Abstract
The maltose transport system in Escherichia coli is a member of the ATP-binding cassette superfamily of transporters that is defined by the presence of two nucleotide-binding domains or subunits and two transmembrane regions. The bacterial import systems are unique in that they require a periplasmic substrate-binding protein to stimulate the ATPase activity of the transport complex and initiate the transport process. Upon stimulation by maltose-binding protein, the intact MalFGK2 transport complex hydrolyzes ATP with positive cooperativity, suggesting that the two nucleotide-binding MalK subunits interact to couple ATP hydrolysis to transport. The ATPase activity of the intact transport complex is inhibited by vanadate. In this study, we investigated the mechanism of inhibition by vanadate and found that incubation of the transport complex with MgATP and vanadate results in the formation of a stably inhibited species containing tightly bound ADP that persists after free vanadate and nucleotide are removed from the solution. The inhibited species does not form in the absence of MgCl2 or of maltose-binding protein, and ADP or another nonhydrolyzable analogue does not substitute for ATP. Taken together, these data conclusively show that ATP hydrolysis must precede the formation of the vanadate-inhibited species in this system and implicate a role for a high-energy, ADP-bound intermediate in the transport cycle. Transport complexes containing a mutation in a single MalK subunit are still inhibited by vanadate during steady-state hydrolysis; however, a stably inhibited species does not form. ATP hydrolysis is therefore necessary, but not sufficient, for vanadate-induced nucleotide trapping.
Maltose transport across the cytoplasmic membrane of Escherichia coli is mediated by a periplasmic maltose-binding protein (MBP) that directs maltose to a membrane-associated transport complex (MalFGK2) that contains two transmembrane-spanning proteins, MalF and MalG, and two copies of an ATP-binding protein, MalK (4, 10). Transport activity has been reconstituted in proteoliposomes from purified components, demonstrating that ATP is hydrolyzed by MalFGK2 during translocation (10). The MalFGK2 complex is a member of the ATP-binding cassette (ABC) superfamily, also known as traffic ATPases (13, 18). ABC proteins serve important functions in both prokaryotes and eukaryotes, and mutations in genes encoding ABC proteins are associated with several disease states, including cystic fibrosis, macular dystrophy, adrenoleukodystrophy, and hyperinsulinemia (1, 19, 24, 26). The development of multiple drug resistance in tumors following chemotherapy is sometimes associated with overproduction of another ABC protein, P-glycoprotein (Pgp), which mediates the ATP-dependent efflux of chemotherapeutic drugs from tumor cells (6).
The conservation of two nucleotide-binding domains or subunits in the ABC family suggests that both are required for function. In the maltose transport system, mutations in a single MalK ATPase subunit reduce both maltose transport and ATPase activities to less than 10% of the wild-type levels (11). ATP is hydrolyzed by MalFGK2 with positive cooperativity (8), indicating that the two subunits interact.
Further insight into the structure and function of the maltose transport system can be gained by analyzing the mechanism of inhibition by vanadate. Orthovanadate is an analog of inorganic phosphate, and previous work delineated two different mechanisms by which vanadate inhibits ATPases. In the myosin and dynein ATPases, vanadate acts by trapping, or occluding, nucleotide at the active site (15, 29). In the three-dimensional structure of myosin complexed with vanadate, ADP lies in the nucleotide-binding pocket with vanadate in the position where the gamma phosphate of ATP would normally lie (31). The vanadium appears to interact with five oxygens, mimicking the gamma phosphate during the transition state for ATP hydrolysis. In the Na+/K+-transport ATPase, vanadate inhibits by binding to the phosphorylation site in the absence of nucleotide, stabilizing a low-energy conformation of the transporter (22). As with myosin, inhibition of Pgp by vanadate involves trapping of nucleotide (37). Nucleotide can be trapped in either of the two nucleotide-binding sites, suggesting that both are catalytic. Furthermore, complete inhibition of ATPase activity occurs when only one nucleotide-binding site is occupied, which suggests that when one site takes on the catalytic conformation, the other cannot hydrolyze ATP (36). The cystic fibrosis transmembrane regulator exhibits vanadate-induced trapping of nucleotide only in the carboxyl-terminal nucleotide-binding site, and the amino-terminal site binds nucleotide tightly in the absence of vanadate, suggesting that the two sites function differently in this ABC transporter (32).
In this study, we investigated the interaction between vanadate and the maltose transport system and found that nucleotide occlusion also occurs in MalFGK2 but only in the presence of vanadate. Because the maltose transport complex is catalytically inactive in the absence of the periplasmic MBP, we can for the first time directly assess the requirement for ATP hydrolysis in vanadate trapping. The results are consistent with the trapping of a transition-state analogue in the nucleotide-binding site and provide evidence for a high-energy ADP-bound intermediate in the translocation cycle.
MATERIALS AND METHODS
Purification and reconstitution of the maltose transport complex.
The maltose transport complex MalFGK2, modified with a polyhistidine tag at the N terminus of MalK, was overexpressed in E. coli essentially as described previously (10, 11), except that expression was induced with isopropyl-β-d-thiogalactopyranoside (IPTG) at a final concentration of 0.01 mM and cells were grown overnight at 23°C. The complexes were purified by metal affinity chromatography as described previously (11) with the following modifications. Detergent-solubilized transport complexes were bound to Talon affinity resin (Clontech) equilibrated with buffer containing 20 mM sodium HEPES (pH 8), 100 mM NaCl, 10% glycerol, and 0.01% n-dodecyl-β-d-maltoside. The column was washed with the equilibration buffer, and then the transport complex was eluted in the same buffer containing 100 mM imidazole. Preparations were >90% pure as judged by Coomassie blue staining of sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels (data not shown). For reconstitution, purified complexes were dialyzed to remove imidazole and mixed with sonicated Escherichia coli phospholipid at a lipid-to-protein ratio (milligrams/milligrams) of 50 in the presence of 1% octyl-β-d-glucopyranoside. Proteoliposomes were formed following dilution of detergent, as described previously (10), and either used fresh or stored frozen at −70°C in aliquots until use.
The isolated MalK protein was purified from strain BL21(DE3) carrying plasmid pMF8 by the procedure of Walter et al. (38). Plasmid pMF8 is a derivative of pT7-7 (33) with malK under control of pT7.
