Abstract
Nanoliposomal irinotecan with fluorouracil and folinic acid (NFF) is a standard regimen after gemcitabine-based therapy for patients with unresectable or recurrent pancreatic cancer. However, there are limited clinical data on its efficacy and safety in the real-world. We therefore initiated a retrospective and prospective observational study (NAPOLEON-2). The results of the retrospective part were reported herein. In this retrospective study, we evaluated 161 consecutive patients who received NFF as second-or-later-line regimen. The main endpoint was overall survival (OS), and the other endpoints were response rate, disease control rate, progression-free survival (PFS), dose intensity, and adverse events (AEs). The median age was 67 years (range, 38–85 years). The median OS and PFS were 8.1 and 3.4 months, respectively. The objective response and disease control rates were 5% and 52%, respectively. The median relative dose intensity was 81.6% for nanoliposomal irinotecan and 82.9% for fluorouracil. Grade 3 or 4 hematological and nonhematological AEs occurred in 47 and 42 patients, respectively. Common grade 3 or 4 AEs included neutropenia (24%), anorexia (12%), and leukocytopenia (12%). Subanalysis of patients treated with second-line and third-or-later-line demonstrated no statistical significant difference in OS (7.6 months vs. 9.1 months, respectively; hazard ratio, 0.92; 95% confidence interval, 0.64–1.35; p = 0.68). In conclusion, NFF has acceptable efficacy and safety profile even in real-world clinical settings. The prospective study is in progress to validate these findings.
Keywords: Nanoliposomal irinotecan, Pancreatic cancer, Second- or later-line, Chemotherapy, Retrospective
Subject terms: Cancer therapy, Pancreatic cancer, Pancreatic cancer, Clinical trial design
Introduction
Pancreatic cancer (PC) is one of the deadliest solid cancers, with a 5-year survival rate of < 10%1–3, although overall survival (OS) has markedly improved over the past two decades. In Japan, approximately 44,000 new cases of PC were reported in 2019, and 37,677 (18,880 men and 18,797 women) deaths were reported in 2020. Both age-specific morbidity and mortality rates of PC have increased, and the overall 5-year (2014–2015) survival rate is approximately 11.8%4, an extremely poor prognosis among malignant tumors.
Most patients with PC have unresectable disease at the time of diagnosis1. These patients are not eligible for surgery and are instead recommended for systemic chemotherapy. Gemcitabine (GEM) was initially the standard first-line chemotherapy regimen for patients with advanced PC5. However, FOLFIRINOX (FFX; a combination of oxaliplatin, leucovorin, irinotecan, and fluorouracil)6 and GEM with nab-paclitaxel (GnP)7 have also shown significant survival benefits in patients with metastases and thus become the most common first-line therapy. Despite these benefits, almost all patients develop disease progression, and the 5-year survival rate remains to be poor1–4. Hence, the demand for second-line chemotherapy has been increasing. However, although there have been several studies on second-line treatment for advanced PC, the benefits of second-line treatment remain limited8–12.
Nanoliposomal irinotecan and fluorouracil with folinic acid (NFF) is currently the standard regimen after GEM-based therapy for unresectable or recurrent PC (urPC) based on the results of the phase 3 (NAPOLI-1)13. In Japan, a randomized phase 2 trial (331,501 study)14 demonstrated significantly better progression-free survival (PFS) with NFF than with fluorouracil with folinic acid (FF) . However, only 40 patients were treated with NFF in the 331,501 study, and thus, we conducted this observational study to evaluate the efficacy and safety of NFF in a real-world setting (NAPOLEON-2 study). We report herein the results of the retrospective part of the NAPOLEON-2 study.
Results
Patient characteristics
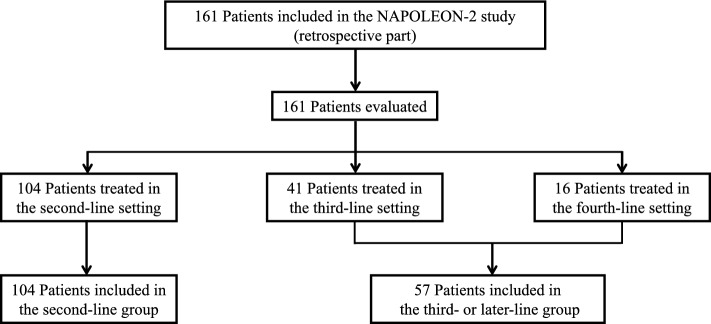
A total of 161 patients with urPC who received NFF treatment were evaluated (Fig. 1). The median follow-up period was 7.3 months (95% confidence interval (CI), 5.6–8.9 months). The patient characteristics are shown in Table 1. The median patient age was 67 years (range, 38–85 years), and 88 patients (55%) were male. All patients had received GEM previously. Overall, 104 (65%) and 57 (35%) patients received NFF as second- or third- or later-line treatment, respectively. The median duration of prior chemotherapy was 9.8 months (range, 1.4–45.0 months). Three patients were homozygous for uridine diphosphate glucuronosyltransferase 1a1 (UGT1A1) *6, two were homozygous for UGT1A1*28, and three were heterozygous for UGT1A128* and *6.
