Abstract
Simultaneous inhibition of angiotensin II AT1 and endothelin ETA receptors has emerged as a promising approach for treatment of chronic progressive kidney disease. This therapeutic approach has been advanced by the introduction of sparsentan, the first dual AT1 and ETA receptor antagonist. Sparsentan is a single molecule with high affinity for both receptors. It is US Food and Drug Administration approved for immunoglobulin A nephropathy (IgAN) and is currently being developed as a treatment for rare kidney diseases, such as focal segmental glomerulosclerosis. Clinical studies have demonstrated the efficacy and safety of sparsentan in these conditions. In parallel with clinical development, studies have been conducted to elucidate the mechanisms of action of sparsentan and its position in the context of published evidence characterizing the nephroprotective effects of dual ETA and AT1 receptor inhibition. This review summarizes this evidence, documenting beneficial anti-inflammatory, antifibrotic, and hemodynamic actions of sparsentan in the kidney and protective actions in glomerular endothelial cells, mesangial cells, the tubulointerstitium, and podocytes, thus providing the rationale for the use of sparsentan as therapy for focal segmental glomerulosclerosis and IgAN and suggesting potential benefits in other renal diseases, such as Alport syndrome.
Keywords: Angiotensin II, Endothelin Type A receptor, Endothelin-1, FSGS, IgA nephropathy, Sparsentan
Introduction
Evidence collected over three decades supports the use of dual inhibition of the renin–angiotensin–aldosterone (RAAS) and endothelin (ET) systems in the treatment of proteinuric chronic kidney disease (CKD), including rare glomerular disorders. This therapeutic approach has been facilitated by the development of sparsentan, the first dual ET and angiotensin receptor antagonist (DEARA) [1–4].
In parallel with clinical development, studies have been conducted to elucidate the mechanisms of action of this drug and its position in the context of published evidence characterizing the nephroprotective effects of dual ET receptor type A (ETAR) and angiotensin receptor type 1 (AT1R) inhibition. This review summarizes this evidence. In addition, we discuss the rationale for the use of sparsentan as therapy for focal segmental glomerulosclerosis (FSGS) and immunoglobulin A nephropathy (IgAN) and potential benefits in other renal diseases, such as Alport syndrome (AS).
Role of ET and RAAS in renal physiology and pathophysiology
Use of sparsentan to treat glomerular diseases requires in-depth understanding of the impact of the ET and RAAS signaling systems in regulating kidney function in health and disease. Basic renal physiology and pathophysiology of these systems have been extensively studied and are the subject of multiple reviews [5–7]. In this section, we briefly summarize the evidence for nephroprotective effects of simultaneous inhibition of these systems and the use of sparsentan.
RAAS
Angiotensin II (Ang II) and aldosterone, the main effectors of RAAS, have well-known functions in the kidney in health and disease [8,9]. Ang II affects nearly all kidney compartments and cell types through AT1R, including hemodynamic actions leading to predominant vasoconstriction of the efferent arteriole and increases in intraglomerular pressure, stimulation of cell growth and extracellular matrix (ECM) production, inflammatory and pro-oxidant activities, and direct effects on podocyte pathophysiology and the pathogenesis of proteinuria [9–14]. Together, these effects ultimately result in glomerulosclerosis and tubulointerstitial fibrosis [8,9]. Additional information on renal Ang II actions is also provided in the context of ET effects below. Traditional physiologic actions of aldosterone are to stimulate sodium reabsorption and potassium excretion in aldosterone-sensitive cells of the distal nephron, thus contributing to the control of blood pressure (BP) and extracellular volume control [15]. Aldosterone also has prosclerotic, fibrogenic, and proteinuric effects [16,17]; thus, the RAAS regulates not only BP but also renal and cardiovascular pathophysiologic processes.
Activation of alternative branches in the RAAS cascade leads to signals that oppose the above-mentioned spectrum of effects of Ang II and aldosterone, which can be viewed as protective in the kidney and cardiovascular system [18]. Among those mechanisms, generation of Ang (1-7) by angiotensin-converting enzyme (ACE) 2 [19] is elevated during AT1R or ACE inhibition. Ang (1-7), acting via Mas receptors, has been shown to have vasodilator, antitrophic properties and natriuretic actions [18,20]. Similarly, activation of the type 2 angiotensin II receptor (AT2R) by Ang II has protective effects, albeit the physiologic and pathophysiologic relevance of these findings has not been well established. The few known physiologic actions, including natriuresis/diuresis and vasodilation, have been summarized previously by Bader and colleagues [18].
RAS inhibitors (RASis) have been considered standard of care for most causes of CKD [21], including the conditions discussed in this review. To enhance therapeutic efficacy of ACE inhibitors (ACEis) or angiotensin receptor blockers (ARBs), aliskiren was introduced to inhibit the system on the renin level, i.e., at the rate-limiting step of the system. Interestingly, these approaches did not lead to the desired greater treatment effect, particularly in the kidney. Multiple factors might be responsible [22], but the inhibition of the system on the renin level, resulting in the suppression of the aforementioned branches of RAAS considered to be protective in the kidney, may play an important role [18]. However, it should be noted that despite this evidence, RASi represented by ACEis or ARBs remains a mainstay of nonimmunosuppressive nephroprotective treatment [21].
ET-1 overview
Endothelin-1 (ET-1) is a potent peptide that activates multiple signaling pathways and is strongly implicated in renal pathophysiology. Its synthesis and release are stimulated by numerous factors that trigger or contribute to the development or progression of kidney disease [6,7]. Many actions of ET-1 implicated in renal pathophysiology resemble those of Ang II [23].
Similar to Ang II, ET-1 is involved in the control of renal and glomerular hemodynamics. Actions of ET peptides in the kidney are mediated by ETAR and ETB receptors (ETBR) [24]; most of the pathophysiological actions of ET-1 in the kidney are mediated via ETAR [6]. Much of the evidence for ET-1–induced glomerular injury has been provided by studies demonstrating beneficial effects of ET receptor inhibition or kidney cell–specific ET receptor knockout in models of diseases that lead to glomerulosclerosis [6].
Effects of ET-1 in the renal vasculature
Functional and binding studies indicate that the renal vasculature expresses both ETAR and ETBR. In general, ETAR are located on renal vascular smooth muscle, while ETBR are found on endothelial cells [25,26]. However, vasoconstrictive ETBR are found in the renal vasculature of rodent kidneys, while only vasoconstrictive ETAR have been reported in rabbit, dog, and human kidneys [27–32]. This species-specific ET receptor expression has led to confusion about the role of ET-1 in the renal vasculature, particularly since most studies on ET-1 vascular actions have been conducted in rats [28,31,32].
ET-1 administered systemically or via the renal artery elicits prompt and sustained whole kidney vasoconstriction [33]. Numerous studies have examined ET-1 actions in the renal microvasculature. Initial studies in rat and rabbit models found that ET-1 constricted isolated afferent and efferent arterioles, with up to 10-fold greater sensitivity in the efferent versus afferent arteriole [34–36]. Subsequent studies in rats using a variety of techniques with intact glomeruli and arterioles (hydronephrotic kidney, isolated perfused kidney, blood-perfused juxtamedullary nephron, micropuncture, and intravital video microscopy of surface glomeruli) largely found that ET-1 resulted in greater constriction in afferent versus efferent arterioles [33,37,38]. However, ET-1–mediated arteriolar constriction in at least two of these systems (hydronephrotic kidney and blood-perfused juxtamedullary nephron) was partly or wholly mediated by ETBR [39–42]. In contrast, in the canine kidney, intrarenal ET-1 infusion increased efferent resistance two times more than afferent resistance; of note, this was attributed to direct effects of ET-1 on the microvasculature as well as ET-1 stimulation of other vasoactive mediators (e.g., thromboxane and Ang II) [43].
In healthy humans and animals, ETAR do not appear to significantly contribute to BP or renal hemodynamics [43–46], while ETBR blockade increases BP and constricts the renal vasculature [44]. However, in healthy individuals given RASi, acute ETAR blockade increased renal blood flow and reduced filtration fraction, suggesting that the RAAS and ET systems can interact to regulate renal microvascular function [45,47]. This interaction between RAS and ETAR is most apparent in individuals with CKD. Acute ETAR blockade increased renal blood flow and decreased filtration fraction in patients with CKD treated with RASi, while acute ETBR blockade prevented the renal hemodynamic effects of ETAR blockade [44,48]. Similarly, chronic ETAR blockade reduced filtration fraction, glomerular filtration rate (GFR), and proteinuria in patients with CKD [49]. Thus, ETAR blockade in CKD (in the setting of RAS inhibition) helps restore renal blood flow; this effect is likely mediated by afferent, and relatively greater efferent, arteriolar relaxation.
Effects of ET-1 in the glomerulus
ET-1 causes mesangial cell contraction and proliferation [50,51] and ECM production [52–55]. This is associated with increased expression and activity of proinflammatory and profibrotic signaling molecules, including nuclear factor κB [55–57], monocyte chemoattractant-1, interleukin-6, adhesion molecules [54,58–60], transforming growth factor ß [52,55,59,61], and connective tissue growth factor [55]. The aforementioned molecules have also been implicated in the renal pathophysiological actions of Ang II [9,12–14] and aldosterone [11,62,63], reflecting extensive overlap and cross talk between the RAAS and ET systems.
ET-1 affects podocytes directly and may play an important role in podocytopathies such as FSGS. ET-1 causes nephrin shedding, loss of synaptopodin, cytoskeletal rearrangement resulting in foot process effacement, and podocyte apoptosis [6,57,59,60,64–66]. These ET-1 actions are mediated via ETAR, β-arrestin-1, and Src kinase activation [65]. Additionally, ET-1 activates the Wnt/β-catenin pathway in podocytes [57,65], which is linked to podocyte dysfunction [67]. Treatment with ET receptor antagonists (ERAs) or genetic deletion of ET receptors in models of podocyte injury ameliorated cytoskeletal changes and restored podocyte structural integrity in parallel with reduced proteinuria and glomerulosclerosis [55,65,66,68].
Podocytes also synthesize and secrete ET-1, which has deleterious paracrine effects on adjacent glomerular endothelial cells (GECs); this manifests as mitochondrial dysfunction and production of reactive oxygen species, which in turn impacts the structural integrity of podocytes (as demonstrated in models of FSGS) [66]. In another example of podocyte-GEC cross talk, ET-1 secreted by podocytes acts as a key negative regulator of the GEC glycocalyx, the mesh-like polyanionic carbohydrate structure on the endothelial cell that forms the internal layer of the glomerular filtration barrier. The ET peptide activates heparanase in endothelial cells (and in glomerular macrophages) and causes glomerular endothelial glycocalyx degradation [69–71]. In the context of podocyte and GEC changes, Saleh and colleagues described BP-independent, ET-1–induced, enhanced glomerular permeability to albumin both in vitro and in vivo [58,72], suggesting that the proteinuric effect is at least partly independent of hemodynamic factors.
Effects of ET-1 in the tubulointerstitial compartment
The spectrum of proinflammatory and profibrotic ET-1 activity in the tubulointerstitial compartment overlap with the prosclerotic actions in the glomeruli described and can be inhibited by ERAs [55,73]. De Miguel et al. [74] demonstrated that stress induced by ETAR stimulation contributes to tubular cell injury and apoptosis. In a recent complex study, Czopek and coworkers [75] showed how multiple ET-1 actions mediated via ETAR act in concert to mediate the transition from acute kidney injury to CKD.
In contrast to the anti-natriuretic actions of Ang II or aldosterone, ET-1 acts as a natriuretic peptide [6]. The major renal site of the natriuretic and diuretic actions of ET-1 is the collecting duct, which is also the predominant site of ET-1 tubular production [24,76]. Most evidence indicates that ETBR is the main receptor responsible for the natriuretic actions of ET-1 in the nephron; however, observations in mice with double ETAR/ETBR knockout in the collecting duct, which display more severe hypertension than ETBR knockout mice, indicate that ETAR contributes to ET-1–induced natriuresis (reviewed by Kohan et al. [33]). Administration of ETAR antagonists causes fluid retention in mice, which is prevented by duct-specific knockout of ETAR [77]. Importantly, edema and fluid retention can complicate clinical use of selective ETAR antagonists [24,78,79], although clinically significant fluid retention has not been reported with sparsentan [4].
