Abstract
Objective:
The immunogenic nature of coronavirus disease 2019 (COVID-19) mRNA vaccines led to some initial concern that these could stimulate the HIV reservoir. We analyzed changes in plasma HIV loads (pVL) and reservoir size following COVID-19 mRNA vaccination in 62 people with HIV (PWH) receiving antiretroviral therapy (ART), and analyzed province-wide trends in pVL before and after the mass vaccination campaign.
Design:
Longitudinal observational cohort and province-wide analysis.
Methods:
Sixty-two participants were sampled prevaccination, and one month after their first and second COVID-19 immunizations. Vaccine-induced anti-SARS-CoV-2-Spike antibodies in serum were measured using the Roche Elecsys Anti-S assay. HIV reservoirs were quantified using the intact proviral DNA assay; pVL were measured using the cobas 6800 (lower limit of quantification: 20 copies/ml). The province-wide analysis included all 290 401 pVL performed in British Columbia, Canada between 2012 and 2022.
Results:
Prevaccination, the median intact reservoir size was 77 [interquartile range (IQR): 20–204] HIV copies/million CD4+ T-cells, compared to 74 (IQR: 27–212) and 65 (IQR: 22–174) postfirst and -second dose, respectively (all comparisons P > 0.07). Prevaccination, 82% of participants had pVL <20 copies/ml (max: 110 copies/ml), compared to 79% postfirst dose (max: 183 copies/ml) and 85% postsecond dose (max: 79 copies/ml) (P > 0.4). There was no evidence that the magnitude of the vaccine-elicited anti-SARS-CoV-2-Spike immune response influenced pVL nor changes in reservoir size (P > 0.6). We found no evidence linking the COVID-19 mass vaccination campaign to population-level increases in detectable pVL frequency among all PWH in the province, nor among those who maintained pVL suppression on ART.
Conclusion:
We found no evidence that COVID-19 mRNA vaccines induced changes in HIV reservoir size nor plasma viremia.
Keywords: coronavirus disease 2019 vaccine, HIV, intact proviral DNA assay, mRNA vaccine, plasma viral load, reservoir size
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination is particularly important for people with HIV (PWH) who are at a higher risk of severe coronavirus disease 2019 (COVID-19) outcomes [1–3]. Reassuringly, a large body of evidence now confirms that PWH receiving suppressive antiretroviral therapy (ART) generally mount robust immune responses to these vaccines [4–12]. Initially however, COVID-19 vaccine confidence was typically lower among PWH compared to the general population [13,14], with possible effects of vaccination on viral rebound cited among the concerns [15,16]. Such concerns are not entirely unfounded, as some vaccines, including those against influenza, hepatitis B, and pneumococcus can induce HIV transcription, leading to transient increases in plasma HIV RNA levels [17–21].
The immunogenic nature of mRNA vaccines, which elicit strong humoral and cell-mediated immune responses by harnessing innate detectors of single-stranded viral RNA [22,23], led to some initial concerns that these might induce HIV expression, and possibly viral release, from the reservoir [24]. This could theoretically occur via direct stimulation of reservoir cells that recognize the vaccine antigen, including naïve CD4+ T-cell reservoirs [25,26] specific for SARS-CoV-2 Spike, or existing cross-reactive memory CD4+ T-cell reservoirs specific for common cold coronavirus antigens [27,28]. Alternatively, reservoir stimulation could occur through a generalized inflammatory response with cytokine production [29,30] that could transiently promote HIV gene expression in bystander reservoir cells that are not specific to the vaccine antigen [31]. Indeed, reports of increased HIV viremia in individuals receiving ART following COVID-19 mRNA vaccination have emerged [31–33], though other studies have observed no such effects [34–36].
Existing studies however are relatively modestly sized. A recent analysis of 35 PWH, which included 15 participants from the present cohort, reported that the frequency of Nef-specific CD8+ T-cells transiently increased after the initial COVID-19 mRNA vaccine dose, consistent with immune sensing of reactivated reservoir cells [37]. Plasma viremia however was not investigated, and no significant changes in reservoir size were observed in the subset of 13 participants analyzed for this outcome [37]. Another analysis of 25 PWH reported no significant changes in reservoir size post-COVID-19 vaccination [35]. A recent analysis of 68 PWH reported a gradual yet not statistically significant increase in pVL after the second vaccine dose with no obvious effects on reservoir size, but nearly half of the participants received the viral vectored ChAdOx1 vaccine (which may be less likely to modulate the reservoir), and pVL and reservoir data were available for fewer than two-thirds of the cohort [33]. To our knowledge, no studies have investigated the effects of COVID-19 mRNA vaccination on the reservoir in an observational cohort while also analyzing population-level trends in pVL test results in a large geographic region following mass COVID-19 vaccination.
Here, we analyzed changes in pVL and reservoir size following the first and second COVID-19 mRNA vaccine doses in 62 PWH receiving ART. Using a longitudinal dataset that captured all PWH in British Columbia (BC), Canada, we also investigated whether the frequency of detectable HIV RNA test results increased at the population level following the mass administration of first, second and booster COVID-19 vaccine doses in the province.
Methods
Cohort and specimen collection
Our cohort of PWH on ART, established at the outset of the mass COVID-19 vaccination campaign in BC, has been described previously [6]. The present analysis includes all PWH who provided a prevaccination sample and who received two mRNA vaccine doses (either BNT162b2 or mRNA-1273). Serum, plasma and peripheral blood mononuclear cells (PBMC; isolated by density gradient separation and cryopreserved at –150°C until analysis) were collected prevaccination, and again one month after the first and second vaccine doses.
Ethics approval
The cohort study was approved by the University of British Columbia/Providence Healthcare and Simon Fraser University Research Ethics Boards. All participants provided written informed consent. The BC Centre for Excellence in HIV/AIDS (BC-CfE) Drug Treatment Program (DTP), the source of the province-wide pVL dataset, is a provincially-funded clinical registry mandated to: deliver healthcare to individuals living with HIV and related diseases, or at risk of HIV infection, implement and support public health initiatives to curb HIV/AIDS, monitor and evaluate these healthcare programs, and support related knowledge translation and educational programs. As a result, the requirement for REB review of the province-wide pVL analysis was waived by the Providence Healthcare/University of British Columbia REB.
