1 Introduction
Genetic modification has not only been applied in bacteria but also in animals. Transgenic mice, sheep, dogs, zebra fish, flies and silkworms have appeared in laboratories, as have transgenic mosquitoes. Anopheline mosquitoes that transmit malaria have been targeted for the insertion of transgenes to reduce their ability to spread disease [1]. Some laboratories, including ours, have succeeded in producing transgenic mosquitoes with lower levels of malaria parasites in the digestive tract after blood meals on malaria -infected animals [2,3]. The goal of such attempts is to control disease transmission through genetic modification of the mosquitoes [4].
As part of our Grand Challenge Exploration grant (Round 1; 2008) we considered producing a pathogen protein in the mosquito salivary glands through insertion of a novel gene into the mosquito genome.
2 The idea
We aimed to put a gene encoding a pathogen protein into a mosquito chromosome, causing it to express the protein in its saliva and inject it into animals or humans during the blood-feeding process. As a result, we anticipated that the host would develop antibodies to the recombinant protein. If transgenic mosquitoes whose saliva contained a vaccine protein against a disease are released in an area where the disease is prevalent, people that are bitten daily by these mosquitoes would develop antibodies to the vaccine protein, and ultimately the entire community would be ‘vaccinated’ against the disease. In this situation, mosquitoes would play the role of vaccine deliverers [5].
We previously discovered a platelet inhibitor in the salivary gland (SG) of the mosquito Anopheles stephensi, cloned its gene, and named the molecule “Anopheline AntiPlatelet Protein” (AAPP) [6]. AAPP is sex-specific and only expressed in the female salivary glands. We used an upstream region of the AAPP gene (aapp) as a promoter for a vaccine protein (Fig. 1).
Figure 1.
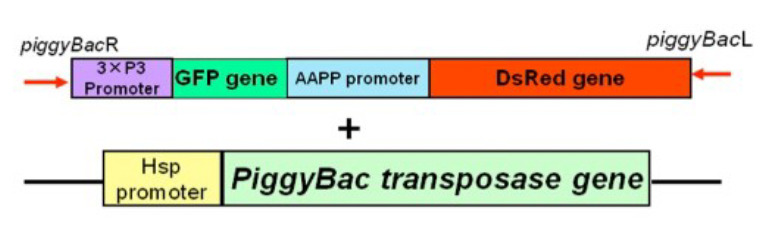
DNA construct injected into mosquito eggs. 3xP3: A protein expressed in the eyes; GFP: green fluorescent protein as an eye marker; AAPP: Anopheline Anti-Platelet Protein [6]; DsRed: Saliva marker; HSP: Heat Shock Protein.
3 Results
We succeeded to produce transgenic mosquitoes that not only express DsRed (Fig. 2a) but also secreting it in their saliva. The recombinant protein (DsRed) was secreted in the lumen of the salivary gland (Fig. 2b). The recombinant protein (DsRed) was exuded from the mosquito’s proboscis (Fig. 3).
Figure 2.

A: GFP and DsRed expression in the abdomen and salivary glands in the thorax of Anopheles stephensi, respectively. B: Expression of GFP in salivary gland cells (left), of DsRed in the lumen of the salivary gland (middle), and a combined image (right).
Figure 3.