Assay of ATP hydrolysis.
Proteoliposomes containing purified, wild-type transport complexes were assayed at 37°C in the presence of 10 mM MgCl2, 5 μM MBP, 0.1 mM maltose, 20 mM sodium HEPES (pH 8), 1 mM dithiothreitol, and 1 mM [γ-32P]ATP. Liu et al. (21) established that proteoliposomes are freely permeable to binding protein and ATP in the presence of 10 mM MgCl2 at 37°C, allowing the efficient stimulation of ATP hydrolysis by MBP. Proteoliposomes containing mutant transport complexes that can transport maltose and hydrolyze ATP in the absence of binding protein (binding-protein-independent transport complexes) were assayed either at 23°C as described previously (8) or at 37°C with 10 mM MgCl2, as described for the wild type. A continuous coupled assay utilizing pyruvate kinase and lactate dehydrogenase (28) was used to assess the time course of inhibition of ATPase activity in the presence of vanadate. (Vanadate, under the conditions used, did not significantly affect the activities of the coupling enzymes.)
Vanadate-induced inhibition of ATPase activity.
Stock solutions of vanadate were prepared and used as described previously (15, 37). To induce the formation of a stably inhibited transport complex, proteoliposomes containing purified MalFGK2 were preincubated with the indicated concentrations of vanadate and nucleotide in 20 mM sodium HEPES (pH 8.0) for 20 min with either 2 mM MgCl2 at 23°C (nonpermeabilizing conditions) or 10 mM MgCl2 at 37°C (permeabilizing conditions). Prior to the assay, free vanadate and nucleotide concentrations were reduced either by diluting samples at least 100-fold in 20 mM sodium HEPES (pH 8)–0.1 mM EDTA, by gel filtration in the same buffer, or by dilution and centrifugation of the proteoliposomes at 400,000 × g for 10 min in a Beckman TL-100 ultracentrifuge. Following removal of vanadate, the samples were maintained at 4°C until used in the assay.
Estimation of the molar ratio of nucleotide to protein in the vanadate-trapped species.
To determine whether nucleotide was tightly bound to the transport complex, either [α-32P]ATP or [γ-32P]ATP was used in place of nonradioactive ATP and complete separation of bound from unbound nucleotide was obtained by gel filtration through 0.5- by 15-cm Sephadex G-50 columns. The concentration of ATP used was determined from the optical density of the sample at 258 nm, using an extinction coefficient of 15,400 M−1 cm−1. The specific radioactivity of ATP was determined by counting aliquots of the same stock solution. Protein concentrations were determined as described previously (10) by the method of Schaffner and Weissmann (27), assuming Mr values of 170,000 for MalFGK2 and 40,000 for MalK. The concentrations obtained by this method agreed well with an estimate of protein concentration made from the total amino acid analysis of purified transport complex in buffer with 0.01% n-dodecyl-β-d-maltoside (Protein Chemistry Core Facility, Baylor College of Medicine).
Thin-layer chromatography was performed on Cellulose PEI plates (J. T. Baker), using 1 M LiCl to separate ATP from ADP.
RESULTS
Vanadate inhibition of a binding-protein-independent transport complex.
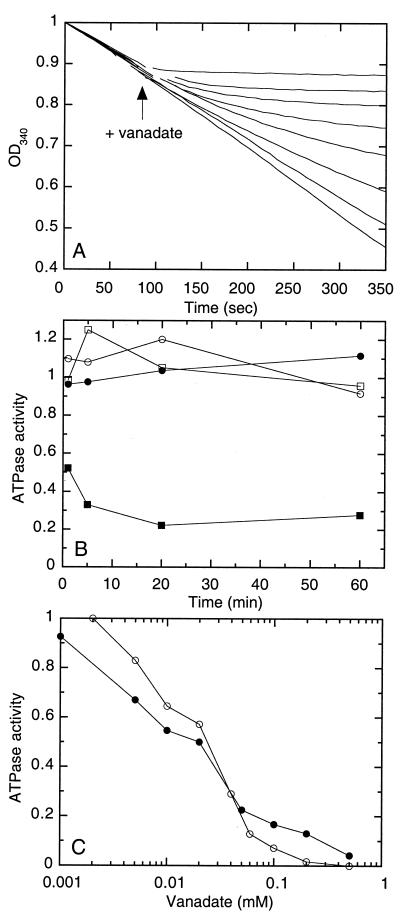
The purified maltose transport complex MalFGK2 is readily reconstituted into proteoliposomes where both maltose transport and ATPase activities can be measured (9, 12). In a wild-type system, both maltose transport and ATPase activities are tightly controlled by the soluble MBP. Mutations which allow maltose transport and ATP hydrolysis to occur in the absence of MBP have been isolated in the malF and malG genes (12, 30, 34). These mutant transport complexes, termed binding-protein-independent transport complexes, are hypothesized to stabilize the transport complex in a catalytically active conformation that is normally induced on interaction of MBP with the wild-type MalFGK2, and they have greatly facilitated the characterization of the ATPase activity of this transporter (8). Both the wild-type and binding-protein-independent transport complexes are inhibited by vanadate (8, 17), and a binding-protein-independent complex that displayed high ATPase activity, MalF500GK2, was used in the initial characterization of vanadate inhibition. The MalF500 protein contains two amino acid substitutions in the putative transmembrane domains, Gly to Arg at position 338 and Asp to Ile at position 501 (7), which allow transport to occur in the absence of MBP. Figure 1A shows the progress curves for ATP hydrolysis by the binding-protein-independent complex, utilizing a coupled assay. In the absence of vanadate, the rate of ATP hydrolysis was essentially linear until NADH was depleted. Addition of increasing concentrations of vanadate to the assay mixture leads to progressively greater inhibition of ATPase activity. Although mixing was complete within 10 s of the addition of vanadate, the reaction slowed in a time-dependent fashion, which is more characteristic of a slow-acting, irreversible inhibitor than of a classical reversible inhibitor.
FIG. 1.
