
Urinary symptoms |
17 (18.1%) |
15 (7.6%) |
0.018 |
|
|
Sepsis |
17 (8.7%) |
3 (3%) |
0.036 |
|
|
Neutrophil count |
8835±5424 |
7165±4358 |
0.014 |
|
|
Renal ultrasonography
|
|
|
|
0.158 |
|
VCUG
|
|
|
|
0.018 |
|
DMSA renal scan
|
|
|
|
0.108 |
|
ESBL: extended-spectrum beta-lactamase |
VCUG: voiding cystourethrogram |
DMSA: 99mTc dimercaptosuccinic acid |
Table 4 : Multivariate results predicting ESBL-caused urinary tract infections .
|
ESBL (n = 92) |
Non-ESBL (n = 198) |
Adjusted OR (95% CI) |
P-value |
|
|
Recent Antibiotherapy |
56 (60,8%) |
104 (52,5%) |
5,626 [1,72-18,36] |
<0,001 |
|
Antibioprophylaxis |
16 (17,3%) |
23 (11,6%) |
1,56 [1,06-2,3] |
0,037 |
|
Hospitalization during the last year |
58 (63%) |
61 (30,8%) |
1,5 [1,07-2,09] |
<0,001 |
|
Outpatient healthcare during the last 6 months |
30 (32,6%) |
35 (17,6%) |
1,83 [1,32-2,55] |
0,005 |
ESBL: extended-spectrum beta-lactamase; CI: confidence interval; OR: odds ratio
Table 5 : Antibiotic susceptibility according to the presence of ESBL isolates .
|
Antibiotic |
ESBL (n = 92) |
Non-ESBL (n = 198) |
Adjusted OR (95% CI) |
P-value |
|
Ampicillin (AMP) |
0 |
0 |
- |
- |
|
Amoxicillin/clavulanic acid (AMC) |
50 (54.34.5%) |
114 (57.57%) |
- |
0.566 |
|
Cefotaxime (CTX) |
44 (47.82%) |
143 (72.22%) |
1.87 [1.29-2.72] |
0.001 |
|
Ceftazidime (CAZ) |
44 (47.82%) |
132 (81.5%) |
1.85 [1.27-2.71] |
0.002 |
|
Piperacillin/tazobactam (TZP) |
29 (31.52%) |
59 (29.8%) |
- |
0.326 |
|
Ticarcillin (TIC) |
27 (29.34%) |
61 (30.8%) |
- |
0.751 |
|
Cefixime (CFM) |
46 (50%) |
151 (76.26%) |
2.23 [1.53-3.23] |
<0.001 |
|
Imipenem (IPM) |
68 (74%) |
172 (86.86%) |
- |
- |
|
Ertapenem (ETP) |
71 (77.17%) |
177 (89.40%) |
- |
- |
|
Gentamicin (GM) |
51 (55.43%) |
167 (84.34%) |
2.95 [1.91-4.57] |
0.001 |
|
Amikacin (AN) |
69 (75%) |
175 (88.38%) |
- |
- |
|
Ofloxacin (OFX) |
57 (62%) |
167 (84.34%) |
2.22 [1.45-3.39] |
0.002 |
|
Ciprofloxacin (CFX) |
58 (63.04%) |
168 (84.84%) |
1.81 [1.01-3.26] |
0.0128 |
|
Trimethoprim/sulfamethoxazole (SXT) |
36 (39.13%) |
94 (47.47%) |
- |
0.608 |
|
Fosfomycin (FOS) |
39 (42.40%) |
105 (53.03%) |
- |
0.474 |
|
Colistin (CT/CL) |
92 (100%) |
198 (100%) |
- |
0.672 |
DISCUSSION AND CONCLUSION
Febrile UTI is one of the most common bacterial infections in children and usually causes irreversible renal damage when not diagnosed and treated early. Timely medication and adequate antibiotic duration can considerably improve the disease outcome [13,14].
In the current study we found that young age, vesicoureteral reflux, posterior ureteral valves, congenital solitary kidney, Sepsis, increased neutrophil count, and history of previous hospitalization within the last three months were associated with the occurrence of ESBL-E coli associated UTI.
