Oxygen is widely available and commonly prescribed by medical and paramedical staff. When administered correctly it may be life saving, but oxygen is often given without careful evaluation of its potential benefits and side effects. Like any drug there are clear indications for treatment with oxygen and appropriate methods of delivery. Inappropriate dose and failure to monitor treatment can have serious consequences. Vigilant monitoring to detect and correct adverse effects swiftly is essential.
In a recent hospital survey 21% of oxygen prescriptions were inappropriate and 85% of patients were inadequately supervised. Similar studies report that oxygen is prescribed inappropriately in general practice. To ensure safe and effective treatment prescriptions should cover the flow rate, delivery system, duration, and monitoring of treatment.
Recognising inadequate tissue oxygenation
Tissues require oxygen for survival. Delivery depends on adequate ventilation, gas exchange, and circulatory distribution. Tissue hypoxia occurs within 4 minutes of failure of any of these systems because the oxygen reserves in tissue and lung are relatively small. The physiological and pathological mechanisms that result in tissue hypoxia will be discussed in later articles. They can be classified into two main groups: those causing arterial hypoxaemia and those causing failure of the oxygen-haemoglobin transport system without arterial hypoxaemia. More than one mechanism may contribute to tissue hypoxia, and predicting the response to supplemental oxygen requires careful evaluation of these functions.
Checklist for safe prescribing of oxygen
How can inadequate tissue oxygenation be recognised?
When is acute oxygen therapy appropriate and at what dose?
Is outcome of disease improved?
How is oxygen best delivered and is humidification necessary?
What are the dangers of oxygen treatment?
What assessment and monitoring are necessary?
When should oxygen therapy be stopped?
Pathophysiological mechanisms of tissue hypoxia
Arterial hypoxaemia
Low inspired oxygen partial pressure (high altitude)
Alveolar hypoventilation (sleep apnoea, opiate overdose)
Ventilation-perfusion mismatch (acute asthma, atelectatic lung zones)
Right to left shunts
Failure of oxygen-haemoglobin transport system
Inadequate tissue perfusion
Low haemoglobin concentration
Abnormal oxygen dissociation curve (haemoglobinopathies, high carboxyhaemoglobin)
Histotoxic poisoning of intracellular enzymes (cyanide poisoning, septicaemia)
Successful treatment of tissue hypoxia requires early recognition. This can be difficult because the clinical features are often non-specific and include altered mental state, dyspnoea, cyanosis, tachypnoea, arrhythmias, and coma. Hyperventilation due to carotid chemoreceptor stimulation becomes pronounced when the arterial partial pressure of oxygen (Pao2) falls to 5.3 kPa. Peripheral vasodilation with consequent systemic hypotension and eventually coma occurs if the Pao2 falls below 4 kPa.
Central cyanosis is an unreliable indicator of tissue hypoxia. It is detectable when the concentration of reduced haemoglobin is about 15 g/l of blood rather than the widely quoted erroneous value of 50 g/l. At a haemoglobin concentration of 150 g/l cyanosis can be detected if the a haemoglobin saturation is 90%, but it is often absent in hypoxaemic patients with anaemia and more obvious in patients with polycythaemia.
Arterial oxygen saturation (Sao2) and Pao2 are readily measured and remain the principal clinical indicators for initiating, monitoring, and adjusting oxygen treatment. However, Pao2 and Sao2 can be normal when tissue hypoxia is caused by low output cardiac states, anaemia, and failure of tissue to use oxygen. In these circumstances mixed venous oxygen partial pressure (Pvo2), which is measured in pulmonary artery blood, approximates to mean tissue Po2 and is a better index of tissue oxygenation. Even in the presence of a normal Pao2 and Pvo2 severe hypoxia in a single organ may result in death. Measurement of individual tissue oxygenation is difficult and requires specialised techniques including tonometry and oxygen probes.
In chronically hypoxaemic patients adequate delivery of oxygen to tissues is achieved by compensatory mechanisms, including polycythaemia, a shift in the haemoglobin-oxygen dissociation curve, and increased extraction of oxygen.
