Summary
The mechanisms underlying the onset and progression of vasculitis remain poorly understood. This condition is characterized by damage to the vascular wall, recruitment of inflammatory cells, and subsequent structural remodeling, which are hallmarks of vasculitis. The histopathological classification of vasculitis relies on the size of the affected vessel and the predominant type of inflammatory cell involved – neutrophils in acute cases, lymphocytes in chronic conditions, and histiocytes in granulomatous forms. Pathological changes progress in every context, and a single vasculitic pattern can be associated with various systemic conditions. Conversely, a single causative agent may lead to multiple distinct clinical and pathological manifestations of vasculitis. Moreover, many cases of vasculitis have no identifiable cause. A foundational understanding of the normal structure of the cutaneous vascular network is crucial. Similarly, identifying the cellular and molecular participants and their roles in forming the “dermal microvascular unit” is propedeutical.
This review aims to elucidate the complex mechanisms involved in the initiation and progression of vasculitis, offering a comprehensive overview of its histopathological classification, underlying causes, and the significant role of the cutaneous vascular network and cellular dynamics. By integrating the latest insights from studies on NETosis and the implications of lymphocytic infiltration in autoimmune diseases, we seek to bridge gaps in current knowledge and highlight areas for future research. Our discussion extends to the clinical implications of vasculitis, emphasizing the importance of identifying etiological agents and understanding the diverse histopathological manifestations to improve diagnostic accuracy and treatment outcomes.
Key words: vasculitis pathogenesis, vasculitis histopathology, dermal microvascular unit, leukocytoclastic, NETosis
Definition and classification of vasculitis
Vascular wall damage, inflammatory cell recruitment and subsequent structural remodelling define a vasculitic process 1. This sequence of events underscores the pathology of the disease, highlighting the progressive nature of the vascular damage and its systemic implications.
The histopathological classification of vasculitis is based primarily by the size of affected vessels and the predominant type of inflammatory cells involved 2. Neutrophils are typically associated with acute forms of vasculitis, while lymphocytes are more commonly seen in chronic conditions 2. In granulomatous forms of vasculitis, histiocytes are the predominant cell type 2. It is important to note that acute forms of vasculitis are generally the most common pattern observed in these diseases 3.
The dermal microvasculature consists of two parallel networks: a deep network and a superficial one, each consisting of paired arterioles and venules 4.
These networks run parallel to the epidermal surface. Arterioles and arteries that cross the subcutaneous septa contribute to the formation of the deep arteriolar plexus. Venules from this deep vascular plexus subsequently drain into larger veins located within the subcutaneous layer 5-7.
The vertical vessels, which serve as crucial connectors between the superficial and deep plexuses in the skin, are strategically oriented 5-7. They often run alongside the excretory ducts of sweat glands, illustrating a sophisticated integration of different skin structures 5-7. In the deeper layers of the skin, the capillaries of the deep vascular plexus form collateral networks around pilosebaceous units and the erector muscles of hair follicles, known as vasa muscolorum 5-7. Additionally, it is noteworthy that the venular components of the papillary capillaries exhibit a higher permeability to inflammatory cells 5-7. Alterations observed at this level of microvasculature are typically not categorized as vasculitis. Instead, they are more accurately described within the framework of dermatitis, reflecting their role in inflammatory skin conditions. This distinction is important for understanding the specific changes that occur in various skin disorders and their implication for skin health and disease.
Cellular players
Recent literature has been shedding light on the complex pathophysiology of the cellular components of the vessel wall, emphasizing the importance of understanding the “dermal microvascular unit” in the context of vasculitis.
The concept of the “dermal microvascular unit” is useful in the understanding of vasculitis process. This unit is characterized by distinct cellular elements that not only express common antigens, but are also spatially interconnected and perform integrated functions. Interestingly, these cells have the potential to transdifferentiate from one form to another, underscoring the dynamic nature of the dermal microvascular unit 8-10.
The dermal microvascular unit includes a variety of cell types, each playing a vital role in its overall function: endothelial cells, pericytes, veil cells/dendritic cells, dermal dendrocytes, mast cells, macrophages, neutrophils, eosinophils, T and B lymphocytes and plasma cells. The collaborative interaction among these cells is essential for the normal functioning of the microvascular system and becomes particularly significant in the context of vasculitis and other related pathologies 8,9,11.
Endothelial cells
Endothelial cells (ECs) play a crucial role in regulating the transport of macromolecules and oxygen to organs and tissues, while also preventing the formation of fibrin. Although there is no universally recognized marker to differentiate between their subsets, and it is important to understand that ECs are not a homogeneous population of cells. Instead, they exhibit significant diversity, with distinct characteristics varying both between and within organs. This heterogeneity makes them variably responsive to specific stimuli, such as inflammatory cytokines and effector immune cells, as well as to damaging factors 12,13.
For example, ECs interaction with natural killer cells (NKc) is different in renal, lung and skin vasculature 14,15.
The glycocalyx (GCX), a component of the apical surface of ECs, plays a pivotal role in regulating the movement of cells across the vascular wall. It serves as a mechanotransducer, converting mechanic stimuli through the cytoskeleton into biochemical response (mechanotransduction) 16. Although studies have established a link between shear stress and the inflammatory processes involved in atherogenesis 17, the impact of these mechanisms in the context of cutaneous vasculitis remains less clear 15,16,18.
The remodeling of intercellular adhesion molecules (ICAMs) is a critical process in various inflammatory conditions and is significantly influenced by shear stress 19,20.
This remodeling may alter the dynamics and efficiency of diapedesis, the passage of blood cells through the intact walls of capillaries, accommodating the traffic of immune cells across the vascular barrier 21. Additionally, these changes can impact the phenotype of ECs, affecting their function and behavior in response to inflammatory signals 22.
Neutrophils and ECs interact to form synapse-like structures, enabling the direct transfer of myeloperoxidase (MPO) into the cytoplasm of the ECs. This mechanism significantly contributes to vascular damage. Moreover, ECs that detach during this process circulate in the bloodstream and are characterized as inflammatory ECs, serving as a potential biomarker for disease activity. The cells exhibit increased expression of vascular-adhesion protein-1 (VAP-1) and MHC class I-related chain A (MICA), which are crucial for their adhesion and signaling roles in the inflammatory process.
ECs exhibit a heightened sensitivity to vascular endothelial growth factor (VEGF), a pivotal regulator of several vital processes including angiogenesis, vascular permeability, chemotaxis, cell migration, production of interstitial collagenase, release of von Willebrand factor, procoagulant activity and the remodeling of the extracellular matrix. Given that a diverse array of cell types, including activated T lymphocytes, activated platelets, activated neutrophils, epidermal cells, monocytes, dermal fibroblasts, eosinophils, can produce VEGF, is not wrong to say that this molecule plays a key role in modulating immune responses, maintaining vascular permeability, and sustaining inflammatory conditions 23-25.
Furthermore, genetic polymorphisms in VEGF genes, may predispose certain ethnic groups to the development of vasculitis 26. An illustrative case of this is leukocytoclastic (hypersensitivity) vasculitis, where VEGF expression is significantly increased 27-29.
High-mobility group box-1 (HMGB1), a nonhistone nuclear protein that binds with DNA, regulates transcription and shapes chromosomal architecture. It is recognized as a key mediator in the inflammatory pathways common to various forms of vasculitis 30. Transendothelial migration of monocytes activated by immunocomplexes in lupus is regulated by cellular signalling involving HMGB1-RAGE axis 31.
ECs are thought to originate from hemangioblasts, which serve as a common precursor for hematopoietic and ECs lines. The process of endothelial-to-mesenchimal transition (EndMT) is a critical cellular transformation wherein ECs lose their characteristic markers, such as CD31 and vascular endothelial cadherin (VE-cadherin) and express mesenchymal markers including procollagen-1, fibroblast-specific protein-1, and alpha-smooth muscle actin (α-SMA.) 32-34. This transition is intricately regulated by inflammatory cytokines, notably fibroblast growth factor (FGF) and transforming growth factor-beta (TGF-B), suggesting a significant role in the fibrotic phase that follows an active vasculitis process 32-34.
Microvesicles (MVs), encompassing both microparticles (MPs) and exosomes, are released by activated ECs and serve as carriers for inflammatory molecules within the vascular system. These vesicles mediate the spread of endothelial damage, activate the complement system, and promote the generation of thrombin 35.
Pericytes
Pericytes (PCs), a type of stromal cells, are strategically situated around ECs, bearing a functional resemblance to the glomerular podocytes found in the kidneys. These cells are characterized by their elongated cytoplasmic processes that reach out towards the endothelial surface, facilitating close cellular interactions. Notably, ultrastructural alterations in both PCs and ECs have been observed in conditions such as allergic vasculitis and erythema elevatum diutinum (EED), indicating their involvement in these diseases 36,37.
The phenomenon of pericyte wrapping, or lamination, around the vessel wall is indicative of chronic damage and serves as an important diagnostic marker for lymphocytic vasculitis 2.
Furthermore, in cases of leukocytoclastic vasculitis triggered by herpesvirus infection, electron microscopic studies have revealed the presence of viral inclusion in both pericytes and ECs, highlighting a direct viral impact on these cellular components 38.
Veil cells/dendritic cells
Veil cells, encircling vessels completely, share a structural similarity with fibroblasts in their ultrastructure.5 It is theorized that the abnormal collagen production by these veil cells is a key factor contributing to perivascular fibrosis observed in conditions such as collagenous vasculopathy 10,39-41.