Figure 1.
Flow diagram of the NAPOLEON-2 study (retrospective part).
Table 1.
Patient characteristics (n = 161).
| Characteristic | Value | |
|---|---|---|
| Age, years | Median (range) | 67 (38–85) |
| ≥ 75 years, n (%) | 26 (16) | |
| Sex, n (%) | Male | 88 (55) |
| ECOG PS, n (%) | 0 | 74 (46) |
| 1 | 76 (47) | |
| ≥ 2 | 11 (7) | |
| Stage, n (%) | Locally advanced | 19 (12) |
| Metastatic | 142 (88) | |
| History of pancreatomy, n (%) | 40 (25) | |
| History of radiation, n (%) | 5 (3) | |
| History of biliary drainage, n (%) | 41 (26) | |
| Duration time of previous chemotherapy, months | Median (range) | 9.8 (1.4–45.0) |
| Treatment line of Nal-IRI, n (%) | 2nd | 104 (65) |
| 3rd or later | 57 (35) | |
| Previous drugs, n (%) | Gemcitabine | 161 (100) |
| Fluoropyrimidine | 52 (32) | |
| Platinum | 20 (12) | |
| Irinotecan | 18 (11) | |
| Histology, n (%) | Adenocarcinoma | 148 (91) |
| Others | 7 (4) | |
| Uncertified | 6 (4) | |
| Tumor location, n (%) | Head | 67 (42) |
| Body/tail | 94 (58) | |
| Site of metastasis, n (%) | Liver | 89 (55) |
| Peritoneum | 44 (27) | |
| Ascites, n (%) | None | 137 (85) |
| Intrapelvic | 17 (11) | |
| Extrapelvic | 7 (4) | |
| CA19-9, U/ml | Median (range) | 1015 (1–543,522) |
| ≥ 37 U/ml, n (%) | 130 (81) | |
| NLR | Median (range) | 3.16 (0.48–21.86) |
| ≥ 3.16, n (%) | 81 (50) | |
| CAR | Median (range) | 0.079 (0.003–11.542) |
| ≥ 0.079, n (%) | 79 (49) | |
| UGT1A1, n (%) | Wild | 80 (50) |
| -/*6 or -/*28 | 64 (40) | |
| *6/*6, *6/*28 or *28/*28 | 8 (5) | |
| Unknown | 9 (6) | |
| Complication, n (%) | Any | 94 (58) |
| Diabetes | 48 (30) | |
| Hypertension | 37 (23) | |
| Cardiovascular disease | 10 (6) | |
| Stroke | 7 (4) |
Abbreviations: ECOG PS Eastern Cooperative Oncology Group performance status, Nal-IRI nanoliposomal irinotecan, CA19-9 carbohydrate antigen 19–9, NLR neutrophil-to-lymphocyte ratio, CAR C-reactive protein-albumin ratio, UGT1A1 uridine diphosphate glucuronosyltransferase family 1 member A1.
Treatment outcomes and safety
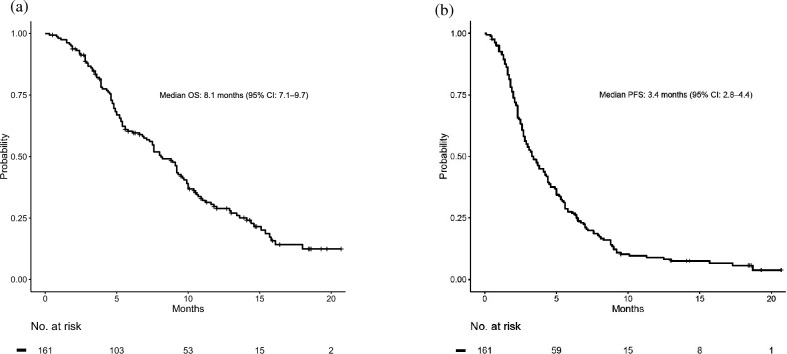
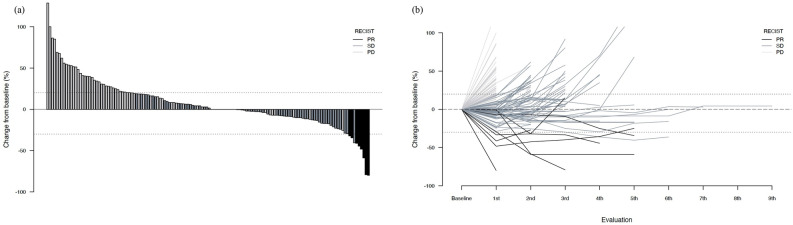
The median OS (mOS) was 8.1 months (95% CI 7.1–9.7 months), and the median PFS (mPFS) was 3.4 months (95% CI 2.8–4.4 months) (Fig. 2). Figure 3a shows the response to treatment by waterfall plot. The objective response rate was 5%, and disease control rate was 52% (Table 2). The spider plot during the clinical course of NFF is shown in Fig. 3b. Table 3 shows the results with respect to treatment intensity. The median number of treatment cycles was 5 (range, 1–38). The initial doses and reasons for dose reduction during treatment are also summarized in Table 3. In total, 104 (65%) patients initially received a full dose of nanoliposomal irinotecan (nal-IRI). The median relative dose intensities (RDIs) for nal-IRI and 5-fluorouracil (5-FU) were 81.6% and 90.7%, respectively. The most common reason for dose-reduction during treatment was myelosuppression, followed by anorexia (Table 3).
