Abstract
Porphyromonas gingivalis is a gram-negative anaerobic bacterium and an etiologic agent of adult periodontitis. By inducing a dysbiotic state within the host microbiota it contributes to a chronic inflammatory environment in the oral cavity. Under some circumstances, the oral bacteria may gain access to systemic circulation. While the most widely recognized function of platelets is to reduce hemorrhage in case of vascular damage, it is known that platelets are also involved in the hematologic responses to bacterial infections. Some pathogenic bacteria can interact with platelets, triggering their activation and aggregation. The aim of this study was to assess platelet responses to the presence of P. gingivalis in whole blood. Human whole blood was pretreated with P. gingivalis and then platelet plug formation was measured under high shear conditions using the PFA-100. In the presence of P. gingivalis, time for a platelet plug to occlude the aperture in the collagen/ADP cartridge was shortened in a manner dependent on bacterial concentration and the duration of bacterial preincubation of blood. P. gingivalis enhances thrombus forming potential of platelets in whole blood.
Keywords: PFA-100, platelets, platelet plug formation, Porphyromonas gingivalis, thrombosis, whole blood
Introduction
Hemostasis involves a highly regulated equilibrium between pro and anticoagulant processes. It is a dynamic system comprising cellular and plasma factors that interact with damaged vascular tissue to minimize blood loss. Platelets are anucleate cellular fragments derived from mature megakaryocytes via a process called thrombopoiesis (Stegner et al., 2017). They circulate throughout the vascular system in a quiescent discoid form, interacting minimally with healthy endothelial cells. Following injury to the endothelium, platelets serve as key effector cells for the hemostatic response. They rapidly adhere to exposed extracellular matrix via tether receptor complexes such as collagen–glycoprotein (GP) VI and von Willebrand factor–GPIb–V–IX (Canobbio et al., 2004, Chen et al., 2002). Platelet attachment triggers downstream effects including the release of platelet-specific α granules and dense granules (Li et al., 2010). Contained within these granules are important membrane bound receptors (GPIIb/IIIa and P-selectin), platelet mediators (adenosine 5’- diphosphate (ADP) and calcium ions) (Blair et al., 2009, Sharda et al., 2018). Release of ADP, a potent platelet agonist, is crucial for the formation of an effective platelet plug. Such ADP mediated activation leads to morphological changes, transforming resting discoid platelets to an amoeboid state with numerous pseudopodia that facilitate platelet interdigitation and stabilize the developing aggregate (White, 1968). Additionally, ADP promotes further platelet recruitment and aggregation at the injury site by increasing expression of GPIIb/IIIa receptors on platelet surfaces (Shattil et al., 1985). Altogether, this signal amplifying mechanism sustains platelet aggregation and plays a central role in platelet plug development at the injury site.
Pathogenic infection can disrupt host hemostasis, as invading bacteria may exploit the circulatory system to reach distant areas within the host, a process termed bacteremia. In healthy individuals, bacterial presence in the blood stream is usually transient. However, serious complications can occur including infective endocarditis, sepsis–associated disseminated intravascular coagulation, or venous thromboembolism (Bergin et al., 2017, Mejer et al., 2014, Semeraro et al., 2010). These and other similar clinical conditions are often characterized by a variety of platelet responses to the pathogenic bacteria (Hurley et al., 2016, Icli et al., 2013, Kim et al., 2015). The interplay between platelets and bacteria can trigger platelet activation and subsequent aggregation even in the absence of vascular injury (Arman et al., 2014, Kerrigan et al., 2002).
Two primary interaction mechanisms between bacteria and platelets are described: a) indirect bacterial binding via a bridging plasma protein and b) direct bacterial binding to platelet receptors. Examples of indirect binding to GPIIb/GPIIIa receptors include Staphylococcus aureus associated clumping factor (Clf) A and B (ClfA and ClfB) (O’Brien et al., 2002). In contrast, Streptococcus gordonii expressed sialic acid-binding protein (Hsa) was observed to directly interact with sialic acid residues located in the amino-terminus of GPIbα, while platelet adherence protein A (PadA) bound to GPIIb/GPIIIa receptors (Bensing et al., 2004, Petersen et al., 2010). A third mode of interaction between platelets and bacteria involves secreted bacterial products which interact with platelets. Even though the exact mechanisms are still poorly understood and not yet widely characterized, several secreted agents/toxins from some bacterial strains are now believed to affect platelets. For example, Porphyromonas gingivalis produces and releases outer membrane vesicles (OMVs) in the local oral cavity environment. Within these released OMVs are important virulence factors including gingipains, which contribute toward platelet activation and aggregation (Haurat et al., 2011, Klarström et al., 2015, Sharma et al., 2000).
P. gingivalis is identified as a keystone pathogen, capable of inducing dysbiosis in the oral microbiota (Hajishengallis et al., 2012). It is implicated as a causative factor for the pathogenesis and progression of periodontitis. Clinical research suggests a link between P. gingivalis mediated periodontitis and an increased risk for systemic diseases such as atherosclerosis (Chukkapalli et al., 2015, Hayashi et al., 2011, Rivera et al., 2011). P. gingivalis expresses a broad spectrum of virulence factors, the most notable are gingipains (Chen et al., 2001). Released or surface bound gingipains are capable of cleaving protease-activated receptors (PARs) on platelet surface (Lourbakos et al., 2001). Activation of these receptors elicits downstream signaling that induces platelet aggregation (Lourbakos et al., 2001). P. gingivalis is also an effective activator of platelets via a gingipain-independent mechanism (Naito et al., 2006). Toll-like receptor dependent platelet activation was reported in the presence of P. gingivalis (Blair et al., 2009). However, the physiological relevance of these studies remains unclear.
P. gingivalis is a gram-negative anaerobe that primarily infects the oral cavity. Oral bacteria can gain access to systemic circulation during dental procedures or routine oral hygiene. Upon entry into blood, P. gingivalis can trigger functional responses from several types of blood cells including platelets. In addition to their hemostatic and pro-thrombotic roles, platelets can contribute to host immune responses by preventing pathogenic dissemination and localizing infections (Engelmann et al., 2013). Dysregulated activation of platelets in this setting can promote thrombosis. In turn, formation of intravascular thrombi is a contributing risk factor for atherosclerosis (Li et al., 2000).
The objective of this study was to evaluate the relationship between P. gingivalis and platelet function in the context of human whole blood. After exposing platelets to bacterial suspensions of varying concentrations and over several different time periods, we assessed the effect of this periodontopathogen on platelet plug formation. Plug formation was measured as the time taken to occlude a standardized aperture as provided in the commercially available PFA-100 diagnostic instrument.