Anti-severe acute respiratory syndrome coronavirus 2 antibody assays
Total binding antibodies against SARS-CoV-2 nucleocapsid (N) and spike receptor binding domain (RBD) in serum were measured using the Roche Elecsys anti-SARS-CoV-2 and anti-SARS-CoV-2 S assays, respectively. Both are electro-chemiluminescence sandwich immunoassays. The presence of anti-N antibodies identified participants with prior SARS-CoV-2 infection. The S assay reports results in arbitrary units/ml (U/ml) calibrated against an external standard, where the measurement range is from 0.4 to 25 000 U/ml.
Plasma HIV RNA quantification
Plasma HIV RNA levels were quantified using the cobas Ampliprep/Taqman HIV-1 Test v2.0 (during the period March 7, 2012–June 4, 2018) or the cobas HIV-1 Test on a cobas 6800 (from June 5, 2018 to present; Roche Diagnostics). The lower limit of quantification (LLOQ) of this test is 20 HIV RNA copies/ml. This threshold defined undetectable viremia, unless otherwise indicated.
HIV reservoir quantification
CD4+ T-cells were isolated from PBMCs via negative selection using the EasySep Human CD4+ T-cell Enrichment Kit (STEMCELL Technologies). Median CD4+ T-cell purity, assessed flow cytometrically postisolation, was 97%. Genomic DNA was extracted from a median of 2.9 [interquartile range (IQR) 2.2–3.8] million CD4+ T-cells using the QIAamp DNA Mini Kit (QIAGEN). HIV reservoir quantification was performed using the intact proviral DNA assay (IPDA) [38] as described previously [39]. Briefly, this droplet digital PCR assay distinguishes genomically-intact proviruses from the vast background of defective ones by simultaneously targeting two HIV regions, the Packaging Signal (Ψ) near the 5′ end of the viral genome and the Rev responsive element (RRE) within envelope (env), that together strongly predict genomic intactness. An unlabeled competitive RRE probe specific for hypermutated proviruses ensures that these are not counted as intact. Occasionally, the published IPDA primers/probes fail to detect a participant's proviral pool due to sequence polymorphism [39], which occurred in 14 (23%) of participants. For these, we employed a secondary env reaction [39] or custom autologous primers/probes. The assay also quantifies human genomic DNA in an independent parallel reaction, using primer/probe sets in the human RPP30 gene that are spaced the same distance apart as the HIV target regions. This spacing also allows each sample's data to be corrected for the DNA shearing that occurs during extraction, by measuring the frequency whereby the RPP30 targets are decoupled. The assay reports the number of intact HIV genomes (those positive for both Ψ and RRE), and the overall number of proviral copies (those positive for at least one target), per million CD4+ T-cells.
A median of 290 000 (IQR 255 000–325 000) cells were assayed per participant across four replicate wells, which were merged to generate the final reservoir measurement. Genomic DNA from J-Lat 9.2 cells, which harbor a single integrated HIV copy per cell, was used as a positive control, while genomic DNA from donors without HIV, and water, were used as negative controls. Droplets were read using the QX200 Droplet Reader (BioRad) and analyzed using QuantaSoft (BioRad, version 1.7.4). Wells containing fewer than 10 000 droplets were excluded from analysis. The median DNA shearing was 0.37 (IQR 0.35–0.39), well within the acceptable range [38].
Temporal analysis of HIV plasma viral load test results in British Columbia
The BC Centre for Excellence in HIV/AIDS (BC-CfE) provides care and treatment for all PWH in the province. The BC-CfE's Drug Treatment Program (DTP) database captures all HIV plasma viral load (pVL) tests and ART information for all PWH in BC. We analyzed all 290 401 pVL tests performed between January 2012 and December 2022 to determine whether the frequency of detectable pVL test results increased following each “wave” of mass COVID-19 vaccination in the province. Though the LLOQ of the pVL assay is 20 copies/ml, the results are clinically reported (and thus stored in the DTP database) with a LLOQ of 40 copies/ml. COVID-19 vaccine distribution data for BC up to December 2022, where 97% of vaccines administered were mRNA [40], were retrieved from the Public Health Agency of Canada and COVID-19 Vaccine Tracker [40–42].
Statistical analysis
Comparisons of continuous variables between groups were performed using the Mann–Whitney U-test (for unpaired data) or Wilcoxon test (for paired data). Correlations between continuous variables were performed using Spearman's correlation. Frequency comparisons were performed using the Fisher's exact test. Where appropriate, multiple comparisons were addressed using a false-discovery rate (q-value) approach [43]. All statistical tests were performed using R (version 4.3.1).
Results
Participant characteristics
The 62 participants in the observational cohort study were a median 43 years old and 55 (89%) were male. Participants had been receiving ART for a median of 6 years, with 74% on integrase inhibitor-based regimen at enrolment (Table 1). The most recent CD4+ T-cell count, measured a median of 40 (IQR 15–159) days before enrolment, was 725 (IQR 475–915; range 130–1800) cells/mm3. The estimated nadir CD4+ T-cell count, recorded a median of 5.6 (IQR 2.8–13) years before enrolment, was 305 (IQR 160–498; range <9–970) cells/mm3. At the baseline (prevaccination) visit, 51 (82%) of participants had pVL below the LLOQ of 20 copies HIV RNA/ml. The highest pVL observed at baseline was 110 copies/ml. Overall, 69% of participants received two doses of the BNT162b2 COVID-19 mRNA vaccine, 26% received two doses of mRNA-1273, and 5% received a mixed mRNA regimen. Note that the interval between first and second COVID-19 doses was extended to up to 112 days in Canada due to initially limited vaccine supplies [44]. The vast majority (57/62; 92%) of participants remained COVID-19 naïve throughout follow-up, four experienced COVID-19 before vaccination, and one acquired COVID-19 between the first and second vaccine doses.
Table 1.
Participant characteristics.
| Characteristic | N = 62 |
| Age at enrolment in years, median (IQR) | 43 (35, 56) |
| Sex at birth | |
| Male, n (%) | 55 (89%) |
| Female, n (%) | 7 (11%) |
| Nadir CD4+ T-cell count in cells/mm3, median (IQR) | 305 (160, 498) |
| Baselinea CD4+ T-cell count in cells/mm3, median (IQR) | 725 (475, 915) |
| Baselinea plasma viral load in copies HIV RNA/ml, median (IQR) | <20 (<20, <20) |
| Years on ART, median (IQR) | 6 (3, 14) |
| Current ART regimen | |
| INSTI-based, n (%) | 46 (74%) |
| NNRTI-based, n (%) | 6 (9.7%) |
| PI-based, n (%) | 5 (8.1%) |
| Intensiveb, n (%) | 4 (6.5%) |
| CCR5 antagonist-based, n (%) | 1 (1.6%) |
| Initial COVID-19 vaccine regimen | |
| BNT162b2 + BNT162b2, n (%) | 43 (69%) |
| mRNA-1273 + mRNA-1273, n (%) | 16 (26%) |
| BNT162b2 + mRNA-1273, n (%) | 3 (4.8%) |
| COVID-19 exposure history | |
| COVID-19 naïve throughout follow-up, n (%) | 57 (92%) |
| COVID-19-experienced prior to vaccination, n (%) | 4 (6.5%) |
| COVID-19 between first and second vaccine doses, n (%) | 1 (1.6%) |
At study entry (i.e. prior to COVID-19 vaccination).
