Summary
Vasculitides are diseases that can affect any vessel. When cardiac or aortic involvement is present, the prognosis can worsen significantly. Pathological assessment often plays a key role in reaching a definite diagnosis of cardiac or aortic vasculitis, particularly when the clinical evidence of a systemic inflammatory disease is missing. The following review will focus on the main histopathological findings of cardiac and aortic vasculitides.
Key words: vasculitis, cardiovascular pathology, aorta, coronary arteries, autopsy
ABBREVIATION LIST:
- AECVP
Association for European Cardiovascular Pathology
- ANCA
anti-neutrophil cytoplasmic antibodies
- EGPA
eosinophilic granulomatosis with polyangiitis
- GCA
giant cell arteritis
- GPA
granulomatosis with polyangiitis
- KD
Kawasaki disease
- PAN
polyarteritis nodosa
- SCVP
Society for Cardiovascular Pathology
- TA
Takayasu’s arteritis
- TAA
thoracic aortic aneurysm
Introduction
Vasculitides are a heterogeneous group of rare disorders characterized by inflammation of blood vessels. Inflammation may result in vessel stenosis, thrombotic occlusion, aneurysm, dissection or even full thickness rupture of the arterial wall 1,2. Besides vessel inflammation, vasculitides may impact the cardiovascular system by inducing accelerated atherosclerosis, hypertension, thromboembolic events, and as such, may end up in life threatening diseases such as myocardial infarction and stroke 3.
Because of the heterogeneous pathogenesis and clinical presentation, the classification of vasculitides has always been challenging. According to the Chapel Hill Consensus Conferences, vasculitides are categorized according to the diameter of the blood vessel most often involved as either large sized, involving the aorta and its branches of large distributing arteries (Takayasu’s arteritis, TA and Giant Cell Arteritis, GCA); medium-sized, involving medium-sized distributing and parenchymatous arteries and veins (Polyarteritis Nodosa, PAN and Kawasaki Disease, KD); and the various types of small vessel vasculitis, involving small distal parenchymatous arteries, arterioles, venules, and capillaries 4. The latter group is subdivided into immune complex- and antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis, according to the presence or absence of specific ANCA autoantibodies [proteinase 3 (PR3)-ANCA and myeloperoxidase (MPO)-ANCA] 5. However, considering the evolving spectrum of clinical, laboratory, genetic, and imaging findings, efforts are still underway to improve the classification criteria 1,6.
The prototype of larger vessel vasculitis, aortitis, can be associated with significant morbidity and mortality through the development of aortic aneurysm, rupture, and dissection, and is often identified only by histopathology after surgical management of aneurysms/dissections or at autopsy 7. A consensus statement proposed by the Society for Cardiovascular Pathology and the Association for European Cardiovascular Pathology (SCVP/AECVP) in 2015 provided a histology-based classification scheme, with recommendations on surgical sample processing, interpretation, and reporting 8. However, histological diagnosis may be challenging because of the overlapping histological features between different entities (see below) and because inflammation may be scarce and obscured by medial degenerative changes and fibrosis. Thus, correlation of histopathology with clinical features and blood and imaging tests is mandatory for proper diagnosis.
Coronary artery vasculitis is a relatively rare form of medium sized arteritis, and because of its vital topographic location in the heart, it can also be associated with significant morbitiy and death. Most reported cases occur in children with KD, and its final diagnosis requires a strict clinicopathological correlation with the extracardiac manifestations of the disease 9. However, and usually at older ages, coronary vasculitis could represent also as an underdiagnosed condition in the clinical setting of myocardial infarction without occlusive coronary artery disease, occurring in patients with an autoimmune systemic disease or with an isolated inflammatory coronary disorder 10-13.
AORTITIS AND PERIAORTITIS
The terms aortitis and periaortitis refer to a spectrum of inflammatory disorders involving the media and/or intima (i.e., aortitis) and/or to the adventitia (i.e., periaortitis), which cannot be attributed to atherosclerosis, or at most atherosclerosis alone 8,14-16. Notably, atherosclerosis is often associated with some degree of inflammation, and in certain settings (e.g., vulnerable plaques or plaque rupture and luminal thrombosis) inflammation may be prominent, although mostly associated with the atherosclerotic lipid cores. A specific entity with unclear etiological backgrounds is the inflammatory atherosclerotic aneurysm of the abdominal aorta (IAA), in which severe atherosclerosis is associated with destruction of the media and marked (peri)adventitial inflammatory infiltrate, primarily composed of lymphocytes and plasma cells. In this setting, a differential diagnosis with periaortitis is mandatory. IAA occurs most often at sites of extensive atherosclerosis, which is the abdominal aorta, whereas most other forms of aortitis or periaortitis localize mostly in the thoracic aorta and its major branches. However, also at the level of thoracic aorta, aortitis may occur in association with atherosclerosis and medial degenerative changes. The evidence of mixed patterns (i.e. aortitis and atherosclerosis or aortitis and degenerative features) has been recently recognized as a frequent morphologic substrate of thoracic aortic aneurysm (TAA) in surgical specimens of the aorta, especially in the elderly female (up to 25% of cases with mixed phenotype versus 3% in which aortitis appears as the unique morphologic substrate of TAA) 7,17,18. Degenerative changes, namely elastic fiber fragmentation and/or loss, smooth muscle nuclei loss, mucoid extracellular matrix accumulation, and medial laminar collapse can be observed both in inflamed and noninflamed areas, probably because of inflammation-induced injury to smooth muscle cells and extracellular matrix components 7. When inflammation is scant and medial degenerative changes are prominent, diagnosis of aortitis may be overlooked 19. Based on the main histopathologic pattern observed, aortitis accounts for 9 to 14% of TAA in two different case series 7,17.
According to the etiology, aortitis may be classified as infectious and non-infectious. Infectious causes are rare, and more often observed after invasive procedures or surgery (including aortic graft infections). Non-infectious aortitis are mostly auto immune mediated and the most common forms are TA (especially in younger patients) and GCA (in older patients) (Tab. I). Several other rheumatologic diseases may involve the aorta including rheumatoid arthritis, ankylosing spondylitis, Reiter’s syndrome, Behçet’s disease, granulomatosis with polyangiitis (GPA), eosinophilic granulomatosis with polyangiitis (EGPA) and IgG4-related disease. A third diagnostic category, isolated aortitis without systemic involvement, has also been recognized 4.
Table I.
Summary of the main features of the most frequently encountered vasculitides in the aorta and coronary arteries.
| Epidemiology | Aortic involvement (% of cases; main features) | Coronary artery involvement (% of cases; main features) | Histology | Preferential site | |
|---|---|---|---|---|---|
| Giant cell arteritis (GCA) | Most common cause of aortitis > 50; F > M | 30; aneurysm, dissection | < 1; stenosis | Lymphoplasmacytic infiltrate, epithelioid macrophages, giant cells, elastic fragmentation | Thoracic aorta, extracranial branches of the carotid artery |
| Takayasu’s arteritis (TA) | Most common cause of aortitis < 50; F > M; Asian descent | 80-100; stenosis, aneurysm | 30; stenosis, occlusion | Compact granulomas, necrosis, scarring | Whole length of the aorta and most of its branches, including the coronary ostia |
| IgG4-Related Disease | Adults > 50; M > F | 10-30; aneurysm, periaortitis | < 10; periarteritis, stenosis | Lymphoplasmacytic infiltrate, IgG4+ plasma cells, storiform fibrosis | Abdominal aorta, rarely thoracic aorta |
| Kawasaki Disease (KD) | Young children; Asian descent | The aorta is usually spared | 20-30; aneurysm, thrombosis | Polymorphous inflammatory infiltrate with histiocytes and granulocytes | Epicardial coronary arteries |
| Polyarteritis nodosa (PAN) | Adults; M > F | The aorta is usually spared | < 20; stenosis, occlusion, aneurysm | Panarteritis with fibrinoid necrosis and polymorphous inflammatory infiltrate | Epicardial coronary arteries and myocardial arterioles |
| Granulomatosis with polyangiitis (GPA) | Adults | The aorta is usually spared | < 30; stenosis, occlusion | Necrotizing granulomatous inflammation +/- eosinophils | Myocardial arterioles |
| Eosinophilic granulomatosis with polyangiitis (EGPA) |
GIANT CELL AORTITS/ARTERITIS
Epidemiology and clinical features
GCA is an immune-mediated vasculitis characterized by granulomatous inflammation involving mainly large- and medium-sized arteries. It is the most common systemic vasculitis involving the aorta in adults over the age of 50, especially females, with an estimated annual incidence of 1-3 per million 20. Involvement of extracranial branches of the carotid artery resulting in headache, jaw claudication, or visual impairment and loss is the most common presentation, often associated with constitutional symptoms. In these cases, the arteritis is clinically frequently recognized and diagnosed at the level of the temporal arteries, and referred to as Horton’s temporal arteritis. Aortitis, usually involving the thoracic aorta, is present in about 30% of cases of GCA, and may precede, be concomitant, or follow the involvement of cranial arteries. To recognize the broad spectrum of clinical presentations, the nomenclature has evolved to reflect the site of inflammation, with the term large vessel GCA, cranial GCA, and large vessel GCA with cranial involvement 21,22. There are no specific serologic markers of disease. Aortic aneurysms and dissections may be the first clinical manifestation when the aorta is involved, and the pathologic diagnosis is rendered at autopsy or after surgery. When cranial arteries are involved, histologic diagnosis by a superficial temporal artery biopsy is considered the gold standard, even though the discontinuous involvement of the vessel (“skip lesions”) is common, possibly leading to false-negative results. A consensus statement by the SCVP on the processing, interpretation, and reporting of temporal artery biopsy for arteritis was recently published 23.