Dermal dendrocytes
Dermal dendrocytes (DCs) are spatially related to microvessels, possessing unique laminar cytoplasmic extension knowing as membranous flaps that envelop neighbouring cells and structures to varying extents 42. This anatomical and functional proximity suggests that these cells are integral to the vascular inflammatory response, acting as key components within the dermal microvascular unit. DCs are critical for the induction of primary immune responses 43.
Specifically, in the context of EED, a condition characterized by leukocytoclastic vasculitis followed by a fibrotic process, an increased number of FXIIIa-positive dermal dendritic cells has been observed, indicating their significant involvement in the pathogenesis of the disease 44.
Additionally, the phenomenon of dendrocytoclasis, the fragmentation of perivascular dendrocytes, has been noted in Henoch-Schonlein purpura (HSP), further highlighting the contribution of DCs alongside neutrophils to the formation of nuclear fragments and the overall inflammatory cascade 45.
CD1a+ Langerhans-like cells, a subset of dendritic cells, not related to other DCs populations, have been observed to accumulate in the perivascular dendritic meshwork during pathological conditions, notably in the advanced stages of leukocytoclastic vasculitis and in lymphocytic vasculitis 46.
These cells are implicated in the immune response mechanisms, potentially by presenting antigens derived from neutrophil cytoplasm to T cells. This antigen presentation process may play a pivotal role in sustaining and exacerbating the inflammatory vicious cycle inherent to the vasculitic process 47.
Mast cells
Mast cells (MCs) serve as vital histamine reservoirs, contributing to the development and preservation of the endothelium and small blood vessels, which are essential components of the vascular unit 48,49.
These cells are intricately associated with DCs, partially enveloped by DC membrane extensions in a ball and socket arrangement 42.
In conditions such as leukocytoclastic vasculitis, activated MCs contribute to enhancing vascular permeability and facilitating the migration of leukocytes. An increased perivascular presence of MCs has been demonstrated in skin lesions characteristic of cutaneous allergic vasculitis and in urticarial vasculitis associated with systemic sclerosis 50,51.
In drug-induced vasculitis, MCs may be directly activated by the offending drug, functioning as key effectors cells alongside neutrophils, lymphocytes, and macrophages 52. Moreover, in experimental models of anti-neutrophil cytoplasmic antibody-associated vasculitis (AAV), activated MCs have been shown to mitigate inflammatory responses and protect against glomerular damage by IL-10 dependent mechanism.
Macrophages
Macrophages (MPs) differentiate from blood monocytes, which are recruited into inflamed tissue. Upon arrival, they undergo a transformation characterized by the loss of myeloperoxidase (MPO) and proteinase 3 (PR3), rendering them insensitive to activation by anti-neutrophil cytoplasmic antibodies (ANCA). MPs main task is to engulf and remove apoptotic neutrophils and neutrophil extracellular traps (NETs) that accumulate around vessels in vasculitis, a process known as efferocytosis (from the latin term effero, “to carry to the grave”, or “to bury”) 53.
MPs express Fc gamma receptors III and I (FcγRIII and FcγRI), enabling them to recognize and bind immune complexes associated with hypersensibility vasculitis 54.
In certain forms of vasculitis, an inductive microenvironment promotes the formation of granulomas which are highly organized structures that facilitate interactions between T cell and MPs, leading to reciprocal activation. Within these granulomas, aberrant Th1 and Th17 cells can occur, contributing to systemic inflammation and the perpetuation of the disease process 55.
Neutrophils
Neutrophils (NPs), the most abundant white blood cells (WBCs), originate from bone marrow and are primarily known for phagocytosis. However, their ability to undergo NETosis, forming neutrophil extracellular traps (NETs), has gained significant interest in the last decade. NETosis consist in expelling DNA and nuclear proteins, mainly histones, as a trap for pathogens 56,57.
While beneficial against infections, in autoimmune or autoinflammatory conditions, spontaneous NETs formation and impaired clearance can lead to autoantibodies formation consequent to cryptic antigen exposition 58,59 and amplification of inflammatory response.
In vasculitis, NETs, induced by activated ECs contribute to the pathology, including procoagulant activities exacerbating vascular damage. Dysregulated neutrophils abnormally interacting with T lymphocytes are responsible of disease progression in Giant Cell Arteritis (GCA) despite immunosuppression 60-62.
Due to their antigenic content NETs are implicated in the pathogenesis of ANCA-associated vasculitides (AAV) 63,64.
Eosinophils
Eosinophils, known for their cytoplasmic granules filled with protein pool with toxic activity, are primarily regulated by IL-5, which is influenced by Type2 innate lymphoid cells (ILC2) near blood vessels.
In eosinophilic granulomatosis with polyangiitis EGPA), overproduction of IL-5 by Th2 lymphocytes mobilizes and activates eosinophils 65,66.
Eosinophils, triggered by IL-5, IFN-γ, LPS, eotaxin, complement factor 5a (C5a) or infection with Gram-negative bacteria, rapidly release extracellular traps. Eosinophils are represented in various proportions in many cutaneous vasculitis forms: drug related leukocytoclastic vasculitis, eosinophilic vasculitis, urticarial vasculitis, granuloma faciale (GF), EED, EGPA, and granulomatosis with polyangiitis (GPA) 66,68. They also significantly contribute to plasma cell survival factors APRIL and IL-6, aiding in plasma cell niche formation 69.
Testing for gene mutation in PDGFRA helps to distinguish systemic vasculitis-associated eosinophilia from clonal hypereosinophilia 67.
Lymphocytes
In the superficial dermis, T cells are represented mainly by CD4+ lymphocytes with an effector memory phenotype (CD45RA-; CD45RO, CCR7), primarily found near dermal blood vessels, integrating into the microvascular unit. These T cells engage in a unique interaction with the endothelial barrier, a process not fully understood involves “tenertaxis” where T cells extend invadosome-like protrusions (ILPs) towards and into endothelial cell surface, facilitating their passage through the vascular barrier 70. T cell-mediated immunity is implicated in various forms of vasculitis 71. A significant aspect of this immunological landscape in vasculitis is the dysfunction of regulatory T cells (Tregs) or an imbalanced ratio between Th17 cells and Tregs 72.
This imbalance or dysfunction has been consistently observed in several vasculitis conditions, including GPA, Kawasaki disease (KD), GCA, Churg-Strauss syndrome (CSS), and AAV 73-78.
Additionally, a distinct population of CD4+ T cells resistant to Treg-mediated suppression has been described 78.
Natural killer (NK) cells, as components of the innate immune system, have the capability to recognize different organ-specific endothelial cells 15.
This recognition ability is particularly noted in the context of AAV, where an increased expression of Toll-like receptors (TLR) on NK cells has been documented, suggesting a significant role in enhancing immune response 79.
B lymphocytes, commonly considered only as plasma-cell precursors, have shown a broader range of functions in immune regulation. For example, a novel population of B cells characterized by long dendrites, not committed to the formation of follicular structures, has recently been identified in GPA 80.
Plasma cells
Plasma cells represent a heterogeneous population of antibody-producing cells derived from different subclasses of B lymphocytes, capable of generating either short-lived or long-lived plasma cells. The latter are particularly resilient to immunosuppressive therapies, thriving in survival niches formed within inflamed tissues, by stromal cells 81.
Plasma cells play a significant role in certain forms of small-vessel neutrophilic vasculitis characterized by patterned fibrosis, such as GF and EED, and are also observed in a subset of patients with GPA and GCS.2 In the context of GPA, the production of self-reactive B lymphocytes is enhanced due to elevated levels of B cell-stimulatory factors such as B cell activation factor (BAFF) and an increased number of T follicular helper cells (TFH). These B lymphocytes can mature into long-lived plasma cells which secrete ANCA, implicating them in the disease’s pathogenesis 82-84.
Furthermore, plasma cells may be involved in the pathogenesis of some specific subtypes of cutaneous vasculitis, such as IgA vasculitis (Henoch-Schonlein purpura, HSP), although they may be not always be detectable in histological samples.
Histopathology
ACUTE VASCULITIS
Acute vasculitis, characterized by leukocytoclastic inflammation, is a histological pattern frequently associated with numerous systemic conditions 85.
Acute vasculitis represents a highly dynamic pathological process, wherein the timing of evaluation plays a crucial role in accurately assessing the inflammatory component. Lesions that have persisted for 18 to 24 hours typically display the most diagnostic characteristics, underscoring the importance of timely examination for effective diagnosis 86.
In acute vasculitis, the small venules, particularly postcapillary venules within the superficial dermal vascular plexus, are primarily affected. Neutrophils dominate the cellular response, with endothelial cells exhibiting swelling and signs of degeneration. These neutrophils are prominently visible within the lumen, traversing a thickened vascular wall to extend into the perivascular zone and interstitial dermis in varying degrees. The presence of nuclear dust is a hallmark of leukocytoclasis (Fig. 1), indicating the breakdown of neutrophils. However, leukocytoclasis itself is not a definitive marker for acute vasculitis and must be interpreted within the appropriate context. For example, leukocytoclasis is commonly observed beneath cutaneous ulcers.
Figure 1.
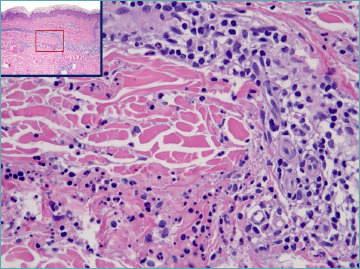
Leukocytoclastic vasculitis: a postcapillary venule in the mid dermis present degenerative changes of endothelial cells. A subtle rim of fibrin is visible. Neutrophils infiltrating the vessel wall and the perivascular stroma are associated with nuclear dust (leukocytoclasis).
