Abstract
Atrial fibrosis serves as an arrhythmogenic substrate in atrial fibrillation (AF) and contributes to AF persistence. Treating atrial fibrosis is challenging because atrial fibroblast activity is multifactorial. We hypothesized that the primary cilium regulates the profibrotic response of AF atrial fibroblasts, and explored therapeutic potentials of targeting primary cilia to treat fibrosis in AF. We included 25 patients without AF (non-AF) and 26 persistent AF patients (AF). Immunohistochemistry using a subset of the patients (non-AF: n = 10, AF: n = 10) showed less ciliated fibroblasts in AF versus non-AF. Acetylated α-tubulin protein levels were decreased in AF, while the gene expressions of AURKA and NEDD9 were highly increased in AF patients’ left atrium. Loss of primary cilia in human atrial fibroblasts through IFT88 knockdown enhanced expression of ECM genes, including FN1 and COL1A1. Remarkably, restoration or elongation of primary cilia by an AURKA selective inhibitor or lithium chloride, respectively, prevented the increased expression of ECM genes induced by different profibrotic cytokines in atrial fibroblasts of AF patients. Our data reveal a novel mechanism underlying fibrotic substrate formation via primary cilia loss in AF atrial fibroblasts and suggest a therapeutic potential for abrogating atrial fibrosis by restoring primary cilia.
Subject terms: Cell biology, Molecular biology
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia associated with a five-fold increased risk of stroke1,2 and heart failure3, and has been associated with a substantial deterioration of patients’ quality of life4. AF is an independent risk factor of all-cause mortality, increasing it by 1.5–2 fold5. The prevalence of AF is projected to increase in the next decades6. In 2010, the number of patients suffering from AF was estimated at approximately 8.8 million in the European Union alone7 and 33.5 million worldwide8. Antiarrhythmic drugs (AADs) and catheter or surgical ablation may be employed to restore sinus rhythm, but these remain modestly successful9–12.
Atrial fibrosis promotes both ectopy and conduction abnormalities13,14 and serves as an essential substrate that sustains and perpetuates AF. As a result, atrial fibrosis is a predictor of poor outcomes of ablative and pharmacologic rhythm control strategies for AF15–17. At the moment, attempts to prevent or reduce atrial fibrosis by interfering with profibrotic pathways, such as the renin–angiotensin–aldosterone system using angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, have been only partially effective18,19. An explanation of the limited efficacy of current strategies to treat fibrosis may lie in targeting a specific profibrotic pathway rather than targeting the process of fibrosis formation as a whole.
Atrial fibrosis results from the aberrant accumulation of extracellular matrix (ECM). ECM is a highly dynamic structure that is constantly remodeled through the deposition of ECM proteins such as collagens and degradation by matrix metalloproteases20. Fibroblast activation is key in both processes. A dynamic ECM balanced by the activities of fibroblasts is necessary for tissue plasticity and integrity20. However, in a pathological condition such as AF, fibroblasts undergo increased proliferation and differentiation into α-smooth muscle actin (αSMA)-expressing myofibroblasts21, which causes discord in ECM dynamics, ultimately culminating in interstitial fibrosis within the atria. Targeting fibroblast activation is challenging because it can be induced by various stimuli within intricate biological processes involving a large number of proteins22. Importantly, our knowledge of what drives fibroblasts to translate the wide variety of stimuli into an exaggerated fibrotic response in AF is limited.
The primary cilium is a cellular sensory organelle composed of acetylated microtubules that exists in nearly all mammalian cells, including human atrial fibroblasts (Fig. 1a, white box)23,24. It protrudes from the cellular membrane into the extracellular space and functions as a signaling hub that transduces external mechanical and chemical cues into intracellular signals25–28. The primary cilium appears only at the G0 phase29,30, whereas its assembly is systematically restricted during the growth phase23,30. This dynamic morphology qualifies the primary cilium to serve as a spatiotemporal sensor that fine-tunes transduction from the external milieu into the cell31. Accordingly, loss of the primary cilium induces abnormal proliferation24 and affects various signal transduction, including renin-angiotensin32 and TGF-β1 signaling33, both of which are crucial for fibrosis formation in AF34. Genetic mutations affecting primary cilia induce fibrosis in multiple organs, including the liver and kidneys35. Furthermore, aberrant shortening of the primary cilium has been linked to age-related acquired diseases, such as cancers36 and atherosclerosis37.
Figure 1.
The proportion of fibroblasts with primary cilia is significantly decreased in the left atrial tissue of AF patients possibly via the NEDD9/AURKA/HDAC6 axis. (a) A representative morphology of primary cilium projecting from the apical surface of a cultured fibroblast isolated from the left atrial tissue of a non-AF patient. Red: ac α-tub (acetylated α-tubulin, primary cilium), green: vimentin (intermediate cytoskeleton), blue: DAPI (nucleus). Fibroblasts were starved for 48 h to promote the formation of primary cilia. Scale bar, 25 μm. **p < 0.01 (Student’s unpaired t-test). (b) Representative images of immunohistochemistry performed on the cryosections of left atrial tissue from the non-AF and AF patients. Arrow heads and a void arrowhead indicate the primary cilia of vimentin positive and vimentin negative cells, respectively. Scale bar, 10 μm. (c) The proportion of fibroblasts with primary cilia (%) in the left atrial tissue of non-AF and AF patients. Approximately 300–500 cells/patient were counted. **p < 0.01 (Student’s unpaired t-test). (d) Protein levels of ac α-tub and HDAC6 in the left atrial tissue of non-AF and AF patients. GAPDH serves as a loading control. **p < 0.01 (Mann–Whitney U test). (e) The protein levels of α-tubulin in the left atrial tissue of non-AF and AF patients. N = 6/group. (f) The protein levels of ac α-tub in the fibroblast fraction isolated from left atrial tissue of non-AF and AF patients. The values at the lowest row show the band density of ac α-tub relative to GAPDH in each lane. (g, h) The gene expression of AURKA (g) and NEDD9 (h) in the left atrial tissue of non-AF and AF patients. HPRT serves as an internal control. **p < 0.01, ***p < 0.001 (Mann–Whitney U test).
Key proteins regulate ciliary formation and assembly. The interaction between aurora kinase A (AURKA) and neural precursor cell expressed developmentally down-regulated protein 9 (NEDD9) phosphorylates and activates histone deacetylase 6 (HDAC6)38. HDAC6 then deacetylases acetylated α-tubulin, leading to a rapid collapse of ciliary axoneme38,39. This leads to primary cilia disassembly. A concomitant increase in HDAC6 activity and reduction in acetylated α-tubulin was indeed reported in the left atrial tissue of AF patients40. Furthermore, our recent transcriptomic study performed on the left atrial tissue from patients with or without AF revealed that genes relevant to primary cilia assembly were downregulated (e.g., a number of intraflagellar transport genes) while disassembly genes were upregulated (e.g., NEDD9 and LIMK2) in AF compared to non-AF patients41. Conversely, the presence of primary cilia in cardiac fibroblasts was suggested to contribute to fibrosis formation in infarcted myocardium42.
We hypothesized that the disassembly of primary cilia in fibroblasts is the central link between external profibrotic stimuli and the formation of fibrosis in AF. Therefore, we examined the function of primary cilia in respect to the profibrotic capacity of atrial fibroblasts from patients with AF.
Results
Loss of primary cilia is observed in the atrial tissue of AF patients
In this study, we included patients with persistent AF43 (AF: n = 26) or without a history of AF44,45 (non-AF: n = 25) (Table 1). The tissue from a subgroup of 10 AF patients and 10 non-AF patients was used for immunohistochemical analysis (Supplemental Table 1). The two cohorts were matched in terms of gender and BMI. AF patients were relatively younger than non-AF patients (65.8 ± 9.1 vs. 70.8 ± 7.5, p = 0.04) (Table 1) in the entire cohort, but the age of subgroup cohorts for immunohistochemical analysis was matched (69.2 ± 6.6 vs. 72.5 ± 6.4, p = 0.27) (Supplemental Table 1). The major differences between the AF and non-AF cohorts were the presence of vascular disease and medication (Table 1 and Supplemental Table 1).
