1 Introduction
Existing malaria vector control measures, such as long lasting insecticide treated nets (LLINs) and house spraying with residual insecticides (IRS), have significantly contributed to the decreasing burden of malaria in sub-Saharan Africa [1]. It is estimated that intervention scale-up from the year 2000 to 2010 averted between 563.000 and 1.36 million child deaths in 43 malaria-endemic countries in Africa [2]. Despite these gains, evidence suggests that even in communities where most people already use the current prevention measures, there remains a significant amount of residual transmission, not readily amenable to control [3-5]. For example, while LLINs and IRS are especially effective against indoor-feeding and indoor-biting species such as Anopheles gambiae and An. funestus, these interventions are far less effective against An. arabiensis [5,6], which now dominates many epidemiological niches left by An. gambiae after long-term vector control in endemic countries [4,5,7-10]. Others include An. rivulorum and An. ziemanni, which also play a secondary but vital transmission role in areas with high IRS and LLIN use [11-13].
This situation constitutes a major long-term challenge to the goal of malaria elimination and eventual eradication [14,15], especially where vector species have divergent ecological needs [16], or reduced behavioural susceptibility to indoor insecticidal interventions [17,18]. Even if universal population coverage with LLINs and IRS was to be achieved, as prescribed by the Global Malaria Action Plan (GMAP) [19], Anopheles mosquitoes would still have numerous resources upon which they could survive and potentially transmit disease, albeit at low levels [16, 20]. Moreover, while most malaria transmission in Africa still occurs indoors [21,22], outdoor biting is increasing in both urban and rural settings [7,23,24]. It is therefore reasonable to infer that despite the remarkable successes of the ongoing malaria prevention operations, efforts towards elimination will likely fall short unless complementary strategies are initiated to target disease-transmitting mosquitoes outdoors [15,16,25].
The Outdoor Mosquito Control (OMC) project, at the Ifakara Health Institute (IHI) in Tanzania, is one example of a handful of ongoing studies around the world that address these challenges. The aim of the OMC project at IHI is to develop and test an integrated outdoor mosquito control strategy that can be sustainably used to complement LLINs and IRS and therefore accelerate malaria elimination efforts in sub-Sahara Africa. This article outlines the idea behind this project, and provides an overview of major research activities conducted by the team. It also reviews some important early findings of the project and discusses the overall potential of this strategy in the context of the malaria control and elimination agenda.
2 The idea
To supplement current malaria prevention methods, and accelerate efforts towards elimination, we are exploring the use of outdoor mosquito control devices that mimic real humans, to attract and kill disease-transmitting vectors on a sustained basis. The idea (as envisioned in our GCE Phase I grant (2009) and Phase II grant (2011)) is to lure female Anopheles mosquitoes onto these outdoor ‘pseudo-hosts’, when positioned at optimally selected sites within or around human settlements, and then either trap these mosquitoes, contaminate them with substances that reduce their survival and ability to transmit disease or kill them instantly or within a few days after contact with the devices, thereby reducing exposure to mosquito bites and mosquito-borne pathogens. To be successful, the efforts should be continued consistently throughout the dry and wet seasons over an extended period of time, while at the same time ensuring sustained and consistent LLIN use in the communities.
To ensure sustainability, this project also incorporates the concept of integrated innovation [26], whereby in addition to designing, constructing and testing these outdoor mosquito control devices, we are also exploring: 1) complementary social innovations that would offer a practical means of promoting and implementing the overall strategy, and 2) innovative market-based options that would ensure uninterrupted medium to long term financing of the strategy. The overall goal is to achieve a practical and community-driven outdoor vector control strategy, which will complement rather than replace existing interventions. The combined strategy would ensure enhanced and progressive decreases of both indoor and outdoor malaria transmission and will accelerate malaria control efforts beyond thresholds necessary to achieve elimination in many low and middle-income endemic communities.
This approach differs from the current intradomicilliary and insecticide-based malaria prevention strategies primarily because it targets mosquitoes while they are outdoors as opposed to indoors. However, it also allows for targeting of mosquitoes that would otherwise bite people in the early evening hours and at dawn, during which time LLINs offer no protection. Though not previously evaluated for malaria prevention, similar or related odour-based approaches have been successful against insect vectors of human and animal diseases [27,28], and agricultural crop pests [29-31], providing key indications that this strategy could have potential against insect vectors of disease, and possibly fill the gaps in future vector control operations.
3 Results
Since most of the findings presented here have been published elsewhere or are not ready for release, this section is primarily intended as an overview, rather than a presentation of the actual primary data. Instead, specific references are provided to guide readers on where more details can be obtained. All the field studies described here were conducted in southern Tanzania, where LLINs are widely used and where malaria transmission has been dramatically reduced over the past decade, though there is still significant residual transmission [4,7] mediated by An. arabiensis and An. funestus (Kaindoa et al., unpublished data).
