Imagine that your patient needs a transplant, say a new heart. A specially bred pig carrying human genes may soon supply it. That is the hope and hype of xenotransplantation, the transfer of animal cells, tissues, and organs to humans. Xenotransplantation is not entirely novel, as pig heart valves have been used for many years without apparent ill effect, but they are essentially inert tissue and seldom elicit rejection. There is now considerable excitement that the transplantation of live animal tissues may soon become a practical treatment option, although this is matched by concern over the risk of new zoonotic infections in transplant recipients.1 This article discusses the potential and the problems of xenotransplantation and explains why it faces an uncertain future.
Summary points
Transplantation of pig cells and tissues to treat diabetes and degenerative conditions such as Parkinson’s disease and Huntington’s chorea will become more common
Whole organ transplants from genetically modified pigs could make up the shortfall in human organs if immunological and physiological barriers can be overcome
Xenografts might be used as “bridging” organs to keep patients alive until human organs become available
The risk of zoonotic infections in xenotransplant recipients and their possible spread in the human population cannot be ignored—HIV and new influenza strains are thought to have started as zoonoses before becoming human pandemics
Clear ethical, safety, and monitoring guidelines are needed to control the development of xenotransplantation
Meeting the need
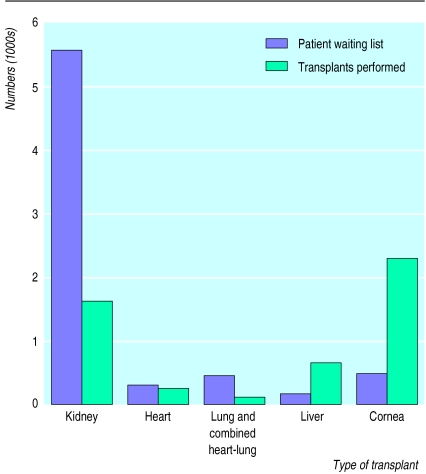
Figure 1 shows the imbalance between the supply and demand for organ transplants, with the largest waiting by far being for kidney transplants. Furthermore, the need for organ donors will continue to rise as more patients and new diseases are deemed eligible for treatment by transplantation. Even if opt out policies were adopted (in which people are assumed to consent unless they state otherwise) the supply of organs and tissues could not match the demand. Could xenografts bridge the gap?
Figure 1.
Major organ transplants in the United Kingdom in 1997 and the size of waiting lists (excluding those who died while waiting) at 31 December 1997. Compiled from data supplied by the UK Transplant Support Service Authority
The quest for suitable animals
From the physiological point of view as well as likely tolerance of the graft, old world monkeys and apes should be the most promising sources of tissue. However, to provide animals uncontaminated by the many viruses that are potentially pathogenic for humans, baby monkeys would have to be born by caesarean section and reared in isolation from other monkeys. This raises ethical concerns, not only for using our near relatives as a “tissue and organ farm,” but also for depriving infant animals of the parental care and behavioural development so important for all primates.
Pigs are the only animals being seriously considered today as a future source of organs for transplantation to humans, although there is speculation about ruminants and even kangaroos. Pigs grow quickly to about the right size, produce large litters, and can be reared in specific pathogen free conditions. Pigs can be genetically manipulated to produce organs less likely to be rejected on transplantation to humans. Several biotechnology companies are investing millions of pounds into developing such genetically modified animals.
The ethical issues about using pigs seem less than those about the use of primates. After all, pigs have been domesticated for thousands of years, they are routinely reared and slaughtered for human consumption, and the medical use of pig heart valves has not raised serious objections from those religions that disapprove of the consumption of pork. Transgenic mice have become a common tool in medical research, and transgenic pigs are not different in principle or practice. What, then, are the medical problems of pig to human transplantation? These are of three kinds—physiological, immunological, and microbiological.
Will pig tissues function in humans?
The physiological problems have not yet been fully unravelled.2,3 Pig insulin has been used for many years, so if pig islet of Langerhans cells could be successfully engrafted they might perform the right function in patients with insulin dependent diabetes. Similarly pig hearts ought to be adequate despite the need to pump blood upwards to the brain. But will pig kidneys, with their rather different structure and function to human ones, respond to the appropriate human diuretic signals?