Table 1.
Clinical characteristics of the patients enrolled in this study.
| Non-AF (n = 25) | Persistent AF (n = 26) | p-value | |
|---|---|---|---|
| Surgery type | |||
| VATS PVI | – | 26 (100) | NA |
| CABG | 22 (88) | – | NA |
| Aortic valve | 5 (20) | – | NA |
| CABG+valve | 4 (16) | – | NA |
| Bentall | 2 (8) | – | NA |
| Baseline | |||
| Sex, male, n(%) | 18 (72) | 19 (73) | 1 |
| Age, years (± s.d.) | 70.8 ± 7.5 | 65.8 ± 9.1 | 0.04 |
| AF duration, years [IQ] | – | 3.0 [1.3–5.0] | NA |
| Previous catheter PVI, n(%) | – | 2 (8) | NA |
| BMI, kg/m2 (± s.d.) | 27.8 ± 2.5 | 28.1 ± 4.2 | 0.753 |
| Creatinine, μl/l (± s.d.) | 85.0 ± 24.6 | 87.5 ± 22.2 | 0.72 |
| CHA2DS2-VASc [IQ] | 3 [2-4] | 1 [1-2] | < 0.001 |
| Vascular disease, n(%) | 22 (88) | 2 (8) | < 0.001 |
| Previous PCI, n(%) | 6 (24) | 0 (0) | 0.026 |
| Myocardial infarction, n(%) | 9 (36) | 1 (4) | 0.011 |
| Hypertension, n(%) | 15 (60) | 12 (46) | 0.478 |
| Diabetes Mellitus, n(%) | 5 (20) | 2 (8) | 0.384 |
| Stroke/TIA/embolus, n(%) | 5 (20) | 3 (12) | 0.656 |
| Medication | |||
| NOAC/vitK antagonist, n(%) | 0 (0) | 26 (100) | < 0.001 |
| Antiplatelet, n(%) | 22 (88) | 1 (4) | < 0.001 |
| Class IA AAD, n(%) | 0 (0) | 1 (4) | 1 |
| Class IC AAD, n(%) | 0 (0) | 7 (27) | 0.017 |
| Class II AAD, n(%) | 19 (76) | 11 (42) | 0.031 |
| Class III AAD, n(%) | 1 (4) | 8 (31) | 0.032 |
| Class IV AAD, n(%) | 0 (0) | 4 (15) | 0.128 |
| Digoxin, n(%) | 0 (0) | 5 (19) | 0.066 |
| ACE inhibitor, n(%) | 9 (36) | 7 (27) | 0.692 |
| Angiotensin II blocker, n(%) | 5 (20) | 6 (23) | 1 |
VATS PVI, video assisted thoracoscopic pulmonary vein isolation; CABG, coronary artery bypass grafting; BMI, body mass index; PCI, percutaneous coronary intervention; TIA, transient ischemic attack; NOAC, non-vitamin K antagonist oral anticoagulants;AAD, antiarrhythmic drugs. The categorical variables (eg. sex, CHA2DS2-VASc, the presence of comorbidities and medication) were compared by Chi-squared test. The continuous variables (eg. age, BMI and creatinine) were compared by Mann–Whitney U test.
Immunostaining of acetylated α-tubulin performed on the subgroup demonstrated a lower proportion of vimentin-positive cells (which are mostly fibroblasts) with the signal of acetylated α-tubulin in the left atrial tissue of AF compared to non-AF patients (Fig. 1b,c). Consistent with this observation, the protein levels of acetylated α-tubulin were dramatically decreased in the left atrial tissue of AF patients compared to non-AF patients (Fig. 1d). The total α-tubulin protein levels were equal between non-AF and AF patients (Fig. 1e). This, in combination with the markedly decreased levels of acetylated α-tubulin, indicates that the acetylation levels but not expression levels of α-tubulin were decreased in AF patients. Importantly, the protein levels of acetylated α-tubulin were also reduced in the fibroblast fraction isolated from left atrial tissue of AF patients compared to non-AF patients (Fig. 1f), suggesting that the loss of primary cilia may occur primarily in the fibroblast fraction. The proportion of vimentin-negative cells (mostly cardiomyocytes) with primary cilia did not significantly differ between the groups (Supplemental Fig. 1c).
The activation of NEDD9/AURKA/HDAC6 in AF is potentially responsible for the loss of cilia
The protein levels of HDAC6 were equivalent between AF and non-AF patients (Fig. 1d). However, the gene expression of AURKA and NEDD9 was significantly upregulated in AF (Fig. 1e,f), suggesting that the activity of HDAC6 is enhanced in AF as was previously reported40. Pitchfork (PIFO) and trichoplein keratin binding (TCHP) are also reported to activate AURKA24,46, but their gene expression was barely altered between AF and non-AF (Supplemental Fig. 1d,e). These results indicate that loss of the primary cilia in the atrial fibroblasts may be induced by the activated NEDD9/AURKA/HDAC6 axis in AF.
Loss of the primary cilia increases the profibrotic capacity of human atrial fibroblasts
Next, we examined the consequences of loss of the primary cilia in human atrial fibroblasts. We observed a trend toward a decrease in gene expression of intraflagellar transport homolog 88 (IFT88), an essential factor for the formation of primary cilia47,48 in the left atrial tissue of AF patients compared to non-AF patients (Fig. 2a). Therefore, we silenced the IFT88 gene by RNAi in primary human atrial fibroblasts isolated from normal adult atrial tissue (hereafter, NHCF-A cells). We observed substantial knockdown efficiency of siRNA targeting IFT88 both at gene and protein levels (Fig. 2b,c). The knockdown of IFT88 significantly decreased the proportion of cells with primary cilia (Fig. 2d). The structure of the primary cilia without IFT88 knockdown in these cells is shown in Supplemental Fig. 1a,b.
Figure 2.
Loss of the primary cilia through knockdown of IFT88 reduces the proportion of NHCF-A cells with primary cilia. (a) The gene expression of IFT88 in the left atrial tissue of non-AF and AF patients tested by Mann–Whitney U test. (b, c) The gene expression (b) and the protein levels (c) of IFT88 in NHCF-A cells transfected with siNC (negative control) or siIFT88. Error bars, mean (SD). N = 3/group. **p < 0.01, ***p < 0.001 (Student’s unpaired t-test). (d) The representative images and quantification of ciliated cells (%) in NHCF-A cells transfected with siNC or siIFT88. Red: acetylated α-tubulin (primary cilia), blue: DAPI (nuclei). Scale bar, 50 μm. *p < 0.05 (Mann–Whitney U test); n = 4/group (101–122 cells/replicate).
The gene expression of ECM components such as fibronectin-1 (FN1), alpha-1 type I collagen (COL1A1), and connective tissue growth factor (CTGF) demonstrated a considerable or trending toward up-regulation in IFT88-silenced NHCF-A cells both in the presence or absence of TGF-β1 (Fig. 3a–c), suggesting that loss of the primary cilia in atrial fibroblasts enhances ECM production. The gene expression of alpha-1 type III collagen (COL3A1) remained unchanged (Fig. 3d).
Figure 3.
Loss of the primary cilia through knockdown of IFT88 enhances the profibrotic capacity of NHCF-A cells and the atrial fibroblasts isolated from non-AF patients. (a–d) The gene expression of FN1 (a), COL1A1 (b), CTGF (c), and COL3A1 (d) in the NHCF-A cells transfected with siNC (negative control) or siIFT88 and cultured in the absence (vehicle) or presence of 2 ng/mL TGF-β1 for 48 h. HPRT serves as an internal control. #p < 0.05, ###p < 0.001 vs. siNC group treated with vehicle, *p < 0.05, ***p < 0.001 (Student’s unpaired t-test or Mann–Whitney U test were employed according to the data distribution, and all the p-values were adjusted by post-hoc analysis). (e, f) The induction of FN1 (e) and COL1A1 (f) gene expression by TGF-β1 in the fibroblasts isolated from the left atrial tissue of three non-AF and three AF patients is shown as a fold-change (TGF-β1 treated/vehicle treated group). The dashed line indicates the baseline. (g, h) The representative images (g) and quantification (h) of αSMA-expressing myofibroblasts isolated from two non-AF (#1 and #2) and two AF patients (#3 and #4). Fibroblasts from non-AF patients were transfected with siNC or siIFT88, and fibroblasts from AF patients were transfected with siNC. Red: αSMA (myofibroblasts), blue: DAPI (nuclei). Scale bar, 50μm. The proportion of αSMA-positive cells was quantified from three technical replicates. Error bar, mean (SD).
