Abstract
Aims
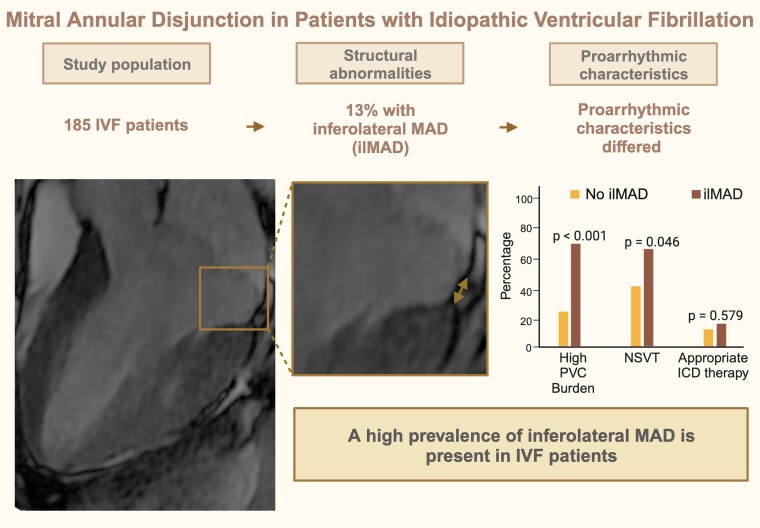
Previously, we demonstrated that inferolateral mitral annular disjunction (MAD) is more prevalent in patients with idiopathic ventricular fibrillation (IVF) than in healthy controls. In the present study, we advanced the insights into the prevalence and ventricular arrhythmogenicity by inferolateral MAD in an even larger IVF cohort.
Methods and results
This retrospective multi-centre study included 185 IVF patients [median age 39 (27, 52) years, 40% female]. Cardiac magnetic resonance images were analyzed for mitral valve and annular abnormalities and late gadolinium enhancement. Clinical characteristics were compared between patients with and without MAD. MAD in any of the 4 locations was present in 112 (61%) IVF patients and inferolateral MAD was identified in 24 (13%) IVF patients. Mitral valve prolapse (MVP) was found in 13 (7%) IVF patients. MVP was more prevalent in patients with inferolateral MAD compared with patients without inferolateral MAD (42 vs. 2%, P < 0.001). Pro-arrhythmic characteristics in terms of a high burden of premature ventricular complexes (PVCs) and non-sustained ventricular tachycardia (VT) were more prevalent in patients with inferolateral MAD compared to patients without inferolateral MAD (67 vs. 23%, P < 0.001 and 63 vs. 41%, P = 0.046, respectively). Appropriate implantable cardioverter defibrillator therapy during follow-up was comparable for IVF patients with or without inferolateral MAD (13 vs. 18%, P = 0.579).
Conclusion
A high prevalence of inferolateral MAD and MVP is a consistent finding in this large IVF cohort. The presence of inferolateral MAD is associated with a higher PVC burden and non-sustained VTs. Further research is needed to explain this potential interplay.
Keywords: idiopathic ventricular fibrillation, cardiac magnetic resonance, mitral valve prolapse, mitral annular disjunction, ventricular arrhythmias
Graphical Abstract
Graphical Abstract.
ICD, implantable cardioverter defibrillator; ilMAD, inferolateral mitral annular disjunction; PVC, premature ventricular complex; NSVT, non-sustained ventricular tachycardia.
See the editorial comment for this article ‘Cardiac arrest, mitral annular disjunction, and mitral valve prolapse: where there is smoke, there is a fire', by K.H. Haugaa and E.W. Aabel, https://doi.org/10.1093/ehjci/jeae079.
Introduction
Improvements in diagnostic techniques and increased knowledge on possible pathological conditions have led to the recognition of novel arrhythmia syndromes in the last decades, thus reducing the number of patients with ‘idiopathic’ ventricular fibrillation (IVF).1,2 Associations between structural abnormalities like mitral valve prolapse (MVP) and arrhythmogenesis have been revealed, resulting in the definition ‘arrhythmic MVP’.3,4 Mitral annular disjunction (MAD) was previously considered a benign structural abnormality, but it is more common in patients with MVP,5 and has been associated with an enhanced risk of ventricular arrhythmias, even without MVP.6 Data on the prevalence of MAD in the general population were scarce until recently. Zugwitz et al. and Toh et al. investigated MAD in the general population using cardiac magnetic resonance (CMR) and computed tomography.7,8 Both studies show that MAD is often found, corroborating its benign appearance. However, inferolateral MAD is uncommon (6.2% inferolateral MAD vs. 61.6% inferior MAD on CMR).7 A comparable prevalence of inferolateral MAD was described in the first autopsy paper from Hutchins et al.9 In line with these findings, our research group previously showed an increased prevalence of inferolateral MAD and MVP in IVF patients compared with an age- and sex-matched control group.10 Recently, the association of MVP with unexplained cardiac arrest was investigated by Alqarawi et al.11 They compared the prevalence of MVP in IVF patients with that of patients with another diagnosis underlying sudden cardiac arrest, and found a prevalence of 6.6%.11 There is, however, still uncertainty on the clinical relevance of MAD, especially in patients without overt MVP.4 With these controversies surrounding MAD and MVP, this study focused on the question if inferolateral MAD should be seen as a possible risk marker for ventricular arrhythmias.