Fibrin deposition serves as a significant indicator of vascular injury in acute vasculitis, detectable within the vascular lumen, in the wall, and the surrounding perivascular area. Thrombosis may also occur under specific conditions. Additionally, the vasculitic process often leads to marked dermal edema, which can result in the formation of vesiculobullous lesions, further complicating the clinical presentation 87.
In lesions that have persisted for an extended period, eosinophils and lymphocytes, along with a presence of plasma cells, may become evident, indicating a more chronic inflammatory phase.
Less common described changes include epidermal lichenoid changes, signs of ischemic damage, squamous sialometaplasia, apoptosis, necrosis, and basal cell hyperplasia.
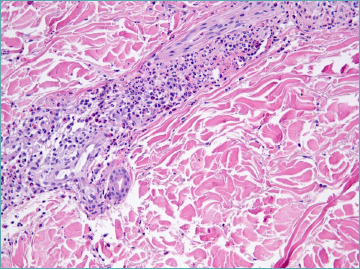
Interstitial macrophages increase in late lesions when neutrophils decrease (Fig. 2). A mild increase in interstitial acid mucopolysaccarides can also be documented 88.
Figure 2.
Leukocytoclastic vasculitis: in late lesion neutrophils are barely visible. Histiocytes with intracytoplasmic nuclear fragments can be documented. Insert: perivascular dermis gains a “busy” aspect.
Some additional clues, when present, may indicate specific conditions:
Presence of neutrophils within the dermal papillae can serve as an indicative clue for dermatitis herpetiformis, in the appropriate clinical context, which may occasionally co-occur with leukocytoclastic vasculitis, thereby suggesting a complex interplay of underlying pathologies 89.
The presence of eosinophils, as illustrated in Figure 3, may be associated with the intake of certain medications, suggesting a potential drug-induced etiology 90.
The detection of IgA deposits can serve as a robust indicator of potential renal pathology 91. Furthermore, IgA deposits have been identified across a diverse array of conditions, including respiratory tract infections, Wegener’s granulomatosis, inflammatory bowel diseases, and various malignancies, highlighting their significance in a broad spectrum of clinical scenarios.
The Henoch-Schönlein purpura (HSP) variant of leukocytoclastic vasculitis is distinguished by the presence of IgA deposits in its early lesions. Despite its histological similarities to general leukocytoclastic vasculitis, the diagnosis of HSP relies on a careful clinical-pathological correlation. The European League Against Rheumatism (EULAR), along with the Pediatric Rheumatology International Trials Organization (PRINTO) and the Pediatric Rheumatology European Society, has established diagnostic criteria for HSP. These include the predominance of purpura and petechiae on the lower limbs, accompanied by at least one of the following: abdominal pain, histopathological evidence of IgA deposition in vessels, arthritis or arthralgia, or evidence of renal involvement. The immune response triggered by the SARS-CoV-2 vaccine or infection may contribute to the onset of HSP, suggesting a potential link between the immune system’s reaction to the virus and the development of this specific form of vasculitis 92.
Eosinophilic vasculitis is characterized by a predominance of eosinophils within the affected tissues. This condition can occur as an idiopathic phenomenon or in association with other medical conditions, such as connective tissue diseases, hypereosinophilic syndrome, or HIV infection.
Granulomas are a hallmark feature of EGPA, previously known as Churg-Strauss disease. However, granulomas are often not observed except in more mature lesions. Typically, EGPA manifests as a small vessel vasculitis, which may or may not include eosinophils. Myeloperoxidase-ANCA (MPO-ANCA) antibodies are detected in 40 to 60% of EGPA patients 93,94. The diagnosis of EGPA is established through a combination of clinical presentation and laboratory findings, emphasizing the necessity of a comprehensive assessment for accurate identification.
-
The involvement of larger blood vessels is a recognized complication in rheumatoid vasculitis, often seen in the context of rheumatoid arthritis or other autoimmune diseases 95.
The clinical manifestations of this condition are diverse, ranging from digital gangrene and cutaneous ulcers to digital nail fold infarctions, palpable purpura, and neuropathy, the latter occurring when the vasa nervorum are affected. There is also a potential for histological overlap with polyarteritis nodosa (PAN) when vessels in the lower dermis are preferentially or exclusively targeted.
Significant edema is observed in the upper layer of the skin, accompanied by a reduced presence of neutrophils, a variable count of lymphocytes, and some eosinophils in the initial stages. These findings are clinically aligned with the appearance of urticarial wheals and/or angioedema, serving as indicators of urticarial vasculitis. This condition is distinguishable from common urticaria by the longer-lasting nature of the wheals, which tend to resolve leaving bruise-like patches. The skin manifestations are more widespread, showing no particular preference for the lower legs. The presence of lowered complement levels (hypocomplementemia) signals a risk for renal failure. Urticarial vasculitis is also associated with several systemic diseases 85.
The presence of hyaline deposits within blood vessels, although not consistently observed, serves as a valuable indicator for the diagnosis of mixed cryoglobulinemia 96.
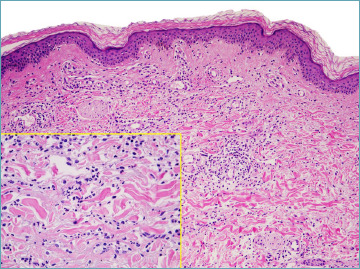
Figure 3.
The presence of eosinophils may suggest a drug-induced case.
In the differential diagnosis of acute leukocytoclastic vasculitis, secondary vasculitis manifests at the base of ulcers or within significant neutrophilic dermal infiltrates, necessitating a thorough examination for vessel alterations beyond the ulcer areas. Conversely, neutrophilic urticaria features neutrophils surrounding dermal vessels without the presence of leukocytoclasis, endothelial harm, or fibrin accumulations, as depicted in Figure 4 97.
Figure 4.
Neutrophilic urticarial dermatosis.
SEPTIC VASCULITIS
Septic vasculitis, a specific form of acute vasculitis, occurs in conjunction with various septicemic conditions. It is characterized by endothelial swelling, focal necrosis, fibrinoid changes, and mild leukocytoclasis in dermal vessels. It is crucial to meticulously document the presence of occlusive thrombi, which are aggregates of platelets, fibrin, red blood cells, and neutrophils. Additional manifestations may include perivascular hemorrhage, degenerative changes in the skin appendages, subepidermal swelling, and pustule formation, as illustrated in Figure 5. In the context of chronic meningococcal or gonococcal septicemia, arterioles may also be affected. Furthermore, the histopathological features of leukocytoclastic vasculitis can be identified in Osler nodes or small, purple-pink, painful lesions typically found on the fingertips, indicative of endocarditis.
Figure 5.
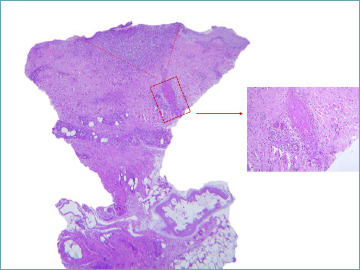
A necrotic vessel is present in the deep dermis. The lumen is obliterated by fibrin cellular debris and nuclear dust. A V shaped ulcer with granulation tissue is present downstream as the result of ischemia. A septicaemic state was documented clinically in this case.
RA-ASSOCIATED VASCULOPATHY
The term “RA-associated”, as proposed by Magro and Crowson 98, encompasses a broad range of inflammatory patterns, including active vasculopathy, lymphocyte-dominant inflammation, neutrophil-rich inflammation, and granulomatous vasculitis. Additionally, this term covers a variety of changes linked to autoimmune diseases, such as extravascular palisading granulomatous inflammation, interstitial and/or subcuticular neutrophilia, pauci-inflammatory vascular thrombosis, glomeruloid neovascularization, folliculocentric vasculitis, benign cutaneous polyarteritis nodosa (PAN) types, and occlusive intravascular histiocytic foci.
ERYTHEMA ELEVATUM DIUTINUM
EED is an uncommon dermatosis characterized by persistent red, violaceous, and yellowish papules, plaques, and nodules. These lesions are typically symmetrically distributed on acral locations, particularly on extensor surfaces and the buttocks. EED presents a unique blend of acute histological features alongside chronic alterations, paralleled by a persistent clinical trajectory.
Early EED lesions prominently feature a perivascular neutrophil infiltrate, accompanied by leukocytoclasis, which is readily identifiable but requires meticulous examination in later stages. In advanced lesions, fibrosis, which concentrically surrounds the vessel lumen, becomes more dominant (Fig. 6). Overlooking neutrophilic vasculitis foci in later stages can lead to an incorrect diagnosis, mistaking it for a purely fibrosing condition.
Figure 6.
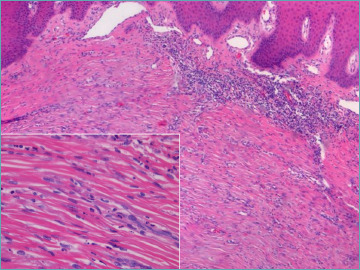
Erythema elevatum diutinum: concentric fibrosis predominates in late lesion. A careful search for nuclear dust (insert) must be done.
Conversely, the early stages of EED may show a significant overlap with neutrophilic dermatoses, such as Sweet’s syndrome, rheumatoid neutrophilic dermatosis, bowel-associated dermatosis–arthritis syndrome, and Behçet’s disease, due to the prominent neutrophilic infiltrate. Additionally, the potential involvement of IgG4 in EED has been explored, placing EED as a mimic when applying stricter criteria for IgG4-related disorders, in comparison to conditions like Granuloma faciale 99,100.
