Abstract
Purpose
We aimed to evaluate the efficacy and safety of re-irradiation stereotactic body radiation therapy (SBRT) in patients with metastatic epidural spinal cord compression (MESCC) following high-dose conventional radiotherapy.
Materials and methods
Twenty-one patients met the following eligibility criteria: with an irradiation history of 50 Gy2 equivalent dose in 2-Gy fractions (EQD2) or more, diagnosed MESCC in the cervical or thoracic spines, and treated with re-irradiation SBRT of 24 Gy in 2 fractions between April 2018 and March 2023. Prior treatment was radiotherapy alone, not including surgery. The primary endpoint was a 1-year local failure rate. Overall survival (OS) and treatment-related adverse events were assessed as the secondary endpoints. Since our cohort includes one treatment-related death (TRD) of esophageal perforation, the cumulative esophageal dose was evaluated to find the dose constraints related to severe toxicities.
Results
The median age was 68, and 14 males were included. The primary tumor sites (esophagus/lung/head and neck/others) were 6/6/7/2, and the median initial radiotherapy dose was 60 Gy2 EQD2 (range: 50–105 Gy2, 60–70/ > 70 Gy2 were 11/4). Ten patients underwent surgery followed by SBRT and 11 SBRT alone. At the median follow-up time of 10.4 months, 17 patients died of systemic disease progression including one TRD. No radiation-induced myelopathy or nerve root injuries occurred. Local failure occurred in six patients, with a 1-year local failure rate of 29.3% and a 1-year OS of 55.0%. Other toxicities included five cases of vertebral compression fractures (23.8%) and one radiation pneumonitis. The cumulative esophageal dose was recommended as follows: Dmax < 203, D0.035 cc < 187, and D1cc < 167 (Gy3 in biological effective dose).
Conclusion
Re-irradiation spine SBRT may be effective for selected patients with cervical or thoracic MESCC, even with high-dose irradiation histories. The cumulative dose assessment across the original and re-irradiated esophagus was recommended to decrease the risk of severe esophageal toxicities.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11604-024-01539-x.
Keywords: Re-irradiation, Stereotactic radiotherapy, Spinal metastases, Spinal cord compression
Introduction
Metastatic epidural spinal cord compression (MESCC) is a major concern but a clinical challenge for the management of advanced cancer patients, leading to significant morbidity, including pain, loss of neurologic function, and poor quality of life. Surgery followed by radiotherapy (RT) or RT alone is recommended for patients presenting with spinal cord compression [1–4]. Especially patients with radiosensitive tumors (e.g., lymphoma, myeloma, small cell lung cancer, germ cell tumor, prostate cancer, and breast cancer) or not severe spinal instability is well expected for pain relief, neurological recovery, and local tumor control even with radiotherapy alone [1, 4, 5].
During follow-up after the initial treatment, salvage treatment is considered if symptomatic or radiographic progression or recurrence is detected [4]. Even if RT or surgery plus RT is performed as the initial treatment, salvage RT is effective [6, 7]. Stereotactic body radiotherapy (SBRT) of the spine has recently emerged as an advanced RT technique expected to be highly effective in pain relief and tumor control [8–10]. Compared to conventional external beam radiotherapy (EBRT), SBRT has demonstrated effective local tumor control and safety due to its dose concentration and risk organ dose sparing [11–13]. International Stereotactic Radiosurgery Society practice guidelines suggest that SBRT can be a recommended treatment option for re-irradiation [14]. In cases of re-irradiation, it is vital to carefully account for the cumulative dose from current and previous treatments for proper risk evaluation and management [15, 16]. Previous reports on re-irradiation SBRT have only provided cases where the initial dose of EBRT was around 30 Gy in 10 fractions—few data exist about re-irradiation spine SBRT with a history of initial EBRT over 50 Gy equivalent dose in 2 Gy fractions (EQD2) [14]. In clinical practice, however, it is not uncommon for patients with a history of high-dose EBRT to undergo re-irradiation SBRT. Administering SBRT to patients with a history of high-dose EBRT carries a potential risk of severe adverse events related to radiation overdose, particularly in the cervical and thoracic areas, affecting critical organs like the esophagus, pharynx, and carotid artery [17–21]. Strict dose constraints and patient selection criteria are needed to minimize the risk of potentially fatal consequences.
We aimed to investigate the outcomes of re-irradiation SBRT for patients with progressive or recurrent spinal cord compression in the cervical and thoracic spines previously irradiated with 50 Gy2 EQD2 or more. The results of this study provide valuable data on the efficacy and safety of re-irradiation SBRT in such challenging situations and may contribute to the expansion of SBRT indications.
Materials and methods
Study design and data source
We retrospectively reviewed our institutional database and identified patients eligible for the following criteria: Patients with MESCC who previously received the conventional radiotherapy with a cumulative dose of 50 Gy2 EQD2 or more. Between April 2018 and March 2023, out of 136 patients treated with spinal SBRT, 21 met the following eligibility criteria: (1) cumulative prior EBRT dose ≥ 50 Gy to the target spines, and (2) re-irradiation of 24 Gy in two fractions of SBRT to the cervical and thoracic spines during the study period. Patients previously receiving SBRT at the current target spine as an initial treatment were excluded from the current study. The institutional ethical review board approved this study, and informed consent was obtained through an opt-out form option displayed on the website.
The patient follow-up for this study ended on September 30, 2023, and patients who were alive or lost follow-up were censored. The primary endpoint was defined as the local failure rate at 1 year. Local failure was defined according to the sc24 protocol as a gross unequivocal increase in tumor volume or linear dimension, any new or progressive tumor within the epidural space, and neurologic deterioration attributable to pre-existing epidural disease with equivocal increased epidural disease dimensions on CT/MRI [12]. As the secondary endpoints, we evaluated overall survival, defined as the survival time from the date of diagnosis of MESCC to the date of death or the last follow-up. Also, treatment-related adverse events in grade 2 or more were recorded and graded according to the Common Terminology Criteria for Adverse Events (CTCAE). Our cohort included one treatment-related death (TRD) of esophageal perforation; the esophageal dose was evaluated to find the biological effective dose (BED) limits related to severe toxicities.