Figure 2.
Kaplan–Meier survival curves for nanoliposomal irinotecan and fluorouracil with leucovorin. (a) Overall survival, (b) progression-free survival. OS overall survival, CI confidence interval, PFS progression-free survival.
Figure 3.
The response to treatment for NFF. (a) The response to treatment for NFF by Waterfall plot of maximum tumor shrinkage, (b) spider plot of treatment with NFF. NFF Nanoliposomal irinotecan and fluorouracil with folinic acid, PR partial response, SD stable disease, PD progressive disease.
Table 2.
Response to NFF in all patients (n = 161).
| Best overall response, n (%) | Value |
|---|---|
| CR | 0 |
| PR | 8 (5) |
| SD | 76 (47) |
| PD | 64 (40) |
| NE | 13 (8) |
| ORR (CR + PR), n (%) | 8 (5) |
| DCR (CR + PR + SD), n (%) | 84 (52) |
Abbreviations: NFF Nanoliposomal irinotecan and fluorouracil with folinic acid, CR complete response, PR partial response, SD stable disease, PD progressive disease, ORR overall response rate, DCR disease control rate.
Table 3.
Relative dose intensity and reasons for dose reduction of NFF.
| Starting dose | Nal-IRI | Fluorouracil | |
|---|---|---|---|
| Full dose | n (%) | 104 (65) | 123 (76) |
| Reduction level −1* | n (%) | 51 (32) | 30 (19) |
| Reduction level −2** | n (%) | 6 (4) | 8 (5) |
| Relative dose intensity | Median (range) | 81.6 (53.0–105.7) | 90.7 (40.8–108.3) |
| Treatment cycle | Median (range) | 5 (1–38) | |
| Reasons for dose reduction during treatment, n (%) | |||
| No reduction | 94 (58) | 99 (61) | |
| Neutropenia | 25 (16) | 23 (14) | |
| Anorexia | 17 (11) | 14 (9) | |
| Nausea/vomiting | 7 (4) | 5 (3) | |
| Diarrhea | 3 (2) | 5 (3) | |
| Fatigue | 2 (1) | 2 (1) | |
| Malaise | 2 (1) | 2 (1) | |
| Others | 10 (6) | 10 (6) | |
*Reduction level −1: Nal-IRI 50 mg/m2, fluorouracil 1800 mg/m2.
**Reduction level −2: Nal-IRI 43 mg/m2, fluorouracil 1,350 mg/m2.
For patients homozygous of UGT1A1*6 or UGT1A1*28 or heterozygous of UGT1A1*6 and UGT1A1*28, the reduction level −1 for Nal-IRI is 43 mg/m2, and the reduction level −2 is 35 mg/m2.
Abbreviations: NFF Nanoliposomal irinotecan and fluorouracil with folinic acid, Nal-IRI nanoliposomal irinotecan, UGT1A1 Uridine diphosphate glucuronosyltransferase Family 1 Member A1.
Treatment-related adverse events (AEs) affecting ≥ 10% of patients are summarized in Table 4. The most common AE was anorexia (73%), followed by general malaise (58%), and anemia (53%). Grade ≥ 3 neutropenia occurred in 38 patients (23%). Nonhematologic AEs of grade ≥ 3 included anorexia in 20 patients (12%); nausea in 9 (6%); general malaise, fatigue, and diarrhea in 5 (3%); vomiting and elevated liver enzymes in 2 (1%); and oral mucositis and peripheral neuropathy in 1 patient (1%). Febrile neutropenia was observed in one patient (1%).
Table 4.
All-grade AEs occurring in ≥ 10% of all patients during NFF.
| All grades, n (%) | Grade ≥ 3, n (%) | |
|---|---|---|
| Hematologic | ||
| Neutropenia | 68 (42) | 38 (23) |
| Leukopenia | 66 (41) | 19 (12) |
| Anemia | 86 (53) | 15 (9) |
| Thrombocytopenia | 20 (12) | 2 (1) |
| Nonhematologic | ||
| Anorexia | 118 (73) | 20 (12) |
| Malaise | 94 (58) | 5 (3) |
| Fatigue | 79 (49) | 5 (3) |
| Nausea | 68 (42) | 9 (6) |
| Vomiting | 32 (20) | 2 (1) |
| Diarrhea | 51 (32) | 5 (3) |
| Constipation | 50 (31) | 0 (0) |
| Liver enzyme elevation | 41 (25) | 2 (1) |
| Peripheral sensory neuropathy | 63 (39) | 1 (1) |
| Oral mucositis | 20 (12) | 1 (1) |
| Alopecia | 36 (22) | 0 (0) |
Abbreviations: AE Adverse Event, NFF Nanoliposomal irinotecan and fluorouracil with folinic acid.
