Abstract
Autoimmune encephalitis (AIE) is a rapid, progressive neurological disorder characterized by nervous system inflammation. While the Graus criteria are the best known criteria for AIE diagnosis, other differential diagnoses meeting the Graus criteria must be considered before management. This narrative review discusses the most common etiologies that resemble AIE. We suggest routine exclusion of mimickers meeting the Graus criteria before confirming an AIE diagnosis. We reviewed 28 studies including 356 patients. The main initial diagnosis was AIE, then paraneoplastic limbic encephalitis and anti-N-methyl-D-aspartate receptor encephalitis. Only 194 patients met the possible Graus criteria. The most frequent conditions among the total population were dementia, other neurodegenerative diseases, and psychiatric and functional neurological disorders. AIE is often misdiagnosed, leading to unnecessary treatment. Despite publication of the Graus criteria, medical cases mimicking this condition are being published. Many neurological diseases entering the differential diagnosis of AIE could be excluded through a detailed history, neurological examination, laboratory analysis, and other investigations, including cerebrospinal fluid and brain magnetic resonance imaging. However, some differential diagnoses complied with the possible Graus criteria, with some having concurrent antineuronal antibodies, which were considered true mimickers. AIE diagnosis suspicion is primarily clinical, but a definitive diagnosis requires various diagnostic tools.
Keywords: Autoimmune encephalitis, mimicker, encephalitis, Graus criteria, literature review, neurological disorder
Introduction
Autoimmune encephalitis (AIE), the third most common cause of encephalitis, with a prevalence of 13.7/100,000), 1 is a rapid, progressive neurological disorder that is characterized by inflammation of the brain and other areas of the nervous system. It is caused by an abnormal immune response 2 with production of antibodies against cell surface, synaptic, or intraneuronal antigens, also known as onconeural antibodies. 3
AIE is classified anatomically depending on its location (limbic, cortical/subcortical, striatal, diencephalic, brainstem, cerebellar, encephalomyelitis, meningoencephalitis or combined) or serologically (antibodies to intracellular antigens, antibodies to surface antigens, seronegative AIE). 2
Despite the progress that has been made in this field, the diagnosis of AIE remains clinical. 2 The Graus criteria for AIE are the most well-known criteria for AIE diagnosis, although two later studies showed that the sensitivity and specificity of these criteria did not exceed 80% and 84% to 94%, respectively.4,5 Moreover, these criteria do not take into account that AIE tends to develop gradually before manifesting the classical clinical, laboratory, and radiological picture that fulfills the diagnosis, 5 and the diffuse inflammation caused by antibodies leads to different syndromes depending on its location. 2 Those syndromes may sometimes overlap, 2 and therefore it is difficult to establish a single criterion encompassing them all. In addition, many studies concluded a final diagnosis of AIE—particularly anti-N-methyl-D-aspartate (NMDA) encephalitis—despite not achieving probable anti-NMDA criteria.6,7 Furthermore, the existence of anti-neuronal antibodies failed to be pathognomonic because 62% of patients tested positive for these antibodies, and these patients had alternative diagnoses in the study by Abboud et al. 2
To increase the complexity, the magnetic resonance imaging (MRI) results, which is the primary tool used for AIE diagnosis, can mimic those of other lesions such as stroke and tumors. Under the appropriate clinical picture, MRI results cannot differentiate between different immune-mediated conditions (AIE, sarcoidosis). 2 Once the diagnosis is made, early initiation of immunotherapy is crucial for the prognosis. 8 However, before starting immunotherapy, other possible diagnoses, some of which may benefit from this treatment such as central nervous system lymphoma, should be excluded.
Because of the above, AIE is a challenging diagnosis as almost any brain lesion can enter its differential diagnosis list, including infections, demyelinating and other autoimmune disorders, nutritional deficiencies, neurodegenerative diseases, primary brain tumors, and even primary psychiatric disorders, paraneoplastic sarcoidosis, and neurosarcoidosis.2,9 Many of these mimickers can be excluded via a detailed history, examinations, cerebrospinal fluid (CSF) findings, and radiological imaging. 8 Furthermore, AIE is usually acute, subacute, or in specific cases chronic and follows a progressive or monophasic pattern. Thus, hyperacute presentation or relapse-remitting patterns should raise suspicion of other entities such as vasculitis or multiple sclerosis. 2
This manuscript is a narrative review aiming to identify the most common etiologies that resemble AIE, focusing on the prevalence of such misdiagnoses, and discussing possible reasons for these medical errors to prevent the inappropriate use of steroids. In this study, we performed a thorough review of mimicking conditions that met the probable AIE Graus criteria and which we believe should be in the forefront of the differential diagnosis of AIE. We also suggest that these conditions should be routinely considered as possible diagnoses when performing a work-up.
Methods
Search strategy
We conducted an electronic database search for AIE mimicker studies in PubMed and Google Scholar using the following search terms: (mimic*) AND (((“Brain Inflammation”) OR (Encephalitis)) OR (encephalomyelitis)). A total of 1589 studies were found. In addition to the references, a manual search was performed. We limited the search results to English-language publications.
Inclusion and exclusion criteria
We included any type of publication (case report, case series, cohort) that included the following:
Patients with disorders that mimicked or resembled the clinical and/or radiological features of AIE
All studies meeting Graus’ possible criteria
People from all age groups
English literature
Exclusion criteria:
Studies that did not fulfill both the clinical and radiological features of AIE
Publications in which AIE was the final diagnosis or studies mentioning cases of AIE presenting as other diseases
The studies included diseases mimicking AIE such as Hashimoto’s encephalopathy, acute disseminated encephalomyelitis, Rasmussen encephalitis, Bickerstaff brainstem encephalitis, Morvan syndrome, stiff person syndrome, and other disease entities that share the same immune backgrounds (see the Limitations section).
