Abstract
In the context of aging, the susceptibility to infectious diseases increases, leading to heightened morbidity and mortality. This phenomenon, termed immunosenescence, is characterized by dysregulation in the aging immune system, including abnormal alterations in lymphocyte composition, elevated basal inflammation, and the accumulation of senescent T cells. Such changes contribute to increased autoimmune diseases, enhanced infection severity, and reduced responsiveness to vaccines. Utilizing aging animal models becomes imperative for a comprehensive understanding of immunosenescence, given the complexity of aging as a physiological process in living organisms. Our investigation focuses on Cisd2, a causative gene for Wolfram syndrome, to elucidate on immunosenescence. Cisd2 knockout (KO) mice, serving as a model for premature aging, exhibit a shortened lifespan with early onset of aging-related features, such as decreased bone density, hair loss, depigmentation, and optic nerve degeneration. Intriguingly, we found that the Cisd2 KO mice present a higher number of neutrophils in the blood; however, isolated neutrophils from these mice display functional defects. Through mass spectrometry analysis, we identified an interaction between Cisd2 and Calnexin, a protein known for its role in protein quality control. Beyond this function, Calnexin also regulates calcium homeostasis through interaction with sarcoendoplasmic reticulum calcium transport ATPase (SERCA). Our study proposes that Cisd2 modulates calcium homeostasis via its interaction with Calnexin and SERCA, consequently influencing neutrophil functions.
Keywords: Aging, Calnexin, Cisd2, Immunosenescence, Neutrophil
INTRODUCTION
The aging process weakens the immune system, leading to immunosenescence, a decline in overall immune function. This decline contributes to increased susceptibility to infectious diseases, reduced responsiveness to vaccines, heightened risk of cancer, and elevated incidence of autoimmune diseases among the elderly (1). Unlike cellular senescence, which entails the cessation of cell proliferation, immunosenescence is characterized by significant immunological changes from the young and elderly individual, including alterations in immune cell composition, such as reduction in naïve T cells, and increase in myeloid/lymphoid cell ratios (2). The functional decline due to aging of innate immune cells, including neutrophil, Natural Killer (NK) cells, macrophages, and dendritic cells, further exacerbates susceptibility to infectious diseases in the elderly (3).
CDGSH iron sulfur domain 2 (Cisd2) has been found to be a causative gene for Wolfram syndrome, and recognized as a pro-longevity gene. Cisd2 knockout (KO) mice exhibit early onset of various signs that are reminiscent of human aging, such as decreased bone density, alopecia, depigmentation, and optic nerve degeneration, coupled with shortened lifespan (4, 5). However, the impact of Cisd2 deficiency on the immune system remains unexplored.
One of the pivotal functions of Cisd2 is the regulation of Ca2+ homeostasis and mitochondrial function. The Cids2 protein is localized on the endoplasmic reticulum (ER), outer mitochondrial membrane (OMM), and Mitochondria-associated membranes (MAMs), collectively playing a central role in intracellular Ca2+ homeostasis (6, 7). Notably, Cisd2 has been identified as interacting with key proteins involved in Ca2+ regulation, which include the Sarco/Endoplasmic Reticulum Calcium ATPase (SERCA), GTPase, IMAP Family Member 5 (Gimap5), and B cell lymphoma-2 (BCL-2) (8-11).
In this study, we utilized Cisd2 knockout (KO) mice as a premature aging model to investigate the aging immune system. We observed that Cisd2 KO mice exhibited a high number of neutrophils in the blood, whereas those neutrophils displayed defective functions. Through affinity purification and subsequent mass spectrometry analysis, we identified that Cisd2 binds to Calnexin, which is known to assist in protein quality control. Further investigation showed that Cisd2 modulates calcium homeostasis by regulating the interaction between Calnexin and SERCA, thus impacting neutrophil function.