Regimen containing at least two of the following drug classes: INSTI, NNRTI, PI, CCR5 antagonist.
COVID-19, coronavirus disease 2019; INSTI: integrase strand transfer inhibitor; IQR, interquartile range; NNRTI: non-nucleoside reverse transcriptase inhibitor; PI: protease inhibitor.
No evidence that coronavirus disease 2019 mRNA vaccination induces detectable viremia
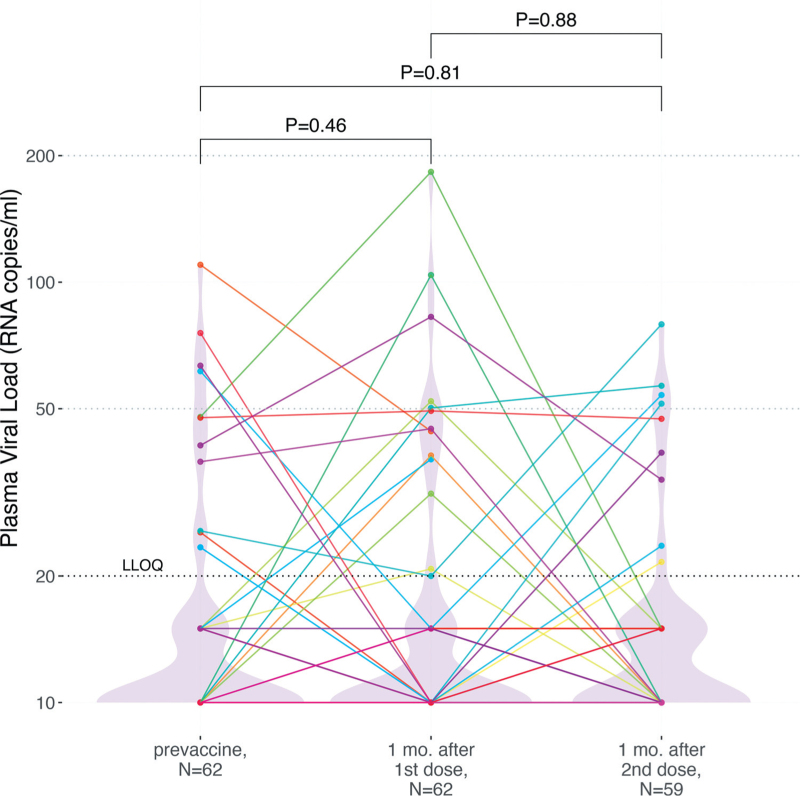
HIV pVL testing was performed at the baseline visit, which occurred a median of 12 (IQR 3–26) days prior to vaccination, one month (a median of 31 [IQR 29–33] days) after the first vaccine dose, and again one month (a median of 30 [IQR 29–30] days) after the second vaccine dose (Fig. 1). At baseline, 82% (51/62) of participants had a pVL <20 copies/ml, where the highest observed value was 110 copies/ml. One month after the first vaccine dose, 79% (49/62) of participants had pVL <20 copies/ml (highest value 183 copies/ml), a difference that was not statistically significant from baseline (Wilcoxon paired test; P = 0.46). One month after the second dose, three participants had temporarily discontinued ART or missed the visit, leaving 59 participants for analysis. Of these, 85% (50/59) had pVL <20 copies/ml (highest value 79 copies/ml), which was not significantly different compared to baseline (P = 0.81), nor one month postfirst dose (P = 0.88). Using a pVL <50 copies/ml threshold produced consistent results: at baseline, 94% (58/62) of participants had a pVL <50 copies/ml, compared to 92% (57/62) one month after the first vaccine dose, and 93% (55/59) after the second (Fisher's exact test P = 1). At no point did any participant experience virologic failure (defined as >200 copies/ml [45,46]). Results also remained consistent after excluding visits from participants who had experienced COVID-19 (all P > 0.58; not shown). Stratification of the data by sex, COVID-19 vaccine regimen, and ART regimen similarly produced no statistically significant differences in pVL between baseline and postvaccination visits for any subgroup (all P > 0.07; q ≥ 0.8; not shown). We also found no association between having a HIV pVL > 20 copies/ml postvaccination and either the nadir or most recent CD4+ T-cell count (P > 0.11; not shown). In contrast, we observed that individuals with a detectable pVL at baseline were more likely to have a detectable pVL on at least one follow-up visit (P < 0.04 not shown), again supporting the notion that these low pVL measurements are unrelated to vaccination.
Fig. 1.
Plasma HIV loads following one- and two-dose COVID-19 vaccination.
HIV plasma viral loads prior to vaccination (left), 1 month after the first dose (middle), and one month following the second dose (right). A black dashed line indicates the assay LLOQ of 20 HIV copies/ml, while light grey dashed lines indicate clinically relevant pVL thresholds of 50 and 200 HIV copies/ml. For graphing purposes, undetectable viral loads were plotted as 10 HIV copies/ml, whereas viral loads that were detectable yet below the LLOQ were plotted as 15 HIV copies/ml. As the vast majority of pVL measurements were below the LLOQ, violin plots help visualize the data distribution. Each participant is identified by a unique color that is consistent throughout all figures. P-values were calculated using the Wilcoxon signed-rank test for paired data. COVID-19, coronavirus disease 2019; LLOQ, lower limit of quantification; pVL, plasma viral load.
