Abstract
The gram-positive endospore-forming bacterium Bacillus subtilis has, under aerobic conditions, a branched respiratory system comprising one quinol oxidase branch and one cytochrome oxidase branch. The system terminates in one of four alternative terminal oxidases. Cytochrome caa3 is a cytochrome c oxidase, whereas cytochrome bd and cytochrome aa3 are quinol oxidases. A fourth terminal oxidase, YthAB, is a putative quinol oxidase predicted from DNA sequence analysis. None of the terminal oxidases are, by themselves, essential for growth. However, one quinol oxidase (cytochrome aa3 or cytochrome bd) is required for aerobic growth of B. subtilis strain 168. Data indicating that cytochrome aa3 is the major oxidase used by exponentially growing cells in minimal and rich medium are presented. We show that one of the two heme-copper oxidases, cytochrome caa3 or cytochrome aa3, is required for efficient sporulation of B. subtilis strain 168 and that deletion of YthAB in a strain lacking cytochrome aa3 makes the strain sporulation deficient.
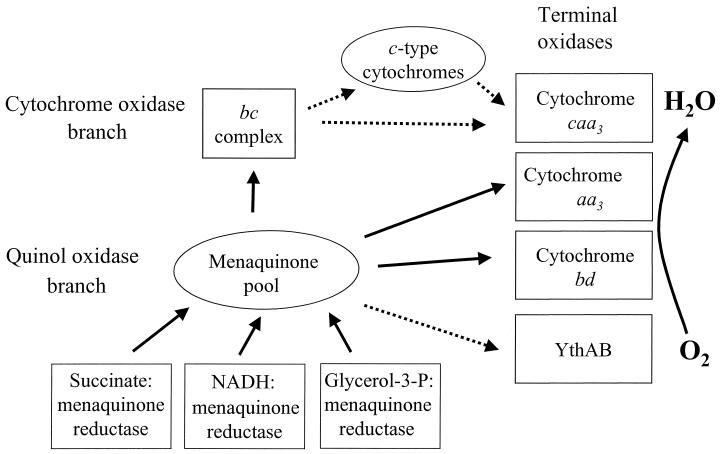
Aerobic and facultative aerobic bacteria can respond to changes within the environment by using different types of respiratory pathways (3, 23). During aerobic growth, the final step in the pathway, the four-electron reduction of dioxygen to two water molecules, is catalyzed by a group of membrane-bound enzymes called terminal oxidases. Many bacteria use more than one terminal oxidase (1, 3, 29). For example in the gram-negative bacterium Escherichia coli, there are two types of terminal oxidases—cytochrome bo3 and cytochrome bd. The former is used under aerobic growth conditions, whereas the latter is induced under microaerobic conditions (7, 30). In the soybean symbiont bacterium Bradyrhizobium japonicum, the main terminal oxidase under free-living conditions is an aa3-type cytochrome c oxidase (20, 21). When B. japonicum lives endosymbiotically, it uses a cbb3-type oxidase. This terminal oxidase has an extremely high affinity for oxygen, which allows it to operate under the low oxygen pressure of the root nodules (21). Another example is from the obligately aerobic, nitrogen-fixing bacterium Azotobacter vinelandii, which has two known terminal oxidases, a cytochrome bo3 and a cytochrome bd. In A. vinelandii, cytochrome bd with its high oxygen affinity protects the oxygen-labile nitrogenase by keeping the oxygen levels sufficiently low (19). The gram-positive endospore-forming soil bacterium Bacillus subtilis synthesizes under aerobic growth conditions a branched electron transport chain comprising three or possibly four terminal oxidases (Fig. 1) (33, 34). The physiological role(s) of the specific terminal oxidases in B. subtilis is unknown. The long-term objective of our work is to define the physiological roles of the terminal oxidases in B. subtilis.
FIG. 1.
Aerobic respiratory pathways in B. subtilis strain 168. Solid arrows, known electron pathways; dashed arrows, tentative pathways.
The electron transport chain in B. subtilis contains two major branches, one quinol oxidase branch and one cytochrome oxidase branch (Fig. 1). Three known terminal oxidases are present. Cytochrome caa3 is a cytochrome c oxidase, whereas cytochrome aa3 and cytochrome bd are quinol oxidases (16, 34). Both a-type oxidases belong to the well-characterized heme-copper oxidase superfamily of respiratory oxidases (4, 6, 34). Characteristic for the bacterial heme-copper oxidases is that they have a subunit homologous to subunit I of the mitochondrial cytochrome c oxidase, contain copper, and pump protons across the cytoplasmic membrane in response to electron transfer (4, 34).
Four structural genes, qoxABCD, are required for expression of B. subtilis cytochrome aa3 (26). Cytochrome caa3 is encoded by the ctaCDEF genes (27). Two additional genes, ctaA and ctaB, are also required for production of both cytochrome caa3 and cytochrome aa3 (28, 31). The ctaA and ctaB gene products are involved in the biosynthesis of the heme a prosthetic group (28). The bd-type of oxidases is a distinct group of terminal oxidases, not related to the heme-copper oxidases. They do not pump protons or contain copper (14). As there is no proton pumping, less energy is conserved by cytochrome bd compared to the heme-copper oxidases.
Expression of cytochrome bd requires cydA and cydB, which code for the two subunits of the enzyme as well as two additional genes, cydC and cydD (33). The latter two genes encode a putative ATP-binding-cassette (ABC) type of transporter. In B. subtilis, the presence of a fourth terminal oxidase can be predicted from the genome sequence (15, 33). A gene cluster containing three genes, ythA, ythB, and ythC, has been identified. The translated sequences of ythA and ythB are closely related to Bacillus stearothermophilus CbdA and CbdB, which constitute a terminal oxidase of bd type (24). No homologue of ythC has been found in B. stearothermophilus. The ythA and ythB genes might encode a terminal oxidase related to the bd-type oxidases. However, there is no direct experimental evidence for the presence of this terminal oxidase in B. subtilis. Throughout this article, the product of these genes is referred to as YthAB. In addition, there is spectroscopic evidence for a putative terminal oxidase of bb′ type. The genes encoding this oxidase have not been identified, but it is not the product of ythA and ythB (2).