Patient selection criteria for re-irradiation SBRT
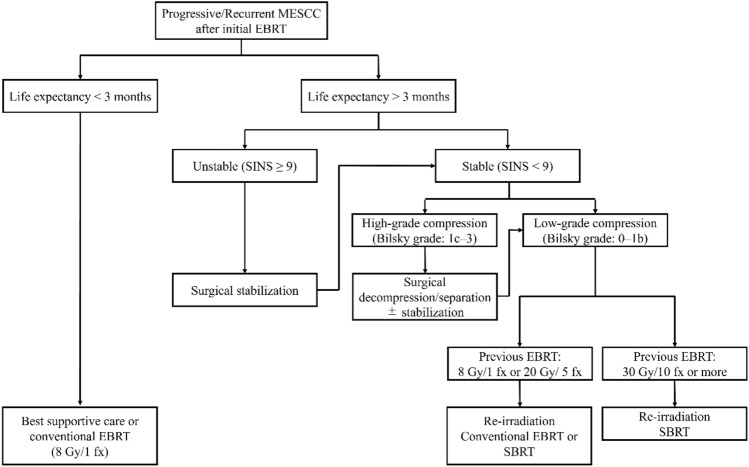
Patients with a history of initial EBRT more than 6 months before and diagnosed with MESCC, expected to survive for at least the next three months, were eligible for re-irradiation SBRT. Figure 1 shows the institutional treatment algorithm for MESCC patients with a history of initial EBRT. This algorithm was created in reference to the International Spine Consortium report [5]. A multidisciplinary approach involving spinal surgeons, radiation oncologists, and physicians for primary cancer was used to determine whether re-irradiation SBRT or surgery followed by re-irradiation SBRT was acceptable based on the severity of the spinal cord compression and spinal instability. All the patients involved in this study were assessed before treatment. The severity of the cord compression is determined by Bilsky grade [22]: if grade 0–1b (low grade), consider re-irradiation SBRT alone, and if grade 1c–3 (high grade), consider decompression/separation (± stabilization) followed by re-irradiation SBRT. The severity of the spinal instability was determined based on the Spine Instability Neoplastic Score (SINS) [23]: if SINS < 9 (low-grade instability), consider re-irradiation SBRT alone, and if SINS is 9 or more (high-grade instability), consider stabilization (± decompression) followed by re-irradiation SBRT. If surgery was considered but not medically possible, only re-irradiation SBRT was performed. After treatment, all patients were followed up every 2–3 months using CT or MRI.
Fig. 1.
Algorithm for management of progressive or recurrent spinal cord compression received by the initial conventional radiotherapy. MESCC metastatic epidural spinal cord compression, EBRT external beam radiation therapy, SBRT stereotactic body radiation therapy, fx fractions, SINS spinal instability neoplastic score
Treatment planning for SBRT
Patients were immobilized in a supine position using an immobilization device (a vacuum bag for the thoracic spine and a thermoplastic head-neck mask for the cervical to upper thoracic spine). Treatment planning CT was performed with an Aquilion LB CT system (Canon Medical Systems, Tochigi, Japan), and CT slice thicknesses were 1 mm (pixel size 512 × 512). MRI scans for fusion to the planning CT were obtained with patients immobilized in the same simulation position using the same device. A 1 mm slice of T1-, T2-weighted, and T1 post-gadolinium axial MRI images were obtained. The MRI images were used for delineating targets and the spinal cord in planning CT images. Postoperative patients with metal prostheses underwent CT myelogram: an intrathecal injection of iohexol contrast (Omnipaque 240; GE Healthcare, Princeton, New Jersey, USA) was performed by the neurosurgeon 2 h before planning CT simulation. To spread the contrast medium around the target spine, injected patients are placed in a high pelvic position until CT scanning.
The gross tumor volume (GTV) was defined as the disease visible on CT and fused MRI images. The clinical target volume (CTV) was delineated based on the international consortium guidelines [24, 25]. The guidelines recommend using the proposed anatomic classification system, which divides each vertebral body into six sectors (the vertebral body, the left/right pedicles, the left/right transverse processes and laminas, and the spinous process). CTV contour generation was determined depending on which sectors GTV was involved in. The entire sector was included in the CTV if any portion of these regions contained the GTV. Additionally, the sectors next to the GTV-involved sectors on both sides were included in the CTV (e.g., If the vertebral body is involved with GTV the entire vertebral body, and the left and right pedicles are included in the CTV). A uniform margin of 2 mm with CTV is required for planning target volume (PTV). We defined PTVeval as the volume obtained by subtracting the spinal cord PRV from the PTV. The prescription was set at 24 Gy in two fractions (12 Gy per fraction) for PTVeval, delivered daily on Monday–Friday. The beam delivery technique was used volumetric modulated arc therapy (VMAT) in a flattening filter-free mode using 10-MV photon on the Varian TrueBeam system (Varian Medical Systems, Palo Alto, CA). The treatment planning was RayStation version 10.0 (RaySearch Laboratories) using the collapsed cone dose algorithm for dose calculation. Table S1 shows dose specification for PTVeval.
Organs at risk (OAR) in this study included the spinal cord, pharynx, larynx, esophagus, trachea, main bronchus, stomach, lungs, carotid arteries, and aorta. The spinal cord was delineated based on T2-weighted MRI fused to the planning CT. In case of postoperative patients with metal prostheses, we used CT myelogram for spinal cord delineation [26, 27]. The other OARs were delineated based on the planning CT alone. Planning organ at risk volume (PRV) margin of 1.5 mm is required for the spinal cord.
Table S2 shows dose constraints for specified OARs. The maximum dose of 0.035 cc (D0.035 cc) to the spinal cord PRV was determined with the highest priority based on prior EBRT dose (BED ≤ 90 Gy2, 90–100 Gy2, > 100 Gy2): D0.035 cc ≤ 12.2 Gy in case of prior EBRT dose ≤ 90 Gy2 in BED, D0.035 cc ≤ 10.8 Gy in case of prior EBRT dose > 100 Gy2 in BED. For past EBRT doses of 90–100 Gy2 in BED, D0.035 cc was calculated based on the report of Sahgal et al., assuming a linear fall from 12.2 Gy to 10.8 Gy (e.g., D0.035 cc ≤ 11.4 Gy in the case of 95 Gy2 < BED ≤ 96 Gy2). D0.035 cc of the other specified OARs and unspecified tissues should be ≤ 20 Gy.
Post-SBRT dosimetric analysis and statistical analysis
This study strongly required the spinal cord and other OARs to adhere to dose constraints. The cumulative doses of the esophagus, spinal cord, and other specified OARs were evaluated retrospectively. The cumulative doses of the SBRT and the initial EBRT plans were calculated using MIM Maestro (MIM Software Inc., Cleveland, USA): Dmax, D0.035 cc, and D1cc of each specified OAR with α/β = 2, 3, and 10 were displayed in the EQD2 and BED dose, respectively. The cumulative doses may be low if some organs are distant from the target vertebrae or surgically removed or implanted, and not all OAR doses are evaluated in each patient. The criteria for inclusion in the dosimetric analysis were a maximum cumulative dose of 50 Gy2 EQD2 or more for the spinal cord (12 patients), 60 Gy2 EQD2 or more for the esophagus, trachea, and aorta (15, 15, and 17 patients), and 66 Gy or more for the carotid artery and pharynx (12 and 7 patients).