No changes in HIV reservoir size after coronavirus disease 2019 mRNA vaccination
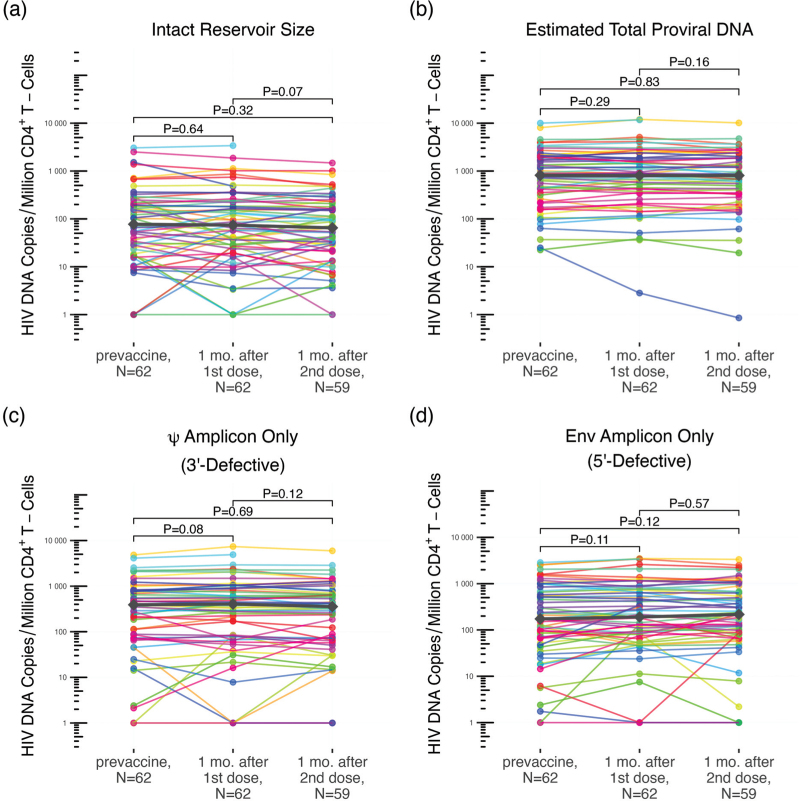
To determine whether COVID-19 mRNA vaccination induced changes in HIV reservoir size (defined as genome-intact proviral load), defective proviral burden or total HIV DNA load, we quantified the number of intact, defective and total proviral copies per million CD4+ T-cells (Fig. 2) [38]. At baseline, the median number of intact HIV copies per million CD4+ T-cells was 77 (IQR 20–204). One month following the first vaccine dose it was 74 (IQR 27–212), a difference that was not statistically significant (Wilcoxon paired test, P = 0.64) (Fig. 2a). One month following the second vaccine dose, the median intact reservoir size was 65 (IQR 22–174), which was not significantly different from baseline (P = 0.32), nor one month after the first dose (P = 0.07) (Fig. 2a). Likewise, 5’-defective, 3’-defective, and total proviral burdens did not change significantly between baseline and either postvaccine visit (Fig. 2a–d; all P > 0.08). These results remained consistent after excluding data from participants who experienced COVID-19 (all q ≥ 0.76 after correction for multiple comparisons; not shown). Stratification of the data by sex, COVID-19 vaccine regimen, and ART regimen similarly produced no statistically significant differences in intact reservoir size, nor in the total, 5’-defective nor 3’-defective proviral burdens for any subgroup, after adjusting for multiple comparisons (all q ≥ 0.32; not shown). Fold-changes in intact reservoir size between the pre and postvaccination timepoints were also not found to be associated with either nadir or most recent CD4+ T-cell count, nor plasma viral load at baseline (P > 0.1; not shown).
Fig. 2.
Measures of intact reservoir size, and total, 5’-defective, 3’-defective proviral burdens after one- and two-dose COVID-19 vaccination.
Intact reservoir size (panel a), total proviral burden (panel b), 3’-defective proviral burden (panel c), and 5’-defective proviral burden (panel d) measured at baseline (prevaccine), one month after the first vaccine dose, and one month after the second vaccine dose, using the intact proviral DNA assay (IPDA). Each participant is identified by a unique color that is consistent throughout all figures. Median values at each time point are depicted as grey diamonds. P-values were calculated using the Wilcoxon signed-rank test for paired data. COVID-19, coronavirus disease 2019.
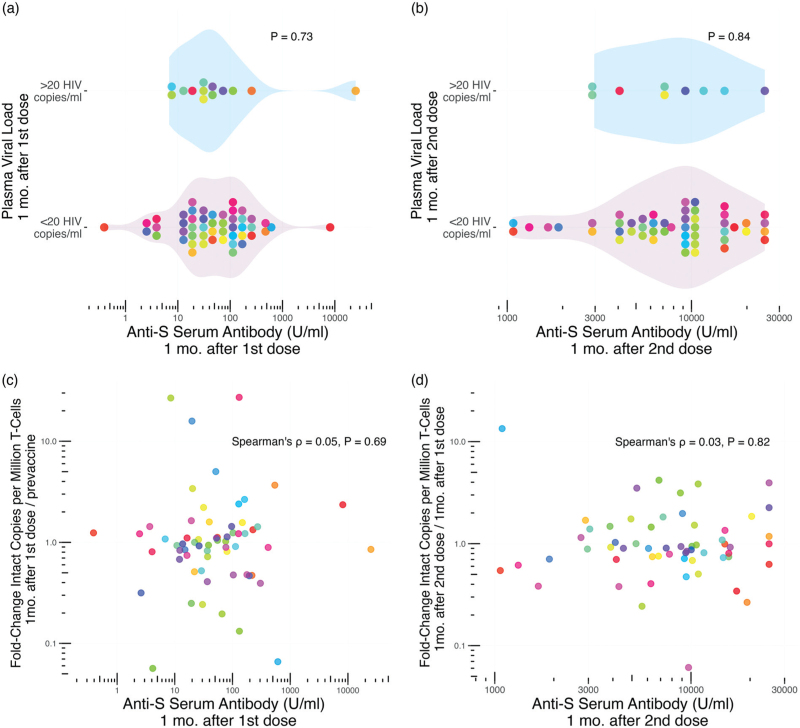
No evidence that the magnitude of the coronavirus disease 2019-vaccine-induced immune response increases the likelihood of HIV reservoir perturbation
Based on the observation that PWH on ART who mounted strong immune responses to influenza vaccination were more likely to show transient increases in HIV pVL [20], we investigated whether the magnitude of the COVID-19-vaccine-induced immune response increased the likelihood of HIV reservoir perturbation. Here, we used SARS-CoV-2-Spike serum antibody concentrations postvaccination as our measure of immune response magnitude. Overall, we found no evidence of a link. One month following the first vaccine dose, anti-SARS-CoV-2-Spike serum antibody concentrations were a median of 51.4 (19.4–130.6 U/ml) in participants who maintained pVL <20 copies/ml versus a median of 36.6 (16.4–80.7 U/ml) among participants with a pVL >20 HIV copies/ml, a difference that was not statistically significant (Mann–Whitney P = 0.73; Fig. 3a). Similarly, one month after the second vaccine dose, anti-SARS-CoV-2-Spike serum antibody concentrations were median of 8970 (5019–13544 U/ml) and 7205 (4163–11 638 U/ml) respectively in participants with pVL <20 versus >20 copies/ml, a difference that was not statistically significant (P = 0.84; Fig. 3b).