Kidneys, as well as producing urine, synthesise erythropoietin, a hormone essential for regulating the production and maturation of red blood cells. Porcine erythropoietin does not function in humans because human erythropoietin receptors on red cell precursors in the bone marrow do not recognise the pig version of the hormone. Thus human recipients of pig kidneys would need to be treated with recombinant human erythropoietin. There may be many other discordant physiological signals between pigs and humans, including the factors that control the health and function of the bone marrow and the liver. We do not yet know which tissues will function properly in the cross species setting. Experimental xenotransplantation of porcine tissues into monkeys as surrogate humans may provide an indication of the likely effects.
The challenge to prolong graft survival
The immunological problems that threaten xenograft survival are greater than those of allografts—human transplantation from an unmatched donor—because the graft is destroyed in three ways: hyperacute, acute, and cell mediated rejection.2–4
Mechanisms of xenograft rejection
Hyperacute rejection
Complement mediated lysis of vascular lining cells in donor organ. Natural human antibodies to foreign αGal sugar moieties on pig endothelium attract complement and destroy the organ’s vasculature within minutes of exposure to human blood. The mechanism is akin to the haemolysis of red blood cells in ABO or Rh mismatched transfusion. It can be suppressed by expressing human complement modulating proteins in transgenic pigs. Hyperacute rejection does not involve T cells and is not a problem for non-vascular xenografts or for human allografts
Acute vascular rejection
Local activation of inflammatory responses leading to platelet coagulation and extravasation of leucocytes in blood capillaries of donor organs. Also called delayed xenograft rejection, it affects the organ’s vasculature within 3-5 days. Like hyperacute rejection, it mainly results from αGal antibodies naturally present in human blood before exposure to the foreign organ. It can be suppressed in the same way and is not a major problem for non-vascular xenografts or allografts
Cell mediated rejection
Antigens on the surface of engrafted cells are perceived as foreign and are subsequently attacked by specific cytotoxic T lymphocytes. As this is a de novo immune response, rejection starts 1-2 weeks after transplantation. As with human allografts, cell mediated rejection of xenografts can be partially suppressed by immunosuppressive drugs such as steroids or cyclosporin A
Preventing acute rejection
Humans, in common with apes and old world monkeys (but unlike pigs), lack the carbohydrate antigen galactose-α(1-3)galactose (αGal). Because many gut bacteria express αGal, all humans make αGal antibodies, which will bind to pig endothelium in xenografts. One way to prevent hyperacute rejection and acute vascular rejection would be to breed pigs that resembled humans in lacking the enzyme α(1-3)galactosyl transferase, which synthesises the major xenoantigen αGal. However, the technology to “knockout” specific genes in pigs does not yet exist.
An alternative approach is to breed transgenic pigs that either make a competing sugar, α fucose, or possess human genes for the cell membrane proteins CD55 (DAF), CD46 (MCP), or CD59, which inhibit the cascade of events triggered by human complement activation that result in acute rejection. It is claimed that hearts transplanted from such pigs survive much longer (up to 40 days) than standard pig hearts in immunosuppressed monkeys.5 Thus, even if the xenograft were eventually rejected, it could serve as a “bridging” organ to keep the patient alive until a human organ became available. However, that would surely increase the demand for human organs.
Hyperacute and acute rejection are less of a problem with xenografted cells and tissues that do not require an intact vasculature. That is why xenotransplantation is progressing for diseases not previously treated by transplantation, such as degenerative brain conditions and diabetes. Phase I clinical trails have been conducted with two pig tissues: islet of Langerhans cells for treating diabetes6 and fetal brain cells for Parkinson’s disease and Huntington’s chorea7 as pig brain cells secreting neurotrophic factors may halt the degenerative processes.
Cell mediated rejection
This is the same mechanism as rejection of human allografts (HLA mismatched transplants). Cell mediated rejection also plays a crucial role in recognising virus infections. Some common viruses, such as cytomegalovirus, persist life long in our bodies but rarely cause harm because they are permanently kept under control by T cell immunity. In immunosuppressed recipients of transplants or AIDS patients, however, such viruses can become lethal. Prophylactic antiviral treatment may help to control these infections, but we do not know whether xenotransplant recipients might need to receive lifelong treatment with immunosuppressive drugs and thus be perpetually open to risk.