Methods
Materials.
The following reagents were used: Brain Heart Infusion (BHI) broth (Difco Laboratories, Detroit, MI), yeast extract (Difco Laboratories, Detroit, MI), DL-cysteine (Sigma-Aldrich, St. Louis, MO), haemin (Sigma-Aldrich, St. Louis, MO), vitamin K (Sigma-Aldrich, St. Louis, MO), defibrinated sheep blood (Hemostat Laboratories, Dixon, CA). The following supplies were used: Vacuette® Blood Collection system with vacuum tubes containing 3.2% sodium citrate (Greiner Bio-one, Kremsmunster, Austria), polypropylene tubes (Sarstedt Inc., Nümbrecht, Germany), and phosphate buffer saline (PBS, Sigma‐Aldrich, St. Louis, MO). PFA-100 collagen/ADP cartridges were purchased from Siemens Healthcare Diagnostics (Tarrytown, NY).
Selection of healthy blood donors.
Recruited donors were generally healthy adults without previous history of bleeding or thrombophilic disorders. However, prior to blood donation, volunteers were not examined for their oral health and periodontal status. Potential donors were excluded from this study if they: a) had a family history of hemostatic blood disorders, such as hemophilia or von Willebrand Disease, b) had recognized cardiovascular disorders requiring medical care, c) had taken any drugs that affected platelet function within a period of 72 hours prior to donation including, but not limited to aspirin, aspirin-containing compounds, and ibuprofen, and d) were unable or unwilling to give informed consent. Before the blood draw, the objective of the study was explained, and each donor signed a voluntary informed consent. This study was approved by the Institutional Review Board of Loma Linda University.
Blood collection and processing.
Fresh human blood was drawn by a qualified phlebotomist to avoid trauma damage at the draw site, to prevent hemolysis, and to minimize artifactual platelet activation. Whole blood was drawn using the Vacuette® Blood Collection system with a vacuum tube containing 3.2% sodium citrate. After the blood draw, all samples were immediately transferred to separate polypropylene tubes for incubation with the several bacterial suspensions. Then, these tubes were placed in 37°C block heaters and kept there throughout the duration of testing. The contents of the tubes were gently mixed several times during the preincubation process and again just before analysis to eliminate temperature gradients within the sample and to ensure uniform cellular distributions throughout. The platelet plug formation process in the PFA-100 is sensitive to sample sedimentation—in high hematocrit conditions the process proceeds more rapidly. All samples were processed within four hours of the blood draw as recommended by the manufacturer.
Bacterial growth conditions.
P. gingivalis wild-type strain, W83, was used for this study and grown as previously described (McKenzie et al., 2016). Briefly, P. gingivalis was first streaked onto BHI agar supplemented with 5% (v/v) sheep blood and incubated at 37°C for 5–7 days under anaerobic conditions (10% H2, 10% CO2, 80% N2). After bacterial colonies formed, a single colony was harvested and cultured in BHI broth, supplemented with yeast extract (0.5% or 5 mg/mL), DL-cysteine (0.1% or 1 mg/mL), haemin (5 μg/mL), and vitamin K (0.5 μg/mL), at 37°C in an anaerobic chamber (Coy Manufacturing, Grass Lake, MI). For all platelet function assays, P. gingivalis cultures were grown overnight to exponential growth-phase in BHI broth prior to dilution and use. Bacterial concentrations were measured by optical density at 600 nm (OD600) using a spectrophotometer (DU-650, Beckman Coulter, Brea, CA). Lastly, P. gingivalis was anaerobically streaked onto BHI agar plates supplemented with 5% (v/v) sheep blood and incubated at 37°C for 5–7 days, to ensure viability and purity.
Platelet function assay.
Platelet function assays were performed using the PFA-100 (Siemens Healthcare Diagnostics, Tarrytown, NY) with collagen/ADP test cartridges, according to manufacturer’s instructions with slight modifications. The PFA-100 is an automated system that simulates the in vivo vascular injury environment under high shear conditions (Kundu et al., 1995). Citrated whole blood was placed into disposable test cartridges and aspirated through a capillary towards a membrane aperture. The instrument measures the time for platelets to adhere and aggregate at the aperture following platelet activation. Formation of a platelet plug will occlude the opening and prevent blood from flowing through the capillary. The end point is defined as the aperture closure time (CT), in seconds, due to the obstructing platelet plug. The reported range of CTs was 62–100 seconds with a mean of 78 seconds for collagen/ADP (Böck et al., 1999). This method is commonly used in clinical laboratories to detect platelet dysfunction and because whole blood can be used it requires minimal manipulation of the sample.
Briefly, sample preparation began ~1 hour after blood draw to allow any physiological antiplatelet molecules to disappear and to stabilize whole blood (Favaloro, 2008). During this wait period, all test samples were maintained at 37°C in block heaters and gently mixed to prevent red cell sedimentation. P. gingivalis suspensions were serially diluted with calcium-free and magnesium-free PBS (pH 7.4) to a final volume of 200 μL. This was then added to 800 μL of citrated whole blood and preincubated for varying periods at 37°C. These samples were gently pipette mixed, before 800 μL was loaded into collagen/ADP cartridges and tested in the PFA-100. All cartridges were kept at room temperature (22°C) for at least 15 minutes before use. Samples were retested if flow obstruction was detected or if closure times (CTs) were non-measurable. OD600 for the P. gingivalis culture was measured using a spectrophotometer and the calculation of colony forming units (CFU) was based on the formula derived from previous P. gingivalis W83 colony count assays:
Two studies were undertaken. The first of these focused on describing the impact of preincubation duration while maintaining a fixed bacterial concentration. The second study investigated the impact of varying bacterial concentrations on platelet plug formation, as reported by CTs, for several fixed preincubation lengths. Highest bacterial concentrations would likely not be compatible with longest preincubation because they would trigger thrombus formation prior to PFA-100 assay. Similarly, it would be of limited value to repeat every bacterial concentration below threshold level for each fixed preincubation. As a result, the intervening bacterial concentrations were chosen with particular spacing to best demonstrate the prolongation and shortening of the CTs for each fixed preincubation.
Statistical Analysis.