Survival analysis was performed using the Kaplan–Meier method, and differences between survival curves were assessed using the log-rank test. Local failure was estimated using cumulative incidence functions, accounting for death without tumor recurrence as a competing risk. In additional concern, local failure and survival were compared between patients distinguished with the oligo-recurrence disease or not using the following criteria: (1) one to several distant metastases/recurrences in one to several organs, (2) primary site of the cancer controlled, (3) one to several distant metastases/recurrences can be treated with local therapy, and (4) no other distant metastases/recurrences other than those in (3) [28–30]. A p value less than 0.05 was considered statistically significant. All statistical analyses were performed using R statistical software version 4.2.2 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient
Table 1 shows the patient characteristics. The 21 eligible patients included 14 males and 7 females with a median age of 68 (interquartile range, IQR: 56–72). Each patient had an initial EBRT history of median 60 Gy/30 fractions (120 Gy2 BED, range: 100–210 Gy2 BED), including 15 patients (71%) who received more than 60 Gy/30 fractions (120 Gy2 BED) and 4 patients (19%) with more than 70 Gy/35 fractions (140 Gy2 BED). Fifteen patients received initial EBRT as definitive and six patients as palliative therapy. Five patients had more than two courses of previous irradiation treatments and were categorized based on their cumulative doses. At the time of diagnosis of recurrent MESCC, 17 patients (81%) had neurologic symptoms or pains, and 4 (19%) were asymptomatic but diagnosed based on radiological findings. The median interval between the last EBRT and recurrent MESCC diagnosis was 13.7 months (IQR: 8.6–30.8 months).
Table 1.
Patient characteristics
| Factor | Overall (N = 21) |
|---|---|
| Age | |
| Median [IQR] | 68 [56, 72] |
| Sex (%) | |
| Female | 7 (33.3) |
| Male | 14 (66.7) |
| ECOG performance status (%) | |
| 0 | 4 (19.0) |
| 1 | 15 (71.4) |
| 2 | 0 |
| 3 | 2 (9.5) |
| 4 | 0 |
| Primary lesion (%) | |
| Esophageal cancer | 6 (28.6) |
| Head and neck cancer | 7 (33.3) |
| Lung cancer | 6 (28.6) |
| Others (Hepatocellular carcinoma, Sarcoma) | 2 (9.5) |
| Initial RT prescription dose | |
| 50–60 Gy2 EQD2 | 6 (28.6) |
| 60–70 Gy2 EQD2 | 11 (52.4) |
| ≥ 70 Gy2 EQD2 | 4 (19.0) |
| Global maximum dose | |
| Maximum dose [IQR] | 90.6 [63.0, 72.3], Gy2 EQD2 |
| Spinal cord maximum dose | |
| Maximum dose [IQR] | 53.4 [31.8, 47.9], Gy2 EQD2 |
| < 40 Gy2 EQD2 | 12 (57.1) |
| 40–45 Gy2 EQD2 | 1 (4.8) |
| 45–50 Gy2 EQD2 | 5 (23.8) |
| ≥ 50 Gy2 EQD2 | 3 (14.3) |
| Esophageal maximum dose* | |
| Maximum dose [IQR] | 72.6 [51.7, 63.1], Gy2 EQD2 |
| < 50 Gy2 EQD2 | 3 (18.8) |
| 50–60 Gy2 EQD2 | 4 (25.0) |
| 60–70 Gy2 EQD2 | 8 (50.0) |
| ≥ 70 Gy2 EQD2 | 1 (6.2) |
| Symptom at diagnosis of recurrent MESCC | |
| Symptomatic | 17 (81.0) |
| Asymptomatic (Diagnosis by radiographic imaging) | 4 (19.0) |
| Surgery | |
| Surgery followed by SBRT | 10 (47.6) |
| SBRT alone | 11 (52.3) |
| Interval between initial RT and SBRT, months | |
| Median [IQR] | 13.7 [8.6, 30.8] |
| Target spinal levels (%) | |
| Cervical spine (C1–5) | 3 (14.3) |
| Cervical-thoracic junctional spine (C6–Th3) | 8 (38.1) |
| Thoracic spine (Th4–Th12) | 10 (47.6) |
| Number of target vertebrae (%) | |
| 1 | 10 (47.6) |
| 2–3 | 8 (38.1) |
| ≧ 4 | 3 (14.3) |
| Systemic disease | |
| Controlled | 6 (28.6) |
| Active | 15 (71.4) |
| SINS (%) | |
| 0–6 (stable) | 7 (33.3) |
| 7–12 (potentially unstable) | 7 (33.3) |
| 13–18 (unstable) | 7 (33.3) |
| Bilsky grade (%) | |
| 0 (Bone only disease) | 3 (14.3) |
| 1a (Epidural impingement, without thecal sac deformation) | 4 (19.0) |
| 1b (Thecal sac deformation, without spinal cord abutment) | 5 (23.8) |
| 1c (Spinal cord abutment, without compression) | 3 (14.3) |
| 2 (Spinal cord compression, CSF visible) | 3 (14.3) |
| 3 (Spinal cord compression, no CSF visible) | 3 (14.3) |
| Frankel classification (%) | |
| E (normal motor) | 18 (85.7) |
| D (preserved motor function) | 1 (4.8) |
| C (preserved motor non-functional) | 2 (9.6) |
IQR interquartile range, ECOG eastern cooperative oncology group, EQD2 equivalent dose at 2 Gy, MESCC metastatic epidural spinal cord compression, SBRT stereotactic body radiation therapy, SINS spinal instability neoplastic score, CSF cerebrospinal fluid
*The esophageal doses at initial RT for 16 patients are shown, excluding five patients who had primary head and neck cancer or underwent esophagectomy
Eleven patients (52.4%) received SBRT alone, and 10 (47.6%) received SBRT following surgery: six received decompression plus stabilization surgery, and four received stabilization surgery. Although four patients in the SBRT alone group had met the institutional criteria for surgery depending on their spinal instability and spinal cord compression, they were considered non-surgical candidates due to poor performance status (N = 1) or risk of discontinuing systemic therapy for advanced systemic disease (N = 3). All SBRT plans achieved the pre-specified institutional dose constraints (Table S1–2), and all patients completed SBRT on schedule.
Local failure rate and survival
The median follow-up time from the diagnosis of recurrent or progressive spinal metastases was 10.4 months (range: 2.9–57.5 months). Seventeen patients died at a median of 10.3 months (2.9–22.8), and the median follow-up time of 4 alive patients was 18.0 months (5.8–57.5 months). As shown in Fig. 2A, the median survival time was 12.4 months (95% CI 5.8–14.3), and the 1-year overall survival rate was 55.0% (95% CI 31.1–73.7). Until the last follow-up, the local tumor was controlled in 15 patients (71%), and six experienced local failures: 1 in the surgery followed by the SBRT group and 5 in the SBRT alone group. The 1-year local failure rate was 29.3% (95% CI 11.4–50.0) in the entire cohort (Fig. 2B). All four unstable but non-surgical patients developed early local failure (median 3.7 months to failure, range 2.0–7.4). If the cohort excluded them, 1-year local failure rate was 12.3% (95% CI 1.8–33.3). As for the oligo-recurrence subgroup, one patient (20%) out of 5 oligo-recurrence patients experienced local recurrence; in contrast, 5 local recurrences (31%) in the other 16 patients. Although it was not statistically significant, the oligo-recurrence patients tended to have longer survival (MST: 10.3 months vs. 18.2 months, P = 0.084).
Fig. 2.
