Abstract
Azotobacter vinelandii produces the exopolysaccharide alginate, which is essential for its differentiation to desiccation-resistant cysts. In different bacterial species, the alternative sigma factor ςE regulates the expression of functions related to the extracytoplasmic compartments. In A. vinelandii and Pseudomonas aeruginosa, the ςE factor (AlgU) is essential for alginate production. In both bacteria, the activity of this sigma factor is regulated by the product of the mucA, mucB, mucC, and mucD genes. In this work, we studied the transcriptional regulation of the A. vinelandii algU-mucABCD gene cluster, as well as the role of the mucA and mucC gene products in alginate production. Our results show the existence of AlgU autoregulation and show that both MucA and MucC play a negative role in alginate production.
Azotobacter vinelandii is a gram-negative soil bacterium which under adverse environmental conditions undergoes a differentiation process leading to the formation of desiccation-resistant cysts (37). The mature cysts are surrounded by two capsule-like layers containing a high proportion of the exopolysaccharide alginate (40). This exopolysaccharide is essential for the encystment process, since nonmucoid strains fail to encyst (6, 31, 34).
Considerable information about alginate biosynthesis and its regulation is available based on the studies of Pseudomonas aeruginosa (4, 5, 11, 12, 16, 19, 27, 28, 42–46). The interest in this bacterium is motivated by the role that alginate plays in the pathogenesis of the lung of cystic fibrosis patients. Respiratory tract infections with mucoid P. aeruginosa strains, which produce copious amounts of alginate, are the major contributing factor causing high morbidity and mortality in cystic fibrosis (17). The alginate biosynthetic pathways are very similar in A. vinelandii (38) and P. aeruginosa (28).
In A. vinelandii, as in P. aeruginosa, the algD gene, coding for the rate-limiting enzyme GDP-mannose dehydrogenase, is located in a biosynthetic cluster which contains the genes coding for the enzymes involved in alginate synthesis, with the exception of algC, which codes for the second enzyme in this biosynthetic route (28). In A. vinelandii, the biosynthetic gene cluster is arranged in three operons, one of which transcribes the algD gene alone (6, 24, 30), while in the latter bacterium, this gene cluster is transcribed as a single operon, whose transcription is started from a promoter upstream of the algD gene (9). The alternative sigma factor ςE (also known as AlgU or AlgT) is responsible for the transcription of the algD gene in P. aeruginosa (44), as well as in Pseudomonas syringae (21). In A. vinelandii, algD is transcribed from three promoters, only one of which is ςE dependent (34), but algU mutants are completely abrogated in alginate production (26, 34), presumably due to the ςE dependence of other genes involved in exopolysaccharide synthesis.
In different bacterial species, the alternative sigma factor ςE regulates the expression of functions related to the extracytoplasmic compartments (33). This sigma factor is similar to the Escherichia coli and Salmonella enterica serovar Typhimurium ςE protein (3, 10, 20, 29, 39). In E. coli, the ςE factor is absolutely required for growth at high temperatures (13, 14). In E. coli, genes encoding the heat shock proteins are transcribed by RNA polymerase holoenzyme containing the alternative sigma factor ς32, encoded by the rpoH gene. At 30°C, the rpoH transcripts originate from two promoters, p1 and p2, which are recognized by ς70 RNA polymerase (13). At 42°C or higher temperatures, almost all transcription of rpoH comes from the p3 promoter, which is ςE dependent (13, 14).
In P. aeruginosa and A. vinelandii, the mucABCD genes are located downstream of the algU gene, forming part of the same transcriptional unit (26, 43). It has been clearly shown elsewhere for P. aeruginosa that the AlgU activity is negatively regulated by the anti-sigma factor MucA (11, 12, 16, 19, 27, 43, 45) and, in an indirect manner, by MucB (27) and also that the mucD gene encodes a periplasmic protease which plays a central role in AlgU activation (4). MucC has been shown to play a role in AlgU regulation for P. aeruginosa, but its mechanism has not been elucidated (5). For Photobacterium strain SS9, a gene cluster carrying homologs of algU, mucA, mucB, and mucC has been described elsewhere (8). The mucC homolog (ORF4) has been reported to code for a protein which seems to participate in the control of adapted growth at cold temperature and high pressure (8). In serovar Typhimurium, the gene homologous to mucC has been shown to be involved in biotin synthesis (3). It has been reported that alginate production in P. syringae is also regulated by the AlgU-MucA sigma factor–anti-sigma factor (21).
P. aeruginosa AlgU and E. coli ςE are interchangeable in the P. aeruginosa background (46). The E. coli chromosome contains, downstream of rpoE, two genes (rseA and rseB) encoding proteins with regulatory functions similar to those of MucA and MucB proteins (10). In A. vinelandii (26), the genetic arrangement of algU-mucABCD is the same as in P. aeruginosa (43), showing high sequence similarity (26). We have previously reported that the A. vinelandii and P. aeruginosa algU-mucABCD gene products play similar regulatory roles in alginate biosynthesis since they are functionally interchangeable (26). It is also clear that in A. vinelandii AlgU is absolutely required for cyst formation, independently of its role in alginate production (34).
Overproduction of alginate by P. aeruginosa is an important virulence determinant expressed by this organism in the lungs of cystic fibrosis patients (17). Although the initial colonizing P. aeruginosa strains are nonmucoid, they undergo conversion to a highly mucoid phenotype in later stages of the disease. Loss-of-function mutations in either mucA or mucB have been reported to convert P. aeruginosa to mucoidy, by increasing AlgU activity (11, 12, 25). In contrast, A. vinelandii strains produce alginate even in the absence of mutations in the mucABCD operon.