3.1 Design, construction and small-scale efficacy tests of experimental proto-types of outdoor mosquito control devices
We initially designed and field tested two different experimental prototypes of outdoor mosquito control devices namely: a) the odour-baited station, now commonly referred to as the Ifakara odour-baited station or the Ifakara OBS, and b) the odour-baited mosquito landing box (Fig. 1). These devices were constructed using materials locally available in Tanzania (wood, canvas and netting) and were baited either with a long-range synthetic mosquito lure [32], or with worn nylon socks (a common reservoir for smelly foot odour and a proven lure for mosquitoes [33]), supplemented with carbon dioxide (CO2) gas. The attractant dispensing units in these experimental prototypes consist of a battery driven fan fitted onto the upper ends of a dispensing unit, inside which the mosquito attractants are inserted (Fig. 1B). Host-seeking mosquitoes lured to these decoy hosts are trapped, contaminated or killed.
Figure 1.

Diagrammatic and pictorial representations of the Ifakara odour baited station (A) and the odour-baited mosquito landing box (B). Panel A of this figure was adapted from Okumu et al. [34].
Before applying any mosquito-killing agents, we first demonstrated that wild mosquitoes (including An. arabiensis and An. funestus) visit these devices. To do this the Ifakara OBS was fitted with interception traps on two sides to catch exiting mosquitoes (Fig. 1A), while the mosquito landing box (Fig. 1B), which does not have any trapping mechanism, was evaluated simply by intermittently covering it with a large netting cage and then collecting the transient mosquitoes found on its surfaces or inside the netting cage at different times during the night (Matowo et al., unpublished data).
3.2 Proof that host-seeking mosquitoes visiting these devices can be contaminated or killed
We used two different mosquito-killing agents to test this concept, namely a non-repellent organophosphate insecticide (pirimiphos methyl), and a mosquito-killing fungus (Metarhizium anisopliae). The tests were done by treating the devices and then sampling mosquitoes that visited them each night. The collected mosquitoes were maintained on 10% sugar meals and their daily survival observed every 24 hrs over several days. The tests with the Ifakara OBS were conducted against wild mosquitoes in a rural village [34]. However, the mosquito landing box was evaluated against laboratory-reared mosquitoes inside a ‘semi-field’ system (a 100 m long screened-tunnel), so that we could recapture and observe any mosquitoes landing on the device and then flying away afterwards (Fig. 2).
Figure 2.

Pictorial representation of semi-field assessment of fungus-treated mosquito landing boxes on female An. arabiensis mosquitoes inside the screened tunnel.
In the field experiments using the Ifakara OBS, we observed that in addition to trapping, it was possible to contaminate and slowly kill between 74% (using pirimiphos methyl [34]) and 95% (using M. anisopliae IP 46 [35]) of the free-flying wild malaria vectors that passed through the device. The mean survival time of wild adult An. arabiensis mosquitoes after exposure to M. anisopliae IP46 in the OBS was reduced five-fold, i.e. from 10.0 (2.8 - 14.3) days to 2.0 (1.0 - 4.0) days, Hazard Ratio = 2.65, P < 0.0001 [35].
In the semi-field system, we placed two fungus-treated mosquito landing boxes (baited with worn socks and CO2 gas from a yeast-sugar solution [36]), and released 400 laboratory-reared female An. arabiensis mosquitoes nightly (Fig. 2). Two exposure-free tent traps [37] with sleeping adult male volunteers were also placed in the tunnel, so that mosquitoes had a choice between real human odours and the outdoor devices. Each morning, mosquitoes were recaptured from the tent traps and from the general area within the tunnel, and monitored for survival and fungal growth on cadavers. Up to 42% of mosquitoes recaptured inside the tent traps and 26% of catches outside the traps had fungus growth on their cadavers, confirming that even where there are competing cues from real humans, mosquitoes still visit the outdoor devices and get contaminated or killed, and that this can happen even before the mosquitoes reach their human hosts (Lwetoijera et al., unpublished data). In this study we also observed that fungus-contaminated mosquitoes survived relatively fewer days (8 and 10 days for mosquitoes collected inside and outside the tents, respectively) compared to those that did not have any signs of fungal contact (10 and 12 days for those mosquitoes collected inside and outside the tents, respectively).
The field and semi-field tests therefore demonstrated that these experimental prototypes of outdoor devices can be used to attract, trap, contaminate and kill mosquitoes, including the main malaria vectors, An. arabiensis and An. funestus [34, 35]. However, despite the efficacies, our experience during these initial trials, and our ongoing field studies suggest that the greatest challenges that will face this technology include: 1) lack of locally produced attractants, including CO2 gas, which is arguably the ‘universal’ attractant of Anopheles mosquitoes [38] and is now considered an essential additive to most other known attractants, including the most potent synthetic lures we currently have [32], and 2) lack of a highly effective mosquito killing agents that cannot be affected by physiological or behavioural resistance in mosquito vector populations [17,18,39-41].