In this work, we show that, in B. subtilis, cytochrome aa3 is the most important terminal oxidase during the exponential-growth phase. Moreover, we show that no single terminal oxidase is essential for aerobic growth of B. subtilis. However, the presence of one of the quinol oxidases, cytochrome aa3 or cytochrome bd, is essential for aerobic growth. In addition, we show that one of the heme-copper oxidases, cytochrome caa3 or cytochrome aa3, is required for normal sporulation of B. subtilis.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Bacterial strains and plasmids used in this work are listed in Table 1. Escherichia coli strains were kept on Luria agar (25). B. subtilis strains grown aerobically were kept on tryptose blood agar base (TBAB) (Difco) plates, which when indicated were supplemented with 1% (wt/vol) glucose. Liquid media were inoculated with B. subtilis cells grown on TBAB plates over night. The cultures were grown at 37°C in an orbital shaker at 200 rpm in nutrient sporulation medium phosphate (NSMP) (5) or in NSMP supplemented with 0.5% (wt/vol) glucose (NSMPG) or in minimal medium supplemented with 0.5% (wt/vol) glucose (MM) (36). The doubling times in the exponential-growth phase were calculated as follows: doubling time equals (t2 − t1) × log 2]/[log optical density at 600 nm (OD600) at t2 − log OD600 at t1], where t1 and t2 are the times of measurement. B. subtilis cells were also grown on minimal medium plates supplemented with 0.5% (wt/vol) of one of the following carbon sources: glucose, malate, glutamate, or succinate. For the sporulation frequency experiment, strains were grown in NSMP at 37°C for 30 h. The number of viable cells per milliliter of culture was determined as the total number of CFUs on TBAB plates. The number of spores per milliliter of culture was determined as the number of CFUs after heat treatment at 80°C for 10 min.
TABLE 1.
List of strains and plasmids used in this work
| Strain or plasmid | Relevant characteristicsa | Source or referenceb |
|---|---|---|
| Strainsc | ||
| Escherichia coli XL1-Blue | endA1 gyrA96 thi hsdR17(rK− mK+) supE44 relA1 lac/F′ proAB+ lacIqlacZΔM15 Tn10 | Stratagene, Inc. |
| B. subtilis JH642 | pheA1 trpC2 | BGSCd |
| Δqox | trpC2 ΔqoxABCD::kan | 32 |
| Δspo0A | pheA1 trpC2 Δspo0A::erm | 13 |
| 168A | trpC2 | Laboratory stock |
| Strains derived from 168A | ||
| LUH14 | ΔqoxABCD::kan | Δqox→168A |
| LUH15 | ΔctaCD::ble | 33 |
| LUH17 | ΔqoxABCD::kan ΔctaCD::ble | 33 |
| LUW10 | ΔcydABCD::cat | 33 |
| LUW20 | ΔcydABCD::tet | 33 |
| LUW22 | ΔcydABCD::tet qox::pSPOX | pSPOX→LUW20 |
| LUW23 | ΔctaCD::ble ΔcydABCD::tet | LUW20→LUH15 |
| LUW24 | ΔctaCD::ble ΔcydABCD::tet, qox::pSPOX | pSPOX→LUW23 |
| LUW29 | ΔcydABCD::cat ΔqoxABCD::kan | LUW10→LUH14 |
| LUW32 | qox::pSPOX | pSPOX→168A |
| LUW33 | ΔctaCD::ble ΔcydABCD::tet ΔqoxABCD::kan | LUH14→LUW23 |
| LUW42 | ΔctaCD::ble qox::pSPOX | pSPOX→LUH15 |
| LUW122 | ΔythAB::tet | 2 |
| LUW128 | ΔcydCD::cat | pCYD24→168A |
| 1A1 | trpC2 | BGSCd |
| Strains derived from 1A1 | ||
| LUW34 | ΔcydABCD::cat | LUW10→1A1 |
| LUW46 | ΔqoxABCD::kan | LUH14→1A1 |
| LUW112 | Δspo0A::erm | Δspo0A→1A1 |
| LUW137 | ΔythAB::tet | LUW122→1A1 |
| LUW138 | ΔqoxABCD::kan ΔythAB::tet | LUW122→LUW46 |
| LUW142 | ΔctaCD::ble | LUH15→1A1 |
| LUW143 | ΔctaCD::ble ΔqoxABCD::kan | LUW46→LUW142 |
| LUW145 | ΔctaCD::ble ΔcydABCD::cat | LUW34→LUW142 |
| LUW147 | ΔctaCD::ble ΔythAB::tet | LUW122→LUW142 |
| LUW148 | ΔctaCD::ble ΔqoxABCD::kan ΔythAB::tet | LUW46→LUW147 |
| LUW196 | ΔctaCD::ble ΔcydABCD::cat ΔythAB::tet | LUW10→LUW147 |
| LUW198 | ΔcydABCD::cat ΔythAB::tet | LUW10→LUW137 |
| Plasmids: | ||
| pDH88 | Cmr Amr | 11 |
| pSPOX | qoxA′ in pDH88 | This work |
| pHP13 | Cmr Emr | 8 |
| pCYD13 | cydA′ and cydD′ in pHV32 | 33 |
| pCYD22 | cydCD in pHP13 | 33 |
| pCYD23 | cydABCD and the cyd promoter in pHP13 | 33 |
| pCYD24 | cydC′ and cydD′ in pHV32 | This work |
| pCYD25 | cydCD and the cyd promoter in pHP13 | This work |
Amr, Cmr, and Emr indicates resistance to ampicillin, chloramphenicol, and erythromycin, respectively.
Arrows indicate transformation and point from donor to recipient.
All B. subtilis strains derived from the parental strains 168A and 1A1 contain the trpC2 mutation. During this work, strain 168A was found to be oligosporogenic, which affects growth in liquid media. Therefore, doubling times of oxidase mutants were calculated for strains derived from the parental strain 1A1.
Bacillus Genetic Stock Center. Department of Biochemistry, Ohio State University, Columbus.
B. subtilis strains were grown anaerobically on TBAB plates, supplemented with 20 mM KNO3 and 1% (wt/vol) glucose, at 37°C. The plates were incubated for 24 h in an anaerobic cabinet (Don Whitley Scientific). The gas composition in the anaerobic cabinet was 10% H2–10% CO2–80% N2. For B. subtilis, the following concentrations of antibiotics were used: chloramphenicol, 5 g/liter; kanamycin, 5 g/liter; and tetracycline, 15 g/liter. For E. coli, ampicillin was used at 100 g/liter.