This result was in accordance with some recent studies, in which UTIs with ESBL-E.coli were found to be significantly higher in patients with urinary system anomalies in USG [8,13,14].
In many studies, inflammation markers such as WBC, CRP, PCT, NLR, PLR were evaluated in patients with UTIs. These markers were used to differentiate lower UTIs and acute pyelonephritis and to predict renal complications with non-invasive and widely used biomarkers rather than invasive screening methods such as dimercaptosuccinic acid scintigraphy (DMSA)
or voiding cystourethrography in most of the studies [ 14 , 15 - 18 ]. In our study, we evaluated these inflammation markers to predict infections with ESBL- E. coli, which have been known to have a higher risk for renal complications, to initiate the appropriate treatment, until the urine culture results are out. Shaikh et al. have identified urinary and serum markers that appear promising in differentiating children with pyuria who do and do not have UTI. Particularly promising single markers included urinary IL-9, IL-2, IL-8, and NGAL [ 19 ].
Analysis of the β-lactam family shows that all strains of E. coli producing ESBLs are resistant to amoxicillin (AMX), 67.7% of strains are resistant to ticarcillin (TIC), and from 56% to 68% of strains are resistant to third generation cephalosporins.
Penicillin protected by beta-lactamase inhibitors such as piperacillin/tazobactam (TZP) and amoxicillin/clavulanic acid is no longer effective against ESBL-EC with resistance ranging from 40% to 67%.
However, the analysis shows that the carbapenems are very effective against ESBL-E. coli with a 100% sensitivity. Our findings are similar to some other studies [ 20 , 25 ]
Presence of urinary symptoms suggestive of pyelonephritis was predictive of ESBL UTI
Several studies have concluded that the clinical picture of UTI cannot exactly predict whether it is ESBL UTI or not (26).
In our study, intravenous antibiotic therapy was prescribed in 74.1% of patients (n=218)
Cefotaxime was prescribed in 33% of patients (n=72/218) and ceftriaxone in 4.1% of patients (n=9/218). Imipenem was prescribed in 6.4% (n=14/218), ceftazidime in 0.45% (n=1), and AAC in 0.45% (n=1).
Aminoglycosides were combined with Third generation cephalosporins in 42.6% of cases (n=93), with imipenem in 8.7% (n=19) and with ceftazidime in 4.1% (n=9).
Carbapenems are currently the first line treatment for ESBL-producing isolates, some authors have concluded that there is an increase in morbidity and mortality with other treatments.
Two studies of pediatric UTIs, in Greece and Korea, found no significant difference in outcomes with or without the use of carbapenems [27 , 28]
Some authors have proposed the use of a β-lactamine-β-lactamase combination, such as cefixime and amoxicillin-clavulanic acid, as an alternative to carbapenems after verification of the of the minimal inhibitory concentration (MIC) [29,30]
There is also strong in vitro evidence for the efficacy of cephalosporin-clavulanic acid combination for the treatment of febrile UTIs (31 ).
The objective is to reduce the prescription of carbapenems, which are the most reliable treatment for ESBL-producing bacilli ( 32 ) and to reserve them for more severe infections.
Poey et al, prescribed amikacin 25mg/kg/day IV for an average of three days as first-line treatment instead of Third generation cephalosporins to prevent the emergence of carbapenemase-producing organisms ( 33 ).
This choice was because early prescription of an antibiotic effective against ESBL pathogens helps to prevent later renal scarring [ 34 ]
Furthermore, for lower urinary tract infections, a metaanalysis reported that nitrofurontoine ´effectiveness ranged from 79% to 92%, and the incidence of side effects ranged from 5% to 16%. These effects were limited to mild and reversible gastrointestinal disorders [35]
In light of the growing incidence of E. coli ESBL and the emergence of carbapenem-resistant bacteria, which were once considered a primary treatment for E. coli ESBL, we suggest the following recommendations:
• Limiting the prescription of antibiotics for established UTIs • Customizing antibiotic treatment based on the results of antibiograms • Reserving treatment of asymptomatic bacteriuria for children scheduled for surgery or endoscopic procedures • Restricting antibiotic prophylaxis to children with recurrent UTIs who are awaiting a surgical or endoscopic procedure on the urinary tract.