Data were analyzed with a two-way ANOVA to examine the effect of P. gingivalis preincubation time on PFA-100 CTs. These CTs were analyzed with the Kolmogorov-Smirnov two-sample test to determine whether the CT measurements followed a normal distribution, and if the samples came from single or multiple populations. The results were then analyzed with a two-way ANCOVA test to identify effect of the preincubation time while adjusting for P. gingivalis concentration. Blood was drawn from 6 different donors for each of the platelet plug formation assays. PFA-100 CT data are presented as mean ± SEM for both assays. P-values of P < 0.05 (*) and P < 0.01 (**) were considered statistically significant. All statistical analyses were performed using the statistical software R, version 3.6.3 (The R Project for Statistical Computing, Vienna, Austria).
Results
Platelet plug closure times are shortened in the presence of P. gingivalis.
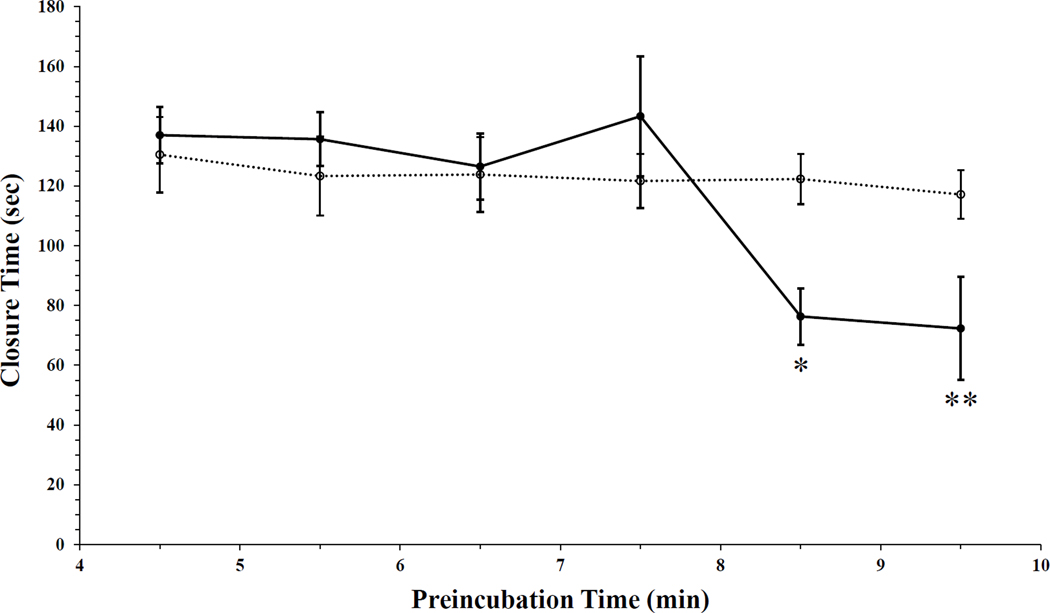
We assessed the effects of P. gingivalis on platelet function in whole blood. Previously it was observed that preincubation of P. gingivalis (1.87 × 107 CFU/mL) in whole blood significantly shortened the clotting time of whole blood (unpublished results). Consequently, we undertook to find the optimal bacterial preincubation time that would maximally shorten the whole blood PFA-100 CTs. Whole blood was pretreated with P. gingivalis for varying durations, followed by measurements of aperture CTs using the PFA-100 (Fig. 1). Similar CTs were observed for preincubation times of 4.5, 5.5, and 6.5 minutes between blood samples preincubated with PBS and those preincubated with suspensions of P. gingivalis. A slight increase in CTs was observed following a 7.5-minute bacterial preincubation, but this did not achieve statistical significance (Fig. 1). However, the duration of whole blood preincubation with P. gingivalis did significantly impact CTs (Fig. 1, P = 0.003). Compared to the 7.5-minute preincubation, significant reductions in CTs followed the 8.5-minute (P = 0.017) and 9.5-minute (P= 0.008) preincubation treatments (Fig. 1). Lastly, a significant interaction effect was also measured between P. gingivalis and preincubation time (Fig. 1, P = 0.019).
Figure 1. In the presence of P. gingivalis whole blood closure times are reduced.
P. gingivalis wild type strain, W83, was preincubated in whole blood at 37°C for six different time points (●). Following pretreatment with the oral pathogen, time to form a platelet plug and occlude the kit aperture was assessed via PFA-100, n = 6. The concentration of P. gingivalis added into whole blood was 1.87 × 107 CFU/mL for all time points. In control samples, whole blood was pre-incubated with only PBS for all six time points (○). Time for the platelet plug to occlude the cartridge aperture is presented as mean ± SEM. Analysis demonstrated preincubation time significantly impacted CTs (Two-way ANOVA, P = 0.003). A significant interaction effect was measured between P. gingivalis and preincubation time (Two-way ANOVA, P = 0.019). Comparison of 8.5 or 9.5-minute preincubation to 7.5 minutes demonstrated significantly decreased CTs (Two-way ANOVA, *P = 0.017 and **P = 0.008).
Differential responses of platelets to P. gingivalis exposure.
Previous studies demonstrated that a minimal bacterial concentration was necessary to elicit platelet aggregation (Kerrigan et al., 2010). Such concentration dependent platelet aggregation was observed for isolated platelets stimulated with P. gingivalis, so that a 1:1 platelet-bacteria ratio induced 80% aggregation (Klarström et al., 2015). Furthermore, platelet aggregation is also characterized by a distinct lag phase in response to bacterial stimulation. Diluting P. gingivalis increased lag times and reduced platelet aggregation in platelet rich plasma (PRP), further supporting bacterial concentration dependence for aggregation (Pham et al., 2002). We investigated the role of bacterial concentration and preincubation time on platelet plug formation in whole blood under shear stress. Varying concentrations of P. gingivalis were preincubated with donor blood for one of three different durations: 4.5, 8.5 and 15.5 minutes. Six different bacterial concentrations were tested for each preincubation time.
