Abstract
Key clinical message
Ayurveda Rasayana Therapy (ART) may serve as a safe and effective alternative treatment option for chemo‐intolerance high‐grade stage IV follicular lymphoma patients for increasing survival and tumor regression.
Abstract
Follicular lymphoma (FL), also called follicle center lymphoma/nodular lymphoma, observed in the B lymphocytes (B‐cells). Available therapeutic options for follicular lymphoma are associated with various side effects and, patients with co‐morbidities can seldom tolerate the chemotherapy regimens. Rasayana therapy not only resulted in tumor regression and improved survival but also dealt with the adverse effects of previous chemotherapy drugs. Herein, we present a case of a 74‐year‐old female diagnosed with Follicular lymphoma who had undergone three cycles of chemotherapy with unresolved disease outcome and serious adverse events. The patient refused to undergo further cycles of chemotherapy. Her family decided to start Ayurveda treatment for her as an alternative therapy for cancer care. On thorough case taking considering the Ayurveda parameters personalized Rasayana therapy as planned for the patient with an aim for improvement in Quality of Life (QoL), increasing survival, and optimizing body's immune response to fight the tumor. After treatment of 8 months, this case demonstrated partial tumor response as evidenced by PET‐CT‐scan. Quality of Life as evaluated using FACT‐G was also seen improved besides significant improvement in physical performance status evaluated using ECOG. The patient showed a survival of 3.5 years after starting Ayurveda Rasayana Therapy (ART). Rasayana therapy was well tolerated by the patient. This case report indicates the potential role of ART as a therapeutic option in geriatric cancer patients who are not eligible for cytotoxic interventions. Case warrants further systematic investigation to evaluate the potential role of ART in the treatment of geriatric cancer patients.
Keywords: Ayurvedic Rasayana therapy, CAM (complementary and alternative therapies), chemo‐intolerance, follicular lymphoma, geriatric cancer, quality of life
1. INTRODUCTION
Follicular lymphoma (FL), also known as follicle center lymphoma/nodular lymphoma, comprises a heterogeneous clinical pathological entity. 1 , 2 , 3 Chemotherapy against FL includes the combination of cyclophosphamide, vincristine, and prednisone (CVP). 4 , 5 Rituximab, a monoclonal antibody, has been included in the above treatment regimen. 6 , 7 , 8
Despite its potential activity, Rituximab is associated with severe adverse effects. 9 Various randomized controlled trials and post‐marketing surveillance studies have reported Rituximab‐related SAEs sometimes leading to ICU admission of the patient. Multiple cases of angina, acute coronary syndrome (ACS), and arrhythmias have been reported on an infusion of Rituximab which has been asked to be taken care of by the FDA. 10 , 11 , 12 , 13
To overcome these adverse effects associated with the available treatment regimen, Ayurvedic therapies have emerged as one of the most commonly used complementary and alternative therapies (CAM) for people with cancer. 14 Although personalized medicines, especially in cancer have recently gained a reputation, has been practiced in Ayurveda for ages in the Indian traditional medicinal system.
Rasayu Cancer Clinic focuses on using Rasayana treatment based on the patient's various clinical parameters. 15 Rasayana formulation regimen to be used is decided based on assessment parameters like ayurvedic three dynamic pathophysiological entities viz. Vata, Pitta, and Kapha. Here, we present a case where we have used Rasayana therapy customized to the patient's condition, including nano‐biocompatible herbo‐metallic complexes/bhasma prepared by ayurvedic manufacturing processes and herbal constituents. It was targeted to follicular lymphoma refractory to chemotherapies. Our treatment regimen not only resulted in tumor regression and improved survival but also dealt with the adverse effects of previous chemotherapy drugs. This paper is based on clinical and imaging tests comparison between pre‐ and post‐Rasayana therapy.
2. CASE EXAMINATION
Herein, we are discussing a case of 74 years‐old female patient with a past medical history of Type II Diabetes Mellitus (T2DM), Hypertension, Ischemic Heart disease, dilated cardiomyopathy, chronic atrial fibrillation (AF), she was on diltiazem for rate control for 13 years along with a history of Cardiovascular attack (CVA) and on medications for her concomitant diseases.
2.1. Clinical findings
She presented at the emergency department outside the hospital on October 23, 2015 with transient loss of consciousness, difficulty in walking, proximal weakness in leg, and dysarthria. Her ultrasonography and MRI suggested underlying malignancy or kochs.
2.2. Timeline
The patient timeline is shown in Table 1.
TABLE 1.