Vanadate inhibition as a function of time and vanadate concentration. (A) ATP hydrolysis by proteoliposomes containing the binding-protein-independent MalF500GK2 complex was monitored by observing the decrease in absorbance at 340 nm (OD340) in a coupled assay. After approximately 90 s, vanadate was added directly to the assay cuvette (at the break in the curves). Curves, from top to bottom, are for experiments with 500, 200, 100, 60, 40, 20, 5, or 0 μM vanadate. (B) Proteoliposomes containing MalF500GK2 were incubated with 0.1 mM vanadate, 3 mM MgCl2, 1 mM ATP, and/or 1 mM ADP. At the indicated times, aliquots were removed, diluted 100-fold, and then assayed immediately or after 1 h at 23°C using the radioactive assay. ATPase activity in the absence of vanadate (∼3.0 μmol/min/mg at 23°C) is normalized to 1, and data are presented as a fraction of the uninhibited value. Each point is the mean of three separate determinations that typically varied within 0.1 of the mean. ●, Vanadate only; ○, MgCl2 plus vanadate; ■, MgCl2, ATP, plus vanadate; □, MgCl2, ADP, plus vanadate. (C) ○, Instantaneous velocity estimated 4 min after addition of different concentrations of vanadate to the assay mixtures in panel A. ●, ATPase activity of preparations preincubated in 1 mM MgATP with or without the indicated concentrations of vanadate for 20 min, diluted 100-fold, and assayed for ATPase activity. ATPase activity in the absence of vanadate (2.8 μmol/min/mg of protein) is normalized to 1, and data are presented as a fraction of the uninhibited value. Each point is the mean of three separate determinations that varied within 0.05 of the mean.
To detect the formation of a stably inhibited species, complexes were preincubated with 0.1 mM vanadate for the indicated time and then diluted 100-fold to reduce the effective concentration of vanadate to subinhibitory concentrations. Figure 1B shows that in the presence of ATP, a relatively rapid, time-dependent decrease in ATPase activity was observed, reaching completion within 5 to 20 min. In the absence of ATP, or if ADP was substituted for ATP, pretreatment with vanadate had no inhibitory effect on the ATPase activity, even after 1 h of incubation. As documented below, the inhibition was not reversed by dilution, gel filtration, or centrifugation and washing of proteoliposomes. The extent of inhibition was essentially the same whether the ATPase activity was measured immediately after dilution or following a prolonged incubation (1 to 5 h) at room temperature, indicating that the vanadate-inhibited form of the complex is stable (Fig. 1B and data not shown). An estimate of the actual lifetime of the inhibited species was complicated by the instability of the uninhibited transport complex to prolonged incubation (>24 h).
The fractions of initial activity remaining approximately 4 min after the addition of vanadate to the continuous-assay mixtures in Fig. 1A are plotted as a function of vanadate concentration in Fig. 1C. These data correlate well with estimates of the ATPase activity after proteoliposomes are preincubated with vanadate for 20 min and then diluted 100-fold prior to the assay. In both cases, 50% inhibition was observed at approximately 20 μM vanadate, with maximal inhibition at 100 to 200 μM. These results are similar to those reported previously for unpurified preparations (8).
The conditions for stable inhibition of the ATPase activity of the binding-protein-independent MalF500GK2 complex by vanadate were systematically analyzed by eliminating one or more components of the preincubation medium (Table 1). In addition to ATP, MgCl2 was required for inhibition. The nonhydrolyzable analogs ADP and β,γ-imidoadenosine 5′-triphosphate (AMPPNP) were not able to substitute for ATP in the formation of the stably inhibited species. Neither an increased ADP concentration (5 mM) nor an extended period of preincubation (5 h) resulted in significant inhibition of ATPase activity compared to that produced by control samples (data not shown). The requirements for Mg2+ and a hydrolyzable nucleotide suggest that ATP hydrolysis may be essential for vanadate inhibition.
TABLE 1.
Conditions for stable inhibition of the binding-protein-independent MalF500GK2 complexa
| Addition to preincubation medium
|
Relative ATPase activity after removal of vanadateb | ||
|---|---|---|---|
| MgCl2 | Nucleotide | Vanadate | |
| − | − | − | 1.0 |
| + | − | − | 1.1 |
| + | ATP | − | 1.1 |
| + | ATP | + | 0.20 |
| − | − | + | 1.1 |
| + | − | + | 0.96 |
| − | ATP | + | 0.85 |
| + | ADP | + | 0.96 |
| + | AMPPNP | + | 0.96 |
Proteoliposomes were preincubated at 23°C in 20 mM HEPES buffer (pH 8). Where indicated, 2 mM nucleotide, 0.1 mM vanadate, and/or 2 mM MgCl2 was added. Samples were diluted 100-fold and then assayed for ATPase activity.
The ATPase activity of the untreated sample (2.5 μmol/min/mg of protein at 37°C with 1 mM [32P]ATP) is normalized to 1, and data are presented as a fraction of the untreated sample. Each value is the mean of four separate determinations that varied within 0.1 of the mean.
Vanadate inhibition of the wild-type transport complex.
If ATP hydrolysis is essential for vanadate inhibition, then, in the wild-type system, stable inhibition by vanadate should occur only in the presence of MBP since ATP hydrolysis occurs only in the presence of MBP. Experiments similar to those in Table 1 for the binding-protein-independent transport complexes were repeated using the reconstituted wild-type transporter. In this series of experiments, 10 mM MgCl2 was present in all preincubations and nucleotide and MBP were varied (Table 2). If MBP was not present during pretreatment, no stable inhibition of the transport ATPase was observed either with vanadate alone or with vanadate in combination with ATP. If MBP was added during the preincubation to stimulate ATP hydrolysis, vanadate plus ATP resulted in stable inhibition of the ATPase activity. Like the binding-protein-independent complex, treatment with vanadate alone or ATP alone had no effect and the use of ADP or AMPPNP rather than ATP failed to generate a stably inhibited species. These results demonstrate that inhibition by vanadate requires ATP and suggest that the complex must hydrolyze ATP, or at least be capable of hydrolyzing ATP, to form the inhibited species.
TABLE 2.