In this context, our aim in this work was to evaluate the effect of mucA and mucC mutations on alginate production by A. vinelandii strains producing different exopolysaccharide levels. We show that the transcription of the A. vinelandii algU gene is initiated from two AlgU-dependent promoters, one of which presents a consensus sequence for the recognition of RNA polymerase containing a ςE subunit, and an apparently ςD promoter, which seems to be regulated indirectly by AlgU. It is also shown that the A. vinelandii AlgU sigma factor is functional in an E. coli background, but with a much lower apparent activity than that of the corresponding E. coli protein. We also show that in A. vinelandii the MucA and MucC proteins negatively regulate alginate production.
MATERIALS AND METHODS
Microbiological methods.
Bacterial strains and plasmids used in this work are shown in Table 1. A. vinelandii strains were routinely grown on BS medium (22) at 30°C. Antibiotic concentrations used for A. vinelandii and E. coli were as follows: ampicillin, not used and 200 μg/ml; chloramphenicol, not used and 30 μg/ml; gentamicin, 1.5 and 10 μg/ml; kanamycin, 2 μg/ml and not used; and tetracycline, 20 and 20 μg/ml, respectively.
TABLE 1.
Bacterial strains and plasmids used in this work
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| Strains | ||
| A. vinelandii | ||
| AEIV | Wild type, mucoid | Svein Valla |
| AEA8 | AEIV with a nonpolar mucA::Gm mutation | This work |
| AEA4 | AEIV with a polar mucA::Gm mutation | This work |
| AEC2 | AEIV with a nonpolar mucC::Tc mutation | This work |
| ATCC 9046 | Highly mucoid due to the muc-1 spontaneous mutation | 26 |
| JRA8 | ATCC 9046 with a nonpolar mucA::Gm mutation | This work |
| JRA4 | ATCC 9046 with a polar mucA::Gm mutation | This work |
| MLC2 | ATCC 9046 with a nonpolar mucC::Tc mutation | This work |
| MLC4 | ATCC 9046 with a polar mucC::Tc mutation | This work |
| WI12 | algD::lacZ derivative of ATCC 9046 | 6 |
| WIA8 | WI12 with a nonpolar mucA::Gm mutation | This work |
| WIA4 | WI12 with a polar mucA::Gm mutation | This work |
| WIC2 | WI12 with a nonpolar mucC::Tc mutation | This work |
| WIC4 | WI12 with a polar mucC::Tc mutation | This work |
| E. coli | ||
| DH5α | supE44 ΔlacU169 hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | 18 |
| CAG16037 | MC1061 λ(rpoH p3-lacZ) | 29 |
| CAG22216 | CAG16037; rpoE::ΩCm | 39 |
| Plasmids | ||
| pBluescript SK(+) | Plasmid used for subcloning DNA | Stratagene |
| pMOSBlue | Plasmid used for subcloning PCR products | Amersham |
| pHP45Ω-Tc | Plasmid used to obtain the Tcr cassette | 15 |
| pBSL141 | Plasmid used to obtain the Gmr cassette | 1 |
| pLRA | pMOSBlue derivative carrying a 1.3-kb DNA fragment containing A. vinelandii mucA gene amplified by PCR | This work |
| pLRA8 | pLRA derivative containing mucA::Gm nonpolar mutation | This work |
| pLRA4 | pLRA containing mutation mucA::Gm polar to mucBCD | This work |
| pLRC | pMOSBlue derivative carrying a 1.3-kb DNA fragment containing A. vinelandii mucC gene amplified by PCR | This work |
| pLRC2 | pLRC derivative containing mucC::Tc nonpolar mutation | This work |
| pLRC4 | pLRC derivative containing mutation mucC::Tc polar to mucD | This work |
| pJMSAT1 | Plasmid containing A. vinelandii algU-mucA genes; Ap | 34 |
Triparental or biparental A. vinelandii matings were done as reported previously (6). A. vinelandii transformation was done as reported by Bali et al. in 1992 (2). Alginate production was measured by the method described previously (23).
β-Galactosidase activity was determined as reported by Miller (32); 1 U corresponds to 1 nmol of O-nitrophenyl-β-d-galactosidase hydrolyzed per min and per mg of protein. All measurements were done in triplicate.
Nucleic acid procedures.
DNA isolation, cloning, and sequencing; Southern blotting; and nick translation procedures were carried out as described previously (41). Primer extension analysis of A. vinelandii algU was done with U1 oligonucleotide (5′-CAATTGCTGATCTTGCTCCTGG-3′) located in the 5′ region of this gene. Primer extension of algD was carried out as previously described (6), using an Amersham primer extension kit as instructed by the manufacturer. The sequencing reaction shown in the primer extension analysis was done with the Thermo Sequenase radiolabeled terminator cycle sequencing kit (Amersham Life Science, Inc.).
Construction of plasmids pLRA and pLRC.
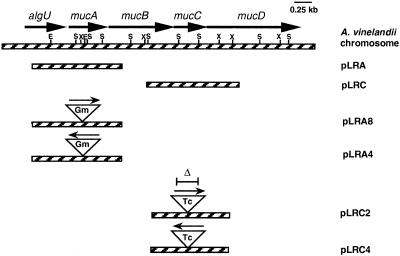
The A. vinelandii mucA and mucC genes were amplified by PCR using ATCC 9046 chromosomal DNA as a template as well as oligonucleotides mucA- 5′ GGCGAGCCTTCGATTTGCTG and mucA-3′ CTGCCGTTACGCTCGTAGA and mucC-5′ GTCCTGCCTGCCAACCTG and mucC-3′ GACTGTGGGGAGCATTCG, respectively. The resulting 1,301- and 1,324-nucleotide PCR products were cloned in pMOSBlue, producing plasmids pLRA and pLRC, respectively (Table 1 and Fig. 1).
FIG. 1.
Physical map of the algU-mucABCD region of A. vinelandii and of plasmids constructed in this study. Arrows indicate the direction of transcription. Antibiotic resistance cassettes, which are represented by inverted triangles, are not shown to scale. Abbreviations: E, EcoRV; S, StyI; X, XhoI.
Construction of polar and nonpolar mucA::Gm and mucC::Tc mutations.