Multivariate analysis using the Cox regression analysis identified that shorter duration of previous chemotherapy, treatment line of NFF, ascites, higher neutrophil-to-lymphocyte ratio (NLR), and high C-reactive protein-to-albumin ratio were independent predictors of survival (Supplemental Table 1). At the final follow-up, 12 (7%) patients continued NFF treatment, and 1 patient (1%) withdrew due to transfer. NFF was discontinued in 148 patients (92%) due to worsening disease (n = 133), AEs (n = 13), and patient request (n = 2). Of them, 95 patients received best supportive care, and 53 patients did post-treatment.
Additional analysis
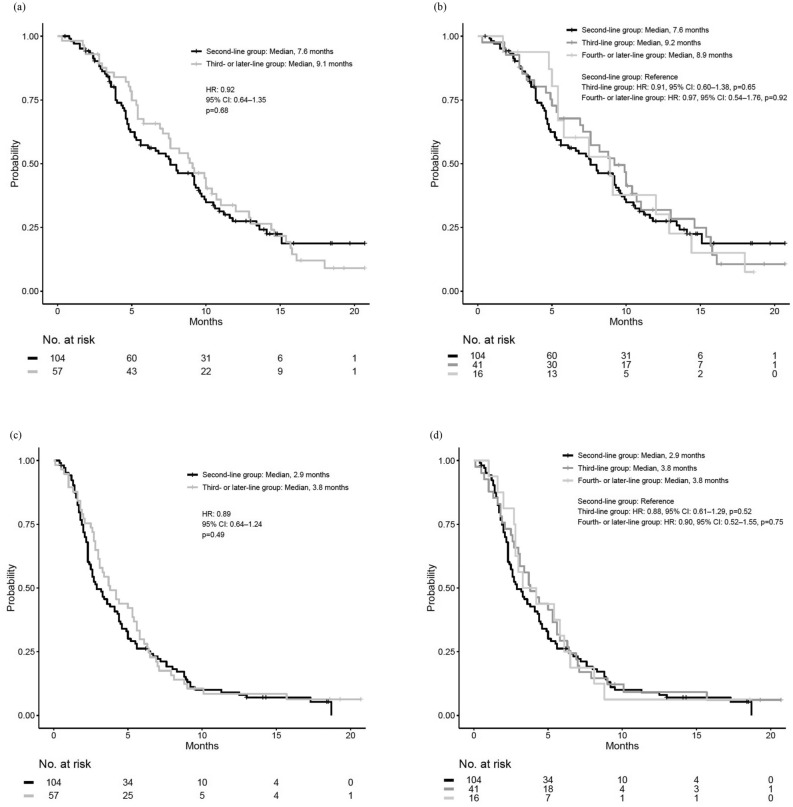
We categorized patients into two groups as the (a) second-line treatment group and (b) third-or-later-line treatment group (Fig. 1) and compared their clinical outcomes. The patient characteristics for this analysis are shown in Supplemental Table 2; there were significant differences in the groups. The median OS values of the second-line group and the third- or later-line group were 7.6 and 9.1 months, respectively (hazard ratio [HR]: 0.92; 95% CI 0.64–1.35; p = 0.68; Fig. 4a). For PFS, the median values of the second-line group and third- or later-line group were 2.9 and 3.8 months, respectively (HR: 0.89; 95% CI 0.64–1.24; p = 0.49; Fig. 4c). The objective response rates and disease control rates were not significantly different between the groups (Supplemental Table 3). The rate of treatment-related AEs affecting ≥ 5% of patients were also not significantly different between the two groups (Supplemental Table 4). Although the starting dose and minimum dose of fluorouracil tended to be lower in the third- or later-line group, those of nal-IRI were not significantly different (Supplemental Table 5).
Figure 4.
Kaplan–Meier survival curves of nanoliposomal irinotecan and fluorouracil with leucovorin in the second-line and third- or later-line groups. (a) Comparison of overall survival between the second-line and the third- or later-line groups, (b) Comparison of overall survival among the second-, third-, and fourth-or-later line groups, (c) Comparison of progression-free survival between the second-line and the third-or later-line groups, (d) Comparison of progression-free survival among the second-, third-, and fourth-or-later line groups. HR hazard ratio, CI confidence interval.
In the third-or-later-line group, 41 and 16 patients were administered NFF as third- and fourth-or-later-line treatment, respectively (Supplemental Table 2). The median OS values in the second-line, third-line, and fourth-or-later-line treatment groups were 7.6, 9.2, and 8.9 months, respectively (Fig. 4b), and the median PFS values were 2.9, 3.8, and 3.8 months, respectively (Fig. 4d). Compared with the second-line group, the third-line and fourth-or-later-line groups did not show significant differences in both PFS (vs third-line group: HR: 0.88, 95% CI 0.61–1.29, p = 0.52; vs fourth- or later-line group: HR: 0.90, 95% CI 0.52–1.55, p = 0.75) and OS (vs third-line group: HR: 0.91, 95% CI 0.60–1.38, p = 0.65; vs fourth-or-later line group: HR: 0.97, 95% CI 0.54–1.76, p = 0.92). Additionally, in the third-or-later-line group, 18 patients received NFF after undergoing treatment with an irinotecan containing regimen, whereas 39 received NFF without prior exposure to an irinotecan containing regimen. The mOS values were 9.2 and 8.9 months, respectively (Supplementary Fig. 1a), and the median PFS values were 2.8 and 5.0 months, respectively (Supplementary Fig. 1b). No significant differences were observed in either OS and PFS. Patient characteristics and response to NFF are presented in Supplementary Table 6 and 7.
