Abstract
Background
In Sudan, the Sterile Insect Technique (SIT) is being developed to suppress populations of Anopheles arabiensis. The present study was carried out to evaluate the impact of long-term colonisation, irradiation, and transportation on male vigour and mating competitiveness under controlled semi-field conditions.
Materials and Methods
Male mosquitoes were irradiated in Khartoum as pupae and transported 400 km to the field site in Dongola. Wild males and females were collected as immature stages (larvae and pupae) from the field site and sexed immediately after adult emergence. Competition experiments were carried out to test the mating competitiveness and vigour of colonised males (non-irradiated or irradiated) against wild conspecifics in the semi-field system.
Results
Mortality resulting from packaging and transportation from Khartoum to Dongola was low for adults (1.1% for irradiated and 1.3% for non-irradiated males). In contrast, all irradiated pupae died on their way to the field site. On average, 54.9% females were inseminated after one night. There were no differences between the number of females inseminated by colony males and those inseminated by wild males. Only a slightly significant difference between the numbers of females inseminated by irradiated males (14.0±1.7) or by wild males (19.7±1.7) was observed. However, the competitive index (CI) for irradiated and colony males when competed with wild males were 0.71 and 0.81 respectively.
Conclusions
Packing and transportation methods for pupae need to be improved. Prolonged colonisation (68 generations), irradiation and transportation of adult males did not affect their ability to locate virgin females and compete against wild conspecifics. Irradiation, in contrast to many reports, only had a marginal effect on released males during the first night after their release. These findings support the feasibility of staging an SIT campaign against this malaria vector.
1 Introduction
Malaria represents a serious health problem with a high number of fatalities, especially in tropical regions of sub-Saharan Africa [1]. In Sudan, it causes an estimated 9 million cases annually with more than 44 thousand deaths, especially in young children [2]. In Sudan, the disease is caused mainly by Plasmodium falciparum and transmitted through the bite of infected Anopheles arabiensis [3]. Anopheles arabiensis is the most widespread member of An. gambiae complex, endemic throughout most of the Afrotropical region, extending northwards along the River Nile to 20°N in Sudan [4]. In northern Sudan, in the Dongola and Karima/Merowe areas, An. arabiensis was found breeding throughout the year in different types of breeding sites including the temporary flood pools created by the Nile River during the flood season when mosquito densities reach their highest level [5]. The presence of this vector represents a serious health threat in this area where the malaria cases occur during most of the year with its peak of incidence in May and June [6].
The control of malaria in Africa faces major difficulties due to the problem of resistance of the parasite to the most commonly used drugs and mosquito vectors to insecticides [1]. This situation emphasises the need for the development of alternative vector control technologies such as those based on the release of sterilised or genetically modified mosquitoes [7]. Major historical successes of the Sterile Insect Technique (SIT) have been obtained against insect pests such as the New World screwworm, fruit flies, tsetse flies and codling moths [9-11]. These successes led to the initiation of a feasibility study for the use of SIT against An. arabiensis in Sudan [12]. Mosquito SIT is thus not new and has been undertaken previously. Trials were conducted in an isolated area (Lake Apastepeque) in El Salvador [13] where a field population of An. albimanus was eliminated for one season after releasing large numbers of sterile males. However, no attempt was made to sustain elimination permanently. Other mosquito SIT trials were unsuccessful in reducing populations, notably the trials in Burkina Faso, Pakistan and USA [14,15]. The main reason for these failures was considered to be the lowered mating competitiveness of the released males [15].
Successful use of SIT to control Anopheles mosquitoes depends on releasing large numbers of sufficiently competitive irradiated males. Released irradiated males should compete with wild conspecifics to induce population level sterility and thus suppress the targeted pest population [16]. However, male vigour (capacity of natural mating of males) and mating competitiveness were thought to be affected by irradiation, which is repeatedly used as an argument to justify the release of genetically-engineered mosquitoes [16]. Only few studies have been conducted on the mating competitiveness of irradiated Anopheles mosquitoes in the field and in cage experiments such as those undertaken with An. culicifacies [18] and An. arabiensis in a semi-field environment [19]. Helinski et al. [19] found that un-irradiated and irradiated laboratory-reared males were able to inseminate wild females in a field cage. Moreover, Helinski and Knols [20] found that irradiated males competed successfully with un-irradiated males for females in small mosquito cages. The authors also reported that the dose used for irradiation of pupae or adults affected male competitiveness. Pupal irradiation with a high dose (120 Gy) resulted in the lowest competitiveness, whereas adult irradiation with a low dose (70 Gy) gave the highest. In another report [21] they showed that the low dose (70 Gy) induced sterility of 83 and 92% in the pupal and adult stage, respectively, whereas the high dose (120 Gy) induced >99% sterility in both stages.