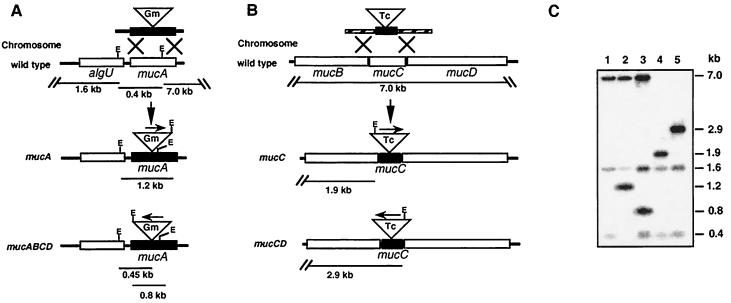
We have previously reported, by Northern blot hybridization assays, that in A. vinelandii the insertion of Ω cassettes into genes with the same orientation as the direction of transcription produces nonpolar mutations which allow expression of the downstream genes in the same operon, whereas the insertion of the cassette in the opossite orientation produces a polar mutation (7, 35, 36). This is also the case for the gentamicin cassette gene described previously (1, 36) and used in the present study. Plasmid pLRA was used to introduce into the unique XhoI site of mucA, a 0.8-kb XhoI fragment containing a gentamicin resistance cassette (1). Clone derivatives containing the gentamicin cassette ligated in both orientations were selected, producing plasmids pLRA8, containing a mucA::Gm nonpolar mutation (mucA), and pLRA4, containing a mucA::Gm polar mutation to mucBCD (resulting in a mucABCD mutation). Plasmid pLRC was cleaved with StyI (releasing a 300-bp DNA fragment of the ampC gene), blunt ended, and ligated to a 2.0-kb SmaI fragment containing a Ω-tetracycline cassette. Clone derivatives containing the Ω-tetracycline cassette ligated in both orientations were selected, producing plasmids pLRC2, containing a mucC::Tc nonpolar mutation (mucC), and pLRC4, containing a mucC::Tc mutation polar to mucD (producing a mucCD mutation). Plasmids pLRA8, pLRA4, pLRC2, and pLRC4 (Fig. 1) were unable to replicate in A. vinelandii and were used to introduce the mucA, mucABCD, mucC, and mucCD mutations into strains ATCC 9046, AEIV, and WI12. Transformants were selected using the corresponding antibiotic and confirmed by Southern blot analysis to carry the desired mutations (Fig. 2).
FIG. 2.
Schematic representation of the strategy followed to construct mucA and mucC mutants. (A and B) Insertional inactivation of the mucA (A) and mucC (B) genes producing the respective polar and nonpolar mutations. (C) Southern blot hybridization of total genomic DNA digested with EcoRV endonuclease with plasmid pRLA4 as a probe. Lanes: 1, ATCC 9046; 2, JRA8 (mucA); 3, JRA4 (mucABCD); 4, MLC2 (mucC); 5, MLC4 (mucCD). Identical hybridization patterns were found for strain AEIV and its corresponding muc mutants (data not shown).
RESULTS AND DISCUSSION
Effect of mucA, mucABCD, and mucC mutations on alginate production in two A. vinelandii strains.
To determine the role of MucA, MucB, MucC, and MucD proteins in alginate production by A. vinelandii, we constructed derivatives of the wild-type strain AEIV carrying mucA, mucABCD, and mucC mutations as described in Materials and Methods and found that the three mutants present a significant increment of alginate production (Table 2). These results reinforce our previous findings (26), based on the complementation of P. aeruginosa mucA mutants, that in A. vinelandii, as in P. aeruginosa, MucA and possibly MucB and MucD products function as negative regulators of AlgU activity. Thus, the disruption of the mucA, mucB, or mucD gene results in an increase of this sigma factor activity, as has been shown for E. coli (10) and P. aeruginosa (27), increasing algD transcription and possibly that of other alg genes and ultimately alginate production. In A. vinelandii, however, MucC seems to function directly as a negative regulator of AlgU activity since mucC mutants have higher alginate production (Table 2), while in P. aeruginosa MucC does not directly affect AlgU activity (5). The mechanism of AlgU activity regulation by MucC in A. vinelandii remains to be determined.
TABLE 2.
Alginate production in different A. vinelandii strains
| Strain | Genotype | Mean alginate concna (mg/mg of protein) ± SEM (%) | Mucoidyb |
|---|---|---|---|
| AEIV | Wild type | 0.9 ± 0.2 (100) | + |
| AEA8 | AEIV, mucA | 5.9 ± 0.4 (651) | +++ |
| AEA4 | AEIV, mucABCD | 4.0 ± 0.15 (444) | ++++ |
| AEC2 | AEIV, mucC | 2.4 ± 0.4 (266) | ++ |
| ATCC 9046 | Wild type, muc-1 | 4.8 ± 0.3 (100) | +++ |
| JRA8 | ATCC 9046, mucA | 6.7 ± 0.8 (139) | +++ |
| JRA4 | ATCC 9046, mucABCD | 8.9 ± 0.7 (185) | +++++ |
| MLC2 | ATCC 9046, mucC | 4.0 ± 0.3 (83) | +++ |
| MLC4 | ATCC 9046, mucCD | 5.1 ± 0.2 (106) | +++ |
Alginate was determined in cells grown for 48 h on liquid Burke's nitrogen-free salts supplemented with 2% sucrose as a carbon source.
+ to +++++, least to most mucoidy, respectively.
The AEIV mucABCD mutant (AEA4) presents the most drastic phenotype as evaluated by alginate production on plates (Table 2). However, when alginate production was quantitated on liquid cultures, this mutant did not show the highest increase in alginate production (Table 2). This lack of correlation is due to the high instability of the mutant due to the selection of spontaneous mutants with a reduced alginate production, possibly affecting AlgU expression. This instability is so high that after four subcultures the mutant AEA4 completely loses its increased alginate production. It is also apparent that the higher the alginate production by any of the muc mutants, the lower the growth rate of the strain (data not shown).