Interactions and cross talk between RAAS and ET-1
In addition to similarities between ET-1 and effectors of RAAS in the pathophysiology of kidney disease, there are also complex interactions and cross talk between these systems. The prosclerotic and inflammatory actions of ET-1 occur as a direct consequence of ETAR stimulation and as part of Ang II signaling [9,54,58]. Ang II stimulates ET-1 release and expression in a variety of cell types, including renal cells [80–82]. ET-1 mediates some of the vascular actions of Ang II, both in vitro [83,84] and in vivo [85], including in the renal circulation [86,87]. In turn, ET-1 stimulates Ang II formation via enhanced action of ACE in vitro in pulmonary endothelial cells [88,89]. In addition, ET-1–induced fibronectin synthesis by mesangial cells is reduced by RAAS inhibition [90].
Similar to Ang II, which inhibits renin expression and synthesis in juxtaglomerular cells via AT1R, ET inhibits renin expression and release by a Ca2+-dependent mechanism [91–93], an effect possibly mediated via both ETAR and ETBR [94]. However, it remains to be established whether the combination of AT1R and ETAR inhibition might have additive effect on renin release and expression and whether this process is mitigated by unopposed stimulation of ETBR. Although the pathophysiological significance is not yet clear, it should be noted that ET-1 also stimulates aldosterone secretion, as well as zona glomerulosa cell growth and proliferation [95,96].
Interactions of ET-1 with another effector of RAAS, Ang (1-7), have also been described in different experimental settings such as mesenteric arteries in spontaneously hypertensive rats during chronic nitric oxide blockade [97] and in renal vasculature of diabetic spontaneously hypertensive rats [98]. In patients with obesity, Ang (1-7) [1–7] blunted ET-1–induced vasoconstriction in forearm arteries [99]. However, the clinical relevance of these observations remains to be established.
Altogether, there is persuasive evidence for similarities of action and cross talk between RAAS effectors and ET-1 in processes that trigger and perpetuate renal injury. This provides a rationale for potential additive effects of inhibition of both systems in the treatment of kidney disease.
Evidence supporting combined inhibition of RAAS and ET-1 in the treatment of kidney disease
Experimental evidence
The concept of dual inhibition of Ang II and ET-1 as nephroprotection has been developing since the 1990s [100,101]. Studies in rodent models have included a passive Heymann nephritis model of idiopathic membranous nephropathy [101] and subtotal nephrectomy [102], a hypertension model involving 5/6 nephrectomy with overexpression of the renin gene [103], and models of diabetic nephropathy [52,61,101]. Across these studies, combining a RASi (using an ARB or ACEi) with an ERA was more effective than monotherapy in attenuating the development and progression of proteinuria, glomerular and tubulointerstitial structural changes, functional deterioration, and molecular changes characteristic of progressive CKD. In uninephrectomized streptozotocin-diabetic rats, the combination restored the number of podocytes, while monotherapies only limited podocyte depletion [52,101].
Clinical evidence
The antiproteinuric effects of combined RAAS-ERA blockade have been most extensively studied in patients with Type 2 diabetes and nephropathy. Phase 2 and 3 studies demonstrated that the ERAs avosentan and atrasentan lowered proteinuria by 30–50% in patients treated with RASis [79,104–106]. In the RADAR phase 2b trial, atrasentan dose dependently reduced proteinuria in patients receiving stable RASis [79]. In the phase 3 SONAR trial [78], the addition of atrasentan in patients with Type 2 diabetes and nephropathy on the maximal tolerated dose of ARBs or ACEis caused a 35% reduction in the risk of the primary renal outcome (a composite of doubling of serum creatinine, onset of end-stage kidney disease [ESKD], or renal death) over a median follow-up period of 2.2 years.
Until recently, the only available data for the additive effects of ERAs on a background of RASis on proteinuria in patients with nondiabetic CKD (≈50% with IgAN) came from a study by Dhaun et al. [49] (more recent sparsentan data are described in this review). Add-on sitaxsentan (ETAR-specific antagonist), but not placebo, lowered proteinuria, BP, and GFR, with unchanged renal plasma flow, resulting in a substantial decrease in filtration fraction in patients treated with RASis. Nifedipine, used for BP control, did not affect proteinuria [49].
Discovery of dual Ang II and endothelin receptor antagonists
The experimental and clinical studies reviewed thus far used a combination of two individual inhibitors of the RAAS and ET systems. As one molecule with high affinity for both AT1 and ETA receptors, sparsentan represents a new approach [2].
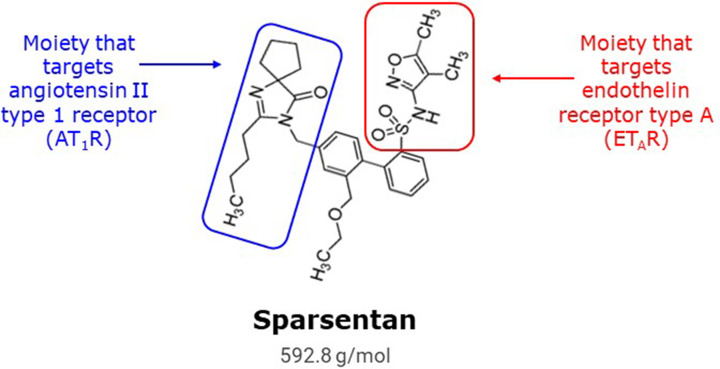
The discovery of dual angiotensin and endothelin receptor antagonists (i.e., single molecules that can inhibit both receptors) was based on recognition of a biphenyl core that some ERAs had in common with several ARBs, including irbesartan [107]. It was hypothesized that merging the active moieties of these two classes of antagonist via this shared biphenyl core would yield a compound with activity for both receptors. This strategy led to the design and synthesis of a novel class of orally administered antagonists of both AT1R and ETAR in a single compound (Figure 1 and Table 1).
Figure 1. Chemical structure of sparsentan.
Chemical structure of the dual endothelin angiotensin receptor antagonist (DEARA), sparsentan, showing the key structural elements of the molecule responsible for antagonism of each receptor, ETAR and AT1R (adapted from [2]). Adapted from Murugesan N, et al. (2005) J. Med. Chem. 48(1):171–179. Copyright © 2005, American Chemical Society.
Table 1. In vitro binding affinities and in vivo pressor inhibition of orally administered ETAR and AT1R antagonists through development and optimization.
| Target receptor (s) | Antagonist name | ETAR Ki (nM) | AT1R Ki (nM) | ETBR Ki (nM) | AT2R Ki (nM) | Oral big ET-1 pressor (30 µM/kg) (AOC units)* | Oral Ang II pressor (30 µM/kg) (AOC units)* |
|---|---|---|---|---|---|---|---|
| ETAR | BMS-193884 | 1.4† | >10,000† | - | - | 10,100* | Inactive* |
| AT1R | Irbesartan | >10,000† | 1.1† | - | - | Inactive* | 11,940* |
| ETAR & AT1R | Unnamed biphenylsulfonamide | 39† | ND† | - | - | - | - |
| ETAR & AT1R | BMS-248360 | 1.9† | 10† | - | - | 7,900* | 6,000* |
| ETAR & AT1R | BMS-346567 (sparsentan) | 9.3* | 0.8* | >10,000* | >10,000* | 15,800* | 18,600* |
The chemical structures of the mono-selective antagonists BMS-193884 and irbesartan were merged to produce the first-generation dual ETAR/AT1R antagonist, an unnamed biphenylsulfonamide. Optimization regained the original target ETAR activity (BMS-248360). Further optimization retained dual specificity while improving bioavailability in dog and monkey and oral pressor inhibition in rat (BMS-346567/sparsentan, bioavailability data not shown).
Abbreviations: ‘-’ = not shown; AOC, area over curve, indicating potency and duration of action at equimolar doses; AT1R, AT1 receptor; ETAR, ET receptor type A; Ki, inhibitory constant; ND, not determined.
Murugesan 2005 [107].
Murugesan 2002 [2].
Subsequent rational drug design to improve pharmacokinetics while maintaining dual receptor potency resulted in the discovery of sparsentan (compound #7) [2]. Receptor affinity constants (Ki) were determined using radioligand binding assays. The affinity of sparsentan was 0.8 nM for human AT1R and 9.3 nM for human ETAR, whereas the affinity toward AT2R and ETBR was negligible (>10 µM) [2]. For comparison, the Ki of irbesartan for AT1R was 1.1 nM [1]. The dual action of sparsentan was demonstrated in vivo by its ability to attenuate the pressor effects of intravenous infusions of either Ang II or big ET-1 in rats [107]. Moreover, sparsentan was more effective in reducing BP than an equimolar dose of irbesartan in models of hypertension such as spontaneously hypertensive rats [1]. Leach and colleagues [108] reported similar sparsentan affinity constants for AT1R (Ki = 0.9 nM) and ETAR (Ki = 13 nM) and also showed selectivity over ETBR (Ki = 6582 nM).
Sparsentan in IgAN
Overview of IgAN
IgAN is the most common diagnosis made using kidney biopsies performed to evaluate glomerular disease in children and adults [109]. For clinical presentation and current treatment options, readers are referred to recent reviews in the field [110]. IgAN is now viewed as a multiple-hit disease process. The initial step is the formation of hypo-galactosylated IgA1 molecules (Gd-IgA1). This leads to the synthesis of anti–Gd-IgA/IgG antibodies and the formation of immune complexes, the second and third hits. The immune complexes are deposited in the mesangial region of the kidney, triggering inflammation and progressive glomerular injury [111–113]. Generation of Gd-IgA1 occurs in the respiratory and gastrointestinal tracts [114–117]. Recent evidence has linked IgAN to the activity of the complement system [118].
RASis were the standard of care for patients with IgAN and proteinuria; however, even with the addition of sodium-glucose cotransporter-2 inhibition or steroids (systemic or gut targeted), significant residual proteinuria, renal functional deterioration, and continued risk of kidney failure occurs [110,119,120]. Activation of the renal ET system in IgAN is supported by several lines of evidence. In addition to a previously mentioned study by Dhaun et al. [49] indicating renal ET system activation in IgAN, Lehrke et al. [121] reported that renal ET-1 gene expression was significantly higher in patients with higher-grade proteinuria compared with patients with lower-grade proteinuria or control participants. Elevated renal ET gene expression was also reported by Tycova et al. [122] in patients with IgAN and was associated with an increased risk of disease progression.
Effect of sparsentan in animal models of IgAN
Sparsentan has been tested in an IgAN mouse model in which engineered immune complexes formed from human Gd-IgA1 and recombinant IgG autoantibody were injected into nude mice to induce glomerular injury mimicking human IgAN. Treatment with sparsentan attenuated both mesangial hypercellularity and proliferation (Ki67 immunoreactivity) in engineered immune complex–treated mice compared with those that received vehicle [123]. RNA sequencing analysis of kidney biopsies taken at the end of the study revealed that up-regulation of key inflammatory and proliferative genes and pathways was markedly attenuated by sparsentan treatment, including complement genes (C1q [complement C1q]), integrin components (Itgb2 [integrin subunit beta 2]), members of the MAP kinase family (MAPK1/3/8/14), and Fc receptor elements (Fcer1g [Fc epsilon receptor Ig]) (date on file; Travere). Moreover, translatability of the transcriptional findings for the immune and inflammatory pathways was supported by partial overlap between differentially expressed murine and human genes in patients with IgAN [124].
The IgAN-prone gddY mouse model spontaneously develops albuminuria by 8 weeks of age; by 12 weeks of age, expression of messenger RNA (mRNA) for ET-1, ETAR and AT1R, and inflammatory pathway genes is up-regulated compared with healthy Balb/c control mice [125]. Sparsentan was compared with an ARB (losartan) in this model. Compared with control gddY mice, mice that received sparsentan and losartan had equivalently reduced BP and ameliorated increases in mRNA for ET-1, ETAR and AT1R, and inflammatory genes. However, sparsentan reduced albuminuria more rapidly than losartan. Moreover, sparsentan protected the kidney from glomerulosclerosis and glycocalyx and podocyte loss to a far greater extent than losartan [125].
Sparsentan has also exerted beneficial effects in the rat anti-Thy1 model [126]. Although not an IgAN model per se, the model exhibits mesangial proliferation and glomerular pathologies that overlap with IgAN, resulting from the binding of anti-Thy1 to the Thy1 antigen on the surface of mesangial cells and subsequent complement activation and mesangiolysis, leading to up-regulation of ET-1 mRNA expression. Sparsentan dose dependently attenuated the increase in proteinuria and mesangial cell proliferation (Ki67 immunoreactivity) and activation (α-smooth muscle actin protein expression) and provided protection from macrophage infiltration, glomerulonephritis, and interstitial myofibroblast activation (α-smooth muscle actin protein expression) (Table 2).