When acute oxygen shortage occurs in chronically hypoxaemic patients Pao2 and Pvo2 are unreliable and must be interpreted in conjunction with acid-base balance and clinical state
Indications for acute oxygen
In acutely ill patients oxygen delivery relies on maintaining a patent airway. This should always be checked first. Give oxygen empirically in patients with cardiac or respiratory arrest or when there is respiratory distress or hypotension. Arterial blood gases should be analysed as soon as possible to assess the degree of hypoxaemia, partial pressure of carbon dioxide (Pco2), and acid-base state.
Guidelines for initial oxygen dose
| Fraction of oxygen in inspired air (%) | |
| Cardiac or respiratory arrest | 100 |
| Hypoxaemia with Paco2<5.3 kPa | 40-60 |
| Hypoxaemia with Paco2>5.3 kPa | 24 initially |
American College of Chest Physicians and National Heart Lung and Blood Institute recommendations for instituting oxygen therapy
Cardiac and respiratory arrest
Hypoxaemia (Pao2<7.8 kPa, Sao2<90%)
Hypotension (systolic blood pressure <100 mm Hg)
Low cardiac output and metabolic acidosis (bicarbonate<18 mmol/l)
Respiratory distress (respiratory rate >24/min)
Increasing the fraction of inspired oxygen (Fio2) increases oxygen transport by ensuring that blood haemoglobin is fully saturated and by raising the quantity of oxygen normally carried in solution in the plasma. However, the solubility of oxygen in blood is low. Even when the inspired oxygen concentration is 100%, dissolved oxygen provides only one third of resting tissue oxygen requirements. Therefore, oxygen treatment must be aimed at correcting arterial hypoxaemia; when tissue hypoxia occurs in the absence of arterial hypoxaemia treatment should always be directed at correcting the underlying cause (that is, heart failure, anaemia).
Clinical efficacy of acute oxygen treatment
Arterial hypoxaemia
Ventilation-perfusion (V/Q) mismatch (pneumonic and atelectatic lung zones)—The response to oxygen depends on V/Q mismatch within individual areas of lung and is unpredictable
Alveolar hypoventilation (drug overdose, neuromuscular disorders)—Oxygen rapidly corrects arterial hypoxaemia, but improving ventilation must be the primary aim of therapy
Right to left shunting (pneumonia, pulmonary embolism, arteriovenous channels)—When the shunt fraction is >20%, arterial hypoxaemia persists despite high inspired Fio2
Tissue hypoxia without arterial hypoxaemia
Myocardial infarction—A controlled double-blind study in uncomplicated myocardial infarction showed no difference in mortality, use of analgesics, or incidence of arrhythmias in patients treated with and without oxygen. If there is any doubt about possible hypoxia oxygen should be given
Low cardiac output states (anaemia, cardiac failure, and hypovolaemic shock)—In the absence of arterial hypoxaemia starting oxygen must not delay correction of the primary clinical problem
Carbon monoxide poisoning—High concentration oxygen treatment is essential despite a normal Pao2 because oxygen competes with carbon monoxide for haemoglobin binding sites and reduces the half life of carboxyhaemoglobin from about 320 to 80 minutes
Chronic lung disease—Subjective relief of breathlessness has been shown in some patients even in the absence of arterial hypoxaemia and a controlled trial of oxygen may be warranted
In the acute situation the dose of oxygen administered may be critical. Inadequate oxygen accounts for more deaths and permanent disability than can be justified by the relatively small risks associated with high dose oxygen. In many acute conditions (for example, asthma, pulmonary embolus), inspired oxygen concentrations of 60-100% for short periods may preserve life until more specific treatment can be instituted. Thereafter oxygen should be given at a dose that will correct hypoxaemia and minimise side effects (increase the Pao2 to 8.0-10.6 kPa). When necessary, oxygen must be given continuously.