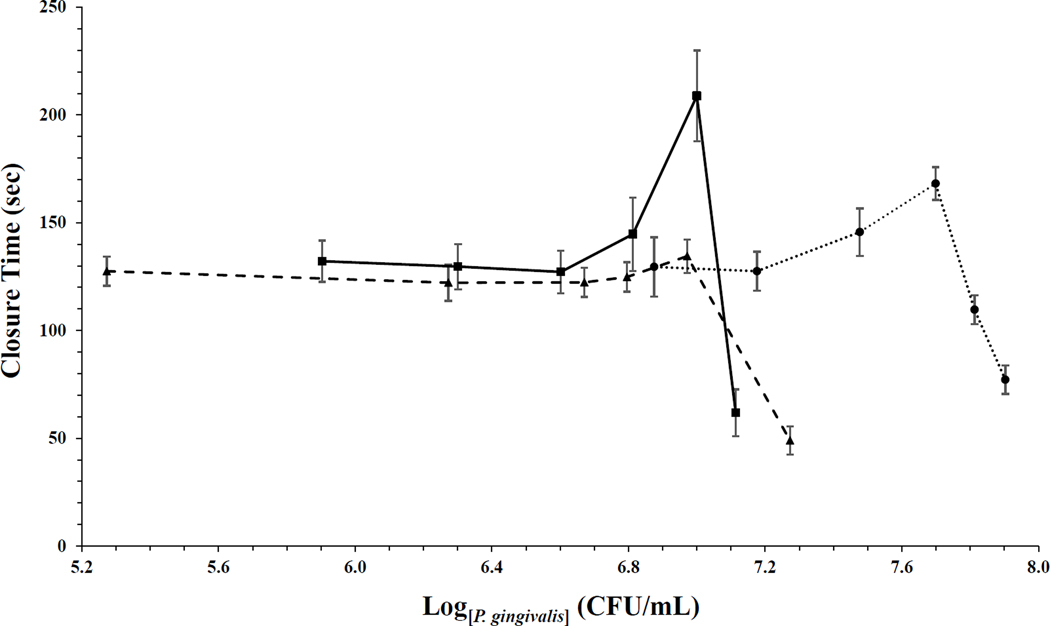
As the bacterial concentration was increased, a prolongation in CTs was unexpectedly observed, before the final significant CT reduction. For the 4.5-minute bacterial pretreatment, CTs gradually increased almost 30% with increasing bacterial burden, prior to the final abrupt reduction. The longest measured CT was almost 170 seconds. This was followed by decreased CTs for the two highest bacterial concentrations, with the shortest CT measuring less than 80 seconds, a reduction by over 50% (Fig. 2). Similar results were observed when P. gingivalis was preincubated in whole blood for 8.5 minutes. A modest increase in the CT, to 135 seconds, was observed immediately before the final CT shortening by 64% to 49 seconds. By extending P. gingivalis preincubation in whole blood to 15.5 minutes the subsequent CTs were prolonged to 209 seconds. Comparison of the data distributions for the 8.5 and 15.5-minute preincubations demonstrated they were significantly different from each other (Fig. 2, P = 0.023). When adjusting for P. gingivalis concentrations, the CTs observed after 15.5 minutes were significantly higher compared to CTs measured after 8.5 minutes preincubation (Fig. 2, P = 0.032).
Figure 2. P. gingivalis reduces whole blood CTs in a concentration and time dependent manner.
Whole blood was pretreated with W83, P. gingivalis wild type strain, at 37°C for three different time points: 4.5 minutes (●), 8.5 minutes (▲), and 15.5 minutes (■). Aperture CTs by a platelet plug was measured with the PFA-100 after bacterial preincubation, n = 6. Six different P. gingivalis concentrations (CFU/mL) was preincubated in whole blood at each time point, concentration calculation was based on previous P. gingivalis W83 colony count assays. CTs are presented as mean ± SEM. The distribution of 8.5-minute preincubation was significant different from 15.5-minute preincubation (Kolmogorov-Smirnov two-sample test, P = 0.023). When adjusting for P. gingivalis, comparison of 8.5 to 15.5-minute preincubation time demonstrated significant differences in CTs (Two-way ANCOVA test, P = 0.032).
Together, prior to shortening, CTs first tended to increase with increasing bacterial load for all preincubation times. However, the most pronounced prolongation of the CT was observed with preincubation of 15.5 minutes. This suggests that distinct or possibly competing time dependent functional processes are occurring, in the context of whole blood, in response to P. gingivalis.
Discussion
P. gingivalis is an opportunistic pathogen, primarily found in the oral cavity. This environment is hostile to bacteria because it is constantly bathed in physiological fluids, such as saliva and gingival crevicular fluid, which contain antimicrobial peptides and proteins (Khurshid et al., 2016, Marsh et al., 2016). This periodontal microbe, in turn, produces a broad spectrum of virulence factors that play a significant role in bacterial survival within this host setting. Through coordinated contributions of these pathogenic agents, P. gingivalis can successfully adhere to and colonize subgingival tissues, while subverting the host immune response to avoid bacterial clearance (Hajishengallis et al., 2014, Zenobia et al., 2015). Chronic infection leads to development of biofilm between the tooth and gingival tissue (Kuboniwa et al., 2010). Subgingival biofilm promotes persistent proinflammatory conditions, clinically characterized by irreversible destruction of periodontal tissues as well as alveolar bone loss (Graves, 2008, Moutsopoulos et al., 2014). Tissue damage from oral hygiene care, or dental procedures, may allow P. gingivalis to access systemic circulation (Forner et al., 2006). It can then easily spread to distant areas inducing further inflammatory responses.
Platelets are among the early responders to endothelial lesions commonly associated with bacterial infections, such as infective endocarditis (Jung et al., 2012). However, platelet responses to P. gingivalis in whole blood are not yet well understood. Platelet plug formation comprises three responses: platelet adhesion, then activation, and finally aggregation (Broos et al., 2011). Adhesion of platelets to the exposed extracellular matrix proteins initiates activation signaling pathways resulting in two important downstream effects. First, platelets undergo a morphological change, transforming from a flat discoid shape into an activated amoeboid-like form with pseudopodia. Activation of platelets triggers a conformational change in GPIIb/IIIa receptors, from an inactive to active state, so that then they can mediate platelet-platelet interactions at vascular damage sites (Bennett, 2015). These physiologic responses contribute to stabilization of the platelet aggregates that develop during platelet plug formation. Second, adherent platelets release crucial physiological agonists, such as ADP, from their granules, creating a positive feedback that amplifies the initial activation signal (Gachet, 2001). This plays a central role in sustaining platelet aggregation by recruiting additional platelets from circulation to the injury site. Previous reports demonstrated that P. gingivalis is an efficient platelet activator under in vitro conditions or for in vivo animal models. When challenged by P. gingivalis, isolated platelet suspensions became activated, as demonstrated by enhanced cytosolic calcium levels (Klarström et al., 2015). Mobilization and release of intracellular calcium is associated with actin reorganization observed in platelet morphological changes (Hartwig, 1992) and with activation of GPIIb/IIIa receptors (Nesbitt et al., 2003). P. gingivalis derived gingipain proteases can activate platelets via cleavage of PARs (Lourbakos et al., 2001). Alternatively, a gingipain independent mechanism was also proposed to induce platelet aggregation in the presence of P. gingivalis (Naito et al., 2006). Pretreatment of PRP with recombinant Hgp44, an adhesin domain expressed on P. gingivalis surface, induces platelet aggregation. PRP treated with monoclonal antibodies, directed against GPIIb/IIIa or FcγRIIa (platelet IgG receptor), also inhibited platelet aggregation following P. gingivalis incubation. Consistent with this, PRP depletion of plasma IgG reactive to P. gingivalis reduced platelet aggregation. Together, these findings imply a contributing role of immune receptor interactions between platelet expressed FcγRIIa and IgG opsonized P. gingivalis for platelet aggregation. Consistent with these reports, increased platelet aggregation was observed in response to collagen stimulation, following intravenous infusion of P. gingivalis into rats (Yu et al., 2011).