RESULTS
Enhanced neutrophil levels in Cisd2 knockout mice
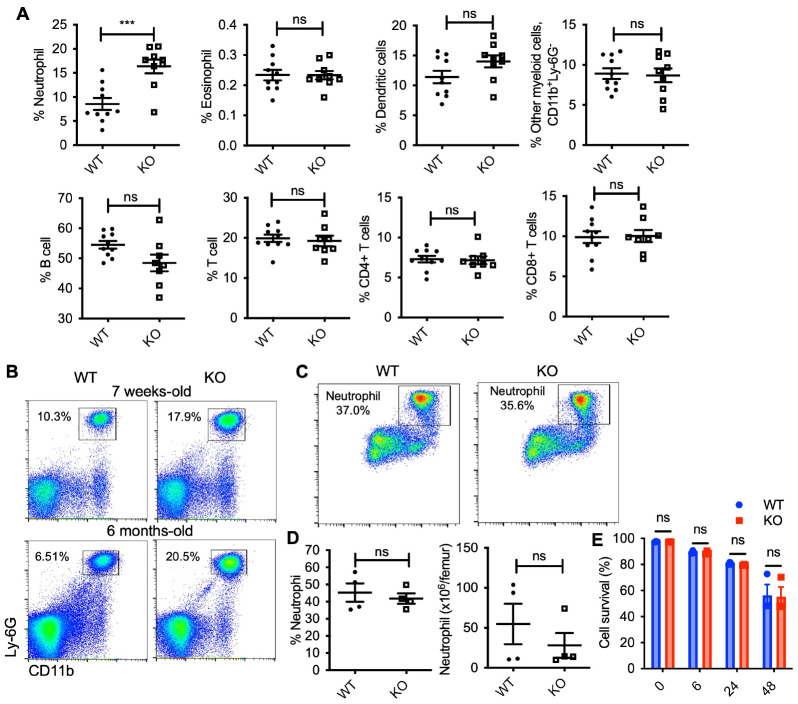
To characterize the immune cell subsets in Cisd2 KO, a premature aging model, blood samples were collected from 6 month-old wild-type (WT) and Cisd2 KO mice, and flow cytometry analysis was performed. Peripheral blood cells were stained with cell-specific markers to identify adaptive and innate immune cells. Gating strategies were applied to distinguish various immune cell populations, including total leukocyte (CD45+), T cells (CD3+), CD4 T cells (CD4+ CD3+), CD8 T cells (CD8+ CD3+), B cells (CD19+), neutrophil (CD11b+, Ly-6G+), eosinophil (CD11c+, Siglec-F+), dendritic cells (CD11c+, Siglec-F−), and other myeloid cells (CD11b+, Ly-6G−), including macrophages and monocytes (Supplementary Fig. 1A).
In Cisd2 KO mice, significantly elevated relative and absolute neutrophil numbers were observed in the blood, while no differences were detected in the percentage and absolute number of total leukocyte and other immune cell populations, compared to WT mice (Fig. 1A and Supplementary Fig. 2). The accumulation of neutrophils in Cisd2 KO mice was detected as early as 7 weeks of age, but as the mice aged, it became more pronounced (Fig. 1B). To determine if there were differences in neutrophil production from bone marrow (BM) in Cisd2 KO mice, we examined the number and relative percentage of neutrophils in BM, and found no difference between WT and Cisd2 KO mice (Fig. 1C, D, and Supplementary Fig. 1B). Additionally, to investigate the role of Cisd2 in neutrophil death, we assessed the death of neutrophils purified from the BM of WT and Cisd2 KO mice cultured for up to 48 h, and observed no difference in spontaneous cell death between WT and Cisd2 KO (Fig. 1E). These results revealed that the absence of Cisd2, a gene implicated in premature aging, elevates neutrophil levels in the peripheral blood; notably, this phenotype becomes more pronounced with aging in mice.
Fig. 1.
Immunophenotyping of blood cells in Cisd2 knockout and WT mice. (A) Quantitative flow cytometric analysis of various myeloid cells and lymphocytes in blood of WT and Cisd2 KO mice (n = 9-10 each group). Each dot plot shows the percentage of immune cell population in CD45+ cell populations. (B) Representative flow cytometric analysis of neutrophils (CD11+ and Ly6G+ cells) neutrophils in WT and Cisd2 KO mice at indicated age. (C) Representative flow cytometric analysis of neutrophils in 6-8 months-old WT and Cisd2 KO bone marrow (n = 4 each group). Percentage of neutrophil in CD45+ cell populations is indicated. (D) Graph showing frequency (left) and absolute (right) number of bone marrow neutrophil in mice of the indicated genotype. (E) The percentage of live neutrophils isolated form bone marrow. Data are presented as the mean ± SEM. ***P = 0.0007. ns, not significant, by Student’s t test.