Timeline of patient.
| October 23, 2015 | She presented at the emergency department outside the hospital with transient loss of consciousness, difficulty in walking, proximal weakness in leg, and dysarthria. |
| October 29, 2015 | Subsequent investigations were s/o‐Grade III Follicular lymphoma, stage IV on the Ann Arbor staging system. FLIPI was 4 |
| November 14, 2015 | Started chemotherapy with Rituximab (Inj. Restova 500 mg) |
| November 24, 2015 | Started Rasayan therapy, received 3 cycles of chemotherapy. However, post cycles suffered from ADRs like cardiac toxicity (CTCAE Grade III), Lung Infection (CTCAE V5 –Grade III), Oral Mucositis (CTCAE V5Gr II), and hematological toxicity (CTCAE Grade II) |
| January 6, 2016 |
Due to intolerance, chemotherapy had to be discontinued. Patient's prognosis was poor. Further, she was treated with exclusive Rasayana therapy from 06 January 2016 for 41 months. ART is tolerated well by the patient. Tumor response was observed along with improvement in quality of life physical performance status. The patient showed a survival of 3.5 years after starting ART. |
2.3. Diagnostic assessment
Her PET/CT scan dated October 26, 2015 showed lymphomatous disease involvement, multiple metabolically active bilateral lung, and left four costovertebral lesions (Figure 1A; Table 2). Her tuberculin test was negative. On examination, the physician found bilateral palpable axillary lymph nodes. Axillary lymph node excision biopsy was done after medical fitness on October 29, 2015, which showed Grade III Follicular lymphoma. Immunohistochemistry report revealed follicles positivity for CD20, CD10, and BCL‐2 and was negative for CD3, CD5, CD23, and cyclin D1, MiB‐1 score was more than 20%. Following the manifestation of PET/CT disease, she was classified as stage IV on the Ann Arbor staging system. The Follicular lymphoma international prognostic index (FLIPI) was 4, indicating the patient at higher risk for rapid disease progression.
FIGURE 1.
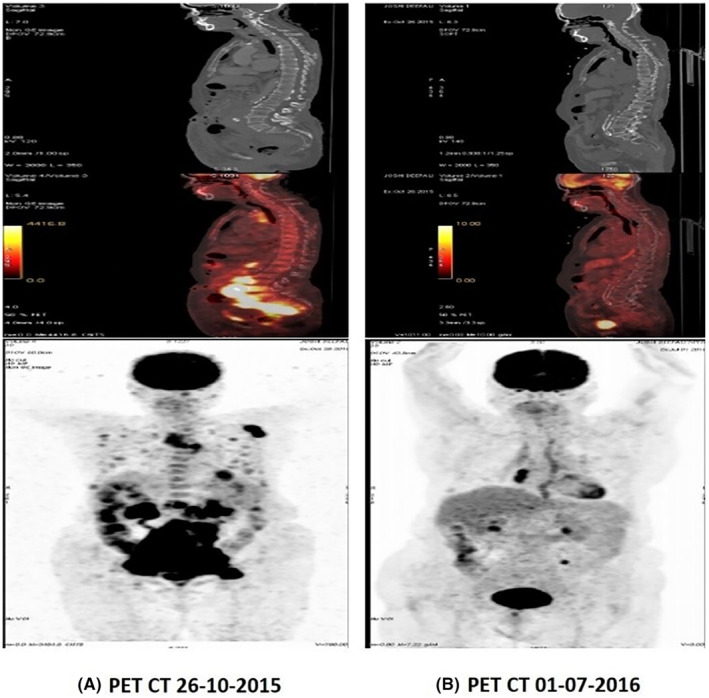
Comparison of PET‐Scan (A) before initiation of chemotherapy (B) patient on both chemotherapy and Rasayana therapy.
TABLE 2.
Observed 2D‐Echo cardiography (2D‐Echo) parameters pre and post‐chemotherapy.