3.3 Initial tests of a participatory geo-location model for identifying the best sites to place the outdoor devices with in and around human settlements
To enhance effectiveness of the outdoor devices, it will be essential to optimally locate them in areas where mosquitoes are most abundant or in locations between mosquito sources and human dwellings. Using the Ifakara OBS as an example, we created the first geo-location test model (Fig. 3A) to identify suitable sites for these devices using the following procedure: 1) community participatory mapping was conducted to identify mosquito breeding habitats in a rural village called Lupiro (8.3854°S; 36.6702°E), 2) entomological field studies were conducted to estimate outdoor mosquito densities and also to determine safe distances from human dwellings at which the odour-baited devices could be placed without increasing exposure to mosquito bites, and 3) field surveys were conducted to map households, roads, landmarks and places where people congregate in the evenings. The data were combined in a Geographical Information Systems (GIS) environment and analysed using ArcGIS 9.2 (ESRI, USA). An easy-to-interpret suitability map showing optimal sites for Ifakara OBS was produced, clearly depicting sites that are least appropriate and sites that are most appropriate for locating the devices [42].
Figure 3.

A: Schematic presentation of the steps in GIS-based location modelling and analysis to determine suitable areas for locating odour-baited lure and kill stations, based on location of mosquito breeding habitats, roads, households and places with high outdoor vector densities within the village. B: Visual representation and comparison of two interpolated mosquito density maps derived from community knowledge and experience (i) and data obtained during outdoor mosquito sampling conducted in the same study area (j). The figures were adapted from Sumaye et al. [42].
Separately, in an attempt to develop a cheaper, quicker and easier geo-location strategy that could be used to scale up this or similar outdoor vector control techniques in future, we conceived and tested a novel geo-targeting method, which primarily exploits knowledge and experiences of community residents to approximate areas where disease-transmitting mosquitoes are most abundant, and therefore the optimal areas where the outdoor devices should be located. Community members were provided with gridded maps of their own village and were asked to rank each grid on a scale of 1-10 based on their knowledge and experiences of mosquito densities, without necessarily paying attention to mosquito taxa. The obtained grid values were interpolated in ArcGIS 9.2 using inverse distance weighted interpolation method (IDW) and the resulting map was visually compared to another map created by interpolating empirical entomological data collected from outdoor mosquito trapping performed throughout the same village (Fig. 3B). The entomological sampling was done during dry season to represent the time when permanent dry season mosquito larval breeding sites (dry-season refugia) are likely to be most important sources of transmission.
Comparative visual interpretation of the two maps derived from interpolating the community data and the entomological data (Fig. 3B) revealed similarities between the maps, with only small positional variations of 75-200 m [42]. This initial small-scale exploration highlighted the possibility that this cheap, quick and easy-to-perform community-based method could in future be applied when implementing outdoor lure-and kill strategies over larger areas. However, since the work was performed in only one village and a single dry season, this initial finding was considered as being merely indicative of the possibility of relying on community knowledge to predict vector densities. Therefore, to validate this methodology prior to any further applications, we are conducting a larger and longer multi-village trial, with a higher geographic resolution and a more rigorous sampling scheme (Mwangungulu et al., unpublished data).
3.4 Mathematical evaluation of potential benefits, limitations and target -product profiles of outdoor mosquito control devices for malaria control and elimination
We have not been able to undertake a rigorous field trial within the scope of this project, which would enable us to examine in detail the benefits of combining LLINs with these outdoor devices on malaria transmission. However, we completed a collaborative mathematical modelling exercise, through which we objectively evaluated the epidemiological potential and limitations of this proposed strategy, and also described essential characteristics that the outdoor devices should have so as to be effective for malaria control and elimination in different scenarios representative of Africa [43].
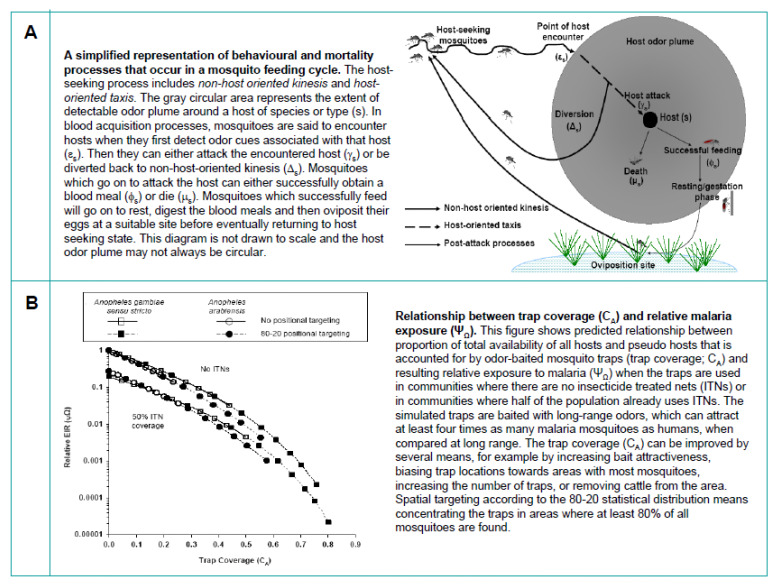
Once again, using the example of Ifakara OBS fitted with exit traps, we first examined whether these devices, when used alone or as a complementary intervention alongside LLINs, can have significant impact on malaria transmission in highly endemic areas. A target product-profile (TPP) that developers of this technology should consider, so as to ensure effectiveness under realistic operational conditions, was also elucidated. In this mathematical assessment, the odour-baited devices were considered as ‘pseudo-hosts’, which unlike humans or cattle cannot provide blood to host-seeking mosquitoes, but which mosquitoes can attack nonetheless (Fig. 4).