Methods
Study population
The study population included patients from the Dutch Idiopathic VF registry and St. George’s University of London. The Dutch Idiopathic VF registry is a large national multi-centre cohort that enrols patients initially diagnosed with IVF. Eligible patients were sudden cardiac arrest survivors, preferably with documented ventricular fibrillation (VF), after exclusion of cardiac, respiratory, metabolic, or toxicological causes, who received CMR imaging as part of the diagnostic work-up. Included patients in this study from the Dutch Idiopathic VF registry were evaluated in any of the participating centres between 2004 and 2022. Patients from St. George’s University of London were IVF patients who presented after their cardiac arrest or were referred to St George’s University Hospitals NHS Trust between 2011 and 2022 who agreed to be enrolled in research studies as per locally approved ethics. Exclusion of specific explainable diagnoses for VF at baseline or during follow-up was based on accepted diagnostic criteria, as described previously.12 Patients from the Dutch Idiopathic VF registry were also excluded if they carried the chromosome 7q36 risk haplotype, harbouring DPP613 and if their CMR was of insufficient quality to determine inferolateral MAD. Patients evaluated in our previous report10 and additional IVF patients were pooled. Supplementary data online, Figure S1 shows the inclusion flowchart. This study was approved by local institutional ethics review boards and complies with the Declaration of Helsinki.
Cardiac magnetic resonance
CMR was performed on either a 1.5- or 3-T scanner using standardized cardiac protocols with electrocardiographic gating and a phased-array cardiac receiver coil. Acquisitions used a breath-hold balanced steady-state free-precession cine sequence [4-chamber long-axis view, 2- and 3-chamber long-axis left ventricle (LV) views, and short-axis multi-slice full coverage of the LV]. Voxel size of cine sequences depended on local scan protocols. Typical voxel size was 1.5 × 1.5 × 5 to 8 mm3. Late gadolinium enhancement (LGE) imaging was performed in identical views, ≥ 10 min after administration of a gadolinium-based contrast agent.
Image analysis
Image analysis was performed by a blinded cardiologist (M.G.) with a level 3 certification in CMR by the European Association for Cardiovascular Imaging and more than 8 years of experience in reporting CMR. CMR images of patients included from our previous study were analyzed as described previously.10 All images were analyzed for the presence of MAD, MVP, and curling. MAD was defined as longitudinal displacement of ≥ 1 mm, measured at end-systole (Figure 1), as proposed by Zugwitz et al.7 Anterolateral MAD was determined on the CMR 4-chamber view, anterior and inferior MAD on the 2-chamber view, inferolateral MAD on the 3-chamber view. To further explore the influence of MAD present at >1 of the four locations, we calculated the total sum of MAD in mm for each patient by adding each measurement of anterolateral, anterior, inferior, or inferolateral MAD when present. Then we stratified this sum based on the mean, median, 75th, 90th, and 95th percentile of the total patient group. MVP was defined as abnormally thickened mitral valve leaflets and systolic displacement of the mitral valve leaflets ≥ 2 mm from the annular plane into the left atrium and determined on 3-chamber view (Figure 1).14 Curling was defined as an abnormal systolic motion of the inferior mitral annulus on the adjacent ventricular wall.15 LGE images were re-evaluated for the presence of any fibrosis (including papillary muscle fibrosis). The pattern was differentiated between an ischaemic or non-ischaemic pattern. A non-ischaemic pattern was further differentiated as junctional, patchy, sub-epicardial, or intra-myocardial. The location was determined as a binary variable using the 17-segment AHA model.16
Figure 1.
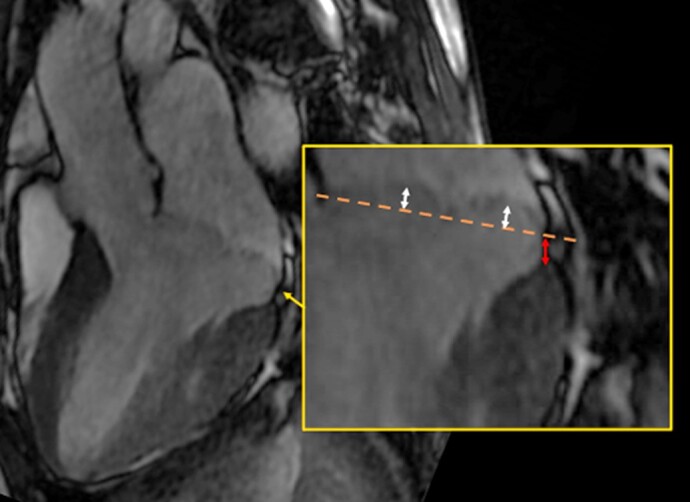
Measurement of MAD and MVP on a 3-chamber view. Red arrow represents the measurement of MAD, white arrows represent the measurement of MVP. MAD, mitral annular disjunction; MVP, mitral valve prolapse.