GRANULOMA FACIALE
Granuloma Faciale (GF) is a rare dermatosis distinguished by solitary or multiple brown-red plaques, nodules, or occasionally papules 101. While the face is the preferred site, it is not the only possible location for these lesions.
Histologically, GF is characterized by mild concentric perivascular fibrosis alongside a mixed cellular infiltrate that includes eosinophils, plasma cells, histiocytes, and neutrophils. The presence of leukocytoclasis and hemosiderin deposits may vary and is time-dependent, similar to other forms of vasculitis.
In the context of diagnosing and understanding GF, inclusion criteria for IgG4-related sclerosing diseases have been identified in a notable portion of GF cases within certain case series, suggesting a potential overlap or association 99. However, no correlation between GF and IgG4 levels was found in another study, indicating variability in the disease’s expression and its association with IgG4-related sclerosing diseases 102.
MICROSCOPIC POLYANGIITIS
Microscopic polyangiitis belongs to the spectrum of ANCA-associated vasculitis, which also includes GPA and EGPA, all sharing a commonality in systemic involvement. The specificity of organ involvement acts as a key diagnostic criterion, indicating a typically progressive clinical trajectory, though cases limited to the skin have been observed. The presence of Anti-Neutrophil Extracellular Trap (NET) antibodies (ANETA) in certain cases correlates with a more limited clinical manifestation, highlighting the significant role of NETs in exacerbating the inflammatory response 103.
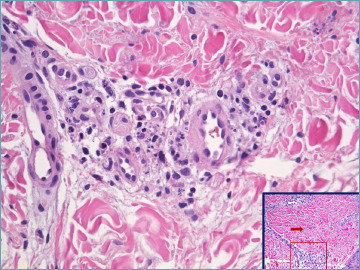
Histologically, microscopic polyangiitis is characterized by a necrotizing vasculitis, often accompanied by neutrophilic leukocytoclastic vasculitis and sporadic fibrinoid degeneration of the vessels, primarily affecting arterioles (Fig 7). Changes in capillaries and postcapillary venules are typically observed. Positivity for p-ANCA antibodies is a hallmark of microscopic polyangiitis, and represents a differential diagnostic criterion with polyarteritis nodosa. Both conditions are indeed characterized by patchy fibrinoid degeneration of the vessel wall.
Figure 7.
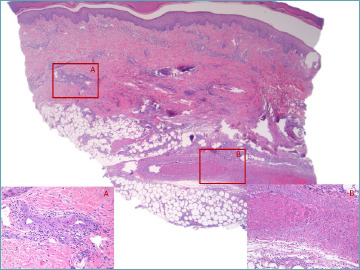
Necrotizing vasculitis in the upper hypodermis associated with subendothelial fibrin deposition in a smaller vessel in deep dermis in a MPO-ANCA+ patient.
POLYARTERITIS NODOSA
PAN affects the skin in a relatively small proportion of cases, more commonly presenting as a systemic condition that impacts multiple organs, including the kidneys, liver, gastrointestinal tract, and nervous system. Notably, a chronic, relapsing form of PAN that is confined to the skin has also been documented. Its pathogenesis is multifaceted, with a significant focus on the role of immune complexes 104. It primarily targets small and medium-sized muscular arteries located at the junction between the dermis and hypodermis.
In patients with vasculitis, although lesions may appear asynchronously, a four-stage progression of the disease has been characterized 105. This progression begins with endothelial loss and the formation of fibrin thrombi accompanied by neutrophil infiltration, yet without significant disruption to the internal elastic lamina or medial fibrinoid necrosis. This phase is followed by the presence of mixed cell infiltrates, which exhibit a distinctive intimal target-like fibrinoid necrosis (as illustrated in Fig. 8), with fibrinoid material leaking through disrupted areas of the internal elastic lamina into the media. Subsequently, the disease enters a reparative stage, marked by intimal fibroblastic proliferation and perivascular neovascularization, with histiocytes and lymphocytes being the predominant infiltrating cells. The final, or healed, stage is characterized by minimal cellular inflammation, resulting in occlusive intimal thickening, signifying the resolution of active inflammation.
Figure 8.
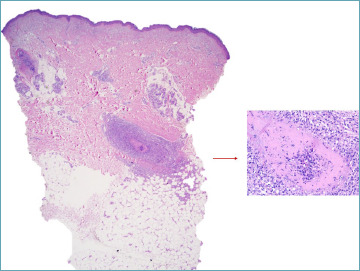
Polyartheritis nodosa: an arteriole in the deep dermis is involved. A complete fibrin ring push endothelial lining narrowing the lumen. Mononuclear cells infiltrate arterial wall and periarterial space. Some nuclear dust is visible.
A potential diagnostic challenge involves distinguishing between vasculitis and superficial thrombophlebitis 105. The differentiation can be nuanced and relies on the identification of a ‘checkerboard’ pattern, characterized by muscle fibers interspersed with collagen within the muscular layer of the vein. In contrast, the presence of a concentric arrangement of smooth muscle fibers is instrumental in identifying arterial structures, particularly in cases of PAN. While the assessment of the internal elastic lamina may provide some insights, it is considered a less definitive histological characteristic for distinguishing between veins and arteries.
Macular arteritis is described as a localized, slow-progressing form of deep arteritis, notable for its hyperpigmented, asymptomatic macules that do not exhibit a tendency to progress 106.
It is considered a latent form of cutaneous PAN 107 marked by an exceptional clinical manifestation and a histological pattern associated with later stages of PAN 108.
The differentiation of macular arteritis and lymphocytic thrombophilic arteritis from cutaneous PAN is facilitated by the absence of disruption in the elastic lamina, as illustrated in Fig 9.
Figure 9.
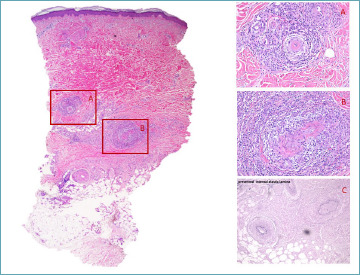
Macular artheritis: more limited vessel wall damage than in polyarthritis nodosa.
Furthermore, the term “lymphocytic thrombophilic arteritis” is used by some researchers to describe a clinically more severe variant, characterized by livedo racemosa and infiltrated plaques 107,109.
LYMPHOCYTIC VASCULITIS
Lymphocytic vasculitis (LV) is characterized by a dominant lymphocytic infiltration within the walls of blood vessels and the surrounding tissues.2 The presence of fibrin extravasation is not a requisite criterion for its diagnosis. The occurrence of lymphocytes within the muscular layer of blood vessels is a significant indicator of lymphocytic vasculitis, given that the migration of lymphocytes (diapedesis) into the muscular layers of arteries or veins is not a normal physiological process. To date, no evidence has been established to demonstrate a physiological interaction between smooth muscle cells and lymphocytes. Conversely, endothelial cells may actively recruit T lymphocytes in response to damage or the presence of viruses and bacteria. Instances of vasculitis induced by the COVID vaccine may exhibit patterns characteristic of lymphocytic vasculitis 110,111.
For practical diagnostic purposes, lymphocytic vasculitis should be considered within a temporal context, and additional histological indicators must be sought (Figs. 10, 11). The simultaneous involvement of both superficial and deep vascular plexuses is characteristic of collagen vascular diseases. Furthermore, a lichenoid tissue reaction pattern is commonly observed in conjunction with conditions such as chilblain lupus, pityriasis lichenoides, drug-induced reactions, and persistent viral infections. This pattern underscores the necessity of a comprehensive histological examination to accurately diagnose and differentiate between these conditions.
Figure 10.
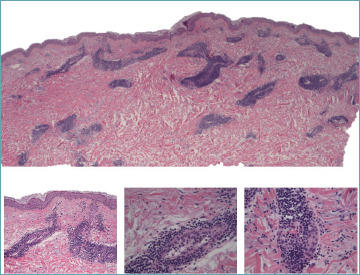
Lupus tumidus. Lymphocytic vasculitis is defined by a heavy sleeve shaped perivascular lymphocytic infiltrate involving the superficial and deep plexus. Lymphocytes are present trough the vessel wall and in contact with plump endothelial cells. Subtle involvement of perieccrine connective and interstitial mucin are diagnostic clues (inserts).
Figure 11.
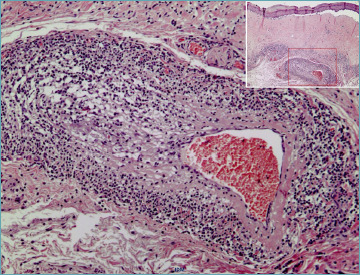
Lymphocytic vasculitis in lichen sclerosus: intramural lymphocytic infiltrates in large muscular vessels associated with classic pathological changes at the junctional level.
The presence of CD123+ plasmacytoid dendritic cells around blood vessels can serve as a significant diagnostic marker for cutaneous lupus 112. Furthermore, lymphocytic vasculitis (LV) has been identified as a potential variant of urticarial vasculitis, wherein wheals typically manifest as a clinical symptom in such cases 113.
During the resolution phase of acute vasculitis, the inflammatory response often shows a marked decrease in neutrophils, with a predominance of mononuclear cells becoming evident 114.
Paraneoplastic vasculitis has been observed both during and after the treatment of myeloid leukemia 115. Instances of vasculitis characterized by infiltration with leukemic cells have been identified in a case series 116. Additionally, the occurrence of chronic lymphocytic leukemia presenting with a vasculitis-like manifestation, akin to leukemia cutis, is rare yet feasible, as demonstrated in Figure 12.
