Abstract
Background
Malaria transmission is heterogeneous. Villages close to each other may have very different transmission characteristics. The presence and abundance of malaria vectors is governed by local ecology and microclimate. Knowledge of the dynamics of transmission is important for planning and evaluation of malaria control strategies. This study investigated the heterogeneity of malaria transmission in preparation for a vaccine trial and offers insights into dynamics of malaria incidence in the forest zone of Ghana.
Methods
Malaria transmission was assessed in four villages with different micro-ecological features in the forest zone of the Akwapim-Mampong Range in Ghana, water shed with rivers flowing north to Lake Volta in the south. Human landing catches (HLC) of mosquitoes were conducted and Plasmodium falciparum circumsporozoite rates were assessed by ELISA. Sporozoite prevalence, annual biting rates (ABR) and entomological inoculation rates (EIR) from the four study sites were compared with climatological and ecological data. Regression analysis was used to compare transmission data and blood parasite prevalence, parasite density (PD) and malaria episodes from children in the study area. Additionally we examined trends in confirmed clinical malaria incidence from 2005 -2012.
Results
In total 1307 Anopheles gambiae s.l. and 54 An. funestus females were caught by HLC from November 2003 to August 2005. Sporozoites in Anopheles vectors in four villages ranged from 4.0 to 10.2%, ABR from 371 to 1890 and EIR from 40 to 158. Linear regression on parasitological and clinical data of children from the villages revealed that the ABR significantly influenced the parasite density (PD) of P. falciparum.
Conclusion
Malaria transmission was intense and heterogeneous and corresponded to the micro-ecological differences. Malaria transmission in the early evening hours before people went to sleep was enough to sustain stable malaria. Scaling up preventive measures to reduce exposure to vectors will be effective in reducing parasitemia in children. Variations in transmission intensity must be considered when evaluating impact of control strategies and interventions such as the vaccine trials.
1 Introduction
Malaria is an important cause of global morbidity and mortality, particularly in sub-Saharan Africa where about 90% of the estimated 219 million cases result in over 600,000 deaths annually [1]. The risk of malaria transmission varies markedly across the continent and within countries. Malaria vector distribution, transmission intensity and disease burden can vary over short distances, between neighbouring villages and even within a single settlement [2-4]. Some people receive more infectious bites, whilst others are more susceptible to the disease [5]. This has a large effect on the relationship between the risk factors and the prevalence of malaria in the community. Risk factors for malaria transmission include population associated risk factors such as climatic variability, urban agriculture, proximity to mosquito breeding habitats [6], and urbanization [7]. Other factors influence individuals such as household construction methods (e.g. open eaves) that result in increased exposure to mosquito bites [8], besides individual variation in attractiveness to mosquitoes [9].
Variations in malaria transmission intensities are known to mirror the nature of their ecological zones [10]. For example, in Ghana, the highest transmission is reported in the forest zones, where the annual entomological inoculation rate (AEIR) can be as high as 866 infectious bites per person per year [11]. An AEIR of 418 infective bites has been reported for the northern savannah zone, with 228, 360 and 630 annual infectious bites being reported for the rocky highlands, lowlands and irrigated areas, respectively, reflecting differences in the micro-ecological settings within the northern savannah zone [12]. The forest -savannah transitional ecological zone reports a moderately high AEIR of 269 [13], whilst coastal forest and coastal savannah areas have relatively low AEIRs of 21.9 and 3.7, respectively [14]. The main vectors in the forest zones are Anopheles gambiae and An. funestus [11,13]. Data on the distribution of An. gambiae s.l. in Ghana indicate that An. gambiae s.s. is the only member of the complex in the forest zone with An. arabiensis and An. melas in the northern savannah and coastal savannah zones, respectively [15]. Plasmodium falciparum is the predominant malaria parasite, followed by P. ovale in the forest and P. malariae in the savannah with overall malaria prevalence around 50% [16].
The entomological inoculation rate (EIR) is the most direct way of assessing human exposure to infectious mosquito bites at individual level. It is expressed as the number of infectious bites a person receives per unit time [17]. This study reports the dynamics of malaria transmission intensities from various communities as well as the dynamics of mean monthly confirmed clinical malaria incidence from January 2005 to December 2012. It is believed that this knowledge will be important for the interpretation of results of the ongoing malaria vaccine trial conducted since 2006 [18] and evaluation of control measures, such as the distribution of insecticide-treated bednets to pregnant women provided by the Ghana Health Service.
2 Materials and Methods
2.1 Study area
Four villages in the forest zone of the Ashanti Region of Ghana were selected: Abotanso, in the Sekyere East District (SED), Gyidim and Hwidiem, both belonging to the greater Agogo area, and Low Cost a suburb of Konongo, are located in the Ashanti Akim North Municipal District (AAN) (Fig. 1). Results of a cohort study on prospective clinical malaria episodes in 2002 carried out in the four villages were used for epidemiological comparisons [19].
Figure 1.
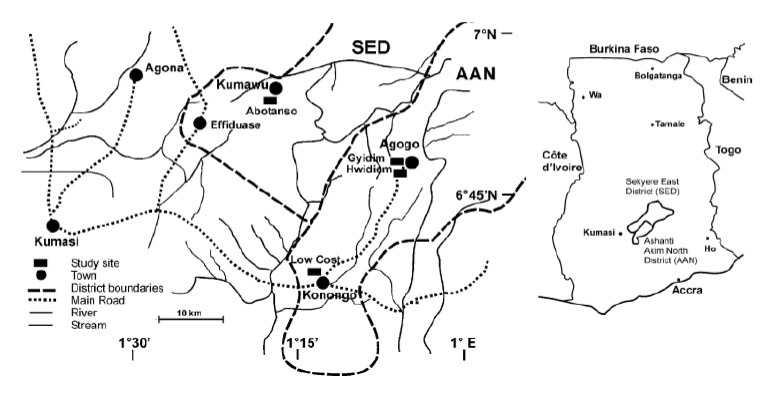
Map of study area in Ghana showing locations of the study sites in Sekyere East District (SED) and Ashanti Akim North District (AAN) and map of Ghana with inset of the two districts.