Figure 4.
Mathematical evaluation of effects of odour-baited mosquito traps (functioning as ‘pseudo-hosts’) on the malaria transmission. A: Illustration of what happens when mosquitoes encounter a host. B: Relationship between trap coverage (which is a function of number of traps, attractiveness of the bait used, the number and types of other vertebrate hosts including humans in the environment, and the location of the traps relative to human dwellings) and relative malaria exposure. Figures are adapted from Okumu et al. [43].
We modified equations from previous mathematical models [44,45] and revised among other features, the description of term ‘host availability’ to mosquitoes so that it was now equivalent to the probability that mosquitoes encounter and then attack a particular host (which could be humans, cattle or ‘pseudo-hosts’ like odour-baited traps) [43]. We determined that malaria transmission would decline if the proportion of total availability of all hosts and ‘pseudo-hosts’ that the traps constitute increased [43]. In other words, transmission would decline with increasing trap coverage in the targeted communities. If the traps are used to complement rather than replace LLINs, transmission could be reduced by more than 99% in most scenarios representative of Africa, by using between 20 and 140 traps for every 1000 people [43], although systems dominated by An. arabiensis would be evidently more challenging than systems dominated by An. gambiae (Fig. 4). In one example, we noted that in hyperendemic settings where unprotected persons are exposed to 200 infectious mosquito bites per person per year, 80% coverage with LLINs combined with about 45 Ifakara OBS devices per 1000 people could reduce relative exposure to malaria from 1 to 0.001, meaning an absolute reduction to 0.2 infectious bites per person per year [43].
The results of this in silico assessment provide a clear indication that by combining optimally located effective outdoor mosquito control devices with high coverage of LLINs, it is possible that malaria transmission intensities can be reduced to, and even below the threshold necessary for malaria elimination in many epidemiological scenarios representative of Africa [43]. However, in order to match cost-effectiveness of LLINs (which are the best malaria prevention technique today and are considered to be the second most cost-effective health product after childhood immunisations [19]), we estimated that the entire operation and maintenance of a single device would have to cost less than 28 US dollars per unit per year [43]. Besides, effective delivery would require vertical government support of organised local communities and public health administration. We also proposed that future development of similar strategies should target highly attractive mosquito lures, optimal geographical positioning, as well as reducing costs and bulk of the devices.
3.5 Social and business innovations to ensure acceptability and sustainability of the outdoor mosquito control strategy
To improve the likelihood of this strategy being successful and sustainable we have incorporated, from the beginning, specific research tasks that focus on examining effects of human behaviour on outdoor malaria transmission, as well as perspectives of communities to outdoor mosquito bites and how best to control these bites (Moshi et al., unpublished data). Specifically, we are assessing community views and behaviours on outdoor transmission and its prevention, and catalogue different outdoor activities done by children and adult household members at different times of the night including early evening hours before people go to bed and in the early morning hours when people wake up.
The study involves both qualitative cross sectional surveys conducted in villages in the Kilombero valley and quantitative assessments using structured observation sheets to capture information on what people say they do and what they actually do, followed by mixed methods analysis [46]. This information will enable us to assess and quantify the risk of outdoor transmission in situations where LLINs are already widely used such as our study sites in southeast Tanzania [4,7]. It will also support our ongoing improvement of the prototype devices, and more importantly become the basis of any future community sensitisation and behaviour change programmes, which will be necessary to ensure that the overall strategy is effective and sustainable.
We are also exploring various options through which this strategy can be effectively financed on a medium to long-term basis (Moshi et al., unpublished data). Specific examples being addressed include: 1) the possibility of using the same solar energy system that powers the odour-dispensing units inside our devices (Fig. 1B) to also provide basic lighting to nearby households, and 2) the possibility of individual families or groups of families directly contributing towards a subsidised cost of these devices as a regular consumer product. Our specific goal in this regard is a market-focused programme that can allow local manufacturers to earn direct financial income from the business of vector control, thus improving local economies as well.
4 Discussion
Malaria control in the past decade has seen major successes, but these can be sustained only if the current goodwill from international partners and endemic country governments is sustained [47]. While, these achievements have convinced the global community to re-consider attempting malaria elimination and eradication [19], there are important challenges that must be addressed effectively in the long-term, so that gains so far accumulated are not reversed.