Figure 12.
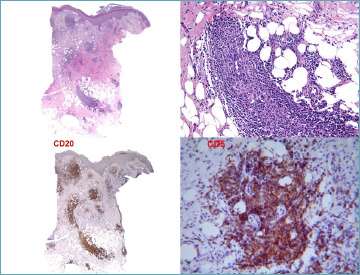
Leukemia cutis with a lymphocytic vasculitis-like pattern. Co-expression of CD20 and CD5 in small lymphocytes is a diagnostic clue for chronic lymphocitic leukemia.
Conclusions
Currently, the initiation and progression of vasculitis remain enigmatic for the complex and dynamic interaction between cellular players and a changing microenvironment.
Classification of vasculitis according to the dimension of vessels represents a pragmatic method in histological evaluation although many clinical entities may present with the same histological pattern during their course. With the advent of extensive research, outdated paradigms are being challenged, highlighting the importance of understanding the pathophysiology to make recognition and therapy more effective.
Dermatopathologists must be aware of this interesting and evolving field of medicine as skin biopsy is more easily to achieve compared with kidney and lung biopsies and can be the first step in detecting and categorizing a vasculitis process. Clinico-pathological correlation is always mandatory when a vasculitic process is identified in a skin biopsy and must support the therapeutic decision.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest. Date: 19/02/2024
FUNDING
This study did not receive any funding from the National Institutes of Health (NIH), the Wellcome Trust and the Howard Hughes Medical Institute (HHNI) and other(s). Internal funding from Department of Clinical Services has been used in part for study-related facilities.
AUTHORS’ CONTRIBUTIONS
AC conceived the study, collected data, drafted and edited the manuscript. LC managed the data and drafted the manuscript. Both authors have read and approved the final version of the manuscript.
ETHICAL CONSIDERATION
As a review article, the present study is exempt from ethical approval.
Figures and tables
References
- 1.Lyle AN, Taylor WR. The pathophysiological basis of vascular disease. Lab Invest. 2019;99(3):284-289. https://doi.org/10.1038/s41374-019-0192-2 10.1038/s41374-019-0192-2 [DOI] [PubMed] [Google Scholar]
- 2.Carlson JA, Mihm MC, Jr., LeBoit PE. Cutaneous lymphocytic vasculitis: a definition, a review, and a proposed classification. Semin Diagn Pathol. 1996;13(1):72-90. [PubMed] [Google Scholar]
- 3.Watts RA, Hatemi G, Burns JC, et al. Global epidemiology of vasculitis. Nat Rev Rheumatol. 2022;18(1):22-34. https://doi.org/10.1038/s41584-021-00718-8 10.1038/s41584-021-00718-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braverman IM, Keh-Yen A. Ultrastructure of the human dermal microcirculation. III. The vessels in the mid- and lower dermis and subcutaneous fat. J Invest Dermatol. 1981;77(3):297-304. https://doi.org/10.1111/1523-1747.ep12482470 10.1111/1523-1747.ep12482470 [DOI] [PubMed] [Google Scholar]
- 5.Braverman IM. The cutaneous microcirculation: ultrastructure and microanatomical organization. Microcirculation. 1997;4(3):329-40. https://doi.org/10.3109/10739689709146797 10.3109/10739689709146797 [DOI] [PubMed] [Google Scholar]
- 6.Braverman IM. Ultrastructure and organization of the cutaneous microvasculature in normal and pathologic states. J Invest Dermatol. 1989;93(2 Suppl):2S-9S. https://doi.org/10.1111/1523-1747.ep12580893 10.1111/1523-1747.ep12580893 [DOI] [PubMed] [Google Scholar]
- 7.Braverman IM. The role of blood vessels and lymphatics in cutaneous inflammatory processes: an overview. Br J Dermatol. Jul. 1983;109 Suppl 25:89-98. [PubMed] [Google Scholar]
- 8.Arrese Estrada J, Pierard GE. Factor-XIIIa-positive dendrocytes and the dermal microvascular unit. Dermatologica. 1990;180(1):51-3. https://doi.org/10.1159/000247986 10.1159/000247986 [DOI] [PubMed] [Google Scholar]
- 9.Sontheimer RD. Perivascular dendritic macrophages as immunobiological constituents of the human dermal microvascular unit. J Invest Dermatol. 1989;93(2 Suppl):96S-101S. https://doi.org/10.1111/1523-1747.ep12581078 10.1111/1523-1747.ep12581078 [DOI] [PubMed] [Google Scholar]
- 10.Sueki H, Whitaker D, Buchsbaum M, et al. Novel interactions between dermal dendrocytes and mast cells in human skin. Implications for hemostasis and matrix repair. Lab Invest. 1993;69(2):160-72. [PubMed] [Google Scholar]
- 11.Manole CG, Simionescu O. The Cutaneous Telocytes. Adv Exp Med Biol. 2016;913:303-323. https://doi.org/10.1007/978-981-10-1061-3_20 10.1007/978-981-10-1061-3_20 [DOI] [PubMed] [Google Scholar]
- 12.Ribatti D, Nico B, Vacca A, et al. Endothelial cell heterogeneity and organ specificity. J Hematother Stem Cell Res. 2002;11(1):81-90. https://doi.org/10.1089/152581602753448559 10.1089/152581602753448559 [DOI] [PubMed] [Google Scholar]
- 13.Gifre-Renom L, Daems M, Luttun A, et al. Organ-Specific Endothelial Cell Differentiation and Impact of Microenvironmental Cues on Endothelial Heterogeneity. Int J Mol Sci. 2022;23(3)https://doi.org/10.3390/ijms23031477 10.3390/ijms23031477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tognarelli S, Gayet J, Lambert M, et al. Tissue-specific microvascular endothelial cells show distinct capacity to activate NK cells: implications for the pathophysiology of granulomatosis with polyangiitis. J Immunol. 2014;192(7):3399-408. https://doi.org/10.4049/jimmunol.1301508 10.4049/jimmunol.1301508 [DOI] [PubMed] [Google Scholar]
- 15.Bielawska-Pohl A, Crola C, Caignard A, et al. Human NK cells lyse organ-specific endothelial cells: analysis of adhesion and cytotoxic mechanisms. J Immunol. 2005;174(9):5573-82. https://doi.org/10.4049/jimmunol.174.9.5573 10.4049/jimmunol.174.9.5573 [DOI] [PubMed] [Google Scholar]
- 16.Tarbell JM, Pahakis MY. Mechanotransduction and the glycocalyx. J Intern Med. 2006;259(4):339-50. https://doi.org/10.1111/j.1365-2796.2006.01620.x 10.1111/j.1365-2796.2006.01620.x [DOI] [PubMed] [Google Scholar]
- 17.Helderman F, Segers D, de Crom R, et al. Effect of shear stress on vascular inflammation and plaque development. Curr Opin Lipidol. 2007;18(5):527-33. https://doi.org/10.1097/MOL.0b013e3282ef7716 10.1097/MOL.0b013e3282ef7716 [DOI] [PubMed] [Google Scholar]
- 18.Askari H, Sadeghinejad M, Fancher IS. Mechanotransduction and the endothelial glycocalyx: Interactions with membrane and cytoskeletal proteins to transduce force. Curr Top Membr. 2023;91:43-60. https://doi.org/10.1016/bs.ctm.2023.02.003 10.1016/bs.ctm.2023.02.003 [DOI] [PubMed] [Google Scholar]
- 19.Nagel T, Resnick N, Atkinson WJ, et al. Shear stress selectively upregulates intercellular adhesion molecule-1 expression in cultured human vascular endothelial cells. J Clin Invest. 1994;94(2):885-91. https://doi.org/10.1172/JCI117410 10.1172/JCI117410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morigi M, Zoja C, Figliuzzi M, et al. Fluid shear stress modulates surface expression of adhesion molecules by endothelial cells. Blood. 1995;85(7):1696-703. [PubMed] [Google Scholar]
- 21.Bouillet L, Baudet AE, Deroux A, et al. Auto-antibodies to vascular endothelial cadherin in humans: association with autoimmune diseases. Lab Invest. 2013;93(11):1194-202. https://doi.org/10.1038/labinvest.2013.106 10.1038/labinvest.2013.106 [DOI] [PubMed] [Google Scholar]
- 22.Russo TA, Banuth AMM, Nader HB, et al. Altered shear stress on endothelial cells leads to remodeling of extracellular matrix and induction of angiogenesis. PLoS One. 2020;15(11):e0241040. https://doi.org/10.1371/journal.pone.0241040 10.1371/journal.pone.0241040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freeman MR, Schneck FX, Gagnon ML, et al. Peripheral blood T lymphocytes and lymphocytes infiltrating human cancers express vascular endothelial growth factor: a potential role for T cells in angiogenesis. Cancer Res. 1995;55(18):4140-5. [PubMed] [Google Scholar]
- 24.Mohle R, Green D, Moore MA, et al. Constitutive production and thrombin-induced release of vascular endothelial growth factor by human megakaryocytes and platelets. Proc Natl Acad Sci U S A. 1997;94(2):663-8. https://doi.org/10.1073/pnas.94.2.663 10.1073/pnas.94.2.663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Viac J, Palacio S, Schmitt D, et al. Expression of vascular endothelial growth factor in normal epidermis, epithelial tumors and cultured keratinocytes. Arch Dermatol Res. 1997;289(3):158-63. https://doi.org/10.1007/s004030050172 10.1007/s004030050172 [DOI] [PubMed] [Google Scholar]