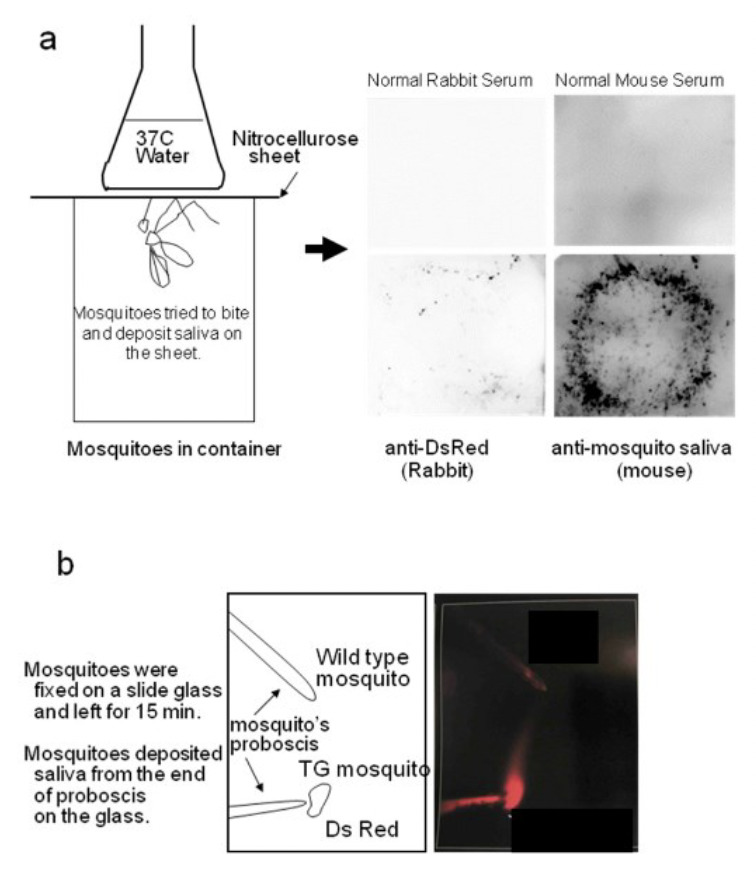
DsRed secretion in the saliva of transgenic mosquitoes.
After repeated biting on mice, the mice developed anti-DsRed and anti-saliva antibodies (Fig. 4). Furthermore, once we injected DsRed into a mouse to prepare memory B cells and exposed the mouse to transgenic (DsRed) mosquito bites, the mouse produced a higher titer of antibodies to DsRed (Fig. 5). Thus the transgenic mosquito bites acted as a booster.
Figure 4.
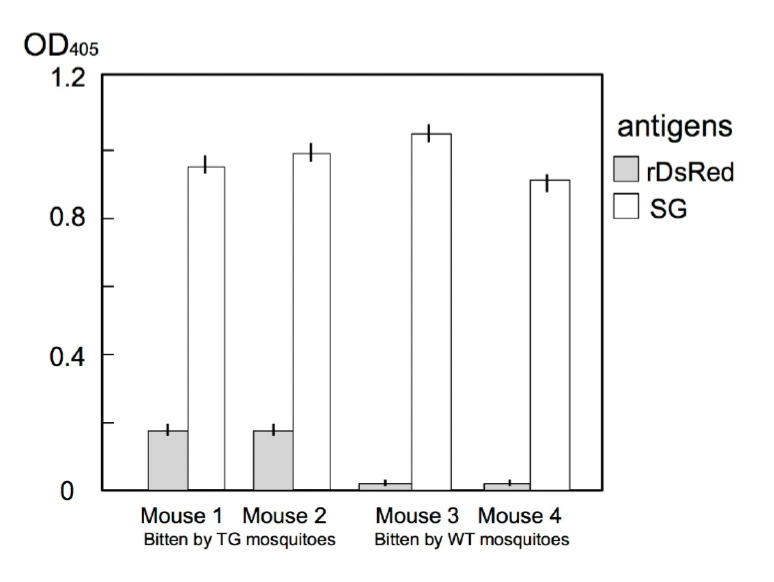
Development of anti-DsRed and anti-saliva (SG) antibodies following bites from DsRed-transgenic mosquitoes. Mouse 1 and 2 were each bitten by 50 DsRed-transgenic mosquitoes five times over a 2-week interval. In both mice anti-DsRed antibody production was induced. Mouse 3 and 4 were each bitten five times by 50 wild-type mosquitoes.
Figure 5.
