Abstract
Benign metastasizing leiomyoma (BML) is a rare disease that results from metastasis of uterine leiomyoma to distant sites with benign pathologic features. The lung is the most common metastatic site for BML. This report describes the case of a 49-year-old woman who presented with a mass in the abdominal wall with a surgical history of uterine myomectomy. Ultrasound and Magnetic resonance imaging (MRI) revealed multiple mass lesions. The histopathology of the mass specimen indicated BML. The imaging and clinical features of BML are discussed based on the characteristics of this case and related literature reports.
Keywords: benign metastasizing leiomyoma, myomectomy, abdominal wall, smooth muscle cell, CEUS
Introduction
BML is a rare disease, first described by Steiner in 1939 (1). Although the disease appears benign in histomorphology, the biological behavior of the tumor is invasive. The most commonly involved organ is the lung, and others include lymph nodes, heart, skeletal muscle and pelvic cavity (2–4). BML is usually associated with a history of hysterectomy or myomectomy (5). A patient with BML in the abdominal wall is described in this study.
Case presentation
A 49-year-old woman, gravida 1 para 1, accidentally presented with an egg-sized abdominal mass. Symptoms of fever, abdominal pain, and weight loss were not present, and she had previously undergone myomectomy. Physical examination revealed a rigid mass, with moderate motion and no tenderness.
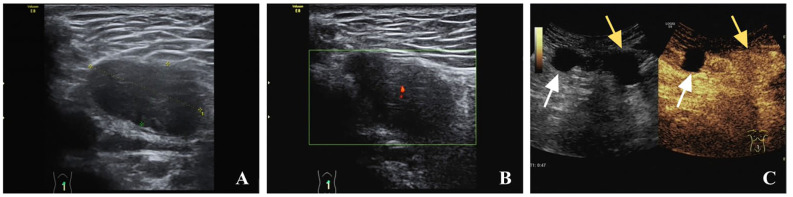
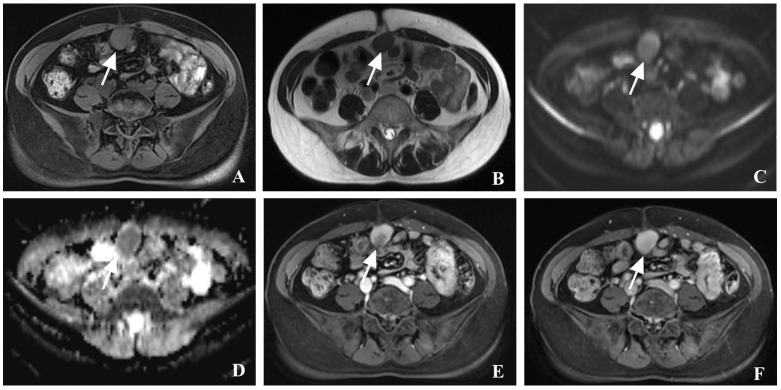
Ultrasound examination showed multiple hypoechoic masses in the subcutaneous fascia layer. The two larger ones are approximately 41 x 25mm and 20 x 16mm with clear boundaries that were connected ( Figure 1A ). Color Doppler flow imaging (CDFI) showed a punctate blood flow signal within the mass ( Figure 1B ), and resistance index of 0.74. Contrast-enhanced ultrasound (CEUS) was performed using the contrast agent SonoVue. Simultaneously, an ultrasound-guided abdominal wall mass core needle biopsy was performed on the two masses. The larger mass showed relatively high enhancement in both arterial and venous phases. The smaller mass showed relatively no enhancement in the arterial phase and low enhancement around in venous phase ( Figure 1C ). An MRI was performed, which showed similar circular abnormal signal shadows on the anterior abdominal wall and abdominal cavity, with sizes of 29 x 16mm and 31 x 21mm, a slightly longer T1 and slightly longer T2, limited diffusion, and progressive enhancement after enhancement ( Figures 2A–F ). Chest Computed Tomography (CT) examination showed no abnormality. CEA, AFP, CA724, CA199 and CA125 markers were all normal.
Figure 1.
Abdominal ultrasonography, contrast-enhanced ultrasound (CEUS) of case. (A) Gray scale ultrasound showed a hypoechoic mass in the abdominal wall; (B) color Doppler flow imaging (CDFI) showed the mass with little blood flow signal; (C) CEUS showed hyper-enhancement of the large mass (yellow arrow) and no enhancement of the small mass (white arrow) in the arterial stage.
Figure 2.
