ABSTRACT
Background
The high risk of major adverse cardiovascular events (MACE) in patients with chronic kidney disease (CKD) has been well described. However, the efficacy of fibrates on the risk of MACE in patients with CKD remains unclear.
Methods
We conducted a nested case–control study using data from a large administrative database that included more than 1.5 million Japanese patients. We defined cases as CKD patients with incidences of MACE and matched them with controls based on age, sex, calendar year of cohort entry and CKD stage. Fibrate exposure timing was categorized as current, recent or past. A conditional logistic regression analysis was used to investigate the association between fibrate use and the risk of MACE.
Results
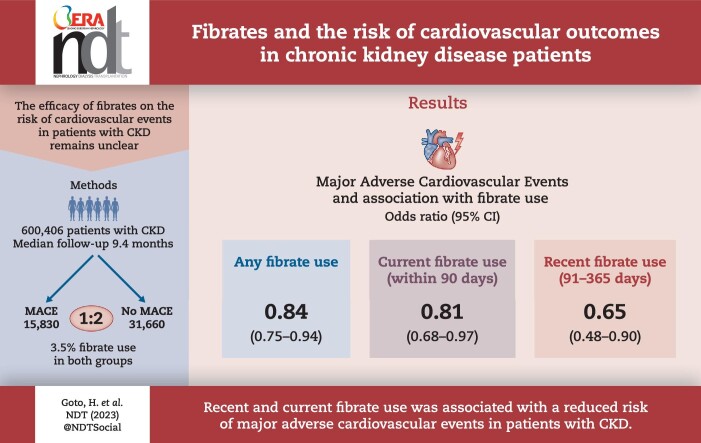
Our study included 47 490 patients with CKD, with 15 830 MACE identified during a median follow-up of 9.4 months. The numbers of fibrates used during the study period were 556 (3.5%) in the case group and 1109 (3.5%) in the control group. Fibrate use was significantly associated with a decreased risk of MACE [odds ratio (OR) 0.84; 95% confidence interval (CI) 0.75–0.94], particularly for current (OR 0.81; 95% CI 0.68–0.97) and recent use (OR 0.65; 95% CI 0.48–0.90). Regarding the class effect of fibrates, pemafibrate use, but not bezafibrate or fenofibrate use, was significantly associated with a decreased risk of MACE (OR 0.73; 95% CI 0.528–0.997).
Conclusion
Recent and current fibrate use, especially pemafibrate use, was associated with a reduced risk of MACE in patients with CKD. This suggests the potential benefits of continuous fibrate therapy and the possible superiority of pemafibrate over other fibrates. However, further investigations in different populations are required to confirm the generalizability of these findings.
Keywords: cardiovascular events, chronic kidney disease, dyslipidemia, fibrates, triglycerides
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What was known:
Patients with chronic kidney disease (CKD) have a higher risk of major adverse cardiovascular events (MACE) than the general population.
Information regarding the association between fibrate use and the risk of MACE in patients with CKD is limited.
Furthermore, the class effect of fibrates on MACE is not fully understood.
This study adds:
Recent and current use of fibrates, but not past use, was significantly associated with reduced risks of MACE in patients with CKD.
Among the classes of fibrates, a significant association was observed between current pemafibrate use, but not bezafibrate and fenofibrate use, and a reduced risk of MACE.
Potential impact:
The optimal use of fibrates in patients with CKD may lead to better clinical outcomes.
Among fibrates, pemafibrate may be a better choice than other fibrates.
INTRODUCTION
Declining kidney function results in various complications, such as vascular calcification and atherosclerosis, as the kidney plays a crucial role in the management of systemic mineral metabolism through the direct and indirect production of various hormones [1]. The development of atherosclerosis can lead to an increase in cardiovascular events, which remain the leading causes of morbidity and mortality in patients with chronic kidney disease (CKD) [1]. The risk of developing cardiovascular events in patients with CKD is 1.3–4 times higher than that in the general population [2, 3]. The prevention of cardiovascular events is important for improving the prognosis of patients with CKD.