High dose oxygen given to patients with chronic obstructive pulmonary disease who have type II respiratory failure can reduce the hypoxic drive to breathe and increase ventilation-perfusion mismatching. This causes carbon dioxide retention and a respiratory acidosis that may be lethal. In these patients initial treatment with low oxygen concentrations (24-28%) should be progressively increased on the basis of repeated blood gas analysis with the aim of correcting hypoxaemia to a Pao2>6.65 kPa without decreasing arterial pH below 7.26. Non-invasive positive pressure ventilation and respiratory stimulants may help achieve adequate oxygenation and prevent carbon dioxide retention by raising minute ventilation in patients with type II respiratory failure. It is more effective and safer than respiratory stimulation and should be used when available. Type II respiratory failure occurs in 10-15% of patients with chronic obstructive pulmonary disease. In patients without type II respiratory failure the risk of hypercapnia is often overstressed, and undertreatment of serious hypoxaemia can result in unnecessary death.
Oxygen delivery systems
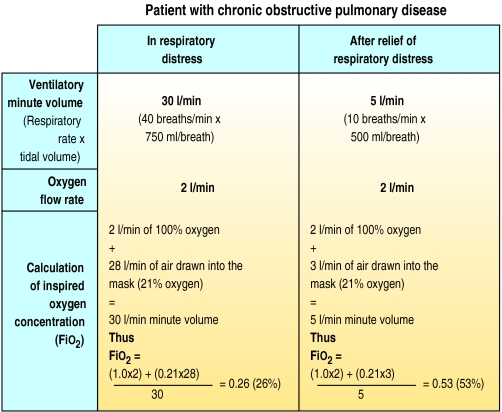
A wide variety of cheap oxygen delivery systems are available. The mask and valve design and oxygen flow rate allows delivery of an inspired oxygen of 24-90% (Fio2 0.26-0.90). The concentration of oxygen that patients inspire depends on the ventilatory minute volume (MV) and the flow rate of oxygen. The greater the ventilation, the lower the Fio2 for a given flow rate of supplemental oxygen. It is impossible to provide a fixed Fio2 to a patient with a varying ventilatory requirement unless the total ventilatory minute volume is provided at the required Fio2.
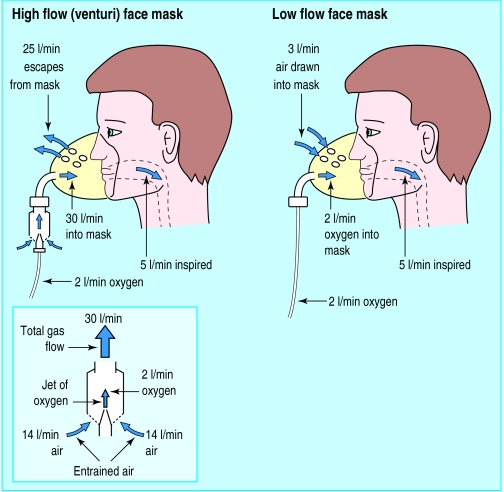
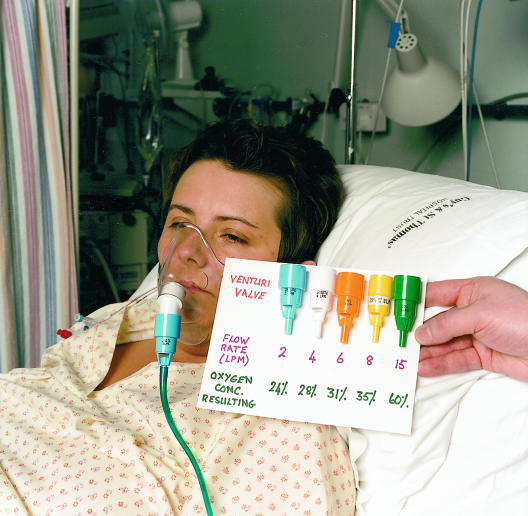
There are two basic types of oxygen mask which deliver either the entire (high flow mask) or a proportion (low flow mask) of the ventilatory requirement. High flow systems deliver about 40 l/min of gas through the mask, which is usually sufficient to meet the total respiratory demand. This ensures that the breathing pattern will not affect the Fio2. The masks contain venturi valves, which use the principle of jet mixing (Bernoulli effect). When oxygen passes through a narrow orifice it produces a high velocity stream that draws a constant proportion of room air through the base of the venturi valve. Air entrainment depends on the velocity of the jet (the size of orifice and oxygen flow rate) and the size of the valve ports. It can be accurately controlled to give inspired oxygen levels of 24-60%.