Contrast-enhanced magnetic resonance imaging (CE-MRI) of case. (A–D) MRI showed a slightly high signal in T1-weighted imaging, and a slightly high signal in T2-weighted imaging, diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC); (E, F) CE-MRI showed that the mass progressive enhancement in the arterial phase and the delayed phase (see arrows).
Pathology of the biopsy suggested a spindle cell tumor, and combined with the patient’s surgical history, a fibromatosis in the abdominal wall was considered. The patient underwent the elective resection of the abdominal wall and abdominal cavity tumors. Two masses of 4 x 3cm and 2 x 2cm were observed during the operation, the larger one protruding into the abdominal cavity and the smaller one located in the anterior sheath of the rectus muscle. Two masses, about 2/3 of the rectus abdominis muscle and part of the posterior sheath, were removed completely. The surgeon performed tension-free repair of an abdominal wall hernia, inserting the patch into the abdominal cavity under the incision, fixing it on the abdominal wall, and suturing the rectus abdominis muscle and anterior sheath of the rectus abdominis muscle.
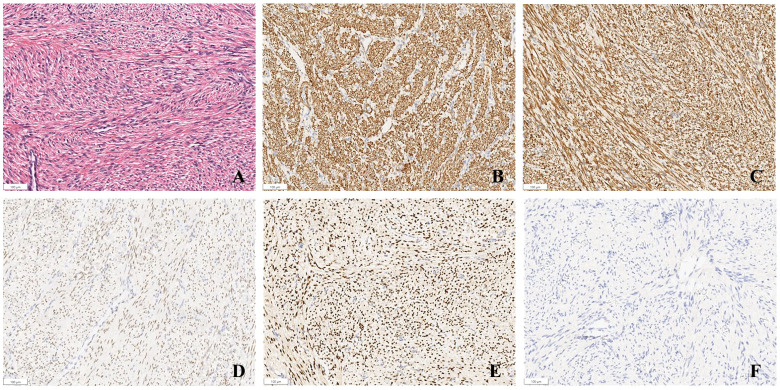
The histopathological examination showed spindle cells. Immunohistochemical staining showed that smooth muscle actin (SMA), desmin, estrogen receptor (ER) and progesterone receptor (PR) were positive, and cell proliferation antigen Ki-67 was less than 1% ( Figures 3A–F ). Based on these findings, the tumor was diagnosed as BML.
Figure 3.
Pathology of case. (A) mass stained with hematoxylin and eosin (original magnification x 10); (B–F) SMA (+), DES (+), PR (+), ER (+), Ki-67 (<1%) was detected by immunohistochemical staining (original magnification x 10).
Discussion
The mechanism of BML has been reported in various reports and remains controversial. Most instances of BML occur after uterine myomectomy or hysterectomy. The mechanism may be that surgery increases surgically induced hematogenous spread. In this case, iatrogenic peritoneal seeding theory seems to be a more reasonable explanation for the occurrence of BML (6, 7). Immunohistochemistry showed that ER and PR of tumor cells were positive, suggesting that BML may also be induced by hormone stimulation. Chromosomal abnormalities such as 19q, 22q, 1p, 13q deletion and 6p21 rearrangement can be found in these tumors (8). Other possible mechanisms include mesothelial metaplasia and misdiagnosed low-grade uterine leiomyosarcoma metastasis (9).
The clinical symptoms of BML are related to the affected sites. The most common site of metastasis is the lung, and the patients have no obvious clinical manifestations in the early stage, with a few having cough, chest pain, dyspnea and other symptoms (10, 11). In patients with BML involving the spine, leg pain and paresthesia have been reported (5). Patients with metastasis to the femur and muscles may have difficulty walking (12). BML involving the uterus, heart, and lymph nodes may be asymptomatic. Multiple abdominal wall metastases are rare, as reported in this case. BML with abdominal wall metastases has been reported in the literature ( Table 1 ). Although most cases of BML occur following myomectomy or hysterectomy, some cases of BML are not related to prior surgical history (22). Studies have shown that BML can occur in premenopausal or postmenopausal women, and up to 30% of cases may have respiratory symptoms (23).
Table 1.