Managing lipid levels, including the level of triglycerides, is beneficial in reducing cardiovascular events in patients with atherogenic dyslipidemia. Various national and international guidelines for lipid management have set triglyceride control targets, and the use of fibrates is recommended in patients with high levels of triglycerides [4–6]. Fibrates are effective in reducing triglyceride levels and increasing high-density lipoprotein cholesterol levels [7]. However, as most fibrates are excreted renally, its use in CKD patients receiving statins was contraindicated until 2018 due to a potentially increased risk of rhabdomyolysis. However, considering its beneficial effects in combination therapy, this contraindication was lifted in Japan in 2018, even for patients with CKD [8]. The prescription of fibrates in Japan may increase following the lifting of contraindications for the combined use of fibrates and statins.
Gemfibrozil use is significantly associated with a reduced incidence of major adverse cardiovascular events (MACE) in the general population and patients with type 2 diabetes [9, 10]. A population-based study in Taiwan showed that fibrates reduced the risk of cardiovascular events and delayed dialysis initiation [11]. However, the data are limited and inconsistent in the CKD population [12]. Fibrate use is poorly studied in the Japanese population, especially in patients with CKD, as its use was limited in the past because of the contraindication for combined use with statins. Furthermore, information regarding the class effect of fibrates on the risk of MACE is sparse [13].
This study aimed to investigate the association between fibrate use and the risk of MACE in patients with CKD using a large national database.
MATERIALS AND METHODS
Patients and data sources
Medical Data Vision Co., Ltd (MDV) maintains one of the largest hospital claims registries in Japan, which contains records of individual prescriptions, procedures, examinations, surgeries, hospitalizations and clinical diagnoses based on the International Classification of Disease 10th Revision (ICD-10) codes from more than 460 hospitals. This database includes data from more than 40 million people, accounting for 31% of Japan's total population (https://en.mdv.co.jp/). As the data used in this study were already anonymized, institutional review board approval and patient consent were not required in accordance with Japanese ethical guidelines [14]; the utilization of de-identified data complied with local regulations.
For our nested case–control study, we extracted data from the MDV database for 1 527 181 patients (aged ≥18 years) who had undergone plasma or serum creatinine measurement at least once between 1 November 2018 and 31 October 2022. We defined CKD as having at least two consecutive creatinine measurements, with a time interval of >3 months and <2 years, indicating estimated glomerular filtration rates (eGFR) ≥15 mL/min/1.73 m2 and <90 mL/min/1.73 m2. The date of the second measurement meeting this criterion was considered the cohort entry date (Fig. 1).
Figure 1:
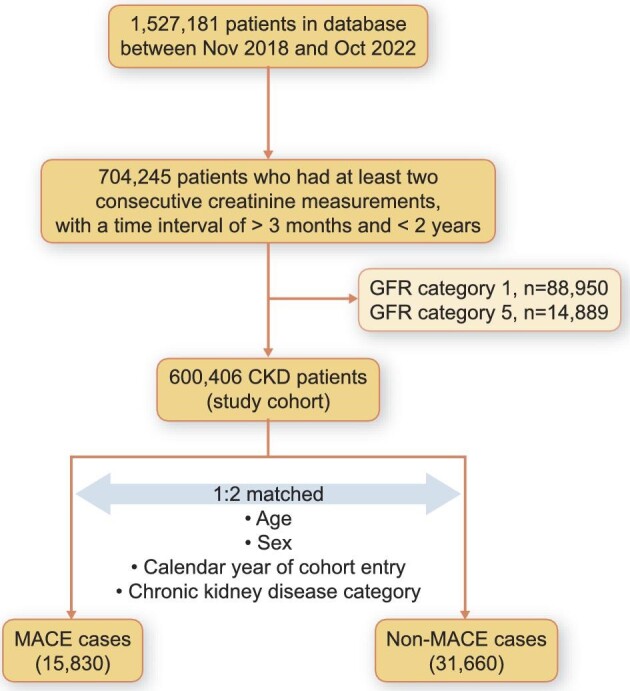
Flow chart of the study.