Clinical characteristics
Medical history, medication use, physical examination, 12-lead electrocardiogram (ECG), Holter monitoring, laboratory testing, echocardiography, coronary imaging, exercise treadmill testing, sodium channel blocker provocation, and genetic testing were collected for all patients. T-wave abnormalities on ECGs were defined as T-wave inversion of ≥ 1 mm or biphasic T-waves. Inferior T-wave abnormalities were present when T-wave inversion or biphasic T-waves were identified in any of the three inferior leads (II, III, aVF). Available ECGs, Holter/telemetry documentation, and exercise treadmill testing ECGs were evaluated to determine premature ventricular complex (PVC) burden and PVC morphology. Patients with either >1000 PVCs per 24 h on Holter monitoring, >20 PVCs during exercise treadmill test, or bigeminy or trigeminy on ECG/exercise treadmill test/telemetry/Holter were considered as patients with a high PVC burden. Non-sustained ventricular tachycardia (VT) was defined as ≥ 3 ventricular beats with a duration of ≤ 30 s.12 Outcome was defined as appropriate implantable cardioverter defibrillator (ICD) therapy (anti-tachycardia pacing or shock) for VT or VF.
Statistical analysis
Data were analyzed with SPSS version 27.0. Categorical variables were analyzed using χ2 or Fisher’s exact tests, as appropriate. The Shapiro–Wilk test was used to determine if continuous variables were normally distributed. Continuous variables were analyzed using Student’s t-test or Mann–Whitney U test, as appropriate. P values <0.05 were considered significant.
Results
Study population
The total study population included 185 IVF patients, with 51 patients included from our previous report and the additional 134 patients entered from 9 collaborating centres (see Supplementary data online, Figure S1). Patients experienced their index event at a median age of 39 (27, 52) years and 40% of the patients were female (Table 1). The minority of patients experienced arrhythmia symptoms (palpitations or syncope) before their event. Median follow-up duration was 5 (2, 8) years. During follow-up, 18% received appropriate ICD therapy.
Table 1.
Clinical characteristics of IVF patients
| IVF patients (n = 185) | |
|---|---|
| Age at event, years | 39 (27, 52) |
| Female, n (%) | 74 (40%) |
| Event during exercise, n (%) | 38/182 (21%) |
| History of palpitations, n (%) | 21/167 (11%) |
| History of syncope, n (%) | 19/167 (10%) |
| Family history of SCDa, n (%) | 22/170 (12%) |
| ICD implantation, n (%) | 182 (99%) |
| Follow-up duration, years | 5 (2, 8) |
| Appropriate ICD therapy, n (%) | 32/182 (18%) |
| Death, n (%) | 3 (2%) |
IVF, idiopathic ventricular fibrillation; SCD, sudden cardiac death; ICD, implantable cardioverter defibrillator.
aFamily history of SCD is defined as a first-degree family member with SCD < 50 years or multiple second-degree family members with SCD.
CMR analysis
Table 2 shows the results from CMR analysis of the 185 IVF patients. MAD in any of the four locations was present in 61% IVF patients, and inferolateral MAD was identified in 24 (13%) IVF patients. Median inferolateral MAD length was 3.8 (2.8, 5.8) mm. The median of the total sum of MAD was 3 (0, 6) mm. MVP was present in 13 (7%) IVF patients, curling was visual in 11 (6%) IVF patients.
Table 2.
CMR findings in IVF patients
| IVF patients (n = 185) | |
|---|---|
| BSA, kg/m2 | 1.94 (±0.22) |
| LVEDV, mL | 171 (±40) |
| LVEDVi, mL/m2 | 88 (±16) |
| LVEF, % | 57 (±7) |
| Mitral valve prolapse | |
| Any MVP, n (%) | 13/182 (7%) |
| Posterior leaflet, n (%) | 7 (4%) |
| Bileaflet, n (%) | 5 (3%) |
| Prolapse, mm | 4.2 (±2.4) |
| Mitral annular disjunction | |
| Any MAD, n (%) | 112 (61%) |
| Anterolateral, n (%) | 32/182 (18%) |
| Anterolateral, mm | 3 (2, 5) |
| Anterior, n (%) | 50/174 (29%) |
| Anterior, mm | 3.8 (2, 4) |
| Inferior, n (%) | 86/180 (47%) |
| Inferior, mm | 3.6 (3, 5) |
| Inferolateral, n (%) | 24 (13%) |
| Inferolateral, mm | 3.8 (2.8, 5.8) |
| Total MAD sum, mm | 3 (0, 6) |
| Curling sign, n (%) | 11/181 (6%) |
BSA, body surface area; LVEDV, left ventricular end diastolic volume; LVEDVi, indexed left ventricular end diastolic volume; LVEF, left ventricular ejection fraction; MAD, mitral annular disjunction; MVP, mitral valve prolapse; IVF, idiopathic ventricular fibrillation.
