Mesalazine is widely prescribed for the treatment of inflammatory bowel disease. It is a single molecule of 5-aminosalicylic acid (5-ASA), and is structurally similar to phenacetin and aspirin. Occasionally, treatment with mesalazine may lead to a severe indolent interstitial nephritis causing appreciable morbidity. Unless detected and treated early this may progress to end stage renal failure despite withdrawal of the drug.1 It is obvious from the increasing number of reports of nephritis and renal failure occurring after treatment with mesalazine that the premise that “there is no need for routine monitoring of renal function”2 needs to be reviewed; the need for a review has been suggested by a number of recent reports.1,3–9 We report two cases of late onset interstitial nephritis induced by mesalazine (Asacol); the first presented after at least 5 years of continuous treatment with the drug and the second after 1 year.
Case reports
Case 1
A 38 year old laboratory technician began taking mesalazine for ulcerative colitis. After 2 years of continuous treatment he remained well with normal renal function (serum creatinine concentration 76 μmol/l; normal range 71-133 μmol/l) and negative results on urinalysis. He had an exacerbation of his colitis during the third and fourth years of treatment. On each occasion he responded to a combination of oral prednisolone treatment and an increase in the dose of mesalazine to 1.2 g twice a day. Each time, steroid treatment lasted for 3 months and began with 40 mg a day of prednisolone which was rapidly tapered down to a maintenance dose of 10 mg a day. Repeat serum creatinine concentration measured after 3 years of mesalazine treatment was 79 μmol/l. Thereafter the dose of mesalazine fluctuated between 800 mg and 1.2 g twice a day depending on the severity of his colitis.
After 5 years of treatment he noticed that his urine was frothy. He carried out his own urinalysis; protein was +++. Mesalazine treatment was discontinued, low dose prednisolone and azathioprine treatment were introduced to control his colitis, and he was referred to the nephrology clinic. At this stage his serum creatinine concentration had risen to 246 μmol/l, his creatinine clearance was 41ml/min (normal range 110-152 ml/min), and his 24 hour urinary protein excretion was 0.28 g (normal range <0.1g/24 h). Both kidneys were of normal size and shape with increased cortical echogenicity on ultrasound scanning. Immunoglobulin and complement concentrations were normal, and the results of screening for autoantibodies and antineutrophil cytoplasmic antibody were negative.
Renal biopsy was performed 8 weeks after mesalazine treatment ended at which time his serum creatinine concentration remained 243 μmol/l. On biopsy 29 glomeruli were identified. Six were globally sclerosed. All glomeruli had ischaemic shrinkage and periglomerular fibrosis (figure). There was extensive destructive lymphocytic tubulitis with areas of tubular atrophy (figure). The interstitial infiltrate comprised lymphocytes, plasma cells, and some eosinophils (figure). The results of immunofluorescence microscopy were negative. The appearance was of florid tubulointerstitial nephritis involving 80% of the biopsy specimen.
Prednisolone was increased to 60 mg a day for 6 weeks and then reduced to 10 mg a day. Three months after biopsy the patient was taking prednisolone 10 mg daily and azathioprine 100 mg daily. Serum creatinine had fallen to 170 μmol/l and his colitis remained in remission. Six months after biopsy he underwent panproctocolectomy and permanent ileostomy creation at his own request after which immunosuppressive treatment was withdrawn without further detriment to his renal function.
Case 2
A 25 year old man began taking mesalazine 400 mg three times a day after being diagnosed with ulcerative colitis. During the first 5 weeks of treatment he also took oral steroids, beginning with 30 mg a day of prednisolone and rapidly tapering down to 5 mg a day on alternate days. After 5 months of treatment he had a relapse and required a further course of oral prednisolone; mesalazine was increased to 800 mg twice a day. After 12 months of mesalazine treatment his serum creatinine concentration had risen to 302 μmol/l from a normal baseline value of 94 μmol/l. Mesalazine was discontinued and he was referred to the nephrology clinic.
Routine immunological screening was unremarkable, and the appearance of the kidneys on ultrasound scanning was similar to that in the first case. Renal biopsy was performed 1 month after withdrawal of mesalazine. The histological appearance of the sample was less severe than in the previous patient; none of the 13 glomeruli on the specimen submitted for light microscopy were globally sclerosed. Nevertheless there was an active tubulointerstitial nephritis with considerable tubule damage and periglomerular fibrosis affecting one third of the biopsy specimen. The patient began taking prednisolone 60 mg a day tapering down to 45 mg a day after one week, and to 30 mg a day a week later. He continued this dose for 1 month after which it was reduced to 10 mg a day over a 4 week period. After 4 months of steroid treatment his serum creatinine had fallen to 183 μmol/l and his colitis remained in remission.