Oxygen masks
Although many different designs of high and low flow systems are available, only a few are used regularly.
High flow, jet mixing masks are useful for accurately delivering low concentrations of oxygen (24-35%). They provide the total ventilatory requirement unaffected by the pattern of ventilation. In patients with chronic obstructive pulmonary disease and type II respiratory failure these masks reduce the risk of carbon dioxide retention while improving hypoxaemia. They are loose fitting and comfortable to wear. Rebreathing of expired gas is not a problem because the mask is flushed by the high flow rates.
Low flow masks—A concentration of up to 60% can be achieved with moderate oxygen flow rates (6-10 l/min), and these masks are used mainly in type I respiratory failure (for example, pulmonary oedema, pulmonary embolus). At low oxygen flow rates (<5 l/min) significant rebreathing may occur because exhaled air is not adequately flushed from the face mask. This makes it difficult to acheive a low inspired oxygen concentration and prevent retention of carbon dioxide.These masks are generally not suitable for patients with type II respiratory failure.
Rebreathing and anaesthetic type oxygen masks—Partial rebreathing masks incorporating non-rebreathing valves and reservoir bags are not in common use but can provide concentrations greater than 60% at low oxygen flow rates. In cardiac or respiratory arrest, tight fitting anaesthetic-type masks can achieve 100% oxygen, but prolonged use risks oxygen toxicity and reabsorption atelectasis.
Nasal prongs are simple and convenient to use. The Fio2 depends on the flow rate of oxygen (1-6 l/min) and varies according to ventilatory minute volume. At an oxygen flow rate of 2 l/min the oxygen concentration in the hypopharynx of a resting subject is 25-30%. Nasal prongs prevent rebreathing, are comfortable for long periods, and allow oxygen to be continued during talking and eating. Local irritation and dermatitis may occur with high flow rates.
Non-invasive assisted ventilation—Supplemental oxygen may be provided through tight fitting nasal or full face masks during nasal intermittent positive pressure ventilation and continuous positive airways pressure. These techniques have been used to support ventilation in sleep associated hypoventilation, during weaning from mechanical ventilation, and in respiratory failure associated with chronic obstructive pulmonary disease.
Other delivery systems
Hyperbaric oxygenation—At a pressure of 300 kPa the small quantity of oxygen in solution in the blood can be increased by up to 300% and diffusion through tissues may be improved. Advice is best sought on an individual basis from the specialist centres providing this service.
Humidification of oxygen—When oxygen is delivered at a flow rate of 1-4 l/min by mask or nasal prongs, the oropharynx or nasopharynx provides adequate humidification. At higher flow rates or when oxygen is delivered directly to the trachea humidification is necessary.
Recommendations for monitoring oxygen therapy
Arterial blood gas analysis should be performed before oxygen therapy if possible
Arterial blood gases should be measured or oximetry done within 2 hours of starting oxygen therapy and Fio2 adjusted accordingly. (An adequate response is defined as Pao2>7.8 kPa or Sao2>90%)
Hypoxaemic patients at risk of arrhythmias or respiratory failure should be monitored continuously by oximetry
In patients at risk of type II respiratory failure, arterial blood gases should be measured more frequently to assess Pao2 and Sao2 should be monitored continuously by oximetry
In the acute stage response should be assessed daily by arterial blood gas analysis or oximetry and Fio2 adjusted accordingly
Monitoring oxygen treatment
Oxygen treatment can be monitored by blood gas measurements or non-invasively by pulse oximetry. Blood gas analysis provides accurate information on the pH, Pao2, and Paco2. Oximetry provides continuous monitoring of the state of oxygenation.