To move beyond the confines of the laboratory and small-cage trials, this study was conducted in a large contained semi-field system in northern Sudan. In it, the mating competitiveness of non-irradiated or irradiated colony An. arabiensis males for wild virgin females was tested against wild males.
2 Materials and Methods
2.1. Study area
The study was conducted in Dongola, which is located on the west bank of the Nile river (19° 10’ N, 30° 28’ E), 560 km from the capital Khartoum. The ecology of the area was described in detail by Malcolm et al. [6]. The soil on either side of the river is rocky and sandy with silt brought down by seasonal alluvial floods that spread on the narrow flood plains (1-4 km from the river). The area lies within the scrub vegetation belt on the Nubian Desert. Crops including vegetables and grains are cultivated on the rich alluvial deposits when the floodwater recedes. The climate of the area is characterised by extreme temperatures and minimal rainfall (≈10 mm per annum). Maximum temperature reaches 47°C in summer (May to August), the minimum is 7-10 °C in winter (December to February) and the mean monthly temperature is around 43°C. The residential area is restricted to the narrow banks of the River Nile where residents mainly depend on irrigated agriculture.
2.2. Mosquitoes
Colonised mosquitoes used in these experiments were from the 68th generation of a colony started from specimens collected from the same area in 2004. This colony was maintained at the Tropical Medicine Research Institute in Khartoum and rearing procedures were described by Helinski et al. [19]. Wild specimens were collected as immature stages at 3rd, 4th larval instars and/or pupal stage from breeding sites near Dongola and reared to adults in the Dongola insectary under optimal conditions. Adult sexing was done as described by Helinski et al. [19]. To ensure virginity of the wild females prior to sexing, 30 specimens were randomly selected from the emergence cage, dissected under dissection microscope and examined under a phase contrast microscope for their insemination status.
2.3. Irradiation
Male An. arabiensis were irradiated as described by Helinski et al. [21] using a Cobalt-60 Gammacell (Nordion 220). Sexing of pupae was done by observing the shape of terminalia under a dissection microscope. Male pupae at 22-26 hrs of age were transferred into an irradiation container in batches of ca. 500 individuals. Irradiation was performed at the Soba laboratory (45 km south of Khartoum) of the Sudan Atomic Energy Commission (SAEC) with a partially sterilising dose of 70 Gy/56 sec which induced 83% sterility in pupal stages [20]. In this study, the low sterilising dose (70 Gry) was selected because it has less effect on male mating competitiveness than that induced by the full sterilising dose (120 Gy) [20]. A dosimetry system was used to measure the dose received by every batch of irradiated pupae using the Gafchromic HD-810 film (International Specialty Products, NJ, USA). One dosimeter was used with each batch of pupae and read after irradiation at the Seibersdorf laboratory (by M. Helinski) with a Radiachromic reader (FarWest Technology Inc., California, USA). Pupae were then kept in cups and left to emerge in a cage. Adult males were maintained on a 10% sucrose solution. Pupae were transported to the irradiation source in a small holding container with a screw top to prevent spilling and the adults were transported in a normal rearing cage, placed in a cardboard box covered with wet towels to maintain a high humidity level.
2.4. Packaging and transportation
Mosquitoes were transported from Khartoum to Dongola as adults or pupae. Batches of fifty adult males were transferred into paper cups and provided with sugar solution. The cups were then placed in a cardboard box and fixed by pieces of packing material. Small wetted towels were placed on the top of the cups to maintain high humidity. The box was sealed by tape and transported to Dongola by aircraft. The trip from the laboratory in Khartoum to Dongola took two hrs, i.e. 20 min by road from the laboratory to Khartoum airport, 50 min waiting at the airport, 40 min by flight to Dongola and 10 min to TMRI station in Dongola. Alternatively, irradiated mosquitoes were transported to Dongola by either public transport (bus) or by project vehicle which took 6-7 hrs. Irradiated pupae were transported only once by public transport; irradiated adults were transported by aircraft and/or project vehicles. The irradiated pupae were placed in cups containing small amounts of water and packed in a box as described above. All mosquitoes were transferred to mosquito cages upon arrival in Dongola until used in the experiments.
2.5. Semi-field system
A contained semi-field system was constructed in Dongola in 2006, consisting of a metal frame (18×8×2.75 m) covered with dark-green shade netting that permits airflow and light entry to simulate ambient conditions. The system was divided into three equal sections (6×8 m2) (Fig. 1) each with a separate outside door to carry out replicate experiments simultaneously. The partitions between the sections were made by gluing mosquito netting to the metal structure and fixing it at ground level with clay and bricks. To simulate the natural ecosystem of An. arabiensis, each section of the contained semi-field system was equipped with different resting sites and plants (Fig. 1) (for additional details see Helinski et al. [19]).