Discussion
There have been limited clinical data on the usefulness and safety of NFF in the real-world setting. In the current study, the median OS and PFS of NFF were 8.1 and 3.4 months, respectively, which were more favorable than those reported in NAPOLI-1. The NAPOLI-1 study was the first prospective trial to demonstrate the efficacy of NFF for metastatic PC patients with refractory to GEM-based therapy13. The National Comprehensive Cancer Network guidelines also recommend NFF as a Category 1 regimen for second-or-later treatment and for recurrent cases15. In addition, NFF was generally well tolerated without any unexpected adverse events in the practical clinical setting. The median observation period was 7.3 months, which is shorter than the mOS in this study, but we consider this may have been influenced by the fact that the mOS for confirmed deaths was 5.6 months.
In the real-world application of regimens that have proven efficacy and safety in clinical trials, efficacy16 and treatment intensity are often reduced for safety reasons17. This is because clinical trials usually involve patients who are relatively young, in good general health, free from serious complications, and different from those in clinical practice18. The median age in the current study was higher than that in the NAPOLI-1 study (67 years vs. 63 years), and the proportion of patients with ECOG PS ≥ 1 was also higher (54% vs. 41%), However, we consider that these characteristics reflect the real-world. The proportions of patients with prior 5-FU and platinum were also lower, but there was no significant difference when compared with other clinical trials13. In addition, there was no significant difference in patient backgrounds compared to other real-world clinical observational studies19–30. Therefore, our study cohort is reasonable and similar with those in previous reports. The current sample size was 161 patients, and there have been a few reports presenting the efficacy of NFF with > 150 patients; thus, we consider that the study results are meaningful.
The mOS was 8.1 months, longer than that of the NAPOLI-1 study (median, 6.1 months) and of many retrospective and prospective studies19–30. This can be attributed to several reasons. First, the proportion of patients who had already received 5-FU in this study was slightly lower than that in others, and this might have contributed to a longer survival. Second, our study included some patients with locally advanced PC in the daily practice. Third, the proportion of patients receiving post-nal-IRI treatment was relatively higher (36%) than that in the NAPOLI-1 study (31%)13. Finally, the rate of AE-related treatment discontinuation was not high (8%) when compared with that in the NAPOLI-1 study (11%), and this might also have influenced the better OS. Given that AE is an important factor for continuing with subsequent chemotherapy, adequate management of AEs might help improve prognosis. In this aspect, approximately 40% of patients in the current study underwent dose reductions to reduce AEs. Dose reductions reportedly does not impact survival22,25, suggesting that appropriate dose modifications should be considered for longer-term continuation of treatment. We will also report the sub-analysis data on the RDI and dose modification in the future.
This study also compared the efficacy of NFF among the different treatment lines. There were no significant differences in PFS, OS, and safety between the second-line group and third-or-later-line groups, although some significant differences in patient backgrounds were observed. The third-or-later-line groups had significantly higher rates of prior treatments with FU, platinum, and irinotecan than had the second-line group; however, the third-or-later-line groups also included significantly younger patients and had lower rates of postoperative recurrence and liver metastases. Patients with postoperative recurrent PC have favorable prognosis, while liver metastases patients have poor prognosis31–33. Our study suggested that NFF might remain effective and safe even in the third-or-later-line setting if the general condition of the patients make them eligible for NFF23,26.
The safety profile of NFF in the current study appears acceptable in comparison with that in previous reports19–30. The incidence rates of grade 3 or 4 AEs were < 30%. Furthermore, grade 5 AEs were not observed. The most common grade 3 or 4 AEs was neutropenia (24%); however, febrile neutropenia occurred only in one patient (1%). Meanwhile, although grade 3 or 4 anorexia was only observed in 12% of the patients, this rate might still be slightly high. It is possible that the RDIs of nal-IRI and FU were higher than those in other reports13,30. Meanwhile, our study cohort included more patients who had undergone prior biliary drainage (26%) compared with the NAPOLI-1 trial cohort (13%)13. Our results suggest that NFF might be a safe treatment even in patients with prior biliary drainage, although further investigation is needed. In the subgroup analysis, the toxicity profile did not significantly differ between the second-line and third-or-later-line groups.