Do pig viruses pose a risk to human health?
Some proponents of xenotransplantation argue that, as pigs have lived alongside humans for so long, we would by now have picked up any of their microbes capable of infecting us. But xenotransplantation will afford a much easier passage for animal viruses. Firstly, the physical barriers are broken if porcine tissue is placed within the human body. Secondly, the immune suppression needed to prevent graft rejection will help a virus to propagate and adapt to its new host. Thirdly, the human genes bred into transgenic donor pigs could promote preadaptation of animal viruses for human infection.
Pig viruses may not be recognised if they do not cause disease in pigs. For example, a pig calicivirus, closely related to human hepatitis E virus, was discovered only last year.8 It might cause no harm in pigs but be pathogenic in humans, just as herpes virus B of macaques gives the monkeys nothing worse than cold sores but causes fatal encephalitis in humans.
Designer pigs, genetically modified to allow organs to survive as xenografts, may allow pig viruses to infect humans more readily.9 The human proteins expressed on the surface of transgenic pig cells can also act as receptors for viruses. CD55 is a receptor for human Coxsackie B and ECHO viruses (relatives of poliovirus), which cause myocarditis. CD46 can act as a receptor for measles virus, so it is possible that related morbilliviruses of animals (such as distemper and rinderpest viruses) could become preadapted in transgenic pigs for human infection. Morbilliviruses can jump host species in any case, as we learned from the deaths of a veterinarian and a stable hand in Australia after a postmortem examination of a horse, which in turn probably acquired the virus from a fruit bat.10 A related but distinct virus has already spread from bats to pigs and affected their human contacts.11 So we should be wary of offering viruses a helping hand by breeding animals with human receptors.
Another way transgenic pigs may heighten the risk is that viruses with lipid envelopes, derived from the host cell membranes from which they bud, will be less likely to be inactivated by human complement. What may be a natural protective mechanism against human infection by viruses of farm animals could be broken down by attempts to make xenografts survive in humans.9
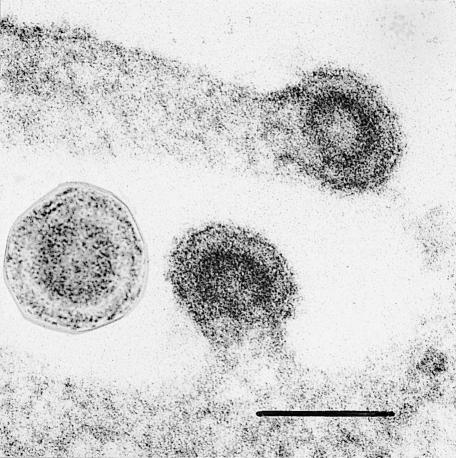
All these arguments would be academic if we could ensure that no pig viruses were present in the transgenic herds to be used for xenotransplantation. Many viruses will be eliminated by breeding pigs free of specific pathogens, but we cannot screen for viruses not yet discovered. Moreover, pigs contain “endogenous” retrovirus genomes that are inherited as Mendelian traits in the DNA of normal pig chromosomes and therefore cannot be eliminated.12 These viral sequences in host DNA can be activated to produce infectious viruses that are closely related to leukaemia viruses of mice, cats, and gibbons and are second cousins to HIV. Last year, we reported that two of three pig endogenous retroviruses can infect human cells in culture (fig 2).13,14 These retroviruses with a potential human host range are released from normal lymphocytes and endothelial cells taken from healthy pigs.15,16 Our findings led Fritz Bach, an expert on the immunology of xenotransplant rejection, to call for a moratorium on further human xenotransplantation until it is known whether pig retroviruses can infect primates and whether they cause disease.17
Figure 2.