Microscopic features
Histologically, GCA shows granulomatous inflammation of the media, mostly the inner half, with epithelioid macrophages and occasional giant cells associated with lymphoplasmacytic infiltrate, usually in the adventitia, in the absence of prominent necrosis (Fig. 1). The latter, if present, should prompt consideration of other entities. Inflammation affects the elastic lamina, which appears fragmented. Well-formed granulomas are usually absent. Intimal hyperplasia is common. In more advanced stages of the disease, extensive fibrosis may be present, with a “tree bark” appearance of the intimal surface, as can be noticed also macroscopically in all forms of aortitis, but the adventitia is relatively spared. Stenosis of the coronary artery ostia and the proximal coronary tract may result from the involvement of the parietal wall by the inflammatory process. Aortic valve insufficiency may result from the dilation of the aortic root/ascending aorta.
Figure 1.
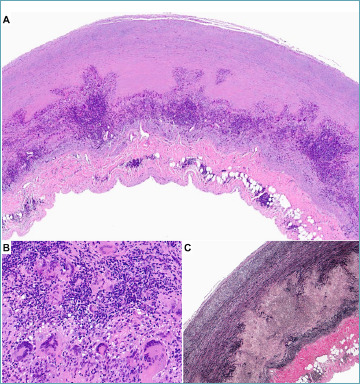
Aortitis in giant cell arteritis. A 73-year-old male with aneurysmatic dilation of the ascending aorta. (A) The panoramic view of the aorta reveals a massive granulomatous inflammation located in the outer third of the media, coupled with mild adventitial inflammation and thickening. (B) At higher magnification, numerous giant cells are admixed with activated macrophages and lymphocytes. (C) Elastic stain demonstrates the complete disruption of the tunica media. (A, B) Hematoxylin-eosin stain, (C) Weigert van Gieson stain.
In the setting of temporal artery biopsy, medial involvement by inflammation is necessary to warrant a diagnosis of active arteritis. The finding of inflammation limited to the adventitia is considered non-specific, however it should prompt the pathologist to obtain additional histological sections 23. The term “healed arterial injury” should be reserved for cases showing intimal thickening with smooth muscle cells and collagen deposition, extensive loss of the internal elastic lamina, medial fibrosis, and fibrous thickening of the adventitia with fragmentation and loss of the external elastic lamina, in absence of granulomatous inflammation. The clinical significance of “healed arterial injury” is uncertain because of the histomorphology overlap between trauma, age-related changes, and healed arteritis 23,24.
TAKAYASU’S ARTERITIS
Epidemiology and clinical features
TA, also known as “pulseless disease”, is the most common granulomatous systemic vasculitis affecting patients below the age of 50, with the majority being women 4,25. Age of onset is the major epidemiological feature that distinguishes TA from GCA. TA is a rare entity in Western Countries; it is most common in Japan, Southeast Asia, and India. The disease leads to luminal narrowing and occlusion of the proximal segments of the aortic arch branches, and aneurysms of the aorta may occur. Clinically TA manifests with an acute phase characterized by systemic symptoms such as fever, fatigue, arthralgia, etc., and a chronic phase which may occur after years whose most common presentation symptoms include impalpable pulses and stroke.
Microscopic features
Although the histologic pattern may overlap with GCA, aortic wall thickness is generally more pronounced; moreover, adventitial scarring and compact granulomas are more common in TA than in GCA. In the acute phase, the disease is characterized by diffuse inflammatory infiltrates involving the media, the media-intimal junction, and the adventitia, including the vasa vasorum. There are compact granulomas composed of macrophages with variable amounts of lymphocytes, plasma cells, and giant cells with medial necrosis. In the late phase of the disease, marked adventitial scarring and intimal thickening are typical lesions. Prominent fibrosis on the long term may lead to diffuse or multisegmented stenosing lesions interchanged by areas of diffuse dilatation or aneurysms.
IGG4-RELATED DISEASE
Epidemiology and clinical features
IgG4-related disease is a recently recognized chronic fibro-inflammatory disorder of unknown aetiology that may cause aortitis and/or periaortitis, among potential manifestations of the disease in several other organs such as pancreas, lacrimal glands, kidneys, lymph nodes and retroperitoneum. The disease was initially identified in the pancreas as autoimmune pancreatitis. It is characterized by increased serum levels of IgG4 and histopathological finding of lymphoplasmacytic infiltrate with IgG4-positive plasma cells, and a storiform pattern of fibrosis. The majority of affected patients are men older than 50 years of age. Although autoimmune pancreatitis in Japan has been estimated as 2,2 cases per 100,000 population, the true prevalence of IgG4-related disease is probably underestimated. As mentioned above, many other organs can be affected by the fibro-inflammatory lesions and presenting symptoms typically vary depending on the involved organ. Interestingly some overlap has been noted between IgG4-related retroperitoneal fibrosis and IAA, in which a small subpopulation of patients displays high numbers of IgG4+ plasma cells in the thick peri-aneurysmal fibrous layer 26. A biopsy is often required to reach a definite diagnosis. The condition generally responds well to immunosuppression, particularly in the inflammatory stage 27-29.
Microscopic features
As for other conditions, IgG4-related aortitis is often an unexpected finding at surgery or autopsy. International consensus guidelines for the pathologic diagnosis of IgG4-related aortitis/periaortitis require the presence of a lymphoplasmacytic aortitis/periaortitis with more than 50 IgG4+ plasma cells per 400 × HPF and an IgG4/IgG ratio greater than 50%, when counting the three HPFs with the highest degree of IgG4 positivity 8,30. Infections or granulomatous inflammation should be excluded. Lymphoplasmacytic infiltration, lymphoid follicle formation, obliterative adventitial phlebitis, and fibrosis, which may have a storiform pattern, are the main histopathological features (Fig. 2). Both the thoracic and abdominal aorta can be affected 31,32.
Figure 2.
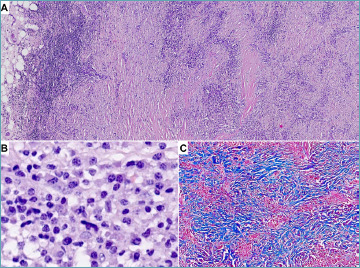
Aortitis in Ig4-related disease. A 56-year-old woman underwent surgery for an abdominal aortic aneurysm. (A) Marked adventitial thickening consistent in lymphoplasmacytic inflammation coupled with fibrosis in a storiform pattern is demonstrated at histology. (B) Abundant plasma cells are evident at higher magnification. C) Masson trichrome stain highlights storiform fibrosis that completely replaced tunica media. (A, B) Hematoxylin-eosin stain, (C) Masson trichrome stain.
CLINICALLY ISOLATED AORTITIS
The term refers to a histopathologically proven aortitis in the absence of systemic involvement by a rheumatologic disease, included in the Chapel Hill nomenclature under the category of single organ vasculitis 4. Isolated aortitis involves the ascending aorta. Histologically, the inflammation is most often granulomatous, like GCA, but can also be purely lymphoplasmacytic 33. It is not clear whether isolated aortitis represents a single entity, or should be regarded as a heterogenous group of vasculitides (involving only the aorta). Nevertheless, in every case a potential clue to further diagnosis should be evaluated with clinicopathological correlations.