There were several limitations in this study. First, this is a retrospective observational study, which might have introduced selection bias and some difficulties in accurately assessing safety profiles. Therefore, we are now conducting a prospective study in another cohort, and the efficacy and safety will be validated. Second, this study included some patients who were clinically diagnosed with PC without histopathologic confirmation. Although histopathological diagnosis is recommended for patients undergoing chemotherapy34, there are some patients whose chemotherapy is initiated based on biochemical and/or radiological features only because of the difficult anatomical position of the pancreas or because of a clinical emergency. Third, NFF was administered for patients in both the second-line and third-or-later-line settings. Given that NFF is generally indicated for cases refractory to GEM, the treatment line of NFF in actual clinical practice is heterogeneous. However, we conducted a subgroup analysis and confirmed the safety and efficacy in the both the second-line and third-or-later-line settings.
In conclusion, NFF for PC is effective and safe in the second- and third-or later-line setting in the real-world. Our study is probably the most realistic observational study to date, as NFF was administered in patients with varying pretreatment histories, and efficacy and safety were retrospectively evaluated in a relatively large population.
Methods
Study design
The NAPOLEON-2 was a multicenter observational study comprised of a retrospective part and a prospective part to investigate the efficacy and safety of NFF as evaluated by specialists in gastroenterology and medical oncologists in Japan. The study population involved patients with urPC treated with NFF after previous GEM-based therapy. In the retrospective part, we reviewed the medical records of consecutive patients who received NFF for urPC at any of the 20 study hospitals between June 2020 and May 2021. Data on patient characteristics, treatment efficacy, and adverse events (AEs) were analyzed. Additionally, the survival efficacies of NFF were compared according to the treatment line, that is, between second-line and third- or later-line, and between after irinotecan containing regimen and not containing in third- or later-line.
This study was approved by each participating institution’s review board or ethics committee and was conducted according to the Declaration of Helsinki. Because this study was a retrospective observational study carried out in Japan, informed consent was obtained using the opt-in/opt-out approach according to each participating institution’s policy.
NFF protocol
NFF was administered as follows: a 90-min intravenous (i.v.) infusion of nal-IRI (70 mg/m2), 46-h continuous i.v. infusion of 5-FU (2400 mg/m2), and 2-h i.v. infusion of levofolinate (200 mg/m2) every 2 weeks. At the discretion of the physician in charge, dose reduction at initiation and dose modification during treatment due to toxicities were allowed. Treatment was discontinued when disease progression or unacceptable AEs occurred even with dose adjustment, or at the patient’s request. Continuation of NFF treatment regimen after disease progression was permitted if deemed feasible by the physician.
Assessments
The main endpoint of the retrospective part was OS. Other endpoints included the objective response rate, disease control rate, PFS, dose intensity, and AEs. Objective response and disease control were evaluated by computed tomography or magnetic resonance imaging according to the Response Evaluation Criteria in Solid Tumors (RECIST, version 1.135). An objective response was defined as a complete or partial response, and disease control was defined as a complete or partial response with stable disease as the best response. OS was calculated from the date of administration of NFF to the date of death from any cause, or was censored at the final follow-up examination.
PFS was calculated from the date of administration of NFF to the date of progression or death from any cause, whichever was earlier, or was censored at the final follow-up examination. Treatment-related AEs were assessed according to the Common Terminology Criteria for Adverse Events, version 5.036. Patient characteristics during NFF treatment were evaluated; these included age, sex, Eastern Cooperative Oncology Group performance status (ECOG PS), stage, medical history, previous chemotherapy information, histology, primary tumor site, metastatic site, ascites, carbohydrate antigen 19–9, NLR, C-reactive protein to albumin ratio, and UGT1A1 status.
Statistical analyses
The proportions and antitumor effects were compared using the Mann–Whitney test for continuous data and the χ2 test for categorical data. PFS and OS were estimated using the Kaplan–Meier method, and the probability of survival were compared using the log-rank test and the Cox proportional hazards model. Univariate and multivariate Cox proportional hazards models were used at the start of NFF treatment. The HR was expressed with 95% CI. Statistical significance was set at p < 0.05. All statistical analyses were performed using R version 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria).
Ethical approval and consent to participate
This study was conducted in accordance with the ethical guideline of the Declaration of Helsinki and was centrally approved by the Institutional review board of Sasebo Kyosai Hospital (study ID 2021–08), and also approved by the Institutional Review Boards or Ethics Committee of following institutions: Kagoshima City Hospital, National Cancer Center Hospital East, Kagoshima University Hospital, Kurume University Hospital, Saga Medical Center Koseikan, Japanese Red Cross Kumamoto Hospital, Karatsu Red Cross Hospital, Japan Community Healthcare Organization Kyushu Hospital, Japanese Red Cross Nagasaki Genbaku Hospital, Oita University Hospital, University of Miyazaki Hospital, National Hospital Organization Kumamoto Medical Center, Saiseikai Kumamoto Hospital, Kagoshima Kouseiren Hospital, Miyazaki Prefectural Miyazaki Hospital, Nagasaki University Hospital, Izumi General Medical Center, Asakura Medical Association Hospital, and Saiseikai Sendai Hospital prior to the study. Because this study was a retrospective observational study carried out in Japan, informed consent was obtained using the opt-in/opt-out approach according to each participating institution’s policy.