References
- Millner R, Becknell B. Urinary Tract Infections. Pediatr Clin North Am. 2019;66(1):1–13. doi: 10.1016/j.pcl.2018.08.002. [DOI] [PubMed] [Google Scholar]
- Simões e Silva AC, Oliveira EA. Update on the approach of urinary tract infection in childhood. J Pediatr. 2015;91(1):2–10. doi: 10.1016/j.jped.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Karmazyn BK, Alazraki AL, Anupindi SA, Dempsey ME, Dillman JR, Dorfman SR, et al. Expert panel on pediatric imaging: ACR appropriateness criteria, urinary tract infection-child. J Am Coll Radiol. 2017;14(5):362–371. doi: 10.1016/j.jacr.2017.02.028. [DOI] [PubMed] [Google Scholar]
- Stephens GM, Akers S, Nguyen H, Woxland H. Evaluation and management of urinary tract infections in the school-aged child. 2015;42(1):33–41. doi: 10.1016/j.pop.2014.09.007. [DOI] [PubMed] [Google Scholar]
- ‘t Hoen LA, Bogaert G, Radmayr C, Dogan HS, Nijman RJM, Quaedackers J, Rawashdeh YF, Silay MS, Tekgul S, Bhatt NR, Stein R. Update of the EAU/ESPU guidelines on urinary tract infections in children. J Pediatr Urol. 2021;17(2):200–207. doi: 10.1016/j.jpurol.2021.01.037. [DOI] [PubMed] [Google Scholar]
- Wagenlehner FME, Bjerklund Johansen TE, Cai T, Koves B, Kranz J, Pilatz A, Tandogdu Z. Epidemiology, definition and treatment of complicated urinary tract infections. Nat Rev Urol. 2020;17(10):586–600. doi: 10.1038/s41585-020-0362-4. [DOI] [PubMed] [Google Scholar]
- Çiçek AÇ, Şemen V, Ejder NA, Gündoğdu DZU, Kalcan SK, Köse FT, et al. Molecular epidemiological analysis of integron gene cassettes and tetA/tetB/tetD gene associations in Escherichia coli strains producing extended-spectrum β-lactamase (ESBL) in urine cultures. Adv Clin Exp Med. 2022;31(1):71–79. doi: 10.17219/acem/142333. [DOI] [PubMed] [Google Scholar]
- Islam MA, Islam MR, Khan R, Amin MB, Rahman M, Hossain MI, et al. Prevalence, etiology and antibiotic resistance patterns of community-acquired urinary tract infections in Dhaka, Bangladesh. PLoS One. 2022;17(9):e0274423. doi: 10.1371/journal.pone.0274423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoban DJ, Nicolle LE, Hawser S, Bouchillon S, Badal R. Antimicrobial susceptibility of global inpatients urinary tract isolates of Escherichia coli: results from the study of monitoring antimicrobial resistance trends (SMART) program: 2009-2010. Diag Microbiol Infect Dis. 2011;70:507–511. doi: 10.1016/j.diagmicrobio.2011.03.021. [DOI] [PubMed] [Google Scholar]
- Hsueh PR, Hoban DJ, Carmeli Y, Chen SY, Desikan S, Alejandria M, et al. Consensus review of the epidemiology and appropriate antimicrobial therapy of complicated urinary tract infections in Asia-Pacific region. J Infect. 2011;63:114–123. doi: 10.1016/j.jinf.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Hawser SP, Bouchillon SK, Hoban DJ, Badal RE, Hsueh PR, Paterson DL. Emergence of high-levels of extended-spectrum beta-lactamase-producing Gram-negative bacilli in the Asia-Pacific region: data from the Study for Monitoring Antimicrobial Resistance Trends (SMART) program, 2007. Antimicrob Agents Chemother. 2009;53:3280. doi: 10.1128/AAC.00426-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffernan HM, Woodhouse RE, Pope CE, Blackmore TK. Prevalence and types of extended-spectrum beat-lactamases among urinary Escherichia coli and Klebsiella spp. in New Zealand. Int J Antimicrob Agents. 2009;34:544–549. doi: 10.1016/j.ijantimicag.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Zhu FH, Rodado MP, Asmar BI, Salimnia H, Thomas R, Abdel-Haq N. Risk factors for community acquired urinary tract infections caused by extended spectrum β-lactamase (ESBL) producing Escherichia coli in children: a case control study. Infect Dis (Lond) 2019;51(11-12):802–809. doi: 10.1080/23744235.2019.1654127. [DOI] [PubMed] [Google Scholar]