- 26.Song GG, Kim JH, Lee YH. Vascular endothelial growth factor gene polymorphisms and vasculitis susceptibility: A meta-analysis. Hum Immunol. 2014;75(6):541-8. https://doi.org/10.1016/j.humimm.2014.02.022 10.1016/j.humimm.2014.02.022 [DOI] [PubMed] [Google Scholar]
- 27.Viac J, Pernet I, Schmitt D, et al. Overexpression of circulating vascular endothelial growth factor (VEGF) in leukocytoclastic vasculitis. Arch Dermatol Res. 1999;291(11):622-3. https://doi.org/10.1007/s004030050464 10.1007/s004030050464 [DOI] [PubMed] [Google Scholar]
- 28.Melincovici CS, Bosca AB, Susman S, et al. Vascular endothelial growth factor (VEGF) - key factor in normal and pathological angiogenesis. Rom J Morphol Embryol. 2018;59(2):455-467. [PubMed] [Google Scholar]
- 29.Scaldaferri F, Vetrano S, Sans M, et al. VEGF-A links angiogenesis and inflammation in inflammatory bowel disease pathogenesis. Gastroenterology. 2009;136(2):585-95 e5. https://doi.org/10.1053/j.gastro.2008.09.064 10.1053/j.gastro.2008.09.064 [DOI] [PubMed] [Google Scholar]
- 30.Paudel YN, Angelopoulou E, Piperi C, et al. Enlightening the role of high mobility group box 1 (HMGB1) in inflammation: Updates on receptor signalling. Eur J Pharmacol. 2019;858:172487. https://doi.org/10.1016/j.ejphar.2019.172487 10.1016/j.ejphar.2019.172487 [DOI] [PubMed] [Google Scholar]
- 31.Sun W, Jiao Y, Cui B, et al. Immune complexes activate human endothelium involving the cell-signaling HMGB1-RAGE axis in the pathogenesis of lupus vasculitis. Lab Invest. 2013;93(6):626-38. https://doi.org/10.1038/labinvest.2013.61 10.1038/labinvest.2013.61 [DOI] [PubMed] [Google Scholar]
- 32.Piera-Velazquez S, Jimenez SA. Endothelial to Mesenchymal Transition: Role in Physiology and in the Pathogenesis of Human Diseases. Physiol Rev. 2019;99(2):1281-1324. https://doi.org/10.1152/physrev.00021.2018 10.1152/physrev.00021.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Y, Kovacic JC. Endothelial to Mesenchymal Transition in Health and Disease. Annu Rev Physiol. 2023;85:245-267. https://doi.org/10.1146/annurev-physiol-032222-080806 10.1146/annurev-physiol-032222-080806 [DOI] [PubMed] [Google Scholar]
- 34.Yun E, Kook Y, Yoo KH, et al. Endothelial to Mesenchymal Transition in Pulmonary Vascular Diseases. Biomedicines. 2020;8(12)https://doi.org/10.3390/biomedicines8120639 10.3390/biomedicines8120639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zaldivia MTK, McFadyen JD, Lim B, et al. Platelet-Derived Microvesicles in Cardiovascular Diseases. Front Cardiovasc Med. 2017;4:74. https://doi.org/10.3389/fcvm.2017.00074 10.3389/fcvm.2017.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dobrescu G, Dobrescu A, Stoica L. Ultrastructural aspects in skin allergic vasculitis. Morphol Embryol (Bucur). 1983;29(1):31-4. [PubMed] [Google Scholar]
- 37.Wolff HH, Maciejewski W, Scherer R, et al. Immunoelectronmicroscopic examination of early lesions in histamine induced immune complex vasculitis in man. Br J Dermatol. Jul 1978;99(1):13-24. https://doi.org/10.1111/j.1365-2133.1978.tb01955.x 10.1111/j.1365-2133.1978.tb01955.x [DOI] [PubMed] [Google Scholar]
- 38.Cohen C, Trapuckd S. Leukocytoclastic vasculitis associated with cutaneous infection by herpesvirus. Am J Dermatopathol. 1984;6(6):561-5. https://doi.org/10.1097/00000372-198412000-00008 10.1097/00000372-198412000-00008 [DOI] [PubMed] [Google Scholar]
- 39.Salama S, Rosenthal D. Cutaneous collagenous vasculopathy with generalized telangiectasia: an immunohistochemical and ultrastructural study. J Cutan Pathol. 2000;27(1):40-8. https://doi.org/10.1034/j.1600-0560.2000.027001040.x 10.1034/j.1600-0560.2000.027001040.x [DOI] [PubMed] [Google Scholar]
- 40.Salama S, Chorneyko K, Belovic B. Cutaneous collagenous vasculopathy associated with intravascular occlusive fibrin thrombi. J Cutan Pathol. 2014;41(4):386-93. https://doi.org/10.1111/cup.12285 10.1111/cup.12285 [DOI] [PubMed] [Google Scholar]
- 41.Stavrou C, Uthayakumar A, Calonje JE, et al. Cutaneous collagenous vasculopathy. BMJ Case Rep. 2021;14(3)https://doi.org/10.1136/bcr-2020-241434 10.1136/bcr-2020-241434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sueki H, Telegan B, Murphy GF. Computer-assisted three-dimensional reconstruction of human dermal dendrocytes. J Invest Dermatol. 1995;105(5):704-8. https://doi.org/10.1111/1523-1747.ep12324502 10.1111/1523-1747.ep12324502 [DOI] [PubMed] [Google Scholar]
- 43.Guillevin L, Dorner T. Vasculitis: mechanisms involved and clinical manifestations. Arthritis Res Ther. 2007;9 Suppl 2(Suppl 2):S9. https://doi.org/10.1186/ar2193 10.1186/ar2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pacheco LS, Sotto MN. Factor XIIIa+ dermal dendrocytes in erythema elevatum diutinum and ordinary cutaneous leukocytoclastic vasculitis lesions. J Cutan Pathol. 2000;27(3):136-40. https://doi.org/10.1034/j.1600-0560.2000.027003136.x 10.1034/j.1600-0560.2000.027003136.x [DOI] [PubMed] [Google Scholar]
- 45.Estrada JA, Goffin F, Cornil F, et al. Dendrocytoclasis in Henoch-Schonlein purpura. Acta Derm Venereol. 1991;71(4):358-9. [PubMed] [Google Scholar]
- 46.Lotti TM, Ghersetich I, Comacchi C, et al. Langerhans’ cells and cutaneous necrotizing vasculitis. Clin Dermatol. 1999;17(5):591-6. https://doi.org/10.1016/s0738-081x(99)00065-6 10.1016/s0738-081x(99)00065-6 [DOI] [PubMed] [Google Scholar]
- 47.Romagnoli P, Ghersetich I, Lotti T. Langerhans cells and vasculitis. Int Angiol. 1995;14(2):113-8. [PubMed] [Google Scholar]
- 48.Zhang J, Alcaide P, Liu L, et al. Regulation of endothelial cell adhesion molecule expression by mast cells, macrophages, and neutrophils. PLoS One. 2011;6(1):e14525. https://doi.org/10.1371/journal.pone.0014525 10.1371/journal.pone.0014525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kunder CA, St John AL, Abraham SN. Mast cell modulation of the vascular and lymphatic endothelium. Blood. 2011;118(20):5383-93. https://doi.org/10.1182/blood-2011-07-358432 10.1182/blood-2011-07-358432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Inamura H, Igarashi Y, Kashiwase Y, Morioka J, Suzuki K, Kurosawa M. Mast cells in cutaneous allergic vasculitis: a case report. Allergol Int. 2006;55(3):343-5. https://doi.org/10.2332/allergolint.55.343 10.2332/allergolint.55.343 [DOI] [PubMed] [Google Scholar]
- 51.Kato Y, Aoki M, Kawana S. Urticarial vasculitis appearing in the progression of systemic sclerosis. J Dermatol. 2006;33(11):792-7. https://doi.org/10.1111/j.1346-8138.2006.00181.x 10.1111/j.1346-8138.2006.00181.x [DOI] [PubMed] [Google Scholar]
- 52.Zhang J, Hanig JP, De Felice AF. Biomarkers of endothelial cell activation: candidate markers for drug-induced vasculitis in patients or drug-induced vascular injury in animals. Vascul Pharmacol. 2012;56(1-2):14-25. https://doi.org/10.1016/j.vph.2011.09.002 10.1016/j.vph.2011.09.002 [DOI] [PubMed] [Google Scholar]
- 53.deCathelineau AM, Henson PM. The final step in programmed cell death: phagocytes carry apoptotic cells to the grave. Essays Biochem. 2003;39:105-17. https://doi.org/10.1042/bse0390105 10.1042/bse0390105 [DOI] [PubMed] [Google Scholar]
- 54.Heller T, Gessner JE, Schmidt RE, et al. Cutting edge: Fc receptor type I for IgG on macrophages and complement mediate the inflammatory response in immune complex peritonitis. J Immunol. 1999;162(10):5657-61. [PubMed] [Google Scholar]
- 55.Hilhorst M, Shirai T, Berry G, et al. T cell-macrophage interactions and granuloma formation in vasculitis. Front Immunol. 2014;5:432. https://doi.org/10.3389/fimmu.2014.00432 10.3389/fimmu.2014.00432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532-5. https://doi.org/10.1126/science.1092385 10.1126/science.1092385 [DOI] [PubMed] [Google Scholar]