Fig. 3.
Relationship between reservoir size, plasma viral load and COVID-19 vaccine immune response magnitude.
Panel a: Anti-SARS-CoV-2-Spike (S) serum antibody levels one month following the first COVID-19 vaccine dose in participants with pVL >20 HIV RNA copies/ml (top group) versus <20 HIV RNA copies/ml (bottom group) at this time point. P-value calculated using the Mann–Whitney U-test. Panel b: same as panel a, but for anti-S serum antibody levels and pVL one month after the second dose. Panel c: Relationship between anti-S serum antibody levels one month following the first COVID-19 vaccine dose, and the fold-change in intact reservoir size from baseline, assessed using Spearman's correlation. Panel d: same as panel c, but depicting the relationship between anti-S serum antibody levels one month following the second COVID-19 vaccine dose, and the fold-change in intact reservoir size since the previous time point. Each participant is identified by a unique color that is consistent throughout all figures. COVID-19, coronavirus disease 2019; pVL, plasma viral load.
Likewise, the magnitude of anti-SARS-CoV-2-Spike serum antibody levels one month after the first vaccine dose did not correlate with the fold-change in reservoir size from baseline (Spearman ρ = 0.05, P = 0.69; Fig. 3c). Similarly, the magnitude of anti-SARS-CoV-2-Spike serum antibody levels one month after the second vaccine dose did not correlate with the fold-change in reservoir size from the prior visit (Spearman ρ = 0.03, P = 0.82; Fig. 3d). Both the pVL and reservoir size results remained consistent after excluding the participants who experienced COVID-19 (all P > 0.51; not shown). Overall, these observations indicate that the magnitude of the vaccine-elicited anti-SARS-CoV-2-Spike immune response did not influence the likelihood of reservoir perturbation.
Taken together, the results from our observational cohort do not provide any evidence that COVID-19 mRNA vaccines modulated the HIV reservoir nor induced plasma viremia.
Province-wide analysis of trends in HIV plasma viral loads before and after mass coronavirus disease 2019 vaccination
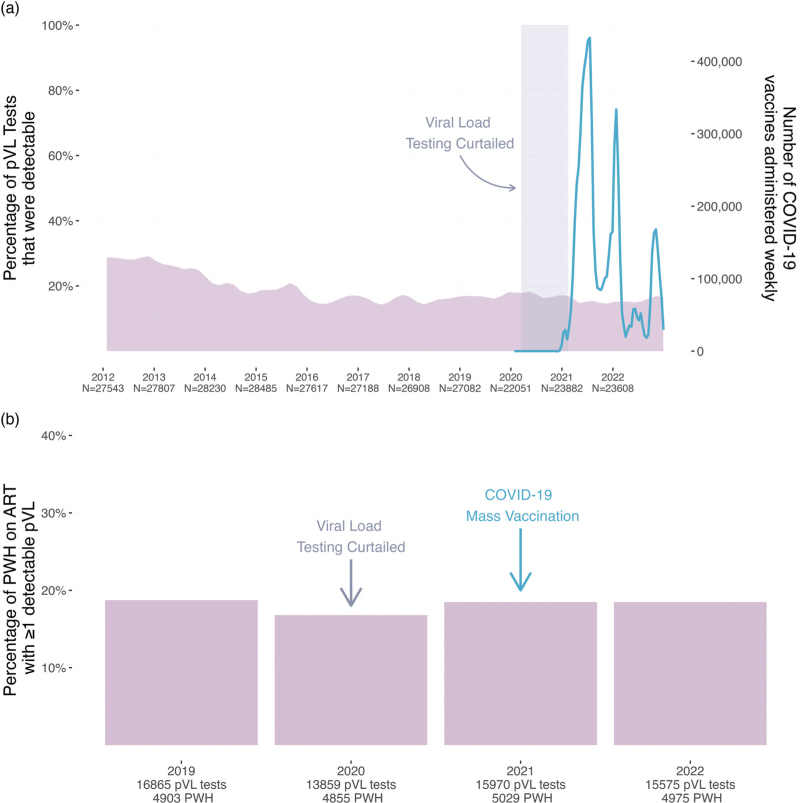
We next investigated whether BC's mass COVID-19 vaccination campaign was associated with an increase in the frequency of detectable pVL test results (defined here as pVL > 40 copies HIV RNA/ml; see Methods) at the population level. We began by analyzing all 290 401 pVL tests performed in BC since 2012. These data represented all pVL test results from the ∼7100–8100 PWH undergoing routine clinical testing annually in BC, where all measurements were included regardless of the individual's ART status at the time of testing (Fig. 4a). Between 2012 and approximately 2016, the percentage of detectable pVL test results declined from nearly 29% to an average of 16% as a result of a province-wide implementation of widespread HIV testing and immediate initiation of free-of-charge ART that began in 2013 [47]. Since 2016, the overall percentage of detectable pVL has remained relatively stable, though there was a slight uptick in the proportion of detectable pVL tests during 2020 because care providers were asked to reduce the frequency of routine pVL testing for PWH with long-term viremia suppression, to preserve laboratory capacity for COVID-19 diagnostic testing (which was also performed on the cobas 6800 in BC).
Fig. 4.
Population-level analysis of pVL test results in BC.
Panel a: The percentage of all pVL tests performed in BC that returned a detectable result (defined as >40 HIV RNA copies/ml; see methods) was computed for every month between 2012 and 2022 and depicted as a smoothed mauve curve. The numbers underneath the x-axis denote the total number of pVL tests performed each year. The smoothed blue line depicts the number of COVID-19 vaccine doses administered in BC weekly, as retrieved from the COVID-19 Vaccine Tracker [42]. The shaded rectangle denotes the period when pVL load testing of long-term ART-suppressed PWH was temporarily curtailed to preserve capacity for COVID-19 diagnostic testing. Panel b: The percentage of PWH in BC receiving uninterrupted ART who experienced at least one detectable pVL measurement (defined as >40 HIV RNA copies/ml; see methods) for the given year. The total number of pVL tests included in each year's analysis, as well as the total number of PWH from whom these pVL data are derived, are shown on the x-axis. BC, British Columbia; COVID-19, coronavirus disease 2019; pVL, plasma viral load.