DNA techniques.
E. coli cells were transformed using the electroporation method described by Hanahan et al. (9). Chromosomal DNA was isolated and competent B. subtilis cells prepared essentially as described by Hoch (12). General DNA techniques were performed as described by Sambrook et al. (25). PCR was performed essentially as described previously (35), using Taq DNA polymerase. The primers used to amplify a 269-bp fragment of qoxA were QoxA1 (5′-GCAAGCTTTTGAGGAAGTATGCACTTCAGA-3′) and QoxA2 (5′-GCTCTAGAGTCGCGGTATTTTACTAAAATAATGG-3′). Chromosomal DNA (0.1 ng) from B. subtilis 1A1 was used as a template. To construct double or triple mutants, B. subtilis strains were transformed with nonsaturating amounts of chromosomal DNA.
Spectral analysis on membranes.
Membranes were prepared as described previously (10) and suspended in 20 mM sodium morpholinic propane sulphonic buffer (pH 7.4). Reduced minus oxidized difference light absorption spectra were recorded as described previously (33).
Construction of a cydCD expression plasmid.
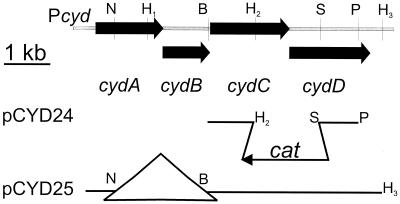
A plasmid containing the cydC and cydD genes under control of the cyd promoter was constructed by removing the 2-kb BglII and NdeI fragment containing cydA and cydB from plasmid pCYD23. The remaining part of pCYD23 was treated with the large (Klenow) fragment of E. coli DNA polymerase I, self-ligated, and used to transform B. subtilis 168A to chloramphenicol resistance. This resulted in plasmid pCYD25 containing cydC and cydD under the control of their native promoter (Fig. 2).
FIG. 2.
Restriction map of the cyd region and plasmids carrying different parts of this region. At the top, the physical map of the B. subtilis cyd region is shown. The restriction sites are abbreviated as follows; B, BglII; N, NdeI; H, HindIII; P, PstI; and S, SphI. Plasmid pCYD24 is a derivative of pCYD13 (33), and plasmid pCYD25 is a derivative of pCYD23 (33). Construction of plasmids is described in Materials and Methods.
Construction of a cydCD null mutant.
To make a cydCD deletion-insertion mutant, the 0.5-kbp EcoRI-HindIII fragment of pCYD13 was replaced by a 1-kb EcoRI-HindIII fragment of pCYD22 carrying a part of the cydC gene. The resulting plasmid, pCYD24 (Fig. 2), was used to transform strain 168A to chloramphenicol resistance. The deletion-insertion within the chromosomal cydC and cydD genes arising from a double-crossover recombination event was confirmed by Southern blot analysis (data not shown).
Construction of conditional qoxABCD mutant strains (Pspac-qoxABCD).
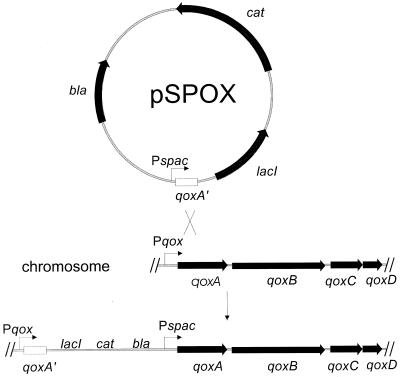
A 269-base-pair fragment (qoxA′) of the 5′ region (−48 to +221 relative to the putative qox translational start site) of qoxA was amplified by PCR. The resulting fragment contains a part of qoxA and includes a putative ribosome-binding site but lacks the promoter region. Plasmid pDH88 contains the artificial hybrid promoter spac, which can be induced by the addition of 1 mM isopropyl β-d-thiogalactoside (IPTG) to the growth medium. The amplified qoxA′ fragment was cleaved with restriction enzymes HindIII and XbaI and inserted into plasmid pDH88, cleaved with the same enzymes. The resulting plasmid was used to transform E. coli XL1-Blue to ampicillin resistance, creating plasmid pSPOX, containing the spac promoter followed by the qoxA′ fragment (Fig. 3). When pSPOX was used to transform B. subtilis strains to chloramphenicol resistance, the plasmid was integrated into the chromosome by a single homologous recombination event in front of the qoxABCD genes. This resulted in strains in which expression of the qoxABCD operon could be controlled by IPTG (Fig. 3).
FIG. 3.
Construction of B. subtilis strains with the qoxABCD operon under control of Pspac. The integrative plasmid pSPOX contains the inducible promoter spac and a 269-bp fragment of qoxA (qoxA′), including the ribosome-binding site but not the promoter region. Integration of pSPOX into the B. subtilis chromosome results in control of the qoxABCD genes by the spac promoter and control of the truncated qoxA by the native qox promoter. The genes for β-lactamase, chloramphenicol resistance, and the lac repressor are indicated as bla, cat, and lacI, respectively.
RESULTS AND DISCUSSION
Growth properties of single oxidase mutants.
Doubling times and growth yields of B. subtilis strains lacking one of the terminal oxidases, cytochrome caa3, cytochrome aa3, cytochrome bd, or YthAB, were compared with the doubling time and growth yield of the wild-type strain in different media (Table 2). In a broth medium (NSMP) or in MM, the doubling times and growth yields of the strains lacking cytochrome caa3, cytochrome bd, or YthAB did not differ from those of the wild type. However, the strain lacking cytochrome aa3 grew significantly more slowly and reached the stationary phase at a lower cell density compared to the wild type in both media (Table 2). When grown in NSMP supplemented with 0.5% glucose, all four mutant strains showed a doubling time and growth yield similar to those of the wild-type strain (Table 2). We concluded that none of the terminal oxidases are, by themselves, essential for aerobic growth. The significantly reduced growth rate of the cytochrome aa3 mutant in NSMP and MM media suggests that cytochrome aa3 in these growth media is the most important oxidase in exponentially growing B. subtilis cells.
TABLE 2.