Cases and controls
Cases were defined based on the incidence of MACE, which are a composite of myocardial infarction, stroke, heart failure and death due to cardiovascular diseases (Supplementary data, Table S1). The diagnostic accuracy of the ICD-10 records in this database has been validated [15]. The index date was the first date of a MACE. The cases and controls were matched in a 1:2 ratio based on age, sex, calendar year of cohort entry and CKD stage at cohort entry. To ensure a comparable follow-up period between the cases and controls, each matched control was assigned the same index date as their corresponding case and met the criteria of being alive and not having experienced MACE. Using this approach, the risk set for a particular case comprised all at-risk individuals. Under this definition, another case could be considered a potential control if it developed MACE at a later date.
Fibrate exposure
The cases and controls were assigned to two distinct and mutually exclusive groups based on their exposure to fibrates (clofibrate, clinofibrate, bezafibrate, fenofibrate and pemafibrate) before the index date, as assessed using Anatomical Therapeutic Chemical Classification (ATC) codes (Supplementary data, Table S2). We determined the timing of fibrate use prior to the index date by analyzing the last date of the dispensed drug. Furthermore, we classified fibrate use into three distinct groups based on the timing with regard to the index date: (i) current use (within 90 days before the index date), (ii) recent use (between 91 and 365 days before the index date) and (iii) past use (more than 366 days before the index date).
Study covariates
For all cases and controls, study covariates included comorbid conditions and medications. Comorbidities within 1 year before the index date, including diabetes, atrial fibrillation/flutter, ischemic heart disease, cerebrovascular disease, peripheral vascular disease, and chronic pulmonary disease, were assessed using ICD-10 codes (Supplementary data, Table S1). Medications included angiotensin-converting–enzyme inhibitors/angiotensin-receptor blockers, β-blockers, calcium channel blockers, statins, diuretics, anticoagulants, antiplatelet agents, sodium-glucose cotransporter 2 inhibitor, glucagon-like peptide-1 receptor agonist, glucocorticoid inhalant, steroids, non-steroidal anti-inflammatory drugs (NSAIDs), opioids and antidepressants, which were assessed using ATC codes (Supplementary data, Table S2).
Statistical analysis
Data are expressed as median (25th–75th percentile), percentage or odds ratio (OR), as appropriate. Comparisons between the two groups were performed using the non-parametric Wilcoxon test for continuous variables and the chi-squared test for nominal variables. To explore the correlation between the use of fibrates and the risk of MACE, we employed conditional logistic regression, which allowed us to calculate the ORs for MACE. The time-matched nested case–control methodology adopted in this study delivers unbiased estimations of the rate ratio and 95% confidence intervals (CIs); all previously mentioned covariates were adjusted as clinically relevant confounding variables. Statistical significance was set at P < .05. Statistical analyses were performed using Stata 16.0 (Stata Corporation, College Station, TX, USA).
RESULTS
Baseline characteristics
In our cohort of 47 490 patients with CKD, we identified 15 830 cases of MACE during a median follow-up of 9.4 months (interquartile range 3.5–19.1). Table 1 shows the demographic and clinical characteristics of the patients, and their matched controls at the index date. The median patient age was 81 years, and 57% of the patients were male. The frequencies of comorbidities and co-medications (except opioids) listed in Table 1 were significantly higher for cases than for controls. The types of last-prescribed fibrates before the index data were comparable between cases and controls. Bezafibrate (48%) was the most used fibrate. When sorting as per the timing of prescriptions, pemafibrate was used less frequently in the past use than in recent and current use times (Fig. 2).
Table 1:
Baseline characteristics of the study participants.