| Parameters | 2D‐Echo before chemotherapy‐October 29, 2015 | 2D‐Echo after 3 cycles of chemotherapy‐January 24, 2016 |
|---|---|---|
| LV–in Diastole | Left ventricular diastolic dysfunction | Grade III left ventricular diastolic dysfunctions/restrictive physiology |
| LV | Mild concentric LVH | Mild concentric LVH |
| LV–in Systole | Normal LV systolic function | Normal LV Systolic Function |
| Ectopics | Observed | Not observed |
| Pulmonary HTN | Moderate pulmonary hypertension | Moderate pulmonary hypertension |
| Valvular regurgitation |
Mild aortic regurgitation Mild mitral regurgitation Mild tricuspid regurgitation |
Trivial aortic regurgitation Mild mitral regurgitation Mild tricuspid regurgitation |
| Interventricular septum | Mildly dilated sclerosed AV | Sclerocalcific AV |
| RA/LA/LV | RA/RV Mildly dilated | LA/RA Mildly dilated |
| EF | 60% | 60% |
2.4. Treatment protocol
2.4.1. Previous chemotherapy sessions (from November 14, 2015 to January 5, 2016)
She was treated with three cycles of chemotherapy with Rituximab (Inj. Restova 500 mg) started on November 14, 2015. However, post chemotherapy cycles she, complains of increased fatigue, general weakness, low physical performance, palpitations, and stomatitis. Hence, she visited Rasayu Cancer Clinic, Pune, on November 24, 2015 and opted for ART (Table 3) to maintain a good Quality of Life (QoL). Treatment at Rasayu was initiated on the same day along with the continuation of chemotherapy. The second cycle of chemotherapy was received on December 7, 2015. On January 7, 2016 patient suffered from retrosternal pain and palpitations and was admitted in a hospital. Her ECG revealed Atrial fibrillation with VR −130/min with no ST‐T changes, and 2 D Echo revealed Grade III left ventricular diastolic dysfunction with restrictive physiology. Conservative management was done, and the patient was discharged on January 10, 2016. After the third cycle of chemotherapy with Rituximab 500 mg and Bendamustine 90 mg on January 6, 2016, the patient was admitted to the hospital on January 24, 2016 with complaints about palpitations, shortness of breath after minimal physical exertion, and general weakness. Her hemogram revealed anemia (Hb 7.3gm %) and leukocytosis (WBC‐26,871/cumm). 2 D Echo revealed Atrial fibrillation with controlled VR (Table 2). Chest X‐ray revealed consolidation in the right mid zone, which suggests infective pneumonitis with pleural effusion. She was treated for LRTI with middle zone pneumonia, CCF, along with anemia and discharged on 2 February 2016. She was also cautioned about the poor prognosis of the disease. Due to intolerance, chemotherapy had to be discontinued. Other conventional chemotherapeutic therapeutic options were discussed with the patient by the treating oncologist, but the patient refused to opt for any further chemotherapy. Repeated hospitalization was one reason for the refusal and abandonment of different chemotherapy cycles by patient and she additionally opted for exclusive ART from January 6, 2016.
TABLE 3.
Ayurvedic Rasayana therapy details.
| Medicines | Main ingredients | Dose and Anupan (to be taken with) |
|---|---|---|
| Navjeevan Rasayana (proprietary ayurvedic formulation) | Purified biocompatible Hartal bhasma–12 mg (An Arsenic preparation) | 1 cap early in the morning with Honey |
| Combination of medicines (powder of classical ayurved formulation) | Tribhuvankirti Ras (70 mg), Tankan bhasma (70 mg), Shwaskuthar Ras (200 mg), Aarogyawardhini (200 mg), Kanchnar Guggul (200 mg), Tapyadiloha (200 mg), Ashwagandha powder (200 mg) | 1 sachet two times in a day after having food |
| Hirak (proprietary ayurved formulation) | Purified biocompatible HirakBhasma 12.5 mg | 1 cap before bed with Honey |
| Dasma Rasayan (Proprietary ayurved formulation) | Purified biocompatible copper formulation 15 mg | 1 cap before bed with Honey |
| Sindoorbhushan (proprietary ayurved formulation) | Purified mercury 16 mg, Purified Sulphur16 mg, Purified biocompatible Goldbhasma1 mg | 1 cap before bed with Honey |
| Sutendra Rasayan (proprietary ayurved formulation) | Sootshekhar 115 mg (Herbomineral Classical Preparation purified silver 3 mg, and Gold 2 mg preparation) | 1 cap before bed with Honey |
| Arpisa Rasayan (proprietary ayurved formulation) |
Purified biocompatible Gold 4 mg Purified biocompatible Silver 5 mg Purified biocompatible Iron 25 mg Purified biocompatible Gold+Mercury 25 mg preparation |
1 cap before bed with Honey |
Note: All ayurvedic formulations used are FDA approved AND CGMP‐mfg facility.
2.4.2. Ayurvedic Rasayana therapy
Treatment at Rasayu Cancer Clinic was started after consideration of patient disease and clinical status. The patient started ayurvedic Rasayana therapy on November 24, 2015 along with chemotherapy (Figure 1B). Discontinued chemotherapy on January 6, 2016, and the patient was solely on Rasayana treatment for a further 41 months (Table 3).
Various cohort studies and oncologists opined that geriatric patients who suffer from such acute decompensated heart failure have a survival of less than 1 year. It can state that the mortality rate is very high due to other co‐morbidities/adverse effects. 16
Considering her co‐morbidities and all her clinical signs and symptoms, she was prescribe the treatment protocol as per Table 3. The therapy for this patient involved a combination of diverse elements and herbs, which were selected based on the principles of Ayurveda. The aim of the therapy was to restore a physiological equilibrium in the patient, which refers to correcting “doshadushyavaishyamya” as per the principles of Ayurveda. ART also aims toward increasing life span (Overall survival) besides improving quality of life and performance status. Considering this, any further clinical studies on ART should consider a whole system‐based pragmatic approach.