The Sekyere East District (SED), with an estimated population of 157,396 lies in the North-Eastern part of the Ashanti Region. The district lies between 6°45’ - 7°32’ N and 0°22 W, and covers an area of 4231 km2. The southern part of the district is covered with moist semi-deciduous forest; the northern part with guinea savannah of deciduous fire resistant trees [20].
Abotanso is a rural village in the SED near Kumawu located on a mountain ridge (6°53’N - 1°17’W) at an altitude of 435 m, with mud houses roofed with thatch or corrugated iron sheets. Water is collected from two springs emanating from rock formations at the base of the valley. Farmers produce tomatoes and cocoa for the market and plantain, cocoyam and cassava for subsistence. Some farmers raise goats, sheep and poultry. Abotanso is about 20km from Gyidim which lies within the Ashanti Akim North District.
The Ashanti Akim North Municipal District (AAN) in the eastern part of Ashanti Region (6°30’ - 7°30’N, 0°15’ - 1° 20’W) shares boundaries with Sekyere East to the west and north and covers an area of 1,160 km2 of generally undulating country (305 to 762 m) interrupted by the Akwapim-Mampong Range (610 to 762 m). The estimated population is 142,434.
Gyidim (06°48’N, 01°05’W) is a rural suburb of Agogo. Most of its population belongs to the Saviour Mission Church group. Farming is the main activity with large banana and plantain plantations along the Kwarire River, which transcends the edge of the village. Agogo, with a population of 28,271, is an important market place for agricultural goods. From west to east it slopes from 442 m to 390 m. The majority of houses in the Gyidim area are mud covered with cement coating with corrugated iron sheets. The Agogo Presbyterian Hospital where data based on monthly confirmed clinical malaria cases was obtained is located in the Agogo township. This is the biggest hospital in the Greater Agogo area with a catchment population of 64,858 (based on 2010 population census data).
Hwidiem (06°46’N, 01°06’W) is a village located on a mountain slope (445-489 m), only 2 km southwest of Gyidim and is surrounded by shrubs and trees. Most houses are made of bricks and are roofed with corrugated iron sheets. Window screens were often found damaged or not in place. The people are mainly farmers. Rainwater drains away quickly through crevices in the soil causing considerable erosion in the village.
Low Cost (06°38’N, 01°33W, altitude 244 m) is a rapidly developing peri-urban suburb of the district capital Konongo. The houses are made of bricks and have generally well screened windows. Inhabitants are engaged in small-scale vegetable farming, trading, and constructional trade. It is about 20km from Agogo (Gyidim). The river Owire transcends the edge of the town.
2.2 Meteorological data
Rainfall data for Agogo (Hwidiem, Gyidim), Konongo (Low Cost) and Kumawu (Abotanso) were provided by the Ghana Meteorological Agency, Kumasi, for September 2003 to December 2005 (Fig. 2). The major rainy season starts in April and ends in July, the minor rainy season begins in September and ends in early November. There was no month without rainfall. Rainfall distribution is not the same in the districts; it is heavier in the southern than in the northern parts.
Figure 2.
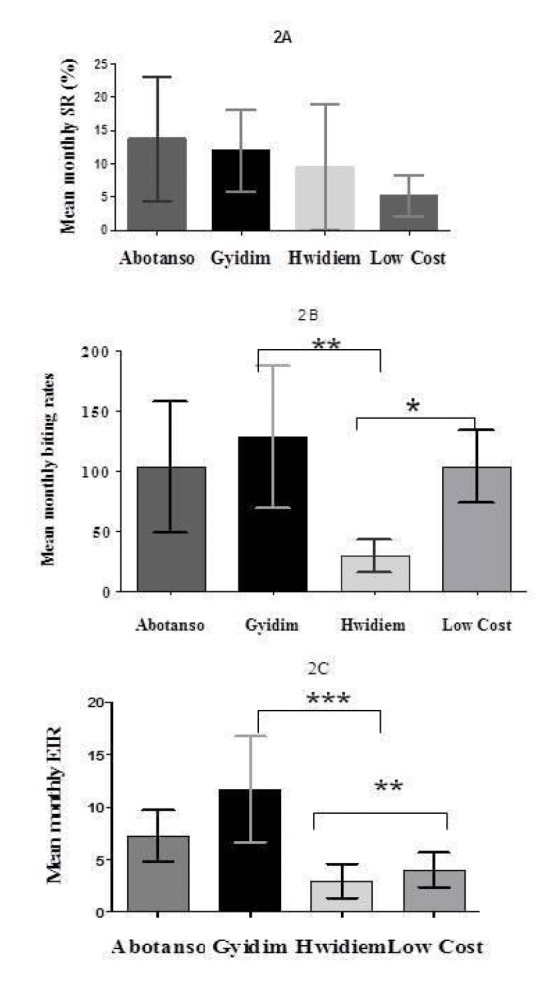
Mean monthly sporozoite rates (SR), biting rates and entomological inoculation rates (EIR) observed at Abotanso, Gyidim, Hwidiem and Low Cost. The error bars indicate 95% CI. Significant differences were investigated by HSD Tukey’s test (*:p<0.05; **: p<0.001).
2.3 Mosquito sampling and identification
Adult female Anopheles were collected twice a month from November 2003 to September 2005 in Gyidim, Hwidiem and Low Cost and from September 2004 to September 2005 in Abotanso. Collections were made by human landing catches (HLC) with volunteers stationed indoor and outdoor from 18:00-06:00 hrs following the standard procedure of WHO [21]. Three collection sites were used per village and two collectors were stationed per site, one indoor and one outdoor. Meetings were held with the assembly men and the unit committee members of the villages who assisted in explaining the goals of the study. The volunteers for the HLC (adult males) gave their verbal consent before participating. Malaria prophylaxis was given, and treatment (at no cost to the volunteers) was arranged with the local hospital but none became sick during the study period. The collected mosquitoes were kept in cold boxes and transported to the laboratory for identification. Anopheles mosquitoes were identified morphologically using the keys of Gillies and Coetzee [22]. Before dissection of mosquitoes, legs and wings were removed for DNA extraction [23] and species identification by PCR [24].