| Characteristic | Cases | Controls | P-value |
|---|---|---|---|
| Sample size | 15 830 | 31 660 | |
| Sex, n (%) | |||
| Women | 6794 (42.9) | 13 588 (42.9) | |
| Age | 81 (74–87) | 81 (74–87) | 1.00 |
| Age categories, n (%) | 1.00 | ||
| 18–49 years | 232 (1.5) | 464 (1.5) | |
| 50–59 years | 554 (3.5) | 1108 (3.5) | |
| 60–69 years | 1615 (10.2) | 3230 (10.2) | |
| 70–79 years | 4663 (29.5) | 9326 (29.5) | |
| 80–89 years | 6407 (40.5) | 12 814 (40.5) | |
| ≥90 years | 2359 (14.9) | 4718 (14.9) | |
| eGFR | 50 (36, 64) | 50 (36, 64) | .79 |
| GFR categories, n (%) | .86 | ||
| Category 2 | 5242 (33.1) | 10 505 (33.2) | |
| Category 3a | 4528 (28.6) | 8976 (28.4) | |
| Category 3b | 3801 (24.0) | 7698 (24.3) | |
| Category 4 | 2259 (14.3) | 4481 (14.2) | |
| Comorbid conditions, n (%) | |||
| Diabetes | 6797 (42.9) | 9956 (31.4) | <.001 |
| Atrial fibrillation/flutter | 2924 (18.5) | 1823 (5.8) | <.001 |
| Ischemic heart disease | 3015 (19.0) | 2676 (8.5) | <.001 |
| Cerebrovascular disease | 3373 (21.3) | 3695 (11.7) | <.001 |
| Peripheral vascular disease | 1810 (11.4) | 2271 (7.2) | <.001 |
| Chronic pulmonary disease | 2159 (13.6) | 2521 (8.0) | <.001 |
| Medications, n (%) | |||
| Fibrate | 556 (3.5) | 1109 (3.5) | .93 |
| Bezafibrate | 273 (1.7) | 521 (1.6) | |
| Fenofibrate | 171 (1.1) | 373 (1.2) | |
| Pemafibrate | 112 (0.7) | 215 (0.7) | |
| ACEi/ARB | 9786 (61.8) | 14 832 (46.8) | <.001 |
| β-blocker | 7521 (47.5) | 8177 (25.8) | <.001 |
| CCB | 9732 (61.5) | 16 449 (52.0) | <.001 |
| Statins | 6225 (39.3) | 10 674 (33.7) | <.001 |
| Diuretics | 9430 (59.6) | 10 955 (34.6) | <.001 |
| Anticoagulants warfarin | 2767 (17.5) | 2438 (7.7) | <.001 |
| Anticoagulants DOAC | 4909 (31.0) | 4819 (15.2) | <.001 |
| Antiplatelet agents | 7521 (47.5) | 10 149 (32.1) | <.001 |
| SGLT2 | 1654 (10.4) | 1699 (5.4) | <.001 |
| GLP1 | 350 (2.2) | 439 (1.4) | <.001 |
| Glucocorticoid inhalant | 1539 (9.7) | 2450 (7.7) | <.001 |
| Steroids | 2362 (14.9) | 4436 (14.0) | <.001 |
| Nsaids | 7863 (49.7) | 15 523 (49.0) | <.001 |
| Opioids | 1181 (7.5) | 3205 (10.1) | <.001 |
| Antidepressant | 1890 (11.9) | 2961 (9.4) | <.001 |
| Calendar year of cohort entry, n (%) | 1.00 | ||
| 2019 | 8362 (52.8) | 16 724 (52.8) | |
| 2020 | 3087 (19.5) | 6174 (19.5) | |
| 2021 | 3481 (22.0) | 6962 (22.0) | |
| 2022 | 900 (5.7) | 1800 (5.7) |
Values are mean (standard deviation), median (interquartile range) or n (%).
ACEi/ARB, angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers; CCB, calcium-channel blockers; SGLT2, sodium-glucose cotransporter 2 inhibitor; GLP1, glucagon-like peptide-1 receptor agonist
Figure 2:
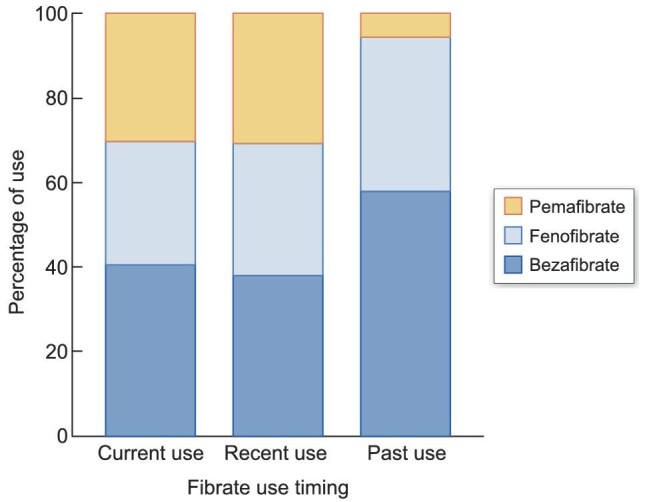
Stacked bar chart showing the percentage of bezafibrate, fenofibrate and pemafibrate use at each prescription timepoint.