Cisd2-deficient neutrophils exhibit impaired function
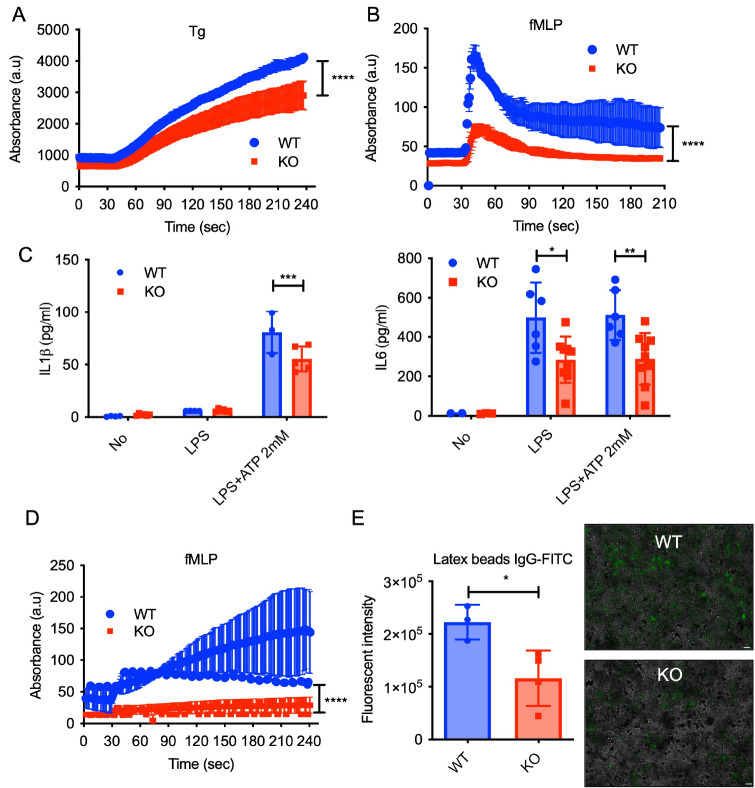
While our data revealed an increase in neutrophil numbers in the Cisd2 KO mice, the functional implications of the Cisd2-deficient neutrophils remained unclear. To clarify this, we isolated neutrophil from both WT and Cisd2 KO, and investigated calcium flux using thapsigargin (Tg), an irreversible SERCA inhibitor (Fig. 2A). Cisd2-deficient neutrophils exhibited a significant reduction in ER store release induced by Tg. To assess whether Cisd2 regulates calcium response to physiological agonist, we exposed cells to the neutrophil-stimulating bacterial peptide N-formyl-methionyl-leucyl-phenylalanine (fMLP). Calcium response was nearly abrogated in Cisd2-deficient neutrophils with fMLP stimulation (Fig. 2B), indicating that Cisd2 plays a role in regulating effective calcium signaling in neutrophils. We also examined the expression of pro-inflammatory cytokines, such as interleukin 1β (IL-1β), and interleukin 6 (IL-6). Fig. 2C shows that compared to the WT, following ATP stimulation post-LPS priming, the secretion of IL-1β in Cisd2-deficient neutrophils was diminished. Additionally, when treated with either LPS alone, or following ATP stimulation, the secretion of IL-6 consistently occurred at lower levels in Cisd2-deficient neutrophils.
Fig. 2.
Neutrophil dysfunction of Cisd2 KO mice. Calcium influx in blood neutrophils from WT and Cisd2 KO mice measured by flow cytometry over time after (A) thapsigargin (TG; 1 μM) or (B) fMLP (1 μM) treatment. Baseline calcium level was established for 30 sec prior to addition of stimuli. (C) Levels of IL-1β or IL-6 in the cell supernatants of blood neutrophils from WT and Cisd2 KO mice primed with LPS (500 ng/ml) alone for 4 h or further stimulated with ATP (2 mM) for 30 min. Each point represents data from single mouse. (D) ROS in blood neutrophils from WT and Cisd2 KO mice measured by flow cytometry over time after fMLP (1 μM) treatment. Baseline ROS level was established for 30 sec prior to addition of stimuli. (E) Phagocytic activity of blood neutrophils from WT and Cisd2 KO mice using IgG-coated latex beads. Each point represents data from single mouse (left). The fluorescence intensity was read in a fluorescence plate reader at an excitation of 485 nm and an emission of 535 nm. Scale bar, 40 μm. Data are presented as the mean ± SEM. *P = 0.0286 by Student’s t test (right). Representative image of three independent experiments.