Conditions for stable inhibition of the wild-type MalFGK2 complexa
| Addition to preincubation medium
|
Relative ATPase activity after removal of vanadateb | ||
|---|---|---|---|
| Nucleotide | Vanadate | MBP | |
| − | − | − | 1.0 |
| ATP | − | − | 1.1 |
| − | + | − | 0.88 |
| − | − | + | 1.1 |
| − | + | + | 0.91 |
| ATP | − | + | 1.1 |
| ATP | + | − | 0.99 |
| ATP | + | + | 0.50 |
| ADP | + | − | 1.0 |
| ADP | + | + | 1.0 |
| AMPPNP | + | − | 1.0 |
| AMPPNP | + | + | 0.94 |
Proteoliposomes were preincubated for 20 min at 37°C in 20 mM HEPES buffer (pH 8) with 10 mM MgCl2 and 0.1 mM maltose. Where indicated, 2 mM nucleotide, 0.1 mM vanadate, and/or 5 μM MBP was added. All samples were diluted 100-fold prior to assay of ATPase activity.
ATPase activity, measured in the presence of MBP, of the uninhibited sample (0.47 μmol/min/mg of protein at 37°C with 0.2 mM [32P]ATP) is normalized to 1, and values are reported as a fraction of the uninhibited sample. In the absence of MBP, the activity of this preparation was <0.02 μmol/min/mg. Each value is the mean of four separate determinations that varied within 0.1 of the mean.
Dependence of vanadate inhibition on nucleotide concentration.
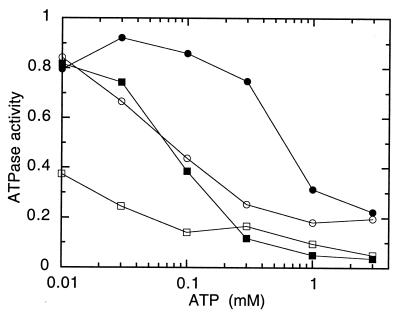
The ATP concentration dependence of vanadate inhibition is shown in Fig. 2 for the binding-protein-independent MalF500GK2 transporter. Concentration curves are depicted for preincubation with two different vanadate concentrations, 0.1 and 0.5 mM, and at two different protein concentrations, 0.15 or 1.5 μM. After treatment and before the assay, proteoliposomes were diluted and collected by centrifugation to remove vanadate from solution. The concentration of ATP required to achieve half-maximal inhibition was a function of both vanadate concentration and protein concentration, varying from less than 10 μM (at low protein and high vanadate concentrations) to 500 μM (at high protein and low vanadate concentrations). Clearly, factors other than the half-saturation constant for ATP hydrolysis (50 to 100 μM [8]) dominate the nucleotide concentration dependence of vanadate inhibition.
FIG. 2.
Dependence of vanadate inhibition on ATP concentration. Proteoliposomes containing MalF500GK2 were preincubated for 20 min at 23°C in the presence of vanadate and nucleotide, diluted 60-fold, and collected by centrifugation. Preincubations contained 3 mM MgCl2, ATP, and, in addition, 1.5 μM MalFGK2 and 0.1 mM vanadate (●); 1.5 μM MalFGK2 and 0.5 mM vanadate (○); 0.15 μM MalFGK2 and 0.1 mM vanadate; (■) or 0.15 μM MalFGK2 and 0.5 mM vanadate (□). ATPase activity is presented as a fraction of the rate seen in the absence of vanadate (3.2 μmol/min/mg of protein). Each point is the mean of three separate determinations that varied within 0.05 of the mean.
Binding of nucleotide to the vanadate-inhibited species.
To determine whether the maltose transport complex contains tightly bound nucleotide either before or after vanadate treatment, proteoliposomes were preincubated for 20 min at 23°C with 3 mM MgCl2 in the presence of either [α-32P]ATP or [γ-32P]ATP and then separated from unbound nucleotide by gel filtration. Some radioactivity became tightly associated with proteoliposomes in the absence of vanadate; however, this binding was nonspecific as evidenced by the failure of excess unlabeled ATP to compete for binding and by the tight association of similar amounts of radioactivity with liposomes lacking MalFGK2. Between 4 and 10 times more radioactivity became associated with proteoliposomes in the presence of vanadate and [α-32P]ATP, indicating that vanadate does lead to nucleotide trapping in the transport complex. No increase over background radioactivity was observed with [γ-32P]ATP, suggesting that ADP rather than ATP was trapped by vanadate. Thin-layer chromatography was used to confirm that the tightly bound nucleotide had been hydrolyzed (data not shown). In preliminary experiments using 0.1 mM [α-32P]ATP and proteoliposomes containing the binding-protein-independent MalF500GK2 complex, 80% of the ATPase was stably inhibited by vanadate yet only 0.12 mol of nucleotide was incorporated per mol of protein. This discrepancy is due in part to the use of intact proteoliposomes. Only transport complexes oriented with their nucleotide-binding sites exposed on the surface can be inhibited and assayed, while all transport complexes were detected in the protein assay. This limitation is overcome by adding MgCl2 at a concentration high enough to render the proteoliposomes permeable to hydrophilic solutes (21). Because the MgCl2 treatment results in some aggregation of vesicles, proteoliposomes are solubilized to obtain complete separation of protein from free nucleotide; the vanadate-trapped complex proved to be stable to solubilization and gel filtration in the detergent n-dodecylβ-d-maltoside. A second factor that increased vanadate-induced nucleotide trapping was the use of higher concentrations of ATP, although nonspecific binding of nucleotide was also increased. Using a concentration of 0.4 mM ATP, a ratio of 0.77 mol of ADP bound per mol of MalF500GK2 was obtained (Table 3). Near-stoichiometric amounts of nucleotide also became tightly bound to the wild-type transport complex when MBP was present (Table 3). In the absence of MBP, no nucleotide was trapped and, as shown previously, the ATPase activity was not inhibited (Table 3).
TABLE 3.