Doubling times and relative yield of oxidase mutants grown in liquid media
| Strain | Relevant genotype | Doubling time (min)a
|
Relative yieldb
|
||||
|---|---|---|---|---|---|---|---|
| NSMP | NSMPG | MM | NSMP | NSMPG | MM | ||
| 1A1 | Wild type | 41 ± 0.7 | 33 ± 1.7 | 49 ± 3.1 | 1.0 | 1.0 | 1.0 |
| LUW34 | ΔcydABCD | 42 ± 0.9 | 34 ± 1.2 | 48 ± 1.1 | 0.97 | 0.95 | 1.0 |
| LUW46 | ΔqoxABCD | 57 ± 0.9 | 32 ± 0.1 | 65 ± 2.0 | 0.57 | 0.95 | 0.78 |
| LUW137 | ΔythAB | 41 ± 0.9 | 31 ± 1.3 | 50 ± 2.1 | 1.0 | 0.96 | 0.98 |
| LUW142 | ΔctaCD | 39 ± 1.8 | 29 ± 1.9 | 50 ± 1.4 | 1.0 | 0.97 | 1.0 |
| LUW148 | ΔqoxABCD ΔctaCD ΔythAB | 59 ± 2.3 | 32 ± 0.5 | 63 ± 5.8 | 0.48 | 0.76 | 0.53 |
| LUW196 | ΔcydABCD ΔctaCD ΔythAB | 42 ± 1.5 | 32 ± 1.9 | 44 ± 0.8 | 1.0 | 1.0 | 0.99 |
Doubling times were calculated for strains in the exponential growth phase, grown at 37°C in different media. Values are the mean of at least three experiments. The degree of spread is indicated as the standard error of the mean.
Relative yield is defined as the highest optical density in early stationary phase in terminal oxidase mutant strain cultures relative to that in the wild-type strain cultures. Values are the means of at least three experiments. The variation is less than 6%.
Mutants defective in multiple terminal oxidases.
Next, we attempted to make B. subtilis mutant strains lacking two, three, or four terminal oxidases. To make a strain lacking both cytochrome aa3 and cytochrome bd, LUW20 (ΔcydABCD::tet) was transformed with nonsaturating amounts of chromosomal DNA (0.2 mg/liter) from LUH14 (ΔqoxABCD::kan), and transformants were selected on TBAB plates containing kanamycin, with and without glucose. However, no transformants were obtained. The reverse experiment, i.e., transformation of a strain lacking cytochrome aa3 with chromosomal DNA from a strain lacking cytochrome bd, was performed with similar results. The experiment was also done with strains derived from 1A1 and JH642, with similar results. As a control, strain LUW20 (ΔcydABCD::tet) harboring plasmid pCYD23, which carries a functional set of the cydABCD genes, was transformed with LUH14 (ΔqoxABCD::kan) chromosomal DNA. Transformants (7.5 × 106/mg of DNA) were obtained, showing that the actual transformation event works in this strain. From this we concluded that a strain lacking both cytochrome aa3 and cytochrome bd is not viable under the conditions employed.
Strains lacking two or three terminal oxidases were made by transformation of a recipient strain with donor chromosomal DNA as indicated in Table 1. All combinations that did not include deletion of both cytochrome bd and cytochrome aa3 could be made. Mutants containing only one terminal oxidase, either cytochrome aa3 or cytochrome bd, were further characterized. The strains were grown in liquid media and the doubling times calculated. In NSMPG, the doubling time of a strain containing only cytochrome bd did not differ from that of the wild type, but in NSMP and in MM, the doubling times were significantly longer, and the mutant strain reached the stationary phase at about half the cell density relative to that of the wild type (Table 2). In contrast, the doubling times and growth yields of a strain containing only cytochrome aa3 did not differ from those of the wild type in either of the media (Table 2).
To further study the growth properties of oxidase mutants, strains were grown on plates containing minimal medium and one of the following carbon sources: glucose, malate, glutamate, or succinate. The strains lacking cytochrome caa3 (LUH15), cytochrome bd (LUW10), or YthAB (LUW122) grew as well as the wild-type strain on all of the tested carbon sources. The same was observed for the strain containing only cytochrome aa3 (LUW196). The strain lacking cytochrome aa3 (LUH14) showed growth properties similar to those of the wild-type strain on glucose, but this strain grew more slowly and formed smaller colonies on the other carbon sources. The strain containing only cytochrome bd (LUW148) grew more slowly and formed colonies significantly smaller than those of the wild-type strain on glucose. This indicates that either cytochrome caa3 or YthAB is required for optimal growth on glucose in a QoxABCD− mutant background. LUW148 did not grow on the nonfermentative substrates malate, glutamate, or succinate. Taken together, our data further indicate that cytochrome aa3 is sufficient to support maximal growth rates in broth and defined media. It is likely that the other terminal oxidases play minor roles in exponentially growing, aerobic wild-type cells.
Anaerobic growth of double and triple mutants.
Under anaerobic conditions, B. subtilis is able to utilize nitrate as a terminal electron acceptor (18). To find out if a strain lacking cytochrome aa3 in combination with cytochrome bd is viable under anaerobic nitrate-respiratory conditions, the transformation experiments were carried out in an anaerobic atmosphere. LUH14 (ΔqoxABCD::kan) was transformed with chromosomal DNA from LUW10 (ΔcydABCD::cat). Transformants were selected on TBAB plates containing chloramphenicol, 20 mM KNO3, and 1% (wt/vol) glucose, incubated at 37°C in an anaerobic cabinet for 24 h. Strain LUW29, lacking both cytochrome aa3 and cytochrome bd, was obtained. A similar procedure was used to construct strain LUW33, lacking cytochrome caa3, cytochrome aa3, and cytochrome bd. The mutant strains LUW29 and LUW33 were streaked on two new plates of which one was incubated aerobically and the other one was incubated in the anaerobic cabinet. The strains grew well in the anaerobic cabinet but could not grow in an aerobic atmosphere. If the anaerobically incubated cells were exposed to oxygen, they could not resume growth in the anaerobic cabinet. The results showed that a strain lacking both cytochrome bd and cytochrome aa3 grows under anaerobic, nitrate-respiratory conditions.
Construction of strains with the qoxABCD genes under control of an IPTG-inducible promoter.