- 57.Brinkmann V, Zychlinsky A. Neutrophil extracellular traps: is immunity the second function of chromatin? J Cell Biol. 2012;198(5):773-83. https://doi.org/10.1083/jcb.201203170 10.1083/jcb.201203170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee KH, Kronbichler A, Park DD, et al. Neutrophil extracellular traps (NETs) in autoimmune diseases: A comprehensive review. Autoimmun Rev. 2017;16(11):1160-1173. https://doi.org/10.1016/j.autrev.2017.09.012 10.1016/j.autrev.2017.09.012 [DOI] [PubMed] [Google Scholar]
- 59.Radic M. Clearance of Apoptotic Bodies, NETs, and Biofilm DNA: Implications for Autoimmunity. Front Immunol. 2014;5:365. https://doi.org/10.3389/fimmu.2014.00365 10.3389/fimmu.2014.00365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gupta AK, Joshi MB, Philippova M, et al. Activated endothelial cells induce neutrophil extracellular traps and are susceptible to NETosis-mediated cell death. FEBS Lett. Jul 16 2010;584(14):3193-7. https://doi.org/10.1016/j.febslet.2010.06.006 10.1016/j.febslet.2010.06.006 [DOI] [PubMed] [Google Scholar]
- 61.Zawrotniak M, Rapala-Kozik M. Neutrophil extracellular traps (NETs) - formation and implications. Acta Biochim Pol. 2013;60(3):277-84. [PubMed] [Google Scholar]
- 62.Nadkarni S, Dalli J, Hollywood J, et al. Investigational analysis reveals a potential role for neutrophils in giant-cell arteritis disease progression. Circ Res. 2014;114(2):242-8. https://doi.org/10.1161/CIRCRESAHA.114.301374 10.1161/CIRCRESAHA.114.301374 [DOI] [PubMed] [Google Scholar]
- 63.Kessenbrock K, Krumbholz M, Schonermarck U, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15(6):623-5. https://doi.org/10.1038/nm.1959 10.1038/nm.1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soderberg D, Segelmark M. Neutrophil Extracellular Traps in ANCA-Associated Vasculitis. Front Immunol. 2016;7:256. https://doi.org/10.3389/fimmu.2016.00256 10.3389/fimmu.2016.00256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vaglio A, Buzio C, Zwerina J. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): state of the art. Allergy. 2013;68(3):261-73. https://doi.org/10.1111/all.12088 10.1111/all.12088 [DOI] [PubMed] [Google Scholar]
- 66.Khoury P, Grayson PC, Klion AD. Eosinophils in vasculitis: characteristics and roles in pathogenesis. Nat Rev Rheumatol. 2014;10(8):474-83. https://doi.org/10.1038/nrrheum.2014.98 10.1038/nrrheum.2014.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tefferi A, Gotlib J, Pardanani A. Hypereosinophilic syndrome and clonal eosinophilia: point-of-care diagnostic algorithm and treatment update. Mayo Clin Proc. 2010;85(2):158-64. https://doi.org/10.4065/mcp.2009.0503 10.4065/mcp.2009.0503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Diny NL, Rose NR, Cihakova D. Eosinophils in Autoimmune Diseases. Front Immunol. 2017;8:484. https://doi.org/10.3389/fimmu.2017.00484 10.3389/fimmu.2017.00484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chu VT, Frohlich A, Steinhauser G, et al. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat Immunol. 2011;12(2):151-9. https://doi.org/10.1038/ni.1981 10.1038/ni.1981 [DOI] [PubMed] [Google Scholar]
- 70.Martinelli R, Zeiger AS, Whitfield M, et al. Probing the biomechanical contribution of the endothelium to lymphocyte migration: diapedesis by the path of least resistance. J Cell Sci. 2014;127(Pt 17):3720-34. https://doi.org/10.1242/jcs.148619 10.1242/jcs.148619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.de Menthon M, Lambert M, Guiard E, et al. Excessive interleukin-15 transpresentation endows NKG2D+CD4+ T cells with innate-like capacity to lyse vascular endothelium in granulomatosis with polyangiitis (Wegener’s). Arthritis Rheum. Jul 2011;63(7):2116-26. https://doi.org/10.1002/art.30355 10.1002/art.30355 [DOI] [PubMed] [Google Scholar]
- 72.Knochelmann HM, Dwyer CJ, Bailey SR, et al. When worlds collide: Th17 and Treg cells in cancer and autoimmunity. Cell Mol Immunol. 2018;15(5):458-469. https://doi.org/10.1038/s41423-018-0004-4 10.1038/s41423-018-0004-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wilde B, Thewissen M, Damoiseaux J, et al. Th17 expansion in granulomatosis with polyangiitis (Wegener’s): the role of disease activity, immune regulation and therapy. Arthritis Res Ther. 18 2012;14(5):R227. https://doi.org/10.1186/ar4066 10.1186/ar4066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abdulahad WH, Stegeman CA, van der Geld YM, et al. Functional defect of circulating regulatory CD4+ T cells in patients with Wegener’s granulomatosis in remission. Arthritis Rheum. 2007;56(6):2080-91. https://doi.org/10.1002/art.22692 10.1002/art.22692 [DOI] [PubMed] [Google Scholar]
- 75.Takahashi K, Oharaseki T, Yokouchi Y. Update on etio and immunopathogenesis of Kawasaki disease. Curr Opin Rheumatol. 2014;26(1):31-6. https://doi.org/10.1097/BOR.0000000000000010 10.1097/BOR.0000000000000010 [DOI] [PubMed] [Google Scholar]
- 76.Samson M, Audia S, Fraszczak J, et al. Th1 and Th17 lymphocytes expressing CD161 are implicated in giant cell arteritis and polymyalgia rheumatica pathogenesis. Arthritis Rheum. 2012;64(11):3788-98. https://doi.org/10.1002/art.34647 10.1002/art.34647 [DOI] [PubMed] [Google Scholar]
- 77.Saito H, Tsurikisawa N, Tsuburai T, et al. The proportion of regulatory T cells in the peripheral blood reflects the relapse or remission status of patients with Churg-Strauss syndrome. Int Arch Allergy Immunol. 2011;155 Suppl 1:46-52. https://doi.org/10.1159/000327265 10.1159/000327265 [DOI] [PubMed] [Google Scholar]
- 78.Free ME, Bunch DO, McGregor JA, et al. Patients with antineutrophil cytoplasmic antibody-associated vasculitis have defective Treg cell function exacerbated by the presence of a suppression-resistant effector cell population. Arthritis Rheum. Jul 2013;65(7):1922-33. https://doi.org/10.1002/art.37959 10.1002/art.37959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tadema H, Abdulahad WH, Stegeman CA, et al. Increased expression of Toll-like receptors by monocytes and natural killer cells in ANCA-associated vasculitis. PLoS One. 2011;6(9):e24315. https://doi.org/10.1371/journal.pone.0024315 10.1371/journal.pone.0024315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao Y, Odell E, Choong LM, et al. Granulomatosis with polyangiitis involves sustained mucosal inflammation that is rich in B-cell survival factors and autoantigen. Rheumatology (Oxford). 2012;51(9):1580-6. https://doi.org/10.1093/rheumatology/kes123 10.1093/rheumatology/kes123 [DOI] [PubMed] [Google Scholar]
- 81.Shapiro-Shelef M, Calame K. Regulation of plasma-cell development. Nat Rev Immunol. 2005;5(3):230-42. https://doi.org/10.1038/nri1572 10.1038/nri1572 [DOI] [PubMed] [Google Scholar]
- 82.Lutalo PM, D’Cruz DP. Diagnosis and classification of granulomatosis with polyangiitis (aka Wegener’s granulomatosis). J Autoimmun. 2014;48-49:94-8. https://doi.org/10.1016/j.jaut.2014.01.028 10.1016/j.jaut.2014.01.028 [DOI] [PubMed] [Google Scholar]
- 83.Merino-Vico A, van Hamburg JP, Tas SW. B Lineage Cells in ANCA-Associated Vasculitis. Int J Mol Sci. 2021;23(1)https://doi.org/10.3390/ijms23010387 10.3390/ijms23010387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hiepe F, Radbruch A. Plasma cells as an innovative target in autoimmune disease with renal manifestations. Nat Rev Nephrol. 2016;12(4):232-40. https://doi.org/10.1038/nrneph.2016.20 10.1038/nrneph.2016.20 [DOI] [PubMed] [Google Scholar]
- 85.Patterson J. Weedon’s Skin Pathology. Fifth Edition ed. Elsevier; 2020. [Google Scholar]
- 86.Zax RH, Hodge SJ, Callen JP. Cutaneous leukocytoclastic vasculitis. Serial histopathologic evaluation demonstrates the dynamic nature of the infiltrate. Arch Dermatol. 1990;126(1):69-72. https://doi.org/10.1001/archderm.126.1.69 10.1001/archderm.126.1.69 [DOI] [PubMed] [Google Scholar]