Next, we silenced IFT88 in the atrial fibroblasts isolated from non-AF patients (hereafter, non-AF fibroblasts) to examine if the knockdown of IFT88 in non-AF fibroblasts induces a phenotype switch to atrial fibroblasts isolated from AF patients (hereafter, AF atrial fibroblasts). As expected, when IFT88 was silenced in non-AF fibroblasts, the gene expression of FN1 and COL1A1 was induced by TGF-β1 at similar to or even higher levels than AF atrial fibroblasts (Fig. 3e,f). The capacity of non-AF fibroblasts to differentiate into myofibroblasts was also enhanced when IFT88 was silenced, although the degree was slightly less than that of AF fibroblasts (Fig. 3g,h). Taken together, our results imply that loss of the primary cilia observed in AF patients induces an enhanced differentiation of atrial fibroblasts into myofibroblasts and increases the expression of genes that are components of ECM.
Restoration of the primary cilium blunts the profibrotic response of human atrial fibroblasts
To explore the effects of protecting primary cilia from disassembly on the activation of atrial fibroblasts, we inhibited the NEDD9/AURKA/HDAC6 axis with PHA-680632 (PHA), a selective inhibitor of AURKA. PHA dose-dependently restored the primary cilia in NHCF-A cells cultured in the growth medium (supplemented with 10% FBS, hFGF-B, and insulin), where the formation of primary cilia is systematically restricted (Fig. 4a). On the other hand, in the medium supplemented with 0.5% FBS, where many cells exit from the cell cycle, PHA treatment didn’t dramatically increase ciliated NHCF-A cells (%) (Fig. 4b, the left two bars). However, it counteracted the decreased tendency of ciliated NHCF-A cells (%) in TGF-β1 treatment (Fig. 4b, the right two bars). Furthermore, PHA significantly increased the length of primary cilia both in the presence and absence of TGF-β1. However, its effect was partially but significantly inhibited by TGF-β1 (Fig. 4c,d).
Figure 4.
PHA promotes the cilia formation and increase the length of primary cilia in NHCF-A cells. (a) The proportion of NHCF-A cells with primary cilia cultured in the growth medium supplemented with 10%FBS, hFGF-B and insulin following a PHA treatment at different doses (0, 0.5, 1.0 or 2.0 μM) for 48 h. N = 2/treatment. Error bars, mean (SD). (b–d) The proportion of ciliated NHCF-A cells (b), the representative morphology (c) and the length of primary cilia (d) when NHCF-A cells were cultured in 0.5% FBS medium with vehicle or 2 ng/mL TGF-β1 in the absence (DMSO) or presence of 2 μg/mL PHA. Arrow heads indicate primary cilia. Red: ac α-tub (primary cilia), blue: DAPI (nuclei). Scale bar, 20 μm. In total, > 100 primary cilia/treatment from three technical replicates were measured. Error bars, mean (SD). **p < 0.01 in (b) (Student’s unpaired t-test). ***p < 0.001 in (c) (Mann–Whitney U test).
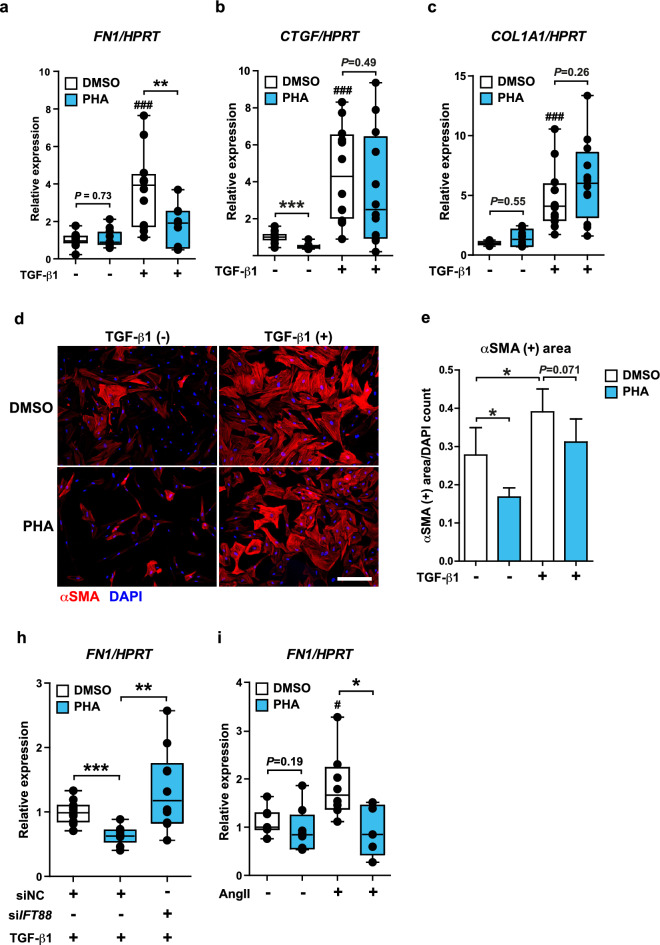
Next, we attempted to abrogate the profibrotic response of AF fibroblasts isolated from AF patients by restoring their primary cilia using PHA. In atrial fibroblasts isolated from the left atrial tissue of AF patients, the expression of ECM genes at baseline and in the presence of TGF-β1 was dramatically suppressed in the presence of PHA (Fig. 5a–d). This effect could be partly due to a suppression of fibroblast differentiation into myofibroblasts, as the proportion of αSMA-positive cells was notably lower when the cells were treated with TGF-β1 in the presence of PHA (Fig. 5e,f).
Figure 5.
PHA (AURKA selective inhibitor PHA-680632) inhibits the profibrotic response of the fibroblasts isolated from the left atrial tissue of AF patients. (a–d) The gene expression of FN1 (a), COL1A1 (b), CTGF (c) and COL3A1 (d) in the fibroblasts isolated from AF patients and cultured with vehicle or 2 ng/mL TGF-β1 in the absence (DMSO) or presence of 2 μg/mL PHA. The dashed line indicates the baseline. ##p < 0.01, ###p < 0.001 vs. PHA (−) TGF-β1 (−) group, *p < 0.05, **p < 0.01, ***p < 0.001 (paired t-test or Wilcoxon signed-rank test according to the data distribution). (e, f) The representative images (e) and the quantification (f) of fibroblasts from AF patients that differentiated into αSMA-expressing myofibroblasts in respective treatments. Red: αSMA (myofibroblasts), blue: DAPI (nuclei). Scale bar, 100 μm. *p < 0.05 (paired t-test).
In NHCF-A cells, PHA suppressed the gene expression of FN1 (Fig. 6a) but not of CTGF and COL1A1 in the presence of TGF-β1 (Fig. 6b,c). Consistently, the differentiation of NHCF-A cells into myofibroblasts was incompletely inhibited (Fig. 6d,e). The limited efficacy of PHA on NHCF-A from a healthy donor suggests that PHA may be more effective in atrial fibroblasts from AF patients due to their supraphysiological activity elicited in the diseased status. Importantly, the inhibitory effect of PHA on FN1 gene induction by TGF-β1 was completely abolished when the formation of primary cilia was inhibited by siIFT88 in NHCF-A cells (Fig. 6h). Thus, the mechanism of action of PHA appears to depend on primary cilia. Remarkably, PHA also suppressed FN1 gene induction by angiotensin II (AngII), another important inducer of atrial fibrosis in AF49 (Fig. 6i), indicating that PHA is potentially capable of suppressing the profibrotic reaction of atrial fibroblasts in response to multiple profibrotic stimuli.
Figure 6.