Figure 1.
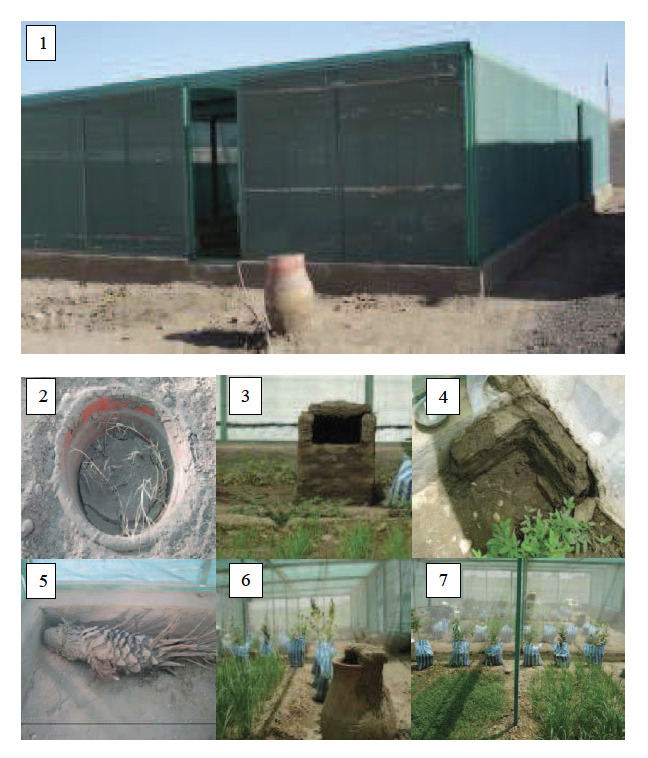
View of the contained semi-field system (1), different resting places: artificial breeding site (2, plastic basin), kuseba (3), corner site (4, made of mud bricks), palm tree trunk (5) and zir (6); Picture 7 shows plant beds seeded with bean and maize and plants rooted in plastic bags.
Climatic conditions inside the system were monitored using HOBO H8 data loggers at one meter height above ground level fixed on stands inside and outside the system to record ambient temperature and relative humidity. Additional data loggers were fitted in different microhabitats inside the system and recorded temperature and relative humidity at 15 min intervals.
2.6. Marking of males
Three hours before releasing mosquitoes in the semi-field system fifty males were selected from cages and placed in a paper cup. The netting mesh of the cup was covered with a piece of plastic to prevent the dispersal of fluorescent powder during marking. The males were then dusted with the powder using a syringe with a long needle. The plastic was removed after 5 minutes and the dusted males were provided sucrose solution until release. Two fluorescent colours were used to discriminate colony (green) and wild males (pink).
2.7. Competition assays
Before running the competition experiments, the relative humidity inside each section of the semi-field system was increased by watering the plant beds every morning. The artificial resting sites were wetted with water and a container of sugar solution (a sucrose solution and a drop of honey to serve as an attractant) was placed in or near each resting place in each section.
Three replicates of mating experiments were done using 3-5 day old irradiated males, wild males and wild virgin females (Ratio 1:1:1). The first replicate consisted of 150, the second of 100, and the third of 75 specimens for each of the three groups. Each experiment was started by releasing the dusted males of the two types and females in a compartment before sunset (16.30 hrs) and leaving them for one night to mate. Recapturing of the released mosquitoes started the following morning at 07.30 hrs and continued until 15.30 hrs. Females were recaptured using clean aspirators and transferred immediately to paper cups covered with fine mesh. To avoid contamination of captured females by the dust particles, aspirators were washed (two to three times) with absolute ethanol and dried before and after the recapturing process. Subsequently, the recaptured females were dissected using a dissection microscope and examined under phase contrast to confirm their insemination status. Upon female dissection, only the two last segments were immersed in a drop of normal saline on a glass slide. The numbers of inseminated females were recorded. Then the dead (dissected) females were left for 6 hrs to air dry and then kept individually in 50 ml tubes.
In Khartoum all the dissected females (un-inseminated and inseminated females) were examined under an ultraviolet light source for the presence of fluorescent dust particles on their bodies in order to identity with which male group she had mated. When the dust particles were detected, the female mosquitoes with pigments of each dust colour (pink or green pigments) were put individually on a glass and were photographed using a gel documentation system. Consideration was given to the presence of the two colours on a single female (double mating), and un-inseminated fmales with or without colour (to estimate false-positives).