Furthermore, we assessed the production of reactive oxygen species (ROS) in response to fMLP in Cisd2-deficient neutrophils. Compared to the WT, ROS production was markedly reduced in Cisd2 KO neutrophils (Fig. 2D). Additionally, we investigated the phagocytic activity, another crucial microbicidal function of neutrophils, which is regulated by calcium and ROS (12). Analysis of phagocytic ability using FITC-labeled IgG latex beads revealed a significant decrease in phagocytic activity in Cisd2-deficient neutrophils, compared to the WT counterparts (Fig. 2E). These results suggest that the neutrophils derived from Cisd2 KO mice exhibit increased abundance, but impaired functionality that is critical for effective pathogen-fighting capabilities.
Cisd2 interacts with Calnexin and SERCA
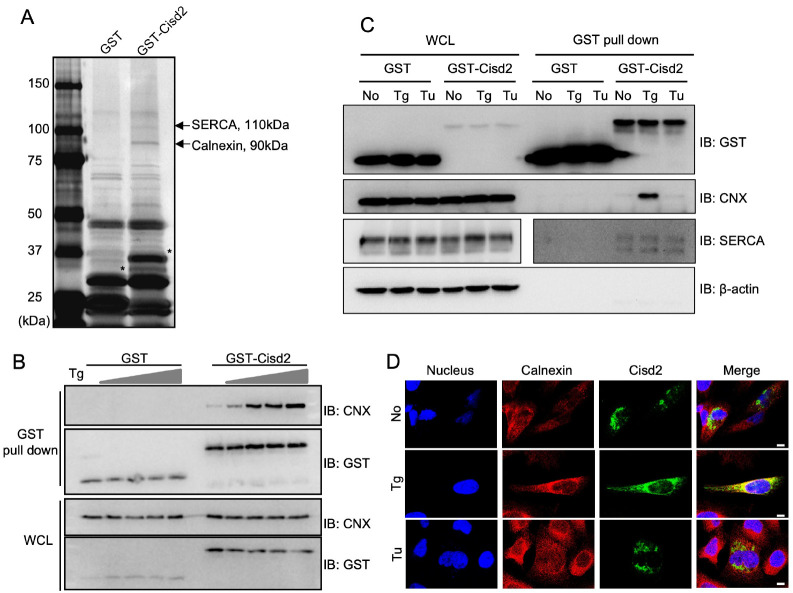
To elucidate the molecular role of Cisd2, we conducted GST pull-down experiments by incubating GST-conjugated Cisd2 (GST-Cisd2) with HEK293T cell lysate to identify its interacting partners. The subsequent mass spectrometry analysis revealed the binding of Cisd2 to 110 kDa SERCA and 90 kDa Calnexin proteins (Fig. 3A). While the interaction between Cisd2 and SERCA had previously been established (8), the newly discovered interaction with Calnexin prompted a focused investigation. To validate this interaction observed in the mass spectrometry results, we performed immunoprecipitation experiments. Remarkably, upon thapsigargin (Tg) treatment, the binding of GST-Cisd2 to endogenous Calnexin increased in a concentration-dependent manner (Fig. 3B). Thapsigargin is a substance that has previously been shown to cause differences in calcium release from neutrophil (Fig. 2A), and is also known to induce stress in the endoplasmic reticulum (ER), where both Cisd2 and Calnexin are located.
Fig. 3.
Cisd2 interacts with Calnexin and SERCA. (A) Silver-stained SDS-PAGE gel showing proteins recovered from GST pull down assay. HEK293T cells were transfected GST-only or GST-Cisd2 constructs, lysed, and subjected to GST pull down. Bands corresponding to 110-kDa and 90-kDa proteins (indicated by arrows) were excised and identified by mass-spectrometry. Asterisks indicate GST and GST-Cisd2. (B) Western blot analysis of whole cell lysates (WCL) and GST pull down products from HEK293T cells transfected with GST-only or GST-Cisd2 constructs and treated with thapsigargin (Tg) at varying doses (0, 0.01, 0.05, 0.1, and 0.3 μM) for 12 h before cell harvest. (C) Western blot analysis of whole cell lysates (WCL) and GST pull down products from HEK293T cells transfected with GST-only or GST-Cisd2 constructs and treated with 0.1 μM of thapsigargin (Tg) or 1 μg/ml of Tunicamycin (Tu) for 12 h before cell harvest. (D) Confocal image representing HeLa cell cultures transfected with GFP-Cisd2. The cells were treated with Tg (2 μM) or Tu (2 μg/ml) for 4 h. Cells were immunostained for Calnexin (red) and nuclei were stained by DAPI (blue). Scale bar, 10 μm.