Strain ATCC 9046 is highly mucoid, due to the presence of a spontaneous regulatory mutation, called muc-1, which upregulates AlgU activity (26). The effect of the mutations on the muc genes is different in the highly mucoid strain ATCC 9046, since neither mucC nor mucCD mutations increased alginate production (Table 2). The different response of the two studied strains is very probably due to a high basal level of AlgU activity in strain ATCC 9046, caused by the muc-1 mutation (26).
An increase of approximately twofold was observed in the ATCC 9046 mucABCD mutant strain JRA4, giving the A. vinelandii strain the highest specific alginate production, to our knowledge. The selection of spontaneous mutations that presented a reduced level of alginate production (2.5 mg/mg of protein) was apparent in mutant JRA4 as well as in mutant AEA4.
The ATCC 9046 mucA nonpolar mutant (JRA8) showed a low, but significant, increase of alginate production (Table 2). It is apparent from this result that in A. vinelandii MucA by itself plays an important role in the negative regulation of AlgU activity, even in a strain with elevated basal AlgU activity (26). The difference in levels of alginate production between mutants JRA4 (mucABCD) and JRA8 (mucA) show that MucB, MucC, and/or MucD affects AlgU activity by a different signaling mechanism than that of the anti-sigma factor MucA.
Even though mutant JRA8 presented a considerably lower increase in alginate production than that of mutant JRA4, the former mutant is also unstable with respect to hyperproduction of alginate. The instability of these mutants suggests that the elevated AlgU activity or the increased alginate production might be deleterious to A. vinelandii.
Effect of mucA, mucABCD, and mucC mutations on algD transcription.
Most of the molecular genetics analysis of alginate production in A. vinelandii has been carried out in the highly mucoid strain ATCC 9046 (6, 7, 24, 26, 30, 31, 34, 35, 36). The detailed analysis of the structure of the regulatory region of the AEIV algD gene is currently being performed. At present, we have only preliminary evidence suggesting that the regulatory elements participating in AEIV algD transcriptional regulation are the same as those involved in the regulation of this gene in strain ATCC 9046 (6, 36) and that the main difference between the strains seems to be the high AlgU activity in the latter strain due to an uncharacterized muc-1 mutation (26). In order to further characterize the effect of the inactivation of the muc genes on the algD transcriptional regulation, we focused our research on strain ATCC 9046 and its derivatives.
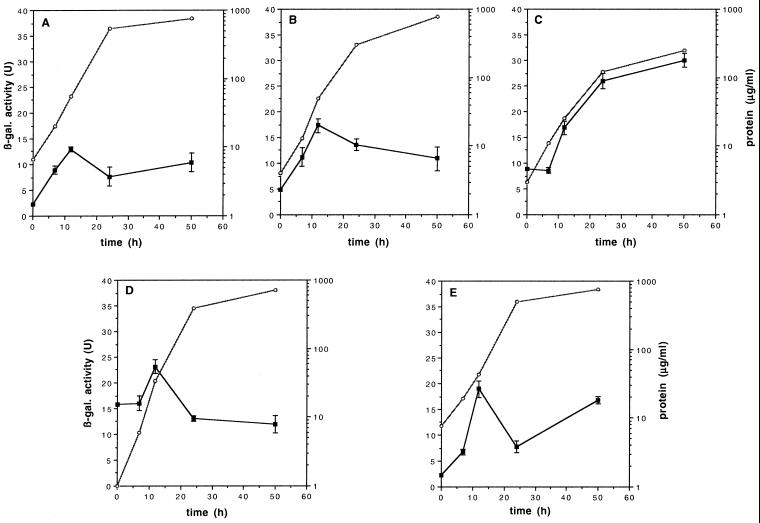
To evaluate the effect of the polar and nonpolar mucA and mucC mutations on algD transcription, they were transferred, as described in Materials and Methods, to strain WI12, an ATCC 9046 derivative which carries an algD-lacZ transcriptional fusion. We have previously reported that in strain WI12 the transcription of algD increased during early exponential phase and declines in prestationary phase (7). As shown in Fig. 3, mucA and mucC mutations increased algD transcription from one- to twofold along the entire growth curve, with a kinetics similar to that observed in the parental strain WI12, whereas the mucABCD mutant shows an approximately fourfold increase of algD transcription during the stationary phase of growth. A deregulation of algD transcription along the entire growth curve was observed in the WIA4 strain, which carries a mucABCD mutation, even though the maximum upregulation of algD transcription was observed during stationary phase (Fig. 3C). The increased algD expression in the ATCC 9046 mucC and mucCD mutant background (mutants WIC2 and WIC4 [Fig. 3D and E]) may indicate that in this highly mucoid strain MucC also exerts a direct negative role on AlgU activity, even though this effect is not so strong as to be reflected in the amount of alginate produced by the mutants.
FIG. 3.
Growth (open symbols) and β-galactosidase activity (closed symbols), on Burke's medium supplemented with 2% sucrose, of strains. WI12 (parental strain) (A), WIA8 (mucA) (B), WIA4 (mucABCD) (C), WIC2 (mucC) (D), and WIC4 (mucCD) (E).
Mutant WIA4 (mucABCD) presented the highest increase in algD expression (Fig. 3C), in accordance with the highest alginate production being that of mutant JRA4 (mucABCD). The pronounced increase in algD expression for WIA4, in contrast to the lower increase observed in mutant WIA8 (Fig. 3B), further supports the involvement of MucB, MucC, and/or MucD in a signaling cascade that affects AlgU activity through a different route from that of MucA.