2.4 Sporozoite and parity rates
Anopheles females were dissected and ovaries examined for parity by inspection of the ovarian tracheoles [25]. Head and thorax were tested for the presence of P. falciparum circumsporozoite protein (CSP) using ELISA [26]. Laboratory-reared Anopheles females served as negative controls. The sporozoite rate was calculated by dividing the number of positive mosquitoes by the total number of mosquito tested.
2.5 Human biting and entomological inoculation rates
The human biting rate is the number of vectors biting an individual over a fixed period of time. The EIR is the product of the human biting rate and the sporozoite rate. The annual EIR (AEIR) is the sporozoite rate multiplied by the mean number of female Anopheles mosquitoes caught per person per night multiplied by 365 [17].
2.6 Clinical investigations for a cohort of children
In 2002, a prospective study including 250 children from the four study villages was carried out. During 31 weekly visits the children were characterised for their prevalence of blood trophozoites of P. falciparum (PP), their blood parasite density measured as 75% percentile (PD), and their prevalence of malaria episodes (ME), defined by fever and/or reported fever and a positive blood smear [27].
2.7 Annual malaria incidence
We collected annual malaria incidence data from the Agogo hospital, where data on the number of confirmed cases out of total tests performed monthly was available. The hospital began this system of reporting only from January 2005 and thus all data from 2005 up until 2012 was obtained for analysis. This data was obtained in order to ascertain whether malaria incidence has changed over the past eight years.
2.8 Statistical analysis
Differences between proportions of mosquitoes caught indoors and outdoors were analysed with Chi2 distribution, with a significance threshold of p<0.05. Analysis of variance was used to test if monthly biting rates, sporozoite rates and the EIRs were different between the four study sites, and the Student t-test to test the differences between mosquito numbers caught indoors and outdoors. A post-hoc analysis of ANOVA was performed using HSD Tukey’s multiple comparison tests to evaluate differences in study sites. The data were analysed and graphed using GraphPad Prism software (San Diego, CA, USA). The analysis of the influence of village-specific sporozoite prevalence in mosquitoes, HBR, EIR on PP, PD and ME of the children was assessed by linear regression (JMP 5.0 software, SAS, Cary NC, USA). Reported significance levels were corrected for 9-fold testing. Monthly malaria incidence per 1000 of the population was calculated by dividing the total number of confirmed malaria cases per catchment population (with yearly population adjustment) multiplied by 1000. ANOVA was used to examine differences in mean monthly malaria incidence. Trend analysis was done using linear regression (Ordinary Least Squares) to fit malaria incidence data to the equation y = mx + C to examine if there was decreasing or increasing trend in malaria incidence during the period 2005-2012.
2.9 Ethical clearance
Ethical approval was obtained from the Committee for Research, Publications and Ethics of the School of Medical Sciences, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana.
3 Results
A total of 1307 An. gambiae s.l. and 54 An. funestus females were collected during 145 full-night catches in the four study sites. Most of the Anopheles were identified as An. gambiae s.l.; only 2%, 3.1%, 4.4% and 5.3% of the anophelines caught at Gyidim, Hwidiem, Low Cost and Abotanso were An. funestus, respectively (Table 1). Out of 300 randomly selected An. gambiae s.l. 297 were identified as An. gambiae s.s. by PCR. DNA of three specimens failed to amplify.
Table 1.
Total number of Anopheles caught per site, parity rates, sporozoite prevalence, annual biting rates (ABR) and annual entomological inoculation rates (AEIR).
| Site | Species; Total # caught | No (%) Parous | Sporozoite prevalence (%) | ABR (both species) | AEIR (both species) |
|---|---|---|---|---|---|
| Abotanso | An. gambiae; 251 | 226 (90) | 15/226 (6.6) | 1285 | 93 |
| An. funestus; 20 | 14 (70) | 2/14 (14.3) | |||
| Gyidim | An. gambiae; 495 | 440 (89) | 39/440 (8.9) | 1890 | 158 |
| An. funestus; 10 | 3 (30) | 0 | |||
| Hwidiem | An. gambiae; 126 | 118 (94) | 12/118 (10.2) | 371 | 40 |
| An. funestus; 4 | 3 (75) | 0 | |||
| Low Cost | An. gambiae; 435 | 402 (92) | 16/402 (4.0) | 1258 | 54 |
| An. funestus; 20 | 12 (60) | 0 | |||
| Total | 1361 | 1187 (87.2) | 84/1200 (7.0) | ||
3.1 Parity, sporozoite, biting rates, and EIR
Parity rates of An. gambiae were generally high and ranged from 89% in Gyidim to 94 % in Hwidiem (Table 1). The overall sporozoite rates per village ranged from 4.0% in Low Cost to 10.2% in Hwidiem, but there were no statistically significant differences between their respective mean monthly rates (F=1.115, df = 3, p>0.05) (Fig. 2A, 2B, 2C).
The Annual Biting Rate (ABR) was lowest in Hwidiem (371), highest in Gyidim (1890) with Abotanso and Low Cost being intermediary (Table 1). They were statistically different from each other (F=5.504, df= 3, p<0.01). Post-hoc HSD Tukey’s comparison test revealed highly significant differences between Gyidim and Hwidiem, (q=5.391, P<0.01) as well as between Low Cost and Hwidiem (q=4.152, P<0.05). No differences were found between Abotanso and the other sites (Fig. 2B). The Anopheles biting rates at the four locations were highest in the rainy season and lowest from November to January, but varied from year to year, though not significantly (Fig. 5).
Figure 5.