Vanadate-induced trapping of nucleotide in transport proteinsa
| Protein sample | Nucleotide (concn, mM) | Vanadate-induced trappingb (mol of nucleotide/mol of protein) | Relative ATPase activityc |
|---|---|---|---|
| MalF500GK2 − MBP | [α-32P]ATP (0.4) | 0.77 | 0.66 |
| MalFGK2 − MBP | [α-32P]ATP (0.1) | 0.03 | 1.2 |
| MalFGK2 + MBP | [α-32P]ATP (0.1) | 0.53 | 0.34 |
| [γ-32P]ATP (0.1) | 0 | NDe | |
| [α-32P]ATP (0.4) | 0.99 | 0.45 | |
| [α-32P]ATP (0.4) | 0.80 | 0.2 | |
| [α-32P]ATP (0.8) | 1.1 | 0.2 | |
| MalFGK(KK42N) + MBP | [α-32P]ATP (0.4) | 0 | ND |
| MalK − MBPd | [α-32P]ATP (0.4) | 0 | 1.1 |
Proteoliposomes were incubated with or without 0.5 mM vanadate in the presence or absence of 5 μM MBP, with 0.1 mM maltose, 10 mM MgCl2, and the indicated concentration of nucleotide for 20 min at 37°C. Unlabeled ATP (10 mM) was added, and proteins were solubilized in 0.1% n-dodecyl-β-d-maltoside and passed over a gel filtration column to separate bound from unbound nucleotide. Prior to solubilization, aliquots of proteoliposomes were diluted 100-fold for assay of ATPase activity.
The molar ratio of nucleotide to protein in the vanadate-trapped species was calculated from the difference between moles of nucleotide bound in the presence and absence of vanadate. The moles bound in the absence of vanadate represented 10 to 20% of the total at 0.1 mM ATP, 25% at 0.4 mM ATP, and 30% at 0.8 mM ATP. Results of individual experiments are shown, and data are reported as averages of duplicate or triplicate determinations.
ATPase activities are reported as a fraction of the activities of the untreated samples. Activities of the untreated species at 37°C with 1 mM [32P]ATP are as follows: MalF500GK2, 8 μmol/min/mg; MalFGK2 with MBP, 4 μmol/min/mg; MalFGK(KK42N) with MBP, 0.04 μmol/min/mg; and MalK, 0.1 μmol/min/mg.
Samples of purified MalK protein were incubated with or without 0.5 mM vanadate, 3 mM MgCl2, and the indicated concentration of nucleotide for 20 min at 23°C in buffer containing 20 mM HEPES (pH 8), 50 mM NaCl, 20% glycerol, and 0.1% lauryldimethylamine oxide. Both the molar ratio and ATPase were determined after gel filtration.
ND, not determined.
Inhibition of ATPase activity and trapping of nucleotide in transport complexes containing a mutation in a single MalK subunit.
Substitution of an Asp for Lys in the nucleotide-binding site of just one of the two MalK subunits in MalFGK2 greatly reduces but does not eliminate the maltose transport and ATPase activities of the transport complex (11). To determine whether the ATPase activity of this mutant transport complex is still sensitive to vanadate inhibition, proteoliposomes containing the purified mutant transport complexes (11) were incubated in the presence or absence of vanadate. The MBP-stimulated ATPase activity of this mutant was inhibited when vanadate was present during the assay (Table 4); however, no stable inhibition of ATPase activity is observed following treatment with vanadate in the presence of MBP (Table 4) and no trapping of nucleotide is observed (Table 3). For comparative purposes, the effects of vanadate on the isolated MalK protein were also examined. The rates of ATPase activity by the isolated MalK protein and the mutant transport complex are comparable, but the isolated MalK protein is reportedly not inhibited by vanadate (23). In agreement with the published data, we observed only a modest ability of vanadate to inhibit the ATPase activity when present in the assay medium, even at the relatively high concentration of 0.5 mM, and no stable inhibition of ATPase activity or nucleotide trapping was detected (Tables 3 and 4).
TABLE 4.
Effect of vanadate on the ATPase activity of MalFGK(KK42N) and the isolated MalK subunit
| Protein sample | Conditions
|
Relative ATPase activity ± vanadate in assayab | Relative ATPase activity following preincubation ± vanadatebc | |
|---|---|---|---|---|
| MBP | Vanadate concn (mM) | |||
| MalFGK(KK42N) | + | 0 | 1.0 | 1.0 |
| MalFGK(KK42N) | + | 0.1 | 0.11 | 0.94 |
| MalK | − | 0 | 1.0 | 1.0 |
| MalK | − | 0.1 | 0.85 | NDd |
| MalK | − | 0.5 | 0.75 | 0.94 |
ATPase activity of proteoliposomes containing MalFGK(KK42N) was assayed at 37°C in 20 mM HEPES buffer (pH 8) with 5 μM MBP, 0.1 mM maltose, 10 mM MgCl2 and 1 mM ATP in the presence or absence of vanadate. MalK was assayed at 37°C with 3 mM MgCl2, 1 mM ATP, 20 mM HEPES (pH 8), 50 mM NaCl, 20% glycerol, and 0.1% lauryldimethylamine oxide in the presence or absence of the indicated concentration of vanadate.
ATPase activities of the uninhibited samples are 0.1 μmol/min/mg for MalK and 0.04 μmol/min/mg for MalFGK(KK42N) with MBP present. These values are normalized to 1, and data from the treated samples are presented as a fraction of the uninhibited values. Each point is the mean of three determinations that varied within 0.15 of the mean. Without MBP present, the MalFGK(KK42N) preparation hydrolyzed only 0.002 μmol ATP/min/mg.
Proteoliposomes containing MalFGK(KK42N) were preincubated for 20 min at 37°C in 20 mM HEPES buffer (pH 8) containing 10 mM MgCl2, 5 μM MBP, and 1 mM ATP in the presence or absence of vanadate and then diluted and collected by centrifugation to remove vanadate. MalK was incubated for 20 min at 23°C in buffer containing 20 mM HEPES (pH 8), 3 mM MgCl2, 1 mM ATP, 50 mM NaCl, 20% glycerol, and 0.1% lauryldimethylamine oxide in the presence or absence of the indicated concentration of vanadate and then subjected to gel filtration prior to assay.
ND, not determined.