- Collingwood JD, Wang L, Aban IB, Yarbrough AH, Boppana SB, Dangle PP. Risk factors for community acquired pediatric urinary tract infection with extended-spectrum-β-lactamase Escherichia coli - A case-control study. J Pediatr Urol. 2023;19(1):129.e1–129.e7. doi: 10.1016/j.jpurol.2022.10.020. [DOI] [PubMed] [Google Scholar]
- Koufadaki AM, Karavanaki KA, Soldatou A, Tsentidis Ch, Sourani MP, Sdogou T, et al. Clinical and laboratory indices of severe renal lesions in children with febrile urinary tract infection. Acta Paediatr. 2014;103:404–409. doi: 10.1111/apa.12706. [DOI] [PubMed] [Google Scholar]
- Kim YH, Yang EM, Kim CJ. Urinary tract infection caused by community-acquired extended-spectrum β-lactamase-producing bacteria in infants. J Pediatr (Rio J) 2017;93(3):260–266. doi: 10.1016/j.jped.2016.06.009. [DOI] [PubMed] [Google Scholar]
- Leroy S, Fernandez-Lopez A, Nikfar R, Romanello C, Bouissou F, Gervaix A, et al. Association of procalcitonin with acute pyelonephritis and renal scars in pediatric UTI. Pediatrics. 2013:870–879. doi: 10.1542/peds.2012-2408. [DOI] [PubMed] [Google Scholar]
- Bouguila J, Khalef I, Charfeddine B, Ben Rejeb M, Chatti K, Limam K, et al. Comparative study of C-reactive protein and procalcitonin in the severity diagnosis of pyelonephritis in children. Pathol Biol (Paris) 2013;61:93–98. doi: 10.1016/j.patbio.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Shaikh N, Martin JM, Hoberman A, Skae M, Milkovich L, McElheny C, et al. Biomarkers that differentiate false positive urinalyses from true urinary tract infection. Pediatr Nephrol. 2020;35(2):321–329. doi: 10.1007/s00467-019-04403-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki S, Le TD, Vien MQ, Van Dang C, Yamamoto Y. Prevalence of extended-spectrum β-lactamase-producing Escherichia coli and residual antimicrobials in the environment in Vietnam. Anim Health Res Rev. 2017;18(2):128–135. doi: 10.1017/S1466252317000160. [DOI] [PubMed] [Google Scholar]
- El bouamri MC, Arsalane L, Kamouni Y, Yahyaoui H, Bennouar N, Berraha M, et al. Current antibiotic resistance profile of uropathogenic Escherichia coli strains and therapeutic consequences. Prog Urol. 2014;24(16):1058–1062. doi: 10.1016/j.purol.2014.09.035. [DOI] [PubMed] [Google Scholar]
- Sbiti M, Lahmadi K, Louzi M. Epidemiological profile of uropathogenic enterobacteria producing extended spectrum beta-lactamases. Pan Afr Med J. 2017;28:1–8. doi: 10.11604/pamj.2017.28.29.11402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravey F, Loggia G, de la Blanchardière A, Cattoir V. Bacterial epidemiology and antimicrobial resistance profiles of urinary specimens of the elderly. Med Mal Infect. 2017;47(4):271–278. doi: 10.1016/j.medmal.2017.03.002. [DOI] [PubMed] [Google Scholar]