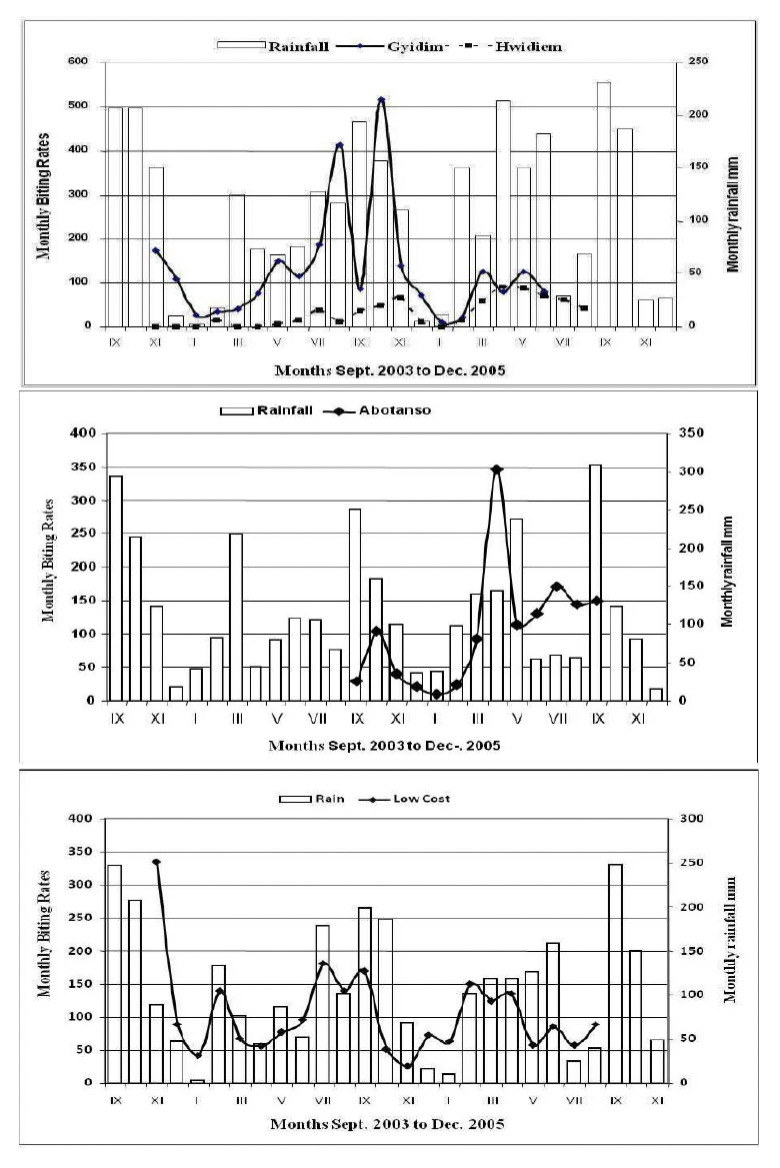
Rainfall patterns and monthly biting rates for Gyidim and Hwidiem. Low Cost and Abotanso.
Comparison of monthly EIRs followed similar trends as biting rates; interestingly Gyidim had the highest monthly EIR whilst the closest community in terms of proximity, Hwidiem, had the lowest EIR. Post-hoc analysis revealed highly significant differences between Gyidim and Hwidiem as well as between Hwidiem and Low Cost (F= 7.799, df= 3, p<0.001) (Fig. 2C). The AEIR was low in Hwidiem (40) and Low Cost (54), higher at Abotanso (93) and highest in Gyidim (158) (Table 1).
3.2 Clinical data
Clinical data revealed the lowest frequency for malaria episodes and P. falciparum parasite prevalence in Low Cost and the highest in Gyidim (Table 2). The results of pair-wise regression analyses of village-specific sporozoite prevalence in mosquito ABR, EIR versus PP, PD and ME of children living in these villages are shown in Table 3. The most significant finding (P= 0.0054) showed a proportion of explained variance (R2) of 0.060 for the regression of ABR versus PD, and a high significance (P = 0.0063) and R2 of 0.045 for the regression of sporozoite rate versus trophozoite prevalence.
Table 2.
Age distribution and frequencies of malaria episodes (ME), P. falciparum blood trophozoite prevalence (PP), and their blood parasite densities (PD), 0.75 quantiles of log parasite counts/μl, from children during a 31-week prospective study on mild malaria (see [19]).
| Village | Number children | Age/years (range) | ME (range) | PP (range) | PD (range) |
|---|---|---|---|---|---|
| Gyidim | 167 | 6 (1-11) | 0.065 (0-0.39) | 0.55 (0-0.94) | 2.87 (0.9-4.2) |
| Hwidiem | 20 | 5.5 (1-11) | 0.048 (0-0.23) | 0.39 (0.03-0.71) | 2.06 (0.6-3.6) |
| Abotanso | 45 | 5 (1-11) | 0.032 (0-0.13) | 0.52 (0-1) | 3.11 (1.6-4.1) |
| Low Cost | 18 | 6 (1-11) | 0.016 (0-0.16) | 0.10 (0-0.68) | 2.38 (1.2-2.8) |
Table 3.
Linear regression of village-specific transmission data (ABR, AEIR and sporozoite rate), versus median of individual frequencies of malaria episodes (ME): P. falciparum blood trophozoite prevalence (PP), and parasite densities (PD) within 31 weeks
| ME | PP | PD | ||
|---|---|---|---|---|
| ABR | R2 | 0.0075 | 0.023 | 0.060 |
| P* | 1 | 0.14 | 0.0054 | |
| Sporozoite rate | R2 | 0.021 | 0.045 | 0.0017 |
| P* | 0.19 | 0.0063 | 1 | |
| AEIR | R2 | 0.017 | 0.042 | 0.048 |
| P* | 0.37 | 0.010 | 0.018 |
R2, explained variance calculated by the square of the regression coefficient R;
*, corrected for 9-fold testing.