Table 2. Summary of disease mechanisms impacted by sparsentan in nonclinical models.
| Nonclinical model | ||||||
|---|---|---|---|---|---|---|
| Disease mechanism | gddY IgAN [125] | EIC model of IgAN [123,124] | Mesangioproliferative GN (anti-Thy1) [126] | Adriamycin rat FSGS [144] | TRPC6-Tg FSGS [138,139] | Alport mouse [154] |
| Proteinuria | √ | Not tested | √ | √ | √ | √ |
| Glomerulosclerosis | √ | NA | √ | √ | √ | √ |
| Podocytes | √ | Not tested | Not tested | √ | √ | √ |
| Mesangial cells | NA | √ | √ | NA | √ | NA |
| mGFR | Not tested | Not tested | Not tested | Not tested | √ | √ |
| Glycocalyx | √ | Not tested | Not tested | √ | √ | Not tested |
| Glomerular basement membrane | Not tested | Not tested | Not tested | √ | Not tested | √ |
| Vasoconstriction | Not tested | Not tested | Not tested | Not tested | √ | Not tested |
| Inflammation | √ | √ | √ | √ | √ | √ |
| Tubules/interstitium | Not tested | Not tested | √ | Not tested | √ | √ |
| Extracellular matrix | Not tested | Not tested | √ | Not tested | Not tested | √ |
Abbreviations: EIC, engineered immune complex; FSGS, focal segmental glomerulosclerosis; GN, glomerulonephritis; IgAN, immunoglobulin A nephropathy; mGFR, measured glomerular filtration rate; NA, not applicable; Tg, transgenic.
Sparsentan in FSGS
Overview of FSGS
FSGS is a renal histological lesion triggered by podocyte injury and characterized by segmental accumulation of glomerular ECM, resulting in glomerular scarring and capillary obliteration [127,128]. A range of heterogeneous clinical conditions and stimuli lead to podocyte injury and consequently to FSGS-type lesions [129], which are classified as primary, genetic, secondary, or forms with unknown cause [110]. Primary FSGS has no identifiable cause but may result from the actions of putative circulating permeability factors that cause podocyte injury [129]. The rapidly expanding list of genetic causes includes mutations in genes encoding proteins required for normal podocyte structure or function. Heterozygous mutations of Col4a3, Col4a4, and Col4a5 genes or high-risk APOL1 alleles are associated with increased risk of FSGS and lead to the characteristic histopathological lesions [128–131]. There are numerous secondary forms of FSGS. Renal lesions in secondary FSGS are due to adaptive structural-functional responses (i.e., a mismatch between metabolic and hemodynamic load and glomerular capacity), such as in patients with congenital or acquired reduction of renal mass, obesity, metabolic derangements, other antecedent diseases, drug use, or infections [128,129].
The presentation of FSGS features varying levels of proteinuria, including severe nephrotic syndrome [132–134]. RASis are used in most patients with FSGS for their antiproteinuric and nephroprotective actions [135,136]. Glucocorticoids remain the mainstay of treatment for patients with FSGS presenting with nephrotic syndrome or rapid loss of kidney function [110]. Additional immunosuppressive agents, such as calcineurin inhibitors, mycophenolate mofetil, or rituximab, are used in patients with steroid-resistant disease to achieve remission and clinically meaningful reduction in proteinuria, albeit with limited success.
Considering the multiple roles of Ang II and ET-1 in podocyte pathophysiology and the development of glomerulosclerosis, as previously discussed, dual ETAR and AT1R inhibition seems to be a logical approach for treatment of most forms of FSGS. Moreover, a recent study demonstrated an increased percentage of glomeruli with ETAR-positive endothelial cells in patients with primary FSGS compared with healthy control participants. Glomerular ETAR-positive endothelium was strongly associated with nephrin loss, glomerular markers of oxidative stress, and proteinuria, further supporting the role of the ET system in the development of FSGS [137].
Effect of sparsentan in models of FSGS
Transgenic mice overexpressing wild-type TRPC6, specifically in podocytes, develop kidney disease similar to human FSGS. In untreated FSGS transgenic mice, the detectable signs of ongoing FSGS pathology include segmental elevations in podocyte calcium, the development of parietal podocytes and adhesions between parietal and visceral layers of Bowman’s capsule, and albumin leakage through the glomerular filtration barrier. In studies by Gyarmati et al. [138,139], treatment with sparsentan for 6 weeks ameliorated glomerulosclerosis and tubulointerstitial fibrosis in this model to a similar degree as losartan. In contrast, sparsentan was more effective in preservation of podocyte number and inhibition of podocyte calcium influx. Multiphoton imaging in intact living kidneys demonstrated greater afferent and efferent arteriole diameters, increased single-nephron GFR and blood flow, and reduced urine albumin/creatinine ratio and albumin sieving coefficient in mice that received 6 weeks of sparsentan treatment compared with losartan-treated TRPC6 mice [138,139]. Parallel experiments demonstrated that the beneficial effects of sparsentan in glomeruli in this model were linked to attenuation of mitochondrial stress in podocytes, restoration of the integrity of the glomerular endothelial surface layer (glycocalyx), and reduction in CD44+ immune cell homing [139].
Further studies by this group focused on tissue remodeling. In this model, sparsentan increased the frequency of larger multi-cell clones in both the renin and endothelial lineage in glomeruli and arterioles. Losartan had a similar effect, but with a reduced magnitude compared with sparsentan. In addition, various renal cortical and medullary tubule segments including cells of the proximal tubule, the distal convoluted tubule and the collecting duct also showed active cellular (clonal) remodeling in response to sparsentan, and, to a lesser extent, losartan [139]. The renin cell lineage is known to have the ability to function as progenitor cells for parietal and epithelial cells as well as podocytes during glomerular structural and functional repair [140,141]. Altogether, this multidirectional evidence supports sparsentan's potential to preserve glomerular structural integrity in addition to its hemodynamic actions.
Of note, several studies have demonstrated concentric hypertrophy of intrarenal arterioles caused by multiclonal expansion of renin cells both in mice and humans in response to RAS inhibition, resulting in glomerular blood flow obstruction, downstream ischemia, and even development of renal fibrosis [142,143]. Our observations in TRPC6 mice treated with sparsentan indicate opposite effects on glomerular hemodynamics and structural benefit [138,139]. Whether these opposite effects are mediated via concomitant ET receptor inhibition remains to be established.
The adriamycin (ADR) murine model is characterized by rapid podocyte injury and proteinuria followed by glomerulosclerosis, tubulointerstitial inflammation, and fibrosis with lesions reflective of human FSGS. Sparsentan treatment attenuated proteinuria and podocyte loss, maintained glomerular basement membrane width, protected the glycocalyx, and reduced glomerular macrophage infiltration [144]. Data from an ADR rat study were used to establish a disease and treatment profile using differential gene expression mapped to data from a cohort of patients with FSGS in the NEPTUNE registry [145]. A series of genes were identified that showed dysregulated expression in the diseased rats and expression changes that were reversed by sparsentan treatment. Human orthologs of these genes showed altered expression in the FSGS cohort compared with healthy controls that correlated with disease severity as measured by proteinuria and estimated glomerular filtration rate (eGFR).
An interesting novel mechanism of action for sparsentan was recently published [146]. The authors found increased numbers of CD8+ tissue-resident memory T (TRM) cells in the glomeruli and tubulointerstitial compartment in the ADR model and in patients with clinical FSGS, as well as in models of and patients with lupus nephritis and Type 2 diabetes. Levels of interleukin-15 (IL-15) were increased in ADR mice and correlated with albuminuria. IL-15 promoted renal CD8+ TRM development and interferon‐γ (IFN-γ) secretion. Inhibition of IL-15 with an antibody reduced renal CD8+ TRM formation and IFN-γ generation, with a beneficial impact on albuminuria, glomerulosclerosis, podocyte morphology, and nephrin/podocin mRNA expression. Considering their parallel observation that IL-15 in renal cells can be induced by Ang II/ET-1, the authors treated ADR mice with sparsentan. The intervention mimicked the IL-15 inhibition described, suggesting a new potential anti-inflammatory and podocyte-protective mechanism for the drug [146].
Sparsentan in Alport syndrome
Overview of AS
AS is an inherited progressive form of glomerular disease that is often associated with extra-renal manifestations, including sensorineural hearing loss and ocular abnormalities [147–149]. AS is a primary basement membrane disorder that results from mutations in genes encoding the alpha-3-5 chains of type IV collagen. In early childhood, hematuria and microalbuminuria are detected. Over time, proteinuria, nephrotic syndrome, hypertension, and progressive renal insufficiency develop at a rate that depends on the type of mutation, but ESKD may occur as early as the third decade of life in patients with X-linked or autosomal recessive disease [147–149].
The treatment of AS is currently based on RASi initiation at the time of diagnosis, especially in patients with proteinuria [150–152]. Data suggest that ACEis postpone ESKD and improve life expectancy in patients with AS, although disease progression continues [151]. A role for ET-1 has also emerged in AS. Dufek and colleagues showed that activation of CDC42/RAC1 leads to mesangial filopodial invasion of glomerular capillaries, resulting in the deposition of mesangial proteins, including laminin α2, in the glomerular basement membrane in a mouse model of autosomal recessive AS (COL4A3 knockout on an SV/129 background) [153]. Laminin α2 directly injures podocytes by activating focal adhesion kinase and nuclear factor κB. Importantly, ETAR blockade prevented mesangial filopodial invasion and ameliorated glomerular disease in these AS mice.
Effect of sparsentan in experimental AS
Sparsentan and losartan were compared in the COL4A3 knockout mice studied by Dufek et al. [153], in which proteinuria and renal pathology typically begin at 5 weeks of age and lifespan is approximately 10 weeks [154] (Table 2). The mice also develop susceptibility to hearing loss when exposed to metabolically stressful noise between 8 and 9 weeks of age. Sparsentan administered from 4 to 7 weeks of age significantly delayed the onset of glomerulosclerosis and interstitial fibrosis, proteinuria, and GFR decline (assessed using a transdermal device to follow the change in fluorescence of fluorescein isothiocyanate–labeled sinistrin over time). Sparsentan attenuated glomerular basement membrane dysmorphology and foot process effacement, blunted mesangial filopodial invasion into the glomerular capillaries, and increased lifespan to a greater extent than losartan despite equivalent BP changes. Sparsentan also lessened changes in the mRNA expression of proinflammatory (Ccr2 and IL-1β), profibrotic (Mmp2 and Serbp1), proliferative (Myc), and adhesion pathway genes (Itga2) that were associated with AS. Notably, sparsentan, but not losartan, prevented accumulation of ECM in the strial (cochlear) capillary basement membranes in the inner ear and reduced susceptibility to hearing loss. Improvements in lifespan and in renal and strial pathology were also observed even when sparsentan was administered following development of renal pathology in mice at 5 weeks of age.
Clinical experience with sparsentan
Sparsentan was approved in 2023 by the US Food and Drug Administration to reduce proteinuria in adults with primary IgAN who are at risk of rapid disease progression [155,156]. This approval was based on the PROTECT study, a phase 3 clinical trial in which 404 adult patients with IgAN with persistent proteinuria of >1 g/day at high risk for kidney failure despite maximal tolerated ACE or ARB therapy were randomized to either sparsentan or irbesartan. The interim analysis demonstrated that patients treated with sparsentan experienced rapid and sustained proteinuria reduction compared with those who received a maximum tolerated dose of irbesartan (−49.8% vs −15.1%, respectively; P<0.0001). Sparsentan was well tolerated, with a safety profile comparable to that of irbesartan [157].
The 2-year data from the PROTECT trial demonstrated marked and sustained reductions in proteinuria, associated with slower kidney function decline, in sparsentan-treated compared with irbesartan-treated patients with IgAN [4].
Sparsentan has also been tested in ongoing phase 2 and 3 randomized controlled trials in patients with FSGS. Both the phase 2 DUET and phase 3 DUPLEX trials demonstrated a significantly greater and sustained reduction in proteinuria in patients with FSGS treated with sparsentan compared with those treated with irbesartan [158,159], although in DUPLEX the effect on eGFR slopes over 2 years between the sparsentan and irbesartan arms was not statistically significant [3,156].