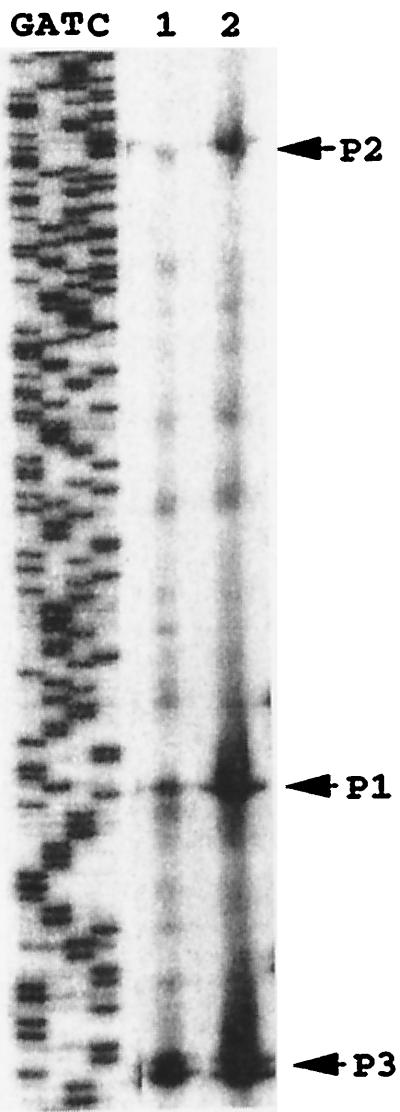
The A. vinelandii ATCC 9046 algD gene is transcribed from three promoters: p1, a ςD promoter; p2, an AlgU (ςE)-dependent promoter; and a p3 promoter which shows no recognized consensus sequences (6, 34, 35). It thus seemed likely that the absence of the Muc products in the mucABCD mutant would upregulate AlgU activity, which in turn would increase algD transcription from the p2 promoter. To verify this hypothesis, primer extension analysis of the algD gene was carried out on the mucABCD mutant. As shown in Fig. 4, mucABCD mutation increased algD transcription from its three promoters and not only from the p2 AlgU-dependent promoter. We have previously reported that an ampDE mutation in the ATCC 9046 background resulted in an increased algD initiation of transcription from its three promoters (36) and that an ATCC 9046 gacS mutant presented a decreased transcription from the three algD promoters (7). These results, together with the data presented here, strongly suggest the existence of a common level of regulation of the three algD promoters.
FIG. 4.
Primer extension analysis of algD transcription. Lanes correspond to RNA extracted from strain ATCC 9046 (lane 1) and JRA4 (lane 2). Each reaction contained as template 50 μg of RNA isolated from bacterial cultures grown for 48 h in Burke's medium supplemented with 2% sucrose.
Transcriptional regulation of the algU gene.
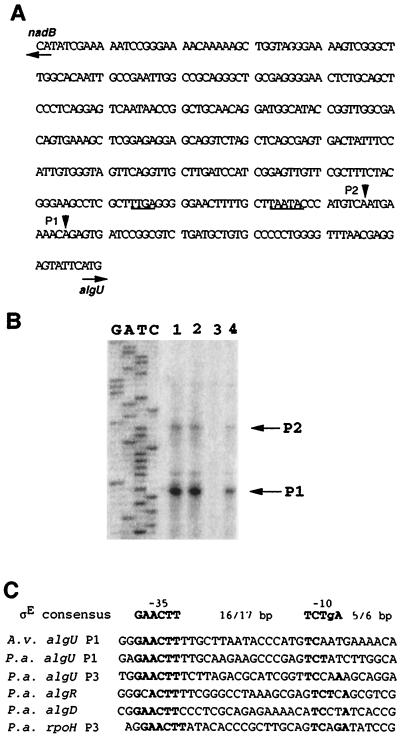
In order to investigate the nature and regulation of the A. vinelandii sigma factor AlgU, primer extension analysis of the algU gene was carried out on strains AEIV and ATCC 9046. As shown in Fig. 5B, in both strains there is a putative transcriptional start site (p1) located 54 nucleotides upstream of the ATG start codon and another putative promoter (p2) starting transcription 62 nucleotides upstream of the translational start site. As shown in Fig. 5C, the p1 −10 and −35 DNA sequences correspond very well to the ςE-dependent promoter consensus sequences. The −10 and −35 p2 sequences suggest that this is a ςD promoter (Fig. 5A).
FIG. 5.
Primer extension analysis of algU transcription. (A) DNA sequence of the 5′ region of algU. p1 and p2 mRNA initiation sites are indicated. The −10 and −35 regions of the p2 promoter are underlined. (B) Primer extension analysis of the algU gene. Lanes correspond to RNA extracted from the following strains: ATCC 9046 (lane 1), JRA4 (mucABCD) (lane 2), SMU88 (algU) (lane 3), and AEIV (lane 4). (C) Sequence alignment of several AlgU (ςE)-dependent promoters. Abbreviations: P.a., P. aeruginosa; A.v., A. vinelandii.
To further characterize these promoters, primer extension analysis was carried out using RNA derived from ATCC 9046 algU mutant SMU88 (34). As expected, the primer extension product corresponding to p1 was not observed in strain SMU88, confirming the dependence of this promoter on the AlgU sigma factor. Unexpectedly, the p2 start site was also abrogated, suggesting that the transcription from this promoter is also regulated by AlgU, but in an indirect manner. These results thus show that algU transcription is autoregulated, as it is in P. aeruginosa (42). All genes which are activated by autoregulation need a constitutive promoter to maintain a basal level of expression; we could not detect the promoter responsible for the basal expression of the algU-mucABCD operon.
We reported previously that the ATCC 9046 algD p2 promoter was AlgU dependent based on its lack of expression on an algU mutant (34) and on the presence of sequences in the −10 and −35 regions showing a reduced similarity with the ςE consensus sequences (6). The algD p2 promoter was the first reported AlgU-dependent promoter in A. vinelandii, and so there was no other sequence for comparison and validation of the significance of the detected homology. The high sequence similarity of algU p1 with ςE-dependent promoters from different bacteria (Fig. 5C) strongly suggests that the algD p2 promoter is not directly recognized by AlgU.