DISCUSSION
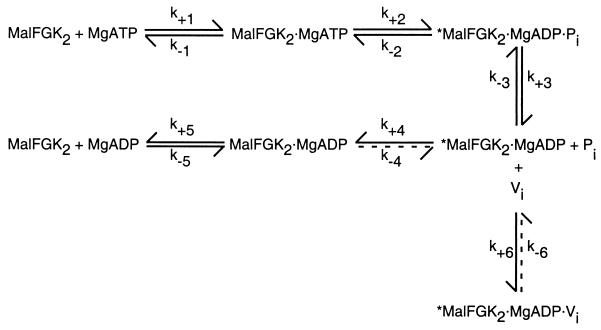
In this study, we found that MalFGK2 is inhibited by vanadate and that this inhibition continues even after vanadate is removed from solution. In contrast to the uninhibited transport complex, which does not bind nucleotide tightly, the vanadate-inhibited species binds ADP tightly. Given that an enzyme functions as a catalyst because it can bind tightly to and stabilize the transition-state conformation of its substrate (14), the ability of vanadate and ADP to form a transition-state analogue provides a rational explanation for the stability of the vanadate-inhibited MalFGK2 complex. In support of this hypothesis, we found that in the maltose transport system, ATP hydrolysis is an obligatory step in the formation of the inhibited species. A periplasmic binding-protein-dependent transport system is uniquely suited to demonstrate a requirement for ATP hydrolysis because the ATPase activity is tightly regulated by MBP. In the presence of MBP, incubation with vanadate leads to formation of the inhibited species. In the absence of MBP or of MgCl2 and hence of ATP hydrolysis, the vanadate-inhibited species does not form, as expected if the transport complex is unable to adopt or stabilize the transition-state conformation. Further support for this hypothesis comes from the observation that ATP but not ADP supports the formation of the vanadate-inhibited species; ATP must be hydrolyzed to ADP before trapping can occur.
The failure of ADP to support formation of the vanadate-inhibited species in MalFGK2 is significant because, in some systems, the addition of either ATP or ADP induces the formation of the inhibited species and, in dynein and myosin, ADP is a better substrate for vanadate trapping (15, 29). These differences are likely to reflect differences in the way that the free energy of ATP hydrolysis is coupled to the work performed by the enzyme. In the basic scheme proposed for inhibition, vanadate binds to a MgADP-bound form of the enzyme that can be generated either from ATP following ATP hydrolysis and dissociation of inorganic phosphate (Pi) or from ADP via direct binding of ADP to the enzyme (15, 29, 36). Given that ADP is a good inhibitor of MalFGK2 ATPase activity and therefore binds to MalFGK2 (A. L. Davidson, unpublished data), our data provide clear evidence of more than one ADP-bound intermediate in the pathway of ATP hydrolysis by MalFGK2. As shown in Fig. 3, we suggest that a high-energy ADP-bound intermediate is formed immediately following ATP hydrolysis and Pi release and that vanadate binds to this intermediate to form the trapped species. In the noninhibited case, ADP would be briefly occluded in this high-energy form, unable to dissociate until the protein relaxes to a lower energy form. The low-energy form would be equivalent to that formed from the binding of ADP directly, and it appears that vanadate cannot easily trap nucleotide by binding to this species. In the special case of the ABC protein Pgp, even though both ATP and ADP support the formation of the vanadate-inhibited species, the rate of formation of the inhibited species was higher with ATP than with ADP (37). Thus, the reaction scheme presented in Fig. 3 can readily describe ATP hydrolysis and vanadate inhibition by both ABC proteins if we assume that step 4 is more readily reversible in Pgp than in MalFGK2. For Pgp, it had been postulated that step 3, the release of Pi, would be associated with a large free energy decrease that could be coupled to transport (36). Given the complexity of active transport, the free energy associated with ATP hydrolysis may actually be released in increments (steps 3 and 4) that are coupled to different conformational changes that mediate translocation.
FIG. 3.
Scheme for vanadate inhibition of the MalFGK2 transport complex. This scheme, describing the inhibition of ATP hydrolysis by vanadate (Vi), has been adapted from other work (15, 29, 36) and is modified to contain both high-energy (∗) and low-energy ADP-bound species. In this scheme, step 4, conversion from the high- to low-energy forms of the ADP-bound species, and step 6, formation of the *MalFGK2 · MgADP · Vi complex, are not readily reversible, as indicated by the dashed arrows.
The scheme in Fig. 3 can also account for the time dependence of vanadate inhibition observed in Fig. 1. Since step 4 (relaxation) and step 6 (vanadate binding) compete for the same substrate (*MalFGK2 · ADP), multiple turnovers can occur before a protein binds to and is ultimately inhibited by vanadate. The appearance of multiple turnovers and hence of a time lag in the formation of the inhibited species could potentially be eliminated by higher concentrations of vanadate; however, the use of higher concentrations is contraindicated since orthovanadate is converted to potentially inhibitory higher-order vanadium species (15). This inefficiency in vanadate trapping would also account for the variation in ATP concentration required to obtain 50% inhibition under different conditions, as observed in Fig. 2. At 1.5 μM MalFGK2, ATP is hydrolyzed at a rate of 0.8 mM/min, and rapid depletion of ATP and accumulation of ADP may halt hydrolysis prior to complete formation of the vanadate-inhibited species.
In a complex containing a mutation in a single MalK subunit, the ability of vanadate to inhibit ATPase activity is conserved; however, stable inhibition of ATPase activity following removal of free vanadate is not observed. According to the model, the simplest explanation for these results is to assume that vanadate still inhibits by interacting with ADP to form a transition-state analog but that the stability of the transition-state complex (*MalFGK2 · MgADP · Vi) is reduced in proportion to the reduction of catalytic activity (step 2 of Fig. 3). Under these circumstances, once vanadate is removed from solution, vanadate and ADP would dissociate from the active site. Given that the mutant complex still displayed significant MBP-stimulated ATPase activity (1 to 2% of the wild-type activity), it is clear that while ATP hydrolysis is necessary, it is not sufficient for vanadate-induced nucleotide trapping. These data offer an alternative to the conclusion that the failure of vanadate to trap 8-azido-ATP in a mutant Pgp is synonymous with the lack of even a single turnover event (35).