3.3 Hourly biting activity
Biting activity of An. gambiae at the four sites began as early as 18:00 hrs and peaked between 23.00 and 01.00 hrs. There were no statistically significant differences for hourly biting activities between Hwidiem, Gyidim and Abotanso (P=0.997), but there was a significant shift at Low Cost where biting activity peaked two hours later (P=0.035) (Fig. 3). Transmission started in the early evening hours from 18:00 to 21:00 hrs, when 10.4% of Anopheles caught were infected. The early evening AEIRs were 2.0 in Hwidiem, 2.5 in Low Cost, 5.0 in Abotanso and 8.0 in Gyidim.
Figure 3.
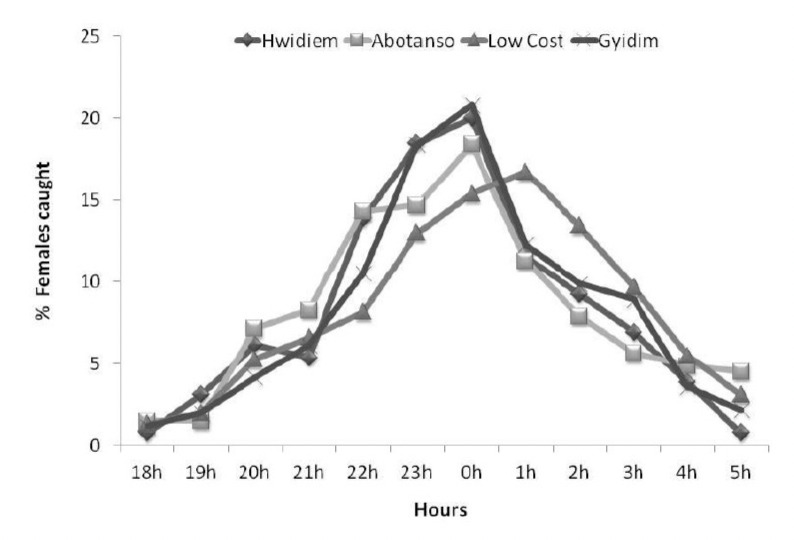
Hourly biting activities of An. gambiae (N=1307) caught at Abotanso, Gyidim, Hwidiem and Low Cost.
There were no significant differences between indoor and outdoor biting activities at all sites (P=0.924) (Fig. 4).
Figure 4.
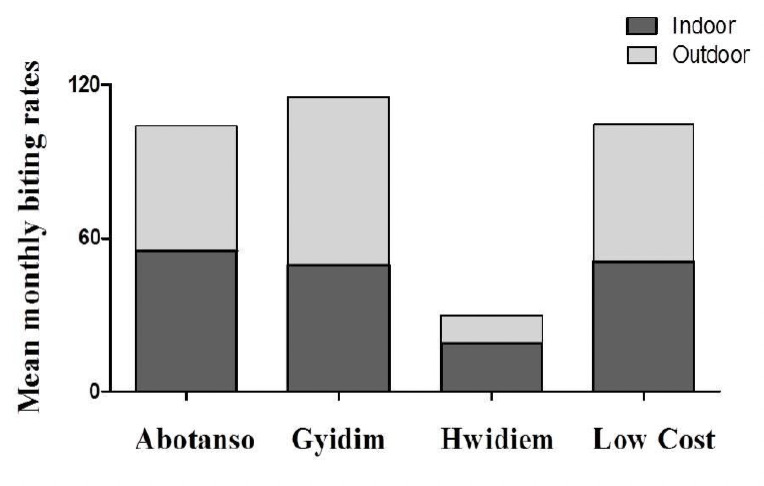
Monthly biting rates (MBR) between indoor and outdoor sites.
3.4 Clinical malaria incidence
No specific seasonal patterns were observed when monthly malaria incidences were compared from 2005 to 2012 (Fig. 6). ANOVA revealed no significant differences between mean monthly clinical malaria incidence from 2005 to 2012 (F=1.95, df=94, p=0.17). Linear regression analysis could not identify significance in terms of decreasing or increasing trends in malaria incidence between 2005 and 2012 (y=-0.0002x+12.63; R2=0.0204, t=-1.40, p=0.17, 95% CI: -210.10-36.50).
Figure 6.
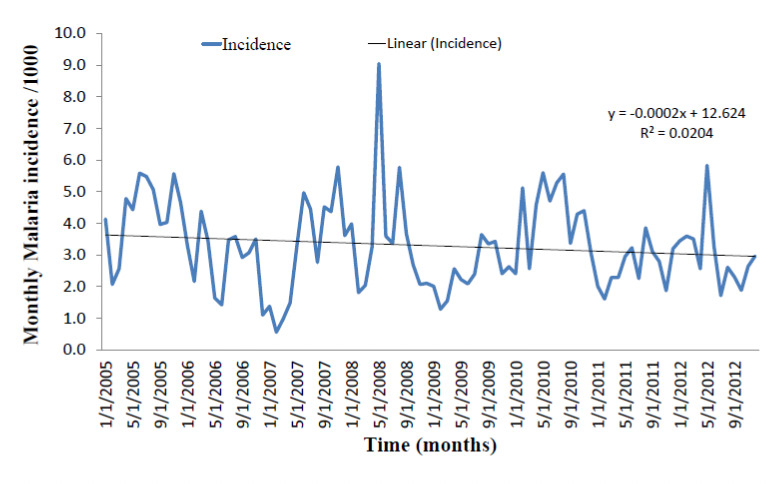
Monthly clinical malaria incidence from 2005-2012 in the Ashanti Akim North District as reported by the Agogo Presbyterian Hospital, Agogo.
4 Discussion
Malaria has a broad range of different epidemiological profiles, depending on the distribution and vectorial capacity of the local anopheline population, environmental conditions, and the degree of protective immunity acquired by the exposed population. Heterogeneity in malaria transmission is known to occur due to varying factors such as distance to larval breeding sites [6], land cover, [28], differences in house design [8], bednet usage [29], and individual differential attractiveness to mosquitoes [9]. Anopheles gambiae s.s. was identified as the main vector in all sites and is the only member of the complex so far reported in the forest zone of Ghana [11,15]. Anopheles funestus is regarded as a secondary vector [11,13]. Malaria transmission in the mountainous area of the Ashanti Region (AEIR 40 –158) was intense and highly heterogeneous in spite of the proximity of the villages to each other. Infectious mosquitoes were caught throughout the year, except during the dry months of December and January at Hwidiem.