PHA suppresses the gene expression of FN1 induced by TGF-β1 in a primary cilia-dependent manner. (a–c) The gene expression of FN1 (a), COL1A1 (b), CTGF (c) in NHCF-A cells when cells were cultured with vehicle or 2 ng/mL TGF-β1 for 48 h in the absence (DMSO) or presence of 2 μM PHA in respective treatments. ###p < 0.001 vs. PHA (−) TGF-β1 (−) group, **p < 0.01 (Mann–Whitney U test). (d, e) The representative images of NHCF-A cells that differentiated into αSMA positive myofibroblasts (d) and the quantification of αSMA positive area (e). Red: αSMA (myofibroblasts), blue: DAPI (nuclei). Scale bar, 100 μm. αSMA positive area is normalized by DAPI count (a.u.). N = 5/group. Error bars, mean (SD). *p < 0.05 (Student’s unpaired t-test). (h) The gene expression of FN1 when NHCF-A cells were first transfected with siNC or siIFT88 and then cultured with 2 ng/mL TGF-β1 in the absence (DMSO) or presence of 2 μg/mL PHA. **p < 0.01, ***p < 0.001 (Student’s unpaired t-test). (i) The gene expression of FN1 in NHCF-A treated with vehicle or 10−6 M AngII (angiotensin II) for 48 h in the absence of presence of 2 μg/mL PHA. #p < 0.05, vs. PHA (−) AngII (−) group, *p < 0.05 (Mann–Whitney U test).
To further test our hypothesis that primary cilia protect against fibrosis formation, we used lithium chloride (LiCl), a well-known compound to elongate the primary cilia in various cell types50,51, and widely used as a mood stabilizer in clinical practice52. Consistent with the previous reports, LiCl elongated the primary cilia in NHCF-A cells (Fig. 7a). LiCl strongly suppressed the gene expression of ECM genes induced by TGF-β1 in NHCF-A cells (Fig. 7b–d). Furthermore, NHCF-A cells that had differentiated into myofibroblasts following long culture periods (> eight to 10 passages) dedifferentiated into αSMA-negative cells upon treatment with LiCl (Fig. 7e,f).
Figure 7.
LiCl elongates primary cilia and inhibits the profibrotic response of the NHCF-A and dedifferentiates them into αSMA-negative cells. (a) The representative images and the quantification of cilia length with LiCl treatment. Red: acetylated α-tubulin (primary cilia), blue: DAPI (nuclei). Scale bar, 20 μm *p < 0.05 (Mann–Whitney U test); n = 4/group (20–29 cells/sample). (b–d) The gene expression of FN1 (b), CTGF (c) and COL1A1 (d) when NHCF-A cells were cultured with vehicle or 2 ng/mL TGF-β1 for 48 h in the absence (water) or presence of 50 mM LiCl. ##p < 0.01, ###p < 0.001 vs. LiCl (−) TGF-β1 (−) group, *p < 0.05, ***p < 0.001 (Student’s unpaired t-test or Mann–Whitney U test according to the data distribution). (e, f) The representative images of NHCF-A cells that differentiated into αSMA-positive myofibroblasts (e) and the quantification of αSMA-positive area (f). NHCF-A cells were passaged more than five times and then cultured with or without 50 mM LiCl for 48 h. Red: αSMA (myofibroblasts), blue: DAPI (nuclei). Scale bar, 100 μm. αSMA-positive area is normalized by DAPI count (a.u.); n = 3–4/group. Error bar, mean (SD). *p < 0.05 (Student’s unpaired t-test).
Our data suggest that preserving primary cilia by either inhibiting its resorption mechanism or increasing its length can blunt the profibrotic response of atrial fibroblasts to multiple profibrotic stimuli and that targeting the primary cilia may form a therapeutic approach to disarm atrial fibrosis in AF.
Discussion
We revealed a novel mechanism underlying the formation of atrial fibrosis in AF via loss of the primary cilia in atrial fibroblasts. Furthermore, we demonstrated that preserving the primary cilia in atrial fibroblasts from AF patients considerably blunted their profibrotic capacity in response to TGF-β1. Importantly, this approach was also shown to be efficacious in the profibrotic response of the fibroblasts induced by angiotensin II. These data suggest that our approach to regulating fibroblast activity by modifying their sensing mechanism via primary cilia rather than targeting a single profibrotic pathway has the potential to control atrial fibrosis formation as a whole in AF. This approach may be of importance when considering the multifactorial processes in fibrosis formation22. As ECM is a highly dynamic structure that is constantly metabolized by fibroblasts, targeting the primary cilia of atrial fibroblasts may halt or even reverse the atrial fibrosis in AF.
Role of primary cilia in fibrosis formation
Loss or dysfunction of the primary cilium has been implicated in fibrosis formation in various tissues53,54. For instance, hepatic periportal fibrosis develops in the Ift88Orpk mouse55, a congenital model of impaired ciliogenesis. Loss of Ift88 in cardiac endothelial cells increases ECM production during the development of the aortic valve56. During the valve development, premature loss of primary cilia due to a missense mutation of a ciliary gene DZIP1 or by IFT88 conditional knockout in the valve interstitial cells induces an enhanced ECM synthesis, resulting in dysmorphic leaflets and, eventually, in mitral valve prolapse in adult56,57. Moreover, mutations of the ciliary proteins polycystin-1 and 2 (PKD1 and PKD2), the main genes causing polycystic kidney disease, results in major pathological changes, including interstitial inflammation and renal fibrosis58–60, where TGFβ-Smad signaling is enhanced61. Recently, loss of pkd1 in activated fibroblasts was shown to attenuate TGF-β1 signaling and suppress ECM production following myocardial injury in mice42. This seemingly contradicting observation may lie in the biphasic mechanism of the transition from fibroblasts to myofibroblasts via primary cilia; once the critical signaling is initiated in epithelial cells and fibroblasts through intact primary cilia, shortening or loss of primary cilia facilitates the transition and sustains the activity of myofibroblasts62. Therefore, deleting pkd1 in activated fibroblasts isolated from neonatal or relatively young mice possibly impairs the initial signaling required to differentiate fibroblasts into myofibroblasts. The fibroblasts isolated from relatively aged AF patients, on the other hand, may long have been exposed to the pathological stimuli, which may have resulted in a systematic shortening of primary cilia over time, thus making these cells prone to eliciting profibrotic responses to various profibrotic stimuli. Further studies focusing on the spatiotemporal regulation of fibroblasts into myofibroblasts’ transition via primary cilia in AF pathology will explain these ostensibly inconsistent cellular responses.
We recently showed that culturing cardiomyocytes with fibroblasts lacking primary cilia reduced the speed of activation wave propagation across the monolayer. This was mediated by extracellular matrix remodeling rather than changes to cardiomyocyte action potential properties such as depolarized resting membranes, likely precluding oxidative stress-mediated electrical remodeling or cell death63.
Furthermore, the shortened primary cilia observed in the AF fibroblasts may alter the Hedgehog signaling, whose function is largely unknown in AF pathology. Hedgehog signaling is crucial for cell differentiation and tissue formation, and primary cilia are central transducers of the Hedgehog signaling pathway. Hedgehog dysregulation has been implicated in pulmonary fibrosis64. Activation of the Hedgehog signaling leads to effects similar to those observed, namely ECM accumulation and myofibroblast differentiation. Further studies are, however, required to illustrate the involvement of this pathway in AF.
AURKA and NEDD9 axis: a potential druggable target in AF
We demonstrated that PHA successfully restored the formation of primary cilia in human atrial fibroblasts. Furthermore, PHA suppressed the profibrotic response of atrial fibroblasts from AF patients, and its mechanism of action was cilia-dependent. Alisertib (MLN8237) is an oral selective inhibitor of AURKA, under investigation and developed for the treatment of malignancies65. Hence, AURKA is a druggable target. At this stage, specific chemotherapy is not indicated for AF patients. However, as AURKA and NEDD9 are highly expressed in the left atrial tissue of AF patients, and Alisertib has been described to have no clinically relevant effects on cardiac repolarization or ECG parameters66, targeting NEDD9/AURKA/HDAC6 axis is attractive as a novel treatment for fibrosis in AF. To note, functions of AURKA extend beyond cilia disassembly to regulating mitochondrial function and DNA repair67,68. Accordingly, it is worthwhile to further examine the contribution of other downstream pathways to AF pathogenesis.