Stopping oxygen treatment
Oxygen should be stopped when arterial oxygenation is adequate with the patient breathing room air (Pao2>8 kPa, Sao2>90%). In patients without arterial hypoxaemia but at risk of tissue hypoxia, oxygen should be stopped when the acid-base state and clinical assessment of vital organ function are consistent with resolution of tissue hypoxia.
Dangers of oxygen treatment
Fire: Oxygen promotes combustion. Facial burns and deaths of patients who smoke when using oxygen are well documented
Pulmonary oxygen toxicity: High concentrations of oxygen (>60%) may damage the alveolar membrane when inhaled for more than 48 hours. Progression to the adult respiratory distress syndrome with high protein alveolar oedema and pulmonary radiographic infiltrates is associated with high mortality
Paul-Bert effect: Breathing hyperbaric oxygen (for example, when diving) can cause severe cerebral vasoconstriction and epileptic fits
Summary
Oxygen is a life saving treatment. It should be treated like any other drug; it should be prescribed in writing, with the required flow rate and the method of delivery clearly specified. Failure to correct hypoxaemia (Pao2>8 kPa) for fear of causing hypoventilation and carbon dioxide retention is unacceptable clinical practice. Careful monitoring of treatment is essential and will detect those patients at risk of carbon dioxide retention.
Figure.
Oxygen is often poorly prescibed. This drug chart was for a severely hypoxaemic patient with pneumonia and mild chronic obstructive pulmonary disease. The risk of carbon dioxide retention was secondary to the danger of severe hypoxaemia and inadequate oxygen was life threatening