IVF patients with or without inferolateral MAD
Clinical characteristics stratified between patients with or without inferolateral MAD are depicted in Table 3. Patients with inferolateral MAD more often had a high PVC burden (67 vs. 23%, P < 0.001) and non-sustained VTs (63 vs. 41%, P = 0.046) at baseline or during follow-up. Appropriate ICD therapy during follow-up was comparable between groups. Additional mitral valve abnormalities were more common in patients with inferolateral MAD than in other IVF patients. MVP was present in 42% of patients with inferolateral MAD, compared with 2% in patients without inferolateral MAD (P < 0.001). Patients with inferolateral MAD more often had MAD in multiple areas (83 vs. 21%, P < 0.001). LGE of non-specific pathogenesis was identified in 13 IVF patients. Papillary muscle LGE was not identified. A detailed description of LGE patterns can be found in Supplementary data online, Table S1. The presence of LGE in any segment did not differ between patients with or without inferolateral MAD (9 vs. 8%, P = 0.693).
Table 3.
Comparison of 185 IVF patients with and without inferolateral MAD
| IVF patients with inferolateral MAD (n = 24) | IVF patients without inferolateral MAD (n = 161) | P-value | |
|---|---|---|---|
| Age, yearsa | 29 (22, 49)] | 39 (28, 53) | 0.140 |
| Female, n (%) | 10 (42%) | 64 (40%) | 0.858 |
| History of syncope, n (%) | 3/18 (17%) | 16/149 (11%) | 0.436 |
| History of palpitations, n (%) | 3/18 (17%) | 18/149 (12%) | 0.704 |
| Family history of SCDb, n (%) | 2/20 (10%) | 20/150 (13%) | 1.000 |
| Arrhythmia characteristics | |||
| Inverted/biphasic T-waves inferior, n (%) | 9 (38%) | 25/159 (16%) | 0.021 |
| High PVC burden, n (%) | 16 (67%) | 36 (23%) | <0.001 |
| Multi-form PVCs | 9/15 (60%) | 12/31 (39%) | 0.174 |
| Non-sustained VT, n (%) | 15 (63%) | 62/152 (41%) | 0.046 |
| Appropriate ICD therapy, n (%) | 3 (13%) | 29 (18%) | 0.579 |
| Death, n (%) | 0 (0%) | 3 (2%) | 1.000 |
| CMR characteristics | |||
| LVEF, %c | 55 (±7) | 58 (±8) | 0.108 |
| Mitral valve prolapse, n (%) | 10 (42%) | 3 (2%) | <0.001 |
| MAD present in multiple areas, n (%) | 20 (83%) | 33 (21%) | <0.001 |
| LGE present, n (%) | 2/23 (9%) | 11/145 (8%) | 0.693 |
CMR, cardiac magnetic resonance; IVF, idiopathic ventricular fibrillation; ICD, implantable cardioverter defibrillation; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction; MAD, mitral annular disjunction; SCD, sudden cardiac death; PVC, premature ventricular complexes; VT, ventricular tachycardia.
aValues are presented as median (interquartile range).
bDefinition as used in Table 1.
cValues are presented as mean (standard deviation).
Influence of inferolateral MAD on pro-arrhythmic parameters and MVP
The length of inferolateral MAD (in mm) did not influence pro-arrhythmic characteristics in terms of high PVC burden, non-sustained VT, and appropriate ICD therapy. IVF patients with MVP demonstrated significantly more annular displacement than those without MVP (see Supplementary data online, Figure S2). Patients with multiple mitral valve abnormalities more often had a high PVC burden and non-sustained VTs (see Supplementary data online, Table S2). Appropriate ICD therapy during follow-up remained comparable. Patients with or without MVP showed similar results when comparing pro-arrhythmic characteristics (see Supplementary data online, Table S3), and pro-arrhythmic characteristics were more often found when MAD sum increased (see Supplementary data online, Table S4). None of the patients with MVP had moderate or severe mitral regurgitation. The presence of mild mitral regurgitation, bileaflet prolapse, and flail in patients with MVP is described in Supplementary data online, Table S5.
Inferolateral MAD patients
Table 4 provides a detailed overview of all patients with inferolateral MAD. Among patients with a high PVC burden, multi-form PVCs were abundant (9/15, 60%). The morphology and most likely origin are depicted in Table 4. When compared with patients without inferolateral MAD with a high PVC burden, the prevalence of multi-form PVCs did not differ (Table 3). Many patients with inferolateral MAD received pharmaceutical therapy, primarily beta blockers. Compared to patients without inferolateral MAD, patients with inferolateral MAD more often received pharmaceutical treatment (see Supplementary data online, Table S6). Two patients underwent radiofrequency ablation of dominant PVCs. Genetic test results of patients with inferolateral MAD can be found in Supplementary data online, Table S7.
Table 4.