COVID-19 vaccines were first made available to priority populations in BC, namely frontline health workers, long-term care residents and individuals with certain clinical conditions (which did not include HIV infection) starting in late December 2020. The age-based mass immunization campaign began in April 2021, which is when the majority of PWH became eligible for COVID-19 vaccination. Peak administration of first doses occurred from approximately May through September 2021, with second doses largely administered from February to March 2022 (Fig. 4a). By April 10, 2022, 89% of all adult British Columbians had received at least two COVID-19 vaccine doses [41]. Notably, we observed no obvious evidence of population-level increases in detectable pVL during or immediately following the peak vaccine administration periods in the province (Fig. 4a).
As vaccine-induced viremia may only be observable in PWH on suppressive ART, we next restricted the province-wide analysis to PWH whose ART prescription refill records were uninterrupted in a given year, and calculated the percentage of these PWH who experienced at least one detectable pVL that year. We began the analysis in 2019, the year before the COVID-19 pandemic was declared and the first full year that the cobas 6800 HIV Test was implemented in BC (Fig. 4b). Between 4903 and 5975 PWH were included in each year's analysis. In 2019, 18.7% of PWH receiving uninterrupted ART experienced at least one detectable pVL. In 2020, the percentage was 16.8%, though this reduction may be attributable to the temporary reduction in pVL testing that year. By 2021, pVL testing frequencies had returned to prepandemic levels. Despite the more than 9 million COVID-19 vaccine doses administered province-wide that year, the percentage of PWH receiving uninterrupted ART who experienced at least one detectable pVL measurement was essentially identical to that observed in 2019 prior to the pandemic, at 18.5%. The percentage for 2022 was similarly 18.5%. Taken together, these results reveal no evidence linking the provincial COVID-19 vaccination campaign to increases in detectable pVL at the population level.
Discussion
The mass rollout of COVID-19 mRNA vaccines provided an opportunity to study the potential stimulatory effects of this new vaccine modality on the HIV reservoir. Though a number of studies have now investigated this topic [33–35,37], their results have not been entirely conclusive. While one study found evidence of a gradual, though not statistically significant increase in the rate of detectable viremia peaking 4 weeks after the second vaccine dose [33], two others reported no changes in viremia following two-dose vaccination [34,36]. Two studies reported reduced frequencies of detectable viremia after three-dose vaccination [34,35], though in one study this was likely attributable to increased time on ART or switches made to more effective ART regimens [34]. Ours is the first study to our knowledge to combine cohort- and population-level analyses of pVL trends during a mass COVID-19 mRNA vaccination campaign.
Overall, neither the cohort nor population-level analyses identified evidence that COVID-19 mRNA vaccination promotes viral release from the reservoir to detectable levels in plasma. One month after the first and second vaccine doses, the proportion of participants with detectable pVL remained statistically unchanged from baseline, with no participant experiencing virologic failure (defined as pVL > 200 copies/ml [45,46]). Moreover, there was no evidence that the magnitude of the anti-SARS-CoV-2 vaccine-induced immune response influenced the likelihood of experiencing plasma viremia. Our population-level analysis similarly found that the frequency of detectable pVL test results remained stable following the mass administration of first, second and booster COVID-19 vaccine doses in BC. This remained the case whether we considered the overall PWH population, or the subset receiving uninterrupted ART.
Our cohort-based analysis also found no evidence that COVID-19 mRNA vaccination induced changes in intact HIV reservoir size, nor in total, 5’-defective, or 3’-defective proviral loads. This is consistent with findings from of three studies that assessed smaller numbers of participants for this outcome using similar approaches [33,35,37].
Our study has some limitations and caveats. We sampled our cohort one month following each COVID-19 vaccine dose because our primary objective was to evaluate vaccine immune responses in PWH, as previously reported [6]. This timing however would have missed rapid viremia events that had resolved by this time (indeed, such rapid viremia events have been reported following influenza vaccination [20]). That said, in reports describing viremia following COVID-19 vaccination, viremia was detectable one month postvaccination [31,33]. Another limitation is that our cohort also had relatively high CD4+ T-cell counts and controlled viremia on ART, so our results may not be applicable to PWH with lower CD4+ T-cell counts or uncontrolled HIV. Our study did not assess cell-associated HIV RNA levels, which have been observed to increase following vaccination for other diseases [17] and following COVID-19 mRNA vaccination [37]. Our sampling was also limited to peripheral blood and may not reflect reservoir changes that may have occurred in tissues. Another limitation is that our province-wide evaluation represents an ecological analysis that correlated population-level pVL and vaccination data, because COVID-19 vaccination dates of individual British Columbians were not available to us. Thus, even though this analysis captured all HIV pVL tests performed in BC, the variable timing of these tests with respect to the individual's vaccination date would not have allowed us to capture all viremia events that may have occurred. Finally, though neither our cohort nor population-level analyses support frequent nor widespread effects of COVID-19 vaccination on the HIV reservoir, we cannot rule out that such events may occur uncommonly, through mechanisms that remain incompletely understood.
In conclusion, we found no evidence that COVID-19 mRNA vaccines induced changes in HIV reservoir size nor plasma viremia in PWH receiving suppressive ART. Taken together with similar findings from other studies [33–35,37], we conclude that there is now a strong body of evidence indicating COVID-19 immunization is safe and effective in PWH [4–12]. This should provide additional reassurance to PWH and their care providers regarding the safety of COVID-19 mRNA vaccines.
Acknowledgements
M.C.D. and Z.L.B. conceived and designed the study. M.A.B. and Z.L.B. obtained funding. H.R.L., M.H., M.A.B. and Z.L.B. established the cohort. M.C.D., F.H.O., N.N.K., S.S. and N.M.-G. processed specimens and/or collected IPDA data. M.C.D. analyzed data and made figures. T.L., C.F.L. and M.G.R. performed the plasma viral load testing. M.L.D., J.S., D.T.H. and M.G.R. performed the COVID-19 serologic testing. N.B., P.S. and C.J.B. performed the province-wide viral load analysis. J.S.G.M. and R.B. provided data access. M.C.D. wrote the first draft of the paper, with all authors contributing edits.