Bacterial stimuli tend to elicit a distinct platelet response, rapid aggregation in an ‘all-or-nothing’ fashion that develops into maximal aggregation (Watson et al., 2016). Consistent with this, using PFA-100 CTs, platelet plug formation for 80% whole blood was dependent on the duration of bacterial preincubation. CTs became significantly reduced when the preincubation of whole blood with P. gingivalis was extended past 8 minutes. However, some aspects of platelet aggregation are dependent on the blood concentration, bacterial concentration and measurement technique. Using lumiaggregometry, P. gingivalis added to 50% whole blood initiated platelet aggregation in 5–6 minutes, reaching maximal aggregation in approximately 15 minutes (Börgeson et al., 2011). Nevertheless, regardless of the technique employed, pretreating whole blood with P. gingivalis can prime platelets by initiating activation signaling pathways.
When PRP was incubated with P. gingivalis and followed by epinephrine stimulation, elevated cytosolic calcium levels were observed (Nylander et al., 2008). This suggested that the oral pathogen sensitized platelets to epinephrine mediated activation. Similarly, platelets may become predisposed to the activating effects of ADP or other platelet agonists. Prior exposure to the oral pathogen appears to potentiate platelet function following subsequent interactions with a secondary stimulus. A similar priming mechanism was suggested to describe neutrophils and the role of priming in their oxidative burst (El‐Benna et al., 2016).
When platelets were challenged with bacteria, a distinct lag phase preceded platelet aggregation (Kerrigan et al., 2007). This delay in aggregation was affected by the platelet to bacteria ratio, where increasing bacterial concentration reduced lag times (Petersson et al., 2018, Rasmussen et al., 2010). Bacterial interaction with platelets can occur via two main mechanisms: direct and indirect. Direct bacterial binding to platelet receptors is characteristic of shorter platelet aggregation lag times (< 8 min) (Byrne et al., 2003, Kerrigan et al., 2002, O’Brien et al., 2002). Alternatively, indirect binding tends to induce platelet aggregation after an extended lag period (8 min < time < 20 min) (Kerrigan et al., 2002, Kerrigan et al., 2007). This suggests that the observed length of lag phase prior to occlusion of cartridge aperture may be dependent on the mechanism of interaction between P. gingivalis and platelets. However, these effects are likely also regulated by surface expression of platelet receptors and P. gingivalis virulence factors. High receptor-ligand binding affinity and increased density of activated platelet receptors can trigger a more robust platelet response, resulting in a relatively shorter lag time.
Platelet plug formation in whole blood is affected by bacterial concentration and the length of preincubation. To some extent, reducing the preincubation duration may be compensated for by increasing the CFU count. With a relatively short preincubation time of 4.5 minutes, the CTs were reduced when P. gingivalis concentration was relatively higher, at 8.00 × 107 CFU/mL. Significant reductions in CTs were observed at a 77% lower P. gingivalis concentration (1.87 × 107 CFU/mL) following an extension of bacterial pretreatment to 8.5 minutes. Further prolonging P. gingivalis preincubation in whole blood, to 15.5 minutes, also reduced CTs in the presence of 84% lower P. gingivalis concentration (1.30 × 107 CFU/mL). Previous studies suggested a minimum concentration of S. aureus bacterial cells was necessary to trigger platelet aggregation (Miajlovic et al., 2007). Increasing surface expression of ClfA fibrinogen binding receptors was demonstrated to shorten the lag duration prior to S. aureus induced platelet aggregation (Loughman et al., 2005). During the platelet plug formation assay, for each preincubation duration, a bacterial count was reached that led to a reduced CT. However, it is still unclear what threshold level of P. gingivalis is required to effectively trigger platelet activation and platelet plug formation. Future studies investigating additional preincubation durations and bacterial concentrations may provide a deeper understanding into the impact of P. gingivalis on platelet function.
As P. gingivalis concentration increased, a distinct elevation in CTs was measured prior to the sharp decline in CTs observed for all tested preincubation durations. This suggests a more complicated platelet response repertoire as a reaction to the presence of P. gingivalis in whole blood. It may be that the P. gingivalis concentrations leading to prolonged CTs were sufficient to trigger platelet activation during preincubation, but insufficient to reach the threshold necessary to induce aggregation. As a result, some platelets may have been spent during preincubation, becoming unavailable for the functional platelet plug formation assay that followed. This may explain why observed platelet response to bacterial challenge appeared weaker than control CTs at certain bacterial concentrations. Different threshold levels of bacteria may be necessary to elicit platelet activation, compared to the levels needed for sustained platelet aggregation.
The goal of this study was to assess the effects of P. gingivalis on platelet function using a whole blood model with the PFA-100. Preincubation with P. gingivalis enhanced platelet plug formation under high shear conditions. Reduction in occlusion time of the cartridge aperture was dependent on both P. gingivalis concentration and preincubation time. The mechanism of action that P. gingivalis utilizes to modulate platelet function remains to be fully characterized. More detailed time course studies of P. gingivalis effects, while specifically tracking platelet activation marker expression and sustained aggregation events, could help to identify the pathways involved. Moreover, expression of virulence factors, including gingipains, plays a central role in P. gingivalis survival and the pathophysiology of periodontitis. PARs are a superfamily of transmembrane G protein-coupled receptors expressed on the surface of some cells including platelets (Kahn et al., 1999). Activation of PARs via gingipain mediated cleavage can trigger signaling pathways that elicit platelet activation and aggregation (Lourbakos et al., 2001). Future studies are necessary to assess the contributions from P. gingivalis produced virulence factors to platelet plug formation. Using specific gingipain knockout strains may provide further insights into mechanisms by which P. gingivalis impacts human platelet function in whole blood.