2.5. Treatment outcomes
2.5.1. Response to chemotherapy
Patient had developed an intolerance to chemotherapy and suffered from cardiac toxicity (CTCAE Grade III), Lung Infection (CTCAE V5 –Grade III), Oral Mucositis (CTCAE V5Gr II), and hematological toxicity (CTCAE Grade II).
2.5.2. Response to ART
Safety and tolerability of ART
These were monitored by periodically performing complete blood count, liver, and kidney function tests, and urine routine examination and 2 D Echocardiography (Table 4). The patient did not report any adverse drug reactions while on therapy. Details of various biochemical and cardiac parameters suggested that ART was safe with no vital organ deterioration. Significantly increased Hemoglobin from basic 7.3 gm% to 13 gm%. LDH was within a normal range.
TABLE 4.
Comparative 2D‐Echocardiography after 2 months and 39 months of Rasayana therapy.
| Parameters | 2D‐Echocardiography after 2 months of Rasayana therapy | 2D‐Echocardiography after 39 months of Rasayana therapy |
|---|---|---|
| RA/RV | Right atrium and right ventricle were normal. | |
| LV‐in Diastole | Patient is in lateral fibrillation with a controlled VR headset | No diastolic dysfunction |
| LA | Dilated LA | |
| LV | Mild concentric LVH | Mild concentric LVH |
| LVinsystole | Normal LV systolic function | Normal |
| Valvular |
Degenerative changes in cardiac valves Mild mitral regurgitation Mild tricuspid regurgitation |
Normal aortic valve Normal trileaflet valve Mild Tricuspid regurgitation |
| EF | 60% | 55% |
| Pulmonary HTN | moderate pulmonaryhypertension | No pulmonary hypertension |
| Pericardial effusion | No | No |
| Thrombus | No | No |
| Interventricular septum/Interatrial septum | Intact IAS, IVS |
Physical performance status and Quality of Life
These were evaluated in the patient; when came to our clinic, and had an ECOG score of 3 indicating that the patient could not take complete care of herself and was mostly confined to a bed or chair. After 8 months of ART treatment, this score was reduced to 1, which indicated that the patient became self‐capable and resumed all chores normally with minimal restriction. It was maintained throughout the treatment period, followed by deterioration observed in the last two visits. Weight increased significantly from 47.1 to 56.3 kg. Health‐related QoL assessed by Fact G score showed that score significantly increased from 48 to 95 after 8 months of treatment and maintained throughout the treatment period followed by deterioration observed in the last visit.
Tumor response
PET scan carried out after 8 months of treatment in Rasayu Cancer Clinic showed complete resolution of lesions at axillary, mesenteric, intra‐gluteal, and lungs (Figure 2A) Partial response was also seen in retroperitoneal, iliac, and mediastinal nodes. However, after 8 months of follow‐up in the PET scan, we observed new FDG avid lymph nodes in the Right inguinal, Bilateral pelvic, retroperitoneal, new lung nodules, left adrenal deposit & musculoskeletal lesion s/o‐progression. After another 6 months of treatment, partial tumor regression was noted (Figure 2B), whereas saw a mixed response was seen after 8 months of continuation of treatment (Figure 2C; Table 5). Although the first PET scan conducted after ART initiation showed tumor regression, it is unclear that she had an unresolved disease after her initial 3 cycles of chemotherapy. However, further scans show new malignant involvement of Rt external iliac node, Lt proximal common iliac mass, and lung nodules. Partial tumor response was seen in all these new lesions exclusively by ART, indicating the potential of ART, in inducing tumor regression.
FIGURE 2.
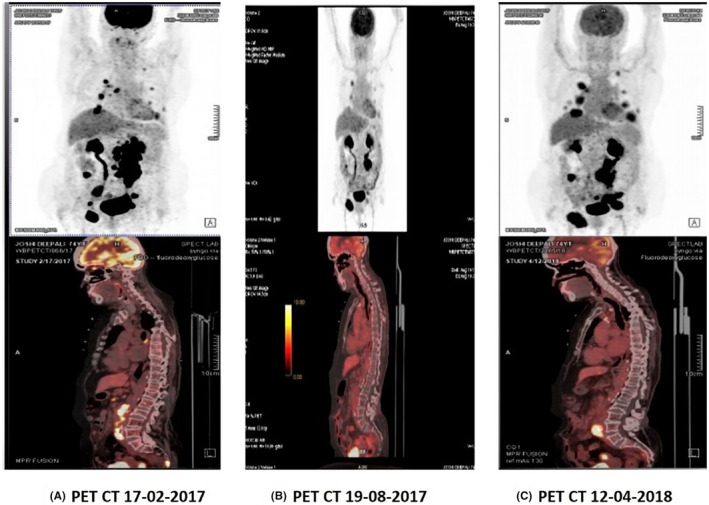
Comparison of PET‐Scan after (A) 8 months of ART alone (B) 6 months of ART alone (C) 14 months of Rasayana therapy alone.