Subsequently, we further examined the impact of different ER stress inducers on the interaction between Cisd2, Calnexin, and SERCA. Treatment with tunicamycin, an inducer of ER stress through N-linked glycosylation degradation, did not significantly alter the interaction of Cisd2 with Calnexin; however, the presence of thapsigargin, which disrupts calcium homeostasis, consistently enhanced the interaction (Fig. 3C). Additionally, we investigated the subcellular localization of Cisd2 and its colocalization with Calnexin under different ER stress conditions. Consistent with our immunoprecipitation results, we observed the colocalization of Cisd2 with Calnexin upon thapsigargin treatment, but not under unstressed or tunicamycin-treated conditions (Fig. 3D). Both thapsigargin and tunicamycin led to increased levels of ER stress-associated proteins, such as Binding Immunoglobulin Protein (BIP) and C/EBP Homologous Protein (CHOP) (Supplementary Fig. 3), despite inducing ER stress through distinct mechanisms—thapsigargin disrupts calcium homeostasis, while tunicamycin inhibits N-glycoprotein synthesis. These findings suggest that the interaction between Cisd2 and Calnexin responds selectively to specific ER stress conditions, in particular, impacting SERCA function in the presence of thapsigargin.
Cisd2 interrupts Calnexin and SERCA complex formation
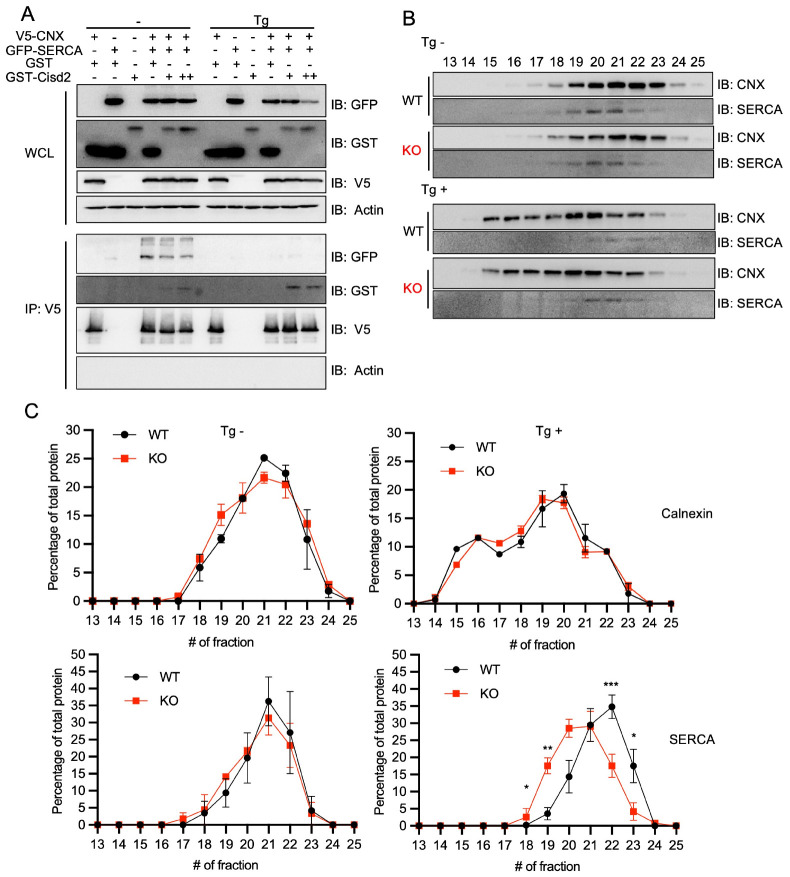
While the interaction between calnexin and SERCA is widely acknowledged, the effect of Calnexin on SERCA function remains open to debate (13, 14). However, recent studies suggest a positive role, indicating that Calnexin interacts with SERCA, and enhances its function (15). To further explore the impact of Cisd2 on the Calnexin-SERCA interaction, we conducted overexpression experiments of the three proteins in HEK293T. Intriguingly, we observed that increased Cisd2 levels reduced the interaction between Calnexin and SERCA (Fig. 4A). Moreover, thapsigargin treatment completely disrupted the Calnexin-SERCA interaction, with Calnexin preferentially binding to Cisd2 instead.
Fig. 4.