INFECTIOUS AORTITIS
Infectious aortitis is a rare but important cause of aortic disease because of the considerable prognostic and therapeutic implications of such a diagnosis 14. In most cases, pathogens seed the vessel wall at sites of pre-existent pathologies, such as atherosclerotic plaques, aneurysms, and areas of medial degenerative changes, or in proximity of an aortic prosthesis. Other known risk factors for infectious aortitis include old age, hypertension, diabetes, recent surgical interventions, vascular malformations and septic embolization in case of endocarditis. Because of the correlation between pre-existent aortic damage and infection, most cases of infectious aortitis tend to involve the abdominal aorta 34. The infection causes the already weakened vessel wall to be further injured by microbial enzymes and host inflammatory cells, with subsequent damage of elastic fibers, formation or expansion of aneurysms or perforating ulcers, which may grow in just weeks. Infectious aortitis can therefore be both a cause and a complication of aortic aneurysms, but is nevertheless a rare finding, reported in around 3% of all abdominal aortic aneurysms 35. The diagnosis is often made after aneurism rupture, the pathologist is, therefore, more likely to face infectious arteritis in the autoptic setting. Pathogens (bacterial, mycobacterial, and fungal organisms) usually colonize the aortic wall directly from the lumen, by septic embolization or by hematogenous seeding; they can also arrive from the vasa vasorum or adjacent infected tissue by direct spread 36 (Tab. II).
Table II.
Summary of the main features of the most frequently encountered infectious aortitis.
| Preferential site | Pathogenetic mechanism | Histology | |
|---|---|---|---|
| Bacterial aortitis | Abdominal aorta | Seeding of aortic wall at previously damaged sites | Diffuse colliquative necrosis |
| Q fever aortitis | Abdominal aorta | Seeding of aortic wall at previously damaged sites | Diffuse colliquative necrosis and non-necrotizing granulomatous inflammation |
| Syphilitic aortitis | Thoracic aorta | Hematogenous spread and colonization from vasa vasorum | Lymphoplasmacytic infiltrate around vasa vasorum with occasional giant cells and microgummas |
| Mycobacterial aortitis | Descending aorta | Direct spread from infected tissue (e.g., lymph nodes) | Granulomatous inflammation with caseous necrosis |
| Fungal aortitis | Abdominal aorta | Seeding of aortic wall at previously damaged sites | Diffuse colliquative necrosis and necrotizing granulomatous inflammation |
Among bacteria, non-typhoidal Salmonella species represent the primary culprit, followed by Staphylococcal and Streptococcal species, and gram-negative species other than Salmonella 14,35,37. Infectious aortitis can also be a manifestation of Q fever, a zoonosis caused by the intracellular bacterium Coxiella burnetii. Cardiovascular involvement by Q fever usually takes the form of endocarditis, but aortitis has also been reported. Histopathologically, bacterial aortitis is characterized by a neutrophilic infiltrate involving most layers of the vessel wall eventually with necrosis and formation of abscesses. In cases of Q fever-related aortitis, non-necrotizing granulomas are also commonly seen 38. Special stains can be used to identify the offending microorganisms, but intraoperative cultures have a much higher yield and allow planning of the antibiotic treatment. Syphilitic (luetic) cardiovascular involvement, exceptionally rare in our current antibiotic era, used to be an important manifestation of tertiary syphilis, with significant morbidity and mortality. Syphilitic cardiovascular pathology has variable manifestations and can present as syphilitic aortitis (usually involving the ascending aorta), with or without syphilitic aortic aneurysms, syphilitic aortic valvulitis with aortic regurgitation, and syphilitic coronary ostial stenosis. Syphilitic aortitis shows the classical pathological finding of the “tree barking” of the aortic intima, which appears irregular and wrinkled, with scarring, depressions, and white, shiny plaques 39-41. At histology, treponematous infection manifests with a lymphoplasmacytic infiltrate surrounding the vasa vasorum at first and extending to the media later, with abundant plasma cells and occasional giant cells. Microgummas can also be observed, consisting of a central area of necrosis and cellular debris, surrounded by palisading histiocytes, lymphocytes, and plasma cells. Special histochemical (Warthin-Starry) and immunohistochemical stains can occasionally allow visualization of the offending Treponema organisms. Tuberculous aortitis has also dropped in relevance recently and is now very uncommon in the developed world. Mycobacterial seeding of the aortic wall usually takes place by direct spread from an adjacent site of infection, such as mediastinal lymph nodes. Tuberculous aneurysms are the typical manifestation of this condition, with the classical histopathological picture of caseating granulomatous inflammation 42. Fungal organisms able to cause infectious aortitis include members of the Candida and Aspergillus species. The pathological picture is like the one of bacterial aortitis, with the common histopathological occurrence of eosinophils.
Coronary arteritis
The term coronary arteritis or “coronaritis” refers to a group of pathological conditions characterized by inflammation of the coronary arteries. Coronary artery vasculitis is rare and may cause myocardial ischemia, angina, infarction, or sudden cardiac death 43.
Most of the reported cases occur in children with KD. Even though the prevalence is rare, the main etiological factors are non-infectious 44 (Tab. I).
CORONARY ARTERITIS IN SYSTEMIC LARGE VESSEL VASCULITIS
In adulthood, TA is the large vessel vasculitis that most often affects the coronary arteries, preferentially the coronary ostia, with an estimated prevalence of 10-45%. Histology of the coronary arteries is characterized by transmural mononuclear cell infiltrate with fragmentation of internal elastic lamina, and granulomatous arteritis. Coronary artery involvement can be diffuse and is often characterized by lumen stenosis or occlusion, with consequent myocardial infarction and ventricular arrhythmias (Fig. 3) 45.
Figure 3.
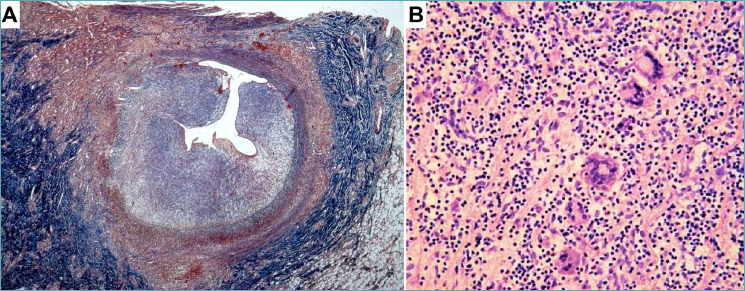
Coronary artery vasculitis in Takayasu’s disease. A 14-year-old girl was rescued after a sudden cardiac arrest. Aortography showed mild aortic dilation with aortic valve insufficiency and sub-occlusion of coronary ostia. The girl died and the autopsy revealed massive myocardial infarction with thickening of the ascending aorta, aortic arch, pulmonary arteries, left external carotid artery, and coronary ostia (modified from 82). (A) The left anterior descending coronary artery shows severe luminal narrowing because of concentric intimal hyperplasia. The tunica media appears destroyed and the adventitia is fibrotic. (B) The involved segments reveal necrotizing granulomatous inflammation with giant cells, consistent with Takayasu’s arteritis. (A) Masson trichrome stain; (B) Hematoxylin-eosin stain.
Involvement of coronary arteries is rare in GCA, being described in less than 1% of cases, mostly at autopsy. Histological features of the coronary arteries in GCA are like those described for the aorta in GCA and are characterized in the acute phase by edema, patchy necrosis, inflammation with scattered giant cells in the outer two-thirds of the media, adventitia, adventitial fat, and vasa vasorum. In the chronic phase, marked intimal and adventitial thickening of the vessels produces multisegmented stenosis with disorganized or absent elastic fibers replaced by collagen and granulation tissue. Coronary aneurysm is rarely observed 43,46.
CORONARY ARTERITIS IN SYSTEMIC MEDIUM VESSEL VASCULITIS
KAWASAKI DISEASE
Epidemiology and clinical features
KD, also known as lymph node mucocutaneous syndrome, is an acute and self-limited systemic vasculitis of the medium and small-sized vessels with unknown etiopathogenesis, which mainly involves the coronary arteries 47,48. It affects male children under 5 years of age with a predilection for Asian origin, especially Japanese, and has a greater incidence in the winter and spring months. The incidence ranges from 10 to 20 per 100,000 children < 5 years of age in the United States and Canada, to 50 to 250 per 100,000 in Japan, Taiwan, or Korea. Clinically, it presents with fever, mucocutaneous rash, palmar and/or plantar erythema, conjunctivitis, and cervical lymphadenopathy. Involvement of coronary arteries is reported in 20-30% of cases and represents the most common cause of coronary artery aneurysms 45.