Overview of patients with inferolateral MAD
| ID | Sex | Age | High PVC burden | PVC morphology | PVC location | ICD therapy | Current medication use | Ablation |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 19 | Yes | RBBB, sup and inf axis | Distal/post-LV | Yes | Atenolol | |
| 2 | M | 58 | No | No | Carvedilol | |||
| 3 | M | 54 | Yes | Multi-form | Basal | No | Bisoprolol | |
| 4 | M | 49 | Yes | Multi-form | RV apex/lateral LV/LV apex | No | Metoprolol | |
| 5 | M | 63 | Yes | Multi-form | LV | Yes | ||
| 6 | M | 27 | Yes | LBBB, inf axis | Basal RV | No | Metoprolol | |
| 7 | M | 15 | No | Yes | ||||
| 8 | M | 53 | No | No | Metoprolol | |||
| 9 | F | 20 | Yes | RBBB, sup axis | LV apex | No | Carvedilol | Yesa |
| 10 | F | 18 | Yes | Multi-form | LV apex | No | Flecainide | |
| 11 | F | 17 | No | No | ||||
| 12 | M | 29 | No | No | ||||
| 13 | M | 47 | No | No | Metoprolol | |||
| 14 | F | 29 | Yes | Multi-form | LVOT | No | Flecainide, bisoprolol | |
| 15 | F | 44 | Yes | Multi-form | LV apex | No | Metoprolol | |
| 16 | M | 25 | Yes | LBBB, inf axis | RVOT | No | Yesb | |
| 17 | F | 21 | Yes | Multi-form | LV basal | No | Metoprolol | |
| 18 | F | 26 | Yes | Multi-form | RVOT | No | Metoprolol | |
| 19 | M | 73 | No | No | Metoprolol | |||
| 20 | M | 29 | Yes | Multi-form | RV basal | No | ||
| 21 | F | 36 | Yes | LBBB, inf axis | RVOT | No | Bisoprolol | |
| 22 | M | 43 | Yes | Unknown | No | Propranolol | ||
| 23 | F | 33 | Yes | RBBB, inf axis | LVOT | No | Bisoprolol | |
| 24 | M | 28 | No | No | Bisoprolol |
ICD, implantable cardioverter therapy; inf, inferior; LBBB, left bundle branch block; LV, left ventricle; LVOT, left ventricular outflow tract; PVC, premature ventricular complexes; RBBB, right bundle branch block; RV, right ventricle; RVOT, right ventricular outflow tract; sup, superior.
aRF ablation dominant PVC inferolateral LV.
bRF ablation monomorphic PVCs in anteroseptal RVOT.
Discussion
With this study, we expanded our previous report on the presence of MAD in patients with IVF.10 This study demonstrates that a high prevalence of inferolateral MAD is a consistent finding in this population. Furthermore, our focus on the pro-arrhythmogenicity of MAD in IVF provided several interesting findings. First, we show that a high PVC burden and non-sustained VTs are more frequently found in IVF patients with inferolateral MAD than in those without. Secondly, these pro-arrhythmic characteristics were more prevalent when additional mitral valve abnormalities (MVP or MAD in multiple areas) were present. Last, multi-form PVCs were abundant in IVF patients with inferolateral MAD. These findings suggest that arrhythmias in these patients might be caused by abnormalities affecting the whole continuum of the mitral valve annulus.
Prevalence of MAD
The first descriptions of MAD date back to before 19909,17,18 MAD has recently regained much interest, which has led to several cohort studies, review articles, and a consensus statement.4,6,10,19–22 Zugwitz et al. shed important light on the prevalence of MAD in the general population and suggest an importance for the location of MAD.7,23 Consistent with our previous findings, anterior and inferior MAD are frequently found, both in IVF patients and in healthy controls.10 Inferolateral MAD was however uncommon in a healthy population and was more frequently found in IVF patients (6.2% in healthy controls vs. 13% in our IVF cohort).7,10 Furthermore, MVP was also found more often in IVF patients (7.1% in IVF patients, compared with 3.4% in the healthy controls).7,11 When comparing our results with the large control group described by Zugwitz et al., the high prevalence of inferolateral MAD in IVF patients appears to be a consistent finding.
Arrhythmogenesis and myocardial fibrosis
One of the first reports on the MAD arrhythmic syndrome by Dejgaard et al. showed that severe arrhythmias in MAD patients were associated with the presence of papillary muscle fibrosis.6 Myocardial fibrosis is also an important predictor for adverse arrhythmic outcomes in MVP patients.24 We did not identify any papillary muscle fibrosis in IVF patients. However, we acknowledge that identifying fibrosis on papillary muscles with CMR is challenging due to the small structures and the relatively low spatial resolution of CMR. In addition, evident pathological LGE patterns fitting a specific diagnosis would have prevented the diagnosis IVF. The presence of any LGE in the LV did not differ between patients with or without inferolateral MAD. T1-mapping has been suggested to be of importance in MVP patients with or without MAD.25,26 As shown by Pavon et al., an increased synthetic myocardial extracellular volume can be present even in the absence of LGE.26 Implementing T1-mapping and CMR feature tracking could reveal sub-clinical abnormalities in IVF patients with inferolateral MAD that might correlate with arrhythmias.25,27
Pro-arrhythmogenicity and ECG abnormalities
A prominent pro-arrhythmic profile, with a higher burden of PVCs and non-sustained VTs, dominates in patients with inferolateral MAD. Studies focusing on patients with MVP and MAD show both similarities and differences.5,6 Essayagh et al. showed that in patients with MVP, MAD was associated with arrhythmic events, without influence on mortality.5,28 The evaluation of 12-lead ECGs with PVCs and non-sustained VTs appeared as polymorphic complexes in 60% of our patients with inferolateral MAD, in line with previous reports showing that polymorphic ectopy can be found in patients with MAD.21,29 The finding supports the hypothesis that an abnormal mechanical motion resulting in conduction abnormalities could be the substrate for arrhythmias in MAD.29 The increased pro-arrhythmic profile when additional mitral valve abnormalities are present further corroborates this hypothesis. However, this is in contrast with the previous report from Dejgaard et al. showing that patients with MAD without MVP had more severe arrhythmic events.6 Furthermore, our pro-arrhythmic characteristics do not reflect on sustained ventricular arrhythmias since appropriate ICD therapy during follow-up did not differ. More research is needed to fully clarify the pro-arrhythmic substrate in MAD with and without additional mitral valve abnormalities.