Interestingly, NEDD9, which stabilizes AURKA38, is highly expressed in endothelial cells and fibroblasts in the heart (The Single Cell Type Atlas, http://www.proteinatlas.org). This expression pattern of NEDD9 may limit the activation of AURKA/HDAC6 and subsequent disassembly of primary cilia to those cell types in the heart. Indeed, primary cilia are primarily formed in the cells that exited from the cell cycle69, such as cardiomyocytes, and their formation is suppressed in actively proliferating cells. Therefore, this cilia biology may allow us only to target the cell population that is largely responsible for fibrosis formation. In either case, more detailed studies focusing on the impact of selective AURKA inhibitors on each cardiac cell population will be required before any clinical application.
Potential therapeutic role of lithium in AF patients
We demonstrated that LiCl, well-known to elongate primary cilia in several cell types50,51, strongly inhibited TGF-β1 induced-upregulation of ECM genes in NHCF-A cells. Furthermore, LiCl induced dedifferentiation of αSMA-expressing myofibroblasts into αSMA-negative fibroblasts. This suggests that the elongation of primary cilia by LiCl may abrogate existing atrial fibrosis in AF patients if the same effect occurs in-vivo. Lithium is reported to promote cilia length by activating α-tubulin acetyltransferase-1 and inhibiting GSK-3β70; however, it has pleiotropic effects on various cellular signaling71, and its exact mechanism of action is not fully understood. From a clinical point of view, lithium may be beneficial for AF patients; lithium at low doses seems to have an antiarrhythmic effect72–74, and, as a potent inhibitor of GSK-3β75, it inhibits myocardial apoptosis in diabetic myocardium76. However, like most antiarrhythmic drugs, lithium intoxication may result in unfavorable electrocardiographic and proarrhythmic changes77.
Limitations
In this study, we studied cardiac tissues from patients who underwent CABG and/or aortic valve replacement without a history of AF as control. The control tissue is, therefore, not of healthy controls. Loss of the primary cilia in fibroblasts from AF patients may also arise from different underlying cardiac diseases between the two biological groups. When we take into account the frequent coexistence of AF with other cardiac conditions such as coronary artery disease78 and heart failure3, as well as the unfeasibility of using healthy heart tissue, we argue that using patients with other cardiac diseases but without AF may be a comparable control in the context of AF to reveal the impact of AF on the cellular physiology of cardiac cells.
A limitation of this study is that the left atrial appendage (LAA) was utilized as a surrogate for left atrial tissue. This is due to the feasibility of obtaining it from live patients during surgery, as was done in previous studies14,79. Although no study comprehensively examined how fibrotic remodeling in the left atrium corresponds to that in the LAA, proportional changes of fibrosis parameters in both tissues, as assessed by CT, predict the same outcome80. We posit that the findings of this study are not limited to fibroblasts residing in the LAA but extend to fibrosis elsewhere in the atrium.
Moreover, the attenuated expression of IFT88 in the left atrial tissue of AF patients cannot be specifically attributed to fibroblasts. This is because IFT88 is essential to the structure of cilia, which are present on virtually all cells. Nevertheless, cardiac fibroblasts, smooth muscle, and endothelial cells more dominantly express IFT88 than cardiomyocytes (The Single Cell Type Atlas, http://www.proteinatlas.org).
Importantly, all the experiments in this study were performed using human tissue and isolated cells from healthy donors and patients with or without AF. The design of this study precluded the demonstration of a cause (cilia loss in fibroblast) and effect (onset of AF) relation in vivo, for which interventions in ex-vivo organ culture or organisms modeling AF in animals such as murine AF models are warranted. Therefore, the causal relationship in-vivo remains uncertain. Nevertheless, we demonstrate that loss of the primary cilia in AF results in increased fibrotic activity, which can be reversed by promoting the formation of primary cilia in the atrial fibroblasts.
Methods
Patient population and materials
A total of 25 patients without AF (non-AF) and 26 patients with persistent AF (AF) were included in this study (Table 1). The 25 non-AF patients were from the 150 participants of the PREDICT AF study (NCT03130985), which aims to discover biomarkers both in cardiac tissue and blood to predict new-onset AF. PREDICT-AF participants underwent cardiac surgery, had a CHA2DS2-VASc stroke score of at least two, and had no history of AF44,45. Persistent AF patients included in this study underwent thoracoscopic ablation for AF consisting of pulmonary vein isolation and additional left atrial lines. From both non-AF and AF patients, the left atrial appendage (hereafter, left atrial tissue) was excised, swiftly washed in the calcium-free buffer (100 mM NaCl, 10 mM KCl, 5 mM MgSO4, 5 mM D-Glucose, 50 mM Taurine, 5 mM MOPS) and immediately snap-frozen in liquid nitrogen on-site. The snap-frozen tissues were stored at − 80 °C. Additionally, 11 AF patients (one paroxysmal, eight persistent, and two long-standing persistent AF) were included in this study (supplemental Table 2) to isolate atrial fibroblasts from their fresh left atrial tissue. The excised atrial tissue was swiftly washed in calcium-free buffer on-site and immediately brought to the laboratory on ice. This study is in accordance with the declaration of Helsinki, and all the procedures, including the use of patients’ materials, were approved by the ethics committee of the Amsterdam University Medical Centers, University of Amsterdam. All the patients enrolled in this study provided written informed consent.
Isolation of atrial fibroblasts from the patients and cell culture
The atrial fibroblasts from non-AF and AF patients were enzymatically isolated from fresh left atrial tissues. Briefly, the fresh tissue brought to the laboratory was dissected into pieces at a size of approximately 1 mm3. After repeated washing followed by a pre-digestion with 1 mg/mL collagenase A (Roche, Cat#. 10103586001) and 500 μg/mL protease XXIV (Sigma, Cat#. P-8038), the tissue pieces were digested with 1.25 mg/mL collagenase A in 10 μM calcium buffer solution for one to two hours. The cell suspension was filtered by a 40 μm-cell strainer (Corning™, Cat#. 15360801) and seeded directly on culture dishes containing DMEM (Gibco, Cat#. 41966-029) supplemented with 10% FBS, 1% penicillin–streptomycin and 0.1% Gentamycin. Twenty-four hours after seeding, the culture dishes were washed twice with PBS to remove floating non-adhesive cells and replenished with fresh medium. The medium was changed every three days, and the fibroblasts were propagated for approximately two weeks before they were used for respective assays without passaging. For RNA interference, cells were cultured with the mixture of Silencer™ Negative Control (Invitrogen™, Cat#. AM4611) or Silencer Select siIFT88 (Invitrogen™, Cat#. 4392420, siRNA ID. s224871) in Lipofectamine RNAiMAX (Invitrogen™, Cat#. 13778150) for 24 h.
Cell culture of normal heart cardiac fibroblasts from atria
Normal Heart Cardiac Fibroblasts from Atria (hereafter NHCF-A cells) were purchased from LONZA (Cat#. CC-2903) and cultured in the growth medium; FGM™-3 added with hFGF-B and insulin (Cat#. CC-4526). To knockdown IFT88, Silencer Negative Control (Invitrogen™, Cat#. AM4611) or Silencer Select siIFT88 (Invitrogen™, Cat#. 4392420, siRNA ID. s224871) were delivered into cells using Lipofectamine RNAiMAX (Invitrogen™, Cat#. 13778150), incubated for 24 h. Following transfection, NHCF-A cells were cultured with DMEM containing 0.5% FBS for six hours to promote the formation of primary cilia. NHCF-A cells were then cultured for 48 h with/without profibrotic cytokines, e.g., TGF-β1 (2 ng/mL) or angiotensin II (10−6 M) in the presence or absence of compounds promoting cilia formation, e.g., 2 μg/mL of PHA-680632 (Selleck Chemicals, Cat#. S1454, purity; 96.62%) or 50 mM of LiCl (Merck, Cat#. L4408, purity; ≥ 99.0%). Most of our experiments used NHCF-A cells of passages three to seven. This is per the manufacturer’s protocol that assures retaining the fibroblast trait in these passages. NHCF-A cells passaged beyond the indicated passage (passages eight to 10) were used only for the purpose of testing the effect of LiCl on the dedifferentiation of αSMA-expressing NHCF-A.
