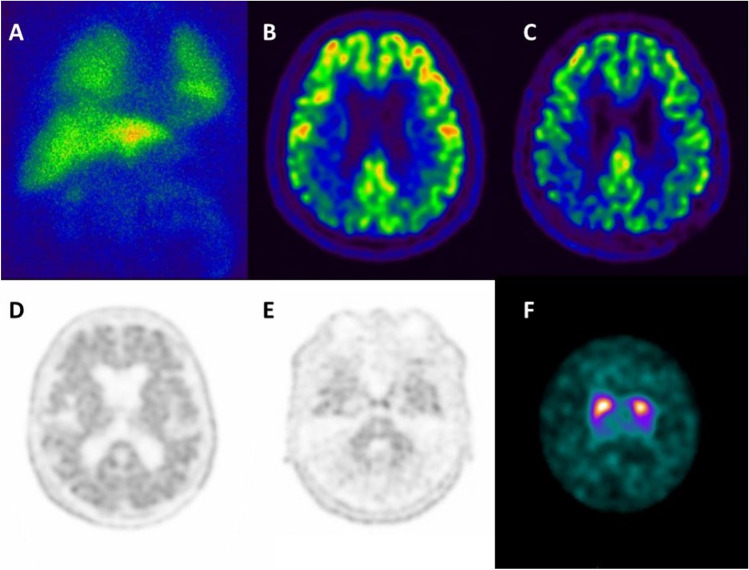
A 72-year-old man was referred to the neurologist for memory, executive, and visuospatial impairment in the last year associated with difficulty in manipulating objects, mild motor slowing, and falls. MRI highlighted temporo-lateral and posterior-parietal cortical atrophy. The syndrome was described as posterior-cortical atrophy (PCA) [1]. To differentiate between AD and DLB, the patient underwent a cardiac-[123I]meta-iodobenzylguanidine (MIBG) scan qualitatively normal with a borderline heart/mediastinum ratio (panel A). [18F]-FDG-PET showed hypometabolism in the bilateral temporo-parietal cortex and precuneus (panel B). Based on the results of MIBG and [18F]-FDG-PET, the patient was labeled as having PCA/AD. He was then recruited into an experimental study on atypical AD and underwent a [18F]-florbetaben-PET scan which confirmed a posterior pattern (early-perfusion-imaging) and was positive for brain amyloidosis (panels C, D, and E) [2]. After 1 year, the patient showed hypomimia and bradykinesia. Given the evolution of the syndrome, to further differentiate between PCA/AD and DLB presenting as PCA, a dopamine transporter imaging (DAT-SPECT) was obtained confirming dopaminergic neurodegeneration leading to a final diagnosis of DLB with comorbid AD (or mixed DLB/AD; panel F). This case emphasizes the impact of biomarkers for the etiological diagnosis of dementia. This case raises the question about the impact of sequence for the use of biomarkers on the final diagnosis: an abnormal DAT-SPECT as first examination would have led to a diagnosis of DLB and the impact of brain amyloidosis could have been neglected [3]. In clinical trials, indicators of co-pathology could be used as exclusionary criteria or to establish efficacy in a broader population in preplanned subset analyses [4–6].
Author contribution
All authors contributed equally to the content and revision of the manuscript. All authors read and approved the final manuscript
Funding
This work was supported by an educational grant from Lilly.
Data Availability
Data are available upon reasonable request to the corresponding author.
Declarations
Competing interests
SM received speaker honoraria from G.E. Healthcare and Life Molecular Imaging and honoraria for participation in advisory boards from Eli-Lilly. MB has received speaker honoraria from G.E. Healthcare. MP received research support from Novartis and Nutricia, and received fees from Novartis, Merck, and Biogen. DA received fees from Fidia, Jazz, and Lundbeck for lectures, consultation, and board participation.
Open access
For related content on neuroimaging for Alzheimer’s disease, please visit: https://neuroimaging-alzheimers-disease-ime.springermedicine.com/.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Crutch SJ, Schott JM, Rabinovici GD, Murray M, Snowden JS, van der Flier WM, Dickerson BC, Vandenberghe R, Ahmed S, Bak TH, Boeve BF, Butler C, Cappa SF, Ceccaldi M, de Souza LC, Dubois B, Felician O, Galasko D, Graff-Radford J, Graff-Radford NR, Hof PR, Krolak-Salmon P, Lehmann M, Magnin E, Mendez MF, Nestor PJ, Onyike CU, Pelak VS, Pijnenburg Y, Primativo S, Rossor MN, Ryan NS, Scheltens P, Shakespeare TJ, Suárez González A, Tang-Wai DF, Yong KXX, Carrillo M, Fox NC; Alzheimer’s Association ISTAART Atypical Alzheimer’s Disease and Associated Syndromes Professional Interest Area. Consensus classification of posterior cortical atrophy.13:870-884Alzheimers Dement. 2017;13:870–884. 10.1016/j.jalz.2017.01.014. [DOI] [PMC free article] [PubMed]
- 2.Haller S, Jäger HR, Vernooij MW, Barkhof F. Neuroimaging in dementia: more than typical Alzheimer disease. Radiology. 2023;308:e230173. doi: 10.1148/radiol.230173. [DOI] [PubMed] [Google Scholar]
- 3.Massa F, Arnaldi D, De Cesari F, Girtler N, Brugnolo A, Grazzini M, Bauckneht M, Meli R, Morbelli S, Pardini M, Sambuceti G, De Carli F, Tiraboschi P, Nobili F. Neuroimaging findings and clinical trajectories of Lewy body disease in patients with MCI. Neurobiol Aging. 2019;76:9–17. doi: 10.1016/j.neurobiolaging.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 4.VandeVrede L, La Joie R, Horiki S, Mundada NS, Koestler M, Hwang JH, Ljubenkov PA, Rojas JC, Rabinovici GD, Boxer AL, Seeley WW. Co-pathology may impact outcomes of amyloid-targeting treatments: clinicopathological results from two patients treated with aducanumab. Acta Neuropathol. 2023;146:777–781. doi: 10.1007/s00401-023-02631-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toledo JB, Abdelnour C, Weil RS, Ferreira D, Rodriguez-Porcel F, Pilotto A, Wyman-Chick KA, Grothe MJ, Kane JPM, Taylor A, Rongve A, Scholz S, Leverenz JB, Boeve BF, Aarsland D, McKeith IG, Lewis S, Leroi I, Taylor JP; ISTAART Lewy body dementias Trial Methods Working Group. Dementia with Lewy bodies: impact of co-pathologies and implications for clinical trial design. Alzheimers Dement. 2023;19:318-332. 10.1002/alz.12814. [DOI] [PMC free article] [PubMed]
- 6.Spina S, La Joie R, Petersen C, Nolan AL, Cuevas D, Cosme C, Hepker M, Hwang JH, Miller ZA, Huang EJ, Karydas AM, Grant H, Boxer AL, Gorno-Tempini ML, Rosen HJ, Kramer JH, Miller BL, Seeley WW, Rabinovici GD, Grinberg LT. Comorbid neuropathological diagnoses in early versus late-onset Alzheimer’s disease. Brain. 2021;144:2186–2198. doi: 10.1093/brain/awab099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request to the corresponding author.