In general, treatment with sparsentan has been well tolerated in both FSGS and IgAN studies [4]. In previous studies investigating ERA, the main safety signal of concern has been fluid retention causing edema or even heart failure (RADAR [79], SONAR [78], and ASCEND [160]). Based on the post hoc analysis of the RADAR and RADAR/JAPAN studies with atrasentan, lower eGFR, higher BP, and higher inhibitor dose have been identified as risk factors for fluid retention in ERA-treated patients [161].
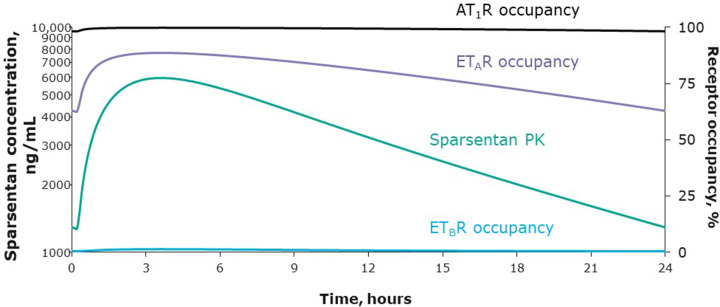
A striking feature of the clinical studies with sparsentan is the lack of clinically significant edema even at doses escalated to 800 mg daily. The PROTECT study shows that sparsentan has actions qualitatively and quantitatively different from a simple increase in angiotensin pathway suppression, supporting ETAR engagement in humans. These actions include strong proteinuria reduction with minimal effects on BP, no exacerbation of hyperkalemia, changes in diastolic BP greater than systolic BP, and a lack of additional acute reductions in eGFR [4,157]. A detailed analysis of the 24-h pharmacokinetic profile and estimation of the ETAR and AT1R occupancies of sparsentan at steady-state 400-mg dosing is shown in Figure 3. ETAR occupancy is in the range of 60–90% at all times, while AT1R occupancy is always above 95%. Substantial ETAR antagonism is therefore achieved, always accompanied by even greater AT1R blockade. This obligatory relationship (as sparsentan possesses dual antagonism in a single molecule) cannot be guaranteed with a single-target ERA combined with an ARB, especially as the dose is increased, and may underlie the edema safety profile of sparsentan. Other strategies have been employed to reduce the risk of edema with ERA administration (dose reduction [162], patient stratification [78], and combination therapy with sodium-glucose cotransporter-2 inhibitors [163,164]) with varying degrees of success. Additional data are needed to elucidate which strategies are best suited to which patient populations.
Figure 3. Sparsentan ETAR and ETBR occupancies (right axis) and plasma concentration* (left axis) over 24 h for a single, daily, 400-mg oral dose at steady-state in the PROTECT study (adapted from [165]).
Steady-state PK parameters calculated using population PK values for sparsentan 400 mg in the PROTECT study were used to estimate diurnal changes in receptor occupancy. Sparsentan AT1R occupancy (>95%) consistently exceeds ETAR occupancy (>60% and <90%) over a full 24-h period. ETBR occupancy never exceeds 2%. *PK data are based on population PK model prediction for a patient with IgAN. AT1R, angiotensin II receptor type 1; ETAR, endothelin receptor type A; ETBR, endothelin receptor type B; PK, pharmacokinetics.
Summary of mechanisms of nephroprotective actions of sparsentan
The protective effects of sparsentan in models of kidney disease corroborate abundant evidence collected over the past decades on the renal pathophysiology of renin–angiotensin and ET systems.
In mesangial cells, sparsentan exerts antiproliferative effects and inhibits production of proinflammatory and profibrotic cytokines and ECM proteins, resulting in amelioration of corresponding glomerular lesions such as mesangio-proliferative glomerulonephritis or glomerulosclerosis [123,126].
In glomerular endothelial cells, sparsentan preserved glycocalyx integrity in several models, a mechanism likely to contribute to its antiproteinuric effect [125,138,139,144].
In podocytes, sparsentan inhibits Ca2+ flux, an established marker of podocyte injury, leading to preservation of nephrin and podocin expression, reduced oxidative stress, attenuation of foot process effacement, and maintenance of the glomerular basement membrane. Given the ability of sparsentan to inhibit IFN-γ [146], which has been shown to be a key regulator of APOL1 synthesis, the drug may be particularly useful in individuals with FSGS and APOL1 risk alleles.
In the tubulointerstitial compartment, sparsentan ameliorated tubulointerstitial fibrosis in association with normalization of proinflammatory cytokine mRNA and protein levels, profibrotic mediators, ECM proteins, and complement components [124,125,144].
In the renal vasculature, sparsentan likely exerts protective glomerular hemodynamic effects through mitigation of Ang II– and ET-1–mediated efferent arteriolar constriction [138,139].
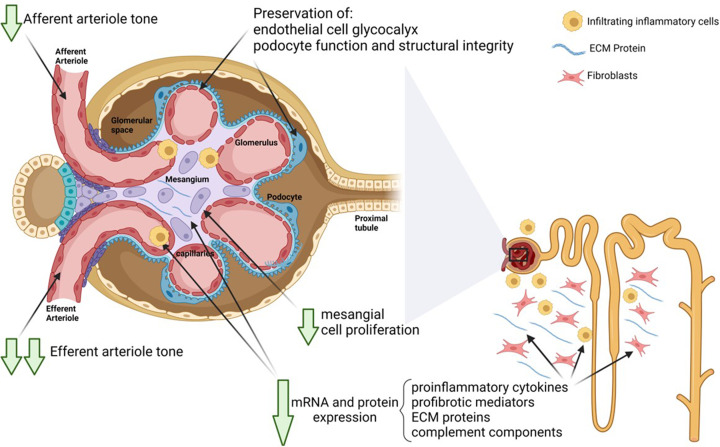
Taken together, the growing body of preclinical data suggest numerous direct cellular, and resulting structural, actions of sparsentan contributing to nephroprotection (Figure 2). The SPARTAN study (NCT04663204), a single-arm interventional study of sparsentan in patients with incident IgAN, is collecting kidney biopsies at baseline and after 24 weeks of treatment and may further inform whether these data translate to the clinical setting.
Figure 2. Mechanisms of action of sparsentan in the kidney.
Schematic presentation of main actions of sparsentan in the kidney associated with long-term nephroprotective effects as observed in preclinical studies. These actions include anti-inflammatory and antifibrotic effects both in glomeruli and the tubulointerstitial compartment, podocyte protection, and preservation of the glomerular glycocalyx, in parallel with beneficial effects on glomerular hemodynamics. Created with Biorender.com by Wilmelenne Clapper. ECM, extracellular matrix; mRNA, messenger RNA.
Conclusion
ET and Ang II are important mediators of renal disease. The two systems closely interact to exert additive pathophysiological effects through cross talk involving multiple signaling pathways that impact the renal vasculature, glomerulus, and tubulointerstitium. Dual blockade of AT1 receptors and ETAR exerts anti-inflammatory, antiproliferative, antifibrotic, and cell protective actions in many forms of kidney disease. Sparsentan, a dual ET and angiotensin receptor antagonist, has been shown in cells, in animal models, and clinically to reduce pathophysiological processes and ameliorate renal injury. As such, sparsentan holds significant promise as a renoprotective agent in CKD.
Acknowledgments
Medical writing support was provided by Nazneen Qureshi, PhD, of Medical Expressions, Inc., a part of Nucleus Global, an Inizio company. We acknowledge Wilmelenne Clapper for assistance with the development of Figure 2.
Abbreviations
- ACE
angiotensin-converting enzyme
- ACEi
ACE inhibitor
- ADR
adriamycin
- Ang II
angiotensin II
- ARB
angiotensin receptor blocker
- AS
Alport syndrome
- AT1R
angiotensin receptor type 1
- BP
blood pressure
- CKD
chronic kidney disease
- DEARA
dual endothelin angiotensin receptor antagonist
- ECM
extracellular matrix
- Gd-IgA1
hypo-galactosylated IgA1 molecules
- GEC
glomerular endothelial cells
- eGFR
estimated glomerular filtration rate
- ERA
endothelin receptor antagonist
- ETAR
endothelin A receptor
- ETBR
endothelin B receptor
- ET-1
endothelin-1
- FSGS
focal segmental glomerulosclerosis
- GED
glomerular endothelial cells
- IFN-γ
interferon‐γ
- IgAN
immunoglobulin A nephropathy
- Ki
affinity constants
- RAAS
renin–angiotensin–aldosterone system
- RASi
RAS inhibitor
Data Availability
N/A - All supporting data are included within the main article.
Competing Interests
D.E.K is a consultant for Chinook Therapeutics and Travere Therapeutics, Inc. P.W.B., C.J., B.H., and R.K. are employees and shareholders of Travere Therapeutics, Inc.
Funding
This study and medical writing support for this manuscript were funded by Travere Therapeutics, Inc.
CRediT Author Contribution
Donald E. Kohan: Conceptualization, Visualization, Writing—original draft, Writing—review & editing. Patricia W. Bedard: Conceptualization, Writing—original draft, Writing—review & editing. Celia Jenkinson: Conceptualization, Writing—original draft, Writing—review & editing. Bruce Hendry: Conceptualization, Writing—original draft, Writing—review & editing. Radko Komers: Conceptualization, Visualization, Writing—original draft, Writing—review & editing.
Disclosures
D.E.K. is a consultant for Chinook Therapeutics and Travere Therapeutics, Inc. P.W.B., C.J., B.H., and R.K. are employees and shareholders of Travere Therapeutics, Inc.