Pig retroviruses growing in a cultured human cell (scale= 100 nm)
Balancing benefit and risk
Investigations of the few patients who have already been transplanted with pig islet of Langerhans cells and brain cells, and two renal dialysis patients whose dialysis tubing was temporarily plumbed into pig kidneys extracorporeally, have so far shown no evidence of retrovirus infection.18,19 If tests on further exposed individuals give similar results, it may seem unnecessarily restrictive to prohibit xenotransplantation. Metamorphosis may no longer be restricted to legend or Kafka’s nightmare.
However, there is a reason to take exceptional care. While the balance of risk of an unwanted pig virus infection may be acceptable to the recipient of a xenotransplant facing probable death through the lack of a human donor organ, the more remote yet plausible risk of such a patient setting off a novel human epidemic is quite a different consideration.9,17 Future decisions about xenotransplantation therefore need to take account of the possible impact on public health. This is why the UK Department of Health accepted the advice of the Kennedy Committee1 to set up the UK Xenotransplantation Interim Regulatory Authority, with a remit to review developments and authorise clinical trials.20 The US Food and Drug Administration has recently decided that trials of xenotransplantation should not be left to the discretion of local ethics committees and is also drawing up new guidelines.21
It is now accepted that xenograft recipients will need long term surveillance. But the need to monitor each patient’s contacts should he or she develop a zoonotic infection raises ethical and practical problems. What obligation will there be for such patients to provide complete information? How will the follow up of contacts be conducted? Surgeons may have to learn from clinics for sexually transmitted diseases.
As a medical procedure, xenotransplantation may benefit the individual patient while threatening harm to the community. This paradox is said to pose a new ethical problem, but it has a familiar ring. If I have convinced you that proceeding with xenotransplantation is reckless, just recall that providing immediate benefit at long term cost is an everyday practice of general practitioners and hospital doctors through the excessive prescribing of antibiotics. While the patient usually recovers, the threat to our future health is a world awash with multiply resistant microbes (see BMJ special issue of 5 September 1998). Perhaps the xenotransplanter’s myopia is no different from that of the rest of us.
Looking further into the future
In the future we can envisage increasing use of “ex vivo” treatments by living cells, for which cultured human tissues may begin to compete with animal sources. The routine preservation of blood stem cells from umbilical cords is being debated, as these could later be used to treat the person from whom they came. In addition, cloning from mature cells, as was done with Dolly the sheep, might allow functional human tissues and, eventually, organs to be regenerated from somatic cells. With advances in reprogramming cellular differentiation, patients may themselves become donors for autografts, making xenografts superfluous. However, these developments in cell therapy22 are likely to be farther away than xenotransplantation.
References
- 1.Department of Health Advisory Group on the Ethics of Xenotransplantation. Animal tissues into humans. London: HMSO; 1997. [Google Scholar]
- 2.Dorling A, Riesbeck K, Warrens A, Lechler R. Clinical xenotransplantation of solid organs. Lancet. 1997;349:867–871. doi: 10.1016/S0140-6736(96)09404-4. [DOI] [PubMed] [Google Scholar]
- 3.Platt JL. Xenotransplantation: recent progress and current perspectives. Curr Opin Immunol. 1996;8:721–728. doi: 10.1016/s0952-7915(96)80091-4. [DOI] [PubMed] [Google Scholar]
- 4.Bach FH, Winkler H, Ferrari C, Hancock WW, Robson SC. Delayed xenograft rejection. Immunol Today. 1996;17:379–384. doi: 10.1016/0167-5699(96)10024-4. [DOI] [PubMed] [Google Scholar]