TABLE 5.
PET‐CT comparison during all treatment periods (preand post‐therapy).
| Site | October 26, 2015 | July 1, 2016 | February 17, 2017 | August 19, 2017 | April 12, 2018 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Size | SUV | Size | SUV | Size | SUV | Size | SUV | Size | SUV | |
| Left axillary‐node | 27 × 14 mm | 11.97 | Resolved | — | — | — | — | |||
| Pre tracheal‐nodes | 25 × 28 mm | 4.78 | Reduced | 2.62 | Resolved | — | — | — | ||
| Lateral segment of Right Middle‐lobe | 16 × 19 mm | 2.84 | reduced | Resolved | — | — | — | |||
| Left paraaortic node | 21 × 21 mm | 11.11 | Reduced in size and metabolic activity | 7.18 | Resolved | — | — | — | ||
| Lesion in B/l ureter | 97 × 75 × 173 mm | 12.75 | Reduced | 9.18 | Resolved | — | — | — | ||
| Left adrenal nodule | 13 × 11 mm | 2.3 | Persistent | 3.83 | Increased | 4.13 | Persistent | 4.13 | Persistent | |
| New Right inguinal node | — | — | 24 × 32 mm | 22.31 | Increased | 12.33 | Persistent | Reduced | ||
| 39 × 35 mm | 9.77 | |||||||||
| Right externa liliac node mass | — | — | 11 mm | 12.54 | Decreased 6 mm | 3.08 | Reduced | 3.7 | ||
| Left proximal common iliac mass | — | — | 42 × 44 mm | 23.08 | Decreased | 10.62 | Decrease | 10.62 | ||
| 41 × 43 mm | 39 × 44 mm | |||||||||
| New deposit in right iliacus | — | — | 16 mm | 13.6 | Persistent | — | Increased | Decreased | ||
| 16 × 24 mm | 7.98 | |||||||||
| Lung nodule in right upper lobe | — | — | — | 8.62 | 5.32 | Increased | Decreased | |||
| 20 mm | 6.63 | |||||||||
| Lung nodule in right lower lobe | 10 | 4.52 | Increased 27 × 23 mm | 5.74 | ||||||
| New deposit posterior to left rectus abdominis | — | — | — | 17 mm | 4.31 | |||||
| Conclusion | Partial tumor response (PR) | Progression of disease (PD) | Partial tumor response (PR) | Mixed response overall disease progression | ||||||
Survival outcome
The patient was under treatment at Rasayu Cancer Centre for nearly 41 months which indicates that survival time has increased significantly after treatment at Rasayu in comparison to predictions by oncologists. The latest information as available is that the patient expired on April 12, 2019 after nearly 3.5 years of ART.
3. DISCUSSION
Available therapeutic regimen for follicular lymphoma is associated with various side effects and, patients with co‐morbidities can seldom tolerate the chemotherapy regimens. Here in this study, we discuss a case of 74‐year‐old female patient who had follicular lymphoma with discontinuation of chemotherapy due to severe adverse effects. The patient came to Rasayu Cancer Clinic with no other therapeutic option as suggested by her oncologist. Various such cases have been reported wherein, the patient is left with no choice after chemotherapy and has developed severe adverse effects. One such case of rituximab treatment was reported by Aagre et al., wherein a young patient of 33 years, was treated for stage IIIB follicular lymphoma with rituximab. Due to chemotherapy, the patient developed exertional dyspnea and cough, resulting in Rituximab discontinuation. 17 Similar side effects were noticed in a 65‐year‐old female treated with Rituximab‐CHOP chemotherapy for non‐Hodgkin lymphoma, which started with a dry cough and increased significantly after three chemotherapies that the patient needed hospitalization. The patient's respiratory status deteriorated so much that she was ventilated, had a cardiac arrest after an episode of self‐extubation, and died of multi‐organ failure. An autopsy revealed intra‐alveolar pulmonary hemorrhage. 18 Rituximab is the first monoclonal antibody designed for the treatment of B cell lymphomas. Randomized trials confirmed the efficacy of Rituximab in diffuse large B‐cell lymphoma (DLBCL), follicular lymphoma (FL), and mantle cell lymphoma. Several other clinical studies have shown that it has high activity and low toxicity ratio, and also it has activity in different types of lymphomas. Rituximab in combination with chemotherapy gives the highest efficacy than any chemotherapy described for DLBCL and FL. 19 Phase 3 clinical trials of idelalisib and rituximab have significantly improved overall survival in patients with chronic lymphocytic leukemia patients who were unable to undergo chemotherapy. 20 Another phase 3 showed the superiority of combining rituximab with the immunomodulatory drug than rituximab monotherapy in patients with recurring non‐Hodgkin lymphoma; however, some hematologic adverse events were seen like grade 3/4 neutropenia; gastrointestinal adverse events (AEs), and lowgrade cutaneous reactions such as rash. 21 Though Rituximab has demonstrated efficacy in various clinical trials on Lymphoma patients, some patients have reported being resistant to rituximab therapy. 22 Phase II trial of Rituximab in patients of follicular demonstrated that Grade 1 or 2 adverse events were the majority of reported toxicities and happened most frequently with the first infusion, decreasing with a subsequent infusion. 23