Immunohistochemistry and immunocytochemistry
The snap-frozen left atrial tissues from the patients were cryo-sectioned (5 μm in thickness) and fixed with 4% PFA. The sections were blocked using SuperBlock™ (Thermo Scientific™, Cat#. 37515) for 10 min at room temperature and incubated with anti-acetylated α-tubulin (Abcam, Cat#. 24610) and anti-vimentin antibody (Abcam, Cat#. ab92547) overnight at 4 °C. Anti-mouse IgG conjugated with Alexa-568 (Invitrogen™, Cat#. A-11004) and anti-rabbit IgG conjugated with Alexa-488 (Invitrogen™, Cat#. A-11008) were used as second antibodies. The sections were mounted with ProLong™ Gold Antifade Mountant with DAPI (Invitrogen™, P36931). The section of each patient was anonymized, and the images were captured by Leica TCS SP8 X confocal microscope. The proportion of the fibroblasts with primary cilia in the tissue was quantified blinded for study groups using ImageJ.
For immunocytochemistry, the cells were washed twice with PBS and subsequently fixed with 4% PFA for 10 min at room temperature. The cells were blocked with 5% BSA in PBS for one hour at room temperature, permeabilized with 1% Triton X-100 and incubated with anti-acetylated α-tubulin and anti-vimentin antibody or anti-α-tubulin (Abcam, Cat#. Ab11317), or anti-α-smooth muscle actin antibody (Dako, Cat#. M0851) overnight at 4 °C. The images of primary cilia were captured by Leica TCS SP8 X confocal microscope, and the images of αSMA-expressing cells were captured by Leica DM6000. Almost all the NHCF-A cells treated with TGF-β1 or passaged beyond passage seven turned into αSMA-expressing cells, resulting in difficulty in quantifying αSMA (+) cells (%). Therefore, αSMA-positive area was measured as a surrogate parameter of αSMA (+) cells (%). The proportion of αSMA-positive cells, the proportion of ciliated cells (counted visually with count tracking), and the length of primary cilia (using the straight line tool) were analyzed by ImageJ, and the anonymized sections were unblinded after analysis.
Quantitative polymerase chain reaction (qPCR)
First, snap-frozen left atrial tissues were mechanically crushed into fine powder in liquid nitrogen (cryo-milling). Total RNA was then extracted from the cryo-milled patient samples or the cultured fibroblasts using TRIzol Reagent (Invitrogen™, Cat. No. 15596018), and 500 ng of total RNA was reverse transcribed by SuperScript II Reverse Transcriptase (Invitrogen™, Cat. No. 18064022). qPCR was performed on the platform of Light Cycler 480 using SYBR Green I Master (Roche, Cat. No. 04707516001), and the obtained data was analyzed by LinRegPCR method (Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data).
Western blot
Proteins were extracted from cryo-milled left atrial tissues of the patients. The protein concentration of the samples was determined by Pierce BCA protein assay kit (Thermo Scientific™, Cat No. 23225). Thirty-μg of total protein was separated in the 4–20% gradient precast gel (Bio-Rad, Cat. No. 456-1094) by electrophoresis and transferred to the 0.2 μm PVDF membrane (Bio-Rad, Cat. No. 1704272). The membrane was then incubated with primary antibody solution containing anti-acetylated α-tubulin, anti-HDAC6 (Proteintech, Cat#. 12834-1-AP), anti-α-tubulin (Santa Cruz, Cat#. Sc-5286), anti-GAPDH (Fitzgerald, Cat#. 10R-G109a), and anti-IFT88 antibody (Proteintech, Cat#. 13967-1-AP). Amersham ECL HRP-conjugated Mouse or Rabbit IgG antibody (GE Healthcare, Cat#. NA-931 and Cat#. NA-934) was used as a second antibody, and the signals were detected using ECL™ Prime (GE Healthcare, Cat#. RPN2232). The density of the protein bands was quantified by densitometry of ImageJ and normalized relative to GAPDH.
Statistics
SPSS (version 26) was used for the statistical analyses. The normality assumption was examined using a histogram and the Shapiro–Wilk test. The comparisons between the two groups were tested by unpaired Student’s t-test or Mann–Whitney U test according to the data distribution. Statistical significance between more than two groups was assessed by one-way ANOVA or the Kruskal–Wallis test according to the data distribution, and the p-values of each comparison were corrected by post-hoc analysis. The comparisons between the treatments using the fibroblasts isolated from the patients were tested by paired t-test or Wilcoxon signed-rank test. The comparison was regarded as significant when the p-value was < 0.05.
Supplementary Information
Author contributions
M.K. and J.R.G. designed the study. M.K., R.A.S., F.A.N., and B.F. conducted the experiments. R.A.S., N.W.E.B., R.W., J.N., E.R.M., S.W.E.B., A.H.G.D. and J.R.G. collected the biological materials and clinical characteristics of the patients enrolled in this study. M.K., R.A.S. and J.R.G. wrote the manuscript.
Funding
This study was supported by the Netherlands Organization for Health Research and Development (ZonMW/NWO, 106.146.310) awarded to JRdG.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Competing interests
JRdG received research grants through his institution from Abbott, Atricure, Boston Scientific, Bayer, Daiichi Sankyo, Johnson&Johnson, Medtronic Servier, and received speaker/consultancy fees from Atricure, Bayer, Daiichi Sankyo, Johnson&Johnson and Medtronic outside the submitted work. AHGD is a consultant for Atricure. The other authors report no disclosures.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Makiri Kawasaki and Rushd F. M. Al-Shama.
Contributor Information
Makiri Kawasaki, Email: makiri.kawasaki@gmail.com.
Rushd F. M. Al-Shama, Email: r.f.alshama@amsterdamumc.nl
Joris R. de Groot, Email: j.r.degroot@amsterdamumc.nl
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-60298-x.