Microscopic features
Histologically KD is characterized by a predominantly lymphohistiocytic inflammatory infiltrate leading to destruction of the internal elastic lamina and necrosis. Different stages of the disease have been recognized, particularly in relationship with coronary artery involvement. During the first 6-9 days, small arteries are commonly involved and macrophages, neutrophils, and lymphocytes appear in the intima and adventitia of coronary arteries. From day 10, inflammation starts to involve the entire vessel wall with internal elastic lamina disruption. Aneurysms may develop from day 12 of illness, with possible rupture or thrombosis and subsequent occlusion of the coronary vessels (Fig. 4). Persistence of inflammation may last for up to 25 days, after which the inflammatory cells gradually decline. If a myocardial infarction develops related to coronary artery occlusion, the myocardial scar will persist after the disease 49-51. Moreover, the inflammatory injury to the arterial wall with formation of aneurysms and followed by scarring repair in childhood can be a risk factor for development of premature coronary atherosclerosis later in life.
Figure 4.
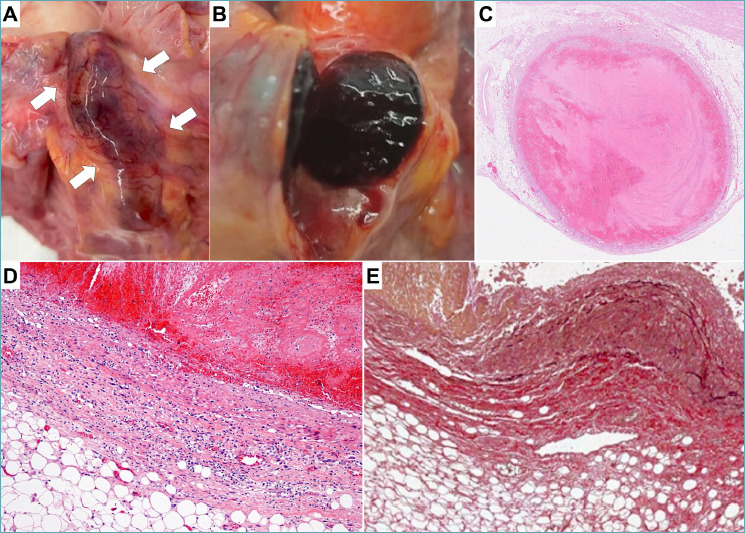
Coronary artery vasculitis in Kawasaki disease. A 6-year-old child recently diagnosed with Hodgkin lymphoma died after some episodes of chest pain. The autopsy revealed aneurysmal dilatation of both the left and right coronary arteries, with suspected luminal thrombosis. (A) A tortuous and dilated left descending coronary artery is indicated by arrows. (B-C) The lumen of coronary artery is occluded by a thrombus. (D-E) Histological analysis confirms the presence of aneurysm with thinning of the vessel wall, polymorphic inflammation and elastic disruption (D). (C, D) Hematoxylin-eosin stain; (E) Weigert van Gieson stain.
POLYARTERITIS NODOSA
PAN is a rare systemic necrotizing vasculitis not associated with ANCA, which involves medium and small-sized vessels. It affects middle-aged and older adult men 52. Most cases are idiopathic, and less commonly it is associated with hepatitis B, hepatitis C, and other microorganisms. It may also be associated with Sjogren’s syndrome, rheumatoid arthritis, and hairy cell leukemia. Pathological features are those of a segmental vasculitis, showing a mixed inflammatory infiltrate composed of lymphocytes, macrophages, neutrophils, and eosinophils, with a resultant destruction of the vessel wall and massive fibrinoid necrosis. Unlike KD, coronary involvement in PAN is rare, and occurs in the contest of systemic disease, with peripheral nerve and skin as the most frequently affected tissues. It can take the form of stenosis, occlusion, aneurysm, or dissection (Fig. 5) 45,53,54.
Figure 5.
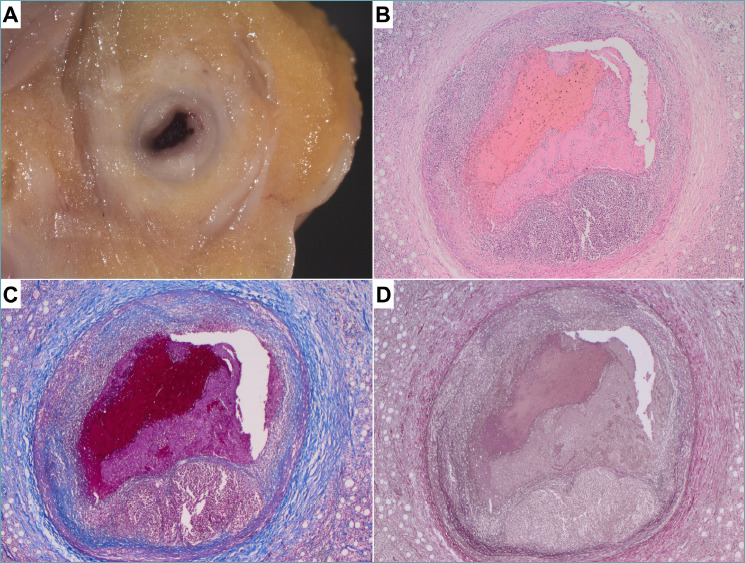
Coronary artery vasculitis in panarteritis nodosa. A 49-year-old man presenting with chest pain due to a suspected myocardial infarction died after a short hospitalization. Panarteritis nodosa was diagnosed only at autopsy. (A) Gross analysis of coronary arteries revealed diffuse concentric lesions. (B-D) At histology, transmural necrotizing polymorphic inflammation was detected with thrombotic occlusion of the right coronary artery. (B) Hematoxylin-eosin stain, (C) Heidenhain’s trichrome stain, (D) Weigert van Gieson stain.
CORONARY ARTERITIS IN SMALL VESSEL VASCULITIS
Small vessel vasculitis, particularly ANCA-associated, may involve the heart, resulting in myocarditis, pericarditis, valvulitis, and rarely coronary arteritis and myocardial infarction. Among this group, EGPA, formerly Churg Straus syndrome, is clinically characterized by the coexistence of asthma, rhinosinusitis, and peripheral eosinophilia, with positive ANCA in about 40% of cases. The average age of onset is between 38-54 years, peaking at 40 years. Cardiac involvement is reported in about one-third of patients, in the form of endo-myocarditis, especially in cases with negative ANCA tests and high eosinophil counts 55. Coronary artery disease has been reported in anecdotal cases and is histologically characterized by eosinophil-rich necrotizing granulomatous inflammation of the vessel wall.
To be considered in the differential diagnosis with EGPA is the isolated eosinophilic coronary periarteritis, a condition associated with spontaneous coronary artery dissection and sudden death in adult women, which rarely occurs in the peripartum period. In such cases, a mixed, predominantly eosinophilic inflammatory infiltrate confined to the periadventitial space of epicardial coronary arteries (usually the left anterior descending artery) is associated with dissection and occlusion of the vascular lumen due to compression by massive dissection hematoma (Fig. 5). This condition is not related to EGPA, although it may be associated with peripheral eosinophilia 56. The mechanism linking the eosinophilic infiltrate to dissection has not yet been elucidated, although an increase in eosinophil activity due to hormonal interactions during pregnancy has been suggested 57,58.
GPA, also known as Wegener’s granulomatosis, is the most frequent ANCA vasculitis, characterized clinically by a pneumo-renal syndrome associated with otorhinolaryngologic manifestations; cardiac involvement is less frequent and involves valves, pericardium, and rarely coronary arteries. Microscopically it is characterized by the early formation of neutrophilic microabscesses leading to poorly formed granulomas consisting of giant cells surrounded by plasma cells, lymphocytes, and dendritic cells, with central necrosis; granulomas in turn cause partial or total occlusion of blood vessels 8,59.
INFECTIVE CORONARY VASCULITIS
Infective coronary vasculitis is very rare, typically identified posthumously. Various pathogens are reported to induce coronary arteritis, including viruses, bacteria, and fungi. Vasculitis due to bacteria commonly results from a direct extension, hematogenous spread or embolization (endocarditis), sometimes leading to the formation of “mycotic aneurysms”.