Acknowledgements
This project was supported in part by the Department of Basic Sciences, School of Medicine, Loma Linda University and Public Health Services Grants R-56-DE13664, DE019730, DE022508, DE022724 from NIDCR (to H.M.F). We would like to thank all the volunteers for taking time out of their busy schedule and donating blood to this study. We would like to thank Yuetan Dou for providing technical support on culturing methods for P. gingivalis. We also thank Karen Hay and Sheryl Aka, for assisting with and providing education on phlebotomy.
Footnotes
Disclosure of interest
The authors declare no conflict of interests.
References
- Arman M, Krauel K, Tilley DO, Weber C, Cox D, Greinacher A, et al. (2014). Amplification of bacteria-induced platelet activation is triggered by FcγRIIA, integrin αIIbβ3, and platelet factor 4. Blood, 123(20), 3166–3174. doi: 10.1182/blood-2013-11-540526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett JS (2015). Regulation of integrins in platelets. Biopolymers, 104(4), 323–333. doi: 10.1002/bip.22679 [DOI] [PubMed] [Google Scholar]
- Bensing BA, López JA, & Sullam PM (2004). The Streptococcus gordonii surface proteins GspB and Hsa mediate binding to sialylated carbohydrate epitopes on the platelet membrane glycoprotein Ibα. Infect Immun, 72(11), 6528–6537. doi: 10.1128/IAI.72.11.6528-6537.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergin SP, Holland TL, Fowler VG Jr., & Tong SYC (2017). Bacteremia, Sepsis, and Infective Endocarditis Associated with Staphylococcus aureus. Curr Top Microbiol Immunol, 409, 263–296. doi: 10.1007/82_2015_5001 [DOI] [PubMed] [Google Scholar]
- Blair P, & Flaumenhaft R (2009). Platelet alpha-granules: basic biology and clinical correlates. Blood Rev, 23, 177–189. doi: 10.1016/j.blre.2009.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair P, Rex S, Vitseva O, Beaulieu L, Tanriverdi K, Chakrabarti S, et al. (2009). Stimulation of Toll‐like receptor 2 in human platelets induces a thromboinflammatory response through activation of phosphoinositide 3‐kinase. Circ Res, 104, 346–354. doi: 10.1161/CIRCRESAHA.108.185785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böck M, De Haan J, Beck KH, Gutensohn K, Hertfelder HJ, Karger R, et al. (1999). Standardization of the PFA-100 platelet function test in 105 mmol/l buffered citrate: effect of gender, smoking and oral contraceptives. Br J Haematol, 106, 898–904. doi: 10.1046/j.1365-2141.1999.01660.x [DOI] [PubMed] [Google Scholar]
- Börgeson E, Lönn J, Bergström I, Brodin VP, Ramström S, Nayeri F, et al. (2011). Lipoxin A4 inhibits Porphyromonas gingivalis-induced aggregation and reactive oxygen species production by modulating neutrophil-platelet interaction and CD11b expression. Infect Immun, 79(4), 1489–97. doi: 10.1128/IAI.00777-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broos K, Feys HB, De Meyer SF, Vanhoorelbeke K, & Deckmyn H (2011). Platelets at work in primary hemostasis. Blood Rev, 25(4), 155–167. doi: 10.1016/j.blre.2011.03.002 [DOI] [PubMed] [Google Scholar]
- Byrne MF, Kerrigan SW, Corcoran PA, Atherton JC, Murray FE, Fitzgerald DJ, et al. (2003). Helicobacter pylori binds von Willebrand factor and interacts with GPIb to induce platelet aggregation. Gastroenterology, 124, 1846–1854. doi: 10.1016/s0016-5085(03)00397-4 [DOI] [PubMed] [Google Scholar]
- Canobbio I, Balduini C, & Torti M (2004). Signalling through the platelet glycoprotein Ib-V-IX complex. Cell Signal, 16, 1329–1344. doi: 10.1016/j.cellsig.2004.05.008 [DOI] [PubMed] [Google Scholar]
- Chen H, Locke D, Liu Y, Liu C, & Kahn ML (2002). The platelet receptor GPVI mediates both adhesion and signaling responses to collagen in a receptor density-dependent fashion. J Biol Chem, 277, 3011–3019. doi: 10.1074/jbc.M109714200 [DOI] [PubMed] [Google Scholar]
- Chen T, Nakayama K, Belliveau L, & Duncan MJ (2001). Porphyromonas gingivalis gingipains and adhesion to epithelial cells. Infect Immun, 69, 3048–3056. doi: 10.1128/IAI.69.5.3048-3056.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chukkapalli SS, Velsko IM, Rivera-Kweh MF, Zheng D, Lucas AR, & Kesavalu L (2015). Polymicrobial oral infection with four periodontal bacteria orchestrates a distinct inflammatory response and atherosclerosis in ApoEnull mice. PLoS One, 10(11), e0143291. doi: 10.1371/journal.pone.0143291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Benna J, Hurtado‐Nedelec M, Marzaioli V, Marie JC, Gougerot‐Pocidalo MA, & Dang PM (2016). Priming of the neutrophil respiratory burst: role in host defense and inflammation. Immunol Rev, 273(1), 180–93. doi: 10.1111/imr.12447 [DOI] [PubMed] [Google Scholar]
- Engelmann B, & Massberg S (2013). Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol, 13(1), 34–45. doi: 10.1038/nri3345 [DOI] [PubMed] [Google Scholar]
- Favaloro EJ (2008). Clinical utility of the PFA‐100. Semin Thromb Hemost, 34, 709–733. doi: 10.1055/s-0029-1145254 [DOI] [PubMed] [Google Scholar]
- Forner L, Larsen T, Kilian M, & Holmstrup P (2006). Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. J Clin Periodontol, 33, 401–407. doi: 10.1111/j.1600-051X.2006.00924.x [DOI] [PubMed] [Google Scholar]
- Gachet C (2001). ADP receptors of platelets and their inhibition. Thromb Haemost, 86, 222–232. doi: 10.1055/s-0037-1616220 [DOI] [PubMed] [Google Scholar]
- Graves D (2008). Cytokines that promote periodontal tissue destruction. J Periodontol, 79, 1585–1591. doi: 10.1902/jop.2008.080183 [DOI] [PubMed] [Google Scholar]