References
- 1.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: The Framingham study. Stroke. 2018;22:983–988. doi: 10.1161/01.STR.22.8.983. [DOI] [PubMed] [Google Scholar]
- 2.Wolf PA, Dawber TR, Thomas HE, Kannel WB. Epidemiologic assessment of chronic atrial fibrillation and risk of stroke: The Framingham study. Neurology. 1978;28:973–977. doi: 10.1212/WNL.28.10.973. [DOI] [PubMed] [Google Scholar]
- 3.Anter E, Jessup M, Callans DJ. Atrial fibrillation and heart failure: Treatment considerations for a dual epidemic. Circulation. 2009;119:2516–2525. doi: 10.1161/CIRCULATIONAHA.108.821306. [DOI] [PubMed] [Google Scholar]
- 4.Thrall G, Lane D, Carroll D, Lip GYH. Quality of life in patients with atrial fibrillation: A systematic review. Am. J. Med. 2006;119(448):e1–448.e19. doi: 10.1016/j.amjmed.2005.10.057. [DOI] [PubMed] [Google Scholar]
- 5.Andersson T, et al. All-cause mortality in 272,186 patients hospitalized with incident atrial fibrillation 1995–2008: A Swedish nationwide long-term case-control study. Eur. Heart J. 2013;34:1061–1067. doi: 10.1093/eurheartj/ehs469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krijthe BP, et al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur. Heart J. 2013;34:2746–2751. doi: 10.1093/eurheartj/eht280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zoni-Berisso M, Lercari F, Carazza T, Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clin. Epidemiol. 2014;6:213–220. doi: 10.2147/CLEP.S47385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chugh SS, et al. Worldwide epidemiology of atrial fibrillation: A global burden of disease 2010 study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Driessen AHG, et al. Ganglion plexus ablation in advanced atrial fibrillation the AFACT study. J. Am. Coll. Cardiol. 2016;68:1155–1165. doi: 10.1016/j.jacc.2016.06.036. [DOI] [PubMed] [Google Scholar]
- 10.Castellá M, et al. Thoracoscopic vs. catheter ablation for atrial fibrillation: long-term follow-up of the FAST randomized trial. EP Eur. 2019;21:325. doi: 10.1093/europace/euy325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirchhof P, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. EP Eur. 2016;18:1609–1678. doi: 10.1093/europace/euw295. [DOI] [PubMed] [Google Scholar]
- 12.Sultan A, et al. Predictors of atrial fibrillation recurrence after catheter ablation: Data from the German ablation registry. Sci. Rep. 2017;7:16678. doi: 10.1038/s41598-017-16938-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kazbanov IV, ten Tusscher KHWJ, Panfilov AV. Effects of heterogeneous diffuse fibrosis on arrhythmia dynamics and mechanism. Sci. Rep. 2016;6:20835. doi: 10.1038/srep20835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krul SPJ, et al. Atrial fibrosis and conduction slowing in the left atrial appendage of patients undergoing thoracoscopic surgical pulmonary vein isolation for atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2018;8:288–295. doi: 10.1161/CIRCEP.114.001752. [DOI] [PubMed] [Google Scholar]
- 15.Marrouche NF, et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: The DECAAF study. JAMA. 2014;311:498–506. doi: 10.1001/jama.2014.3. [DOI] [PubMed] [Google Scholar]
- 16.Chelu MG, et al. Atrial fibrosis by late gadolinium enhancement magnetic resonance imaging and catheter ablation of atrial fibrillation: 5-year follow-up data. J. Am. Heart Assoc. 2018;7:e006313. doi: 10.1161/JAHA.117.006313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma N, et al. Left atrial appendage fibrosis and 3-year clinical outcomes in atrial fibrillation after endoscopic ablation: A histologic analysis. Ann. Thorac. Surg. 2020;109:69–76. doi: 10.1016/j.athoracsur.2019.05.055. [DOI] [PubMed] [Google Scholar]
- 18.Investigators G-A, et al. Valsartan for prevention of recurrent atrial fibrillation. New Engl. J. Med. 2009;360:1606–1617. doi: 10.1056/NEJMoa0805710. [DOI] [PubMed] [Google Scholar]
- 19.Yamashita T, et al. Randomized trial of angiotensin II-receptor blocker vs. dihydropiridine calcium channel blocker in the treatment of paroxysmal atrial fibrillation with hypertension (J-RHYTHM II Study) EP Eur. 2011;13:473–479. doi: 10.1093/europace/euq439. [DOI] [PubMed] [Google Scholar]
- 20.Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. CSH Perspect. Biol. 2011;3:a005058. doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kendall RT, Feghali-Bostwick CA. Fibroblasts in fibrosis: Novel roles and mediators. Front. Pharmacol. 2014;5:123. doi: 10.3389/fphar.2014.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wynn T. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sánchez I, Dynlacht BD. Cilium assembly and disassembly. Nat. Cell Biol. 2016;18:711–717. doi: 10.1038/ncb3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inoko A, et al. Trichoplein and Aurora A block aberrant primary cilia assembly in proliferating cells. J. Cell Biol. 2012;197:391–405. doi: 10.1083/jcb.201106101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berbari NF, O’Connor AK, Haycraft CJ, Yoder BK. The primary cilium as a complex signaling center. Curr. Biol. 2009;19:R526–R535. doi: 10.1016/j.cub.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pala R, Alomari N, Nauli S. Primary cilium-dependent signaling mechanisms. Int. J. Mol. Sci. 2017;18:2272. doi: 10.3390/ijms18112272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wheway G, Nazlamova L, Hancock JT. Signaling through the primary cilium. Front. Cell Dev. Biol. 2018;6:8. doi: 10.3389/fcell.2018.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nachury MV, Mick DU. Establishing and regulating the composition of cilia for signal transduction. Nat. Rev. Mol. Cell Biol. 2019;20:389–405. doi: 10.1038/s41580-019-0116-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tucker RW, Pardee AB, Fujiwara K. Centriole ciliation is related to quiescence and DNA synthesis in 3T3 cells. Cell. 1979;17:527–535. doi: 10.1016/0092-8674(79)90261-7. [DOI] [PubMed] [Google Scholar]
- 30.Pan J, Snell W. The primary cilium: Keeper of the key to cell division. Cell. 2007;129:1255–1257. doi: 10.1016/j.cell.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 31.Malicki JJ, Johnson CA. The cilium: Cellular antenna and central processing unit. Trends Cell Biol. 2017;27:126–140. doi: 10.1016/j.tcb.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saigusa T, et al. Activation of the intrarenal renin-angiotensin-system in murine polycystic kidney disease. Physiol. Rep. 2015;3:e12405. doi: 10.14814/phy2.12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawasaki M, et al. TGF-β suppresses Ift88 expression in chondrocytic ATDC5 cells. J. Cell Physiol. 2015;230:2788–2795. doi: 10.1002/jcp.25005. [DOI] [PubMed] [Google Scholar]
- 34.Burstein B, Nattel S. Atrial fibrosis: Mechanisms and clinical relevance in atrial fibrillation. J. Am. Coll. Cardiol. 2008;51:802–809. doi: 10.1016/j.jacc.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 35.Seeger-Nukpezah T, Golemis EA. The extracellular matrix and ciliary signaling. Curr. Opin. Cell Biol. 2012;24:652–661. doi: 10.1016/j.ceb.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuan K, et al. Primary cilia are decreased in breast cancer: Analysis of a collection of human breast cancer cell lines and tissues. J. Histochem. Cytochem. 2010;58:857–870. doi: 10.1369/jhc.2010.955856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dinsmore C, Reiter JF. Endothelial primary cilia inhibit atherosclerosis. EMBO Rep. 2016;17:156–166. doi: 10.15252/embr.201541019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pugacheva EN, Jablonski SA, Hartman TR, Henske EP, Golemis EA. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell. 2007;129:1351–1363. doi: 10.1016/j.cell.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ran J, Yang Y, Li D, Liu M, Zhou J. Deacetylation of α-tubulin and cortactin is required for HDAC6 to trigger ciliary disassembly. Sci. Rep. 2015;5:12917. doi: 10.1038/srep12917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang D, et al. Activation of histone deacetylase-6 induces contractile dysfunction through derailment of α-tubulin proteostasis in experimental and human atrial fibrillation. Circulation. 2014;129:346–358. doi: 10.1161/CIRCULATIONAHA.113.005300. [DOI] [PubMed] [Google Scholar]
- 41.van den Berg NWE, et al. Epicardial and endothelial cell activation concurs with extracellular matrix remodeling in atrial fibrillation. Clin. Transl. Med. 2021;11:e558. doi: 10.1002/ctm2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Villalobos E, et al. Fibroblast primary cilia are required for cardiac fibrosis. Circulation. 2019;139:2342–2357. doi: 10.1161/CIRCULATIONAHA.117.028752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krul SPJ, et al. Thoracoscopic video-assisted pulmonary vein antrum isolation, ganglionated plexus ablation, and periprocedural confirmation of ablation lesions: first results of a hybrid surgical-electrophysiological approach for atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2011;4:262–270. doi: 10.1161/CIRCEP.111.961862. [DOI] [PubMed] [Google Scholar]