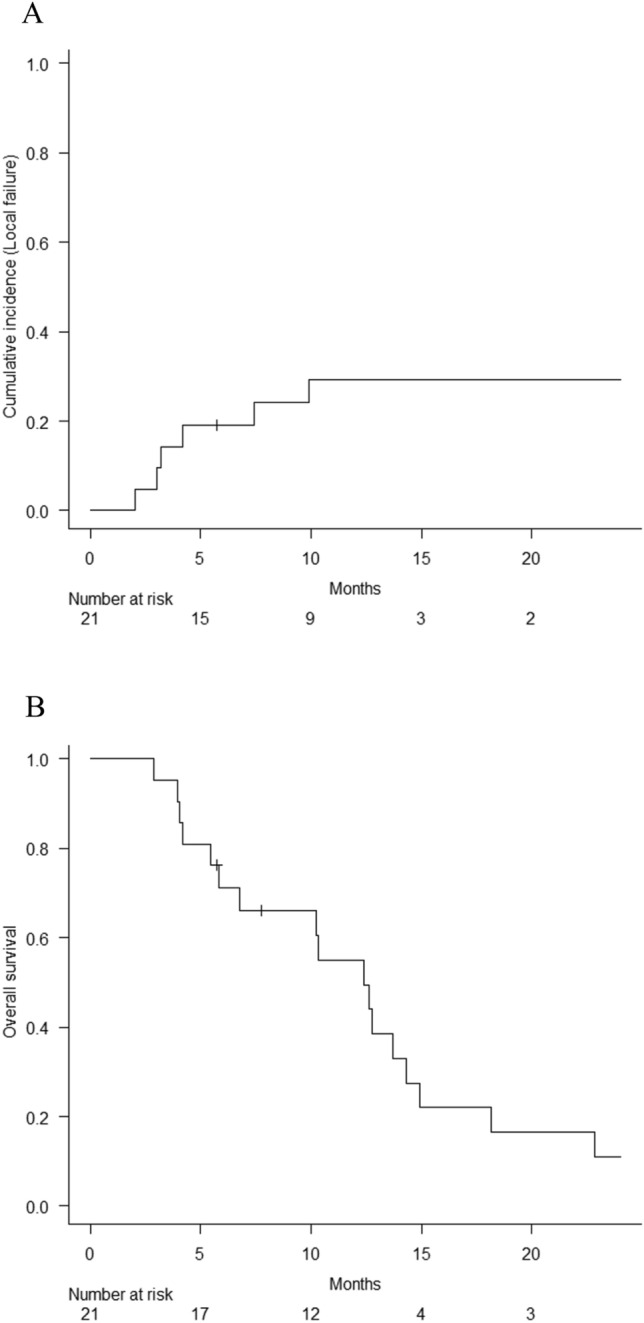
Local failure rate and overall survival in the entire cohort
Of the 6 patients with local failure, 5 received additional salvage treatment: surgery in 2 patients, surgery plus repeat SBRT in 1, and repeat SBRT alone in 2. Although 3 patients achieved local tumor control with avoided neurological deficiency, 2 developed progressive disease with leptomeningeal dissemination. One patient was in poor general condition and was administered supportive care without salvage treatment.
Adverse events
Table 2 summarizes grade 2–5 radiation-induced adverse events. Treatment-related mortality occurred in 1 patient (4.8%) due to mediastinitis and epidural abscess from esophageal perforation. The patient who died had active systemic disease due to recurrent esophageal cancer and received re-irradiation SBRT for Th4–5 MESCC (Bilsky grade 1b and SINS 5) 7.8 months after the initial chemoradiotherapy (60 Gy in 30 fractions). Two weeks after SBRT, systemic chemotherapy (5-fluorouracil and cisplatin) was resumed. After the systemic disease progression, the treatment was switched to weekly paclitaxel until 25 days before death. The patient was urgently hospitalized 18 days before death due to a fever and developed paraplegia 12 days before death. CT scans (Fig. 3A) diagnosed esophageal perforation, mediastinitis, and spinal cord compression due to an epidural abscess (Esophageal perforation diagnosed 154 days after SBRT). Initially, the esophageal perforation was attributed to the progression of the mediastinal tumor. However, fusing the dose distributions of initial EBRT and SBRT to the diagnostic CT (Fig. 3A) showed that the esophageal perforation occurred where the location irradiated high doses in both plans (Fig. 3B-C). Eventually, the death was highly suspected to be a treatment-related death (TRD) due to an overdose of esophagus from SBRT. The patient died 166 days after the diagnosis of the spinal metastases and 124 days after SBRT.
Table 2.
Incidence of grade 2 or higher treatment-related adverse events
| Adverse events | Overall (N = 21) | ||||
|---|---|---|---|---|---|
| Grade 2–5 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | |
| Dysphagia | 2 (10%) | 1 (5%) | 1 (5%) | 0 | 0 |
| Nausea | 2 (10%) | 1 (5%) | 1 (5%) | 0 | 0 |
| Esophagitis | 2 (10%) | 1 (5%) | 0 | 0 | 1 (5%) |
| Vertebral compression fracture | 5 (24%) | 3 (14%) | 2 (10%) | 0 | 0 |
| Radiation pneumonitis | 1 (5%) | 1 (5%) | 0 | 0 | 0 |
Fig. 3.
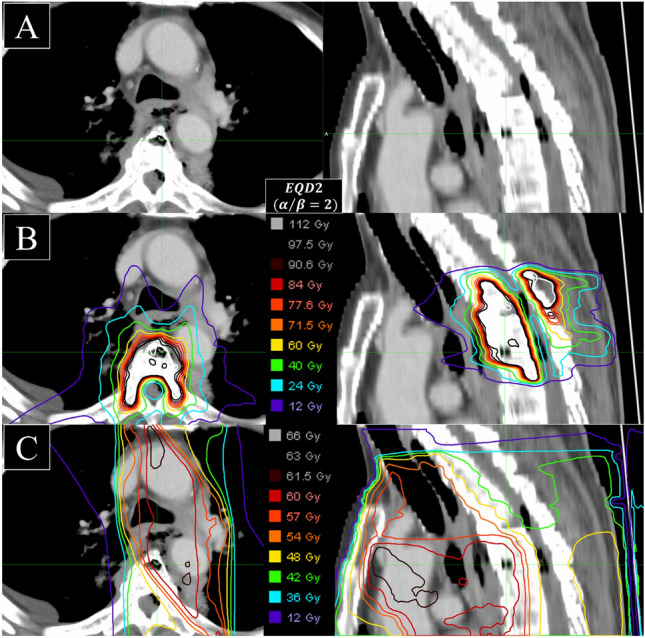
The images of diagnostic CT of radiation-induced esophageal perforation. A The diagnostic image. The patient was urgently hospitalized 18 days before death due to a fever and developed paraplegia 12 days before death. The image shows esophageal perforation, mediastinitis, and spinal cord compression due to an epidural abscess. B Dose distribution of re-irradiation SBRT on the fused-CT image (same CT as Fig. 2A). C Dose distribution of initial EBRT on the fused-CT image (same CT as Fig. 2A). CT computed tomography, SBRT stereotactic body radiation therapy, EBRT external beam radiation therapy
Other identified adverse events included vertebral compression fractures (VCF) of any grade in 5 (23.8%) of the entire patients: they were all treated with SBRT alone, including 3 of 4 patients (75%) who had spinal instability but not performed surgery prior to SBRT. Two patients with grade 3 VCF underwent stabilization. Radiation pneumonitis (grade 2) occurred in one patient (4.8%). No cases of radiation-induced myelopathy or nerve root injury or carotid artery rupture were observed.