To be able to study the growth properties of mutants lacking both cytochrome aa3 and cytochrome bd, we constructed strains in which expression of the qoxABCD operon was controlled by the IPTG-inducible spac promoter. An integrative plasmid (pSPOX) carrying Pspac was constructed and used to transform different B. subtilis strains to chloramphenicol resistance as described in Materials and Methods and in Figure 3.
Plasmid pSPOX was used to transform B. subtilis 168A (wild type), LUW20 (ΔcydABCD), LUW23 (ΔctaCD ΔcydABCD), and LUH15 (ΔctaCD) to chloramphenicol resistance. Transformants were selected on TBAB plates with or without IPTG and incubated aerobically or anaerobically. Transformation of the wild-type strain and the strain lacking cytochrome caa3 resulted in transformants under all growth conditions (Table 3). To confirm that cytochrome aa3 was only synthesized in the presence of IPTG, the wild-type strain carrying pSPOX in its chromosome was grown in NSMP supplemented with 0.5% glucose, with or without IPTG. Spectral analysis of membranes from this strain showed that cytochrome aa3 could be detected only in membranes from cells grown in the presence of IPTG (data not shown). When transforming the strain lacking cytochrome bd or the strain lacking both cytochrome bd and cytochrome caa3, transformants were obtained only on plates incubated anaerobically or on plates incubated aerobically and supplemented with IPTG (Table 3). When colonies from plates supplemented with IPTG were streaked on new plates without IPTG and incubated aerobically, no growth was seen.
TABLE 3.
Relative frequency of transformants obtained in different B. subtilis strains transformed with plasmid pSPOX
| Strain | Relative frequency of transformants under culture conditionsa
|
|||
|---|---|---|---|---|
| +O2
|
−O2
|
|||
| +IPTG (+ cyt. aa3) | −IPTG (− cyt. aa3) | +IPTG (+ cyt. aa3) | −IPTG (− cyt. aa3) | |
| 168A (wild-type) | 0.95 | 1.0 | 1.1 | 1.1 |
| LUH15 (ΔctaCD) | 1.1 | 1.1 | n.d. | n.d. |
| LUW20 (ΔcydABCD) | 1.1 | 0 | 0.98 | 0.88 |
| LUW23 (ΔctaCD ΔcydABCD) | 1.2 | 0 | 1.1 | 0.98 |
Transformants were selected on plates containing chloramphenicol, with (+ cytochrome aa3) or without (− cytochrome aa3) 1 mM IPTG, incubated with (+O2) or without (−O2) oxygen. A value of 1.0 corresponds to 1,102 transformants. n.d., not done.
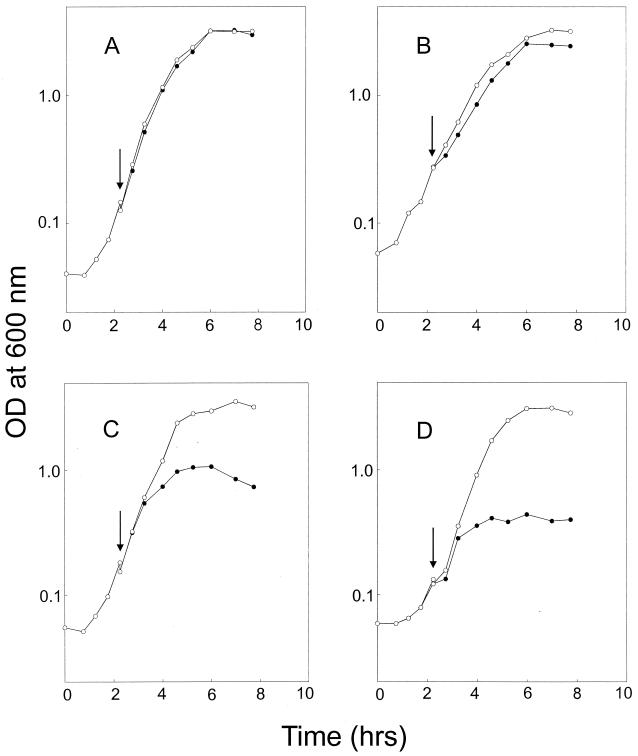
To study the growth properties in liquid cultures, LUW32 (Pspac-qoxABCD), LUW22 (Pspac-qoxABCD ΔcydABCD), LUW24 (Pspac-qoxABCD ΔctaCD ΔcydABCD), and LUW42 (Pspac-qoxABCD ΔctaCD) were grown in NSMPG in the presence of IPTG. After 2.25 h, the cells were harvested, washed, and resuspended in NSMPG, with or without IPTG. In the presence of IPTG, the growth rates of the strains did not differ from that of the wild-type (Fig. 4). When IPTG was removed, no effect was seen in LUW32 (Fig. 4A). Growth of LUW42 (lacking cytochrome caa3) was slightly poorer than that of the wild type (Fig. 4B), whereas in LUW22 (lacking cytochrome bd) and LUW24 (lacking cytochrome bd and cytochrome caa3), the growth rate was significantly decreased by about 1 h after removal of IPTG (Fig. 4C and D). Moreover, LUW22 and LUW24 failed to reach the final optical density exhibited by the LUW32 strain. The decrease in growth rate did not occur immediately after removal of IPTG, probably because cytochrome aa3 was present in the cell membrane at the time of IPTG removal. The data further confirmed our results that a B. subtilis strain lacking both cytochrome aa3 and cytochrome bd cannot grow vegetatively in an aerobic atmosphere.
FIG. 4.
B. subtilis strains carrying pSPOX grown in NSMPG with and without IPTG. Cells were grown in NSMPG supplemented with IPTG. After 2.25 h (OD600 between 0.15 and 0.24, indicated by arrows), cells were harvested, washed, and resuspended in fresh media. Open circles show cells grown in NSMPG containing 1 mM IPTG. Solid circles show cells grown in NSMPG without IPTG. (A) LUW32 (Pspac-qoxABCD); (B) LUW42 (Pspac-qoxABCD ΔctaCD); (C) LUW22 (Pspac-qoxABCD ΔcydABCD); (D) LUW24 (Pspac-qoxABCD ΔctaCD ΔcydABCD).
Sporulation of oxidase mutants.