Transmission of P. falciparum varied on a micro-geographical scale in Abotanso/Gyidim and Hwidiem/Low Cost. Abotanso has spring water emanating from rock formations creating breeding habitats for mosquitoes throughout the year, leading to perennial transmission. Gyidim, a rural village in a valley close to a small stream, had the highest AEIR observed. Krefis et al. [28] associated the high incidence of malaria infection with the proximity of Gyidim to extensive plantain and banana plantations as a risk factor. This corroborates with an observation by Anosike et. al. [30] from the rainforest of south-eastern Nigeria that leaf axils of plantain and banana were a micro-habitat for breeding An. gambiae and a risk factor for malaria. The plantain and banana farms in the vicinity of the village may therefore explain the higher risk of malaria in Gyidim. Hwidiem in contrast, although in close proximity to Gyidim, is located on a mountain slope where rainwater does not collect and does not create Anopheles breeding sites which explains the low mosquito density and malaria transmission. Low Cost, a rapidly expanding suburb of Konongo with modern brick houses, screened windows, better personal protection against mosquito bites and access to antimalarial drugs, recorded the lowest sporozoite rate (4.0%) contrasting with a high mosquito abundance due to the vicinity of lowland swampy areas and house construction activities with open pits and ditches. The relationship between rainfall and mosquito biting densities and transmission parameters (Fig. 5) were similar to those described from the Agona area [11]. Krefis et. al. [31] reported a high association between rainfall and malaria incidence at the village clusters of Agogo and Konongo. Rainfall preceded higher incidence of malaria by a time lag of around 9 weeks. Few An. funestus were collected, and infected ones only in Abotanso. In contrast, in the low-land forest area of Agona, the higher transmission in one of two neighbouring villages could be attributed to the abundance of the secondary vector An. funestus [11].
The hourly biting activities with peaks around midnight and declining towards dawn are typical for An. gambiae and have been described in several areas [32, 33]. In contrast, Appawu et al. [12] observed that night biting activities of vectors in northern Ghana peaked at day break (04:00-06:00 hrs). The significant shift of the peak of transmission by two hours in Low Cost (Fig. 3) occurred probably as a result of the expanding peri-urban environment.
Transmission in the early evening hours (18:00-21:00 hrs) may be a serious problem, specifically for children when not protected [12,34,35]. In our study, the number of infectious bites individuals received in the early evening before bedtime (AEIR 2-7.8) was lower than that observed in the low land forest area (AEIR 39 and 57) [11], but was still sufficient to maintain a stable level of malaria. Similarly, studies from Tanzania reported infectious bites during hours when people are unlikely to be sleeping under insecticide-treated bednets; 12% of all infectious bites occurred between 19:00–22:00 hrs and 05:00-06:00 hrs, which translated into 0.5 infectious bites per person per night [35]. It was also observed that An. gambiae s.s. did not have a strong endophagic behaviour and was readily biting outside. This plasticity in biting behaviour has also been reported from coastal Ghana [36]. Thus it is important for any malaria control programme to take into account the biting behaviour of local malaria vectors before choosing appropriate interventions that will be most effective.
The comparison of ABR, AEIR, and sporozoite rates from 2003 to 2005 against clinical data from a prospective study on mild malaria in children (Table 3) demonstrated a direct influence of transmission intensity on parasite prevalence and density but there was no significant correlation between transmission intensity (EIR) and the frequency of clinical malaria episodes in the children. It should be noted that the human biting rate (a key component of the EIR) depends on the individual’s body mass [37]; the volunteers used in this study were male adults and thus may resulted in an overestimation of the challenge faced by children. It is known that the relationship between EIR and disease outcome is not directly proportional; the outcome of a given infectious bite could be anything from a mild discomfort to death [38,39]. Disease outcome among other factors depends on the immune status, age [38], and genetic polymorphism within human hosts [40].
Fundamental to the development of sound malaria control programmes is a basic understanding of the relationships between malaria transmission by vector populations and malaria outcomes. Beier et. al. [41] observed that sites with AEIRs of 5, 15 and 200 infectious bites had levels of P. falciparum prevalence exceeding 40%, 50% and 80%, respectively. The ideal situation would be to compare our data with clinical malaria data obtained in the same year but this was not available. When comparing our data with a prospective clinical malaria study among a cohort of children over a 31-week period from the same study sites in the previous year, we observed a significant linear relationship between ABR and parasite density among the children. Additionally a significant correlation between the sporozoite infection rate in the mosquito population and parasite prevalence among the children in the same study sites was observed. Beier et al. [38] reported from western Kenya that annual biting rates alone accounted for 68% of the variation in malaria attack rates in children. Measurements of either the EIR or the human-biting rate can thus be used to predict corresponding attack rates in children.
The malaria burden in Ghana is largely perennial with marked seasonal fluctuations restricted to the northern savannah zones (PMI Ghana report 2013). Additionally, data available from 2005-2012 showed no specific seasonal patterns and no significant increasing or decreasing trend in monthly malaria incidence. It is therefore plausible to propose that malaria episodes obtained by the active case surveillance among children in the study community represent a longer term transmission potential as a result of cumulative exposure to the local malaria vectors, the distribution of which is driven by micro-ecological differences rather than monthly climatic variation. The entomological study therefore provides useful data that relates to the variation in clinical malaria in the same study sites.
5 Conclusions
Malaria transmission in the mountainous rainforest area was intense but highly heterogeneous corresponding with micro-ecological differences. The early evening transmission between 18:00-21:00 hrs is sufficient to maintain stable malaria. AEIR proved to be a good tool to explain malaria exposure and parasite densities within a population. The variation in transmission intensities observed will be informative in the evaluation of vaccine efficacy and the distribution of insecticide-treated bednets that are currently ongoing in the study area.