A careful examination of current malaria prevention targets suggest that even if universal population coverage as currently prescribed by GMAP [19] were achieved with indoor interventions like LLINs and IRS, effective biological coverage of all potential vertebrate hosts from which disease transmitting mosquitoes can possibly obtain blood meals would remain sub-optimal [20]. Yet, expert opinion suggests that for malaria elimination to be achieved, it will be essential to also identify and cover essential extra-domiciliary and non-anthropological resources that Anopheles mosquitoes depend upon for survival [15,16]. This challenge of sub-optimal biological coverage occurs primarily because: 1) LLINs and IRS primarily target mosquitoes that enter and those that attempt to enter human dwellings, yet mosquitoes also obtain significant proportions of essential resources outdoors, including biting human and non-human blood hosts, and 2) some mosquito populations either naturally bite outdoors or have developed behaviouristic resistance against insecticides commonly used to control them [40, 41].
The current action plan, which consolidates opinions of 250 experts from a wide range of fields, encourages countries to scale up existing malaria prevention measures, appropriate diagnosis and treatment options, so as to reach universal coverage of at-risk populations, and to sustain these universal levels in the long term [19]. Very importantly, however, this plan together with other expert reports from consultative groups, such as the malaria eradication research agenda initiative [15], recognise that the current stated goals are unlikely to be achieved in most endemic countries, unless additional complementary interventions are introduced. Research for development of these new tools thus remains crucial and is recommended at all levels [15,19]. Our efforts to develop and test new ways for targeting malaria vectors outdoors are therefore perfectly in line with the ongoing global health initiatives.
By targeting vector outdoors, in places and at times when LLINs and IRS are less effective, our proposed strategy will enhance the pressure that these current strategies are already having on malaria transmission, and will greatly diminish the remaining opportunities available for pathogen transmission. If we supplement rather than supplant the existing interventions, this strategy would offer an option to further shrink the residual malaria transmission and potentially accelerate elimination efforts in Africa. Obviously, there are still some specific challenges that must be addressed and we duly recognise that the overall strategy has still a long way to go. For example, the available findings reviewed here and our ongoing studies indicate that we will have to explore options to circumvent issues such as: 1) the current lack of locally produced attractants, including CO2 gas [38], which is necessary as additive to most known attractants for malaria vectors, and 2) lack of a highly effective mosquito killing agent that is unlikely to be affected by physiological or behavioural resistance in mosquito vector populations [39,40].
Other than vector behaviour and the efficacy of interventions, human behaviour is known to be a major determinant of the overall effectiveness of interventions [48]. Risk behaviours associated with outdoor malaria transmission should therefore be identified and carefully examined to determine where and when new approaches could have the greatest impact [48]. Even though we have determined that outdoor mosquito control devices can have significant impact [43], it is critical to also consider the perspectives of typical users and to adequately address the numerous important questions. In this regard, we believe that the most important of these questions will be: 1) whether people appreciate the fact that mosquitoes also bite outdoors and that malaria transmission can actually happen when people are outside their houses, 2) whether people know the risk factors associated with such transmission, 3) how they currently protect themselves from such transmission, 4) what measures they would propose against such outdoor biting and transmission, 5) what strategies would they prefer as a means to complement LLINs, and 6) whether they would be willing to participate in the implementation of the strategy or contribute resources towards the strategy. The project described here is therefore designed with components to answer these and similar questions.
We also expect that the integrated innovation approach that has been adopted here [26] will ensure that technologies being developed are not only effective, but also that the overall strategy is acceptable to communities, and sustainable in the long term. Evidence suggests that vector control programmes can be more effectively conducted through supervised community-based programmes [49,50] and also that community involvement increases acceptability and sustainability. Besides, health promotion efforts with substantial community participation are more likely to succeed than those that focus on reproducing external technologies and practices [50-53]. Moreover, community level strategies that allow local financing of such initiatives, while at the same time improving local household economies could potentially increase uptake and sustainability.
5 Future perspectives
This research is one example of the many new approaches that scientists across the world are exploring to address important global health challenges. It focuses on outdoor mosquito control to address limitations of LLINs and IRS, which are the current malaria vector control methods of choice but are inadequate for achieving the goals of full transmission control in many endemic countries in sub-Sahara Africa. The project has already made significant progress and we have a clear strategy going forward. We recognise, however, that it will take significant time and resources, along with well co-ordinated partnerships with other researchers and industrial partners, to eventually achieve the final goal of a practical and community-driven outdoor vector control strategy to sustainably complement indoor interventions such as LLINs. Nevertheless, we already have clear indications from our studies, that this or similar strategies could have substantial impact in future malaria control and elimination efforts, if the target product profiles are met. Also important is that this research is now enabling identification of key opportunities and challenges associated with outdoor mosquito control strategies, particularly in low and middle-income countries.