Effect of fibrate use on the MACE risk
The frequencies of fibrate use during the study period were similar between the two groups: 556 cases (3.5%) and 1109 controls (3.5%). A similar tendency was observed for the differences in the frequencies of fibrate use between the two groups according to prescription timing.
Based on the conditional logistic regression analysis after adjusting for potential confounders, fibrate use during the study period was significantly associated with a decreased risk of MACE (OR 0.84; 95% CI 0.75–0.94). Similarly, significant associations were observed between recent and current use and the risk of MACE (current use: OR 0.81; 95% CI 0.68–0.97, recent use: OR 0.65; 95% CI 0.48–0.90). However, past use was not significantly associated with the risk of MACE (OR 0.94; 95% CI 0.79–1.12)(Table 2).
Table 2:
Multivariate adjusted ORs for the risk of MACE.
| n (%) | OR (95% CI) | |||
|---|---|---|---|---|
| Cases | Controls | Adjusted | P-value | |
| No fibrate use | 15 274 (96.5) | 30 551 (96.5) | 1 (reference) | |
| Fibrate use | 556 (3.5) | 1109 (3.5) | 0.84 (0.75–0.94) | <.05 |
| Current use (within 90 days) | 225 (1.4) | 486 (1.5) | 0.81 (0.68–0.97) | <.05 |
| Recent use (91–365 days) | 71 (0.5) | 156 (0.5) | 0.65 (0.48–0.90) | <.05 |
| Past use (over 365 days) | 260 (1.6) | 467 (1.5) | 0.94 (0.79–1.12) | .49 |
Covariates list: age, sex, eGFR category, diabetes, atrial fibrillation/flutter, ischemic heart disease, cerebrovascular disease, peripheral vascular disease, chronic pulmonary disease, angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers, β-blockers, calcium channel blockers, statins, diuretics, anticoagulants, antiplatelet agents, sodium-glucose cotransporter 2 inhibitor, glucagon-like peptide-1 receptor agonist, glucocorticoid inhalant, steroids, NSAIDs, opioids, antidepressant and calendar year of cohort entry.
The class effect of fibrate on the risk of MACE
As we observed significant associations between current and recent use and the risk of MACE, we investigated the class effect of fibrates on the risk of MACE. The frequency of current no-fibrate use was similar between the two groups. Based on a multivariate conditional logistic regression analysis, current pemafibrate use was significantly associated with a decreased risk of MACE (OR 0.73; 95% CI 0.528–0.997) compared with non-current use. However, there was no significant association between current bezafibrate and fenofibrate use and the risk of MACE (Table 3).
Table 3:
Multivariate adjusted ORs for the risk of MACE in relation to the current use of bezafibrate, fenofibrate and pemafibrate compared with non-use.
| n (%) | OR (95% CI) | |||
|---|---|---|---|---|
| Cases | Controls | Adjusted | P-value | |
| No current fibrate use | 15 605 (98.6) | 31 174 (98.5) | 1 (reference) | |
| Current use of bezafibrate | 92 (0.6) | 195 (0.6) | 0.91 (0.692–1.201) | .509 |
| Current use of fenofibrate | 61 (0.4) | 147 (0.5) | 0.77 (0.554–1.082) | .134 |
| Current use of pemafibrate | 72 (0.5) | 144 (0.5) | 0.73 (0.528–0.997) | <.05 |
Covariates list: age, sex, eGFR category, diabetes, atrial fibrillation/flutter, ischemic heart disease, cerebrovascular disease, peripheral vascular disease, chronic pulmonary disease, angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers, β-blockers, calcium channel blockers, statins, diuretics, anticoagulants, antiplatelet agents, sodium-glucose cotransporter 2 inhibitor, glucagon-like peptide-1 receptor agonist, glucocorticoid inhalant, steroids, NSAIDs, opioids, antidepressant and calendar year of cohort entry.