Initiation of algU transcription in the ATCC 9046 background seems to be much more frequent from the p1 promoter than from the p2 initiation site, whereas in strain AEIV both initiation sites show the same intensity (Fig. 5B). These results suggest that the muc-1 mutation present in strain ATCC 9046 increases AlgU activity by increasing algU transcription from p1, the promoter directly recognized by AlgU itself.
The finding of AlgU autoregulation and the increased algU transcription from the p1 promoter in strain ATCC 9046 suggested to us that the different muc mutants might show an increased level of algU transcription. However, we found that, in both strains studied, all the muc mutants showed similar levels of algU transcription (see Fig. 5B for an example). These results further reinforce our previous findings suggesting that, as in P. aeruginosa mucA and mucB (27), MucA, MucB, MucC, and MucD modulate AlgU activity and not the transcription of the algU gene. In contrast, the E. coli rseA mutants present a 12-fold increase in algU transcription (10).
Activation of rpoH p3 initiation of transcription in E. coli by A. vinelandii AlgU.
In order to test whether A. vinelandii AlgU was able to activate the rpoH p3 promoter of E. coli, plasmid pJMSAT1, which carries the ATCC 9046 A. vinelandii algU-mucA genes (34), was transferred by transformation to E. coli strains CAG16037 (rpoH p3::lacZ) and CAG22216 (rpoE::Cmr, rpoH p3::lacZ), and the effect of a heat shock treatment (30 to 42°C) was evaluated by measuring the kinetics of β-galactosidase expression (Table 3). A. vinelandii AlgU restored from 12 to 20% of the rpoH p3 transcription in E. coli (Table 3). This reduced level of expression is sufficient to complement the CAG22216 ability to grow on plates at 42°C (data not shown).
TABLE 3.
Determination of A. vinelandii AlgU activity in the E. coli background
| Strain | Genotype | β-Galactosidase (U)a at min:
|
||
|---|---|---|---|---|
| 0 | 30 | 60 | ||
| CAG16037 | rpoH p3::lacZ | 8.75 | 10.8 | 9.17 |
| CAG22216 | rpoE::CmrrpoH p3::lacZ | ND | ND | ND |
| CAG22216/pJMSAT1 | A. vinelandii algU+ mucA+ | 1.12 | 1.6 | 2.0 |
β-Galactosidase activity reflects the level of E. coli rpoH p3 promoter which is fully dependent on ςE activity and was determined in cultures after shift to 42°C for the indicated time in minutes. Cells were grown on M9 medium as described previously (29). The data presented are the averages of three independent experiments. ND, not detected.
We have thus shown that A. vinelandii AlgU is able to complement an E. coli rpoE mutant for growth on plates at 42°C. This result shows that the function of both proteins in transcription at high temperatures is conserved, at least partially. However, activity of A. vinelandii AlgU in the E. coli background accounts for only around 15% of the detected activity of the E. coli ςE factor in the transcription from rpoH p3 (Table 3). The rate-limiting step for this reduced AlgU activity, whether at the level of expression or the level of protein function, of the A. vinelandii AlgU activity in the E. coli background remains to be determined.
We have previously reported evidence showing that in A. vinelandii AlgU activity is regulated by the muc gene products in a manner similar to that reported previously for P. aeruginosa (26). These data, together with the increase in alginate production and algD expression in mucA and mucABCD mutants reported here, suggest that, in A. vinelandii, AlgU activity is negatively regulated by MucA and possibly also by MucB and MucD. On the other hand, our results show that MucC is by itself a negative regulator of alginate production in A. vinelandii (Table 2), contradicting the previously reported results on the lack of a direct effect of MucC in P. aeruginosa (5). The mechanism of AlgU regulation by MucC in A. vinelandii is presently unknown.
The disruption of any of the muc genes did not affect encystment frequencies or cyst morphology of the two strains studied, AEIV and ATCC 9046 (data not shown). These data show that the level of alginate production does not correlate with the proportion of cells undergoing differentiation and suggest that AlgU activity is not the rate-limiting step in cyst formation. The same two conclusions were attained when cyst formation was evaluated on strains with reduced levels of AlgU expression (34).
ACKNOWLEDGMENTS
We are grateful to Rebeca Nájera, Paul Gaytán, and Eugenio López for expert technical assistance.
This work was supported by grants IN212096 DGAPA-PAPIIT, UNAM, and CONACyT 27767.