Clinical consequences and future directions
In our previous report on the prevalence of MAD in IVF patients, we advocated that examination of the mitral valve deserves attention during the clinical evaluation of patients after an unexplained sudden cardiac arrest. This study further supports this recommendation. Interestingly, IVF patients with inferolateral MAD more often received pharmaceutical therapy, primarily beta blockers, during follow-up. Pharmaceutical therapy is not generally indicated for patients with IVF. This might have lowered the PVC burden during follow-up and also influenced sustained ventricular arrhythmias. Recent studies focused on the indication for flecainide treatment in arrhythmic MVP syndrome, which could also provide interesting findings for MAD patients.30 We did not observe a significant difference in pro-arrhythmic characteristics when stratified by inferolateral MAD length. Previous studies did show an increased risk for arrhythmias with larger MAD length.21,29 More insights into ‘normal’ or ‘benign’ MAD length could lead to a better understanding of the pathogenic mechanisms underlying MAD.
Limitations
The retrospective aspects of this study had limitations. First, we needed to re-evaluate performed CMR images, in which a uniform CMR protocol was not initiated. Artefacts or the absence of LGE sequences might have resulted in missing data. In addition, as T1-mapping was not performed in most patients, analysis for subtle fibrosis was not possible. Because this is a multi-centre study, field strength and vendor-related differences between centres complicates the comparison of T1-mapping results. Secondly, determining the cut-off value of MAD is debatable. In our previous report we used ≥2 mm, however, to enable comparison with the study from Zugwitz et al. we now used ≥1 mm. This definition was based on the consensus statement of CMR.31 Thirdly, information regarding arrhythmia characteristics and pharmaceutical treatment were also retrospectively collected, and registrations of PVCs or non-sustained VT were not uniform across different centres. Furthermore, we were unable to retrieve the specific indication for initiating pharmaceutical treatment in many patients. Due to the lack of uniformity in reporting several variables, information might have been missed that could have influenced our conclusion. Finally, even though our cohort consists of one of the largest number of IVF patients, we were unable to prove causality and can only conclude on a possible association. Future prospective studies should focus on proving causality in this high-risk population.
Conclusion
This study revealed a significant prevalence of inferolateral MAD and MVP among IVF patients. Notably, we observed distinct pro-arrhythmic characteristics in patients with inferolateral MAD compared with those without.
Supplementary Material
Contributor Information
L M Verheul, University Medical Center Utrecht, Heidelberglaan 100, 3584 CX Utrecht, The Netherlands.
M Guglielmo, University Medical Center Utrecht, Heidelberglaan 100, 3584 CX Utrecht, The Netherlands.
S A Groeneveld, University Medical Center Utrecht, Heidelberglaan 100, 3584 CX Utrecht, The Netherlands.
F P Kirkels, University Medical Center Utrecht, Heidelberglaan 100, 3584 CX Utrecht, The Netherlands.
C Scrocco, Cardiology Research Section, St. George University of London, Cranmer Terrace, London SW17 0RE, UK; St George’s University Hospitals NHS Foundation Trust, London SW17 0QT, UK.
M J Cramer, University Medical Center Utrecht, Heidelberglaan 100, 3584 CX Utrecht, The Netherlands.
M Bootsma, Leiden University Medical Center, Albinusdreef 2, 2333 ZA Leiden, The Netherlands.
G F L Kapel, Medisch Spectrum Twente, Koningstraat 1, 7512 KZ Enschede, The Netherlands.
M Alings, Amphia Hospital, Molengracht 21, 4818 CK Breda, The Netherlands.
R Evertz, Radboud UMC, Geert Grooteplein Zuid 10, 6525 GA, Nijmegen, The Netherlands.
B A Mulder, University Medical Center Groningen, Hanzeplein 1, 9713 GZ Groningen, The Netherlands.
N H J Prakken, University Medical Center Groningen, Hanzeplein 1, 9713 GZ Groningen, The Netherlands.
J C Balt, St. Antonius Hospital, Koekoekslaan 1, 3435 CM Nieuwegein, The Netherlands.
P G A Volders, Maastricht University Medical Center+, Peter Debyelaan 25, 6229 HX Maastricht, The Netherlands.
A Hirsch, Thorax Center, Cardiovascular Institute, Erasmus MC, Dr. Molewaterplein 40, 3015 GD Rotterdam, The Netherlands.
S C Yap, Thorax Center, Cardiovascular Institute, Erasmus MC, Dr. Molewaterplein 40, 3015 GD Rotterdam, The Netherlands.
P G Postema, Department of Cardiology, Amsterdam UMC location University of Amsterdam, Meibergdreef 9, Amsterdam, The Netherlands; Amsterdam Cardiovascular Sciences, Heart Failure and Arrhythmias, Amsterdam, The Netherlands.