The principal pathology of cancer is dysregulation of the cell cycle called in Ayurveda terminology as vaishyamya. The main philosophy of Ayurveda shamana treatment is to establish harmony and equilibrium in any body's dysregulated systems. Rasayanachikitsa also acts through the same mechanism. Rasayana medicine potentiates the body's natural mechanism to stop the growth of cancer cells and correct the dysregulation in cancerous cells. ART also aims to qualitatively improve normal cells's function and offers them protection from undergoing mutation which, can cause dysregulation (Vaishyamya) in cell cycle. 24 , 25
Our major aim at Rasayu is to treat such patients with ART personalized for the subject based on the current disease status and other parameters, including patients' lifestyle‐related questionnaire usually studied in Ayurveda. This concept of personalized medicine has gained a lot of attention in modern medicine within the last decade. Still, it is a very familiar concept for Ayurveda. Various parameters of overall health, strength, immunity, and physiology among individuals are considered, which allows a holistic approach to choosing appropriate drug combinations. The medical condition of patient before initiating treatment at Rasayu revealed severe adverse effects in the form of rapid ventricular rate with acute decompensated heart failure after chemotherapy. Left ventricular diastolic dysfunction was observed in a 2D echo. The Oncologist predicted the maximum survival for 2–3 months and the patient was unable to sustain chemotherapy. ART was initiated, as mentioned in Table 1. These are ayurvedic metallic/mineral preparations with herbal extracts or decoctions exposed to heat as per traditional Indian Ayurveda. These bhasmas have been reported for various other medicinal uses such as anti‐oxidant, anti‐bacterial, anti‐inflammatory, and few show anti‐cancer potential against various cancer forms breast, lung, and cervical. 26 , 27 , 28 , 29 , 30 Hirak rasayana used has been studied for its anti‐cancer potential against breast cancer and other constituents. 31 Among these formulations, Suvarna bhasma has been explored widely for its anti‐cancer potential 32 and other medicinal properties, 33 along with its safety. 34 , 35
The results obtained after the above‐discussed treatment protocol were promising after 8 months of treatment of the patient at Rasayu. Complete resolution of lesions at axillary, mesenteric, intra‐gluteal, and lungs along with partial response in retroperitoneal, iliac, and mediastinal nodes was also seen in the PET scan. When assessed by 2D echo and biochemical parameters the abnormalities developed due to chemotherapy were also resolved. Normal aortic valve and normal tricuspid valve were recorded along with normal right atrium and right ventricle.
Real‐world data indicates that Rasayna therapy can have the potential to serve as a safe, alternative therapeutic approach for chemo‐intolerant or chemo‐resistant patients It was significant to observe that with treatment at Rasayu, no adverse effects developed, and in addition those present after chemotherapy were also resolved after discontinuation. In this patient, we also evaluated the quality of life using FACT–G at monthly intervals which showed substantial improvement in quality of life. ECOG score decreased from 3 to 1 indicating, that the patient who was mostly bed/chair ridden was able to independently perform all her day‐to‐day activities. Biochemical and Hematological parameters for safety were evaluated every 2 months, which indicates that our treatment regimen was safe and well tolerated by this patient.
This case study acts as an important documented preliminary source of evidence about the efficacy of ART in the management of high‐grade stage IV follicular lymphoma. However, considering the limitations of anecdotal observations it is not possible to make any further clinical recommendations based on the outcomes of this case study. As such it is important that further pragmatic clinical evaluation of this therapy, based on the leads obtained from this and similar case study is required to generate a high level of evidence for its efficacy and safety in the management of high‐grade stage IV follicular lymphoma.
4. CONCLUSION
Ayurveda Rasayana therapy which used various combinations of nano‐biocompatible herbo‐metallic complexes along with other constituents served as a safe and effective therapeutic approach in this patient. Rasayana treatment leads to tumor regression along with resolving adverse effects on various body systems that developed during chemotherapy. Survival of patients extended to 3.5 years which was significantly high compared to the predicted survival during chemotherapy. Increased survival was also associated with improved quality of life and performance status. More systematic data from well‐controlled clinical trials are required before making any clinical recommendations of ART as an alternative therapeutic option in chemo‐intolerant geriatric patients with lymphoma.