6 Acknowledgments
We are indebted to the mosquito collectors and the people of Gyidim, Hwidiem, Abotanso and Low Cost for continuous cooperation. The support of staff of the Kumasi Centre for Collaborative Research in Tropical Medicine is appreciated. We also acknowledge the support of Mr. Frempong and Dr. T. Rettich both PHS Agogo for hospital based malaria data and Dr Guofa Zhou of the University of California, Irvine, for data analysis. Finally, Dr. Bari Howell is thanked for proof reading of the manuscript. Rolf Garms was supported by the German Senior Experten Service in 2003, 2005 and 2006. The study was partially funded by the German Ministry of Education and Research through the German Human Genome Project (DHGP) and the National Genome Research Project (NGFN) as well as the Bundesministerium für Bildung und Forschung (grant 01KA02)
References
- 1.World Health Organization: World Malaria Report; Geneva, Switzerland,: 2012. [Google Scholar]
- 2.Kreuels B, Kobbe R, Adjei S, Kreuzberg C et al. Spatial variation of malaria incidence in young children from a geographically homogeneous area with high endemicity. J. Infect. Dis. 2008;197:85–93. doi: 10.1086/524066. [DOI] [PubMed] [Google Scholar]
- 3.Mboera LEG, Senkoro P, Mayala BK, Rumisha SF et al. Spatio-temporal variation in malaria transmission intensity in five agro-ecosystems in Mvomero district, Tanzania. Geospat. Health. 2010;4:167–178. doi: 10.4081/gh.2010.198. [DOI] [PubMed] [Google Scholar]
- 4.Greenwood BM: The micro-epidemiology of malaria and its importance to malaria control. Trans. R. Soc. Trop. Med. Hyg. 1989;83:25–29. doi: 10.1016/0035-9203(89)90599-3. [DOI] [PubMed] [Google Scholar]
- 5.Smith DL, Dushoff J, Snow RW, Hay SI: The entomological inoculation rate and Plasmodium falciparum infection in African children. Nature. 2005;438:492–495. doi: 10.1038/nature04024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stoler J, Week JR, Getis A, Hill GA: Distance threshold for the effect of urban agriculture on elevated self-reported malaria prevalence in Accra, Ghana. Am. J. Trop. Med. Hyg. 2009;80:547–554. [PMC free article] [PubMed] [Google Scholar]
- 7.Hay SI, Guerra CA, Tatem AJ, Atkinson PM et al. Urbanization, malaria transmission and disease burden in Africa. Nat. Rev. Microbiol. 2005;3:81–90. doi: 10.1038/nrmicro1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindsay SW, Snow RW: The trouble with eaves - house entry by vectors of malaria. Trans. Roy. Soc. Trop. Med. Hyg. 1988;82:645–646. doi: 10.1016/0035-9203(88)90546-9. [DOI] [PubMed] [Google Scholar]
- 9.Lindsay SW, Adiamah JH, Miller JE, Pleass RJ et al. Variation in attractiveness of human subjects to malaria mosquitoes (Diptera: Culicidae) in The Gambia. J. Med. Entomol. 1993;30:368–373. doi: 10.1093/jmedent/30.2.368. [DOI] [PubMed] [Google Scholar]
- 10.Afari EA, Akanmori BD, Nakano T, Ofori-Adjei D: Plasmodium falciparum: sensitivity to chloroquine in vivo in three ecological zones in Ghana. Trans. R. Soc. Trop. Med. Hyg. 1992;86:231–232. doi: 10.1016/0035-9203(92)90285-k. [DOI] [PubMed] [Google Scholar]
- 11.Abonuusum A, Owusu-Daako K, Tannich E, May J et al. Malaria transmission in two rural communities in the forest zone of Ghana. Parasitol. Res. 2011;108:1465–1471. doi: 10.1007/s00436-010-2195-1. [DOI] [PubMed] [Google Scholar]
- 12.Appawu MA, Owusu-Agyei S, Dadzie S, Asoala V et al. Malaria transmission dynamics at a site in northern Ghana proposed for testing malaria vaccines. Trop. Med. Int. Health. 2004;9:164–170. doi: 10.1046/j.1365-3156.2003.01162.x. [DOI] [PubMed] [Google Scholar]
- 13.Dery DB, Brown C, Asante KP, Adams M et al. Patterns and seasonality of malaria transmission in the forest-savannah transitional zones of Ghana. Malar. J. 2010;9:314. doi: 10.1186/1475-2875-9-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Appawu MA, Baffoe-Wilmot A, Afari EA, Dunyo S et al. Malaria vector studies in two ecological zones in southern Ghana. Afr. Entomol. 2001;9:59–65. [Google Scholar]
- 15.De Souza D, Kelly-Hope L, Lawson B, Wilson M et al. Environmental factors associated with the distribution of Anopheles gambiae s.s. in Ghana; an important vector of lymphatic filariasis and malaria. PLoS ONE. 2010;5:e9927. doi: 10.1371/journal.pone.0009927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Browne EN, Frimpong E, Sievertsen J, Hagen J et al. Malariometric update for the rainforest and savanna of Ashanti region, Ghana. Ann. Trop. Med. Parasitol. 2000;94:15–22. [PubMed] [Google Scholar]
- 17.Hay SI, Rogers DJ, Toomer JF, Snow RW: Annual Plasmodium falciparum entomological inoculation rates (EIR) across Africa: Literature survey, internet access and review. Trans. R. Soc. Trop. Med. Hyg. 2000;94:113–127. doi: 10.1016/s0035-9203(00)90246-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agnandji ST, Lell B, Soulanoudjingar SS, Fernandes JF et al. First results of Phase 3 Trial of RTS,S/AS01 malaria vaccine in African children. N. Engl. J. Med. 2011;365:1863–1875. doi: 10.1056/NEJMoa1102287. [DOI] [PubMed] [Google Scholar]
- 19.Timmann C, Evans JA, König IR, Kleensang A et al. Genome-wide linkage analysis of malaria infection intensity and mild disease. PLoS Genet. 2007;3:e48. doi: 10.1371/journal.pgen.0030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghana Districts-A repository of all districts in the Republic of Ghana: 2006. http://ghanadistricts.com/districts/?news&r=2&_=25http://ghanadistricts.com/districts/?r=2&_=18&sa=5964 and.