Another three replicates of competition assays were conducted to test the mating vigour and competitiveness of the colony males against the wild males for wild virgin females. Procedures were similar to those described above. For each replicate 250 mosquitoes were released for each group (750 per replicate in total).
2.8. Statistical analysis
The data obtained from the mating competitiveness experiments was first normalised by log transformation and then the two means in each experiment were compared using t-tests. All data were analysed using SPSS (version 13) software. The competiveness index (CI) (the ratio of females inseminated by each male type) was calculated for each irradiated and colony males against the wild males in each experiment using the mathematical model: C (CI) = I1/IC, where C is the rate at which experimental males succeed in inseminating females divided by the rate at which those males succeed when in competition with others.
3 Results
The observed mortality due to handling and transportation was very low for adult mosquitoes. It was 1.1% (4/390) for irradiated males and 1.3% (10/1700) for non-irradiated colony males. However, all pupae died during transportation and no emergence were observed.
The average temperature and relative humidity outside (35.7±5.1°C; 12.9±5.2 RH%) and inside (36.2±6.3°C; 12.9±5.3 RH%) the contained semi-field system were similar during the experiments. The temperature and RH% of the artificial brick resting site in the corners (25.1±5.0 °C; 55.0±14.6 RH%) and the Kuseba (23.0±4.5°C; 83.8±19.6 RH%) were cooler and more humid than the palm tree (30.0±6.0°C; 18.0±6.7 RH%), Zir (25.6±5.0°C; 73.7±2.6 RH%) and the artificial breeding site (plastic basins) (25.5±5.9°C; 54.2±14.0 RH%).
Table 1 shows the number and percentages of recaptured mosquitoes and mated females when irradiated males competed against wild males. Recapture rates were high for all groups (Table 1). The results of the competition experiments of irradiated and wild males (at a ratio 1:1) showed that the average insemination rate of the pooled females after one night was 54.6%). Results of the competition experiment showed that the number of females inseminated by irradiated males (14.0±1.7) was lower than those inseminated by wild males (19.7±1.7) although this difference was only marginally significant (t=2.779; P=0.043).
Table 1.
Numbers and % of irradiated and wild Anopheles arabiensis recaptured and mated during mating competitiveness experiments conducted in a semi-field system.
| Replicate | Recapture rate of mosquitoes | Insemination rate of ♀ by | Total | |||
|---|---|---|---|---|---|---|
| ♀wild | ♂wild | ♂irradiated | irradiated ♂ | wild ♂ | ||
| 1 | 68.0 | 80.7 | 90.0 | 42.5 | 57.5 | 58.8 |
| (102/150)* | (121/150) | (135/150) | (17/40) ** | (23/40) | (40/68) *** | |
| 2 | 72.0 | 92.0 | 85.0 | 37.9 | 62.1 | 43.9 |
| (72/100) | (92/100) | (85/100) | (11/29) | (18/29) | (29/66) | |
| 3 | 76.0 | 96.0 | 93.3 | 53.7 | 56.3 | 62.7 |
| (57/75) | (72/75) | (70/75) | (14/32) | (18/32) | (32/51) | |
| Total | 66.5 | 87.7 | 89.2 | 41.9 | 58.1 | 54.6 |
| (216/325) | (285/325) | (290/325) | (42/101) | (59/101) | (101/185) | |
| P value | 0.043 | |||||
* % (recaptured / released)
** % (♀ inseminated by each male type / total ♀ inseminated)
*** % (Total ♀ inseminated / total ♀ dissected)
Table 2 shows the recapture rates of mosquitoes after one night in the semi-field system when non-irradiated colony males were competed against wild males. Recapture rates were high, with a 55.1% average insemination rate by both colony and wild males after one night (Table 2). No significant difference was detected between the number of females inseminated by colony males (18.7±0.3) and those inseminated by wild males (23.0±2.7) (t=2.16; P=0.09).
Table 2.
Numbers and % of colony and wild Anopheles arabiensis recaptured and mated during mating competitiveness experiments conducted in a semi-field system.