Cisd2 interrupts Calnexin and SERCA complex formation. (A) HEK293T cells were transfected with V5-Calnexin (CNX), GFP-SERCA, GST, GST-Cisd2 constructs individually or in combinations. The expression level of GST-Cisd2 was varied between low (+) and high (++) levels. Two days post-transfection, whole cell lysates (WCL) were prepared and immunoprecipitated (IP) with anti-V5 antibodies. Co-immunoprecipitated proteins were detected by Western blotting by using anti-GFP, anti-GST, anti-V5, and anti-β-actin. (B) Representative Western blots of size-exclusion chromatography (SEC) fractions (13-25) from MEF cell lysates, prepared with or without Tg (0.3 μM for 6 h) treatment. Western blotting with anti-Calnexin (CNX), and anti-SERCA. (C) SEC fraction profiles represented as the percentage of Calnexin and SERCA in each fraction, indicating the amount per fraction relative to the total protein amount across all fractions. *P < 0.05, **P = 0.0094, ***P = 0.0009, by two-way ANOVA.
To further validate the observed modulation in the Calnexin-SERCA interaction by Cisd2, we used size-exclusion chromatography (SEC) to investigate Calnexin-SERCA complex in Cisd2-deficient mouse embryonic fibroblasts (MEF) (Fig. 4B). Under basal conditions without ER stress, Calnexin and SERCA co-localize in fractions 20 and 21. Following thapsigargin treatment, Calnexin shifted to a higher molecular weight fraction, while SERCA relocated to a lower molecular weight fraction, resulting in their separation. Intriguingly, in the presence of thapsigargin-induced ER stress, this separation was less pronounced in Cisd2 KO MEFs, indicating that Calnexin and SERCA still formed a complex in the 20 and 21 fractions (Fig. 4C). Similarly, following thapsigargin treatment, we observed a reduced separation of Calnexin and SERCA in Cisd2 knockout neutrophils (Supplementary Fig. 4). These findings suggest that both human and mouse Cisd2 play a role in disrupting the Calnexin and SERCA complex during thapsigargin-induced ER stress. This elucidates the interaction between Cisd2, Calnexin, and SERCA, providing new insights into the regulatory dynamics of calcium homeostasis.
DISCUSSION
Our study explores the intricate interplay between Cisd2, Calnexin, and SERCA, shedding light on their collective role in neutrophil dysfunction. The elevated neutrophil count in the blood of Cisd2 KO mice, reminiscent of premature aging, raises questions concerning the functional implications of these immune cells. Our results suggest that despite the increased abundance of neutrophils, their functionality is markedly compromised, which is consistent with the notion of premature aging and immunosenescence (16, 17).
The discovery of an interaction between Cisd2 and Calnexin, particularly its consistent augmentation under thapsigargin-induced ER stress, unveils a novel regulatory mechanism. Calnexin, primarily residing in the ER, fulfills crucial roles, such as facilitating the N-linked glycosylation of glycoproteins, and modulating ER calcium ion levels. Intriguingly, the inhibition of N-linked glycoprotein synthesis by tunicamycin did not affect the binding strength of Cisd2-Calnexin. However, thapsigargin treatment, inhibiting SERCA and thereby impeding calcium ion reuptake into the ER, enhanced Cisd2 binding to Calnexin. Following the previously identified interaction of Cisd2 and SERCA (8), we found in this study an interaction of Cisd2 and Calnexin, suggesting a possible involvement of Cisd2 as a modulator in the regulation of SERCA by Calnexin during calcium homeostasis. Moreover, observations from Cisd2 KO mouse embryonic fibroblasts (MEFs) and neutrophils using size-exclusion chromatography support the disruption of the Calnexin-SERCA complex during thapsigargin-induced ER stress.
We hypothesize that under normal conditions without ER stress, Calnexin associates with SERCA, facilitating calcium transport into the ER in WT cells. Meanwhile, in Cisd2-deficient cells, the Calnexin-SERCA interaction intensifies, potentially resulting in a slight decrease in cytosolic calcium levels (Fig. 2A, B). However, when SERCA function is inhibited by thapsigargin, Calnexin dissociates from SERCA, and forms a stronger association with Cisd2. This leads to a complete inhibition of SERCA function, and an increase in cytosolic calcium levels. Conversely, in Cisd2-deficient cells lacking Cisd2 to sequester Calnexin from SERCA, Calnexin maintains its binding to and activation of SERCA, even after thapsigargin treatment (Supplementary Fig. 5). These findings, corroborated by our human cell line results, underscore the evolutionarily conserved role of Cisd2 in modulating the Calnexin-SERCA interaction during ER stress triggered by calcium imbalance.