REFERENCES
- 1.Kowala M.C., Murugesan N., Tellew J., Carlson K., Monshizadegan H., Ryan C.et al. (2004) Novel dual action AT1 and ETA receptor antagonists reduce blood pressure in experimental hypertension. J. Pharmacol. Exp. Ther. 309, 275–284 10.1124/jpet.103.055855 [DOI] [PubMed] [Google Scholar]
- 2.Murugesan N., Gu Z., Fadnis L., Tellew J.E., Baska R.A., Yang Y.et al. (2005) Dual angiotensin II and endothelin A receptor antagonists: synthesis of 2'-substituted N-3-isoxazolyl biphenylsulfonamides with improved potency and pharmacokinetics. J. Med. Chem. 48, 171–179 10.1021/jm049548x [DOI] [PubMed] [Google Scholar]
- 3.Rheault M.N., Alpers C.E., Barratt J., Bieler S., Canetta P., Chae D.W.et al. (2023) Sparsentan versus irbesartan in focal segmental glomerulosclerosis. N. Engl. J. Med. 389, 2436–2445 10.1056/NEJMoa2308550 [DOI] [PubMed] [Google Scholar]
- 4.Rovin B.H., Barratt J., Heerspink H.J.L., Alpers C.E., Bieler S., Chae D.W.et al. (2023) Efficacy and safety of sparsentan versus irbesartan in patients with IgA nephropathy (PROTECT): 2-year results from a randomised, active-controlled, phase 3 trial. Lancet 402, 2077–2090 10.1016/S0140-6736(23)02302-4 [DOI] [PubMed] [Google Scholar]
- 5.Siragy H.M. and Carey R.M. (2010) Role of the intrarenal renin-angiotensin-aldosterone system in chronic kidney disease. Am. J. Nephrol. 31, 541–550 10.1159/000313363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kohan D.E. and Barton M. (2014) Endothelin and endothelin antagonists in chronic kidney disease. Kidney Int. 86, 896–904 10.1038/ki.2014.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohan D.E., Inscho E.W., Wesson D. and Pollock D.M. (2011) Physiology of endothelin and the kidney. Compr. Physiol. 1, 883–919 10.1002/cphy.c100039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wennmann D.O., Hsu H.H. and Pavenstadt H. (2012) The renin-angiotensin-aldosterone system in podocytes. Semin. Nephrol. 32, 377–384 10.1016/j.semnephrol.2012.06.009 [DOI] [PubMed] [Google Scholar]
- 9.Ruster C. and Wolf G. (2011) Angiotensin II as a morphogenic cytokine stimulating renal fibrogenesis. J. Am. Soc. Nephrol. 22, 1189–1199 10.1681/ASN.2010040384 [DOI] [PubMed] [Google Scholar]
- 10.Greene E.L., Kren S. and Hostetter T.H. (1996) Role of aldosterone in the remnant kidney model in the rat. J. Clin. Invest. 98, 1063–1068 10.1172/JCI118867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han K.H., Kang Y.S., Han S.Y., Jee Y.H., Lee M.H., Han J.Y.et al. (2006) Spironolactone ameliorates renal injury and connective tissue growth factor expression in type II diabetic rats. Kidney Int. 70, 111–120 10.1038/sj.ki.5000438 [DOI] [PubMed] [Google Scholar]
- 12.Junaid A., Rosenberg M.E. and Hostetter T.H. (1997) Interaction of angiotensin II and TGF-b1 in the rat remnant kidney. J. Am. Soc. Nephrol. 8, 1732–1738 10.1681/ASN.V8111732 [DOI] [PubMed] [Google Scholar]
- 13.Kashiwagi M., Shinozaki M., Hirakata H., Tamaki K., Hirano T., Tokumoto M.et al. (2000) Locally activated renin-angiotensin system associated with TGF-b1 as a major factor for renal injury induced by chronic inhibition of nitric oxide synthase in rats. J. Am. Soc. Nephrol. 11, 616–624 10.1681/ASN.V114616 [DOI] [PubMed] [Google Scholar]
- 14.Yang F., Chung A.C., Huang X.R. and Lan H.Y. (2009) Angiotensin II induces connective tissue growth factor and collagen I expression via transforming growth factor-b-dependent and -independent Smad pathways: the role of Smad3. Hypertension (Dallas, Tex: 1979) 54, 877–884 10.1161/HYPERTENSIONAHA.109.136531 [DOI] [PubMed] [Google Scholar]
- 15.Epstein M., Kovesdy C.P., Clase C.M., Sood M.M. and Pecoits-Filho R. (2022) Aldosterone, mineralocorticoid receptor activation, and CKD: a review of evolving treatment paradigms. Am. J. Kidney Dis.: Off. J. Natl. Kidney Foundation 80, 658–666 10.1053/j.ajkd.2022.04.016 [DOI] [PubMed] [Google Scholar]
- 16.Vallon V., Wyatt A.W., Klingel K., Huang D.Y., Hussain A., Berchtold S.et al. (2006) SGK1-dependent cardiac CTGF formation and fibrosis following DOCA treatment. J. Mol. Med. 84, 396–404 10.1007/s00109-005-0027-z [DOI] [PubMed] [Google Scholar]
- 17.Sun G.P., Kohno M., Guo P., Nagai Y., Miyata K., Fan Y.Y.et al. (2006) Involvements of Rho-kinase and TGF-b pathways in aldosterone-induced renal injury. J. Am. Soc. Nephrol. 17, 2193–2201 10.1681/ASN.2005121375 [DOI] [PubMed] [Google Scholar]
- 18.Bader M., Steckelings U.M., Alenina N., Santos R.A.S. and Ferrario C.M. (2024) Alternative renin-angiotensin system. Hypertension (Dallas, Tex: 1979) 81, 964–976 10.1161/HYPERTENSIONAHA.123.21364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chappell M.C., Modrall J.G., Diz D.I. and Ferrario C.M. (2004) Novel aspects of the renal renin-angiotensin system: angiotensin-(1-7), ACE2 and blood pressure regulation. Contrib. Nephrol. 143, 77–89 10.1159/000078713 [DOI] [PubMed] [Google Scholar]
- 20.Tallant E.A., Diz D.I. and Ferrario C.M. (1999) State-of-the-Art lecture. Antiproliferative actions of angiotensin-(1-7) in vascular smooth muscle. Hypertension (Dallas, Tex: 1979) 34, 950–957 10.1161/01.HYP.34.4.950 [DOI] [PubMed] [Google Scholar]
- 21.Kidney Disease Improving Global Outcomes (KDIGO) (2024) Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 105, S117–S314 10.1016/j.kint.2023.10.018 [DOI] [PubMed] [Google Scholar]
- 22.Komers R. (2013) Renin inhibition in the treatment of diabetic kidney disease. Clin. Sci. (Lond.) 124, 553–566 10.1042/CS20120468 [DOI] [PubMed] [Google Scholar]
- 23.Komers R. and Plotkin H. (2016) Dual inhibition of renin-angiotensin-aldosterone system and endothelin-1 in treatment of chronic kidney disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 310, R877–R884 10.1152/ajpregu.00425.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohan D.E. (2011) Endothelin and collecting duct sodium and water transport. Contrib. Nephrol. 172, 94–106 10.1159/000328687 [DOI] [PubMed] [Google Scholar]
- 25.Wendel M., Knels L., Kummer W. and Koch T. (2006) Distribution of endothelin receptor subtypes ETA and ETB in the rat kidney. J. Histochem. Cytochem. 54, 1193–1203 10.1369/jhc.5A6888.2006 [DOI] [PubMed] [Google Scholar]
- 26.Davenport A.P., Kuc R.E., Hoskins S.L., Karet F.E. and Fitzgerald F. (1994) [125I]-PD151242: a selective ligand for endothelin ETA receptors in human kidney which localizes to renal vasculature. Br. J. Pharmacol. 113, 1303–1310 10.1111/j.1476-5381.1994.tb17140.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barton M. and Sorokin A. (2015) Endothelin and the glomerulus in chronic kidney disease. Semin. Nephrol. 35, 156–167 10.1016/j.semnephrol.2015.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wellings R.P., Corder R., Warner T.D., Cristol J.P., Thiemermann C. and Vane J.R. (1994) Evidence from receptor antagonists of an important role for ETB receptor-mediated vasoconstrictor effects of endothelin-1 in the rat kidney. Br. J. Pharmacol. 111, 515–520 10.1111/j.1476-5381.1994.tb14767.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Just A., Olson A.J. and Arendshorst W.J. (2004) Dual constrictor and dilator actions of ET(B) receptors in the rat renal microcirculation: interactions with ET(A) receptors. Am. J. Physiol. Ren. Physiol. 286, F660–F668 10.1152/ajprenal.00368.2003 [DOI] [PubMed] [Google Scholar]
- 30.Gellai M., Fletcher T., Pullen M. and Nambi P. (1996) Evidence for the existence of endothelin-B receptor subtypes and their physiological roles in the rat. Am. J. Physiol. 271, R254–R261 10.1152/ajpregu.1996.271.1.R254 [DOI] [PubMed] [Google Scholar]
- 31.Cristol J.P., Warner T.D., Thiemermann C. and Vane J.R. (1993) Mediation via different receptors of the vasoconstrictor effects of endothelins and sarafotoxins in the systemic circulation and renal vasculature of the anaesthetized rat. Br. J. Pharmacol. 108, 776–779 10.1111/j.1476-5381.1993.tb12877.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pollock D.M. and Opgenorth T.J. (1993) Evidence for endothelin-induced renal vasoconstriction independent of ETA receptor activation. Am. J. Physiol. 264, R222–R226 10.1152/ajpregu.1993.264.1.R222 [DOI] [PubMed] [Google Scholar]
- 33.Kohan D.E., Rossi N.F., Inscho E.W. and Pollock D.M. (2011) Regulation of blood pressure and salt homeostasis by endothelin. Physiol. Rev. 91, 1–77 10.1152/physrev.00060.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edwards R.M., Trizna W. and Ohlstein E.H. (1990) Renal microvascular effects of endothelin. Am. J. Physiol. 259, F217–F221 10.1152/ajprenal.1990.259.2.F217 [DOI] [PubMed] [Google Scholar]
- 35.Lanese D.M., Yuan B.H., McMurtry I.F. and Conger J.D. (1992) Comparative sensitivities of isolated rat renal arterioles to endothelin. Am. J. Physiol. 263, F894–F899 10.1152/ajprenal.1992.263.5.F894 [DOI] [PubMed] [Google Scholar]
- 36.Ozawa Y., Hasegawa T., Tsuchiya K., Yoshizumi M. and Tamaki T. (2003) Effect of endothelin-1 (1-31) on the renal resistance vessels. J. Med. Invest. 50, 87–94 [PubMed] [Google Scholar]
- 37.Badr K.F., Murray J.J., Breyer M.D., Takahashi K., Inagami T. and Harris R.C. (1989) Mesangial cell, glomerular and renal vascular responses to endothelin in the rat kidney. Elucidation of signal transduction pathways. J. Clin. Invest. 83, 336–342 10.1172/JCI113880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saito M., Homma S., Yamatsu I., Sato M. and Ohshima N. (1994) Visualization of renal microcirculation in isolated Munich-Wistar rat kidneys: effects of endothelin-1 on renal hemodynamic activity. Jpn. J. Pharmacol. 66, 221–229 10.1254/jjp.66.221 [DOI] [PubMed] [Google Scholar]
- 39.Schildroth J., Rettig-Zimmermann J., Kalk P., Steege A., Fahling M., Sendeski M.et al. (2011) Endothelin type A and B receptors in the control of afferent and efferent arterioles in mice. Nephrol., Dialysis, Transplantation: Off. Publ. Eur. Dialysis Transplant Assoc. - Eur. Renal Assoc. 26, 779–789 10.1093/ndt/gfq534 [DOI] [PubMed] [Google Scholar]
- 40.Inscho E.W., Imig J.D., Cook A.K. and Pollock D.M. (2005) ETA and ETB receptors differentially modulate afferent and efferent arteriolar responses to endothelin. Br. J. Pharmacol. 146, 1019–1026 10.1038/sj.bjp.0706412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Imig J.D., Pham B.T., LeBlanc E.A., Reddy K.M., Falck J.R. and Inscho E.W. (2000) Cytochrome P450 and cyclooxygenase metabolites contribute to the endothelin-1 afferent arteriolar vasoconstrictor and calcium responses. Hypertension (Dallas, Tex: 1979) 35, 307–312 10.1161/01.HYP.35.1.307 [DOI] [PubMed] [Google Scholar]
- 42.Endlich K., Hoffend J. and Steinhausen M. (1996) Localization of endothelin ETA and ETB receptor-mediated constriction in the renal microcirculation of rats. J. Physiol. 497, 211–218 10.1113/jphysiol.1996.sp021761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heller J., Kramer H.J. and Horacek V. (1996) Action of endothelin-1 on glomerular haemodynamics in the dog: lack of direct effects on glomerular ultrafiltration coefficient. Clin. Sci. (Lond.) 90, 385–391 10.1042/cs0900385 [DOI] [PubMed] [Google Scholar]
- 44.Goddard J., Johnston N.R., Hand M.F., Cumming A.D., Rabelink T.J., Rankin A.J.et al. (2004) Endothelin-A receptor antagonism reduces blood pressure and increases renal blood flow in hypertensive patients with chronic renal failure: a comparison of selective and combined endothelin receptor blockade. Circulation 109, 1186–1193 10.1161/01.CIR.0000118499.69469.51 [DOI] [PubMed] [Google Scholar]