| Replicate | Recapture rate of mosquitoes | Insemination rate of ♀ by | Total | |||
|---|---|---|---|---|---|---|
| ♀wild | ♂wild | ♂colony | colony ♂ | wild ♂ | ||
| 1 | 70.0 | 78.0 | 80.4 | 42.2 | 55.8 | 56.6 |
| (175/250)* | (195/250) | (201/250) | (19/43) ** | (24/43) | (43/76)) *** | |
| 2 | 66.4 | 84.8 | 79.6 | 40.0 | 60.0 | 66.2 |
| (166/250) | 212/250) | (199/250) | (18/45) | (27/45) | (45/68) | |
| 3 | 72.8 | 74.0 | 70.0 | 51.3 | 48.7 | 44.6 |
| (182/250) | (185/250) | (175/250) | (19/37) | (18/37) | (37/83) | |
| Total | 69 | 78.9 | 76.7 | 48.8 | 51.2 | 55.1 |
| 523/750 | (592/250) | (575/750) | (56/125) | (69/125) | (125/227) | |
| P value | 0.09 | |||||
* % (recaptured / released)
** % (♀ inseminated by each male type / total ♀ inseminated)
*** % (Total ♀ inseminated / total ♀ dissected)
Successful mating in the greenhouse was recorded for the green (wild males) and pink marked males (colony or irradiated males) with the non-marked virgin wild females in two separate experiments in a contained semi-field system. In the first experiment, 101 wild females were inseminated by either irradiated (42 females) or wild males (59 females). In the second experiment, 125 wild females were inseminated by either colony (56 females) or wild males (59 females) (Table 2). The inseminated females that had mated with either green (wild) or pink marked (colony or irradiated) males showed traces of pigmentation of the dust particles on their bodied (tip of the wings, thorax, and last abdominal segments) according to the dust particles of the male groups they mated with (Fig. 2). However, none of the inseminated females showed no coloured pigmentation or showed pigmentations with the two colours. Also, none of the un-inseminated females showed pigmentation with either colour.
Figure 2.
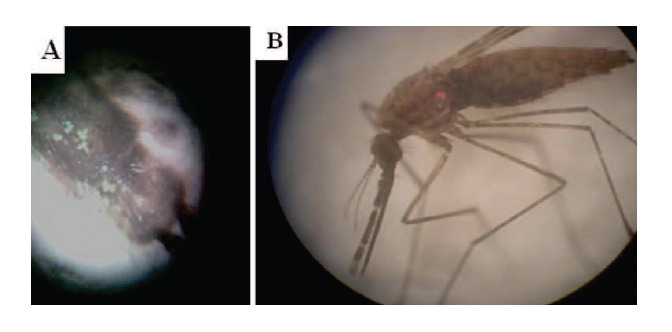
Traces of the fluorecent particles on the body parts of the wild females mated (inseminated) with wild males (green; A) and colony or irradiated males (pink; B) in the contained semi-field system.
Figure 3 shows the competitive indices for the irradiated and colony males which compete with the wild males in two separate experiments (3 replicates) in a contained semi-field system. The competition indices (average CI) were 0.71 and 0.81 for the irradiated and the colony males, respectively (Fig. 3).
Figure 3.
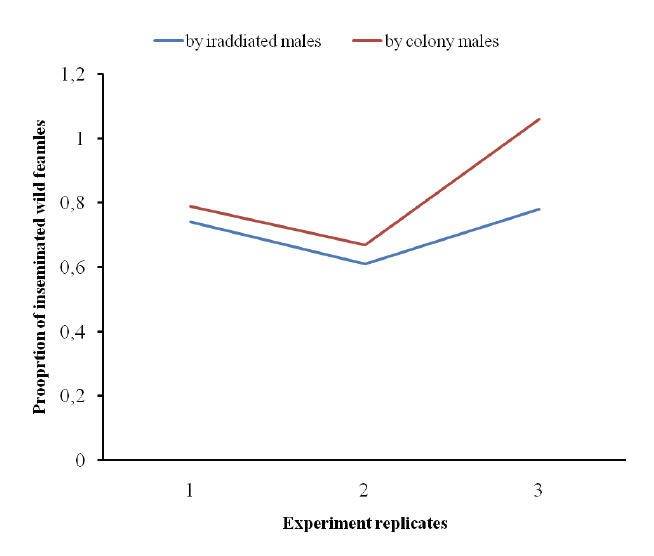
Competetive indices of irradiated and control males in the semi-field
4 Discussion
Knowledge on the methods of packing, transportation survival and mating behaviour of irradiated males is an important pre-requisite for a SIT project to suppress population of malaria vectors. Such information is of value to obtain successful releases with sufficient numbers of irradiated males in the field. However, little information is available on the mating competitiveness of irradiated male of An. arabiensis mosquitoes [19]. The results obtained in this study indicated the efficiency of the method used to transport adult males An. arabiensis by both airplane and road. The mortality rates of adult males recorded in this study were lower than previous results (< 6%) reported in the same area by Helinski et al. [19]. This could be due to the difference in timing of the two studies. However, the methods need to be improved for future releases when much larger numbers of irradiated males and/or pupae will be packed and transported.