- 5.Cozzi E, White DG. The generation of transgenic pigs as potential organ donors for humans. Nature Med. 1995;1:964–966. doi: 10.1038/nm0995-964. [DOI] [PubMed] [Google Scholar]
- 6.Groth CG, Korsgren O, Tibell AJ, Tollemar J, Moller E, Bolinder J, et al. Transplantation of porcine fetal pancreas to diabetic patients. Lancet. 1994;344:1402–1404. doi: 10.1016/s0140-6736(94)90570-3. [DOI] [PubMed] [Google Scholar]
- 7.Deacon T, Schumacher J, Dinsmore J, Thomas C, Palmer P, Kott S, et al. Histological evidence of fetal pig neural cell survival after transplantation into a patient with Parkinson’s disease. Nature Med. 1997;3:350–353. doi: 10.1038/nm0397-350. [DOI] [PubMed] [Google Scholar]
- 8.Meng X-J, Purcell RH, Halbur PG, Lehman JR, Webb DM, Tsareva TS, et al. A novel virus in swine is closely related to the human hepatitis E virus. Proc Natl Acad Sci USA. 1997;94:9860–9865. doi: 10.1073/pnas.94.18.9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss RA. Transgenic pigs and virus adaptation. Nature. 1998;391:327–328. doi: 10.1038/34772. [DOI] [PubMed] [Google Scholar]
- 10.O’Sullivan JB, Allworth AM, Paterson DL, Snow TM, Boots R, Gleeson LJ, et al. Fatal encephalitis due to novel paramyxovirus transmitted from horses. Lancet. 1997;349:93–95. doi: 10.1016/s0140-6736(96)06162-4. [DOI] [PubMed] [Google Scholar]
- 11.Philbey AW, Kirkland PD, Ross AD, Davis RJ, Gleeson AB, Love RJ, et al. An apparently new virus (family Paramyxoviridae) infections for pigs, humans and fruit bats. Emerging Infect Dis. 1998;4:269–271. doi: 10.3201/eid0402.980214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akiyoshi DE, Denaro M, Zhu HH, Greenstein JL, Banerjee P, Fishman JA. Identification of a full-length cDNA for an endogenous retrovirus of miniature swine. J Virol. 1998;72:4503–4507. doi: 10.1128/jvi.72.5.4503-4507.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patience C, Takeuchi Y, Weiss RA. Infection of human cells by an endogenous retrovirus of pigs. Nature Med. 1997;3:282–286. doi: 10.1038/nm0397-282. [DOI] [PubMed] [Google Scholar]
- 14.Le Tissier P, Stoye P, Takeuchi Y, Patience C, Weiss RA. Two sets of human-tropic pig retrovirus. Nature. 1997;389:681–682. doi: 10.1038/39489. [DOI] [PubMed] [Google Scholar]
- 15.Wilson CA, Wong S, Muller J, Davidson CE, Rose TM, Burd P. Type C retrovirus released from porcine primary peripheral blood mononuclear cells infects human cells. J Virol. 1998;72:3082–3087. doi: 10.1128/jvi.72.4.3082-3087.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin U, Kiessig V, Blisch JH, Haverich JH, von der Helm K, Herden T, et al. Expression of pig endogenous retrovirus by primary porcine endothelial cells and infection of human cells. Lancet. 1998;352:692–694. doi: 10.1016/S0140-6736(98)07144-X. [DOI] [PubMed] [Google Scholar]
- 17.Bach FH, Fishman JA, Daniels N, Proimos J, Anderson B, Carpenter CB, et al. Uncertainty in xenotransplantation: individual benefit versus collective risk. Nature Med. 1998;4:141–144. doi: 10.1038/nm0298-141. [DOI] [PubMed] [Google Scholar]
- 18.Heineine W, Tibell A, Switzer WM, Sandstrom P, Rosales GV, Matthews A, et al. No evidence of infection with porcine endogenous retrovirus in recipients of porcine islet-cell xenografts. Lancet. 1998;352:695–698. doi: 10.1016/S0140-6736(98)07145-1. [DOI] [PubMed] [Google Scholar]
- 19.Patience C, Patton GS, Takeuchi Y, Weiss RA, McClure MO, Rydberg L, et al. No evidence of pig DNA or retroviral infection in patients with short-term extracorporeal connection to pig kidneys. Lancet. 1998;352:699–701. doi: 10.1016/S0140-6736(98)04369-4. [DOI] [PubMed] [Google Scholar]
- 20.Warden J. Xenotransplantation moves ahead in UK. BMJ. 1998;317:365. doi: 10.1136/bmj.317.7155.365. [DOI] [PubMed] [Google Scholar]
- 21.Gage F. Cell therapy. Nature. 1998;329(suppl):18–24. [PubMed] [Google Scholar]
- 22.Butler D. Last chance to stop and think on risks of xenotransplants. Nature. 1998;319:320–325. doi: 10.1038/34749. [DOI] [PubMed] [Google Scholar]