Discussion
Since January 1985 there have been 104 adverse drug reactions of a renal or urinary nature associated with mesalazine treatment and reported to the Committee on Safety of Medicines (personal communication). In 35 cases the abnormality reported was interstitial nephritis. The increasing number of reports suggests that this adverse drug reaction may be underrecognised and underreported.1,3–9 We found no reports of interstitial nephritis occurring in association with untreated inflammatory bowel disease. It is unlikely that the association between mesalazine and interstitial nephritis is coincidental; the most recent report of nephritis associated with mesalazine suggested that recurrences occurred on rechallenge with the drug.9 The true incidence of this complication has not been determined, but it has been suggested that renal impairment may occur in up to 1 in 100 patients treated with mesalazine, although clinically important interstitial nephritis occurs in only 1 in 500 patients.1
The most frequent form of interstitial nephritis is severe, chronic, and progressive. It does not present until several months after treatment has begun. Unlike classic drug induced interstitial nephritis, symptoms and signs are scanty and non-specific. Testing urine with reagent strips may identify little abnormality, and the first indication that something is amiss may be the chance finding of a raised serum creatinine concentration. Restoration of renal function may be seen on withdrawal of medication in 85% of cases if the diagnosis of nephrotoxicity is made within 10 months of beginning treatment. If the diagnosis is delayed until 18 months after beginning treatment only partial recovery of renal function is likely to occur and then in only one third of cases.1 The exact mechanism of the induction of interstitial nephritis is unknown. However, the prime mechanism is unlikely to be a type 1 hypersensitivity reaction, as in only a few cases have patients been noted to have fever, arthralgia, eosinophilia, or skin rashes. A delayed, cell mediated response is the more likely mechanism.
The cases of the two patients presented here support earlier findings and show the indolent nature of this form of interstitial nephritis. The first patient had a serum creatinine concentration well within the normal range 3 years after beginning treatment. Despite this he went on to develop severe interstitial nephritis that was associated with mesalazine treatment. His renal biopsy sample showed evidence of severe active tubulointerstitial nephritis 8 weeks after withdrawal of the drug. Although the role of immunosuppression in addition to drug withdrawal is undetermined, functional renal improvement was only achieved with aggressive immunosuppressive treatment. Similarly, in the second case there was evidence of active tubulointerstitial nephritis 4 weeks after withdrawal of the drug; improvement in renal function occurred only after introduction of immunosuppressive treatment.
These two cases emphasise the need for increased awareness among practitioners who prescribe mesalazine of its ability to cause late onset nephrotoxicity and renal impairment. About half of all reported cases of nephrotoxicity associated with mesalazine have presented within a year of starting drug treatment but in some the diagnosis was made after 3½ years of continuous treatment. There are no reported cases presenting after 5 years of continuous mesalazine treatment. It is unclear whether or not the use of intercurrent courses of steroid treatment to control exacerbations of inflammatory bowel disease may have contributed to the delay in presentation in both cases. Using reagent strip urinalysis is not sufficient to monitor possible nephrotoxicity. It is advisable to monitor serum creatinine concentrations at each clinic visit during the first year after starting treatment, and at least annually thereafter for the duration of treatment.
Figure.