In a similar study, with transportation and ground release of adult An. albimanus using a flat transportation cage in El Salvador release trials in the 1970s [22], the mortality of irradiated adult was also very low. Unlike the present finding of high mortality amongst the packed pupae, a successful release of pupae was performed in El Salvador during which large numbers of pupae were released in cups or pans and left to emerge in the field [22-24]. However, the high mortality rates among the irradiated pupae in this study might be due to the cups and the amount of the water used for packing and/or common transportation used. This problem can be resolved in future by improving the packing methods for irradiated pupae, such as packing them in wet filter papers placed on large petri dishes instead of suspending them in water. Also, transportation of irradiated pupae should be done either by aircraft or project vehicles, which will offer more possibilities for careful handling of the irradiated pupae during the journey.
For SIT programmes to be successful they need to establish a suitable ratio of irradiated males versus wild males to enable effective competition with wild males for females from the target population [25]. Unless the released males compete successfully with the wild males, the wild females will continue to support the population with fertile eggs and hence new generations. In contrast to persistent claims that irradiated males have impaired competitiveness, the results from the present study show the ability of colonised and irradiated males to compete effectively for wild females in a contained semi-field system. Similar mating competiveness of released males of An. arabiensis in cage experiments in Sudan [19] and An. culicifacies in the field in Pakistan [18] have been reported. The female insemination rates recorded in this study were similar to those reported by Helinski et al. [19]. These high insemination rates may have been affected by the time of release, at 5.30 pm. It is known that the mating of anopheline mosquitoes is crepuscular [26] as in members of the An. gambiae complex and An. funestus [27,28].
The equal numbers of wild females inseminated by the colony and wild males indicates that the colony males were vigourous enough to compete with wild males under these semi-field conditions. However, the significance of the difference in the number of females inseminated by irradiated males and wild males may indicate that the irradiation affects male vigour [16], although this was by far not as dramatic as reported previously [15].
The mating process in mosquitoes is known to be more complex and the copulation takes place for less than 16 seconds [29]. However, studies on mating competitiveness in the field-cages and the laboratory had shown some difficulties especially in recording copulation of mosquitoes [30]. Therefore, more sensitive techniques should be used to record the mating and insemination in female mosquitoes these techniques like semen labels stable isotopes or (e.g. 13C and 15N) [31] or marking the insect body with florescent dyes [32]. The labels have been reported to be transferred to the females during the mating as an indicator for mating [32,33]. However, both label types have drawbacks. For example, in the case of using stable isotopes, the label does not persist in spermathecae of the females more than 5 days after insemination [33]. In the case of fluorescent dyes, the excessive amount of dust can kill the insect or produce adverse behavioural effects.
In this study, traces of pigmentations of the fluorescent dust particles were detected on the bodies of inseminated females mated with colour-marked males in the mating competitiveness experiments in the semi-field system. The traces of the particles were clearly detected on the female’s thorax, wings and abdomen. This finding might be of value for use of the marker in mating competiveness studies in insect vectors and to estimate the insemination rates in females by different male types after copulation.
During the contained semi-field system experiments, recapture rates of the released mosquitoes were high although difficulties were experienced in collecting the unmarked specimens (wild females). The mosquitoes were collected by active search of the artificial resting sites in the system. More mosquitoes were collected from the corner brick sites and kuseba than from the palm tree, zir and the artificial breeding site (plastic basins) which may reflect the fact that the two sites were much cooler and humid as indicated from the data that recorded by the HOBO data loggers. In all experiments the recapture rates were higher than those reported by Helinski et al. [19]. However, the recapture rates of dusted males (colony and irradiated males) were higher than wild males and wild females and this is in agreement with what was observed by Helinski et al. [19]. The high recapture rates of mosquitoes obtained in this study indicate that the colony and irradiated males can survive under such conditions although information on male survival in the field is scarce and observations beyond one or two days were not performed. Studies elsewhere (Ng’habi, pers. commun.) have shown that an An. arabiensis population can be sustained in semi-field environments for at least twenty generations. Further studies, in which released (irradiated) males are followed for longer periods, should provide further insight in the likely performance of males in SIT campaigns.
5 Conclusions
The results from the competition experiments in this study indicate that colonised and irradiated male An. arabiensis mosquitoes are able to compete successfully against wild males for field-collected virgin females. Colonisation for four years under laboratory conditions had no major impact on the vigour and mating competitiveness of male An. arabiensis compared to wild males. The slight difference in mating competitiveness between irradiated and wild males could be compensated by increasing the numbers of irradiated males to be released.