- Hajishengallis G, Darveau RP, & Curtis MA (2012). The keystone-pathogen hypothesis. Nature Rev Microbiol, 10, 717–725. doi: 10.1038/nrmicro2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, & Lamont RJ (2014). Breaking bad: Manipulation of the host response by Porphyromonas gingivalis. Eur J Immunol, 44(2), 328–38. doi: 10.1002/eji.201344202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig JH (1992). Mechanisms of actin rearrangements mediating platelet activation. J Cell Biol, 118, 1421–42. doi: 10.1083/jcb.118.6.1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haurat MF, Aduse-Opoku J, Rangarajan M, Dorobantu L, Gray MR, Curtis MA, et al. (2011). Selective sorting of cargo proteins into bacterial membrane vesicles. J Biol Chem, 286, 1269–1276. doi: 10.1074/jbc.M110.185744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi C, Viereck J, Hua N, Phinikaridou A, Madrigal AG, Gibson III FC, et al. (2011). Porphyromonas gingivalis accelerates inflammatory atherosclerosis in the innominate artery of ApoE deficient mice. Atherosclerosis, 215(1), 52–9. doi: 10.1016/j.atherosclerosis.2010.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley SM, Lutay N, Holmqvist B, & Shannon O (2016). The dynamics of platelet activation during the progression of streptococcal sepsis. PLoS One, 11(9), e0163531. doi: 10.1371/journal.pone.0163531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Icli A, Tayyar S, Varol E, Aksoy F, Arslan A, Ersoy I, et al. (2013). Mean platelet volume is increased in infective endocarditis and decreases after treatment. Med Princ Pract, 22, 270–273. doi: 10.1159/000345393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung CJ, Yeh CY, Shun CT, Hsu RB, Cheng HW, Lin CS, et al. (2012). Platelets enhance biofilm formation and resistance of endocarditis-inducing streptococci on the injured heart valve. J Infect Dis, 205(7), 1066–1075. doi: 10.1093/infdis/jis021 [DOI] [PubMed] [Google Scholar]
- Kahn ML, Nakanishi‐Matsui M, Shapiro MJ, Ishihara H, & Coughlin SR (1999). Protease‐activated receptors 1 and 4 mediate activation of human platelets by thrombin. J Clin Invest, 103, 879–87. doi: 10.1172/JCI6042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerrigan SW, & Cox D (2010). Platelet–bacterial interactions. Cell Mol Life Sci, 67(4), 513–23. doi: 10.1007/s00018-009-0207-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerrigan SW, Douglas I, Wray A, Heath J, Byrne MF, Fitzgerald D, et al. (2002). A role for glycoprotein Ib in Streptococcus sanguis-induced platelet aggregation. Blood, 100, 509–516. doi: 10.1182/blood.v100.2.509 [DOI] [PubMed] [Google Scholar]
- Kerrigan SW, Jakubovics NS, Keane C, Maguire P, Wynne K, Jenkinson HF, et al. (2007). Role of Streptococcus gordonii surface proteins SspA/SspB and Hsa in platelet function. Infect Immun, 75, 5740–5747. doi: 10.1128/IAI.00909-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurshid Z, Naseem M, Sheikh Z, Najeeb S, Shahab S, & Zafar MS (2016). Oral antimicrobial peptides: types and role in the oral cavity. Saudi Pharm J, 24, 515–524. doi: 10.1016/j.jsps.2015.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Kim SJ, Lee MJ, Kwon YE, Kim YL, Park KS, et al. (2015). An increase in mean platelet volume from baseline is associated with mortality in patients with severe sepsis or septic shock. PLoS One, 10, e0119437. doi: 10.1371/journal.pone.0119437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarström Engström K, Khalaf H, Kälvegren H, & Bengtsson T (2015). The role of Porphyromonas gingivalis gingipains in platelet activation and innate immune modulation. Mol Oral Microbiol, 30(1), 62–73. doi: 10.1111/omi.12067 [DOI] [PubMed] [Google Scholar]
- Kuboniwa M, & Lamont RJ (2010). Subgingival biofilm formation. Periodontol 2000, 52(1), 38–52. doi: 10.1111/j.1600-0757.2009.00311.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu SK, Heilmann EJ, Sio R, Garcia C, Davidson RM, & Ostgaard RA (1995). Description of an in vitro platelet function analyzer PFA-100. Semin Thromb Hemost, 21, 106–12. doi: 10.1055/s-0032-1313612 [DOI] [PubMed] [Google Scholar]
- Li X, Kolltveit KM, Tronstad L, Olsen I (2000) Systemic diseases caused by oral infection. Clin Microbiol Rev, 13(4), 547–58. doi: 10.1128/cmr.13.4.547-558.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Delaney MK, O’Brien KA, & Du X (2010). Signaling during platelet adhesion and activation. Arterioscler Thromb Vasc Biol, 30, 2341–2349. doi: 10.1161/ATVBAHA.110.207522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughman A, Fitzgerald JR, Brennan MP, Higgins J, Downer R, Cox D, et al. (2005). Roles for fibrinogen, immunoglobulin and complement in platelet activation promoted by Staphylococcus aureus clumping factor A. Mol Microbiol, 57, 804–818. doi: 10.1111/j.1365-2958.2005.04731.x [DOI] [PubMed] [Google Scholar]
- Lourbakos A, Potempa J, Travis J, D’Andrea MR, Andrade-Gordon P, Santulli R, et al. (2001). Arginine-specific protease from Porphyromonas gingivalis activates protease-activated receptors on human oral epithelial cells and induces interleukin-6 secretion. Infect Immun, 69, 5121–5130. doi: 10.1128/IAI.69.8.5121-5130.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourbakos A, Yuan YP, Jenkins AL, Travis J, Andrade-Gordon P, Santulli R, et al. (2001). Activation of protease-activated receptors by gingipains from Porphyromonas gingivalis leads to platelet aggregation: a new trait in microbial pathogenecity. Blood, 97, 3790–3797. doi: 10.1182/blood.v97.12.3790 [DOI] [PubMed] [Google Scholar]
- Marsh PD, Do T, Beighton D, & Devine DA (2016). Influence of saliva on the oral microbiota. Periodontol 2000, 70, 80–92. doi: 10.1111/prd.12098 [DOI] [PubMed] [Google Scholar]