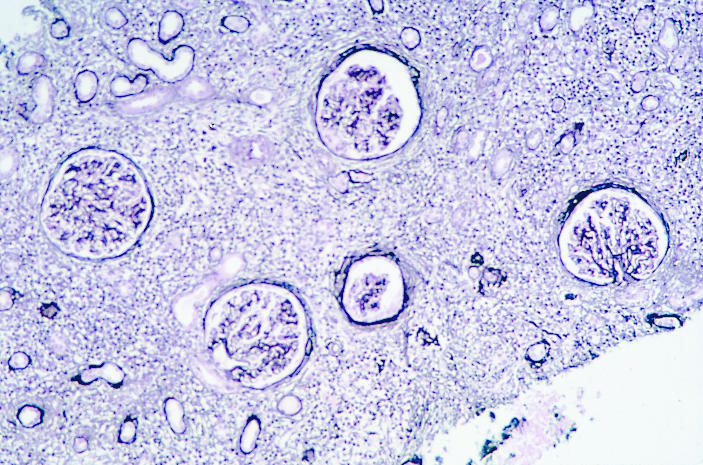
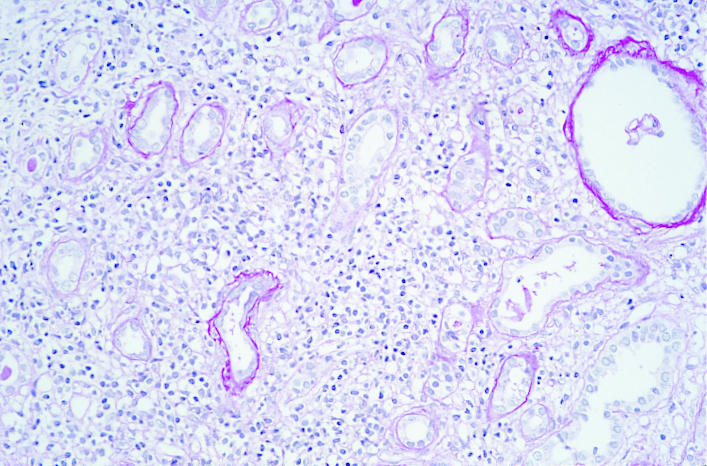
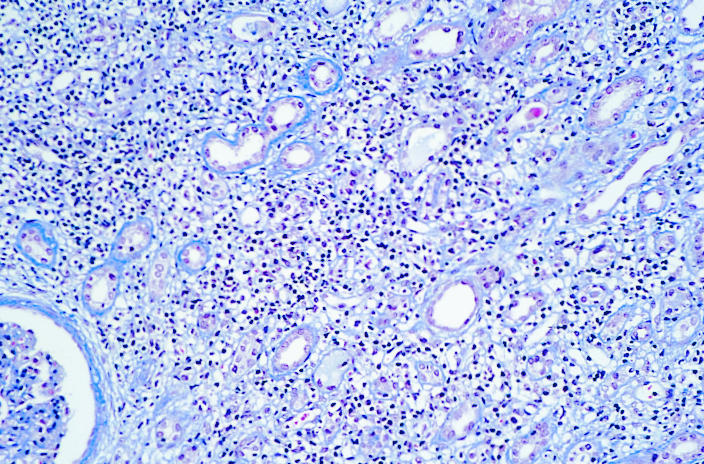
Renal biopsy specimen taken 8 weeks after treatment with mesalazine ended in patient in case 1. Ischaemic shrinkage and periglomerular fibrosis are evident (left) as is destructive lymphocytic tubulitis (centre). The interstitial infiltrate contained lymphocytes, plasma cells, and some eosinophils (right)
References
- 1.World MJ, Stevens PE, Ashton MA, Rainford DJ. Mesalazine-associated interstitial nephritis. Nephrol Dial Transplant. 1996;11:614–621. doi: 10.1093/oxfordjournals.ndt.a027349. [DOI] [PubMed] [Google Scholar]
- 2.Choosing an oral aminosalicylic acid preparation for ulcerative colitis. Drug Ther Bull. 1992;30:50–52. [PubMed] [Google Scholar]
- 3.Thuluvath PJ, Ninkovic M, Calam J, Anderson M. Mesalazine induced interstitial nephritis. Gut. 1994;35:1493–1496. doi: 10.1136/gut.35.10.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta RP. Acute interstitial nephritis due to 5-aminosalicylic acid. Can Med Assoc J. 1990;143:1031–1032. [PMC free article] [PubMed] [Google Scholar]
- 5.Witte T, Olbritcht CJ, Koch KM. Interstitial nephritis associated with 5-aminosalicylic acid. Nephron. 1994;67:481–482. doi: 10.1159/000188024. [DOI] [PubMed] [Google Scholar]
- 6.Smilde TJ, van Liebergen FJ, Koolen MI, Gerlag PG, Assman KJ, Berden JH. Tubulointerstitial nephritis caused by mesalazine (5-ASA) agents. Ned Tijdschr Geneeskd. 1994;138:2557–2561. [PubMed] [Google Scholar]
- 7.Hamling J, Raedler A, Helmchen U, Schreiber S. 5-Aminosalicylic acid-associated renal tubular acidosis with decreased renal function in Crohn’s disease. Digestion. 1997;58:304–307. doi: 10.1159/000201459. [DOI] [PubMed] [Google Scholar]
- 8.De Broe ME, Stolear JC, Nouwen EJ, Elseviers MM. 5-Aminosalicylic acid (5-ASA) and chronic tubulointerstitial nephritis in patients with chronic inflammatory bowel disease: is there a link? Nephrol Dial Transplant. 1997;12:1839–1841. doi: 10.1093/ndt/12.9.1839. [DOI] [PubMed] [Google Scholar]
- 9.Manenti L, De Rosa A, Buzio C. Mesalazine-associated interstitial nephritis: twice in the same patient. Nephrol Dial Transplant. 1997;12:2031. [PubMed] [Google Scholar]