Dosimetric analyses
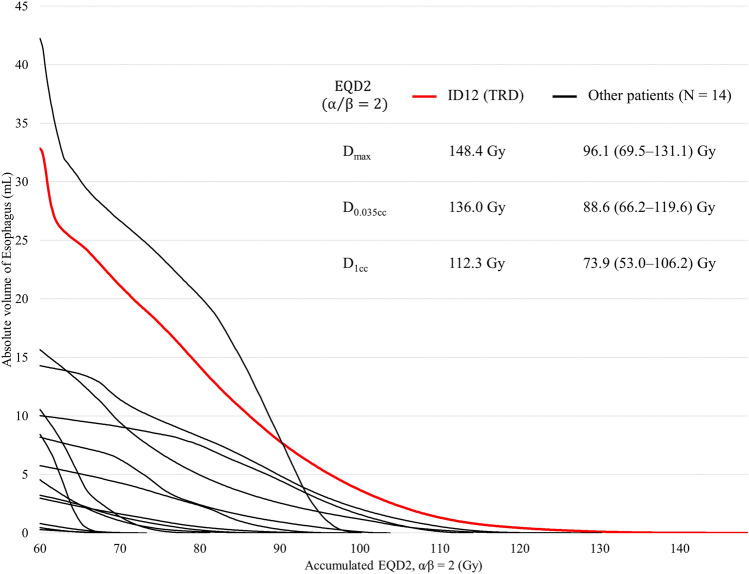
The esophagus of the TRD patient was irradiated with a higher dose per volume for both SBRT alone and the cumulative dose with initial EBRT. Table 3 shows the Dmax, D0.035 cc, and D1cc, which were higher than the maximum values compared to the TRD patient (identification number 12) and other evaluable 15 patients: the SBRT dose was 127 Gy3 vs. 117 Gy3, 114 Gy3 vs. 106 Gy3, 83 Gy3 vs. 82 Gy3 in BED, and the cumulative dose was 227 Gy3 vs. 203 Gy3, 209 Gy3 vs. 187 Gy3, 175 Gy3 vs. 167 Gy3 in BED, respectively. Figure 4 shows the dose-volume histogram of the esophagus. In the TRD patient, the DVH shows that the cumulative esophageal dose curve extends into a higher dose range, showing a longer tail than in other patients.
Table 3.
Esophageal dose comparison between the patient with grade 5 esophageal toxicity and other patients
| The TRD patient (N = 1) vs. other patients (N = 15) | |||
|---|---|---|---|
| Dmax | D0.035 cc | D1cc | |
| SBRT dose, maximum value [IQR] | |||
| EQD2 | |||
| α/β = 2, Gy2 | 89 vs. 82 [32, 66] | 80 vs. 74 [25, 58] | 57 vs. 57 [15, 48] |
| α/β = 3, Gy3 | 76 vs. 70 [28, 57] | 68 vs. 64 [22, 50] | 50 vs. 49 [14, 42] |
| α/β = 10, Gy10 | 46 vs. 43 [20, 36] | 42 vs. 40 [16, 32] | 32 vs. 32 [11, 28] |
| BED | |||
| α/β = 2, Gy2 | 178 vs. 164 [63, 131] | 160 vs. 148 [49, 116] | 114 vs. 114 [29, 95] |
| α/β = 3, Gy3 | 127 vs. 117 [47, 95] | 114 vs. 106 [37, 84] | 83 vs. 82 [23, 69] |
| α/β = 10, Gy10 | 55 vs. 52 [24, 43] | 51 vs. 48 [20, 39] | 38 vs. 38 [13, 33] |
| Cumulative dose, maximum value [IQR] | |||
| EQD2 | |||
| α/β = 2, Gy2 | 148 vs. 131 [76, 103] | 136 vs. 120 [72, 101] | 112 vs. 106 [66, 94] |
| α/β = 3, Gy3 | 136 vs. 122 [73, 96] | 125 vs. 112 [70, 95] | 105 vs. 100 [63, 90] |
| α/β = 10, Gy10 | 106 vs. 102 [74, 88] | 100 vs. 97 [71, 87] | 89 vs. 88 [65, 84] |
| BED | |||
| α/β = 2, Gy2 | 296 vs. 262 [152, 206] | 272 vs. 240 [144, 202] | 224 vs. 212 [132, 188] |
| α/β = 3, Gy3 | 227 vs. 203 [121, 160] | 209 vs. 187 [117, 158] | 175 vs. 167 [104, 150] |
| α/β = 10, Gy10 | 127 vs. 122 [89, 106] | 120 vs. 117 [85, 104] | 107 vs. 106 [78, 101] |
EQD2 equivalent dose at 2 Gy, BED biological effective dose, IQR interquartile range, SBRT stereotactic body radiation therapy, Cumulative dose total dose accumulated SBRT dose and the initial external beam radiation therapy doses, Dmax the maximum dose at one point of the target volume, D0.035 cc the maximum dose that covered 0.035 cc of the target volume, D1cc the maximum dose that covered 1 cc of the target volume
Fig. 4.
A Dose volume histogram for cumulative esophageal dose (equivalent dose at 2 Gy with the α/β ratio of 2). Red line shows a histogram of the patient died of radiation-induced esophageal perforation (grade 5), and Black lines show those of the other patients
Twelve patients met the inclusion criteria for the post-SBRT dosimetric analysis of the esophagus: their prior irradiation included 4 patients of more than two irradiation courses and 8 patients of initial radiotherapy with 120 Gy2 BED prescription doses. Table S3 shows the cumulative doses of the spinal cord and other organs: the maximum value (IQR) of the spinal cord cumulative D0.035 cc was 122 (104, 112) Gy2 BED. For other organs, pharynx, trachea, carotid arteries, and aorta, the maximum value (IQR) of the cumulative Dmax was 200 (127, 173) Gy3 BED, 197 (111, 157) Gy3 BED, 197 (160, 178) Gy3 BED, and 373 (198, 271) Gy3 BED, respectively.
Discussion
The results of our study demonstrate that re-irradiation with 24 Gy in 2 fractions of SBRT can be an effective salvage treatment for selected patients with cervical or thoracic MESCC previously irradiated with 50 Gy2 EQD2 or more. The analysis was limited to patients with a history of high-dose initial EBRT with a median 60 Gy2 EQD2 (α/β = 2, range: 50–105 Gy2), higher than those of any previous studies. Patients who receive high-dose initial EBRT may be at higher risk for overdose and severe toxicity of surrounding organs after salvage irradiation, because the surrounding organs are at least as or more irradiated than the spinal cord. The study has a strength in providing data on the efficacy and safety of salvage SBRT re-irradiation in cases of such challenging patients with a history of high-dose irradiation, such as the profile of radiation-induced toxicity and recommended esophageal dose constraints.