- 45.Montanari A., Carra N., Perinotto P., Iori V., Fasoli E., Biggi A.et al. (2002) Renal hemodynamic control by endothelin and nitric oxide under angiotensin II blockade in man. Hypertension (Dallas, Tex: 1979) 39, 715–720 10.1161/hy0202.104399 [DOI] [PubMed] [Google Scholar]
- 46.Pedersen E.B., Thomsen I.M. and Fjordside L.S. (2005) Effect of BQ-123, an endothelin antagonist, on renal hemodynamics, tubular function, vasoactive hormones, and blood pressure in healthy humans: a dose response study. Am. J. Hypertens. 18, 1578–1585 10.1016/j.amjhyper.2005.06.008 [DOI] [PubMed] [Google Scholar]
- 47.Goddard J., Eckhart C., Johnston N.R., Cumming A.D., Rankin A.J. and Webb D.J. (2004) Endothelin A receptor antagonism and angiotensin-converting enzyme inhibition are synergistic via an endothelin B receptor-mediated and nitric oxide-dependent mechanism. J. Am. Soc. Nephrol. 15, 2601–2610 10.1097/01.ASN.0000141313.84470.4B [DOI] [PubMed] [Google Scholar]
- 48.Dhaun N., Macintyre I.M., Melville V., Lilitkarntakul P., Johnston N.R., Goddard J.et al. (2009) Blood pressure-independent reduction in proteinuria and arterial stiffness after acute endothelin-A receptor antagonism in chronic kidney disease. Hypertension (Dallas, Tex: 1979) 54, 113–119 10.1161/HYPERTENSIONAHA.109.132670 [DOI] [PubMed] [Google Scholar]
- 49.Dhaun N., MacIntyre I.M., Kerr D., Melville V., Johnston N.R., Haughie S.et al. (2011) Selective endothelin-A receptor antagonism reduces proteinuria, blood pressure, and arterial stiffness in chronic proteinuric kidney disease. Hypertension (Dallas, Tex: 1979) 57, 772–779 10.1161/HYPERTENSIONAHA.110.167486 [DOI] [PubMed] [Google Scholar]
- 50.Simonson M.S., Wann S., Mene P., Dubyak G.R., Kester M., Nakazato Y.et al. (1989) Endothelin stimulates phospholipase C, Na+/H+ exchange, c-fos expression, and mitogenesis in rat mesangial cells. J. Clin. Invest. 83, 708–712 10.1172/JCI113935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sorokin A. and Kohan D.E. (2003) Physiology and pathology of endothelin-1 in renal mesangium. Am. J. Physiol. Ren. Physiol. 285, F579–F589 10.1152/ajprenal.00019.2003 [DOI] [PubMed] [Google Scholar]
- 52.Gagliardini E., Corna D., Zoja C., Sangalli F., Carrara F., Rossi M.et al. (2009) Unlike each drug alone, lisinopril if combined with avosentan promotes regression of renal lesions in experimental diabetes. Am. J. Physiol. Ren. Physiol. 297, F1448–F1456 10.1152/ajprenal.00340.2009 [DOI] [PubMed] [Google Scholar]
- 53.Boffa J.J., Tharaux P.L., Dussaule J.C. and Chatziantoniou C. (2001) Regression of renal vascular fibrosis by endothelin receptor antagonism. Hypertension (Dallas, Tex: 1979) 37, 490–496 10.1161/01.HYP.37.2.490 [DOI] [PubMed] [Google Scholar]
- 54.Simonson M.S. and Ismail-Beigi F. (2011) Endothelin-1 increases collagen accumulation in renal mesangial cells by stimulating a chemokine and cytokine autocrine signaling loop. J. Biol. Chem. 286, 11003–11008 10.1074/jbc.M110.190793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watson A.M., Li J., Schumacher C., de Gasparo M., Feng B., Thomas M.C.et al. (2010) The endothelin receptor antagonist avosentan ameliorates nephropathy and atherosclerosis in diabetic apolipoprotein E knockout mice. Diabetologia 53, 192–203 10.1007/s00125-009-1540-3 [DOI] [PubMed] [Google Scholar]
- 56.Gerstung M., Roth T., Dienes H.P., Licht C. and Fries J.W. (2007) Endothelin-1 induces NF-kB via two independent pathways in human renal tubular epithelial cells. Am. J. Nephrol. 27, 294–300 10.1159/000101999 [DOI] [PubMed] [Google Scholar]
- 57.Lenoir O., Milon M., Virsolvy A., Henique C., Schmitt A., Masse J.M.et al. (2014) Direct action of endothelin-1 on podocytes promotes diabetic glomerulosclerosis. J. Am. Soc. Nephrol. 25, 1050–1062 10.1681/ASN.2013020195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saleh M.A., Boesen E.I., Pollock J.S., Savin V.J. and Pollock D.M. (2010) Endothelin-1 increases glomerular permeability and inflammation independent of blood pressure in the rat. Hypertension (Dallas, Tex: 1979) 56, 942–949 10.1161/HYPERTENSIONAHA.110.156570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saleh M.A., Pollock J.S. and Pollock D.M. (2011) Distinct actions of endothelin A-selective versus combined endothelin A/B receptor antagonists in early diabetic kidney disease. J. Pharmacol. Exp. Ther. 338, 263–270 10.1124/jpet.111.178988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saleh M.A., Boesen E.I., Pollock J.S., Savin V.J. and Pollock D.M. (2011) ETA receptor specific stimulation of glomerular inflammation and injury in streptozotocin-induced diabetic rats. Diabetologia 54, 979–988 10.1007/s00125-010-2021-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sasser J.M., Sullivan J.C., Hobbs J.L., Yamamoto T., Pollock D.M., Carmines P.K.et al. (2007) Endothelin A receptor blockade reduces diabetic renal injury via an anti-inflammatory mechanism. J. Am. Soc. Nephrol. 18, 143–154 10.1681/ASN.2006030208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kang Y.S., Ko G.J., Lee M.H., Song H.K., Han S.Y., Han K.H.et al. (2009) Effect of eplerenone, enalapril and their combination treatment on diabetic nephropathy in type II diabetic rats. Nephrol. Dialysis, Transpl.: Off. Publ. Eur. Dialysis Transplant Assoc. - Eur. Renal Assoc. 24, 73–84 10.1093/ndt/gfn448 [DOI] [PubMed] [Google Scholar]
- 63.Shrestha A., Che R.C. and Zhang A.H. (2019) Role of aldosterone in renal fibrosis. Adv. Exp. Med. Biol. 1165, 325–346 10.1007/978-981-13-8871-2_15 [DOI] [PubMed] [Google Scholar]
- 64.Barton M. and Tharaux P.L. (2012) Endothelin and the podocyte. Clin. Kidney J. 5, 17–27 10.1093/ckj/sfs001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buelli S., Rosano L., Gagliardini E., Corna D., Longaretti L., Pezzotta A.et al. (2014) b-arrestin-1 drives endothelin-1-mediated podocyte activation and sustains renal injury. J. Am. Soc. Nephrol. 25, 523–533 10.1681/ASN.2013040362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Daehn I., Casalena G., Zhang T., Shi S., Fenninger F., Barasch N.et al. (2014) Endothelial mitochondrial oxidative stress determines podocyte depletion in segmental glomerulosclerosis. J. Clin. Invest. 124, 1608–1621 10.1172/JCI71195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dai C., Stolz D.B., Kiss L.P., Monga S.P., Holzman L.B. and Liu Y. (2009) Wnt/beta-catenin signaling promotes podocyte dysfunction and albuminuria. J. Am. Soc. Nephrol. 20, 1997–2008 10.1681/ASN.2009010019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Opocensky M., Dvorak P., Maly J., Kramer H.J., Backer A., Kopkan L.et al. (2004) Chronic endothelin receptor blockade reduces end-organ damage independently of blood pressure effects in salt-loaded heterozygous Ren-2 transgenic rats. Physiol. Res. 53, 581–593 [PubMed] [Google Scholar]
- 69.Garsen M., Lenoir O., Rops A.L., Dijkman H.B., Willemsen B., van Kuppevelt T.H.et al. (2016) Endothelin-1 induces proteinuria by heparanase-mediated disruption of the glomerular glycocalyx. J. Am. Soc. Nephrol. 27, 3545–3551 10.1681/ASN.2015091070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boels M.G., Avramut M.C., Koudijs A., Dane M.J., Lee D.H., van der Vlag J.et al. (2016) Atrasentan reduces albuminuria by restoring the glomerular endothelial glycocalyx barrier in diabetic nephropathy. Diabetes 65, 2429–2439 10.2337/db15-1413 [DOI] [PubMed] [Google Scholar]
- 71.Ebefors K., Wiener R.J., Yu L., Azeloglu E.U., Yi Z., Jia F.et al. (2019) Endothelin receptor-A mediates degradation of the glomerular endothelial surface layer via pathologic crosstalk between activated podocytes and glomerular endothelial cells. Kidney Int. 96, 957–970 10.1016/j.kint.2019.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saleh M.A., Sandoval R.M., Rhodes G.J., Campos-Bilderback S.B., Molitoris B.A. and Pollock D.M. (2012) Chronic endothelin-1 infusion elevates glomerular sieving coefficient and proximal tubular albumin reuptake in the rat. Life Sci. 91, 634–637 10.1016/j.lfs.2012.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hocher B., Thone-Reineke C., Rohmeiss P., Schmager F., Slowinski T., Burst V.et al. (1997) Endothelin-1 transgenic mice develop glomerulosclerosis, interstitial fibrosis, and renal cysts but not hypertension. J. Clin. Invest. 99, 1380–1389 10.1172/JCI119297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.De Miguel C., Hamrick W.C., Hobbs J.L., Pollock D.M., Carmines P.K. and Pollock J.S. (2017) Endothelin receptor-specific control of endoplasmic reticulum stress and apoptosis in the kidney. Sci. Rep. 7, 43152 10.1038/srep43152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Czopek A., Moorhouse R., Gallacher P.J., Pugh D., Ivy J.R., Farrah T.E.et al. (2022) Endothelin blockade prevents the long-term cardiovascular and renal sequelae of acute kidney injury in mice. Sci. Transl. Med. 14, eabf5074 10.1126/scitranslmed.abf5074 [DOI] [PubMed] [Google Scholar]
- 76.Kohan D.E. (1991) Endothelin synthesis by rabbit renal tubule cells. Am. J. Physiol. 261, F221–F226 10.1152/ajprenal.1991.261.2.F221 [DOI] [PubMed] [Google Scholar]
- 77.Stuart D., Chapman M., Rees S., Woodward S. and Kohan D.E. (2013) Myocardial, smooth muscle, nephron, and collecting duct gene targeting reveals the organ sites of endothelin A receptor antagonist fluid retention. J. Pharmacol. Exp. Ther. 346, 182–189 10.1124/jpet.113.205286 [DOI] [PubMed] [Google Scholar]
- 78.Heerspink H.J.L., Parving H.H., Andress D.L., Bakris G., Correa-Rotter R., Hou F.F.et al. (2019) Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial. Lancet 393, 1937–1947 10.1016/S0140-6736(19)30772-X [DOI] [PubMed] [Google Scholar]
- 79.de Zeeuw D., Coll B., Andress D., Brennan J.J., Tang H., Houser M.et al. (2014) The endothelin antagonist atrasentan lowers residual albuminuria in patients with type 2 diabetic nephropathy. J. Am. Soc. Nephrol. 25, 1083–1093 10.1681/ASN.2013080830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Emori T., Hirata Y., Ohta K., Shichiri M. and Marumo F. (1989) Secretory mechanism of immunoreactive endothelin in cultured bovine endothelial cells. Biochem. Biophys. Res. Commun. 160, 93–100 10.1016/0006-291X(89)91625-2 [DOI] [PubMed] [Google Scholar]
- 81.Emori T., Hirata Y., Ohta K., Kanno K., Eguchi S., Imai T.et al. (1991) Cellular mechanism of endothelin-1 release by angiotensin and vasopressin. Hypertension (Dallas, Tex: 1979) 18, 165–170 10.1161/01.HYP.18.2.165 [DOI] [PubMed] [Google Scholar]
- 82.Kohno M., Horio T., Ikeda M., Yokokawa K., Fukui T., Yasunari K.et al. (1992) Angiotensin II stimulates endothelin-1 secretion in cultured rat mesangial cells. Kidney Int. 42, 860–866 10.1038/ki.1992.361 [DOI] [PubMed] [Google Scholar]
- 83.Chen L., McNeill J.R., Wilson T.W. and Gopalakrishnan V. (1995) Heterogeneity in vascular smooth muscle responsiveness to angiotensin II. Role of endothelin. Hypertension (Dallas, Tex: 1979) 26, 83–88 10.1161/01.HYP.26.1.83 [DOI] [PubMed] [Google Scholar]
- 84.Webb M.L., Dickinson K.E., Delaney C.L., Liu E.C., Serafino R., Cohen R.B.et al. (1992) The endothelin receptor antagonist, BQ-123, inhibits angiotensin II-induced contractions in rabbit aorta. Biochem. Biophys. Res. Commun. 185, 887–892 10.1016/0006-291X(92)91710-8 [DOI] [PubMed] [Google Scholar]