- Critchley IA, Cotroneo N, Pucci MJ, Mendes R. The burden of antimicrobial resistance among urinary tract isolates of Escherichia coli in the United States in 2017. PloS One. 2019;14(12):1–11. doi: 10.1371/journal.pone.0220265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K, Nguyen J, Nguyen K, Huse HK, Nieberg PH, Wong-Beringer A. Prevalence of the Carbapenem-heteroresistant Phenotype Among ESBL-Producing Escherichia Coli and Klebsiella Pneumoniae Clinical Isolates. J Antimicrob Chemother. 2020;75(6):1506–1512. doi: 10.1093/jac/dkaa048. [DOI] [PubMed] [Google Scholar]
- Priyadharshana U, Piyasiri LB, Wijesinghe C. Prevalence, Antibiotic sensitivity pattern and genetic analysis of extended spectrum beta lactamase producing Escherichia Coli and Klebsiella Spp among patients with community acquired urinary tract infection in Galle District, Sri Lanka. Ceylon Med J. 2019;64(4):140–145. doi: 10.4038/cmj.v64i4.8990. [DOI] [PubMed] [Google Scholar]
- Lignieres G, Birgy A, Jung C, Bonacorsi S, Levy C, Angoulvant F, et al. Relay oral therapy in febrile urinary tract infections caused by extended spectrum beta-lactamase-producing Enterobacteriaceae in children: A French multicenter study. PLoS One. 2021;16(9):e0257217. doi: 10.1371/journal.pone.0257217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Kang SY, Kang HM, Yang NR, Kang HG, Ha IS, et al. Outcome of Antimicrobial Therapy of Pediatric Urinary Tract Infections Caused by Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae. Infect Chemother. 2013;45(4):415–421. doi: 10.3947/ic.2013.45.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson H, Török ME. Extended-spectrum β-lactamase-producing and carbapenemase-producing Enterobacteriaceae. Microb Genom. 2018;4(7):e000197. doi: 10.1099/mgen.0.000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhanel GG, Lawson CD, Adam H, Schweizer F, Zelenitsky S, Lagacé-Wiens PRS, et al. Ceftazidime-avibactam: a novel cephalosporin/β-lactamase inhibitor combination. Drugs. 2013;73(2):159–177. doi: 10.1007/s40265-013-0013-7. [DOI] [PubMed] [Google Scholar]
- Rossi B, Soubirou JF, Chau F, Massias L, Dion S, Lepeule R, et al. Cefotaxime and Amoxicillin-Clavulanate Synergism against Extended Spectrum-β-Lactamase-Producing Escherichia coli in a Murine Model of Urinary Tract Infection. Antimicrob Agents Chemother. 2016;60(1):424–430. doi: 10.1128/AAC.02018-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirano G, Pitout JDD. Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae: Update on Molecular Epidemiology and Treatment Options. Drugs. 2019;79(14):1529–1541. doi: 10.1007/s40265-019-01180-3. [DOI] [PubMed] [Google Scholar]
- Gauzit R, Pean Y, Alfandari S, Bru JP, Bedos JP, Rabaud C, et al. Carbapenem use in French hospitals: A nationwide survey at the patient level. Int J Antimicrob Agents. 2015;46(6):707–712. doi: 10.1016/j.ijantimicag.2015.08.013. [DOI] [PubMed] [Google Scholar]
- Shaikh N, Mattoo TK, Keren R, Ivanova A, Cui G, Moxey-Mims M, et al. Early antibiotic treatment for pediatric febrile urinary tract infection and renal scarring. JAMA Pediatr. 2016;170(9):848–854. doi: 10.1001/jamapediatrics.2016.1181. [DOI] [PubMed] [Google Scholar]
- Zykov IN, Sundsfjord A, Småbrekke L, Samuelsen O. The antimicrobial activity of mecillinam, nitrofurantoin, temocillin and fosfomycin and comparative analysis of resistance patterns in a nationwide collection of ESBL-producing Escherichia coli in Norway 2010–2011. Infect Dis. 2016;48(2):99–107. doi: 10.3109/23744235.2015.1087648. [DOI] [PubMed] [Google Scholar]