REFERENCES
- 1.Alexeyev M F, Shokolenko I, Croughan T P. Improved antibiotic-resistance gene cassettes and omega elements for Escherichia coli vector construction and in vitro deletion/insertion mutagenesis. Gene. 1995;160:63–67. doi: 10.1016/0378-1119(95)00108-i. [DOI] [PubMed] [Google Scholar]
- 2.Bali A, Blanco G, Hill S, Kennedy C. Excretion of ammonium by a nifL mutant of Azotobacter vinelandii fixing nitrogen. Appl Environ Microbiol. 1992;58:1711–1718. doi: 10.1128/aem.58.5.1711-1718.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beck B J, Connolly L E, de las Peñas A, Downs D M. Evidence that rseC, a gene in the rpoE cluster, has a role in thiamine synthesis in Salmonella typhimurium. J Bacteriol. 1997;179:6504–6508. doi: 10.1128/jb.179.20.6504-6508.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boucher J C, Martínez-Salazar J, Schurr M J, Mudd M, Yu H, Deretic V. Two distinct loci affecting conversion to mucoidy in Pseudomonas aeruginosa in cystic fibrosis encode homologs of the serine protease HtrA. J Bacteriol. 1996;178:511–523. doi: 10.1128/jb.178.2.511-523.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boucher J C, Schurr M J, Yu H, Rowen D W, Deretic V. Pseudomonas aeruginosa in cystic fibrosis: role of mucC in the regulation of alginate production and stress sensitivity. Microbiology. 1997;143:3473–3480. doi: 10.1099/00221287-143-11-3473. [DOI] [PubMed] [Google Scholar]
- 6.Campos M-E, Martínez-Salazar J M, Lloret L, Moreno S, Núñez C, Espín G, Soberón-Chávez G. Characterization of the gene coding for GDP-mannose dehydrogenase (algD) from Azotobacter vinelandii. J Bacteriol. 1996;178:1793–1799. doi: 10.1128/jb.178.7.1793-1799.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castañeda M, Guzmán J, Moreno S, Espín G. GacS sensor kinase regulates alginate and poly-β-hydroxybutyrate production in Azotobacter vinelandii. J Bacteriol. 2000;182:2624–2628. doi: 10.1128/jb.182.9.2624-2628.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chi E, Bartlett D H. Characterization of a locus important for high pressure adaptation from the barophilic deep-sea bacterium Photobacterium SS9. Mol Microbiol. 1995;17:713–726. doi: 10.1111/j.1365-2958.1995.mmi_17040713.x. [DOI] [PubMed] [Google Scholar]
- 9.Chitnis C E, Ohman D E. Genetic analysis of the alginate biosynthetic gene cluster of Pseudomonas aeruginosa shows evidence of an operonic structure. Mol Microbiol. 1993;8:583–590. doi: 10.1111/j.1365-2958.1993.tb01602.x. [DOI] [PubMed] [Google Scholar]
- 10.De las Peñas A, Connolly L, Gross C A. The sigma-E-mediated response to extracytoplasmic stress in Escherichia coli is transduced by RseA and RseB, two negative regulators of sigma-E. Mol Microbiol. 1997;24:373–385. doi: 10.1046/j.1365-2958.1997.3611718.x. [DOI] [PubMed] [Google Scholar]
- 11.Deretic V, Martin D W, Schurr M J, Mudd M H, Hibler N S, Curcic R, Boucher J C. Conversion to mucoidy in Pseudomonas aeruginosa. Bio/Technology. 1993;11:1133–1136. doi: 10.1038/nbt1093-1133. [DOI] [PubMed] [Google Scholar]
- 12.Deretic V, Schurr M J, Boucher J C, Martin D W. Conversion of Pseudomonas aeruginosa to mucoidy in cystic fibrosis: environmental stress and regulation of bacterial virulence by alternative sigma factors. J Bacteriol. 1994;176:2773–2780. doi: 10.1128/jb.176.10.2773-2780.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erickson J W, Vaughn V, Walter W A, Neidhardt F C, Gross C A. Regulation of the promoters and transcripts of rpoH, the Escherichia coli heat shock regulatory gene. Genes Dev. 1987;1:419–432. doi: 10.1101/gad.1.5.419. [DOI] [PubMed] [Google Scholar]
- 14.Erickson J W, Gross C A. Identification of the ςE subunit of Escherichia coli RNA polymerase: a second alternate ς factor involved in high-temperature gene expression. Genes Dev. 1989;3:1462–1471. doi: 10.1101/gad.3.9.1462. [DOI] [PubMed] [Google Scholar]
- 15.Fellay R, Frey J, Krisch H. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis. Gene. 1987;52:147–154. doi: 10.1016/0378-1119(87)90041-2. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg J B, Gorman W L, Flynn J L, Ohman D E. A mutation in algN permits trans activation of alginate production by algT in Pseudomonas species. J Bacteriol. 1993;175:1303–1308. doi: 10.1128/jb.175.5.1303-1308.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Govan J R W, Deretic V. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev. 1996;60:539–574. doi: 10.1128/mr.60.3.539-574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanahan D. Studies on transformation of E. coli. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 19.Hershberger C D, Ye R W, Parsek M R, Xie Z-D, Chakrabarty A M. The algT (algU) gene of Pseudomonas aeruginosa, a key regulator involved in alginate biosynthesis, encodes an alternative sigma factor (ςE) Proc Natl Acad Sci USA. 1995;92:7941–7945. doi: 10.1073/pnas.92.17.7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson K, Charles I, Dougan G, Pickard D, O'Gaora P, Costa G, Ali T, Miller I, Hormaeche C. The role of stress response protein in Salmonella typhimurium virulence. Mol Microbiol. 1991;5:401–407. doi: 10.1111/j.1365-2958.1991.tb02122.x. [DOI] [PubMed] [Google Scholar]
- 21.Keith L M, Bender C L. AlgT (ς22) controls production and tolerance to environmental stress in Pseudomonas syringae. J Bacteriol. 1999;181:7176–7184. doi: 10.1128/jb.181.23.7176-7184.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kennedy C, Gamal R, Humphrey R, Ramos J, Brigle K, Dean D. The nifH, nifM and nifN genes of Azotobacter vinelandii: characterization by Tn5 mutagenesis and isolation from pLAFR1 gene banks. Mol Gen Genet. 1986;205:318–325. [Google Scholar]
- 23.Knutson C A, Jeanes A. A new modification of the carbazole reaction: application to heteropolysaccharides. Anal Biochem. 1968;24:470–481. doi: 10.1016/0003-2697(68)90154-1. [DOI] [PubMed] [Google Scholar]