- 85.Wenzel R.R., Ruthemann J., Bruck H., Schafers R.F., Michel M.C. and Philipp T. (2001) Endothelin-A receptor antagonist inhibits angiotensin II and noradrenaline in man. Br. J. Clin. Pharmacol. 52, 151–157 10.1046/j.0306-5251.2001.01422.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Balakrishnan S.M., Wang H.D., Gopalakrishnan V., Wilson T.W. and McNeill J.R. (1996) Effect of an endothelin antagonist on hemodynamic responses to angiotensin II. Hypertension (Dallas, Tex: 1979) 28, 806–809 10.1161/01.HYP.28.5.806 [DOI] [PubMed] [Google Scholar]
- 87.Riggleman A., Harvey J. and Baylis C. (2001) Endothelin mediates some of the renal actions of acutely administered angiotensin II. Hypertension (Dallas, Tex: 1979) 38, 105–109 10.1161/01.HYP.38.1.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kawaguchi H., Sawa H. and Yasuda H. (1991) Effect of endothelin on angiotensin converting enzyme activity in cultured pulmonary rtery endothelial cells. J. Hypertens. 9, 171–174 10.1097/00004872-199102000-00012 [DOI] [PubMed] [Google Scholar]
- 89.Kawaguchi H., Sawa H. and Yasuda H. (1990) Endothelin stimulates angiotensin I to angiotensin II conversion in cultured pulmonary artery endothelial cells. J. Mol. Cell Cardiol. 22, 839–842 10.1016/0022-2828(90)90115-I [DOI] [PubMed] [Google Scholar]
- 90.Gomez-Garre D., Ruiz-Ortega M., Ortego M., Largo R., Lopez-Armada M.J., Plaza J.J.et al. (1996) Effects and interactions of endothelin-1 and angiotensin II on matrix protein expression and synthesis and mesangial cell growth. Hypertension (Dallas, Tex: 1979) 27, 885–892 10.1161/01.HYP.27.4.885 [DOI] [PubMed] [Google Scholar]
- 91.Matsumura Y., Nakase K., Ikegawa R., Hayashi K., Ohyama T. and Morimoto S. (1989) The endothelium-derived vasoconstrictor peptide endothelin inhibits renin release in vitro. Life Sci. 44, 149–157 10.1016/0024-3205(89)90533-X [DOI] [PubMed] [Google Scholar]
- 92.Grünberger C., Obermayer B., Klar J., Kurtz A. and Schweda F. (2006) The calcium paradoxon of renin release: calcium suppresses renin exocytosis by inhibition of calcium-dependent adenylate cyclases AC5 and AC6. Circ. Res. 99, 1197–1206 10.1161/01.RES.0000251057.35537.d3 [DOI] [PubMed] [Google Scholar]
- 93.Ryan M.J., Black T.A., Millard S.L., Gross K.W. and Hajduczok G. (2002) Endothelin-1 increases calcium and attenuates renin gene expression in As4.1 cells. Am. J. Physiol. Heart Circ. Physiol. 283, H2458–H2465 10.1152/ajpheart.00295.2002 [DOI] [PubMed] [Google Scholar]
- 94.Ortiz-Capisano M.C. (2014) Endothelin inhibits renin release from juxtaglomerular cells via endothelin receptors A and B via a transient receptor potential canonical-mediated pathway. Physiol. Rep. 2, 10.14814/phy2.12240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mazzocchi G., Rebuffat P., Meneghelli V., Malendowicz L.K., Kasprzak A. and Nussdorfer G.G. (1990) Effects of prolonged infusion with endothelin-1 on the function and morphology of rat adrenal cortex. Peptides 11, 767–772 10.1016/0196-9781(90)90193-9 [DOI] [PubMed] [Google Scholar]
- 96.Mazzocchi G., Malendowicz L.K., Meneghelli V. and Nussdorfer G.G. (1992) Endothelin-1 stimulates mitotic activity in the zona glomerulosa of the rat adrenal cortex. Cytobios 69, 91–96 [PubMed] [Google Scholar]
- 97.Benter I.F., Yousif M.H., Anim J.T., Cojocel C. and Diz D.I. (2006) Angiotensin-(1-7) prevents development of severe hypertension and end-organ damage in spontaneously hypertensive rats treated with L-NAME. Am. J. Physiol. Heart Circ. Physiol. 290, H684–H691 10.1152/ajpheart.00632.2005 [DOI] [PubMed] [Google Scholar]
- 98.Benter I.F., Yousif M.H., Dhaunsi G.S., Kaur J., Chappell M.C. and Diz D.I. (2008) Angiotensin-(1-7) prevents activation of NADPH oxidase and renal vascular dysfunction in diabetic hypertensive rats. Am. J. Nephrol. 28, 25–33 10.1159/000108758 [DOI] [PubMed] [Google Scholar]
- 99.Schinzari F., Tesauro M., Veneziani A., Mores N., Di Daniele N. and Cardillo C. (2018) Favorable vascular actions of angiotensin-(1-7) in human obesity. Hypertension (Dallas, Tex: 1979) 71, 185–191 10.1161/HYPERTENSIONAHA.117.10280 [DOI] [PubMed] [Google Scholar]
- 100.Barton M., Shaw S., d'Uscio L.V., Moreau P. and Luscher T.F. (1997) Angiotensin II increases vascular and renal endothelin-1 and functional endothelin converting enzyme activity in vivo: role of ETA receptors for endothelin regulation. Biochem. Biophys. Res. Commun. 238, 861–865 10.1006/bbrc.1997.7394 [DOI] [PubMed] [Google Scholar]
- 101.Benigni A., Corna D., Maffi R., Benedetti G., Zoja C. and Remuzzi G. (1998) Renoprotective effect of contemporary blocking of angiotensin II and endothelin-1 in rats with membranous nephropathy. Kidney Int. 54, 353–359 10.1046/j.1523-1755.1998.00011.x [DOI] [PubMed] [Google Scholar]
- 102.Amann K., Simonaviciene A., Medwedewa T., Koch A., Orth S., Gross M.L.et al. (2001) Blood pressure-independent additive effects of pharmacologic blockade of the renin-angiotensin and endothelin systems on progression in a low-renin model of renal damage. J. Am. Soc. Nephrol. 12, 2572–2584 10.1681/ASN.V12122572 [DOI] [PubMed] [Google Scholar]
- 103.Certikova Chabova V., Vernerova Z., Kujal P., Huskova Z., Skaroupkova P., Tesar V.et al. (2014) Addition of ETA receptor blockade increases renoprotection provided by renin-angiotensin system blockade in 5/6 nephrectomized Ren-2 transgenic rats. Life Sci. 118, 297–305 10.1016/j.lfs.2013.12.018 [DOI] [PubMed] [Google Scholar]
- 104.Kohan D.E., Pritchett Y., Molitch M., Wen S., Garimella T., Audhya P.et al. (2011) Addition of atrasentan to renin-angiotensin system blockade reduces albuminuria in diabetic nephropathy. J. Am. Soc. Nephrol. 22, 763–772 10.1681/ASN.2010080869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wenzel R.R., Littke T., Kuranoff S., Jurgens C., Bruck H., Ritz E.et al. (2009) Avosentan reduces albumin excretion in diabetics with macroalbuminuria. J. Am. Soc. Nephrol. 20, 655–664 10.1681/ASN.2008050482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mann J.F., Green D., Jamerson K., Ruilope L.M., Kuranoff S.J., Littke T.et al. (2010) Avosentan for overt diabetic nephropathy. J. Am. Soc. Nephrol. 21, 527–535 10.1681/ASN.2009060593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Murugesan N., Tellew J.E., Gu Z., Kunst B.L., Fadnis L., Cornelius L.A.et al. (2002) Discovery of N-isoxazolyl biphenylsulfonamides as potent dual angiotensin II and endothelin A receptor antagonists. J. Med. Chem. 45, 3829–3835 10.1021/jm020138n [DOI] [PubMed] [Google Scholar]
- 108.Leach K., Pan-Zhou X.R., Deats W. and Beconi M. (2016) Renal pharmacology and preclinical attributes of sparsentan, a dually active endothelin A and angiotensin 1 receptor antagonist. J. Am. Soc. Nephrol. 27 (suppl).132A Abstract TH-PO16626041841 [Google Scholar]
- 109.Schena F.P. and Nistor I. (2018) Epidemiology of IgA nephropathy: a global perspective. Semin. Nephrol. 38, 435–442 10.1016/j.semnephrol.2018.05.013 [DOI] [PubMed] [Google Scholar]
- 110.Kidney Disease Improving Global Outcomes (KDIGO) . Clinical practice guideline for the management of glomerular diseases. Available from: https://kdigo.org/wp-content/uploads/2017/02/KDIGO-Glomerular-Diseases-Guideline-2021-English.pdf (accessed October 2023) [Google Scholar]
- 111.Maixnerova D., Ling C., Hall S., Reily C., Brown R., Neprasova M.et al. (2019) Galactose-deficient IgA1 and the corresponding IgG autoantibodies predict IgA nephropathy progression. PloS ONE 14, e0212254 10.1371/journal.pone.0212254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rodrigues J.C., Haas M. and Reich H.N. (2017) IgA nephropathy. Clin. J. Am. Soc. Nephrol. 12, 677–686 10.2215/CJN.07420716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Neugut Y.D. and Kiryluk K. (2018) Genetic determinants of IgA nephropathy: western perspective. Semin. Nephrol. 38, 443–454 10.1016/j.semnephrol.2018.05.014 [DOI] [PubMed] [Google Scholar]
- 114.Coppo R. (2018) The gut-kidney axis in IgA nephropathy: role of microbiota and diet on genetic predisposition. Pediatr. Nephrol. 33, 53–61 10.1007/s00467-017-3652-1 [DOI] [PubMed] [Google Scholar]
- 115.Coppo R. (2018) The gut-renal connection in IgA nephropathy. Semin. Nephrol. 38, 504–512 10.1016/j.semnephrol.2018.05.020 [DOI] [PubMed] [Google Scholar]
- 116.Yeo S.C., Cheung C.K. and Barratt J. (2018) New insights into the pathogenesis of IgA nephropathy. Pediatr. Nephrol. 33, 763–777 10.1007/s00467-017-3699-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fellstrom B.C., Barratt J., Cook H., Coppo R., Feehally J., de Fijter J.W.et al. (2017) Targeted-release budesonide versus placebo in patients with IgA nephropathy (NEFIGAN): a double-blind, randomised, placebo-controlled phase 2b trial. Lancet 389, 2117–2127 10.1016/S0140-6736(17)30550-0 [DOI] [PubMed] [Google Scholar]
- 118.Maillard N., Wyatt R.J., Julian B.A., Kiryluk K., Gharavi A., Fremeaux-Bacchi V.et al. (2015) Current understanding of the role of complement in IgA nephropathy. J. Am. Soc. Nephrol. 26, 1503–1512 10.1681/ASN.2014101000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kohan D.E., Barratt J., Heerspink H.J.L., Campbell K.N., Camargo M., Ogbaa I.et al. (2023) Targeting the endothelin A receptor in IgA nephropathy. Kidney Int. Rep. 8, 2198–2210 10.1016/j.ekir.2023.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pitcher D., Braddon F., Hendry B., Mercer A., Osmaston K., Saleem M.A.et al. (2023) Long-term outcomes in IgA nephropathy. Clin. J. Am. Soc. Nephrol. 18, 727–738 10.2215/CJN.0000000000000135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lehrke I., Waldherr R., Ritz E. and Wagner J. (2001) Renal endothelin-1 and endothelin receptor type B expression in glomerular diseases with proteinuria. J. Am. Soc. Nephrol. 12, 2321–2329 10.1681/ASN.V12112321 [DOI] [PubMed] [Google Scholar]
- 122.Tycova I., Hruba P., Maixnerova D., Girmanova E., Mrazova P., Stranavova L.et al. (2018) Molecular profiling in IgA nephropathy and focal and segmental glomerulosclerosis. Physiol. Res. 67, 93–105 10.33549/physiolres.933670 [DOI] [PubMed] [Google Scholar]
- 123.Jenkinson C., Moldoveanu Z., Komers R., Hall S., Huang Z.Q., Knoppova B.et al. (2019) Protective effects of sparsentan from proliferative glomerular injury induced by administration of human immune complexes in a murine model of experimental IgA nephropathy. Kidney Int. Rep. 4, S5–S6Abstract SAT-010 10.1016/j.ekir.2019.05.031 [DOI] [Google Scholar]
- 124.Reily C., Moldoveanu Z., Pramparo T., Hall S., Huang Z.Q., Rice T.et al. (2024) Sparsentan ameliorates glomerular hypercellularity and inflammatory-gene networks induced by IgA1-IgG immune complexes in a mouse model of IgA nephropathy. Am. J. Physiol. Ren. Physiol. 3265F862–F875 10.1152/ajprenal.00253.2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nagasawa H., Ueda S., Suzuki H., Jenkinson C., Fukao Y., Nakayama M.et al. (2024) Sparsentan is superior to losartan in the gddY mouse model of IgA nephropathy. Nephrol., Dialysis, Transplantation: Off. Publ. Eur. Dialysis Transplant Assoc. - Eur. Renal Assoc. 10.1093/ndt/gfae021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jenkinson C., Diva U., Liu K., Barnes J.L., Mercer A. and Komers R. (2018) Effect of sparsentan, a dual angiotensin II type 1 (AT1) and endothelin type A (ETA) receptor antagonist, in the rat anti-Thy1 model of glomerulonephritis. Presented at: 15th International Symposium on IgA Nephropathy - IIgANN; September 27-29, 2018. [Google Scholar]