DISCUSSION
Our nested case–control study showed a significant association between recent and current fibrate use and a reduced risk of MACE in patients with CKD. However, this association was not observed in patients treated with fibrates in the past. Regarding the class effect of fibrates on the risk of MACE, only the current use of pemafibrate was significantly associated with a reduced risk of MACE. There were no significant associations between the current use of bezafibrate and fenofibrate and the risks of MACE.
A previous meta-analysis showed that fibrate use was associated with lower cardiovascular events in patients with CKD with 30 mL/min/1.73 m2 ≤ eGFR < 59.9 mL/min/1.73 m2 and eGFR ≥60 mL/min/1.73 m2 [16]. Our study corroborated this observation and expanded the patient population by including those with an eGFR between 15 and 30 mL/min/1.73 m2; this suggests that fibrate use may be effective in a broader range of patients with CKD. The mechanism of the beneficial impact of fibrate on MACE incidence may be explained by various effects such as reduced levels of triglycerides and total cholesterol, increased levels of high-density lipoprotein cholesterol and inhibition of albuminuria progression [16, 17]. Interestingly, a significant effect was observed for recent and current fibrate usage but not for past use, suggesting the importance of continuous fibrate therapy for cardioprotective benefits. This observation aligns with those of previous studies that highlighted the transient nature of the lipid-lowering effects of fibrates [17].
Data regarding the impact of fibrates on MACE is inconsistent among previous studies [9, 10, 17, 18]. Early studies, such as Helsinki Heart Study and Veterans Affairs and High-Density Lipoprotein Cholesterol Intervention Trial Study conducted in the 1990s, showed a reduction of MACE by fibrate alone [9, 10]. Conversely, large interventional trials conducted in the 2000s, such as the ACCORD and PROMINENT trials focusing on patients with type 2 diabetes, did not find a reduction in MACE associated with fibrate use [17, 18]. This discrepancy in the impact of fibrates on MACE could be attributed to differences in the population and study settings. Unlike the populations in the ACCORD and PROMINENT trials, our study focused on patients with CKD, not excluding those with a high risk of MACE. The objective of the present study was to investigate the association between fibrate use and the overall risk of MACE in patients with CKD. The PROMINENT study included patients with a median age of 64 years, and the ACCORD study included patients with a mean age of 62.3 years. This study used real-world data, which reflect the actual patient population, and the median age was 81 years; this may have led to different results, as many of the patients were in an age group that had at high risk of developing MACE.
Another interesting finding—a significant association of current pemafibrate use, but not bezafibrate and fenofibrate use, with the risk of MACE—suggests a possible intra-class difference of fibrates on MACE. Pemafibrate is a newer-generation fibrate compared with fenofibrate and bezafibrate. Pemafibrate, a more potent and selective activator peroxisome proliferator-activated receptor-alpha, may lead to more effective lipid-lowering effects, fewer drug–drug interactions, and fewer side effects compared with older fibrates [3, 19–21]. Furthermore, the PROMINENT study demonstrated a more pronounced decrease in serum C-reactive protein levels in pemafibrate users [18]. These anti-inflammatory effects may also contribute to a reduction in the risk of MACE. Another advantage of pemafibrate over bezafibrate and fenofibrate is the difference in metabolic pathways. While fenofibrate and bezafibrate are mainly excreted via the kidneys, pemafibrate is primarily metabolized in the liver and excreted via bile [22]. This differential metabolism may have influenced the results of our study, which focused on patients with CKD. Pemafibrate was launched in Japan in 2018, but its use remains limited. Therefore, the reported observational studies were mainly trials evaluating blood lipids, and none has investigated their association with MACE. To our knowledge, this is the first observational study of pemafibrate to explore its involvement in the development of MACE. However, because pemafibrate is contraindicated in dialysis patients in Japan, we were unable to examine its impact on the risk of MACE in dialysis patients.