- 21.World Health Organization: Manual on practical entomology in malaria. Methods and techniques. Part II.; Geneva, Switzerland: 1975. [Google Scholar]
- 22.Gillies MT, Coetzee M: A supplement to the Anophelinae of Africa south of the Sahara (Afrotropical Region) South Afr. Inst. Med. Res. 1987;55:1–143. [Google Scholar]
- 23.Collins FH, Mendez MA, Rasmussen M, Mehaffey PC et al. A ribosomal RNA gene probe differentiates member species of the Anopheles gambiae complex. Am. J. Trop. Med. Hyg. 1987;37:37–41. doi: 10.4269/ajtmh.1987.37.37. [DOI] [PubMed] [Google Scholar]
- 24.Scott JA, Brogdon WG, Collins FH: Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am. J. Trop. Med. Hyg. 1993;49::520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- 25.Detinova TS: In: Age grouping methods in dipteral of medical importance, with special reference to some vectors of malaria. World Health Organization,; Geneva,: 1962. Determination of the epidemiological importance of populations of Anopheles maculipennis by their age composition. [PubMed] [Google Scholar]
- 26.Wirtz RA, Burkot TR: Detection of malarial parasites in mosquitoes. Adv. Dis. Vector Res. 1991;8:77–106. [Google Scholar]
- 27.Warrell DA, Gilles HM: Essential malariology. Fourth Edition. New York:: Arnold; 2002. [Google Scholar]
- 28.Krefis AC, Schwarz NG, Nkrumah B, Acquah S et al. Spatial analysis of land cover determinants of malaria incidence in the Ashanti region, Ghana. PLoS ONE. 2011;6:e17905. doi: 10.1371/journal.pone.0017905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ter Kuile FO, Terlouw DJ, Howard PA, Hawley WA et al. Impact of permethrin-treated bed nets on malaria and all-cause morbidity in young children in an area of intense perennial malaria transmission in western Kenya: cross-sectional survey. Am. J. Trop. Med. Hyg. 2003;68:100–107. [PubMed] [Google Scholar]
- 30.Anosike JC, Nwoke BEB, Okere AN, Oku EE et al. Epidemiology of tree-hole breeding mosquitoes in the tropical rainforest of Imo State, South-East Nigeria. Ann. Agric. Environ. Med. 2007;14:31–38. [PubMed] [Google Scholar]
- 31.Krefis AC, Schwarz NG, Krüger A, Fobil J et al. Model-ling the relationship between precipitation and malaria incidence in children from a holoendemic area in Ghana. Am. J. Trop. Med. Hyg. 2011;84:285–291. doi: 10.4269/ajtmh.2011.10-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tuno N, Kjaerandsen J, Badu K, Kruppa T: Blood-feeding behaviour of Anopheles gambiae and Anopheles melas in Ghana, Western Africa. J. Med. Entomol. 2010;47:28–31. doi: 10.1603/033.047.0104. [DOI] [PubMed] [Google Scholar]
- 33.Dossou-Yovo J, Diarrassou S, Doannio JMC, Darriet F et al. Biting indoor cycle of Anopheles gambiae s.s. and malaria transmission on the Bouake region (Cote d’Ivore). It’s importance in the use of impregnated bednets. Bull. Soc. Pathol. Exot. 1999;92:198–200. [PubMed] [Google Scholar]
- 34.Reyburn H, Mbatia R, Drakeley C, Bruce J et al. Association of transmission intensity and age with clinical manifestations and case fatality of severe Plasmodium falciparum malaria. JAMA. 2005;293:1461–7140. doi: 10.1001/jama.293.12.1461. [DOI] [PubMed] [Google Scholar]
- 35.Maxwel CA, Wakibara J, Tho S, Curtis CF: Malaria-infective biting at different hours of the night. Med. Vet. Entomol. 1998;12:325–327. doi: 10.1046/j.1365-2915.1998.00108.x. [DOI] [PubMed] [Google Scholar]
- 36.Tchouassi DP, Quakyi IA, Addison EA, Bosompem KM et al. Characterization of malaria transmission by vector populations for improved interventions during the dry season in the Kpone-on-Sea area of coastal Ghana. Parasit. Vectors. 2012;5:212. doi: 10.1186/1756-3305-5-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Port GR, Boreham PFL, Bryan JH: The relationship of host size to feeding by mosquitoes of the Anopheles gambiae Giles complex (Diptera, Culicidae). Bull. Entomol. Res. 1980;70:133–144. [Google Scholar]
- 38.Beier JC, Oster CN, Onyango FK, Bales JD et al. Plasmodium falciparum incidence relative to entomologic inoculation rates at a site proposed for testing malaria vaccines in western Kenya. Am. J. Trop. Med. Hyg. 1994;50:529–536. doi: 10.4269/ajtmh.1994.50.529. [DOI] [PubMed] [Google Scholar]
- 39.Smith T, Killeen G, Lengeler C, Tanner M: Relationships between the outcome of Plasmodium falciparum infection and the intensity of transmission in Africa. Am. J. Trop. Med. Hyg. 2004;71:80–86. [PubMed] [Google Scholar]
- 40.Williams TN: Human red cell polymorphism and malaria. Curr. Opin. Microbiol. 2006;9:388–394. doi: 10.1016/j.mib.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 41.Beier JC, Killeen GF, Githure JI: Short Report: Entomologic inoculation rates and Plasmodium Falciparum malaria prevalence in Africa. Am. J. Trop. Med. Hyg. 1999;61:109–113. doi: 10.4269/ajtmh.1999.61.109. [DOI] [PubMed] [Google Scholar]