Given that the isolated MalK protein exhibits a rate of ATPase activity similar in magnitude to that of an intact transport complex containing a mutation in a single MalK subunit, the failure of vanadate to inhibit isolated MalK does not result from its low catalytic activity. The scheme in Fig. 3 offers an explanation for the ability of vanadate to inhibit MalK ATPase activity in the intact complex but not in the isolated protein. The high-energy ADP-bound conformation may be stabilized in the intact complex, relative to the isolated MalK subunit, through intersubunit interactions. This stabilization may be achieved by coupling the decay of this species (step 4) to a relatively slow conformational change in the transport complex, allowing vanadate to bind to and inhibit the enzyme. This conformational change may involve binding, transport, or release of the translocated substrate, and/or it may be linked to interactions between the two nucleotide-binding sites within the context of an intact complex. It should be noted that the homologous HisP protein, which is catalytically active only as a dimer, also cannot be inhibited by vanadate in the absence of the membrane-spanning components of its transport complex (25).
One of several models that is consistent with the data might link dissociation of ADP from one MalK subunit to the binding of ATP to the second MalK. From the structure of RAD50cd, a soluble ABC dimer, a loop immediately following the Walker B consensus motif in one nucleotide-binding site has been identified (the D-loop) that appears to contact nucleotide in the second nucleotide-binding site and could mediate such communication (16, 20). ATP hydrolysis by the two nucleotide-binding sites of MalFGK2 and other ABC transporters is clearly coupled since mutations in one nucleotide-binding site severely impair ATP hydrolysis in the second site (2, 3, 11). Similarly, in Pgp, vanadate-induced trapping of nucleotide in a single nucleotide-binding site prevents ATP hydrolysis in the second site (37). We also observe a maximum of one nucleotide bound per MalFGK2 complex, implying that a mechanism similar to that of Pgp may be operating in maltose transport. However, the variation in the amount of ATPase activity remaining after incubation with vanadate (Table 3) weakens our argument. As documented for the FoF1ATPase (5), a mechanism in which nucleotide binding at the second subunit is in some way required to complete a cycle of ATP hydrolysis in the first subunit will also account for the presence of positive cooperativity in ATP hydrolysis by the intact maltose transport system (8).
ACKNOWLEDGMENTS
This work was supported by grants GM49261 from NIH and Q-1391 from the Robert A. Welch Foundation.
We thank Monica Farinas for constructing plasmid pMF8 and Fred Gimble for reading the manuscript.
REFERENCES
- 1.Allikmets R, Singh N, Sun H, Shroyer N E, Hutchinson A, Chidambaram A, Gerrard B, Baird L, Stauffer D, Peiffer A, Rattne R A, Smallwood P, Li Y X, Anderson K L, Lewis R A, Nathans J, Leppert M, Dean M, Lupski J R. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive stargardt macular dystrophy. Nat Genet. 1997;15:236–246. doi: 10.1038/ng0397-236. [DOI] [PubMed] [Google Scholar]
- 2.Azzaria M, Schurr E, Gros P. Discrete mutations introduced in the predicted nucleotide-binding sites of the mdr1 gene abolish its ability to confer multidrug resistance. Mol Cell Biol. 1989;9:5289–5297. doi: 10.1128/mcb.9.12.5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berkower C, Michaelis S. Mutational analysis of the yeast a-factor transporter STE6, a member of the ATP binding cassette (ABC) protein superfamily. EMBO J. 1991;10:3777–3785. doi: 10.1002/j.1460-2075.1991.tb04947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boos W, Lucht J M. Periplasmic binding protein-dependent ABC transporters. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C.: ASM Press; 1996. pp. 1175–1209. [Google Scholar]
- 5.Boyer P D. The binding change mechanism for ATP synthase—some probabilities and possibilities. Biochim Biophys Acta. 1993;1140:215–250. doi: 10.1016/0005-2728(93)90063-l. [DOI] [PubMed] [Google Scholar]
- 6.Chen C-J, Chin J E, Ueda K, Clark D P, Pastan I, Gottesman M M, Roninson I G. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell. 1986;47:381–389. doi: 10.1016/0092-8674(86)90595-7. [DOI] [PubMed] [Google Scholar]
- 7.Covitz K-M Y, Panagiotidis C H, Reyes M, Treptow N A, Shuman H A. Mutations that alter the transmembrane signalling pathway in an ATP binding cassette (ABC) transporter. EMBO J. 1994;13:1752–1759. doi: 10.1002/j.1460-2075.1994.tb06439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davidson A L, Laghaeian S S, Mannering D E. The maltose transport system of Escherichia coli displays positive cooperativity in ATP hydrolysis. J Biol Chem. 1996;271:4858–4863. [PubMed] [Google Scholar]
- 9.Davidson A L, Nikaido H. Overproduction, solubilization, and reconstitution of the maltose transport system from Escherichia coli. J Biol Chem. 1990;265:4254–4260. [PubMed] [Google Scholar]
- 10.Davidson A L, Nikaido H. Purification and characterization of the membrane-associated components of the maltose transport system from Escherichia coli. J Biol Chem. 1991;266:8946–8951. [PubMed] [Google Scholar]
- 11.Davidson A L, Sharma S. Mutation of a single MalK subunit severely impairs maltose transport activity in Escherichia coli. J Bacteriol. 1997;179:5458–5464. doi: 10.1128/jb.179.17.5458-5464.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davidson A L, Shuman H A, Nikaido H. Mechanism of maltose transport in Escherichia coli: transmembrane signalling by periplasmic binding proteins. Proc Natl Acad Sci USA. 1992;89:2360–2364. doi: 10.1073/pnas.89.6.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doige C A, Ames G F-L. ATP-dependent transport systems in bacteria and humans: relevance of cystic fibrosis and multidrug resistance. Annu Rev Microbiol. 1993;47:291–319. doi: 10.1146/annurev.mi.47.100193.001451. [DOI] [PubMed] [Google Scholar]