Acknowledgments
Our special thanks to Dr. Hameed Nugud and Dr. Osman M. Abdel Nour for their help during this study and Sudan Atomic Energy Commission (SAEC) staff at Soba laboratory for their help with gamma radiation. We are grateful to the staff of Northern State Ministry of Health (NSMOH) for their help in the field. Also our thanks to Dalia El-Dirdeiry, Hazar Badawi, Maha A. Abdalla and Tellal B. Ageep for their help in preparing and sending mosquitoes to the field. This study was financially supported by the Tropical Medicine Research Institute and IAEA TC programme and technically by FAO/IAEA as part of the IAEA Regional Project (RAF 5/052).
Author contributions
MMH designed and performed the experiments and drafted the manuscript. WME participated in the experiments. RTA prepared and irradiated the mosquitoes. BBE assisted in the design of the experiments and final manuscript drafting.
References
- 1.WHO. World malaria report WHO/HTM/GMP/2008.1. World Health Organization Geneva; 2008. [Google Scholar]
- 2.Abdalla SI, Malik EM, Ali KM. The burden of malaria in Sudan: incidence, mortality and disability - adjusted life - years. Malar. J. 2007;6:97. doi: 10.1186/1475-2875-6-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamad AA, Nugud AH, Arnot DE, Giha HA et al. A marked seasonality of malaria transmission in two rural sites in eastern Sudan. Acta Trop. 2002;831:71–82. doi: 10.1016/s0001-706x(02)00059-1. [DOI] [PubMed] [Google Scholar]
- 4.Dakeen MY, Omer SM. Ecology of malaria vector Anopheles arabiensis Patton (Diptera: Culicidae) by the Nile of northern Sudan. Bull. Entomol. Res. 1986;76:451–467. [Google Scholar]
- 5.Ageep TB, Cox J, Hassan MM, Knols BGJ et al. Spatial and temporal distribution of the malaria mosquito Anopheles arabiensis in northern Sudan: influence of environmental factors and implications for vector control. Malar. J. 2009;8:123. doi: 10.1186/1475-2875-8-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malcolm CA, El Sayed B, Babiker A, Girod R et al. Field site selection: getting it right first time around. Malar. J. 2009;8(Suppl 2):S7. doi: 10.1186/1475-2875-8-S2-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Catteruccia F, Crisanti A, Wimmer EA. Transgenic technologies to induce sterility. Malar. J. 2009;8(Suppl 2):S7. doi: 10.1186/1475-2875-8-S2-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson AS, Knols BGJ, Voigt G, Hendrichs J. Conceptual framework and rationale. Malar. J. 2009;8(Suppl 2):S1. doi: 10.1186/1475-2875-8-S2-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dyck VA, Hendrichs J, Robinson AS. The Sterile Insect Technique: Principles and practice in area-wide integrated pest management. Springer Publishers; Dordrecht: 2005. [Google Scholar]
- 10.Hendrichs J, Franz G, Rendon P. Increased effectiveness and applicability of the Sterile Insect Technique through male-only release for control of Mediterranean fruit-flies during fruiting seasons. J. Appl. Entomol. 1995;119:371–377. [Google Scholar]
- 11.Vreysen MJ, Saleh KM, Ali MY, Abdulla AM et al. Glossina austeni (Diptera: Glossinidae) eradicated on the island of Unguja, Zanzibar, using the sterile insect technique. J. Econom. Entomol. 2000;93(1):123–35. doi: 10.1603/0022-0493-93.1.123. [DOI] [PubMed] [Google Scholar]
- 12.El Sayed BB, Malcolm CA, Babiker A, Malik EM et al. Ethical, legal and social aspects of the approach in Sudan. Malar. J. 2009;8(Suppl 2):S3. doi: 10.1186/1475-2875-8-S2-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lofgren CS, Dame DA, Breeland SG, Weidhaas DE et al. Release of chemosterilized males for the control of Anopheles albimanus in El Salvador: Field methods and population control. Am. J. Trop. Med. Hyg. 1974;23:288–297. doi: 10.4269/ajtmh.1974.23.288. [DOI] [PubMed] [Google Scholar]
- 14.Reisen WK, Sakai RK, Baker RH, Rathor HR et al. Field competitiveness of Culex tritaeniorhynchus Giles males carrying a complex chromosomal aberration: a second experiment. Ann. Entomol. Soc. Am. 1980;73:479–484. [Google Scholar]
- 15.Benedict MQ, Robinson AS. The first releases of transgenic mosquitoes: an argument for the sterile insect technique. Trends Parasitol. 2003;19:349–355. doi: 10.1016/s1471-4922(03)00144-2. [DOI] [PubMed] [Google Scholar]