Supplementary Information
Acknowledgements
We thank all the patients and their families, and all of the investigators at the 20 institutions that participated in the NAPOLEON 2 study. We would also like to thank the Saga Study Group of Liver Disease (SASLD) for their cooperation. We are indebted to clinical research coordinators and medical office assistants of the NAPOLEON study group for their assistance in the data collection. We also thank Editage for providing medical writing support.
Author contributions
1. T.S., T.O., M.S., T.M., and K.M. created the study concept. 2. T.S., T.O., M.S., J.N., T.S., S.A., F.K., T.M., and K.M. designed the study. 3. T.K., T.I., M.K., J.N., T.H., T.S., S.A., A.I., K.M., Y.O., F.K., Y.U., Y.K., H.S., S.T., K.N., A.K., S.O., A.H., T.S., K.S., H.O., M.K., S.A., T.H., H.T., K.T., Y.K., T.F., T.S., and K.M. acquired the clinical data. 4. T.S., T.O., M.S., and K.M. were involved in the quality control of the data and algorithms. 5. T.K., T.S., T.O., M.S., T.M., and K.M. were involved in the data analysis and interpretation. 6. T.K., T.S., T.O., M.S., and K.M. performed statistical analysis. 7. T.K. prepared the manuscript, and it was edited by T.S., T.O., M.S., T.H., A.I., T.M., and K.M. 8. The manuscript was critically reviewed by all authors, and they approved the final version. The requirement for consent for publication was obtained using the opt-in/opt-out approach according to each participating institution’s policy.
Data availability
All data generated or analyzed in this study are stored in a secured research database. Although they are not publicly available, they are available through the corresponding author upon reasonable request.
Code availability
We used R ver. 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria) for statistical analyses.
Competing interests
SO received a personal fee from IQVIA services Japan. The remaining authors have no competing interests or financial disclosures to declare.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-63172-y.
References
- 1.Sung H, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Egawa S, et al. Japan pancreatic cancer registry; 30th year anniversary: Japan pancreas society. Pancreas. 2012;41:985–992. doi: 10.1097/MPA.0b013e318258055c. [DOI] [PubMed] [Google Scholar]
- 3.Vincent A, et al. Pancreatic cancer. Lancet. 2011;378:607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Editorial Board of the Cancer Statistics in Japan: Cancer Statistics in Japan-2019. Foundation for promotion of cancer research (FPCR) 2020.
- 5.Burris HA, III, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J. Clin. Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 6.Conroy T, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 7.Von Hoff DD, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rahma OE, et al. Second-line treatment in advanced pancreatic cancer: A comprehensive analysis of published clinical trials. Ann. Oncol. 2013;24:1972–1979. doi: 10.1093/annonc/mdt166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pelzer U, et al. Best supportive care (BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC in patients for second-line advanced pancreatic cancer: A phase III-study from the German CONKO-study group. Eur. J. Cancer. 2011;47:1676–1681. doi: 10.1016/j.ejca.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Oettle H, et al. Second-line oxaliplatin, folinic acid, and fluorouracil versus folinic acid and fluorouracil alone for gemcitabine-refractory pancreatic cancer: Outcomes from the CONKO-003 Trial. J. Clin. Oncol. 2014;32:2423–2429. doi: 10.1200/JCO.2013.53.6995. [DOI] [PubMed] [Google Scholar]
- 11.Gill S, et al. PANCREOX: A randomized phase III study of fluorouracil/leucovorin with or without oxaliplatin for second-line advanced pancreatic cancer in patients who have received gemcitabine-based chemotherapy. J. Clin. Oncol. 2016;34:3914–3920. doi: 10.1200/JCO.2016.68.5776. [DOI] [PubMed] [Google Scholar]
- 12.Ioka T, et al. TAS-118 (S-1 plus leucovorin) versus S-1 in patients with gemcitabine-refractory advanced pancreatic cancer: A randomised, open-label, phase 3 study (GRAPE trial) Eur. J. Cancer. 2019;106:78–88. doi: 10.1016/j.ejca.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Wang-Gillam A, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): A global, randomised, open-label, phase 3 trial. Lancet. 2016;387:545–557. doi: 10.1016/S0140-6736(15)00986-1. [DOI] [PubMed] [Google Scholar]
- 14.Ueno M, et al. nal-IRI+5-FU/LV versus 5-FU/LV in post-gemcitabine metastatic pancreatic cancer: Randomized phase 2 trial in Japanese patients. Cancer Med. 2020;9:9396–9408. doi: 10.1002/cam4.3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Margaret AT, et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2021;19:439–457. doi: 10.6004/jnccn.2021.0017. [DOI] [PubMed] [Google Scholar]
- 16.Otsuka T, et al. A multicenter propensity score analysis of FOLFIRINOX vs gemcitabine plus nab-paclitaxel administered to patients with metastatic pancreatic cancer: Results from the NAPOLEON study. Int. J. Clin. Oncol. 2021;26:941–950. doi: 10.1007/s10147-021-01859-2. [DOI] [PubMed] [Google Scholar]