We are grateful to the study participants, without whom this research would not be possible.
This work was supported in part by the Canadian Institutes for Health Research (CIHR) through a project grant (PJT-159625 to ZLB) and a team grant (HB1-164063 to ZLB and MAB). This work was also supported in part by the National Institutes of Health (NIH) through the Martin Delaney “REACH” Collaboratory (NIH grant 1-UM1AI164565-01 to Z.L.B. and M.A.B.), which is supported by the following NIH cofunding institutes: NIMH, NIDA, NINDS, NIDDK, NHLBI and NIAID. MCD was supported by a CIHR CGS-M award. FHO was supported by a PhD fellowship from the sub-Saharan African Network for TB/HIV Research Excellence (SANTHE), a DELTAS Africa Initiative (grant # DEL-15-006). The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS)'s Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa's Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust [grant # 107752/Z/15/Z] and the UK government. N.N.K. was supported by a CIHR Vanier Canada Graduate Scholarship. M.L.D. and Z.L.B. were supported by Scholar Awards from Michael Smith Health Research BC. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other funders.
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1.Geretti AM, Stockdale AJ, Kelly SH, Cevik M, Collins S, Waters L, et al. Outcomes of coronavirus disease 2019 (COVID-19) related hospitalization among people with human immunodeficiency virus (HIV) in the ISARIC World Health Organization (WHO) Clinical Characterization Protocol (UK): a prospective observational study. Clin Infect Dis 2020; 73:e2095–e2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tesoriero JM, Swain C-AE, Pierce JL, Zamboni L, Wu M, Holtgrave DR, et al. COVID-19 outcomes among persons living with or without diagnosed HIV infection in New York state. JAMA Netw Open 2021; 4:e2037069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhaskaran K, Rentsch CT, MacKenna B, Schultze A, Mehrkar A, Bates CJ, et al. HIV infection and COVID-19 death: a population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet HIV 2021; 8:e24–e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021; 397:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruddy JA, Boyarsky BJ, Bailey JR, Karaba AH, Garonzik-Wang JM, Segev DL, et al. Safety and antibody response to two-dose SARS-CoV-2 messenger RNA vaccination in persons with HIV. AIDS 2021; 35:2399–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brumme ZL, Mwimanzi F, Lapointe HR, Cheung PK, Sang Y, Duncan MC, et al. Humoral immune responses to COVID-19 vaccination in people living with HIV receiving suppressive antiretroviral therapy. npj Vaccines 2022; 7:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antinori A, Cicalini S, Meschi S, Bordoni V, Lorenzini P, Vergori A, et al. Humoral and cellular immune response elicited by mRNA vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in people living with human immunodeficiency virus receiving antiretroviral therapy based on current CD4 T-lymphocyte count. Clin Infect Dis 2022; 75:e552–e563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vergori A, Cozzi Lepri A, Cicalini S, Matusali G, Bordoni V, Lanini S, et al. Immunogenicity to COVID-19 mRNA vaccine third dose in people living with HIV. Nat Commun 2022; 13:4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fidler S, Fox J, Tipoe T, Longet S, Tipton T, Abeywickrema M, et al. Booster vaccination against SARS-CoV-2 induces potent immune responses in people with human immunodeficiency virus. Clin Infect Dis 2023; 76:201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costiniuk CT, Singer J, Lee T, Langlois MA, Arnold C, Galipeau Y, et al. COVID-19 vaccine immunogenicity in people with HIV. AIDS 2023; 37:F1–F10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lapointe HR, Mwimanzi F, Cheung PK, Sang Y, Yaseen F, Speckmaier S, et al. Antibody response durability following three-dose coronavirus disease 2019 vaccination in people with HIV receiving suppressive antiretroviral therapy. AIDS 2023; 37:709–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costiniuk CT, Singer J, Lee T, Galipeau Y, McCluskie PS, Arnold C, et al. Antibody neutralization capacity after coronavirus disease 2019 vaccination in people with HIV in Canada. AIDS 2023; 37:F25–F35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaida A, Brotto LA, Murray MCM, Côté HCF, Albert AY, Nicholson V, et al. Intention to receive a COVID-19 vaccine by HIV status among a population-based sample of women and gender diverse individuals in British Columbia, Canada. AIDS Behav 2022; 26:2242–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Han J, Li X, Chen D, Zhao X, Qiu Y, et al. COVID-19 vaccination in people living with HIV (PLWH) in China: a cross sectional study of vaccine hesitancy, safety, and immunogenicity. Vaccines 2021; 9:1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu S, Zhu S, Yan X, Xu Y, Xu H, Yang F, et al. Willingness to receive the COVID-19 vaccine among HIV positive men who have sex with men in China: a cross-sectional study. BMC Public Health 2023; 23:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yao Y, Chai R, Yang J, Zhang X, Huang X, Yu M, et al. Reasons for COVID-19 vaccine hesitancy among Chinese people living with HIV/AIDS: structural equation modeling analysis. JMIR Public Health Surveill 2022; 8:e33995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yek C, Gianella S, Plana M, Castro P, Scheffler K, Garcia F, et al. Standard vaccines increase HIV-1 transcription during antiretroviral therapy. AIDS 2016; 30:2289–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Negredo E, Domingo P, Sambeat MA, Rabella N, Vázquez G. Effect of pneumococcal vaccine on plasma HIV-1 RNA of stable patients undergoing effective highly active antiretroviral therapy. Eur J Clin Microbiol Infect Dis 2001; 20:287–288. [DOI] [PubMed] [Google Scholar]
- 19.Calmy A, Bel M, Nguyen A, Combescure C, Delhumeau C, Meier S, et al. Strong serological responses and HIV RNA increase following AS03-adjuvanted pandemic immunization in HIV-infected patients. HIV Med 2012; 13:207–218. [DOI] [PubMed] [Google Scholar]
- 20.Günthard HF, Wong JK, Spina CA, Ignacio C, Kwok S, Christopherson C, et al. Effect of influenza vaccination on viral replication and immune response in persons infected with human immunodeficiency virus receiving potent antiretroviral therapy. J Infect Dis 2000; 181:522–531. [DOI] [PubMed] [Google Scholar]