Gram-positive pathogens, particularly Staphylococci and Streptococci, are common culprits for large or medium-vessel vasculitis. Further, Streptococcus species have also been linked to PAN and KD 60,61. Within the wide group of gram-negative bacteria, Salmonella and Treponema pallidum are the most common pathogens to cause vasculitis, usually manifesting as aortitis. The involvement of the coronary arteries by Salmonella spp. is almost anecdotal 62, while syphilitic ostial coronaritis has been described also 63. In a large autoptic study on Leptospirosis, coronaritis was a common finding (21/45 cases) 64. The characteristic histological feature was the infiltration of the intima by lymphocytes, macrophages, and/or neutrophils; further involvement of the media and adventitia was present in some cases. Anitschow cells and mononuclear inflammation were documented around the intramural arteries. Thropherima whippeli, the pathogen that causes Whipple’s disease, can infect the arteries of several organs, including the coronary tree. In a case series of patients with fatal Whipple’s disease, there was a broad spectrum of coronary lesions associated with the presence of PAS-positive bacilli, ranging florid inflammation towards scarring 65. Mycobacterium tuberculosis is a well-known cause of aortitis, whereas coronary arteries involvement has been described rarely, and mostly in endemic regions 66,67. Disease extension to the heart often occurs through hematogenous seeding from the lungs or by lymphatic spread. Autopsy findings from a case with sudden cardiac death showed variable features in both right and left coronary arteries, including luminal narrowing, dense transmural lymphohistiocytic infiltrates, focal necrosis, poorly-defined histiocytic aggregates, and occasional Langhans cells 68. Vasculitis associated with invasive fungal infections is rare, occurring mainly in immunocompromised or specific populations, such as intravenous drug users or inhabitants from countries with endemic mycoses. Most cases of invasive fungal vasculitis are caused by Aspergillus and Coccidioides. A wide variety of viruses have been implicated in vasculitis through different mechanisms, ranging from direct endothelial cell invasion to immune complex deposition. Notable pathogens include HBV and HCV, causing forms of PAN, albeit with rare coronary involvement. HIV infection is associated with vasculitis affecting the aorta and major branches, and PAN-like forms have also been described. Chronic active EBV infection is linked to medium- to large-vessel vasculitis, with coronary artery aneurysms as a typical complication 69,70; it is also linked with KD 71. A broad spectrum of coronary changes including aneurysms, chronic vasculitis, and luminal narrowing due to myofibroblastic proliferation, have been described in a young adult suffering from KD associated with Parvovirus 19 infection 72.
The association of New Haven Coronavirus and KD has been known for a long time 73. During the initial phases of the COVID-19 pandemic, it became evident that children infected with SARS-CoV-2 might occasionally exhibit a severe hyperinflammatory state, recognized as pediatric multisystemic syndrome 74. This condition shares similarities with KD and toxic shock syndrome, encompassing artery dilatation/aneurysms in a subgroup of patients. Interestingly, Kawasaki-like disease typically manifests in individuals without apparent COVID-19 pneumonia or ongoing infections; this observation implies that direct viral infection is less likely to be a contributing factor. Aside from this specific population, most people who died from COVID-19 did not exhibit vasculitis of major coronary arteries 75,76. Instead, a post-mortem study focused on cardiac changes showed that COVID-19 leads to a small vessel endothelialitis: arterioles, venules, and epicardial capillaries exhibited variable degrees of lymphomonocytic inflammation, compared to epicardial coronary arteries that appeared almost unharmed 77.
THE ROLE OF THE PATHOLOGIST
Aortic and coronary vascular inflammatory diseases are rare entities that the pathologist can seldom encounters during their routine diagnostic activities. The settings in which the diagnosis of a vasculitis can be performed are the surgical pathology of the aorta, the biopsy of the temporal artery, and the autopsy.
General recommendations for histological processing of aortic and temporal artery specimens are included in the SCVP/AECVP Consensus documents 8,23,78. The most frequently confronted issue when approaching aortic or temporal artery specimens is the differential diagnosis with atherosclerosis and age-related changes (‘arteriosclerosis’). Atherosclerosis in surgically resected segments of the aorta can have different grades ranging from mild intimal thickening to confluent areas of atherosclerotic plaque formation with destruction or loss of 1/3 or more of the media. In addition, aortic atherosclerosis can be associated with a spectrum of different degrees of plaque inflammation with or without plaque complication. In case of an excessive neutrophilic inflammation near an atherosclerotic plaque or excessive adventitial inflammation coupled with severe atherosclerosis, the report can include these findings but it may be not possible to definitively exclude either infection or periaortitis, respectively.
When dealing with suspected aortitis or temporal arteritis, reviewing the clinical, radiologic, and laboratory findings is paramount. In some cases, a conclusive subclassification cannot be obtained, but the main recommendation is to categorize the pathology based on the pattern of inflammation (granulomatous/giant cell pattern, lymphoplasmacytic pattern, mixed inflammatory pattern, suppurative pattern). Each one of these patterns can address a specific systemic disease 8. The most commonly encountered pattern is the granulomatous/giant cell type of inflammation with clusters of activated epithelioid macrophages; however, the finding of giant cells does not warrant a diagnosis of GCA. The presence of additional features (necrosis, well-formed or necrotizing granulomas, extensive neutrophils) should orient towards the specific disease (Tab. III).
Table III.
Histologic differential diagnosis of aortitis with granulomatous inflammation
| Lympho-plasmacytic infiltrate | Eosinophilic infiltrate | Epithelioid macrophages | Compact granuloma | Caseous necrosis | Colliquative necrosis | |
|---|---|---|---|---|---|---|
| Giant cell arteritis (GCA) | + | - | + | -/+ | - | - |
| Takayasu’s arteritis (TA) | + | - | + | + | - | + |
| Granulomatosis with polyangiitis (GPA) | + | - | + | - | - | + |
| Eosinophilic granulomatosis with polyangiitis (EGPA) | + | + | + | - | - | + |
| Mycobacterial aortitis | + | - | + | + | + | - |
| Fungal aortitis | + | + | + | + | - | + |
| Q fever aortitis | + | - | + | + | - | + |
The autopsy setting offers the unique opportunity to have the whole body available for analysis and sampling. The value of autopsy is demonstrated by both the possibility to eventually detect clinically silent diseases and the chance to investigate a selected population for research purposes systemically. Routine sampling of the heart and the coronary arteries is recommended in any case, with additional samples at the discretion of the examiner. In certain settings, evaluation of the heart and aorta by a cardiac pathologist is indicated. Specific recommendations for the investigation of sudden death cases are available 79,80. Sudden death as the first manifestation of coronary artery vasculitis is not infrequent, with TA and KD being the most frequently reported causes. When coronary artery involvement by vasculitis is suspected, careful inspection of the coronary ostia and multiple transverse parallel cuts at the level of each major epicardial branch is required, with mapped histological sampling of the grossly affected segments. In some cases, similarly to aortic vasculitis, histopathology of the coronary artery wall is not diagnostic for a specific type of vasculitis. Therefore, final categorization usually relies on the combination of pathological, clinical, and serological parameters 81.
Conclusions
Inflammation of the aorta and of coronary arteries can progress undetected during the clinical course of a patient, until the moment of histopathological diagnosis in surgical specimen (or at autopsy). Nevertheless, correlation with clinical information is the cornerstone of every pathological diagnosis, particularly in cardiovascular pathology. The knowledge of the commonly accepted nomenclature scheme for vasculitides is the starting point for adopting a global approach not based solely on histopathological features. Although the variability of clinical subclassifications acts as a limitation in identifying the distinctive features of these conditions, any pathologist should be able to recognize specific histological patterns in vascular inflammatory pathologies and to correlate them with the entire clinical setting.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing interests.
FUNDING
MDG, SR, and CB are supported by the Registry for Cardio-cerebro-vascular Pathology, Veneto Region, Italy (DGR n. 151 24/02/2023).
AUTHORS’ CONTRIBUTION
MDG-CG conceptualisation; MDG-CG-CBald-CS-AAsc methodology; CBass, GdA, AAng, CRTdG, ACvdW supervision, MDG-AASc-CBald-CG-CS-AM-AP-SR writing – original draft; CB, GdA, AAng, CRTdG, ACvdW writing – review & editing.
ETHICAL CONSIDERATION
Ethical approval was waived due to the retrospective nature of this work.
Figures and tables
Figure 6.

Coronary artery dissection and eosinophilic coronary periarteritis. A 35-year-old woman was admitted to the emergency room because of acute thoracic pain. She died shortly afterward. (A, B) At autopsy, coronary artery dissection of the left anterior descending and diagonal branches was revealed with serial sections of the coronary tree. (C) Histological analysis demonstrated a marked eosinophilic infiltrate at the level of the adventitia in the culprit vessel (C, Hematoxylin-eosin stain). A diagnosis of eosinophilic coronary periarteritis in the setting of coronary artery dissection was made.