Calcium signaling governs key processes associated with neutrophil functions (18). Disruption of calcium homeostasis due to Cisd2 deficiency ultimately leads to diminished pro-inflammatory cytokine secretion and compromised phagocytic activity. Stimulation with fMLP initiates signaling cascades that involve calcium-dependent enzymes, such as phospholipase C (PLC), which generates inositol trisphosphate (IP3), triggering calcium release from ER stores (19). Increased extracellular calcium concentrations trigger activation of the NLRP3 inflammasome and NFAT transcription factor, and increase proinflammatory cytokines, such as IL-1β and IL-6. We hypothesize that in the absence of Cisd2, there is increased calcium transport through SERCA into the ER (Supplementary Fig. 5), resulting in reduced calcium levels in the cytosol. This reduction in cytosolic calcium may impair cytokine induction. Further elucidation of the molecular mechanisms underlying NLRP3 inflammasome activation and NFAT transcription factor modulation is necessary to fully comprehend the dysregulated immune response observed in Cisd2-deficient conditions.
In addition, the SNARE complex, a family of calcium-binding proteins, plays a crucial role in cytokine secretion via membrane fusion (20). It is plausible that the insufficient calcium levels in the cytosol of Cisd2-deficient neutrophils hinder cytokine release through membrane fusion. Calcium-dependent proteins, like calmodulin, myosin, and gelsolin, also regulate actin dynamics during phagocytosis (21, 22). Thus, the reduced phagocytosis observed in Cisd2-deficient neutrophils may be attributed to calcium dysregulation mediated by Cisd2. These findings underscore the critical role of Cisd2 in orchestrating effective immune responses through calcium homeostasis. To broaden the scope of our findings from the molecular to the physiological level, further investigation into the in vitro bactericidal ability of neutrophils should be conducted to complement these findings. Additionally, conducting in vivo studies utilizing Cisd2 KO mice could provide comprehensive understanding of the physiological implications of the observed molecular interactions.
In summary, our study unveils a previously unrecognized regulatory axis involving Cisd2, Calnexin, and SERCA, which collectively influence neutrophil functions and respond to ER stress. Considering that Cisd2 is a putative gene associated with aging and exhibits an age-dependent decrease in expression, our findings suggest that Cisd2 may hold potential as a therapeutic target to enhance impaired immune function.
MATERIALS AND METHODS
Materials and Methods are available in the Supplementary Data.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Samad Amini-Bavil-Olyaee for initiating the current work. We also thank Dr. Ting-Fen Tsai and Jae U. Jung for providing reagents. This paper was supported by Konkuk University in 2023.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Oh SJ, Lee JK, Shin OS. Aging and the immune system: the impact of immunosenescence on viral infection, immunity and vaccine immunogenicity. Immune Netw. 2019;19:e37. doi: 10.4110/in.2019.19.e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Z, Liang Q, Ren Y, et al. Immunosenescence: molecular mechanisms and diseases. Signal Transduct Target Ther. 2023;8:200. doi: 10.1038/s41392-023-01451-2.29fd65001ed549ba89b568718601163c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaw AC, Goldstein DR, Montgomery RR. Age-dependent dysregulation of innate immunity. Nat Rev Immunol. 2013;13:875–887. doi: 10.1038/nri3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen YF, Kao CH, Kirby R, Tsai TF. Cisd2 mediates mitochondrial integrity and life span in mammals. Autophagy. 2009;5:1043–1045. doi: 10.4161/auto.5.7.9351. [DOI] [PubMed] [Google Scholar]