Figure.

Oxygen saturation is readily measured with pulse oximeters
Figure.
Fio2 depends on ventilatory minute volume and flow rate of oxygen. This is illustrated by calculating the Fio2 at a fixed oxygen flow rate of 2 l/min in a patient with an exacerbation of chronic obstructive pulmonary disease before and after treatment
Figure.
High flow oxygen masks provide the entire ventilatory requirement using a venturi valve. The port size of the valve ensures the correct proportions of oxygen and entrained air are mixed to obtain a fixed oxygen concentration. Low flow oxygen masks do not provide the entire ventilatory requirement. Air is drawn in through the loose fitting mask to supplement the oxygen flow rate
Figure.
High flow oxygen mask and series of venturi valves. The flow rate of oxygen required to provide a fixed concentration of oxygen is shown on each valve
Figure.
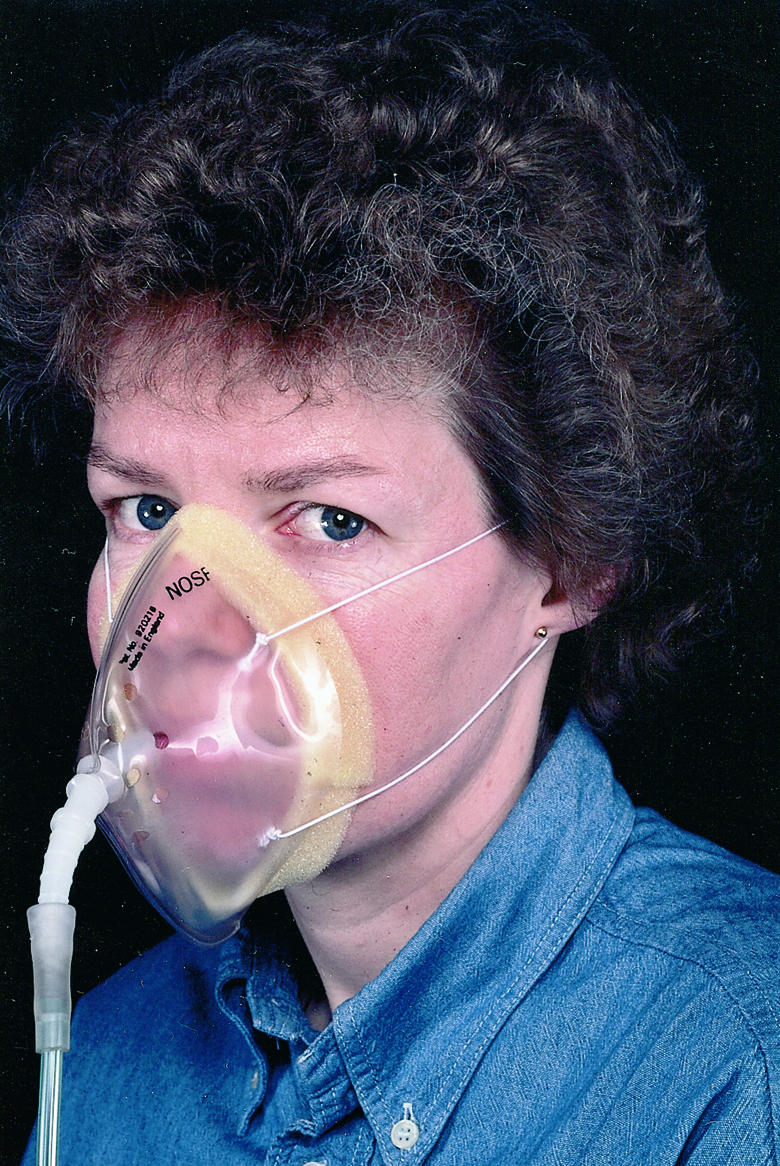
Typical low flow mask. This mask is ideal for providing high concentrations of oxygen (40-60%). The flow rate of oxygen required to achieve an approximate Fio2 is shown on the mask packaging
Figure.
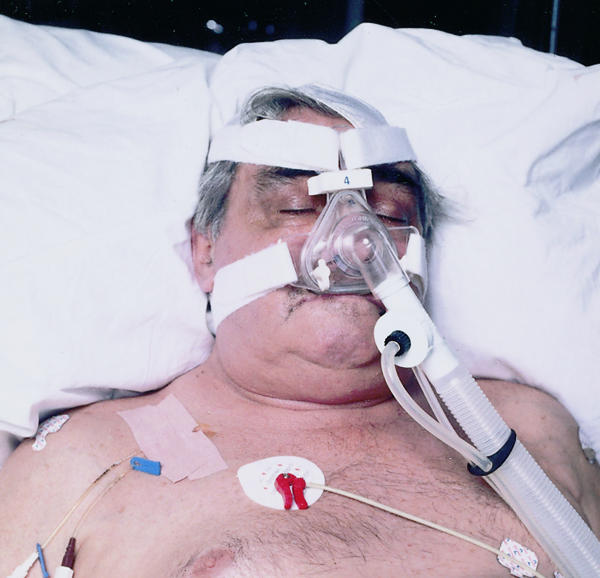
Non-invasive partial pressure ventilation
Figure.
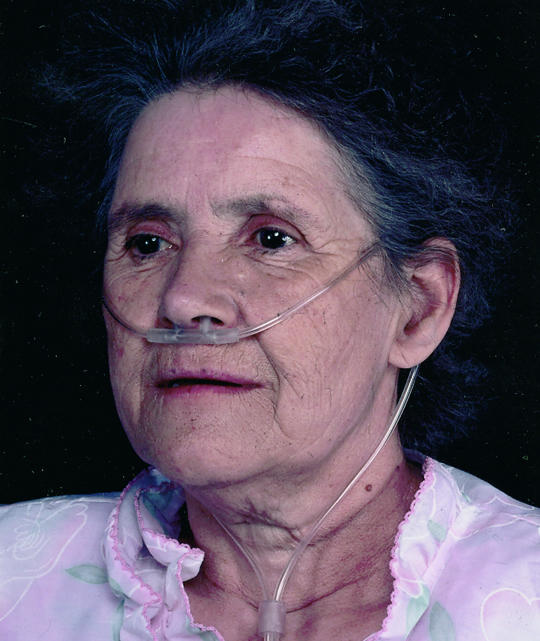
Correct positioning of nasal prongs
Footnotes
N T Bateman is consultant physician, Department of Respiratory Medicine, St Thomas’s Hospital, London.
The ABC of Oxygen is edited by Richard M Leach, consultant physician, and John Rees, consultant physician, Guy’s and St Thomas’s Hospital Trust, London. It will be published as a book later this year