Summary of BML cases with abdominal wall metastasis.
| Metastasis | Age | History of gynecologic surgery | Surgical treatment |
|---|---|---|---|
| abdominal wall and pelvic cavity | 37 (13) | myomectomy 10 years earlier | extirpation of the abdominal and pelvic tumors and partial greater omentum |
| abdominal wall and pelvic cavity | 46 (14) | hysterectomy 8 years earlier | extirpation of the abdominal and pelvic tumors and bilateral oophorectomy |
| abdominal wall and pulmonary | 33 (15) | myomectomy 13 years earlier | extirpation partial tumors |
| abdominal wall | 42 (16) | mysterectomy 8 years earlier | extirpation of the tumors |
| abdominal wall | 44 (17) | hysterectomy | extirpation of the tumors |
| abdominal wall and pulmonary | 44 (18) | myomectomy | N/A |
| abdominal wall and pelvic cavity | 49 (18) | myomectomy 12 years earlier | N/A |
| abdominal wall, pelvic cavity and pulmonary | 47 (19) | twice myomectomy and hysterectomy | N/A |
| abdominal wall and pulmonary | 44 (20) | myomectomy and abdominal hysterectomy | extirpation of the tumors in pulmonary |
| multiple metastasis (abdominal wall, ventricle wall, lungs, liver, muscles, and pelvic cavity) | 37 (21) | twice myomectomy | hysterectomy and resection of the nodules in the mesosalpinx, mesenterium, and abdominal wall |
| abdominal wall, abdominal and pelvic cavity | 54 (9) | abdominal hysterectomy and bilateral salpingo-oophorectomy 6 years earlier | extirpation of the tumors |
BML is radiographically characterized as a benign mass. On ultrasound, BML can be manifested as multiple hypoechoic masses in the abdominal wall or pelvic cavity, and blood flow signals can be seen inside. It can be shown as multiple nodules with clear boundaries on CT, with no significant enhancement on enhanced CT (24). Notably, fluorodeoxyglucose (FDG) -positron emission tomography (PET)/CT showed that FDG uptake by tumors was all within the physiological range (12). According to the review of the FDG-PET/CT examination results of BML, the FDG uptake level of more than 90% of BML patients is lower than the medium level (25), which can be used as one of the crucial bases for the differentiation of benign and malignant tumors. Therefore, physicians must maintain heightened awareness and index of suspicion when approaching a woman with a mass in any region of the abdomen or pelvis. Further investigation with abdominal and pelvic ultrasonography and magnetic resonance imaging or computed tomography is necessary. Benign lesions can be found even in patients presenting with giant masses and higher CA125 than normal levels (26). CA125 and CA199 are considered to be important gynecological tumor indicators, which are commonly seen in malignant tumor diseases, and their elevated levels do not represent absolute malignancy (27, 28).
Regarding pathological diagnostic criteria for BML, BML presents as an extra-uterine smooth muscle cell proliferation with histological features similar to those of primary benign uterine fibroids. Uterine leiomyoblastoma can also exhibit some malignant features, making it difficult for physicians to distinguish (29). Immunohistochemistry is also very helpful in differentiating leiomyoma from leiomyosarcoma. Studies have shown that the positive rate of ER/PR in MBL patients is over 90%, and only 4.4% are negative at the same time (30). ER/PR positivity is specific for uterine leiomyomas and may be helpful in diagnosing BML. In the present case, the abdominal mass was excised, and immunohistochemistry confirmed that the patient’s ER/PR was positive.
There is currently no standardized treatment for BML. Due to the behavioral characteristics of the slow growth of BML, most lesions do not change in size after long-term follow-up, and regular follow-up and periodic imaging examinations can be chosen. Since BML can be hormone-dependent (ER and PR-positive), hormone therapy such as GnRH-a, progesterone antagonists (Mifepristone), selective estrogen receptor modulators (SERMs, tamoxifen) and aromatase inhibitors may be considered, or even surgery to remove the ovaries, particularly for patients with symptomatic lung involvement (24, 30, 31). Although bilateral oophorectomy is commonly used, unilateral oophorectomy is sufficient to promote the reduction of lung BML mass (2). In addition, surgical removal of the tumor is an effective treatment.
Conclusion
The characteristics of low specificity and incidence rate of BML can lead physicians to be unfamiliar with the disease, enabling it to be misdiagnosed as a malignant tumor. Asking operation history of myomectomy or hysterectomy can help us diagnose BML. Pathological diagnosis plays an essential role in determining the follow-up treatment. Obtaining part of the specimens through ultrasound-guided tissue core needle biopsy is effective and safe and can be used as one of the recommended methods.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JH: Writing – original draft. ST: Writing – review & editing. QP: Writing – review & editing. YY: Writing – review & editing.