Our treatment algorithm shows the importance of patient selection for surgery plus SBRT or SBRT alone. A 1-year local failure rate of 29.3% highlights the possibility that SBRT can offer effective local control, even in patients with a history of high-dose radiation therapy. The current cohort had a higher incidence of treatment-related adverse events than in the previous studies, with 5 cases (24%) of VCF of any grade. As three (75%) non-surgical patients with unstable spinal lesions had VCF, the higher incidence of VCF was suspected to be due to the higher proportion of patients with high baseline spinal instability. Table S4 summarizes the previous studies of re-irradiation spine SBRT [31–43]. In the previous studies, the median initial EBRT dose was 30 Gy, and data on the efficacy and safety of cases with higher EBRT doses still need to be investigated. Ito et al. evaluated the efficacy and safety of re-irradiation SBRT (24 Gy/2 fx) stratified by previous EBRT doses (EQD2: < 30 Gy2, 30–40 Gy2, 40–50 Gy2, > 50 Gy2) [43]. The 1-year local failure rate was 25.8% in the entire cohort (123 patients), and the previous EBRT dose was not correlated with the local failure rate (P = 0.13). Their cohort included 17 (12.8%) of previous > 50 Gy2 EQD2 cases, but severe toxicities were not observed. According to two retrospective studies, poor dosimetric coverage of the GTV (Dmin, D98, and D95) may be important risk factors for local failure [44, 45]. However, the trade-off between sacrificing coverage of the target volume to meet critical OAR constraints, such as the spinal cord and esophagus, is more important for patients with a high-dose irradiation history.
SBRT is also an expected strategy to improve disease control and survival outcomes in patients with oligometastatic disease [4]. Although oligo-metastasis and oligo-recurrence have been proposed for many years [28–30, 46], randomized-controlled trials using SBRT have only recently emerged and have achieved positive results. In an open-label randomized phase II SABR-COMET trial, standard palliative radiotherapy was compared to SABR (i.e., SBRT) in 99 patients with 1–5 metastatic lesions and a controlled primary tumor. Five-year OS was significantly greater in the SABR arm than in the palliative radiotherapy arm (42.3% vs. 17.7%; P = 0.006) [11]. However, it should be noted that there were three deaths (4.5%) in the SABR arm related to stereotactic treatment [4], and further studies are required to prevent the severe toxicities of performing SBRT for high-risk patients such as those in this study.
We experienced one TRD case in the present study due to esophageal perforation, even though all specified SBRT dose constraints were met. Retrospective assessment of the SBRT dose and cumulative dose initial EBRT and SBRT revealed that the TRD patient received the highest doses compared to other patients. Treatment-related death due to radiation-induced esophageal toxicity after re-irradiation SBRT is a rare complication, and we found only six papers (seven patients) as far as we reviewed pertinent literature (Table S5): three cases were treated for lung tumors, and four were spine SBRT. TRD occurred in one re-irradiation case and the rest in the initial RT cases [21, 24, 47–51]. The associated risk factors for severe esophageal toxicity identified were esophageal dose, chemotherapy use, re-irradiation, and iatrogenic esophageal manipulation (e.g., biopsy). The patients in our study received higher cumulative esophageal doses, both Dmax and D1cc, compared to those reported for evaluable TRD patients. Concurrently, 71.4% of the patients had chemotherapy for active systemic disease. Although such factors were associated with an increased risk of severe esophageal toxicities, severe ones were absent in our cohort except for one case that irradiated the highest dose. A possible explanation for the acceptable incidence of severe esophageal toxicity from re-irradiation may be the long interval between the initial EBRT and re-irradiation (median 13.7 months). The risk of toxicity increases with shorter intervals between re-irradiations, which is supported both at the experimental level and in several re-irradiation studies [52–54]. The cumulative dose of our TRD patient received the highest cumulative doses (Dmax of 227 Gy3 BED, D0.035 cc of 209 Gy3 BED, and D1cc of 175 Gy3 BED, Table 3), so careful consideration is critical in case of re-irradiation for patients with high-dose EBRT history. According to the results of the esophageal dose assessment, the following esophageal cumulative dose constraints are recommended in addition to dose constraints of only the SBRT to reduce the risk of severe esophageal toxicity: Dmax < 203 Gy3 BED, D0.035 cc < 187 Gy3 BED, D1cc < 167 Gy3 BED, which were based on the highest esophageal cumulative doses in the patients other than TRD. These cumulative esophageal dose constraints, appropriate time to re-irradiation should be determined by each patient and will be updated as more toxicity data are accumulated in the future. The present data did not include severe toxicities of other OARs (pharynx, trachea, carotid arteries, and aorta), and further data are needed to know whether cumulative dose limits of these OARs exist at higher levels.
There are several possible significant limitations in this study. The data were a small sample size from a single institution. Our data were obtained by retrospective observation, and the timing of the evaluation varied. Our recommended cumulative esophageal dose should be validated in future prospective studies with a larger sample size. Also, we note that the study population included 15 patients (71%) who received definitive initial EBRT, not palliative treatment.
In conclusion, in cervical thoracic MESCC patients with a history of irradiation of 50 GyEQD2 or more, SBRT re-irradiation may be expected to have favorable local control similar to that of patients with a lower irradiation history. Cumulative dose constraints for the esophagus (Dmax < 203 Gy3 BED, D0.035 cc < 187 Gy3 BED, D1cc < 167 Gy3 BED) were key findings for safer treatment.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank all the patients, investigators, and institutions involved in this study.
Funding
This work was supported by Japan Society for the Promotion of Science (JSPS, Grant Number 23K14669).
Declarations
Conflict of interest
YK has a speaker’s bureau for Hitachi Co., and received research funding from JSPS (Grant Number 23K14669). TK has a speaker’s bureau for Hitachi Co., Bristol Myers Squibb., Accuray Co., Elekta Co., Ono Pharmaceutical Co., AstraZeneca Co., Taiho Pharmaceutical Co., Canon Co., and Janssen Pharmaceutical Co.
Ethical approval
This study was approved by the institutional review board of our institution.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Oldenburger E, Brown S, Willmann J, et al. ESTRO ACROP guidelines for external beam radiotherapy of patients with complicated bone metastases. Radiother Oncol. 2022;173:240–253. doi: 10.1016/j.radonc.2022.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Lutz S, Balboni T, Jones J, et al. Palliative radiation therapy for bone metastases: update of an ASTRO evidence-based guideline. Pract Radiat Oncol. 2017;7:4–12. doi: 10.1016/j.prro.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomized trial. Lancet. 2005;366:643–648. doi: 10.1016/S0140-6736(05)66954-1. [DOI] [PubMed] [Google Scholar]
- 4.NCCN guidelines available at http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed 16 Aug 2023. cns.