- 24.Lloret L, Barreto R, Campos M-E, Moreno S, Martínez-Salazar J M, Espín G, León R, Soberón-Chávez G. Genetic analysis of the transcriptional arrangement of Azotobacter vinelandii alginate biosynthetic genes: identification of two independent promoters. Mol Microbiol. 1996;21:449–457. doi: 10.1111/j.1365-2958.1996.tb02554.x. [DOI] [PubMed] [Google Scholar]
- 25.Martin D W, Schurr M J, Mudd M H, Govan J R W, Holloway B W, Deretic V. Mechanism of conversion to mucoidy in Pseudomonas aeruginosa infecting cystic fibrosis patients. Proc Natl Acad Sci USA. 1993;90:8377–8381. doi: 10.1073/pnas.90.18.8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martínez-Salazar J, Moreno S, Nájera R, Boucher J C, Espín G, Soberón-Chávez G, Deretic V. Characterization of the genes coding for the putative sigma factor AlgU and its regulators MucA, MucB, MucC, and MucD in Azotobacter vinelandii and evaluation of their roles in alginate biosynthesis. J Bacteriol. 1996;178:1800–1808. doi: 10.1128/jb.178.7.1800-1808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathee K, McPherson C J, Ohman D E. Posttranslational control of the algT (algU)-encoded ς22 for expression of the alginate regulon in Pseudomonas aeruginosa and localization of its antagonist proteins MucA and MucB (AlgN) J Bacteriol. 1997;179:3711–3720. doi: 10.1128/jb.179.11.3711-3720.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.May T B, Chakrabarty A M. Pseudomonas aeruginosa: genes and enzymes of alginate synthesis. Trends Microbiol. 1994;2:151–157. doi: 10.1016/0966-842x(94)90664-5. [DOI] [PubMed] [Google Scholar]
- 29.Mecsas J, Rouviere P E, Erickson J W, Donohue T J, Gross C A. The activity of ςE, an Escherichia coli heat-inducible ς-factor, is modulated by expression of outer membrane proteins. Genes Dev. 1993;7:2618–2628. doi: 10.1101/gad.7.12b.2618. [DOI] [PubMed] [Google Scholar]
- 30.Mejía-Ruíz H, Guzmán J, Moreno S, Soberón-Chávez G, Espín G. The Azotobacter vinelandii alg8 and alg44 genes are essential for alginate synthesis, and they constitute an algD independent operon. Gene. 1997;199:271–277. doi: 10.1016/s0378-1119(97)00380-6. [DOI] [PubMed] [Google Scholar]
- 31.Mejía-Ruíz H, Moreno S, Guzmán J, Nájera R, León R, Soberón-Chávez G, Espín G. Isolation and characterization of an Azotobacter vinelandii algK mutant. FEMS Microbiol Lett. 1997;156:101–106. doi: 10.1111/j.1574-6968.1997.tb12712.x. [DOI] [PubMed] [Google Scholar]
- 32.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. pp. 431–435. [Google Scholar]
- 33.Missiakas D, Raina S. The extracytoplasmic function sigma factors, role and regulation. Mol Microbiol. 1998;28:1059–1066. doi: 10.1046/j.1365-2958.1998.00865.x. [DOI] [PubMed] [Google Scholar]
- 34.Moreno S, Nájera R, Guzmán J, Soberón-Chávez G, Espín G. Role of alternative ς factor AlgU in encystment of Azotobacter vinelandii. J Bacteriol. 1998;180:2766–2769. doi: 10.1128/jb.180.10.2766-2769.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Núñez C, Moreno S, Soberón-Chávez G, Espín G. The Azotobacter vinelandii response regulator AlgR is essential for cyst formation. J Bacteriol. 1999;181:141–148. doi: 10.1128/jb.181.1.141-148.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Núñez C, Moreno S, Cardenas L, Soberón-Chávez G, Espín G. Inactivation of the ampDE operon increases transcription of algD and affects morphology and encystment in Azotobacter vinelandii. J Bacteriol. 2000;182:4829–4835. doi: 10.1128/jb.182.17.4829-4835.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Page W J. Formation of cyst-like structures by iron-limited Azotobacter vinelandii strain UW during prolonged storage. Can J Microbiol. 1983;29:1110–1118. [Google Scholar]
- 38.Pindar D F, Bucke C. The biosynthesis of alginic acid by Azotobacter vinelandii. Biochem J. 1975;152:617–622. doi: 10.1042/bj1520617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rouviére P E, De Las Peñas A, Mecsas J, Lu C Z, Rudd K E, Gross C A. rpoE, the gene encoding the second heat-shock sigma factor, ςE, in Escherichia coli. EMBO J. 1995;14:1032–1042. doi: 10.1002/j.1460-2075.1995.tb07084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sadoff H L. Encystment and germination in Azotobacter vinelandii. Bacteriol Rev. 1975;39:516–539. doi: 10.1128/br.39.4.516-539.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd. ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 42.Schurr M J, Yu H, Boucher J C, Hibler N S, Deretic V. Multiple promoters and induction by heat shock of the gene encoding the alternative sigma factor AlgU (ςE) which controls mucoidy in cystic fibrosis isolates of Pseudomonas aeruginosa. J Bacteriol. 1995;177:5670–5679. doi: 10.1128/jb.177.19.5670-5679.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schurr M J, Yu H, Martínez-Salazar J M, Boucher J C, Deretic V. Control of AlgU, a member of the ςE-like family of stress sigma factors, by the negative regulators MucA and MucB and Pseudomonas aeruginosa conversion to mucoidy in cystic fibrosis. J Bacteriol. 1996;178:4997–5004. doi: 10.1128/jb.178.16.4997-5004.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wozniak D J, Ohman D E. Transcriptional analysis of the Pseudomonas aeruginosa genes algR, algB, and algD reveals a hierarchy of alginate gene expression which is modulated by algT. J Bacteriol. 1994;176:6007–6014. doi: 10.1128/jb.176.19.6007-6014.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie Z-D, Hershberger C D, Shankar S, Ye R W, Chakrabarty A M. Sigma factor–anti-sigma factor interaction in alginate synthesis: inhibition of AlgT by MucA. J Bacteriol. 1996;178:4990–4996. doi: 10.1128/jb.178.16.4990-4996.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu H, Schurr M J, Deretic V. Functional equivalence of Escherichia coli ςE and Pseudomonas aeruginosa AlgU: E. coli rpoE restores mucoidy and reduces sensitivity to reactive oxygen intermediates in algU mutants of P. aeruginosa. J Bacteriol. 1995;177:3259–3268. doi: 10.1128/jb.177.11.3259-3268.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]