Sporulation in B. subtilis is an energy-requiring process. To see whether the absence of any of the terminal oxidases affected sporulation, mutant strains were tested for sporulation efficiency. As shown in Table 4, the sporulation efficiency of the single-oxidase mutants did not differ from that of the wild-type strain. Mutants lacking both cytochrome caa3 and cytochrome bd or both cytochrome caa3 and YthAB or both cytochrome bd and YthAB showed normal sporulation (Table 4). The same was observed for the strain containing only cytochrome aa3 (i.e., a strain lacking cytochrome caa3, cytochrome bd, and YthAB). However, the strain containing only cytochrome bd showed an approximately 5,000-fold reduction of the sporulation frequency relative to that of the wild-type strain (Table 4). Sporulation was also inhibited in the strain lacking both cytochrome aa3 and cytochrome caa3. This is in line with previous data showing that a CtaA− mutant strain, which is unable to make the heme a prosthetic group, is sporulation deficient (17). The strain lacking cytochrome aa3 and YthAB showed a 24-fold decrease in the level of sporulation (Table 4). These results showed that one of the heme copper terminal oxidases, cytochrome aa3 or cytochrome caa3, is required for efficient sporulation of B. subtilis strain 168, probably because at least one proton-pumping oxidase is required to conserve enough energy for sporulation. In addition, our results suggested that YthAB may have a role in sporulation and can compensate for the loss of cytochrome aa3.
TABLE 4.
Sporulation frequencies of oxidase mutants
| Strain | Relevant genotype | Viable count (cells/ml) | Spore count (cells/ml) | Sporulation frequency (%)a |
|---|---|---|---|---|
| 1A1 | Wild-type | 4.6 × 108 | 4.2 × 108 | 91 |
| Single mutants | ||||
| LUW142 | ΔctaCD | 2.9 × 108 | 2.6 × 108 | 90 |
| LUW34 | ΔcydABCD | 4.5 × 108 | 4.4 × 108 | 97 |
| LUW137 | ΔythAB | 2.8 × 108 | 2.7 × 108 | 98 |
| LUW46 | ΔqoxABCD | 4.8 × 108 | 4.5 × 108 | 93 |
| Double mutants | ||||
| LUW145 | ΔctaCD ΔcydABCD | 2.9 × 108 | 2.8 × 108 | 97 |
| LUW147 | ΔctaCD ΔythAB | 4.0 × 108 | 3.6 × 108 | 90 |
| LUW143 | ΔctaCD ΔqoxABCD | 8.7 × 108 | 2.0 × 105 | 0.023 |
| LUW198 | ΔcydABCD ΔythAB | 4.0 × 108 | 3.7 × 108 | 92 |
| LUW138 | ΔqoxABCD ΔythAB | 8.4 × 108 | 3.3 × 107 | 3.8 |
| Triple mutants | ||||
| LUW148 | ΔctaCD ΔythAB ΔqoxABCD | 9.8 × 107 | 1.8 × 104 | 0.018 |
| LUW196 | ΔctaCD ΔcydABCD ΔythAB | 2.7 × 108 | 2.6 × 108 | 97 |
Sporulation frequencies were calculated as spore count divided by viable count. The sporulation mutant strain LUW112 (Δspo0A) was found to have a sporulation frequency of >3.1 × 10−6%. Each experiment was repeated at least twice; the variation was less than 5%. In each case, data from a single experiment are presented.
The role of the CydCD transporter.
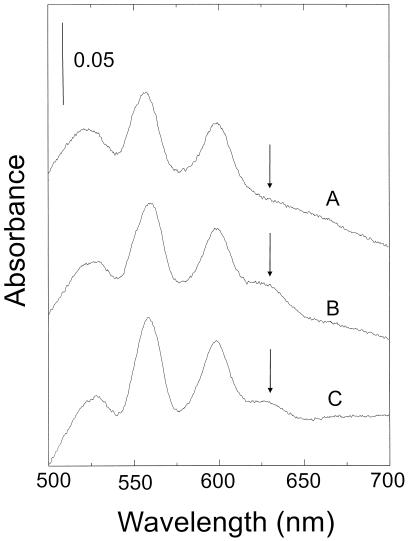
The cydC and cydD gene products are likely to encode a heterodimeric, membrane bound ABC type of transporter that is required for assembly of cytochrome bd (33). To analyze whether the CydCD ABC transporter is also required for the assembly of an additional terminal oxidase in B. subtilis, we constructed a strain containing the CydCD transporter but lacking cytochrome bd. A plasmid, pCYD25, carrying cydCD under control of the cyd promoter, was introduced into LUW20, thus creating a strain lacking the chromosomal cydABCD operon but carrying cydCD on a plasmid. To confirm that pCYD25 contained a functional set of cydCD, strain LUW128 lacking cydCD was constructed, and pCYD25 was introduced into this strain. LUW128 carrying pCYD25 and LUW128 carrying pHP13 were grown in NSMPG, and membranes were studied by light absorption difference (reduced minus oxidized) spectroscopy. Membranes from strain 1A1 (wild type) grown in the same way were used as a control. No cytochrome bd was detected in membranes from LUW128(pHP13). Membranes from LUW128(pCYD25) showed a spectrum similar to that of the wild-type strain, showing that pCYD25 expressed a functional cydCD and that overexpression of cydCD did not result in an increased production of cytochrome bd (Fig. 5).
FIG. 5.
Light absorption difference (dithionite-reduced minus ferricyanide-oxidized) spectra of membranes (3 mg of protein per ml) from strains 1A1 and LUW128 carrying different plasmids. B. subtilis strains were grown in NSMPG and harvested in the stationary-growth phase. Line A, LUW128(pHP13); line B, LUW128(pCYD25); line C, 1A1.
To find out whether there is an additional terminal oxidase present, which requires CydCD and can compensate for the loss of both quinol oxidases in B. subtilis, the following experiment was performed. Chromosomal DNA from LUH14 (ΔqoxABCD::kan) was used to transform LUW20(pCYD25), LUW128(pCYD25), and LUW128. Transformants were selected on TBAB plates containing kanamycin with and without glucose. A few transformants were obtained with LUW20(pCYD25) and LUW128, but these had all became wild type with respect to cytochrome bd; i.e., the antibiotic resistance marker in the cyd locus had been substituted with the cydABCD or the cydCD genes from the LUH14 chromosomal DNA. Several transformants were obtained with LUW128(pCYD25) (data not shown). Our results indicate that there is no additional terminal oxidase in B. subtilis, requiring the CydCD ABC transporter, that could compensate for the loss of cytochrome bd and cytochrome aa3. The results also confirm that no functional cytochrome bd is made if CydCD is not present, and they suggest that none of the other about 80 ABC transporters in B. subtilis (22) can compensate for the loss of CydCD.