- 5.Spratt DE, Beeler WH, de Moraes FY, et al. An integrated multidisciplinary algorithm for the management of spinal metastases: an international spine oncology consortium report. Lancet Oncol. 2017;18:e720–e730. doi: 10.1016/S1470-2045(17)30612-5. [DOI] [PubMed] [Google Scholar]
- 6.Imano N, Saito T, Hoskin P, et al. Pain response rates after conventional radiation therapy for bone metastases assessed using international consensus pain response endpoints: a systematic review and meta-analysis of initial radiation therapy and reirradiation. Int J Radiat Oncol Biol Phys. 2023;116:739–746. doi: 10.1016/j.ijrobp.2023.01.050. [DOI] [PubMed] [Google Scholar]
- 7.Chow E, Hoskin P, Mitera G, et al. Update of the international consensus on palliative radiotherapy endpoints for future clinical trials in bone metastases. Int J Radiat Oncol Biol Phys. 2012;82:1730–1737. doi: 10.1016/j.ijrobp.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Benzil DL, Saboori M, Mogilner AY, et al. Safety and efficacy of stereotactic radiosurgery for tumors of the spine. J Neurosurg. 2004;101:413–418. doi: 10.3171/sup.2004.101.supplement3.0413. [DOI] [PubMed] [Google Scholar]
- 9.Bilsky MH, Yamada Y, Yenice KM, et al. Intensity-modulated stereotactic radiotherapy of paraspinal tumors: a preliminary report. Neurosurgery. 2004;54:823–30. doi: 10.1227/01.NEU.0000114263.01917.1E. [DOI] [PubMed] [Google Scholar]
- 10.Chang EL, Shiu AS, Lii M-F, et al. Phase I clinical evaluation of near-simultaneous computed tomographic image-guided stereotactic body radiotherapy for spinal metastases. Int J Radiat Oncol Biol Phys. 2004;59:1288–1294. doi: 10.1016/j.ijrobp.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 11.Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: long-term results of the SABR-COMET phase II randomized trial. J Clin Orthod. 2020;38:2830–2838. doi: 10.1200/JCO.20.00818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sahgal A, Myrehaug SD, Siva S, et al. Stereotactic body radiotherapy versus conventional external beam radiotherapy in patients with painful spinal metastases: an open-label, multicentre, randomized, controlled, phase 2/3 trial. Lancet Oncol. 2021;22:1023–1033. doi: 10.1016/S1470-2045(21)00196-0. [DOI] [PubMed] [Google Scholar]
- 13.Song X, Wei J, Sun R, et al. Stereotactic body radiation therapy versus conventional radiation therapy in pain relief for bone metastases: a systematic review and meta-analysis. Int J Radiat Oncol Biol Phys. 2022;115:909–921. doi: 10.1016/j.ijrobp.2022.10.017. [DOI] [PubMed] [Google Scholar]
- 14.Myrehaug S, Sahgal A, Hayashi M, et al. Reirradiation spine stereotactic body radiation therapy for spinal metastases: systematic review. J Neurosurg Spine. 2017;27:428–435. doi: 10.3171/2017.2.SPINE16976. [DOI] [PubMed] [Google Scholar]
- 15.Chow E, van der Linden YM, Roos D, et al. Reiradiation: Single versus multiple fractions of repeat radiation for painful bone metastases: a randomized, controlled, non-inferiority trial. Lancet Oncol. 2014;15:164–171. doi: 10.1016/S1470-2045(13)70556-4. [DOI] [PubMed] [Google Scholar]
- 16.Murray L, Thompson C, Pagett C, et al. Treatment plan optimisation for reirradiation. Radiother Oncol. 2023;182:109545. doi: 10.1016/j.radonc.2023.109545. [DOI] [PubMed] [Google Scholar]
- 17.Sahgal A, Chang JH, Ma L, et al. Spinal cord dose tolerance to stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2021;110:124–136. doi: 10.1016/j.ijrobp.2019.09.038. [DOI] [PubMed] [Google Scholar]
- 18.Nieder C, Grosu AL, Andratschke NH, et al. Update of human spinal cord reirradiation tolerance based on additional data from 38 patients. Int J Radiat Oncol Biol Phys. 2006;66(5):1446–9. doi: 10.1016/j.ijrobp.2006.07.1383. [DOI] [PubMed] [Google Scholar]
- 19.Kirkpatrick JP, Van Der Kogel AJ, Schultheiss TE, et al. Radiation dose–volume effects in the spinal cord. Int J Radiat Oncol Biol Phys. 2010;76:S42–S49. doi: 10.1016/j.ijrobp.2009.04.095. [DOI] [PubMed] [Google Scholar]
- 20.Sahgal A, Ma L, Weinberg V, et al. Reirradiation human spinal cord tolerance for stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2012;82:107–116. doi: 10.1016/j.ijrobp.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 21.Cassidy V, Amdur RJ. Esophageal damage from thoracic spine stereotactic body radiation therapy. Pract Radiat Oncol. 2022;12:392–396. doi: 10.1016/j.prro.2022.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Bilsky MH, Laufer I, Fourney DR, et al. Reliability analysis of the epidural spinal cord compression scale: clinical article. J Neurosurg Spine. 2010;13:324–328. doi: 10.3171/2010.3.SPINE09459. [DOI] [PubMed] [Google Scholar]
- 23.Fisher CG, DiPaola CP, Ryken TC, et al. A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine. 2010;35:E1221–E1229. doi: 10.1097/BRS.0b013e3181e16ae2. [DOI] [PubMed] [Google Scholar]
- 24.Cox BW, Spratt DE, Lovelock M, et al. International Spine Radiosurgery Consortium consensus guidelines for target volume definition in spinal stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2012;83:e597–605. doi: 10.1016/j.ijrobp.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Redmond KJ, Lo SS, Soltys SG, et al. Consensus guidelines for postoperative stereotactic body radiation therapy for spinal metastases: results of an international survey. J Neurosurg Spine. 2017;26:299–306. doi: 10.3171/2016.8.SPINE16121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koide Y, Shimizu H, Miyauchi R, et al. Fully automated rigid image registration versus human registration in postoperative spine stereotactic body radiation therapy: a multicenter non-inferiority study. J Radiat Res. 2022;63:115–121. doi: 10.1093/jrr/rrab113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimizu H, Koide Y, Sasaki K, et al. Dosimetric analysis on computed tomography myelography based treatment planning in stereotactic body radiotherapy for spinal metastases. Med Dosim. 2023;48:187–192. doi: 10.1016/j.meddos.2023.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Niibe Y, Hayakawa K. Oligometastases and oligo-recurrence: the new era of cancer therapy. Jpn J Clin Oncol. 2010;40:107–111. doi: 10.1093/jjco/hyp167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niibe Y, Chang JY. Novel insights of oligometastases and oligo-recurrence and review of the literature. Pulm Med. 2012;2012:261096. doi: 10.1155/2012/261096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niibe Y, Jingu K, Onishi H. Oligo-recurrence and Sync-oligometastases. J Thorac Oncol. 2018;13:e59–e60. doi: 10.1016/j.jtho.2017.11.115. [DOI] [PubMed] [Google Scholar]