- 127.Stokes M.B. and D'Agati V.D. (2014) Morphologic variants of focal segmental glomerulosclerosis and their significance. Adv. Chronic Kidney Dis. 21, 400–407 10.1053/j.ackd.2014.02.010 [DOI] [PubMed] [Google Scholar]
- 128.Rosenberg A.Z. and Kopp J.B. (2017) Focal segmental glomerulosclerosis. Clin. J. Am. Soc. Nephrol. 12, 502–517 10.2215/CJN.05960616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.D'Agati V.D., Kaskel F.J. and Falk R.J. (2011) Focal segmental glomerulosclerosis. N. Engl. J. Med. 365, 2398–2411 10.1056/NEJMra1106556 [DOI] [PubMed] [Google Scholar]
- 130.Yao T., Udwan K., John R., Rana A., Haghighi A., Xu L.et al. (2019) Integration of genetic testing and pathology for the diagnosis of adults with FSGS. Clin. J. Am. Soc. Nephrol. 7213–223 10.2215/CJN.08750718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kopp J.B. and Rosenberg A.Z. (2019) One actor, many roles: histopathologies associated with APOL1 genetic variants. Adv. Anat. Pathol. 26, 215–219 10.1097/PAP.0000000000000221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Coppo R. (2013) Different targets for treating focal segmental glomerular sclerosis. Contrib. Nephrol. 181, 84–90 10.1159/000348637 [DOI] [PubMed] [Google Scholar]
- 133.Ponticelli C. and Graziani G. (2013) Current and emerging treatments for idiopathic focal and segmental glomerulosclerosis in adults. Expert Rev. Clin. Immunol. 9, 251–261 10.1586/eci.12.109 [DOI] [PubMed] [Google Scholar]
- 134.Sethna C.B. and Gipson D.S. (2014) Treatment of FSGS in children. Adv. Chronic Kidney Dis. 21, 194–199 10.1053/j.ackd.2014.01.010 [DOI] [PubMed] [Google Scholar]
- 135.Kiffel J., Rahimzada Y. and Trachtman H. (2011) Focal segmental glomerulosclerosis and chronic kidney disease in pediatric patients. Adv. Chronic Kidney Dis. 18, 332–338 10.1053/j.ackd.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hogan J. and Radhakrishnan J. (2014) The treatment of idiopathic focal segmental glomerulosclerosis in adults. Adv. Chronic Kidney Dis. 21, 434–441 10.1053/j.ackd.2014.03.016 [DOI] [PubMed] [Google Scholar]
- 137.van de Lest N.A., Bakker A.E., Dijkstra K.L., Zandbergen M., Heemskerk S.A.C., Wolterbeek R.et al. (2021) Endothelial endothelin receptor A expression is associated with podocyte injury and oxidative stress in patients with focal segmental glomerulosclerosis. Kidney Int. Rep. 6, 1939–1948 10.1016/j.ekir.2021.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gyarmati G., Shroff U., Izuhara A., Komers R., Bedard P.W. and Peti-Peterdi J. (2021) Sparsentan improves glomerular blood flow and augments protective tissue remodeling in mouse models of focal segmental glomerulosclerosis (FSGS). Nephrol., Dialysis, Transplantation: Off. Publ. Eur. Dialysis Transplant Assoc. - Eur. Renal Assoc. 36 (suppl 1).i10.Abstract FC016 [Google Scholar]
- 139.Gyarmati G., Deepak S., Shroff U., Izuhara A., Komers R., Bedard P.W.et al. (2022) Sparsentan improves glomerular endothelial and podocyte functions and augments protective tissue repair in a mouse model of focal segmental glomerulosclerosis (FSGS). Presented at: American Society of Nephrology, Kidney Week, 3-6 November 2022. Abstract FR-OR56 [Google Scholar]
- 140.Lichtnekert J., Kaverina N.V., Eng D.G., Gross K.W., Kutz J.N., Pippin J.W.et al. (2016) Renin-angiotensin-aldosterone system inhibition increases podocyte derivation from cells of renin lineage. J. Am. Soc. Nephrol. 27, 3611–3627 10.1681/ASN.2015080877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pippin J.W., Kaverina N.V., Eng D.G., Krofft R.D., Glenn S.T., Duffield J.S.et al. (2015) Cells of renin lineage are adult pluripotent progenitors in experimental glomerular disease. Am. J. Physiol. Ren. Physiol. 309, F341–F358 10.1152/ajprenal.00438.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Watanabe H., Martini A.G., Brown R.I., Liang X., Medrano S., Goto S.et al. (2021) Inhibition of the renin-angiotensin system causes concentric hypertrophy of renal arterioles in mice and humans. JCI Insight 6, 10.1172/jci.insight.154337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Oka M., Medrano S., Sequeira-Lόpez M.L.S. and Gómez R.A. (2017) Chronic stimulation of renin cells leads to vascular pathology. Hypertension (Dallas, Tex: 1979) 70, 119–128 10.1161/HYPERTENSIONAHA.117.09283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bedard P.W., Jenkinson C. and Komers R. (2022) Sparsentan protects the glomerular basement membrane and glycocalyx, and attenuates proteinuria in a rat model of focal segmental glomerulosclerosis (FSGS). Nephrol., Dialysis, Transplantation: Off. Publ. Eur. Dialysis Transplant Assoc. - Eur. Renal Assoc. 37 (suppl 3).i177.Abstract MO255 10.1093/ndt/gfac067.054 [DOI] [Google Scholar]
- 145.Eddy S., Eichinger F., Godfrey B., Nair V., Ju W., Jenkinson C.et al. (2022) Development of a treatment response prediction strategy for endothelin and angiotensin receptor inhibition in glomerular disease. Presented at: American Society of Nephrology, Kidney Week, November 3-6, 2002. Abtract FR-PO724 [Google Scholar]
- 146.Li L., Tang W., Zhang Y., Jia M., Wang L., Li Q.et al. (2022) Targeting tissue-resident memory CD8(+) T cells in the kidney is a potential therapeutic strategy to ameliorate podocyte injury and glomerulosclerosis. Mol. Ther. 30, 2746–2759 10.1016/j.ymthe.2022.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gross O., Kashtan C.E., Rheault M.N., Flinter F., Savige J., Miner J.H.et al. (2017) Advances and unmet needs in genetic, basic and clinical science in Alport syndrome: report from the 2015 International Workshop on Alport Syndrome. Nephrol., Dialysis, Transplantation: Off. Publ. Eur. Dialysis Transplant Assoc. - Eur. Renal Assoc. 32, 916–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kashtan C.E., Ding J., Garosi G., Heidet L., Massella L., Nakanishi K.et al. (2018) Alport syndrome: a unified classification of genetic disorders of collagen IV a345: a position paper of the Alport Syndrome Classification Working Group. Kidney Int. 93, 1045–1051 10.1016/j.kint.2017.12.018 [DOI] [PubMed] [Google Scholar]
- 149.Kashtan C.E. (1999) Alport syndrome. An inherited disorder of renal, ocular, and cochlear basement membranes. Medicine (Baltimore). 78, 338–360 10.1097/00005792-199909000-00005 [DOI] [PubMed] [Google Scholar]
- 150.Kashtan C.E., Ding J., Gregory M., Gross O., Heidet L., Knebelmann B.et al. (2013) Clinical practice recommendations for the treatment of Alport syndrome: a statement of the Alport Syndrome Research Collaborative. Pediatr. Nephrol. 28, 5–11 10.1007/s00467-012-2138-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Savige J., Gregory M., Gross O., Kashtan C., Ding J. and Flinter F. (2013) Expert guidelines for the management of Alport syndrome and thin basement membrane nephropathy. J. Am. Soc. Nephrol. 24, 364–375 10.1681/ASN.2012020148 [DOI] [PubMed] [Google Scholar]
- 152.Stock J., Kuenanz J., Glonke N., Sonntag J., Frese J., Tonshoff B.et al. (2017) Prospective study on the potential of RAAS blockade to halt renal disease in Alport syndrome patients with heterozygous mutations. Pediatr. Nephrol. 32, 131–137 10.1007/s00467-016-3452-z [DOI] [PubMed] [Google Scholar]
- 153.Dufek B., Meehan D.T., Delimont D., Cheung L., Gratton M.A., Phillips G.et al. (2016) Endothelin A receptor activation on mesangial cells initiates Alport glomerular disease. Kidney Int. 90, 300–310 10.1016/j.kint.2016.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cosgrove D., Gratton M.A., Madison J., Vosik D., Samuelson G., Meehan D.et al. (2023) Dual inhibition of the endothelin and angiotensin receptor ameliorates renal and inner ear pathologies in Alport mice. J. Pathol. 260, 353–364 10.1002/path.6087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Travere Therapeutics . Filspari (sparsentan) product information. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/216403s000lbl.pdf (accessed October 2023) [Google Scholar]
- 156.Travere Therapeutics . Travere Therapeutics announces topline results from two-year primary efficacy endpoint in pivotal phase 3 DUPLEX study of sparsentan in focal segmental glomerulosclerosis. Available from: https://ir.travere.com/news-releases/news-release-details/travere-therapeutics-announces-topline-results-two-year-primary (accessed October 2023) [Google Scholar]
- 157.Heerspink H.J.L., Radhakrishnan J., Alpers C.E., Barratt J., Bieler S., Diva U.et al. (2023) Sparsentan in patients with IgA nephropathy: a prespecified interim analysis from a randomised, double-blind, active-controlled clinical trial. Lancet 401, 1584–1594 10.1016/S0140-6736(23)00569-X [DOI] [PubMed] [Google Scholar]
- 158.Trachtman H., Nelson P., Adler S., Campbell K.N., Chaudhuri A., Derebail V.K.et al. (2018) DUET: A phase 2 study evaluating the efficacy and safety of sparsentan in patients with FSGS. J. Am. Soc. Nephrol. 29, 2745–2754 10.1681/ASN.2018010091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hogan J., Derebail V.K., Murphey E.L., P.C. Raguram, Pergola P.E., Sanghani N.S.et al. (2018) Long-term effects of sparsentan, a dual angiotensin and endothelin receptor antagonist in primary focal segmental glomerulosclerosis (FSGS): Interim 84-Week Analysis of the DUET Trial. J. Am. Soc. Nephrol. 2961.Abstract FR-OR087 [Google Scholar]
- 160.Mann J.F., Green D., Jamerson K., Ruilope L.M., Kuranoff S.J., Littke T.et al. (2010) Avosentan for overt diabetic nephropathy. J. Am. Soc. Nephrol. 21, 527–535 10.1681/ASN.2009060593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kohan D.E., Lambers Heerspink H.J., Coll B., Andress D., Brennan J.J., Kitzman D.W.et al. (2015) Predictors of atrasentan-associated fluid retention and change in albuminuria in patients with diabetic nephropathy. Clin. J. Am. Soc. Nephrol. 10, 1568–1574 10.2215/CJN.00570115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Koomen J.V., Stevens J., Mostafa N.M., Parving H.H., de Zeeuw D. and Heerspink H.J.L. (2018) Determining the optimal dose of atrasentan by evaluating the exposure-response relationships of albuminuria and bodyweight. Diabetes Obes. Metab. 20, 2019–2022 10.1111/dom.13312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Heerspink H.J.L., Kiyosue A., Wheeler D.C., Lin M., Wijkmark E., Carlson G.et al. (2023) Zibotentan in combination with dapagliflozin compared with dapagliflozin in patients with chronic kidney disease (ZENITH-CKD): a multicentre, randomised, active-controlled, phase 2b, clinical trial. Lancet 402, 2004–2017 10.1016/S0140-6736(23)02230-4 [DOI] [PubMed] [Google Scholar]
- 164.Heerspink H.J.L., Greasley P.J., Ahlström C., Althage M., Dwyer J.P., Law G.et al. (2024) Efficacy and safety of zibotentan and dapagliflozin in patients with chronic kidney disease: study design and baseline characteristics of the ZENITH-CKD trial. Nephrol. Dialysis Transpl.: Off. Publ. Eur. Dialysis Transplant Assoc. - Eur. Renal Assoc. 39, 414–425 10.1093/ndt/gfad183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hendry B., Kohan D., Geletka R., Jenkinson C., Chen S.-C. and Bedard P.W. (2023) Sparsentan receptor occupancy modeling, clinical actions, and safety. J. Am. Soc. Nephrol. 34799.Abstract SA-PO276 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
N/A - All supporting data are included within the main article.