Conclusion.
The aerobic respiratory pathways in B. subtilis terminate with one of three or possibly four alternative terminal oxidases, as indicated in Fig. 1. Taken together, our data strongly indicate that one of the quinol oxidases, cytochrome aa3 or cytochrome bd, is essential for aerobic growth of B. subtilis strain 168. The reason that the cytochrome oxidase branch cannot compensate for the loss of the quinol oxidase branch is most probably that the cytochrome oxidase branch is not expressed until the cells enter the stationary phase. This hypothesis is supported by observations that the genes encoding the bc complex and probably also cytochrome caa3 are repressed by the transition state regulator AbrB in the exponential growth phase (37; L. Winstedt and C. von Wachenfeldt, unpublished data). We do not know under which conditions the ythAB genes are expressed. However, it seems likely that they are not expressed in exponentially growing cells. Deletion of ythAB in a strain lacking cytochrome aa3 makes the strain sporulation deficient, indicating a physiological role for YthAB in B. subtilis.
The combined results of this work show that cytochrome aa3 is the most important terminal oxidase contributing to proton motive force generation in exponentially growing cells. The results also demonstrate that one of the proton-pumping heme-copper oxidases, cytochrome caa3 or cytochrome aa3, is required for efficient sporulation. It is likely that B. subtilis cannot conserve enough energy for initiation or completion of the sporulation cycle by using only the nonproton-pumping terminal oxidase, cytochrome bd.
ACKNOWLEDGMENTS
We thank L. Rutberg for valuable comments on the manuscript, E. Holst for help with anaerobic growth of bacteria, and P. A. Levin for the kind gift of the Δspo0A strain.
This work was supported by grants from Crafoordska Stiftelsen and Emil och Wera Cornells Stiftelse.
REFERENCES
- 1.Anraku Y. Bacterial electron transport chains. Annu Rev Biochem. 1988;57:101–132. doi: 10.1146/annurev.bi.57.070188.000533. [DOI] [PubMed] [Google Scholar]
- 2.Azarkina N, Siletsky S, Borisov V, von Wachenfeldt C, Hederstedt L, Konstantinov A A. A cytochrome bb′-type quinol oxidase in Bacillus subtilis strain 168. J Biol Chem. 1999;274:32810–32817. doi: 10.1074/jbc.274.46.32810. [DOI] [PubMed] [Google Scholar]
- 3.Baker S C, Ferguson S J, Ludwig B, Page M D, Richter O M H, van Spanning R J M. Molecular genetics of the genus Paracoccus: metabolically versatile bacteria with bioenergetic flexibility. Microbiol Mol Biol Rev. 1998;62:1046–1078. doi: 10.1128/mmbr.62.4.1046-1078.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferguson-Miller S, Babcock G T. Heme/copper terminal oxidases. Chem Rev. 1996;96:2889–2907. doi: 10.1021/cr950051s. [DOI] [PubMed] [Google Scholar]
- 5.Fortnagel P, Freese E. Analysis of sporulation mutants. II. Mutants blocked in the citric acid cycle. J Bacteriol. 1968;95:1431–1438. doi: 10.1128/jb.95.4.1431-1438.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia-Horsman J A, Barquera B, Rumbley J, Ma J, Gennis R B. The superfamily of heme-copper respiratory oxidases. J Bacteriol. 1994;176:5587–5600. doi: 10.1128/jb.176.18.5587-5600.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gennis R B, Stewart V. Respiration. In: Neidhart F C, editor. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 217–261. [Google Scholar]
- 8.Haima P, Bron S, Venema G. The effect of restriction on shotgun cloning and plasmid stability in Bacillus subtilis Marburg. Mol Gen Genet. 1987;209:335–342. doi: 10.1007/BF00329663. [DOI] [PubMed] [Google Scholar]
- 9.Hanahan D, Jessee J, Bloom F R. Plasmid transformation of Escherichia coli and other bacteria. Methods Enzymol. 1991;204:63–113. doi: 10.1016/0076-6879(91)04006-a. [DOI] [PubMed] [Google Scholar]
- 10.Hederstedt L. Molecular properties, genetics, and biosynthesis of Bacillus subtilis succinate dehydrogenase complex. Methods Enzymol. 1986;126:399–414. doi: 10.1016/s0076-6879(86)26040-1. [DOI] [PubMed] [Google Scholar]
- 11.Henner D J. Inducible expression of regulatory genes in Bacillus subtilis. Methods Enzymol. 1990;185:223–228. doi: 10.1016/0076-6879(90)85022-g. [DOI] [PubMed] [Google Scholar]
- 12.Hoch J A. Genetic analysis in Bacillus subtilis. Methods Enzymol. 1991;204:305–320. doi: 10.1016/0076-6879(91)04015-g. [DOI] [PubMed] [Google Scholar]
- 13.Ireton K, Rudner D Z, Siranosian K J, Grossman A D. Integration of multiple developmental signals in Bacillus subtilis through the Spo0A transcription factor. Genes Dev. 1993;7:283–294. doi: 10.1101/gad.7.2.283. [DOI] [PubMed] [Google Scholar]
- 14.Jünemann S. Cytochrome bd terminal oxidase. Biochim Biophys Acta. 1997;1321:107–127. doi: 10.1016/s0005-2728(97)00046-7. [DOI] [PubMed] [Google Scholar]
- 15.Kunst F, Ogasawara N, Moszer I, Albertini A M, Alloni G, Azevedo V, Bertero M G, Bessieres P, Bolotin A, Borchert S, Borriss R, Boursier L, Brans A, Braun M, Brignell S C, Bron S, Brouillet S, Bruschi C V, Caldwell B, Capuano V, Carter N M, Choi S K, Codani J J, Connerton I F, Danchin A, et al. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 16.Lauraeus M, Haltia T, Saraste M, Wikström M. Bacillus subtilis expresses two kinds of haem-A-containing terminal oxidases. Eur J Biochem. 1991;197:699–705. doi: 10.1111/j.1432-1033.1991.tb15961.x. [DOI] [PubMed] [Google Scholar]