- 31.Sahgal A, Ames C, Chou D, et al. Stereotactic body radiotherapy is effective salvage therapy for patients with prior radiation of spinal metastases. Int J Radiat Oncol Biol Phys. 2009;74:723–731. doi: 10.1016/j.ijrobp.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 32.Choi CYH, Adler JR, Gibbs IC, et al. Stereotactic radiosurgery for treatment of spinal metastases recurring in close proximity to previously irradiated spinal cord. Int J Radiat Oncol Biol Phys. 2010;78:499–506. doi: 10.1016/j.ijrobp.2009.07.1727. [DOI] [PubMed] [Google Scholar]
- 33.Garg AK, Shiu AS, Yang J, et al. Phase 1/2 trial of single-session stereotactic body radiotherapy for previously unirradiated spinal metastases. Cancer. 2012;118:5069–5077. doi: 10.1002/cncr.27530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Damast S, Wright J, Bilsky M, et al. Impact of dose on local failure rates after image-guided reirradiation of recurrent paraspinal metastases. Int J Radiat Oncol Biol Phys. 2011;81:819–826. doi: 10.1016/j.ijrobp.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 35.Mahadevan A, Floyd S, Wong E, et al. Stereotactic body radiotherapy reirradiation for recurrent epidural spinal metastases. Int J Radiat Oncol Biol Phys. 2011;81:1500–1505. doi: 10.1016/j.ijrobp.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 36.Ahmed KA, Stauder MC, Miller RC, et al. Stereotactic body radiation therapy in spinal metastases. Int J Radiat Oncol Biol Phys. 2012;82:e803–e809. doi: 10.1016/j.ijrobp.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 37.Chang U-K, Cho W-I, Kim M-S, et al. Local tumor control after retreatment of spinal metastasis using stereotactic body radiotherapy; comparison with initial treatment group. Acta Oncol. 2012;51:589–595. doi: 10.3109/0284186X.2012.666637. [DOI] [PubMed] [Google Scholar]
- 38.Thibault I, Al-Omair A, Masucci GL, et al. Spine stereotactic body radiotherapy for renal cell cancer spinal metastases: analysis of outcomes and risk of vertebral compression fracture: clinical article. J Neurosurg Spine. 2014;21:711–718. doi: 10.3171/2014.7.SPINE13895. [DOI] [PubMed] [Google Scholar]
- 39.Hashmi A, Guckenberger M, Kersh R, et al. Re-irradiation stereotactic body radiotherapy for spinal metastases: a multi-institutional outcome analysis. J Neurosurg Spine. 2016;25:646–653. doi: 10.3171/2016.4.SPINE151523. [DOI] [PubMed] [Google Scholar]
- 40.Boyce-Fappiano D, Elibe E, Zhao B, et al. Reirradiation of the spine with stereotactic radiosurgery: efficacy and toxicity. Pract Radiat Oncol. 2017;7:e409–e417. doi: 10.1016/j.prro.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 41.Ito K, Nihei K, Shimizuguchi T, et al. Postoperative re-irradiation using stereotactic body radiotherapy for metastatic epidural spinal cord compression. J Neurosurg Spine. 2018;29:332–338. doi: 10.3171/2018.1.SPINE171155. [DOI] [PubMed] [Google Scholar]
- 42.Sasamura K, Suzuki R, Kozuka T, et al. Outcomes after reirradiation of spinal metastasis with stereotactic body radiation therapy (SBRT): a retrospective single institutional study. J Radiat Res. 2020;61:929–934. doi: 10.1093/jrr/rraa058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ito K, Ogawa H, Nakajima Y. Efficacy and toxicity of re-irradiation spine stereotactic body radiotherapy with respect to irradiation dose history. Jpn J Clin Oncol. 2021;51:264–270. doi: 10.1093/jjco/hyaa178. [DOI] [PubMed] [Google Scholar]
- 44.Lovelock DM, Zhang Z, Jackson A, et al. Correlation of local failure with measures of dose insufficiency in the high-dose single-fraction treatment of bony metastases. Int J Radiat Oncol Biol Phys. 2010;77:1282–1287. doi: 10.1016/j.ijrobp.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bishop AJ, Tao R, Rebueno NC, et al. Outcomes for spine stereotactic body radiation therapy and an analysis of predictors of local recurrence. Int J Radiat Oncol Biol Phys. 2015;92:1016–1026. doi: 10.1016/j.ijrobp.2015.03.037. [DOI] [PubMed] [Google Scholar]
- 46.Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13:8–10. doi: 10.1200/JCO.1995.13.1.8. [DOI] [PubMed] [Google Scholar]
- 47.Stephans KL, Djemil T, Diaconu C, et al. Esophageal dose tolerance to hypofractionated stereotactic body radiation therapy: risk factors for late toxicity. Int J Radiat Oncol Biol Phys. 2014;90:197–202. doi: 10.1016/j.ijrobp.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 48.Onimaru R, Shirato H, Shimizu S, et al. Tolerance of organs at risk in small-volume, hypofractionated, image-guided radiotherapy for primary and metastatic lung cancers. Int J Radiat Oncol Biol Phys. 2003;56:126–135. doi: 10.1016/S0360-3016(03)00095-6. [DOI] [PubMed] [Google Scholar]
- 49.Le Q-T, Loo BW, Ho A, et al. Results of a phase I dose-escalation study using single-fraction stereotactic radiotherapy for lung tumors. J Thorac Oncol. 2006;1:802–809. doi: 10.1097/01243894-200610000-00008. [DOI] [PubMed] [Google Scholar]
- 50.Gomez DR, Hunt MA, Jackson A, et al. Low rate of thoracic toxicity in palliative paraspinal single-fraction stereotactic body radiation therapy. Radiother Oncol. 2009;93:414–418. doi: 10.1016/j.radonc.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abelson JA, Murphy JD, Loo BW, Jr, et al. Esophageal tolerance to high-dose stereotactic ablative radiotherapy. Dis Esophagus. 2012;25:623–629. doi: 10.1111/j.1442-2050.2011.01295.x. [DOI] [PubMed] [Google Scholar]
- 52.Das S, Patro KC, Mukherji A. Recovery and tolerance of the organs at risk during re-irradiation. J Curr Oncol. 2018;1:23. doi: 10.4103/jco.jco_2_17. [DOI] [Google Scholar]
- 53.Kim DW, Raoof S, Lamba N, et al. Definitive re-irradiation of locally recurrent esophageal cancer after trimodality therapy in patients with a poor performance status. Mol Clin Oncol. 2020;13:27–32. doi: 10.3892/mco.2020.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao K, Si Y, Sun L, et al. Efficacy and toxicity of re-irradiation for esophageal cancer patients with locoregional recurrence: a retrospective analysis. Radiat Oncol. 2020;15:243. doi: 10.1186/s13014-020-01685-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.