6 Acknowledgements
We thank the following IHI technicians: Edgar Mbeyela, Godfrey Ligamba, Emmanuel Simfukwe, Monica Mpingwa, Hassan Ngonyani, Salum Mapua and Gustave Mkandawile; who occasionally assisted with field experiments and identification of mosquitoes. We also thank Dr. Sarah J. Moore and Dr. Nico J. Govella who offered equipment and supplies for some of the experiments and advised us on proper installation; Dr. Zoe Hildon at IHI for her ongoing support on the various methodological issues associated with the behavioural aspects of the research and Ms. Joyce P. Parshuku who initially managed these human behaviour studies and co-ordinated our project. We further recognise the assistance received from scientists at IHI (Drs. Gerry F Killeen, Nico J. Govella and Sarah J Moore) and the Swiss Tropical Institute (Dr. Nakul Chitnis) on the various aspects of mathematical modelling work presented here. The fungus spores used here were originally provided to IHI by Prof. Christian Luz (Instituto de Patologia Tropical e Saúde Pública, Universidade Federal Goiás, Goiânia, Brazil).
This work is co-funded by the Global Health Discovery programme of the Bill & Melinda Gates Foundation under the Grand Challenges Explorations scheme (Award numbers 53214 and OPP103572), and Low and Middle Income Country Stars in Global Health Programme of The Grand Challenges Canada™ (Award number: 0012-01-01-01-01). The funders have no role in the publication of this article. Ethical approval for the project was obtained from institutional review board of IHI (Ref: IHI/IRB/NO.030) and the Medical Research Coordinating Committee at the National Institute of Medical Research in Tanzania (Ref:NIMR/HQ/R.8a/Vol.IX/1222).
References
- 1.World Health Organization: World Malaria Report. 2011. World Health Organization, 2011. [Google Scholar]
- 2.Eisele TP, Larsen DA, Walker N, Cibulskis RE et al. Estimates of child deaths prevented from malaria prevention scale-up in Africa 2001-2010. Malar. J. 2012;11:93. doi: 10.1186/1475-2875-11-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffin JT, Hollingsworth TD, Okell LC, Churcher TS et al. Reducing Plasmodium falciparum malaria transmission in Africa: a model-based evaluation of intervention strategies. PLoS Med. 2010;7:e1000324. doi: 10.1371/journal.pmed.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Russell TL, Lwetoijera DW, Maliti D, Chipwaza B et al. Impact of promoting longer-lasting insecticide treatment of bed nets upon malaria transmission in a rural Tanzanian setting with pre-existing high coverage of untreated nets. Malar. J. 2010;9:187. doi: 10.1186/1475-2875-9-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitau J, Oxborough RM, Tungu PK, Matowo J et al. Species shifts in the Anopheles gambiae complex: do LLINs successfully control Anopheles arabiensis? PLoS ONE. 2012;7:e31481. doi: 10.1371/journal.pone.0031481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okumu FO, Mbeyela E, Lingamba G, Moore J et al. Comparative field evaluation of combinations of long-lasting insecticide treated nets and indoor residual spraying, relative to either method alone, for malaria prevention in an area where the main vector is Anopheles arabiensis. Para-sit. Vectors. 2013;6:46. doi: 10.1186/1756-3305-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russell TL, Govella NJ, Azizi S, Drakeley CJ et al. Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar. J. 2011;10:80. doi: 10.1186/1475-2875-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bayoh MN, Mathias DK, Odiere MR, Mutuku FM et al. Anopheles gambiae: historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province, Kenya. Malar. J. 2010;9:62. doi: 10.1186/1475-2875-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mwangangi J, Mbogo C, Orindi B, Muturi E et al. Shifts in malaria vector species composition and transmission dynamics along the Kenyan coast over the past 20 years. Malar. J. 2013;12:13. doi: 10.1186/1475-2875-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Derua YA, Alifrangis M, Hosea KM, Meyrowitsch DW et al. Change in composition of the Anopheles gambiae complex and its possible implications for the transmission of malaria and lymphatic filariasis in north-eastern Tanzania. Malar. J. 2012;11:188. doi: 10.1186/1475-2875-11-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gillies M, Smith A: The effect of a residual house spraying campaign in East Africa on species balance in the Anopheles funestus group: the replacement of Anopheles funestus by Anopheles rivulorum. Bull. Entomol. Res. 1960;51:243–253. [Google Scholar]
- 12.Wilkes T, Matola Y, Charlwood JD: Anopheles rivulorum, a vector of human malaria in Africa. Med. Vet. Ent. 1996;10:108–110. doi: 10.1111/j.1365-2915.1996.tb00092.x. [DOI] [PubMed] [Google Scholar]