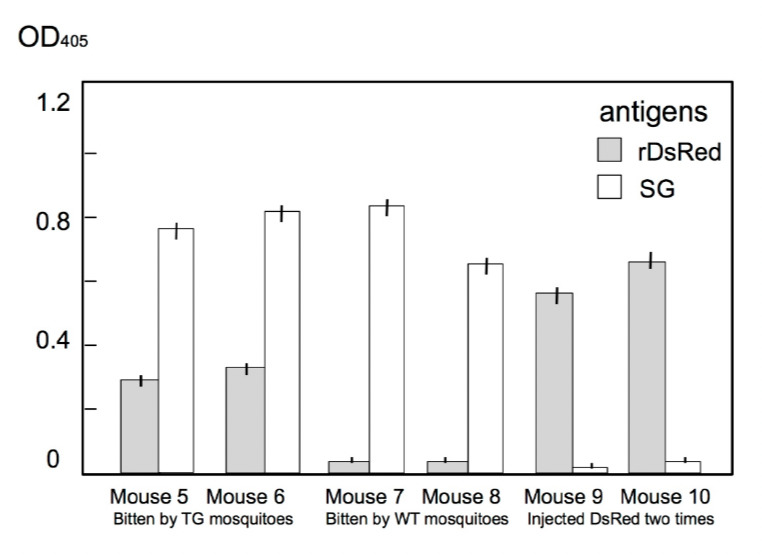
Development of anti-DsRed and anti-saliva (SG) antibodies after DsRed injection or DsRed-transgenic mosquito (TG) bites. All mice were first injected with 1 μg of DsRed. Subsequently, mouse 5 and 6 were each bitten by 50 DsRed-TG mosquitoes five times over a 2-week interval and produced high levels of anti-DsRed antibodies. Mouse 7 and 8 were each bitten five times by 50 wild-type mosquitoes. Following the first injection, mouse 9 and 10 were injected again with 1 μg of DsRed.
4 Discussion
Mosquitoes deposit saliva in the skin when they take a blood meal that induces a variety of physiological responses in the host. Repeated biting leads to the development of anti-saliva antibodies. In areas where malaria is hyper-endemic, more than one hundred anopheline mosquitoes can bite a person in a single hour during the night [7]. In these areas, therefore, most people have high levels of anti-saliva antibodies [8,9]. If we could produce transgenic mosquitoes expressing a vaccine protein in saliva and release millions of these mosquitoes into the field, people would be supplied with the protein when they were bitten, produce antibodies to the protein, and develop immunity to the disease.
We employed a coloured protein, DsRed, so as to observe the location of the alien protein under a fluorescence microscope. As expected, we observed that the transgenic mosquitoes excreted DsRed in the cavity of the salivary glands (Fig. 2). Moreover, these mosquitoes discharged DsRed via their proboscis (Fig. 3). The mice bitten by DsRed-TG mosquitoes produced anti-DsRed antibodies as expected (Fig. 4), however, the titer was not as high as that of the anti-saliva antibody. In the next experiment, we injected 1 μg of DsRed intra-peritoneally once and allowed the mice to be fed upon by DsRed-transgenic mosquitoes. After several rounds of biting, the mice developed a higher concentration of anti-DsRed antibody than did the mice only bitten by DsRed-TG mosquitoes (Fig. 5). Memory B cells might be produced in the mice in response to the first injection of DsRed. The DsRed-transgenic mosquito biting might then act as a booster. This suggests that the primary immunization of infants with a malarial vaccine protein followed by exposure to transgenic mosquitoes excreting the malarial vaccine protein in saliva could produce a synergistic effect.
5 Future perspectives
In our recent publication [10], we exposed mice 20 times to 100 transgenic mosquitoes. Over four months and about 1500 bites were needed to induce sufficiently high antibody titers to inhibit malaria parasite invasion of hepatocytes [10]. However, pre-immunizing methods may reduce the overall number of mosquito bites needed. If one pre-immunization was made in a mouse, five exposures to 50 transgenic mosquitoes might be sufficient to induce similar titers of anti-Plasmodium antibodies in the mouse. Further experiments are needed to confirm this possibility.
Grant recipient publications directly related to this GCE project
Matsuoka H, Ikezawa T, Hirai M: Production of a transgenic mosquito expressing circumsporozoite protein, a malarial protein, in the salivary gland of Anopheles stephensi (Diptera: Culicidae). Acta Med. Okayama 2010, 64:233-241.
Matsuoka H, Sano G, Hattori R, Tomita H, et al.: One injection of DsRed followed by bites from transgenic mosquitoes producing DsRed in the saliva elicits a high titer of antibody in mice. Trop. Med. Health 2012, 40:47-53.