- 5.Chen YF, Kao CH, Chen YT, et al. Cisd2 deficiency drives premature aging and causes mitochondria-mediated defects in mice. Genes Dev. 2009;23:1183–1194. doi: 10.1101/gad.1779509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen ZQ, Huang YL, Teng YC, et al. Cisd2 maintains cellular homeostasis. Biochim Biophys Acta Mol Cell Res. 2021;1868:118954. doi: 10.1016/j.bbamcr.2021.118954. [DOI] [PubMed] [Google Scholar]
- 7.Yeh CH, Chou YJ, Kao CH, Tsai TF. Mitochondria and calcium homeostasis: Cisd2 as a big player in cardiac ageing. Int J Mol Sci. 2020;21:23. doi: 10.3390/ijms21239238.ba59a8f7573a4fc8a38a5b95bf573a79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen ZQ, Chen YF, Chen JR, et al. Cisd2 haploinsufficiency disrupts calcium homeostasis, causes nonalcoholic fatty liver disease, and promotes hepatocellular carcinoma. Cell Rep. 2017;21:2198–2211. doi: 10.1016/j.celrep.2017.10.099.d9514aa1af2d4cba9ff02d52ab7e23ad [DOI] [PubMed] [Google Scholar]
- 9.Yeh CH, Shen ZQ, Hsiung SY, et al. Cisd2 is essential to delaying cardiac aging and to maintaining heart functions. PLoS Biol. 2019;17:e3000508. doi: 10.1371/journal.pbio.3000508.b74c01ffb5e5436ca4e8b4b9cb955d21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang CH, Chen YF, Wu CY, et al. Cisd2 modulates the differentiation and functioning of adipocytes by regulating intracellular Ca2+ homeostasis. Hum Mol Genet. 2014;23:4770–4785. doi: 10.1093/hmg/ddu193. [DOI] [PubMed] [Google Scholar]
- 11.Chang NC, Nguyen M, Bourdon J, et al. Bcl-2-associated autophagy regulator Naf-1 required for maintenance of skeletal muscle. Hum Mol Genet. 2012;21:2277–2287. doi: 10.1093/hmg/dds048. [DOI] [PubMed] [Google Scholar]
- 12.Mayadas TN, Cullere X, Lowell CA. The multifaceted functions of neutrophils. Annu Rev Pathol. 2014;9:181–218. doi: 10.1146/annurev-pathol-020712-164023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lynes EM, Raturi A, Shenkman M, et al. Palmitoylation is the switch that assigns calnexin to quality control or ER Ca2+ signaling. J Cell Sci. 2013;126:3893–3903. doi: 10.1242/jcs.125856. [DOI] [PubMed] [Google Scholar]
- 14.Roderick HL, Lechleiter JD, Camacho P. Cytosolic phosphorylation of calnexin controls intracellular Ca(2+) oscillations via an interaction with SERCA2b. J Cell Biol. 2000;149:1235–1248. doi: 10.1083/jcb.149.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gutierrez T, Qi H, Yap MC, et al. The ER chaperone calnexin controls mitochondrial positioning and respiration. Sci Signal. 2020;13:638. doi: 10.1126/scisignal.aax6660. [DOI] [PubMed] [Google Scholar]
- 16.Drew W, Wilson DV, Sapey E. Inflammation and neutrophil immunosenescence in health and disease: targeted treatments to improve clinical outcomes in the elderly. Exp Gerontol. 2018;105:70–77. doi: 10.1016/j.exger.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 17.Van Avondt K, Strecker JK, Tulotta C, Minnerup J, Schulz C, Soehnlein O. Neutrophils in aging and aging-related pathologies. Immunol Rev. 2023;314:357–375. doi: 10.1111/imr.13153. [DOI] [PubMed] [Google Scholar]
- 18.Hann J, Bueb JL, Tolle F, Brechard S. Calcium signaling and regulation of neutrophil functions: still a long way to go. J leukoc biol. 2020;107:285–297. doi: 10.1002/JLB.3RU0719-241R. [DOI] [PubMed] [Google Scholar]
- 19.Newton K, Dixit VM. Signaling in innate immunity and inflammation. Cold Spring Harb Perspect Biol. 2012;4:3. doi: 10.1101/cshperspect.a006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Othman A, Sekheri M, Filep JG. Roles of neutrophil granule proteins in orchestrating inflammation and immunity. FEBS J. 2022;289:3932–3953. doi: 10.1111/febs.15803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stendahl O, Krause KH, Krischer J, et al. Redistribution of intracellular Ca2+ stores during phagocytosis in human neutrophils. Science. 1994;265:1439–1441. doi: 10.1126/science.8073285. [DOI] [PubMed] [Google Scholar]
- 22.Aslam S, Bhattacharya S, Bhattacharya A. The calmodulin-like calcium binding protein EhCaBP3 of entamoeba histolytica regulates phagocytosis and is involved in actin dynamics. PLoS Pathog. 2012;8:e1003055. doi: 10.1371/journal.ppat.1003055.91240c39b1f04d6ba26857364780612c [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.