Funding Statement
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Steiner PE. Metastasizing fibroleiomyoma of the uterus: report of a case and review of the literature. Am J Pathol. (1939) 15:89–110. [PMC free article] [PubMed] [Google Scholar]
- 2. Pacheco-Rodriguez G, Taveira-DaSilva AM, Moss J. Benign metastasizing leiomyoma. Clin Chest Med. (2016) 37:589–95. doi: 10.1016/j.ccm.2016.04.019 [DOI] [PubMed] [Google Scholar]
- 3. Liu Y, Li D, Li X, Wang J, Wang J. Benign metastasizing leiomyoma: is “Wait and watch” Strategy feasible? Reprod Sci. (2023) 30:3568–77. doi: 10.1007/s43032-023-01314-9 [DOI] [PubMed] [Google Scholar]
- 4. Karnib M, Rhea I, Elliott R, Chakravarty S, Al-Kindi SG. Benign metastasizing leiomyoma in the heart of a 45-year-old woman. Tex Heart Inst J. (2021) 48:e197066. doi: 10.14503/THIJ-19-7066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hur JW, Lee S, Lee JB, Cho TH, Park JY. What are MRI findings of spine benign metastasizing leiomyoma? Case report with literature review. Eur Spine J. (2015) 24 Suppl 4:S600–5. doi: 10.1007/s00586-015-3774-8 [DOI] [PubMed] [Google Scholar]
- 6. Beck MM, Biswas B, D’Souza A, Kumar R. Benign metastasising leiomyoma after hysterectomy and bilateral salpingo-oophorectomy. Hong Kong Med J. (2012) 18:153–5 [PubMed] [Google Scholar]
- 7. Cucinella G, Granese R, Calagna G, Somigliana E, Perino A. Parasitic Myomas after Laparoscopic Surgery: An Emerging Complication in the use of Morcellator? Description of Four Cases. Fertil Steril. (2011) 96:e90–6. doi: 10.1016/j.fertnstert.2011.05.095 [DOI] [PubMed] [Google Scholar]
- 8. Bowen JM, Cates JM, Kash S, Itani D, Gonzalez A, Huang D, et al. Genomic imbalances in benign metastasizing leiomyoma: characterization by conventional karyotypic, fluorescence in situ hybridization, and whole genome SNP array analysis. Cancer Genet. (2012) 205:249–54. doi: 10.1016/j.cancergen.2012.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Awonuga AO, Rotas M, Imudia AN, Choi C, Khulpateea N. Recurrent benign metastasizing leiomyoma after hysterectomy and bilateral salpingo-oophorectomy. Arch Gynecol Obstet. (2008) 278:373–6. doi: 10.1007/s00404-008-0581-z [DOI] [PubMed] [Google Scholar]
- 10. Panagiotou E, Vamvakaris I, Syrigos NK, Kotteas E. Pulmonary benign metastasizing leiomyoma: A case report. Monaldi Arch Chest Dis. (2023) 94:A6925. doi: 10.4081/monaldi.2023.2411 [DOI] [PubMed] [Google Scholar]
- 11. AlQudah MA, Hamouri S, Haddad HK, Tawalbeh R, Haddad HK. Pulmonary benign metastasizing leiomyoma: A report of two cases. Future Sci OA. (2022) 8:FSO814. doi: 10.2144/fsoa-2022-0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aoki K, Yamamoto T, Terauchi R, Mori T, Shirai T, Kitawaki J. Benign metastasizing leiomyoma in femur and thigh with A history of uterine leiomyoma: A case report and literature review. J Obstet Gynaecol Res. (2021) 47:812–7. doi: 10.1111/jog.14545 [DOI] [PubMed] [Google Scholar]
- 13. Li Y, Xu T, Wang M, Jiang L, Liu Q, Liu K. Concurrent benign metastasizing leiomyoma in the abdominal wall and pelvic cavity: A case report and review of the literature. Front Surg. (2022) 9:842707. doi: 10.3389/fsurg.2022.842707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xiao H, Li B, Li W, Feng X, Wu L. A rare case of benign abdominal wall and pelvic metastasizing leiomyomas following hysterectomy. J Obstet Gynaecol. (2012) 32:198–9. doi: 10.3109/01443615.2011.622059 [DOI] [PubMed] [Google Scholar]
- 15. Zhao L, Jiang R, Li S, Gui Q, Wang Z, Yang Y. Clinicopathological study of 6 cases of benign metastasizing leiomyoma. Chin J Clin Exp Pathol. (2017) 33:529–33. doi: 10.13315/j.cnki.cjcep.2017.05.013 [DOI] [Google Scholar]
- 16. Kapila K, Mallik MK, Amanguno HG, Ahmed A. Fine needle aspiration diagnosis of a benign metastasizing leiomyoma of the abdominal wall. J Cytology. (2008) 25:105–7. doi: 10.4103/0970-9371.44047 [DOI] [Google Scholar]