References
- 1.Watts RA, Robson J. Introduction, epidemiology and classification of vasculitis. Best Pract Res Clin Rheumatol. 2018;32(1):3-20. https://doi.org/10.1016/j.berh.2018.10.003 10.1016/j.berh.2018.10.003 [DOI] [PubMed] [Google Scholar]
- 2.Ponte C, Águeda AF, Luqmani RA. Clinical features and structured clinical evaluation of vasculitis. Best Pract Res Clin Rheumatol. 2018;32(1):31-51. https://doi.org/10.1016/j.berh.2018.10.001 10.1016/j.berh.2018.10.001 [DOI] [PubMed] [Google Scholar]
- 3.Silveira LH. Cardiovascular Manifestations of Systemic Vasculitides. Curr Rheumatol Rep. 2020;22(10):72. https://doi.org/10.1007/s11926-020-00952-1 10.1007/s11926-020-00952-1 [DOI] [PubMed] [Google Scholar]
- 4.Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65(1):1-11. https://doi.org/10.1002/art.37715 10.1002/art.37715 [DOI] [PubMed] [Google Scholar]
- 5.Domínguez-Quintana M, Alba MA, Hinojosa-Azaola A. Classification of ANCA-associated vasculitis: differences based on ANCA specificity and clinicopathologic phenotype. Rheumatol Int. 2021;41(10):1717-1728. https://doi.org/10.1007/s00296-021-04966-5 10.1007/s00296-021-04966-5 [DOI] [PubMed] [Google Scholar]
- 6.Torp CK, Brüner M, Keller KK, et al. Vasculitis therapy refines vasculitis mechanistic classification. Autoimmun Rev. 2021;20(6):102829. https://doi.org/10.1016/j.autrev.2021.102829 10.1016/j.autrev.2021.102829 [DOI] [PubMed] [Google Scholar]
- 7.Amemiya K, Ishibashi-Ueda H, Mousseaux E, Achouh P, Ochiai M, Bruneval P. Comparison of the damage to aorta wall in aortitis versus noninflammatory degenerative aortic diseases. Cardiovasc Pathol. 2021;52:107329. https://doi.org/10.1016/j.carpath.2021.107329 10.1016/j.carpath.2021.107329 [DOI] [PubMed] [Google Scholar]
- 8.Stone JR, Bruneval P, Angelini A, et al. Consensus statement on surgical pathology of the aorta from the Society for Cardiovascular Pathology and the Association for European Cardiovascular Pathology: I. Inflammatory diseases. Cardiovasc Pathol. 2015;24(5):267-278. https://doi.org/10.1016/j.carpath.2015.05.001 10.1016/j.carpath.2015.05.001 [DOI] [PubMed] [Google Scholar]
- 9.Yeung RSM. Kawasaki disease: update on pathogenesis. Curr Opin Rheumatol. 2010;22(5):551-560. https://doi.org/10.1097/BOR.0b013e32833cf051 10.1097/BOR.0b013e32833cf051 [DOI] [PubMed] [Google Scholar]
- 10.Chatta P, Park E, Ghatnekar N, Kirk S, Hilliard A, Parwani P. A case report of myocardial infarction with non-obstructive coronary arteries as the initial presentation of eosinophilic granulomatosis with polyangiitis. Eur Heart J Case Rep. 2022;6(1):ytac021. https://doi.org/10.1093/ehjcr/ytac021 10.1093/ehjcr/ytac021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuminetti G, Regazzoni V, Vizzardi E, et al. Cardiac ANCA-associated vasculitis mimicking an acute coronary syndrome. Int J Cardiol. 2016;214:200-201. https://doi.org/10.1016/j.ijcard.2016.03.117 10.1016/j.ijcard.2016.03.117 [DOI] [PubMed] [Google Scholar]
- 12.Rodrigues T, Aguiar-Ricardo I, Menezes MN, et al. Rapidly Progressive Coronary Aneurysm: A Rare Case of Isolated Coronary Vasculitis With Recurrent Myocardial Infarction. JACC Case Rep. 2022;4(9):538-542. https://doi.org/10.1016/j.jaccas.2022.02.022 10.1016/j.jaccas.2022.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tahtouh R, Al Khodari K, Al Ali Alhasan J, Awwad I, Arabi A. Acute Myocardial Infarction Secondary to Triple Coronary Arteries Dissection in a Patient With Takayasu Vasculitis. JACC Case Rep. 2023;18:101905. https://doi.org/10.1016/j.jaccas.2023.101905 10.1016/j.jaccas.2023.101905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gornik HL, Creager MA. Aortitis. Circulation. 2008;117(23):3039-3051. https://doi.org/10.1161/CIRCULATIONAHA.107.760686 10.1161/CIRCULATIONAHA.107.760686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ladich E, Yahagi K, Romero ME, Virmani R. Vascular diseases: aortitis, aortic aneurysms, and vascular calcification. Cardiovasc Pathol. 2016;25(5):432-441. https://doi.org/10.1016/j.carpath.2016.07.002 10.1016/j.carpath.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 16.Saadoun D, Vautier M, Cacoub P. Medium- and Large-Vessel Vasculitis. Circulation. 2021;143(3):267-282. https://doi.org/10.1161/CIRCULATIONAHA.120.046657 10.1161/CIRCULATIONAHA.120.046657 [DOI] [PubMed] [Google Scholar]
- 17.Leone O, Pacini D, Foà A, et al. Redefining the histopathologic profile of acute aortic syndromes: Clinical and prognostic implications. J Thorac Cardiovasc Surg. 2018;156(5):1776-1785.e6. https://doi.org/10.1016/j.jtcvs.2018.04.086 10.1016/j.jtcvs.2018.04.086 [DOI] [PubMed] [Google Scholar]
- 18.Leone O, Corsini A, Pacini D, et al. The complex interplay among atherosclerosis, inflammation, and degeneration in ascending thoracic aortic aneurysms. J Thorac Cardiovasc Surg. 2020;160(6):1434-1443.e6. https://doi.org/10.1016/j.jtcvs.2019.08.108 10.1016/j.jtcvs.2019.08.108 [DOI] [PubMed] [Google Scholar]
- 19.Halushka MK, Angelini A, Bartoloni G, et al. Consensus statement on surgical pathology of the aorta from the Society for Cardiovascular Pathology and the Association For European Cardiovascular Pathology: II. Noninflammatory degenerative diseases — nomenclature and diagnostic criteria. Cardiovascular Pathology. 2016;25(3):247-257. https://doi.org/10.1016/j.carpath.2016.03.002 10.1016/j.carpath.2016.03.002 [DOI] [PubMed] [Google Scholar]
- 20.Salvarani C, Crowson CS, O’Fallon WM, Hunder GG, Gabriel SE. Reappraisal of the epidemiology of giant cell arteritis in Olmsted County, Minnesota, over a fifty-year period. Arthritis Rheum. 2004;51(2):264-268. https://doi.org/10.1002/art.20227 10.1002/art.20227 [DOI] [PubMed] [Google Scholar]
- 21.Dejaco C, Ramiro S, Bond M, et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice: 2023 update. Ann Rheum Dis. Published online August 7, 2023:ard-2023-224543. https://doi.org/10.1136/ard-2023-224543 10.1136/ard-2023-224543 [DOI] [PubMed] [Google Scholar]
- 22.Hellmich B, Agueda A, Monti S, et al. 2018 Update of the EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis. 2020;79(1):19-30. https://doi.org/10.1136/annrheumdis-2019-215672 10.1136/annrheumdis-2019-215672 [DOI] [PubMed] [Google Scholar]
- 23.Nair V, Fishbein GA, Padera R, et al. Consensus statement on the processing, interpretation and reporting of temporal artery biopsy for arteritis. Cardiovasc Pathol. 2023;67:107574. https://doi.org/10.1016/j.carpath.2023.107574 10.1016/j.carpath.2023.107574 [DOI] [PubMed] [Google Scholar]
- 24.Cavazza A, Muratore F, Boiardi L, et al. Inflamed temporal artery: histologic findings in 354 biopsies, with clinical correlations. Am J Surg Pathol. 2014;38(10):1360-1370. https://doi.org/10.1097/PAS.0000000000000244 10.1097/PAS.0000000000000244 [DOI] [PubMed] [Google Scholar]
- 25.Weyand CM, Goronzy JJ. Medium- and large-vessel vasculitis. N Engl J Med. 2003;349(2):160-169. https://doi.org/10.1056/NEJMra022694 10.1056/NEJMra022694 [DOI] [PubMed] [Google Scholar]
- 26.Kasashima S, Zen Y. IgG4-related inflammatory abdominal aortic aneurysm. Curr Opin Rheumatol. 2011;23(1):18-23. https://doi.org/10.1097/BOR.0b013e32833ee95f 10.1097/BOR.0b013e32833ee95f [DOI] [PubMed] [Google Scholar]