AUTHOR CONTRIBUTIONS
Yogesh Narayan Bendale: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; supervision; validation; visualization; writing – original draft; writing – review and editing. Poonam Birari‐Gawande: Conceptualization; data curation; formal analysis; investigation; methodology; validation; visualization; writing – original draft; writing – review and editing. Anandrao Patil: Conceptualization; data curation; formal analysis; investigation; methodology; validation; visualization; writing – original draft; writing – review and editing. Avinash Kadam: Conceptualization; data curation; formal analysis; investigation; methodology; validation; visualization; writing – original draft; writing – review and editing.
FUNDING INFORMATION
None.
CONFLICT OF INTEREST STATEMENT
None.
ETHICS APPROVAL
Personal data have been respected.
CONSENT
Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent is available for review by the Editor in Chief of this journal upon request.
Bendale YN, Birari‐Gawande P, Patil A, Kadam A. Ayurveda Rasayana Therapy (ART) leads to tumor regression and increased survival in chemo‐intolerance high‐grade stage IV follicular lymphoma: A case study. Clin Case Rep. 2024;12:e8076. doi: 10.1002/ccr3.8076
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this published paper.
REFERENCES
- 1. Tan DE, Foo JN, Bei JX, et al. Genome‐wide association study of B cell non‐Hodgkin lymphoma identifies 3q27 as a susceptibility locus in the Chinese population. Nat Genet. 2013;45:804‐807. [DOI] [PubMed] [Google Scholar]
- 2. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375‐2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ott G, Katzenberger T, Lohr A, et al. Cytomorphologic, immunohistochemical, and cytogenetic profiles of follicular lymphoma: 2 types of follicular lymphoma grade 3. Blood. 2002;99(10):3806‐3812. [DOI] [PubMed] [Google Scholar]
- 4. Nickenig C, Dreyling M, Hoster E, et al. Combined cyclophosphamide, vincristine, doxorubicin, and prednisone (CHOP) improves response rates but not survival and has lower hematologic toxicity compared with combined mitoxantrone, chlorambucil, andprednisone (MCP) in follicular and mantle cell ly. Cancer. 2006;107(5):1014‐1022. [DOI] [PubMed] [Google Scholar]
- 5. Mason SL, Grant IA, Elliott J, Cripps P, Blackwood L. Gastrointestinal toxicity after vincristine or cyclophosphamide administered with or without maropitant in dogs: a prospective randomised controlled study. J Small Anim Pract. 2014;55:391‐398. [DOI] [PubMed] [Google Scholar]
- 6. Salles G, Seymour JF, Offner F, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet. 2011;377:42‐51. [DOI] [PubMed] [Google Scholar]
- 7. Van Oers MHJ, Kersten MJ. Treatment strategies in advanced stage follicular lymphoma. Best Pract Res Clin Haematol. 2011;24:187‐201. [DOI] [PubMed] [Google Scholar]
- 8. Cvetković RS, Perry CM. Rituximab: a review of its use in non‐Hodgkin's lymphoma and chronic lymphocytic leukaemia. Drugs. 2006;66:791‐820. [DOI] [PubMed] [Google Scholar]
- 9. Kasi PM, Tawbi HA, Oddis CV, Kulkarni HS. Clinical review: serious adverse events associated with the use of rituximab – a critical care perspective. Crit Care. 2012;16:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dotan E, Aggarwal C, Smith MR. Impact of rituximab (Rituxan) on the treatment of B‐cell non‐Hodgkin's lymphoma. Pharmacol Ther. 2010;35:148‐157. [PMC free article] [PubMed] [Google Scholar]
- 11. Tydén G, Genberg H, Tollemar J, et al. A randomized, double blind, placebo‐controlled, study of single‐dose rituximab as induction in renal transplantation. Transplantation. 2009;87:1325‐1329. [DOI] [PubMed] [Google Scholar]
- 12. Feugier P. A review of rituximab, the first anti‐CD20 monoclonal antibody used in the treatment of B non‐Hodgkin's lymphomas. Future Oncol. 2015;11:1327‐1342. [DOI] [PubMed] [Google Scholar]
- 13. Foran JM, Rohatiner AZ, Cunningham D, et al. European phase II study of rituximab (chimeric anti‐CD20 monoclonal antibody) for patients with newly diagnosed mantle‐cell lymphoma and previously treated mantle‐cell lymphoma, immunocytoma, and small B‐cell lymphocytic lymphoma. J Clin Oncol. 2000;18:317‐324. [DOI] [PubMed] [Google Scholar]