The strength of this study is that patients who underwent serum creatinine testing were selected from a database covering more than 30% of the Japanese population, including those aged ≥75 years, and patients with decreased kidney functions were identified. To the best of our knowledge, no other large administrative database in Japan includes data on serum creatinine levels in elderly patients. In particular, fibrates are thought to elevate the risk of rhabdomyolysis in patients with renal dysfunction. This study is important since no intervention study using such a population has been conducted in Japan.
Some limitations of this study should be considered when interpreting the results. First, as with any observational study, causality could not be established. Second, this study used a large administrative database in Japan, which may have limited the generalizability of the findings. Third, many important confounders related to blood pressure and health status, such as the frailty index, alcohol consumption and smoking, were not accounted for, which may have affected the results. Fourth, our database has no information about urinalysis and renal imaging, which could lead to potential misclassification.
In conclusion, given the significant association between recent and current fibrate use and the reduced risk of MACE in patients with CKD, optimal use of fibrates may lead to better clinical outcomes. Moreover, among the classes of fibrates, pemafibrate may be superior to other fibrates, such as bezafibrate and fenofibrate. However, the findings should also be externally validated in countries other than Japan.
Supplementary Material
Contributor Information
Hirohito Goto, Center for Novel and Exploratory Clinical Trials (Y-NEXT), Yokohama City University Hospital, Kanagawa, Japan; Department of Clinical Pharmacy, Division of Clinical Research and Development, School of Pharmacy, Showa University, Tokyo, Japan.
Ken Iseri, Department of Clinical Pharmacy, Division of Clinical Research and Development, School of Pharmacy, Showa University, Tokyo, Japan.
Noriko Hida, Department of Clinical Pharmacy, Division of Clinical Research and Development, School of Pharmacy, Showa University, Tokyo, Japan.
FUNDING
None declared.
AUTHORS' CONTRIBUTIONS
H.G., K.I. and N.H. contributed to and coordinated the study design. H.G. and K.I. conducted the statistical analyses. H.G. and K.I. wrote the first draft of the manuscript. All authors read, reviewed, edited and approved the final manuscript.
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are not open access.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Drüeke TB, Massy ZA. Atherosclerosis in CKD: differences from the general population. Nat Rev Nephrol 2010;6:723–35. 10.1038/nrneph.2010.143 [DOI] [PubMed] [Google Scholar]
- 2. Go AS, Chertow GM, Fan D et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296–305. 10.1056/NEJMoa041031 [DOI] [PubMed] [Google Scholar]
- 3. Nishimura K, Okamura T, Watanabe M et al. Predicting coronary heart disease using risk factor categories for a Japanese urban population and comparison with the Framingham risk score: the Suita Study. J Atheroscler Thromb 2014;21:784–98. 10.5551/jat.19356 [DOI] [PubMed] [Google Scholar]
- 4. Grundy SM, Stone NJ, Bailey AL et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice guidelines. Circulation 2019;139:e1082–143. 10.1161/CIR.0000000000000625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Authors/Task Force Members, ESC Committee for Practice Guidelines (CPG), ESC National Cardiac Societies . 2019 ESC/EAS guidelines for the management of dyslipidemia: lipid modification to reduce cardiovascular risk. Atherosclerosis 2019;290:140–205. 10.1016/j.atherosclerosis.2019.08.014 [DOI] [PubMed] [Google Scholar]
- 6. Kinoshita M, Yokote K, Arai H et al. ; Committee for Epidemiology and Clinical Management of Atherosclerosis . Japan Atherosclerosis Society (JAS) guidelines for prevention of atherosclerotic cardiovascular diseases 2017. J Atheroscler Thromb 2018;25:846–984. 10.5551/jat.GL2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schoonjans K, Staels B, Auwerx J. Peroxisome proliferator-activated receptors (PPARs) and their effects on lipid metabolism and adipocyte differentiation. Biochim Biophys Acta 1996;1302:93–109. 10.1016/0005-2760(96)00066-5 [DOI] [PubMed] [Google Scholar]