- 27.Frulloni L, Lunardi C, Simone R, et al. Identification of a novel antibody associated with autoimmune pancreatitis. N Engl J Med. 2009;361(22):2135-2142. https://doi.org/10.1056/NEJMoa0903068 10.1056/NEJMoa0903068 [DOI] [PubMed] [Google Scholar]
- 28.Kamisawa T, Zen Y, Pillai S, Stone JH. IgG4-related disease. Lancet. 2015;385(9976):1460-1471. https://doi.org/10.1016/S0140-6736(14)60720-0 10.1016/S0140-6736(14)60720-0 [DOI] [PubMed] [Google Scholar]
- 29.Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012;366(6):539-551. https://doi.org/10.1056/NEJMra1104650 10.1056/NEJMra1104650 [DOI] [PubMed] [Google Scholar]
- 30.Deshpande V, Zen Y, Chan JKC, et al. Consensus statement on the pathology of IgG4-related disease. Modern Pathology. 2012;25(9):1181-1192. https://doi.org/10.1038/modpathol.2012.72 10.1038/modpathol.2012.72 [DOI] [PubMed] [Google Scholar]
- 31.Stone JH, Khosroshahi A, Deshpande V, Stone JR. IgG4-related systemic disease accounts for a significant proportion of thoracic lymphoplasmacytic aortitis cases. Arthritis Care Res (Hoboken). 2010;62(3):316-322. https://doi.org/10.1002/acr.20095 10.1002/acr.20095 [DOI] [PubMed] [Google Scholar]
- 32.Stone JR. Aortitis, periaortitis, and retroperitoneal fibrosis, as manifestations of IgG4-related systemic disease. Curr Opin Rheumatol. 2011;23(1):88-94. https://doi.org/10.1097/BOR.0b013e3283412f7c 10.1097/BOR.0b013e3283412f7c [DOI] [PubMed] [Google Scholar]
- 33.Maleszewski JJ, Younge BR, Fritzlen JT, et al. Clinical and pathological evolution of giant cell arteritis: a prospective study of follow-up temporal artery biopsies in 40 treated patients. Mod Pathol. 2017;30(6):788-796. https://doi.org/10.1038/modpathol.2017.10 10.1038/modpathol.2017.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdelazeem B, Kambalapalli S, Lahmar A, Yousaf A, Kusz H. Infectious Aortitis: Case Report and Literature Review. Cureus. 2022;14(3):e23198. https://doi.org/10.7759/cureus.23198 10.7759/cureus.23198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foote EA, Postier RG, Greenfield RA, Bronze MS. Infectious Aortitis. Curr Treat Options Cardiovasc Med. 2005;7(2):89-97. https://doi.org/10.1007/s11936-005-0010-6 10.1007/s11936-005-0010-6 [DOI] [PubMed] [Google Scholar]
- 36.Miller DV, Oderich GS, Aubry MC, Panneton JM, Edwards WD. Surgical pathology of infected aneurysms of the descending thoracic and abdominal aorta: Clinicopathologic correlations in 29 cases (1976 to 1999). Human Pathology. 2004;35(9):1112-1120. https://doi.org/10.1016/j.humpath.2004.05.013 10.1016/j.humpath.2004.05.013 [DOI] [PubMed] [Google Scholar]
- 37.Gardini G, Zanotti P, Pucci A, et al. Non-typhoidal Salmonella aortitis. Infection. 2019;47(6):1059-1063. https://doi.org/10.1007/s15010-019-01344-z 10.1007/s15010-019-01344-z [DOI] [PubMed] [Google Scholar]
- 38.Botelho-Nevers E, Fournier PE, Richet H, et al. Coxiella burnetii infection of aortic aneurysms or vascular grafts: report of 30 new cases and evaluation of outcome. Eur J Clin Microbiol Infect Dis. 2007;26(9):635-640. https://doi.org/10.1007/s10096-007-0357-6 10.1007/s10096-007-0357-6 [DOI] [PubMed] [Google Scholar]
- 39.Heggtveit HA. Syphilitic Aortitis: A Clinicopathologic Autopsy Study of 100 Cases, 1950 to 1960. Circulation. 1964;29(3):346-355. https://doi.org/10.1161/01.CIR.29.3.346 10.1161/01.CIR.29.3.346 [DOI] [PubMed] [Google Scholar]
- 40.Roberts WC, Ko JM, Vowels TJ. Natural history of syphilitic aortitis. Am J Cardiol. 2009;104(11):1578-1587. https://doi.org/10.1016/j.amjcard.2009.07.031 10.1016/j.amjcard.2009.07.031 [DOI] [PubMed] [Google Scholar]
- 41.Tavora F, Cresswell N, Li L, Ripple M, Solomon C, Burke A. Comparison of necropsy findings in patients with sarcoidosis dying suddenly from cardiac sarcoidosis versus dying suddenly from other causes. The American journal of cardiology. 2009;104(4):571-577. https://doi.org/10.1016/j.amjcard.2009.03.068 10.1016/j.amjcard.2009.03.068 [DOI] [PubMed] [Google Scholar]
- 42.Long R, Guzman R, Greenberg H, Safneck J, Hershfield E. Tuberculous Mycotic Aneurysm of the Aorta: Review of Published Medical and Surgical Experience. Chest. 1999;115(2):522-531. https://doi.org/10.1378/chest.115.2.522 10.1378/chest.115.2.522 [DOI] [PubMed] [Google Scholar]
- 43.Norita K, de Noronha SV, Sheppard MN. Sudden cardiac death caused by coronary vasculitis. Virchows Arch. 2012;460(3):309-318. https://doi.org/10.1007/s00428-011-1173-z 10.1007/s00428-011-1173-z [DOI] [PubMed] [Google Scholar]
- 44.Vaideeswar P, Verma R, Gupta R. Infective coronary arteritis: a pathological analysis at autopsy. Hum Pathol. 2012;43(12):2334-2341. https://doi.org/10.1016/j.humpath.2012.04.005 10.1016/j.humpath.2012.04.005 [DOI] [PubMed] [Google Scholar]
- 45.Khanna S, Garikapati K, Goh DSL, et al. Coronary artery vasculitis: a review of current literature. BMC Cardiovasc Disord. 2021;21(1):7. https://doi.org/10.1186/s12872-020-01813-6 10.1186/s12872-020-01813-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lie JT. Coronary vasculitis. A review in the current scheme of classification of vasculitis. Arch Pathol Lab Med. 1987;111(3):224-233. [PubMed] [Google Scholar]
- 47.Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi. 1967;16(3):178-222. [PubMed] [Google Scholar]
- 48.Kawasaki T. Kawasaki disease. Int J Rheum Dis. 2014;17(5):597-600. https://doi.org/10.1111/1756-185X.12408 10.1111/1756-185X.12408 [DOI] [PubMed] [Google Scholar]
- 49.Fujiwara H, Hamashima Y. Pathology of the heart in Kawasaki disease. Pediatrics. 1978;61(1):100-107. [PubMed] [Google Scholar]
- 50.Fujiwara T, Fujiwara H, Nakano H. Pathological features of coronary arteries in children with Kawasaki disease in which coronary arterial aneurysm was absent at autopsy. Quantitative analysis. Circulation. 1988;78(2):345-350. https://doi.org/10.1161/01.cir.78.2.345 10.1161/01.cir.78.2.345 [DOI] [PubMed] [Google Scholar]
- 51.Takahashi K, Oharaseki T, Yokouchi Y. Histopathological aspects of cardiovascular lesions in Kawasaki disease. Int J Rheum Dis. 2018;21(1):31-35. https://doi.org/10.1111/1756-185X.13207 10.1111/1756-185X.13207 [DOI] [PubMed] [Google Scholar]
- 52.Mahr A, Guillevin L, Poissonnet M, Aymé S. Prevalences of polyarteritis nodosa, microscopic polyangiitis, Wegener’s granulomatosis, and Churg-Strauss syndrome in a French urban multiethnic population in 2000: a capture-recapture estimate. Arthritis Rheum. 2004;51(1):92-99. https://doi.org/10.1002/art.20077 10.1002/art.20077 [DOI] [PubMed] [Google Scholar]
- 53.Mohankumar SP, Das S, Likitha P, et al. Kawasaki disease or polyarteritis nodosa: coronary involvement, a diagnostic conundrum. Rheumatol Int. 2023;43(12):2327-2331. https://doi.org/10.1007/s00296-023-05388-1 10.1007/s00296-023-05388-1 [DOI] [PubMed] [Google Scholar]
- 54.Raman SV, Basso C, Tandri H, Taylor MR. Imaging phenotype vs genotype in nonhypertrophic heritable cardiomyopathies: dilated cardiomyopathy and arrhythmogenic right ventricular cardiomyopathy. CirculationCardiovascular imaging. 2010;3(6):753-765. https://doi.org/10.1161/CIRCIMAGING.110.957563 [doi] 10.1161/CIRCIMAGING.110.957563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neumann T, Manger B, Schmid M, et al. Cardiac involvement in Churg-Strauss syndrome: impact of endomyocarditis. Medicine (Baltimore). 2009;88(4):236-243. https://doi.org/10.1097/MD.0b013e3181af35a5 10.1097/MD.0b013e3181af35a5 [DOI] [PubMed] [Google Scholar]