Sumitani M, Kasashima K, Yamamoto D, Yagi K et al.: Reduction of malaria transmission by transgenic mosquitoes expressing an anti-sporozoite antibody in their salivary glands. Insect Mol. Biol. 2013, 22:in press.
Yamamoto DS, Nagumo H, Yoshida S: Flying vaccinator; a transgenic mosquito delivers a Leishmania vaccine via blood feeding. Insect Mol. Biol. 2010, 19:391-398.
Yamamoto DS, Sumitani M, Nagumo H, Yoshida S et al.: Induction of anti-sporozoite antibodies by biting of transgenic Anopheles stephensi delivering malarial antigen via blood feeding. Insect Mol. Biol. 2012, 21:223-233.
Yoshida S, Shimada Y, Kondoh D, Kouzuma Y et al.: Hemolytic C-type lectin CEL-III from sea cucumber expressed in transgenic mosquitoes impairs malaria parasite development. PLoS Pathog. 2007, 3:e192.
Yoshida S, Sudo T, Niimi M, Tao L et al.: Inhibition of collagen-induced platelet aggregation by anopheline antiplatelet protein, a saliva protein from a malaria vector mosquito. Blood 2008, 111:2007-2014.
References
- 1.Alphey L, Beard CB, Billingsley P, Coetzee M et al. Malaria control with genetically manipulated insect vectors. Science. 2002;298:119–121. doi: 10.1126/science.1078278. [DOI] [PubMed] [Google Scholar]
- 2.Ito J, Ghosh A, Moreira LA, Wimmer EA et al. Transgenic anopheline mosquitoes impaired in transmission of a malaria parasite. Nature. 2002;417:452–455. doi: 10.1038/417452a. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida S, Shimada Y, Kondoh D, Kouzuma Y et al. Hemolytic C-type lectin CEL-III from sea cucumber expressed in transgenic mosquitoes impairs malaria parasite development. PLoS Pathog. 2007;3:e192. doi: 10.1371/journal.ppat.0030192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crampton JM, Warren A, Lycett GJ, Hughes MA et al. Genetic manipulation of insect vectors as a strategy for the control of vector-borne disease. Ann. Trop. Med. Parasitol. 1994;88:3–12. doi: 10.1080/00034983.1994.11812828. [DOI] [PubMed] [Google Scholar]
- 5.Crampton JM, Stowell SL, Karras M, Sinden RE. Model systems to evaluate the use of transgenic haematophagous insects to deliver protective vaccines. Parasitologia. 1999;41:473–477. [PubMed] [Google Scholar]
- 6.Yoshida S, Sudo T, Niimi M, Tao L et al. Inhibition of collagen-induced platelet aggregation by anopheline antiplatelet protein, a saliva protein from a malaria vector mosquito. Blood. 2008;111:2007–2014. doi: 10.1182/blood-2007-06-097824. [DOI] [PubMed] [Google Scholar]
- 7.Harada M, Ikeshoji T, Suguri S: Ishii A, Nihei N, Sasa M . Tokyo:: Inter Group Corporation,; 1998. Studies on vector control by ‘Mosquito Candle’. pp. 120–125. In: Malaria Research in the Solomon Islands ( , Eds). [Google Scholar]
- 8.Waitayakul A, Somsri S, Sattabongkot J, Looareesuwan S et al. Natural human humoral response to salivary gland proteins of Anopheles mosquitoes in Thailand. Acta Trop. 2006;98:66–73. doi: 10.1016/j.actatropica.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Andrade BB, Rocha BC, Reis-Filho A, Camargo LM et al. Anti-Anopheles darlingi saliva antibodies as marker of Plasmodium vivax infection and clinical immunity in the Brazilian Amazon. Malar. J. 2009;8:121. doi: 10.1186/1475-2875-8-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamamoto DS, Sumitani M, Nagumo H, Yoshida S et al. Induction of anti-sporozoite antibodies by biting of transgenic Anopheles stephensi delivering malarial antigen via blood feeding. Insect Mol. Biol. 2012;21:223–233. doi: 10.1111/j.1365-2583.2011.01128.x. [DOI] [PubMed] [Google Scholar]