- 14. Metri K, Bhargav H, Chowdhury P, Koka PS. Ayurveda for chemo‐radiotherapy induced side effects in cancer patients. J Stem Cells. 2013;8:115‐129. [PubMed] [Google Scholar]
- 15. Sumantran VN, Tillu G. Insights on personalized medicine from Ayurveda. J Altern Complement Med. 2013;19:370‐375. [DOI] [PubMed] [Google Scholar]
- 16. Natella PA, Le Corvoisier P, Paillaud E, et al. Long‐term mortality in older patients discharged after acute decompensated heart failure: a prospective cohort study. BMC Geriatr. 2017;17:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aagre S, Patel A, Kendre P, Anand A. Rituximab‐induced interstitial lung disease in a patient with follicular lymphoma: a rare case report. Lung India. 2015;32:620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aagre S, Patel A, Kendre P, Anand A. Fatal intra‐alveolar hemorrhage after rituximab in a patient with non‐Hodgkin lymphoma. Leuk Lymphoma. 2004;32(6):620. [DOI] [PubMed] [Google Scholar]
- 19. Winter MC, Hancock BW. Ten years of rituximab in NHL. Expert Opin Drug Saf. 2009;8:223‐235. [DOI] [PubMed] [Google Scholar]
- 20. Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370:997‐1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cheson BD, Morschhauser F, Martin P. Management of adverse events from the combination of rituximab and lenalidomide in the treatment of patients with follicular and low‐grade non‐Hodgkin lymphoma. Clin Lymphoma Myeloma Leuk. 2020;20:563‐571. [DOI] [PubMed] [Google Scholar]
- 22. Coiffier B. Rituximab therapy in malignant lymphoma. Oncogene. 2007;26:3603‐3613. [DOI] [PubMed] [Google Scholar]
- 23. Piro LD, White CA, Grillo‐Lopez AJ, et al. Extended rituximab (anti‐CD20 monoclonal antibody) therapy for relapsed or refractory low‐grade or follicular non‐Hodgkin's lymphoma. Ann Oncol. 1999;10:655‐661. [DOI] [PubMed] [Google Scholar]
- 24. Tiwari SN, Tiwari RP, Dwivedi SR, Kirar PK, Khodre SK. Therapeutics utilization of Rasayan chikitsa as per principles of Ayurveda. Int J Phytopharm. 2015;5:1‐3. [Google Scholar]
- 25. Sharma V, Chaudhary A. Concepts of Dhatu Siddhanta (theory of tissues formation and differentiation) and Rasayana; probable predecessor of stem cell therapy. Ayu. 2014;35:231‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kaur G, Gupta V, Bansal P. Innate antioxidant activity of some traditional formulations. J Adv Pharm Technol Res. 2017;8:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kumar BS, Saran GS, Mouna A, Kumar CN. In vitro anti–inflammatory activity of Tankana churna. Food Feed Res. 2013;40:17‐20. [Google Scholar]
- 28. Anuroopa HK. Clinical efficacy of Kapha Ketu rasa on Tamaka Swasa. J Ayurveda Integr Med Sci. 2016;1:10‐18. [Google Scholar]
- 29. Sankpal DJ, Takalikar DJ. Comperehencive review of Tankana. J Ayurveda Integr Med Sci. 2018;3:110‐115. [Google Scholar]
- 30. Palliyaguru DL, Singh SV, Kensler TW. Withania somnifera: from prevention to treatment of cancer. Mol Nutr Food Res. 2016;60:1342‐1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bakrania AK, Nakka S, Variya BC, et al. Antitumor potential of herbomineral formulation against breast cancer: involvement of inflammation and oxidative stress. Indian J Exp Biol. 2017;55:680‐687. [Google Scholar]
- 32. Das S, Das M, Paul R. Swarna Bhasma in cancer: a prospective clinical study. Ayu. 2012;33:365‐367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shah ZA, Gilani RA, Sharma P, Vohora SB. Attenuation of stress‐elicited brain Catecholamines, serotonin and plasma corticosterone levels by calcined gold preparations used in Indian system of medicine. Basic Clin Pharmacol Toxicol. 2005;96:469‐474. [DOI] [PubMed] [Google Scholar]
- 34. Sharma V, Hiremath RR, Patil PA, Prasad BS. Safety assessment on chronic administration of Swarna Bindu Prashan—a popular ayurvedic preparation of incinerated gold ashusedas immune booster to children in southern India. Indian J Exp Biol. 2017;55:217‐224. [Google Scholar]
- 35. Patil TS, Wele AA. Review on therapeutic potential of Ayurvedic medicine Swarnabhasma (incinerated gold). Indo Am J Pharm. 2018;5:192‐205. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published paper.