- 56.Robinowitz M, Virmani R, McAllister HA, Jr, U null. Spontaneous coronary artery dissection and eosinophilic inflammation: a cause and effect relationship? Am J Med. 1982;72(6):923-928. https://doi.org/10.1016/0002-9343(82)90853-1 10.1016/0002-9343(82)90853-1 [DOI] [PubMed] [Google Scholar]
- 57.Borczuk AC, van Hoeven KH, Factor SM. Review and hypothesis: the eosinophil and peripartum heart disease (myocarditis and coronary artery dissection)--coincidence or pathogenetic significance? Cardiovasc Res. 1997;33(3):527-532. https://doi.org/10.1016/s0008-6363(96)00257-x 10.1016/s0008-6363(96)00257-x [DOI] [PubMed] [Google Scholar]
- 58.Pitliya A, Datta S, Kalayci A, et al. Eosinophilic inflammation in spontaneous coronary artery dissection: A potential therapeutic target? Med Hypotheses. 2018;121:91-94. https://doi.org/10.1016/j.mehy.2018.09.039 10.1016/j.mehy.2018.09.039 [DOI] [PubMed] [Google Scholar]
- 59.Blockmans D, Baeyens H, Van Loon R, Lauwers G, Bobbaers H. Periaortitis and aortic dissection due to Wegener’s granulomatosis. Clin Rheumatol. 2000;19(2):161-164. https://doi.org/10.1007/s100670050038 10.1007/s100670050038 [DOI] [PubMed] [Google Scholar]
- 60.Belizna CC, Hamidou MA, Levesque H, Guillevin L, Shoenfeld Y. Infection and vasculitis. Rheumatology (Oxford). 2009;48(5):475-482. https://doi.org/10.1093/rheumatology/kep026 10.1093/rheumatology/kep026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Somer T, Finegold SM. Vasculitides associated with infections, immunization, and antimicrobial drugs. Clin Infect Dis. 1995;20(4):1010-1036. https://doi.org/10.1093/clinids/20.4.1010 10.1093/clinids/20.4.1010 [DOI] [PubMed] [Google Scholar]
- 62.McGee MB, Khan MY. Ruptured mycotic aneurysm of a coronary artery. A fatal complication of Salmonella infection. Arch Intern Med. 1980;140(8):1097-1098. [PubMed] [Google Scholar]
- 63.Michaud P, Termet H, Chassignolle J, Rassat JP, Dureau G, Teneriello F. Syphilitic ostial coronaritis. Analysis of 6 observations. J Cardiovasc Surg (Torino). 1971;12(3):254-263. [PubMed] [Google Scholar]
- 64.Chakurkar G, Vaideeswar P, Pandit SP, Divate SA. Cardiovascular lesions in leptospirosis: an autopsy study. J Infect. 2008;56(3):197-203. https://doi.org/10.1016/j.jinf.2007.12.007 10.1016/j.jinf.2007.12.007 [DOI] [PubMed] [Google Scholar]
- 65.James TN. On the wide spectrum of abnormalities in the coronary arteries of Whipple’s disease. Coron Artery Dis. 2001;12(2):115-125. https://doi.org/10.1097/00019501-200103000-00005 10.1097/00019501-200103000-00005 [DOI] [PubMed] [Google Scholar]
- 66.Chan S. An unusual case of mycobacterium tuberculous coronary arteritis and thrombosis resulting in acute myocardial infarction. Forensic Sci Med Pathol. 2018;14(3):390-394. https://doi.org/10.1007/s12024-018-0002-y 10.1007/s12024-018-0002-y [DOI] [PubMed] [Google Scholar]
- 67.Kinare SG, Bhatia BI. Tuberculous coronary arteritis with aneurysm of the ventricular septum. Chest. 1971;60(6):613-616. https://doi.org/10.1378/chest.60.6.613 10.1378/chest.60.6.613 [DOI] [PubMed] [Google Scholar]
- 68.Paliwal P, Jain S, Ahuja A, Mittal S, Chauhan DS. Coronary arteritis as a cause of sudden cardiac death in a young girl. Autops Case Rep. 2021;11:e2021310. https://doi.org/10.4322/acr.2021.310 10.4322/acr.2021.310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jamal O, Sahel N, Saouab R, et al. Fatal Systemic Vasculitis Associated with Chronic Active Epstein-Barr Virus Infection. Mo Med. 2021;118(3):226-232. [PMC free article] [PubMed] [Google Scholar]
- 70.Kang R, Tanaka TD, Ogasawara Y, Yoshimura M. A Rare Complication of Chronic Active Epstein-Barr Virus Infection. JACC Case Rep. 2020;2(5):756-759. https://doi.org/10.1016/j.jaccas.2020.03.022 10.1016/j.jaccas.2020.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miyabe C, Miyabe Y, Miyata R, Ishiguro N. Pathogens in Vasculitis: Is It Really Idiopathic? JMA J. 2021;4(3):216-224. https://doi.org/10.31662/jmaj.2021-0021 10.31662/jmaj.2021-0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Flossdorf S, Schiwy-Bochat KH, Teifel D, Fries JWU, Rothschild MA. Sudden death of a young adult with coronary artery vasculitis, coronary aneurysms, parvovirus B19 infection and Kawasaki disease. Forensic Sci Med Pathol. 2020;16(3):498-503. https://doi.org/10.1007/s12024-020-00263-y 10.1007/s12024-020-00263-y [DOI] [PubMed] [Google Scholar]
- 73.Esper F, Shapiro ED, Weibel C, Ferguson D, Landry ML, Kahn JS. Association between a novel human coronavirus and Kawasaki disease. J Infect Dis. 2005;191(4):499-502. https://doi.org/10.1086/428291 10.1086/428291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jiang L, Tang K, Levin M, et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis. 2020;20(11):e276-e288. https://doi.org/10.1016/S1473-3099(20)30651-4 10.1016/S1473-3099(20)30651-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Basso C, Leone O, Rizzo S, et al. Pathological features of COVID-19-associated myocardial injury: a multicentre cardiovascular pathology study. European Heart Journal. 2020;41(39). https://doi.org/10.1093/eurheartj/ehaa664 10.1093/eurheartj/ehaa664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lindner D, Fitzek A, Bräuninger H, et al. Association of Cardiac Infection With SARS-CoV-2 in Confirmed COVID-19 Autopsy Cases. JAMA Cardiol. 2020;5(11):1281-1285. https://doi.org/10.1001/jamacardio.2020.3551 10.1001/jamacardio.2020.3551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maccio U, Zinkernagel AS, Shambat SM, et al. SARS-CoV-2 leads to a small vessel endotheliitis in the heart. EBioMedicine. 2021;63:103182. https://doi.org/10.1016/j.ebiom.2020.103182 10.1016/j.ebiom.2020.103182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stone JR, Basso C, Baandrup UT, et al. Recommendations for processing cardiovascular surgical pathology specimens: a consensus statement from the Standards and Definitions Committee of the Society for Cardiovascular Pathology and the Association for European Cardiovascular Pathology. Cardiovasc Pathol. 2012;21(1):2-16. https://doi.org/10.1016/j.carpath.2011.01.001 10.1016/j.carpath.2011.01.001 [DOI] [PubMed] [Google Scholar]
- 79.Basso C, Aguilera B, Banner J, et al. Guidelines for autopsy investigation of sudden cardiac death: 2017 update from the Association for European Cardiovascular Pathology. Virchows Arch. 2017;471(6):691-705. https://doi.org/10.1007/s00428-017-2221-0 10.1007/s00428-017-2221-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Basso C, Burke M, Fornes P, et al. Guidelines for autopsy investigation of sudden cardiac death. Virchows Arch. 2008;452(1):11-18. https://doi.org/10.1007/s00428-007-0505-5 [doi] 10.1007/s00428-007-0505-5 [DOI] [PubMed] [Google Scholar]
- 81.van der Wal AC. Coronary artery pathology. Heart. 2007;93(11):1484-1489. https://doi.org/10.1136/hrt.2004.038364 10.1136/hrt.2004.038364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Basso C, Baracca E, Zonzin P, Thiene G. Sudden cardiac arrest in a teenager as first manifestation of Takayasu’s disease. Int J Cardiol. 1994;43(1):87-89. https://doi.org/10.1016/0167-5273(94)90095-7 10.1016/0167-5273(94)90095-7 [DOI] [PubMed] [Google Scholar]