- 17.Mueller J P, Taber H W. Isolation and sequence of ctaA, a gene required for cytochrome aa3 biosynthesis and sporulation in Bacillus subtilis. J Bacteriol. 1989;171:4967–4978. doi: 10.1128/jb.171.9.4967-4978.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakano M M, Hulett F M. Adaptation of Bacillus subtilis to oxygen limitation. FEMS Microbiol Lett. 1997;157:1–7. doi: 10.1111/j.1574-6968.1997.tb12744.x. [DOI] [PubMed] [Google Scholar]
- 19.Poole R, Hill S. Respiratory protection of nitrogenase activity in Azotobacter vinelandii—roles of the terminal oxidases. Biosci Rep. 1997;17:303–317. doi: 10.1023/a:1027336712748. [DOI] [PubMed] [Google Scholar]
- 20.Preisig O, Anthamatten D, Hennecke H. Genes for a microaerobically induced oxidase complex in Bradyrhizobium japonicum are essential for a nitrogen fixing endosymbiosis. Proc Natl Acad Sci USA. 1993;90:3309–3313. doi: 10.1073/pnas.90.8.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Preisig O, Zufferey R, Thöny-Meyer L, Appleby C A, Hennecke H. A high-affinity cbb3-type cytochrome oxidase terminates the symbiosis-specific respiratory chain of Bradyrhizobium japonicum. J Bacteriol. 1996;178:1532–1538. doi: 10.1128/jb.178.6.1532-1538.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quentin Y, Fichant G, Denizot F. Inventory, assembly and analysis of Bacillus subtilis ABC transport systems. J Mol Biol. 1999;287:467–484. doi: 10.1006/jmbi.1999.2624. [DOI] [PubMed] [Google Scholar]
- 23.Richardson D J. Bacterial respiration: a flexible process for a changing environment. Microbiology. 2000;146:551–571. doi: 10.1099/00221287-146-3-551. [DOI] [PubMed] [Google Scholar]
- 24.Sakamoto J, Koga E, Mizuta T, Sato C, Noguchi S, Sone N. Gene structure and quinol oxidase activity of a cytochrome bd-type oxidase from Bacillus stearothermophilus. Biochim Biophys Acta. 1999;1411:147–158. doi: 10.1016/s0005-2728(99)00012-2. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 26.Santana M, Kunst F, Hullo M F, Rapoport G, Danchin A, Glaser P. Molecular cloning, sequencing, and physiological characterization of the qox operon from Bacillus subtilis encoding the aa3-600 quinol oxidase. J Biol Chem. 1992;267:10225–10231. [PubMed] [Google Scholar]
- 27.Saraste M, Metso T, Nakari T, Jalli T, Lauraeus M, van der Oost J. The Bacillus subtilis cytochrome-c oxidase: variations on a conserved protein theme. Eur J Biochem. 1991;195:517–525. doi: 10.1111/j.1432-1033.1991.tb15732.x. [DOI] [PubMed] [Google Scholar]
- 28.Svensson B, Lübben M, Hederstedt L. Bacillus subtilis CtaA and CtaB function in haem A biosynthesis. Mol Microbiol. 1993;10:193–201. doi: 10.1111/j.1365-2958.1993.tb00915.x. [DOI] [PubMed] [Google Scholar]
- 29.Thöny-Meyer L. Biogenesis of respiratory cytochromes in bacteria. Microbiol Mol Biol Rev. 1997;61:337–376. doi: 10.1128/mmbr.61.3.337-376.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Unden G, Bongaerts J. Alternative respiratory pathways of Escherichia coli: energetics and transcriptional regulation in response to electron acceptors. Biochim Biophys Acta. 1997;1320:217–234. doi: 10.1016/s0005-2728(97)00034-0. [DOI] [PubMed] [Google Scholar]
- 31.van der Oost J, von Wachenfeldt C, Hederstedt L, Saraste M. Bacillus subtilis cytochrome oxidase mutants: biochemical analysis and genetic evidence for two aa3-type oxidases. Mol Microbiol. 1991;5:2063–2072. doi: 10.1111/j.1365-2958.1991.tb00829.x. [DOI] [PubMed] [Google Scholar]
- 32.Villani G, Tattoli M, Capitanio N, Glaser P, Papa S, Danchin A. Functional analysis of subunits III and IV of Bacillus subtilis aa3-600 quinol oxidase by in vitro mutagenesis and gene replacement. Biochim Biophys Acta. 1995;1232:67–74. doi: 10.1016/0005-2728(95)00112-5. [DOI] [PubMed] [Google Scholar]
- 33.Winstedt L, Yoshida K, Fujita Y, von Wachenfeldt C. Cytochrome bd biosynthesis in Bacillus subtilis: characterization of the cydABCD operon. J Bacteriol. 1998;180:6571–6580. doi: 10.1128/jb.180.24.6571-6580.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Wachenfeldt C, Hederstedt L. Molecular biology of Bacillus subtilis cytochromes. FEMS Microbiol Lett. 1992;100:91–100. doi: 10.1111/j.1574-6968.1992.tb14025.x. [DOI] [PubMed] [Google Scholar]
- 35.von Wachenfeldt C, Hederstedt L. Physico-chemical characterization of membrane-bound and water-soluble forms of Bacillus subtilis cytochrome c-550. Eur J Biochem. 1993;212:499–509. doi: 10.1111/j.1432-1033.1993.tb17687.x. [DOI] [PubMed] [Google Scholar]
- 36.Yoshida K, Ishio I, Nagakawa E, Yamamoto Y, Yamamoto M, Fujita Y. Systematic study of gene expression and transcription organization in the gntZ-ywaA region of the Bacillus subtilis genome. Microbiology. 2000;146:573–579. doi: 10.1099/00221287-146-3-573. [DOI] [PubMed] [Google Scholar]
- 37.Yu J, Hederstedt L, Piggot P J. The cytochrome bc complex (menaquinone:cytochrome c reductase) in Bacillus subtilis has a nontraditional subunit organization. J Bacteriol. 1995;177:6751–6760. doi: 10.1128/jb.177.23.6751-6760.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]