- 13.Kawada H, Dida GO, Sonye G, Njenga SM et al. Reconsideration of Anopheles rivulorum as a vector of Plasmodium falciparum in Western Kenya: some evidence from biting time, blood preference, sporozoite positive rate, and pyrethroid resistance. Parasit. Vectors. 2012;5:230. doi: 10.1186/1756-3305-5-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Govella NJ, Ferguson H: Why use of interventions targeting outdoor biting mosquitoes will be necessary to achieve malaria elimination. Front. Physiol. 2012;3:199. doi: 10.3389/fphys.2012.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The malERA Consultative Group on Vector Control: A research Agenda for Malaria Eradication: Vector Control. PLoS Med. 2011;8:e1000401. doi: 10.1371/journal.pmed.1000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferguson HM, Dornhaus A, Beeche A, Borgemeister C et al. Ecology: A prerequisite for malaria elimination and eradication. PLoS Med. 2010;7:e1000303. doi: 10.1371/journal.pmed.1000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elliott R: The influence of vector behavior on malaria transmission. Am. J. Trop. Med. Hyg. 1972;21:755–763. doi: 10.4269/ajtmh.1972.21.755. [DOI] [PubMed] [Google Scholar]
- 18.Gatton ML, Chitnis N, Churcher T, Donnelly MJ et al. The Importance of Mosquito Behavioural Adaptations to Malaria Control in Africa. Evolution. 2013;67:1218–1230. doi: 10.1111/evo.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization: Global Malaria Action Plan. World Health Organization, Roll Back Malaria Partnership,; 2008. [Google Scholar]
- 20.Kiware SS, Chitnis N, Devine GJ, Moore SJ et al. Biologically meaningful coverage indicators for eliminating malaria transmission. Biol. Lett. 2012;8:874–877. doi: 10.1098/rsbl.2012.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huho B, Briet O, Seyoum A, Sikaala C et al. Consistently high baseline estimates for the proportion of human exposure to rural African malaria vector populations that occurred indoors. Int. J. Epidem. 2013;42:235–247. doi: 10.1093/ije/dys214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seyoum A, Sikaala CH, Chanda J, Chinula D et al. Human exposure to anopheline mosquitoes occurs primarily indoors, even for users of insecticide-treated nets in Luangwa Valley, South-east Zambia. Parasit. Vectors. 2012;5:101. doi: 10.1186/1756-3305-5-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Govella NJ, Okumu FO, Killeen GF: Insecticide-treated nets can reduce malaria transmission by mosquitoes which feed outdoors. Am. J. Trop. Med. Hyg. 2010;82:415–419. doi: 10.4269/ajtmh.2010.09-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reddy MR, Overgaard HJ, Abaga S, Reddy VP et al. Outdoor host seeking behaviour of Anopheles gambiae mosquitoes following initiation of malaria vector control on Bioko Island, Equatorial Guinea. Malar. J. 2011;10:184. doi: 10.1186/1475-2875-10-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takken W, Knols BGJ: Malaria vector control: current and future strategies. Trends Parasitol. 2009;25:101–104. doi: 10.1016/j.pt.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Grand Challenges Canada. Integrated Innovation. 2010. See: http://www.grandchallenges.ca/integrated-innovation/.
- 27.Day JF, Sjogren RD: Vector control by removal trapping. Am. J. Trop. Med. Hyg. 1994;50:126–133. doi: 10.4269/ajtmh.1994.50.126. [DOI] [PubMed] [Google Scholar]
- 28.Vale GA, Lovemore DF, Flint S, Cockbill GF: Odour-baited targets to control tsetse flies, Glossina spp. (Diptera: Glossinidae), in Zimbabwe. Bull. Entomol. Res. 1988;78:31–49. [Google Scholar]
- 29.Quarles W: Pheromones for aphid control. IPM Practit. 1999;21:1–6. [Google Scholar]
- 30.Khan ZR, Pickett JA, Van den Berg J, Wadhams LJ et al. Exploiting chemical ecology and species diversity: stem borer and striga control for maize and sorghum in Africa. Pest Manag. Sci. 2000;56:957–962. [Google Scholar]
- 31.Van Lenteren J, Overholt W: Ecology and integrated pest management. Insect Sci. Applic. 1994;15:557–582. [Google Scholar]
- 32.Okumu FO, Killeen GF, Ogoma S, Biswaro L et al. Development and field evaluation of a synthetic mosquito lure that is more attractive than humans. PLoS ONE. 2010;5:e8951. doi: 10.1371/journal.pone.0008951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Njiru B.N., Mukabana WR, Takken W, Knols BG: Trapping of the malaria vector Anopheles gambiae with odour baited MM-X traps in semi field conditions in Western Kenya. Malar. J. 2006;5:39. doi: 10.1186/1475-2875-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okumu FO, Madumla EP, John AN, Lwetoijera DW et al. Attracting, trapping and killing disease-transmitting mosquitoes using odor-baited stations-The Ifakara Odor-Baited Stations. Parasit. Vectors. 2010;3:12. doi: 10.1186/1756-3305-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lwetoijera DW, Sumaye RD, Madumla EP, Kavishe DR et al. An extra-domiciliary method of delivering entomopathogenic fungus, Metharizium anisopliae IP 46 for controlling adult populations of the malaria vector, Anopheles arabiensis. Parasit. Vectors. 2010;3:18. doi: 10.1186/1756-3305-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saitoh Y, Hattori J, Chinone S, Nihei N, Tsuda Y, Kurahashi H, Kobayashi M: Yeast-generated CO2 as a convenient source of carbon dioxide for adult mosquito sampling. J. Am. Mosq. Control Assoc. 2004;20:261–264. [PubMed] [Google Scholar]