- 14.Fersht A. Enzyme structure and mechanism. 2nd ed. New York, N.Y: W. H. Freeman & Co.; 1985. [Google Scholar]
- 15.Goodno C C. Myosin active-site trapping with vanadate ion. Methods Enzymol. 1982;85:116–123. doi: 10.1016/0076-6879(82)85014-3. [DOI] [PubMed] [Google Scholar]
- 16.Hopfner K P, Karcher A, Shin D S, Craig L, Arthur L M, Carney J P, Tainer J A. Structural biology of Rad50 ATPase: ATP-driven conformational control in DNA double-strand break repair and the ABC-ATPase superfamily. Cell. 2000;101:789–800. doi: 10.1016/s0092-8674(00)80890-9. [DOI] [PubMed] [Google Scholar]
- 17.Hunke S, Dose S, Schneider E. Vanadate and bafilomycin A1 are potent inhibitors of the ATPase activity of the reconstituted bacterial ATP-binding cassette transporter for maltose (MalFGK2) Biochem Biophys Res Commun. 1995;216:589–594. doi: 10.1006/bbrc.1995.2663. [DOI] [PubMed] [Google Scholar]
- 18.Hyde S C, Emsley P, Hartshorn M J, Mimmack M M, Gileadi U, Pearch S R, Gallagher M P, Gill D R, Hubbard R E, Higgins C F. Structural model of ATP-binding proteins associated with cystic fibrosis, multidrug resistance and bacterial transport. Nature. 1990;346:362–365. doi: 10.1038/346362a0. [DOI] [PubMed] [Google Scholar]
- 19.Inagaki N, Gonoi T, Clement J P t, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- 20.Jones P M, George A M. Subunit interactions in ABC transporters: towards a functional architecture. FEMS Microbiol Lett. 1999;179:187–202. doi: 10.1111/j.1574-6968.1999.tb08727.x. [DOI] [PubMed] [Google Scholar]
- 21.Liu C E, Liu P Q, Ames G F L. Characterization of the adenosine triphosphatase activity of the periplasmic histidine permease, a traffic ATPase (ABC transporter) J Biol Chem. 1997;272:21883–21891. doi: 10.1074/jbc.272.35.21883. [DOI] [PubMed] [Google Scholar]
- 22.Macara I G. Vanadium—an element in search of a role. Trends Biol Sci. 1980;5:92–94. [Google Scholar]
- 23.Morbach S, Tebbe S, Schneider E. The ATP-binding cassette (ABC) transporter for maltose/maltodextrins of Salmonella typhimurium. Characterization of the ATPase activity associated with the purified MalK subunit. J Biol Chem. 1993;268:18617–18621. [PubMed] [Google Scholar]
- 24.Mosser J, Douar A M, Sarde C O, Kioschis P, Feil R, Moser H, Poustka A M, Mandel J L, Aubourg P. Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature. 1993;361:726–730. doi: 10.1038/361726a0. [DOI] [PubMed] [Google Scholar]
- 25.Nikaido K, Liu P Q, Ames G F. Purification and characterization of HisP, the ATP-binding subunit of a traffic ATPase (ABC transporter), the histidine permease of Salmonella typhimurium. Solubility, dimerization, and ATPase activity. J Biol Chem. 1997;272:27745–27752. doi: 10.1074/jbc.272.44.27745. [DOI] [PubMed] [Google Scholar]
- 26.Riordan J R, Rommens J M, Kerem B-S, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou J-L, Drumm M L, Iannuzzi M C, Collins F S, Tsui L C. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 27.Schaffner W, Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973;56:502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- 28.Scharschmidt B F, Keefe E B, Blankenship N M, Ockner R K. Validation of a recording spectrophotometric method for measurement of membrane-associated Mg- and NaK-ATPase activity. J Lab Clin Med. 1979;93:790–799. [PubMed] [Google Scholar]
- 29.Shimizu T, Johnson K A. Presteady state kinetic analysis of vanadate-induced inhibition of the dynein ATPase. J Biol Chem. 1983;258:13833–13840. [PubMed] [Google Scholar]
- 30.Shuman H A. Active transport of maltose in Escherichia coli K-12: role of the periplasmic maltose binding protein and evidence for a substrate recognition site in the cytoplasmic membrane. J Biol Chem. 1982;257:5455–5461. [PubMed] [Google Scholar]
- 31.Smith C A, Rayment I. X-ray structure of the magnesium(II).ADP.vanadate complex of the Dictyostelium discoideum myosin motor domain to 1.9 A resolution. Biochemistry. 1996;35:5404–5417. doi: 10.1021/bi952633+. [DOI] [PubMed] [Google Scholar]
- 32.Szabo K, Szakacs G, Hegedus T, Sarkadi B. Nucleotide occlusion on the human cystic fibrosis transmembrane conductance regulator. Different patterns in the two nucleotide binding domains. J Biol Chem. 1999;274:12209–12212. doi: 10.1074/jbc.274.18.12209. [DOI] [PubMed] [Google Scholar]
- 33.Tabor S, Richardson C C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci USA. 1985;82:1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Treptow N A, Shuman H A. Genetic evidence for substrate and periplasmic-binding-protein recognition by the MalF and MalG proteins, cytoplasmic membrane components of the Escherichia coli maltose transport system. J Bacteriol. 1985;163:654–660. doi: 10.1128/jb.163.2.654-660.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Urbatsch I L, Beaudet L, Carrier I, Gros P. Mutations in either nucleotide-binding site of P-glycoprotein (Mdr3) prevent vanadate trapping of nucleotide at both sites. Biochemistry. 1998;37:4592–4602. doi: 10.1021/bi9728001. [DOI] [PubMed] [Google Scholar]
- 36.Urbatsch I L, Sankaran B, Bhagat S, Senior A E. Both P-glycoprotein nucleotide-binding sites are catalytically active. J Biol Chem. 1995;270:26956–26962. doi: 10.1074/jbc.270.45.26956. [DOI] [PubMed] [Google Scholar]
- 37.Urbatsch I L, Sankaran B, Weber J, Senior A E. P-glycoprotein is stably inhibited by vanadate-induced trapping of nucleotide at a single catalytic site. J Biol Chem. 1995;270:19383–19390. doi: 10.1074/jbc.270.33.19383. [DOI] [PubMed] [Google Scholar]
- 38.Walter C, Honer-zu-Bentrup K, Schneider E. Large scale purification, nucleotide binding properties, and ATPase activity of the MalK subunit of Salmonella typhimurium maltose transport complex. J Biol Chem. 1992;267:8863–8869. [PubMed] [Google Scholar]