- 21.Rey D, Krantz V, Partisani M, Schmitt M-P, Meyer P, Libbrecht E, et al. Increasing the number of hepatitis B vaccine injections augments anti-HBs response rate in HIV-infected patients. effects on HIV-1 viral load. Vaccine 2000; 18:1161–1165. [DOI] [PubMed] [Google Scholar]
- 22.Kowalczyk A, Doener F, Zanzinger K, Noth J, Baumhof P, Fotin-Mleczek M, et al. Self-adjuvanted mRNA vaccines induce local innate immune responses that lead to a potent and boostable adaptive immunity. Vaccine 2016; 34:3882–3893. [DOI] [PubMed] [Google Scholar]
- 23.Iavarone C, O’Hagan DT, Yu D, Delahaye NF, Ulmer JB. Mechanism of action of mRNA-based vaccines. Expert Rev Vaccines 2017; 16:871–881. [DOI] [PubMed] [Google Scholar]
- 24.Frater J, Ewer KJ, Ogbe A, Pace M, Adele S, Adland E, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in HIV infection: a single-arm substudy of a phase 2/3 clinical trial. Lancet HIV 2021; 8:474–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Venanzi Rullo E, Pinzone MR, Cannon L, Weissman S, Ceccarelli M, Zurakowski R, et al. Persistence of an intact HIV reservoir in phenotypically naive T cells. JCI Insight 2020; 5:e133157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zerbato JM, McMahon DK, Sobolewski MD, Mellors JW, Sluis-Cremer N. Naive CD4+ T cells harbor a large inducible reservoir of latent, replication-competent human immunodeficiency virus type 1. Clin Infect Dis 2019; 69:1919–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woldemeskel BA, Garliss CC, Blankson JN. SARS-CoV-2 mRNA vaccines induce broad CD4+ T cell responses that recognize SARS-CoV-2 variants and HCoV-NL63. J Clin Invest 2021; 131:e149335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Becerra-Artiles A, Calvo-Calle JM, Co MD, Nanaware PP, Cruz J, Weaver GC, et al. Broadly recognized, cross-reactive SARS-CoV-2 CD4 T cell epitopes are highly conserved across human coronaviruses and presented by common HLA alleles. Cell Rep 2022; 39:110952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teijaro JR, Farber DL. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol 2021; 21:195–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Howard JN, Bosque A. IL-15 and N-803 for HIV cure approaches. Viruses 2023; 15:1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bozzi G, Lombardi A, Ludovisi S, Muscatello A, Manganaro L, Cattaneo D, et al. Transient increase in plasma HIV RNA after COVID-19 vaccination with mRNA-1272. Int J Infect Dis 2021; 113:125–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levy I, Wieder-Finesod A, Litchevsky V, Biber A, Indenbaum V, Olmer L, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in people living with HIV-1. Clin Microbiol Infect 2021; 27:1851–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matveev VA, Mihelic EZ, Benko E, Budylowski P, Grocott S, Lee T, et al. Immunogenicity of COVID-19 vaccines and their effect on HIV reservoir in older people with HIV. iScience 2023; 26:107915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fusco FM, Carleo MA, Sangiovanni N, D’Abbraccio M, Tambaro O, Borrelli F, et al. Does COVID-19 vaccination with BNT162b2 influence HIV-related immunological and virological markers? Data from 235 persons living with HIV at Cotugno Hospital, Naples, Italy: immune response after second and third doses, and influence on immunovirological markers. Viral Immunol 2023; 36:360–365. [DOI] [PubMed] [Google Scholar]
- 35.Tuttle DJ, Castanha PMS, Nasser A, Wilkins MS, Alaoui-El-Azher M, Cuff DE, et al. SARS-CoV-2 mRNA Vaccine Induced Immune Responses in Men with HIV-1 and Impact on Proviral Reservoirs. https://ssrn.com/abstract=4417076 or http://dx.doi.org/10.2139/ssrn.4417076. [Google Scholar]
- 36.Portillo V, Fedeli C, Ustero Alonso P, Petignat I, Mereles Costa EC, Sulstarova A, et al. Impact on HIV-1 RNA levels and antibody responses following SARS-CoV-2 vaccination in HIV-infected individuals. Front Immunol 2022; 12:820126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stevenson EM, Terry S, Copertino D, Leyre L, Danesh A, Weiler J, et al. SARS CoV-2 mRNA vaccination exposes latent HIV to Nef-specific CD8+ T-cells. Nat Commun 2022; 13:4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bruner KM, Wang Z, Simonetti FR, Bender AM, Kwon KJ, Sengupta S, et al. A novel quantitative approach for measuring the reservoir of latent HIV-1 proviruses. Nature 2019; 566:120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kinloch NN, Ren Y, Conce Alberto WD, Dong W, Khadka P, Huang SH, et al. HIV-1 diversity considerations in the application of the intact proviral DNA assay (IPDA). Nat Commun 2021; 12:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Public Health Agency of Canada. Canadian report on COVID-19 vaccine doses administered. Ottawa: Public Health Agency of Canada; 2023. Available at: https://health-infobase.canada.ca/covid-19/vaccine-administration/ (accessed 20 September 2023). [Google Scholar]
- 41.Public Health Agency of Canada. Canadian COVID-19 vaccination coverage report. Ottawa: Public Health Agency of Canada; 2023. Available at: https://health-infobase.canada.ca/covid-19/vaccination-coverage/ (accessed 18 September 2023). [Google Scholar]
- 42.Little N. COVID-19 Tracker Canada. 2020. COVID19Tracker.ca. [Google Scholar]
- 43.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci USA 2003; 100:9440–9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Public Health Agency of Canada. NACI rapid response: extended dose intervals for COVID-19 vaccines to optimize early vaccine rollout and population protection in Canada. Public Health Agency of Canada; 2021. Available at: https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/rapid-response-extended-dose-intervals-covid-19-vaccines-early-rollout-population-protection.html (accessed 7 September 2023). [Google Scholar]
- 45.The Committee for Drug Evaluation and Therapy. Therapeutic guidelines for antiretroviral (ARV) treatment of adult HIV infection. British Columbia Centre for Excellence in HIV/AIDS; 2023. [Google Scholar]
- 46.Panel on Antiretroviral Guidelines for Adults and Adolescents. HIV clinical guidelines: adult and adolescent ARV – what's new in the guidelines. Department of Health and Human Services; 2023. [Google Scholar]
- 47.Nanditha NGA, Dong X, Tafessu HM, Wang L, Lu M, Barrios R, et al. A province-wide HIV initiative to accelerate initiation of treatment-as-prevention and virologic suppression in British Columbia, Canada: a population-based cohort study. CMAJ Open 2022; 10:E27–E34. [DOI] [PMC free article] [PubMed] [Google Scholar]