- 87.Miyagawa F, Ogawa K, Hashimoto T, et al. A Case of Systemic Lupus Erythematosus with Cutaneous Leukocytoclastic Vasculitis Mimicking Bullous SLE. Case Rep Dermatol. 2021;13(3):464-469. https://doi.org/10.1159/000519022 10.1159/000519022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ragab G, Hegazy MT, Ali M, et al. Three Patterns of Cutaneous Involvement in Granulomatosis with Polyangiitis. J Adv Res. Jul 2020;24:311-315. https://doi.org/10.1016/j.jare.2020.05.009 10.1016/j.jare.2020.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Naylor E, Atwater A, Selim MA, et al. Leukocytoclastic vasculitis as the presenting feature of dermatitis herpetiformis. Arch Dermatol. 2011;147(11):1313-6. https://doi.org/10.1001/archdermatol.2011.293 10.1001/archdermatol.2011.293 [DOI] [PubMed] [Google Scholar]
- 90.Ichiyama S, Funasaka Y, Yamashita H, et al. Leukocytoclastic vasculitis with eosinophilic infiltration associated with thalidomide therapy for multiple myeloma: A case report. Allergol Int. Jul 2017;66(3):497-498. https://doi.org/10.1016/j.alit.2016.12.006 10.1016/j.alit.2016.12.006 [DOI] [PubMed] [Google Scholar]
- 91.Tsai TC, Chen CY, Lin WT, et al. Sjogren’s syndrome complicated with IgA nephropathy and leukocytoclastic vasculitis. Ren Fail. 2008;30(7):755-8. https://doi.org/10.1080/08860220802213054 10.1080/08860220802213054 [DOI] [PubMed] [Google Scholar]
- 92.Di Vincenzo F, Ennas S, Pizzoferrato M, et al. Henoch-schonlein purpura following exposure to SARS-CoV2 vaccine or infection: a systematic review and a case report. Intern Emerg Med. 2024;19(1):13-37. https://doi.org/10.1007/s11739-023-03366-w 10.1007/s11739-023-03366-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu S, Han L, Liu Y, et al. Clinical Significance of MPO-ANCA in Eosinophilic Granulomatosis With Polyangiitis: Experience From a Longitudinal Chinese Cohort. Front Immunol. 2022;13:885198. https://doi.org/10.3389/fimmu.2022.885198 10.3389/fimmu.2022.885198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sinico RA, Di Toma L, Maggiore U, et al. Prevalence and clinical significance of antineutrophil cytoplasmic antibodies in Churg-Strauss syndrome. Arthritis Rheum. 2005;52(9):2926-35. https://doi.org/10.1002/art.21250 10.1002/art.21250 [DOI] [PubMed] [Google Scholar]
- 95.Genta MS, Genta RM, Gabay C. Systemic rheumatoid vasculitis: a review. Semin Arthritis Rheum. 2006;36(2):88-98. https://doi.org/10.1016/j.semarthrit.2006.04.006 10.1016/j.semarthrit.2006.04.006 [DOI] [PubMed] [Google Scholar]
- 96.Liu PY, Prete PE, Kukes G. Leukocytoclastic vasculitis in a patient with type 1 cryoglobulinemia. Case Rep Rheumatol. 2011;2011:124940. https://doi.org/10.1155/2011/124940 10.1155/2011/124940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cassisa A. Neutrophilic Dermatoses in Autoimmune Diseases: Report of Two Cases Associated with Autoimmune Thyroiditis. Dermatopathology (Basel). 2018;5(1):34-37. https://doi.org/10.1159/000486669 10.1159/000486669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Magro CM, Crowson AN. The spectrum of cutaneous lesions in rheumatoid arthritis: a clinical and pathological study of 43 patients. J Cutan Pathol. 2003;30(1):1-10. https://doi.org/10.1034/j.1600-0560.2003.300101.x 10.1034/j.1600-0560.2003.300101.x [DOI] [PubMed] [Google Scholar]
- 99.Cesinaro AM, Lonardi S, Facchetti F. Granuloma faciale: a cutaneous lesion sharing features with IgG4-associated sclerosing diseases. Am J Surg Pathol. 2013;37(1):66-73. https://doi.org/10.1097/PAS.0b013e318271efd0 10.1097/PAS.0b013e318271efd0 [DOI] [PubMed] [Google Scholar]
- 100.Skopec Z, Alsawas M, Maxwell T, et al. Assessment of specificity of dermatopathologic criteria for IgG4-related skin disease. J Cutan Pathol. Feb 2024;51(2):163-169. https://doi.org/10.1111/cup.14548 10.1111/cup.14548 [DOI] [PubMed] [Google Scholar]
- 101.Zargari O. Disseminated granuloma faciale. Int J Dermatol. 2004;43(3):210-2. https://doi.org/10.1111/j.1365-4632.2004.02099.x 10.1111/j.1365-4632.2004.02099.x [DOI] [PubMed] [Google Scholar]
- 102.Kavand S, Lehman JS, Gibson LE. Granuloma Faciale and Erythema Elevatum Diutinum in Relation to Immunoglobulin G4-Related Disease: An Appraisal of 32 Cases. Am J Clin Pathol. 2016;145(3):401-6. https://doi.org/10.1093/ajcp/aqw004 10.1093/ajcp/aqw004 [DOI] [PubMed] [Google Scholar]
- 103.Yoshinari M, Nishibata Y, Masuda S, et al. Low disease activity of microscopic polyangiitis in patients with anti-myosin light chain 6 antibody that disrupts actin rearrangement necessary for neutrophil extracellular trap formation. Arthritis Res Ther. 2022;24(1):274. https://doi.org/10.1186/s13075-022-02974-9 10.1186/s13075-022-02974-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wolff L, Horisberger A, Moi L, et al. Polyarteritis Nodosa: Old Disease, New Etiologies. Int J Mol Sci. 2023;24(23)https://doi.org/10.3390/ijms242316668 10.3390/ijms242316668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ishibashi M, Chen KR. A morphological study of evolution of cutaneous polyarteritis nodosa. Am J Dermatopathol. 2008;30(4):319-26. https://doi.org/10.1097/DAD.0b013e3181766190 10.1097/DAD.0b013e3181766190 [DOI] [PubMed] [Google Scholar]
- 106.Fein H, Sheth AP, Mutasim DF. Cutaneous arteritis presenting with hyperpigmented macules: macular arteritis. J Am Acad Dermatol. 2003;49(3):519-22. https://doi.org/10.1067/s0190-9622(03)00747-3 10.1067/s0190-9622(03)00747-3 [DOI] [PubMed] [Google Scholar]
- 107.Macarenco RS, Galan A, Simoni PM, et al. Cutaneous lymphocytic thrombophilic (macular) arteritis: a distinct entity or an indolent (reparative) stage of cutaneous polyarteritis nodosa? Report of 2 cases of cutaneous arteritis and review of the literature. Am J Dermatopathol. 2013;35(2):213-9. https://doi.org/10.1097/DAD.0b013e31825ba0ec 10.1097/DAD.0b013e31825ba0ec [DOI] [PubMed] [Google Scholar]
- 108.Buffiere-Morgado A, Battistella M, Vignon-Pennamen MD, et al. Relationship between cutaneous polyarteritis nodosa (cPAN) and macular lymphocytic arteritis (MLA): Blinded histologic assessment of 35 cPAN cases. J Am Acad Dermatol. 2015;73(6):1013-20. https://doi.org/10.1016/j.jaad.2015.09.010 10.1016/j.jaad.2015.09.010 [DOI] [PubMed] [Google Scholar]
- 109.Kolivras A, Thompson C. Cutaneous lymphocytic thrombophilic (macular) arteritis. Clin Dermatol. 2021;39(2):278-282. https://doi.org/10.1016/j.clindermatol.2020.10.011 10.1016/j.clindermatol.2020.10.011 [DOI] [PubMed] [Google Scholar]
- 110.Abdelmaksoud A, Wollina U, Temiz SA, et al. SARS-CoV-2 vaccination-induced cutaneous vasculitis: Report of two new cases and literature review. Dermatol Ther. 2022;35(6):e15458. https://doi.org/10.1111/dth.15458 10.1111/dth.15458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ungari M, Pezzarossa E. Cutaneous Lymphocytic Vasculitis After Administration of the Second Dose of AZD1222 (Oxford-AstraZeneca) Severe Acute Respiratory Syndrome Coronavirus 2 Vaccination: Casuality or Causality? Am J Dermatopathol. 2022;44(1):80-82. https://doi.org/10.1097/DAD.0000000000002104 10.1097/DAD.0000000000002104 [DOI] [PubMed] [Google Scholar]
- 112.Tomasini D, Mentzel T, Hantschke M, et al. Plasmacytoid dendritic cells: an overview of their presence and distribution in different inflammatory skin diseases, with special emphasis on Jessner’s lymphocytic infiltrate of the skin and cutaneous lupus erythematosus. J Cutan Pathol. 2010;37(11):1132-9. https://doi.org/10.1111/j.1600-0560.2010.01587.x 10.1111/j.1600-0560.2010.01587.x [DOI] [PubMed] [Google Scholar]
- 113.Aboobaker J, Greaves MW. Urticarial vasculitis. Clin Exp Dermatol. 1986;11(5):436-44. https://doi.org/10.1111/j.1365-2230.1986.tb00490.x 10.1111/j.1365-2230.1986.tb00490.x [DOI] [PubMed] [Google Scholar]
- 114.Smoller BR, McNutt NS, Contreras F. The natural history of vasculitis. What the histology tells us about pathogenesis. Arch Dermatol. 1990;126(1):84-9. [PubMed] [Google Scholar]
- 115.Paydas S, Zorludemir S, Sahin B. Vasculitis and leukemia. Leuk Lymphoma. 2000;40(1-2):105-12. https://doi.org/10.3109/10428190009054886 10.3109/10428190009054886 [DOI] [PubMed] [Google Scholar]
- 116.Reolid A, Rodriguez-Jimenez P, Llamas-Velasco M, Chicharro P, Fraga J, Aragues M. Leukemic Vasculitis: Case Report and Review of the Literature. Am J Dermatopathol. 2019;41(11):826-831. https://doi.org/10.1097/DAD.0000000000001438 10.1097/DAD.0000000000001438 [DOI] [PubMed] [Google Scholar]