- 8. Pharmaceuticals and Medical Device Agency . Pharmaceuticals and Medical Devices Agency Report on Investigation Results Pharmaceuticals and Medical Devices Agency. Published Online 2018. Available at: https://www.pmda.go.jp/files/000226296.pdf (2 June 2023, date last accessed). [Google Scholar]
- 9. Frick MH, Elo O, Haapa K et al. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med 1987;317:1237–45. 10.1056/NEJM198711123172001 [DOI] [PubMed] [Google Scholar]
- 10. Rubins HB, Robins SJ, Collins D et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med 1999;341:410–8. 10.1056/NEJM199908053410604 [DOI] [PubMed] [Google Scholar]
- 11. Yen CL, Fan PC, Lin MS et al. Fenofibrate delays the need for dialysis and reduces cardiovascular risk among patients with advanced CKD. J Clin Endocrinol Metab 2021;106:1594–605. 10.1210/clinem/dgab137 [DOI] [PubMed] [Google Scholar]
- 12. Ho WY, Yen CL, Lee CC et al. Use of fibrates is not associated with reduced risks of mortality or cardiovascular events among ESRD patients: a national cohort study. Front Cardiovasc Med 2022;9:907539. 10.3389/FCVM.2022.907539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jun M, Zhu B, Tonelli M et al. Effects of fibrates in kidney disease: a systematic review and meta-analysis. J Am Coll Cardiol 2012;60:2061–71. 10.1016/j.jacc.2012.07.049 [DOI] [PubMed] [Google Scholar]
- 14. Eba J, Nakamura K. Overview of the ethical guidelines for medical and biological research involving Human subjects in Japan. Jpn J Clin Oncol 2022;52:539–44. 10.1093/jjco/hyac034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nishikawa A, Yoshinaga E, Nakamura M et al. Validation study of algorithms to identify malignant tumors and serious infections in a Japanese administrative healthcare database. Ann Clin Epidemiol 2022;4:20–31. 10.37737/ace.22004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hadjivasilis A, Kouis P, Kousios A et al. Effect of fibrates on kidney function and chronic kidney disease progression: a systematic review and meta-analysis of randomized studies. J Clin Med 2022;11:768. 10.3390/jcm11030768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. ACCORD Study Group ; Ginsberg HN, Elam MB, Lovato LC et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010;362:1563–74. 10.1056/NEJMoa1001282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Das Pradhan A, Glynn RJ, Fruchart JC et al. ; PROMINENT Investigators . Triglyceride lowering with pemafibrate to reduce cardiovascular risk. N Engl J Med 2022;387:1923–34. 10.1056/NEJMoa2210645 [DOI] [PubMed] [Google Scholar]
- 19. Fruchart JC. Selective peroxisome proliferator-activated receptorα modulators (SPPARMα): the next generation of peroxisome proliferator-activated receptor α-agonists. Cardiovasc Diabetol 2013;12:82. 10.1186/1475-2840-12-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yamashita S, Masuda D, Matsuzawa Y. Pemafibrate, a new selective PPARα modulator: drug concept and its clinical applications for dyslipidemia and metabolic diseases. Curr Atheroscler Rep 2020;22:5. 10.1007/S11883-020-0823-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Araki E, Yamashita S, Arai H et al. Effects of pemafibrate, a novel selective PPARα modulator, on lipid and glucose metabolism in patients with type 2 diabetes and hypertriglyceridemia: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care 2018;41:538–46. 10.2337/dc17-1589 [DOI] [PubMed] [Google Scholar]
- 22. Ishibashi S, Arai H, Yokote K et al. ; K-877 Study Group . Efficacy and safety of pemafibrate (K-877), a selective peroxisome proliferator-activated receptor α modulator, in patients with dyslipidemia: results from a 24-week, randomized, double-blind, active-controlled, phase 3 trial. J Clin Lipidol 2018;12:173–84. 10.1016/j.jacl.2017.10.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are not open access.