- 37.Govella NJ, Chaki PP, Geissbuhler Y, Kannady K, Okumu F, Charlwood JD, Anderson RA, Killeen GF: A new tent trap for sampling exophagic and endophagic members of the Anopheles gambiae complex. Malar. J. 2009;8:157. doi: 10.1186/1475-2875-8-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gillies MT: The role of Carbon dioxide in host finding by mosquitoes (Diptera: culicidae): a review. Bull. Entomol. Res. 1980;70:525–532. [Google Scholar]
- 39.Hemingway J, Ranson H: Insecticide resistance in insect vectors of human disease. Ann. Rev. Entomol. 2000;45:371–391. doi: 10.1146/annurev.ento.45.1.371. [DOI] [PubMed] [Google Scholar]
- 40.Muirhead-Thomson RC: The significance of irritability, behaviouristic avoidance and allied phenomena in malaria eradication. Bull. World Hlth Organ. 1960;22:721–734. [PMC free article] [PubMed] [Google Scholar]
- 41.Pates H, Curtis C: Mosquito behaviour and vector control. Ann. Rev. Entomol. 2005;50:53–70. doi: 10.1146/annurev.ento.50.071803.130439. [DOI] [PubMed] [Google Scholar]
- 42.Sumaye RD, Lweitoijera DW, Madumla EP, Okumu F: A geographical location model for targeted implementation of lure and kill strategies against disease-transmitting mosquitoes in rural areas. MWJ. 2012;3:1. [Google Scholar]
- 43.Okumu FO, Govella NJ, Moore SJ, Chitnis N et al. Potential Benefits, Limitations and Target Product-Profiles of Odor-Baited Mosquito Traps for Malaria Control in Africa. PLoS ONE. 2010;5:e11573. doi: 10.1371/journal.pone.0011573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Killeen GF, Smith TA: Exploring the contributions of bed-nets, cattle, repellents and insecticides to malaria control: a deterministic model of mosquito host-seeking behaviour and mortality. Trans. Roy. Soc. Trop. Med. Hyg. 2007;101:867–880. doi: 10.1016/j.trstmh.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Killeen GF, Smith TA, Ferguson HM, Mshinda H et al. Preventing childhood malaria in Africa by protecting adults from mosquitoes with insecticide-treated nets. PLoS Med. 2007;4:e229. doi: 10.1371/journal.pmed.0040229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson RB, Onwuegbuzie AJ: Mixed methods research: A research paradigm whose time has come. Educ. Res. 2004;33:14–26. [Google Scholar]
- 47.World Health Organization: World Malaria Report. World Health Organization,; 2012.. 2012. [Google Scholar]
- 48.Dunn CE, Le Mare A, Makungu C: Malaria risk behaviours, socio-cultural practices and rural livelihoods in southern Tanzania: Implications for bednet usage. Soc. Sci. Med. 2010;72:408–417. doi: 10.1016/j.socscimed.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 49.Ruebush 2nd T, Godoy HA: Community participation in malaria surveillance and treatment. I. The Volunteer Collaborator Network of Guatemala. Am. J. Trop. Med. Hyg. 1992;46:248. doi: 10.4269/ajtmh.1992.46.248. [DOI] [PubMed] [Google Scholar]
- 50.Chaki PP, Dongus S, Fillinger U, Kelly A et al. Community-owned resource persons for malaria vector control: enabling factors and challenges in an operational programme in Dar es Salaam, United Republic of Tanzania. Hum. Resour. Health. 2011;9:21. doi: 10.1186/1478-4491-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manikutty S: Community participation: so what? Evidence from a comparative study of two rural water supply and sanitation projects in India. Dev. Policy Rev. 2003;15:115–140. doi: 10.1111/1467-7679.00029. [DOI] [PubMed] [Google Scholar]
- 52.Minkler M, Wallerstein N, Wilson N: Glantz K, Lewis F, Rimer B . In: Health behavior and health education: Theory, research, and practice ( San Francisco:: Jossey-Bass,; 1997. Improving health through community organization and community building. pp. 279–311. , Eds.), . pp. [Google Scholar]
- 53.Winch PJ, Bagayoko A, Diawara A, Kane M et al. Increases in correct administration of chloroquine in the home and referral of sick children to health facilities through a community-based intervention in Bougouni District, Mali. Trans. R. Soc. Trop. Med. Hyg. 2003;97:481–490. doi: 10.1016/s0035-9203(03)80001-9. [DOI] [PubMed] [Google Scholar]