R Nijveldt, Radboud UMC, Geert Grooteplein Zuid 10, 6525 GA, Nijmegen, The Netherlands.
B K Velthuis, University Medical Center Utrecht, Heidelberglaan 100, 3584 CX Utrecht, The Netherlands.
E R Behr, Cardiology Research Section, St. George University of London, Cranmer Terrace, London SW17 0RE, UK; St George’s University Hospitals NHS Foundation Trust, London SW17 0QT, UK.
A A M Wilde, Department of Cardiology, Amsterdam UMC location University of Amsterdam, Meibergdreef 9, Amsterdam, The Netherlands; Amsterdam Cardiovascular Sciences, Heart Failure and Arrhythmias, Amsterdam, The Netherlands.
R J Hassink, University Medical Center Utrecht, Heidelberglaan 100, 3584 CX Utrecht, The Netherlands.
Supplementary data
Supplementary data are available at European Heart Journal—Cardiovascular Imaging online.
Funding
This work is funded by the Dutch Heart Foundation (CVON2017-13 VIGILANCE). E.R.B., C.S., A.A.M.W., P.G.P. acknowledge the Genomics of Unexplained Cardiac Arrest (The GenUCA) project, funded by the British Heart Foundation Special Project no. SP/20/4/35124, the German Center for Cardiovascular Research and the Dutch Heart Foundation. E.R.B. and C.S. received funding from the Robert Lancaster Memorial fund.
Data availability
Data are available upon reasonable request to the corresponding author.
References
- 1. Conte G, Giudicessi JR, Ackerman MJ. Idiopathic ventricular fibrillation: the ongoing quest for diagnostic refinement. Europace 2021;23:4–10. [DOI] [PubMed] [Google Scholar]
- 2. Visser M, van der Heijden JF, Doevendans PA, Loh P, Wilde AA, Hassink RJ. Idiopathic ventricular fibrillation. Circ Arrhythm Electrophysiol 2016;9:1–11. [DOI] [PubMed] [Google Scholar]
- 3. Basso C, Perazzolo Marra M, Rizzo S, Lazzari M De Giorgi B, Cipriani A, . Arrhythmic mitral valve prolapse and sudden cardiac death. Circulation 2015;132:556–66. [DOI] [PubMed] [Google Scholar]
- 4. Sabbag A, Essayagh B, Barrera JDR, Basso C, Berni A, Cosyns B, et al. EHRA expert consensus statement on arrhythmic mitral valve prolapse and mitral annular disjunction complex in collaboration with the ESC Council on Valvular Heart Disease and the European Association of Cardiovascular Imaging endorsed CBY the Heart Rhythm Society, by the Asia Pacific Heart Rhythm Society, and by the Latin American Heart Rhythm Society. Europace 2022;24:1981–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Essayagh B, Sabbag A, Antoine C, Benfari G, Batista R, Yang LT, et al. The mitral annular disjunction of mitral valve prolapse: presentation and outcome. JACC Cardiovasc Imaging 2021;14:2073–87. [DOI] [PubMed] [Google Scholar]
- 6. Dejgaard LA, Skjølsvik ET, Lie ØH, Ribe M, Stokke MK, Hegbom F, et al. The mitral annulus disjunction arrhythmic syndrome. J Am Coll Cardiol 2018;72:1600–9. [DOI] [PubMed] [Google Scholar]
- 7. Zugwitz D, Fung K, Aung N, Rauseo E, McCracken C, Cooper J, et al. Mitral annular disjunction assessed using CMR imaging. JACC Cardiovasc Imaging 2022;15:1856–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Toh H, Mori S, Izawa Y, Fujita H, Miwa K, Suzuki M, et al. Prevalence and extent of mitral annular disjunction in structurally normal hearts: comprehensive 3D analysis using cardiac computed tomography. Eur Heart J Cardiovasc Imaging 2021;22:614–22. [DOI] [PubMed] [Google Scholar]
- 9. Hutchins GM, Moore GW, Skoog DK. The association of floppy mitral valve with disjunction of the mitral annulus fibrosus. N Engl J Med 1986;314:535–40. [DOI] [PubMed] [Google Scholar]
- 10. Groeneveld SA, Kirkels FP, Cramer MJ, Evertz R, Haugaa KH, Postema PG, et al. Prevalence of mitral annulus disjunction and mitral valve prolapse in patients with idiopathic ventricular fibrillation. J Am Heart Assoc 2022;11:e025364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alqarawi W, Tadros R, Roberts JD, Cheung CC, Green MS, Burwash IG, et al. The prevalence and characteristics of arrhythmic mitral valve prolapse in patients with unexplained cardiac arrest. JACC Clin Electrophysiol 2023;9:2494–503. [DOI] [PubMed] [Google Scholar]
- 12. Zeppenfeld K, Tfelt-Hansen J, de Riva M, Winkel BG, Behr ER, Blom NA, et al. 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death developed by the task force for the management of patients with death of the European Society of Cardiology (ESC). Eur Heart J 2022;43:3997–4126. [DOI] [PubMed] [Google Scholar]