- 44.van den Berg NWE, et al. PREventive left atrial appenDage resection for the predICtion of fuTure atrial fibrillation: Design of the PREDICT AF study. J. Cardiovasc. Med. 2019;20:752–761. doi: 10.2459/JCM.0000000000000868. [DOI] [PubMed] [Google Scholar]
- 45.van den Berg NWE, et al. Extracellular matrix remodeling precedes atrial fibrillation: Results of the PREDICT-AF trial. Heart Rhythm. 2021;18:2115–2125. doi: 10.1016/j.hrthm.2021.07.059. [DOI] [PubMed] [Google Scholar]
- 46.Kinzel D, et al. Pitchfork regulates primary cilia disassembly and left-right asymmetry. Dev. Cell. 2010;19:66–77. doi: 10.1016/j.devcel.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pazour GJ, et al. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene Tg737, are required for assembly of cilia and flagella. J. Cell Biol. 2000;151:709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh S, et al. Endothelial-specific loss of IFT88 promotes endothelial-to-mesenchymal transition and exacerbates bleomycin-induced pulmonary fibrosis. Sci. Rep. 2020;10:4466. doi: 10.1038/s41598-020-61292-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuzniatsova N, Shantsila E, Lip GYH. Atrial fibrillation: Blockade of the renin-angiotensin system in atrial fibrillation. Nat. Rev. Cardiol. 2010;7:428–430. doi: 10.1038/nrcardio.2010.103. [DOI] [PubMed] [Google Scholar]
- 50.Miyoshi K, Kasahara K, Miyazaki I, Asanuma M. Lithium treatment elongates primary cilia in the mouse brain and in cultured cells. Biochem. Biophys. Res. Commun. 2009;388:757–762. doi: 10.1016/j.bbrc.2009.08.099. [DOI] [PubMed] [Google Scholar]
- 51.Thompson CL, Wiles A, Poole CA, Knight MM. Lithium chloride modulates chondrocyte primary cilia and inhibits Hedgehog signaling. FASEB J. 2016;30:716–726. doi: 10.1096/fj.15-274944. [DOI] [PubMed] [Google Scholar]
- 52.Machado-Vieira R, Manji HK, Zarate CA., Jr The role of lithium in the treatment of bipolar disorder: Convergent evidence for neurotrophic effects as a unifying hypothesis. Bipolar Disord. 2009;11:92–109. doi: 10.1111/j.1399-5618.2009.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Teves ME, Strauss JF, Sapao P, Shi B, Varga J. The primary cilium: Emerging role as a key player in fibrosis. Curr. Rheumatol. Rep. 2019;21:29. doi: 10.1007/s11926-019-0822-0. [DOI] [PubMed] [Google Scholar]
- 54.Collins I, Wann AKT. Regulation of the extracellular matrix by ciliary machinery. Cells. 2020;9:278. doi: 10.3390/cells9020278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zimmerman KA, Song CJ, Gonzalez-Mize N, Li Z, Yoder BK. Primary cilia disruption differentially affects the infiltrating and resident macrophage compartment in the liver. Am. J. Physiol.-Gastr. L. 2018;314:G677–G689. doi: 10.1152/ajpgi.00381.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Toomer KA, et al. A role for primary cilia in aortic valve development and disease. Dev. Dyn. 2017;246:625–634. doi: 10.1002/dvdy.24524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Toomer KA, et al. Primary cilia defects causing mitral valve prolapse. Sci. Transl. Med. 2019;11:eaax0290. doi: 10.1126/scitranslmed.aax0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gascue C, Katsanis N, Badano JL. Cystic diseases of the kidney: Ciliary dysfunction and cystogenic mechanisms. Pediatr. Nephrol. 2011;26:1181–1195. doi: 10.1007/s00467-010-1697-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xue C, Mei C-L. Polycystic kidney disease and renal fibrosis. Adv. Exp. Med. Biol. 2019;1165:81–100. doi: 10.1007/978-981-13-8871-2_5. [DOI] [PubMed] [Google Scholar]
- 60.Bergmann C. Early and severe polycystic kidney disease and related ciliopathies: An emerging field of interest. Nephron. 2019;141:50–60. doi: 10.1159/000493532. [DOI] [PubMed] [Google Scholar]
- 61.Hassane S, et al. Elevated TGFbeta-smad signalling in experimental Pkd1 models and human patients with polycystic kidney disease. J. Pathol. 2010;222:21–31. doi: 10.1002/path.2734. [DOI] [PubMed] [Google Scholar]
- 62.Rozycki M, et al. The fate of the primary cilium during myofibroblast transition. Mol. Biol. Cell. 2014;25:643–657. doi: 10.1091/mbc.e13-07-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ernault AC, et al. Knockdown of Ift88 in fibroblasts causes extracellular matrix remodeling and decreases conduction velocity in cardiomyocyte monolayers. Front. Physiol. 2022;13:1057200. doi: 10.3389/fphys.2022.1057200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Effendi WI, Nagano T. The hedgehog signaling pathway in idiopathic pulmonary fibrosis: Resurrection time. Int. J. Mol. Sci. 2021;23:171. doi: 10.3390/ijms23010171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Friedberg JW, et al. Phase II study of alisertib, a selective Aurora A kinase inhibitor, in relapsed and refractory aggressive B- and T-cell non-hodgkin lymphomas. J. Clin. Oncol. 2013;32:44–50. doi: 10.1200/JCO.2012.46.8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou X, et al. Effect of alisertib, an investigational aurora a kinase inhibitor on the QTc interval in patients with advanced malignancies. Invest. New Drug. 2018;36:240–247. doi: 10.1007/s10637-017-0498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Byrum AK, et al. Mitotic regulators TPX2 and Aurora A protect DNA forks during replication stress by counteracting 53BP1 function. J. Cell Biol. 2019;218:422–432. doi: 10.1083/jcb.201803003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bertolin G, et al. Aurora kinase A localises to mitochondria to control organelle dynamics and energy production. Elife. 2018;7:e38111. doi: 10.7554/eLife.38111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Irigoín F, Badano JL. Keeping the balance between proliferation and differentiation: The primary cilium. Curr. Genom. 2011;12:285–297. doi: 10.2174/138920211795860134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nakakura T, et al. The elongation of primary cilia via the acetylation of α-tubulin by the treatment with lithium chloride in human fibroblast KD cells. Med. Mol. Morphol. 2015;48:44–53. doi: 10.1007/s00795-014-0076-x. [DOI] [PubMed] [Google Scholar]
- 71.Lenox RH, Wang L. Molecular basis of lithium action: Integration of lithium-responsive signaling and gene expression networks. Mol. Psychiatr. 2003;8:135–144. doi: 10.1038/sj.mp.4001306. [DOI] [PubMed] [Google Scholar]
- 72.Lee T-M, Lin S-Z, Chang N-C. Effect of lithium on ventricular remodelling in infarcted rats via the Akt/mTOR signalling pathways. Biosci. Rep. 2017;37:BSR20160257. doi: 10.1042/BSR20160257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee T-M, Lin S-Z, Chang N-C. Antiarrhythmic effect of lithium in rats after myocardial infarction by activation of Nrf2/HO-1 signaling. Free Radic. Biol. Med. 2014;77:71–81. doi: 10.1016/j.freeradbiomed.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 74.Polumbo RA, Branzi A, Schroeder JS, Harrison DC. The antiarrhythmic effect of lithium chloride for experimental ouabain-induced arrhythmias. Proc. Soc. Exp. Biol. Med. Soc. Exp. Biol. Med. N.Y. 1973;142:1200–1204. doi: 10.3181/00379727-142-37208. [DOI] [PubMed] [Google Scholar]
- 75.Jope RS. Lithium and GSK-3: One inhibitor, two inhibitory actions, multiple outcomes. Trends Pharmacol. Sci. 2003;24:441–443. doi: 10.1016/S0165-6147(03)00206-2. [DOI] [PubMed] [Google Scholar]
- 76.Wu W, Liu X, Han L. Apoptosis of cardiomyocytes in diabetic cardiomyopathy involves overexpression of glycogen synthase kinase-3β. Biosci. Rep. 2019;39:BSR20171307. doi: 10.1042/BSR20171307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mehta N, Vannozzi R. Lithium-induced electrocardiographic changes: A complete review. Clin. Cardiol. 2017;40:1363–1367. doi: 10.1002/clc.22822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Michniewicz E, Mlodawska E, Lopatowska P, Tomaszuk-Kazberuk A, Malyszko J. Patients with atrial fibrillation and coronary artery disease–double trouble. Adv. Med. Sci. 2018;63:30–35. doi: 10.1016/j.advms.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 79.Kahr PC, et al. Systematic analysis of gene expression differences between left and right atria in different mouse strains and in human atrial tissue. Plos One. 2011;6:e26389. doi: 10.1371/journal.pone.0026389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tian X, et al. Morphological and functional parameters of left atrial appendage play a greater role in atrial fibrillation relapse after radiofrequency ablation. Sci. Rep. 2020;10:8072. doi: 10.1038/s41598-020-65056-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.