- 16.Alphey LS. Re-engineering the Sterile Insect Technique. Insect Biochem. Mol. Biol. 2002;32:1243–1247. doi: 10.1016/s0965-1748(02)00087-5. [DOI] [PubMed] [Google Scholar]
- 17.Howell PI, Knols BGJ. Male mating biology. Malar. J. 2009;8(Suppl 2):S8. doi: 10.1186/1475-2875-8-S2-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baker RH, Reisen WK, Sakai RK, Rathor HK et al. Anopheles culicifacies mating behavior and competitiveness in nature of males carrying a complex chromosomal aberration. Ann. Entomol. Soc. Am. 1980;73:581–588. [Google Scholar]
- 19.Helinski MEH, Hassan MM, El-Motasim WM, Malcom CA et al. Towards a sterile insect technique field release of Anopheles arabiensis in Sudan: Irradiation, transportation, and field cage experiments. Malar. J. 2008;7:65. doi: 10.1186/1475-2875-7-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Helinski MEH, Knols BGJ. Mating competitiveness of male Anopheles arabiensis mosquitoes irradiated with a partially or fully sterilizing dose in small and large laboratory cages. J. Med. Entomol. 2008;45(4):608–705. doi: 10.1603/0022-2585(2008)45[698:mcomaa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 21.Helinski MEH, Parker AG, Knols BGJ. Radiation-induced sterility for pupal and adult stages of the malaria mosquito Anopheles arabiensis. Malar. J. 2006;5:41. doi: 10.1186/1475-2875-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bailey DL, Lowe RE, Fowler JEF, Dame DA. Sterilizing and packaging males of Anopheles albimanus Wiedemann for field release. Am. J. Trop. Med. Hyg. 1979;28:902–908. [PubMed] [Google Scholar]
- 23.Dame DA, Lofgren CS, Ford HR, Boston MD et al. Release of chemosterilized males for the control of Anopheles albimanus in El Salvador. II. Methods of rearing, sterilization, and distribution. Am. J. Trop. Med. Hyg. 1974;23:282–287. doi: 10.4269/ajtmh.1974.23.282. [DOI] [PubMed] [Google Scholar]
- 24.Lowe RE, Bailey DL, Dame, DA, Savage K, Kaiser PE. Efficiency of techniques for the mass release of sterile male Anopheles albimanus Wiedemann in El Salvador. Am. J. Trop. Med. Hyg. 1980;29:695–703. doi: 10.4269/ajtmh.1980.29.695. [DOI] [PubMed] [Google Scholar]
- 25.Richardson RH. The screw worm problem. University of Texas Press; Austin: 1978. [Google Scholar]
- 26.Yuval B. Mating systems of blood-feeding flies. Annu. Rev. Entomol. 2006;51:413–440. doi: 10.1146/annurev.ento.51.110104.151058. [DOI] [PubMed] [Google Scholar]
- 27.Charlwood JD, Pinto J, Sousa CA, Ferreira C, Do Rosario VE. Male size does not affect mating success of Anopheles gambiae in Sao Tome. Med. Vet. Entomol. 2002;16:109–111. doi: 10.1046/j.0269-283x.2002.00342.x. [DOI] [PubMed] [Google Scholar]
- 28.Charlwood JD, Thompson R, Madsen H. Observations on the swarming and mating behaviour of Anopheles funestus from southern Mozambique. Malar. J. 2003;2:2. doi: 10.1186/1475-2875-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang F. No love for Mosquitoes. In the fight against malaria and other insect-borne diseases, scientists take aim at sixteen seconds of mosquito mating. IAEA Bullet. 2008;50:26–27. [Google Scholar]
- 30.Ng’habi KR, John B, Nkwengulila G, Knols BGJ, Killeen GF, Ferguson HM. Effect of larval crowding on mating competitiveness of Anopheles gambiae mosquitoes. Malar. J. 2005;4:49. doi: 10.1186/1475-2875-4-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Helinski MEH, Hood RC, Knols BGJ. A stable isotope dual-labelling approach to detect multiple insemination in unirradiated and irradiated Anopheles arabiensis mosquitoes. Parasites & Vectors. 2008;1:9. doi: 10.1186/1756-3305-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sied-Ahmed OME. On mating biology of male Anopheles arabiensis Patton (Diptera: Culicidae): Implications in the use of sterile insect technique in vector control. University of Khartoum; 2006. p. 136. MSc. thesis submitted for Faculty of Science, [Google Scholar]
- 33.Helinski MEH, Hood RC, Gludovacz D, Mayr L, Knols BGJ. A 15N stable isotope semen label to detect mating in the malaria mosquito Anopheles arabiensis Patton. Parasites & Vectors. 2008;1:19. doi: 10.1186/1756-3305-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]