- 13. Postema PG, Christiaans I, Hofman N, Alders M, Koopmann TT, Bezzina CR, et al. Founder mutations in The Netherlands: familial idiopathic ventricular fibrillation and DPP6. Neth Heart J 2011;19:290–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Han Y, Peters DC, Salton CJ, Bzymek D, Nezafat R, Goddu B, et al. Cardiovascular magnetic resonance characterization of mitral valve prolapse. JACC Cardiovasc Imaging 2008;1:294–303. [DOI] [PubMed] [Google Scholar]
- 15. Perazzolo Marra M, Basso C, De Lazzari M, Rizzo S, Cipriani A, Giorgi B, . Morphofunctional abnormalities of mitral annulus and arrhythmic mitral valve prolapse. Circ Cardiovasc Imaging 2016;9:e005030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. Circulation 2002;105:539–42. [DOI] [PubMed] [Google Scholar]
- 17. Angelini A, Ho SY, Anderson RH, Becker AE, Davies MJ. Disjunction of the mitral annulus in floppy mitral valve. N Engl J Med 1988;318:188–9. [DOI] [PubMed] [Google Scholar]
- 18. Bharati S, Granston AS, Liebson PR, Loeb HS, Rosen KM, Lev M, et al. The conduction system in mitral valve prolapse syndrome with sudden death. Am Heart J 1981;101:667–70. [DOI] [PubMed] [Google Scholar]
- 19. Faletra FF, Leo LA, Paiocchi VL, Schlossbauer SA, Pavon AG, Ho SY, et al. Morphology of mitral annular disjunction in mitral valve prolapse. J Am Soc Echocardiogr 2022;35:176–86. [DOI] [PubMed] [Google Scholar]
- 20. Anderson RH, Garbi M, Zugwitz D, Petersen SE, Nijveldt R. Anatomy of the mitral valve relative to controversies concerning the so-called annular disjunction. Heart 2022;109:734–9. [DOI] [PubMed] [Google Scholar]
- 21. Raina A, Gersh BJ, Asirvatham SJ, Del-Carpio Munoz F. Characterization of ventricular arrhythmias and sudden cardiac death in subjects with mitral valve prolapse and mitral annular disjunction. Heart Rhythm 2023;20:112–21. [DOI] [PubMed] [Google Scholar]
- 22. Bennett S, Tafuro J, Duckett S, Appaji A, Khan JN, Heatlie G, et al. Definition, prevalence, and clinical significance of mitral annular disjunction in different patient cohorts: a systematic review. Echocardiography 2022;39:514–23. [DOI] [PubMed] [Google Scholar]
- 23. Haugaa KH, Aabel EW. Mitral annular disjunction: normal or abnormal: it is all about location. JACC Cardiovasc Imaging 2022;15:1867–9. [DOI] [PubMed] [Google Scholar]
- 24. Figliozzi S, Georgiopoulos G, Lopes PM, Bauer KB, Moura-Ferreira S, Tondi L, et al. Myocardial fibrosis at cardiac MRI helps predict adverse clinical outcome in patients with mitral valve prolapse. Radiology 2023;306:112–21. [DOI] [PubMed] [Google Scholar]
- 25. Guglielmo M, Fusini L, Muscogiuri G, Baessato F, Loffreno A, Cavaliere A, et al. T1 mapping and cardiac magnetic resonance feature tracking in mitral valve prolapse. Eur Radiol 2021;31:1100–9. [DOI] [PubMed] [Google Scholar]
- 26. Pavon AG, Arangalage D, Pascale P, Hugelshofer S, Rutz T, Porretta AP, et al. Myocardial extracellular volume by T1 mapping: a new marker of arrhythmia in mitral valve prolapse. J Cardiovasc Magn Reson 2021;23:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guglielmo M, Arangalage D, Bonino MA, Angelini G, Bonanni M, Pontone G, et al. Additional value of cardiac magnetic resonance feature tracking parameters for the evaluation of the arrhythmic risk in patients with mitral valve prolapse. J Cardiovasc Magn Reson 2023;25:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Essayagh B, Sabbag A, El-Am E, Cavalcante JL, Michelena HI, Enriquez-Sarano M. Arrhythmic mitral valve prolapse and mitral annular disjunction: pathophysiology, risk stratification, and management. Eur Heart J 2023;44:3121–35. [DOI] [PubMed] [Google Scholar]
- 29. Drescher CS, Kelsey MD, Yankey GS, Sun AY, Wang A, Sadeghpour A, et al. Imaging considerations and clinical implications of mitral annular disjunction. Circ Cardiovasc Imaging 2022;15:E014243. [DOI] [PubMed] [Google Scholar]
- 30. Aabel EW, Dejgaard LA, Chivulescu M, Helle-Valle TM, Edvardsen T, Hasselberg NE, et al. Flecainide in patients with arrhythmic mitral valve syndrome: a case series. Heart Rhythm 2023;20:635–6. [DOI] [PubMed] [Google Scholar]
- 31. Garg P, Swift AJ, Zhong L, Carlhäll CJ, Ebbers T, Westenberg J, et al. Assessment of mitral valve regurgitation by cardiovascular magnetic resonance imaging. Nat Rev Cardiol 2020;17:298–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request to the corresponding author.