- 17. Schwarz EI, Ramach C, Mende KA, Strobel K. Physiologic FDG uptake in the ovary together with an abdominal wall leiomyoma mimicking metastasizing ovarian cancer on PET/CT imaging. Clin Nucl Med. (2009) 34:249–50. doi: 10.1097/RLU.0b013e31819a208f [DOI] [PubMed] [Google Scholar]
- 18. Liang Q, Ling M, Yu H. The imaging findings and literature of 4 cases with pulmonary benign metastasizing leiomyoma. J Clin Radiol. (2019) 38:746–50. doi: 10.13437/j.cnki.jcr.2019.04.042 [DOI] [Google Scholar]
- 19. Gosavi A, Shah S, Purandare NC, Puranik A, Agrawal A, Rangarajan V. Benign metastasizing leiomyoma and findings on fluorodeoxyglucose positron emission tomography/contrast-enhanced computed tomography. Indian J Nucl Med. (2022) 37:68–70. doi: 10.4103/ijnm.ijnm_118_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ağaçkiran Y, Findik G, Ustün LN, Aydoğdu K, Kaya S. Pulmonary benign metastasizing leiomyoma: an extremely rare case. Turk Patoloji Derg. (2014) 32:193–5. doi: 10.5146/tjpath.2013.01217 [DOI] [PubMed] [Google Scholar]
- 21. Cai A, Li L, Tan H, Mo Y, Zhou Y. Benign metastasizing leiomyoma. Case report and review of the literature. Herz. (2014) 39:867–70. doi: 10.1007/s00059-013-3904-1 [DOI] [PubMed] [Google Scholar]
- 22. Pitts S, Oberstein EM, Glassberg MK. Benign metastasizing leiomyoma and lymphangioleiomyomatosis: sex-specific diseases? Clin Chest Med. (2004) 25:343–60. doi: 10.1016/j.ccm.2004.01.014 [DOI] [PubMed] [Google Scholar]
- 23. Miller J, Shoni M, Siegert C, Lebenthal A, Godleski J, McNamee C. Benign metastasizing leiomyomas to the lungs: an institutional case series and a review of the recent literature. Ann Thorac Surg. (2016) 101:253–8. doi: 10.1016/j.athoracsur.2015.05.107 [DOI] [PubMed] [Google Scholar]
- 24. Yuan X, Sun Y, Jin Y, Xu L, Dai H, Wang J, et al. Multiple organ benign metastasizing leiomyoma: A case report and literature review. J Obstet Gynaecol Res. (2019) 45:2132–6. doi: 10.1111/jog.14066 [DOI] [PubMed] [Google Scholar]
- 25. Sawai Y, Shimizu T, Yamanaka Y, Niki M, Nomura S. Benign metastasizing leiomyoma and 18-FDG-PET/CT: A case report and literature review. Oncol Lett. (2017) 14:3641–6. doi: 10.3892/ol.2017.6609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mulita F, Tavlas P, Maroulis I. A giant ovarian mass in a 68-year-old female with persistent abdominal pain and elevated serum CA-125 level. Prz Menopauzalny. (2020) 19:108–10. doi: 10.5114/pm.2020.97870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mulita F, Oikonomou N, Tchabashvili L, Liolis E, Kehagias I. A giant ovarian mucinous tumor in a 58-year-old postmenopausal patient with persistent abdominal pain and high serum levels of CA 19-9. Pan Afr Med J. (2020) 37:76. doi: 10.11604/pamj.2020.37.76.25932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mulita F, Liolis E, Kehagias D, Tchabashvili L, Kaplanis C, Iliopoulos F, et al. An Enormous Pelvic Tumor in a 46-year-old Woman with an Elevated Serum CA 125 level, What Lies Beneath it? Investigation of Uterine Tumors in Postmenopausal Women. Prz Menopauzalny. (2021) 20:154–7. doi: 10.5114/pm.2021.109773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mulita F, Iliopoulos F, Plachouri KM, Kehagias I. Uterine leiomyoblastoma. BMJ Case Rep. (2021) 14:e241533. doi: 10.1136/bcr-2020-241533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lewis EI, Chason RJ, DeCherney AH, Armstrong A, Elkas J, Venkatesan AM. Novel hormone treatment of benign metastasizing leiomyoma: an analysis of five cases and literature review. Fertil Steril. (2013) 99:2017–24. doi: 10.1016/j.fertnstert.2013.01.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zong D, He W, Li J, Peng H, Chen P, Ouyang R. Concurrent benign metastasizing leiomyoma in the lung and lumbar spine with elevated standardized uptake value level in positron-emission tomography computed tomography: A case report and literature review. Med (Baltimore). (2018) 97:e11334. doi: 10.1097/MD.0000000000011334 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.