- 17.Hatashima A, et al. First-line gemcitabine plus nab-paclitaxel versus FOLFIRINOX for metastatic pancreatic cancer in a real-world population. Future Oncol. 2022;18:2521–2532. doi: 10.2217/fon-2021-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ford JG, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: A systematic review. Cancer. 2008;112:228–242. doi: 10.1002/cncr.23157. [DOI] [PubMed] [Google Scholar]
- 19.Procaccio L, et al. The role of nanoliposomal irinotecan plus fluorouracil/leucovorin in the continuum of care of patients with metastatic pancreatic ductal adenocarcinoma. Cancer Med. 2023;12:14337–14345. doi: 10.1002/cam4.6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kieler M, et al. A real-world analysis of second-line treatment options in pancreatic cancer: Liposomal-irinotecan plus 5-fluorouracil and folinic acid. Ther. Adv. Med. Oncol. 2019;11:1758835919853196. doi: 10.1177/1758835919853196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoo C, et al. Real-world efficacy and safety of liposomal irinotecan plus fluorouracil/leucovorin in patients with metastatic pancreatic adenocarcinoma: A study by the Korean Cancer Study Group. Ther. Adv. Med. Oncol. 2019;11:1758835919871126. doi: 10.1177/1758835919871126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu KH, et al. Clinical outcomes among patients with metastatic pancreatic ductal adenocarcinoma treated with liposomal irinotecan. Front. Oncol. 2021;11:678070. doi: 10.3389/fonc.2021.678070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glassman DC, et al. Nanoliposomal irinotecan with fluorouracil for the treatment of advanced pancreatic cancer, a single institution experience. BMC Cancer. 2018;18:693. doi: 10.1186/s12885-018-4605-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kasi A, et al. Efficacy and tolerability of the combination of nano-liposomal irinotecan and 5-fluorouracil/leucovorin in advanced pancreatic adenocarcinoma: Post-approval clinic experience. J. Gastrointest. Oncol. 2021;12:464–473. doi: 10.21037/jgo-20-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barzi A, et al. Real-world dosing patterns and outcomes of patients with metastatic pancreatic cancer treated with a liposomal irinotecan regimen in the United States. Pancreas. 2020;49:193–200. doi: 10.1097/MPA.0000000000001479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park SJ, et al. Nanoliposomal irinotecan plus fluorouracil and folinic acid as a second-line treatment option in patients with metastatic pancreatic ductal adenocarcinoma: A retrospective cohort study. BMC Cancer. 2021;21:1176. doi: 10.1186/s12885-021-08887-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chun JW, et al. A real-world analysis of nanoliposomal-irinotecan with 5-fluorouracil and folinic acid as third- or later-line therapy in patients with metastatic pancreatic adenocarcinoma. Ther. Adv. Med. Oncol. 2022;14:17588359221119539. doi: 10.1177/17588359221119539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frampton JE. Liposomal irinotecan: A review in metastatic pancreatic adenocarcinoma. Drugs. 2020;80:1007–1018. doi: 10.1007/s40265-020-01336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tezuka S, et al. Nal-IRI/5-FU/LV versus modified FOLFIRINOX and FOLFIRI as second-line chemotherapy for unresectable pancreatic cancer: A single center retrospective study. Pancreatology. 2022;22:789–796. doi: 10.1016/j.pan.2022.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Miki M, et al. Treatment effect and safety of nanoliposomal irinotecan with fluorouracil and folinic acid after gemcitabine-based therapy in patients with advanced pancreatic cancer: A multicenter prospective observational study. J. Clin. Med. 2022;11:5084. doi: 10.3390/jcm11175084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taguchi H, et al. Gemcitabine plus nanoparticle albumin-bound paclitaxel versus FOLFIRINOX for recurrent pancreatic cancer after resection. Anticancer Res. 2021;41:3573–3582. doi: 10.21873/anticanres.15145. [DOI] [PubMed] [Google Scholar]
- 32.Shibuki T, et al. Prognostic nomogram for patients with unresectable pancreatic cancer treated with gemcitabine plus nab–paclitaxel or FOLFIRINOX: A post hoc analysis of a multicenter retrospective study in Japan (NAPOLEON study) BMC Cancer. 2022;22:19. doi: 10.1186/s12885-021-09139-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Engstrand J, et al. Colorectal cancer liver metastases - a population-based study on incidence, management and survival. BMC Cancer. 2018;18:78. doi: 10.1186/s12885-017-3925-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ducreux M, et al. Cancer of the pancreas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015;26:v56–v68. doi: 10.1093/annonc/mdv295. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz LH, et al. RECIST 1.1—Standardisation and disease-specific adaptations: Perspectives from the RECIST Working Group. Eur. J. Cancer. 2016;62:138–45. doi: 10.1016/j.ejca.2016.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Common Terminology Criteria for Adverse Events (CTCAE) Version 5. Published: November 27. US Department of Health and Human Services, National Institutes of Health, National Cancer Institute (2017).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed in this study are stored in a secured research database. Although they are not publicly available, they are available through the corresponding author upon reasonable request.
We used R ver. 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria) for statistical analyses.