- McKenzie RM, Henry LG, Boutrin MC, Ximinies A, & Fletcher HM (2016). Role of the Porphyromonas gingivalis iron-binding protein PG1777 in oxidative stress resistance. Microbiology, 162, 256–267. doi: 10.1099/mic.0.000213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejer N, Westh H, Schønheyder HC, Jensen AG, Larsen AR, Skov R, et al. (2014). Increased risk of venous thromboembolism within the first year after Staphylococcus aureus bacteraemia: a nationwide observational matched cohort study. J Intern Med, 275, 387–397. doi: 10.1111/joim.12147 [DOI] [PubMed] [Google Scholar]
- Miajlovic H, Loughman A, Brennan M, Cox D, & Foster TJ (2007). Both complement- and fibrinogen-dependent mechanisms contribute to platelet aggregation mediated by Staphylococcus aureus clumping factor B. Infect Immun, 75, 3335–3343. doi: 10.1128/IAI.01993-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutsopoulos NM, Konkel J, Sarmadi M, Eskan MA, Wild T, Dutzan N, et al. (2014). Defective neutrophil recruitment in leukocyte adhesion deficiency type I disease causes local IL-17-driven inflammatory bone loss. Sci Transl Med, 6, 229ra40. doi: 10.1126/scitranslmed.3007696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito M, Sakai E, Shi Y, Ideguchi H, Shoji M, Ohara N, et al. (2006). Porphyromonas gingivalis‐induced platelet aggregation in plasma depends on Hgp44 adhesin but not Rgp proteinase. Mol Microbiol, 59, 152–167. doi: 10.1111/j.1365-2958.2005.04942.x [DOI] [PubMed] [Google Scholar]
- Nesbitt WS, Giuliano S, Kulkarni S, Dopheide SM, Harper IS, & Jackson SP (2003). Intercellular calcium communication regulates platelet aggregation and thrombus growth. J Cell Biol, 160, 1151–1161. doi: 10.1083/jcb.200207119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylander M, Lindahl TL, Bengtsson T, & Grenegård M (2008). The periodontal pathogen Porphyromonas gingivalis sensitises human blood platelets to epinephrine. Platelets, 19, 352–358. doi: 10.1080/09537100802056102 [DOI] [PubMed] [Google Scholar]
- O’Brien L, Kerrigan SW, Kaw G, Hogan M, Penadés J, Litt D, et al. (2002). Multiple mechanisms for the activation of human platelet aggregation by Staphylococcus aureus: roles for the clumping factors ClfA and ClfB, the serine-aspartate repeat protein SdrE and protein A. Mol Microbiol, 44, 1033–1044. doi: 10.1046/j.1365-2958.2002.02935.x [DOI] [PubMed] [Google Scholar]
- Petersen HJ, Keane C, Jenkinson HF, Vickerman MM, Jesionowski A, Waterhouse J et al. (2010). Human platelets recognize a novel surface protein, PadA, on Streptococcus gordonii through a unique interaction involving fibrinogen receptor GPIIbIIIa. Infect Immun, 78(1), 413–422. doi: 10.1128/IAI.00664-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersson F, Kilsgård O, Shannon O, & Lood R (2018). Platelet activation and aggregation by the opportunistic pathogen Cutibacterium (Propionibacterium) acnes. PLoS One, 13, e0192051. doi: 10.1371/journal.pone.0192051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham K, Feik D, Hammond BF, Rams TE, & Whitaker EJ (2002). Aggregation of human platelets by gingipain-R from Porphyromonas gingivalis cells and membrane vesicles. Platelets, 13(1), 21–30. doi: 10.1080/09537100120104863 [DOI] [PubMed] [Google Scholar]
- Rasmussen M, Johansson D, Söbirk SK, Mörgelin M, & Shannon O (2010). Clinical isolates of Enterococcus faecalis aggregate human platelets. Microbes Infect, 12, 295–301. doi: 10.1016/j.micinf.2010.01.005 [DOI] [PubMed] [Google Scholar]
- Rivera MF, Lee JY, Aneja M, Goswami V, Liu L, Velsko IM, et al. (2013). Polymicrobial infection with major periodontal pathogens induced periodontal disease and aortic atherosclerosis in hyperlipidemic ApoEnull mice. PLoS One, 8(2), e57178. doi: 10.1371/journal.pone.0057178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semeraro N, Ammollo CT, Semeraro F, & Colucci M (2010). Sepsis-associated disseminated intravascular coagulation and thromboembolic disease. Mediterr J Hematol Infect Dis, 2, e2010024. doi: 10.4084/MJHID.2010.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharda A, & Flaumenhaft R (2018). The life cycle of platelet granules. F1000Res, 7, 236. doi: 10.12688/f1000research.13283.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Novak EK, Sojar HT, Swank RT, Kuramitsu HK, & Genco RJ (2000). Porphyromonas gingivalis platelet aggregation activity: outer membrane vesicles are potent activators of murine platelets. Oral Microbiol Immunol, 15, 393–396. doi: 10.1034/j.1399-302x.2000.150610.x [DOI] [PubMed] [Google Scholar]
- Shattil SJ, Hoxie JA, Cunningham M, & Brass LF (1985). Changes in the platelet membrane glycoprotein IIb.IIIa complex during platelet activation. J Biol Chem, 260(20), 11107–11114. [PubMed] [Google Scholar]
- Stegner D, vanEeuwijk JMM, Angay O, Gorelashvili MG, Semeniak D, Pinnecker J, et al. (2017). Thrombopoiesis is spatially regulated by the bone marrow vasculature. Nat Commun, 8(1), 127. doi: 10.1038/s41467-017-00201-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CN, Kerrigan SW, Cox D, Henderson IR, Watson SP, & Arman M (2016). Human platelet activation by Escherichia coli: Roles for FcγRIIA and integrin αIIbβ3. Platelets, 27, 535–540. doi: 10.3109/09537104.2016.1148129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JG (1968). Fine structural alterations induced in platelets by adenosine diphosphate. Blood, 31, 604–622. [PubMed] [Google Scholar]
- Yu KM, Inoue Y, Umeda M, Terasaki H, Chen ZY, & Iwai T (2011). The peridontal anaerobe Porphyromonas gingivalis induced platelet activation and increased aggregation in whole blood by rat model. Thromb Res, 127(5), 418–25. doi: 10.1016/j.thromres.2010.12.004 [DOI] [PubMed] [Google Scholar]
- Zenobia C, & Hajishengallis G (2015). Porphyromonas gingivalis virulence factors involved in subversion of leukocytes and microbial dysbiosis. Virulence, 6(3), 236–43. doi: 10.1080/21505594.2014.999567 [DOI] [PMC free article] [PubMed] [Google Scholar]