Data collection and statistical analysis
Data extraction was performed by MZBA and MSA using Endnote X8 and Microsoft Excel to format the table. The extraction table includes the authors’ names, year, study design, number of patients, country, age, sex, presentation, initial diagnosis, etiology or final diagnosis, method of diagnosis, laboratory tests, and imaging investigations.Six authors (MZBA, MSA, MZ, TB, MT, NA) screened the studies according to the inclusion and exclusion criteria to select appropriate articles. Regarding the etiology, the data were collected using two independent methods: general and then only cases that fulfilled the possible Graus criteria.
Results
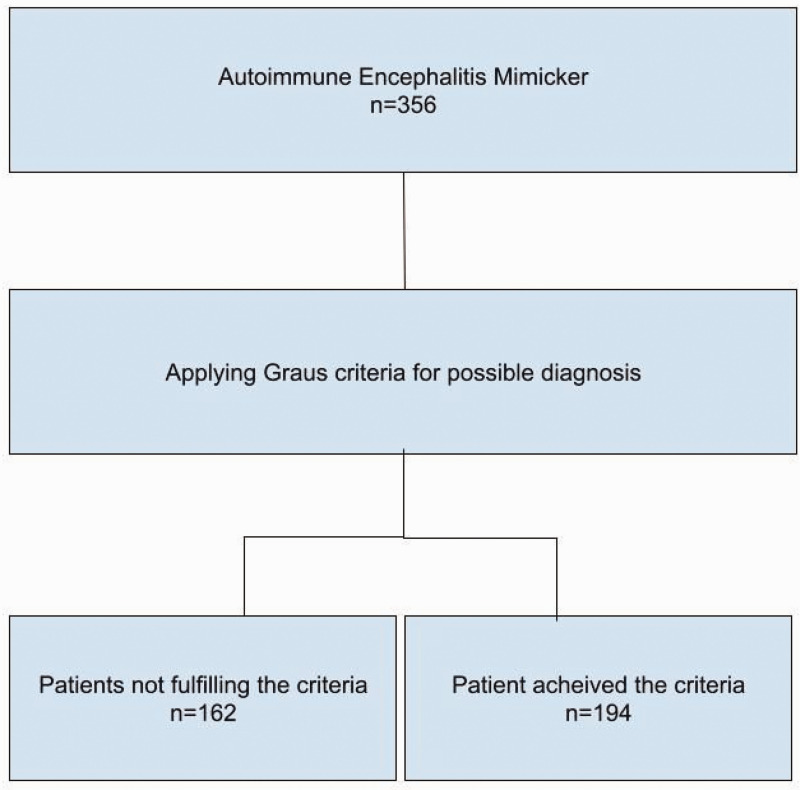
A total of 1589 studies were found. After excluding duplicate papers and applying the inclusion and exclusion criteria, we identified 28 studies for inclusion in our review. Those studies were included and formatted in the extraction table. The included studies were published between 2005 and 2023. These studies included 19 case reports, 1 case series (6 patients), 3 retrospective studies (109 + 109 + 107 patients), 4 letters to the editor, and 1 short communication (Figure 1) (Table 1).
Figure 1.
Flow chart of the patients.
Table 1.
Baseline Information.
| Year | Author | Article design | n | Country | Age group (years) | Sex | Main symptoms |
|---|---|---|---|---|---|---|---|
| 2022 | Diogo Costa et al. 5 | retrospective study | 109 | Portugal | Median age: 61 | m/f | altered mental status (50.8% of all patients).movement disorders (28.2%)new-onset seizures (27.4%) neuropsychiatric symptoms (25.8%) working memory deficits (23.4%) focal neurological signs (22.6%) |
| 2023 | Flanagan et al. 12 | retrospective study | 107 | USA | Median age: 48 | 42 m/65 f | short term memory loss (34%) altered mental status (40%)psychiatric symptoms (39%) |
| 2017 | Budhram et al. 13 | case report | 1 | Canada | 50–54 | m | behavioral or psychiatric symptoms and seizures |
| 2018 | Thomas et al. 14 | case report | 1 | Germany | 60–64 | m | seizures and working memory deficit |
| 2014 | Zuhorn et al. 15 | case report | 1 | Germany | 75–79 | f | memory deficit |
| 2019 | Lu et al. 16 | case report | 1 | China | 65–69 | m | seizures |
| 2023 | Van Steenhoven et al. 18 | retrospective cohort study | 109/239 | Netherlands and others | Median age: 15 | 47 m/62 f | new-onset seizures 39% working memory deficit 59%behavioral disorders 58%sleeping disorders 26% psychiatric symptoms 45%autonomous disorders 16%movement disorders 26% focal deficits 25%decreased level of consciousness 17% |
| 2012 | AbdeleRahman et al. 19 | case report | 1 | USA | 50–54 | m | memory deficit, behavioral or psychiatric symptoms |
| 2018 | Vogrig et al. 20 | case series | 6 | France | 60–64 | f | seizures |
| 30–34 | f | altered mental status, seizures, behavioral or psychiatric symptoms | |||||
| 60–64 | f | seizure and behavioral or psychiatric symptoms | |||||
| 55–59 | m | behavioral or psychiatric symptoms | |||||
| 65–69 | m | subacute onset, and behavioral or psychiatric symptoms | |||||
| 65–69 | m | behavioral or psychiatric symptoms | |||||
| 2020 | Macchia et al. 21 | short communication | 1 | USA | 40–44 | f | subacute onset, seizures, and altered mental status |
| 2009 | Badruddin et al. 25 | case report | 1 | USA | 20–24 | m | behavioral or psychiatric symptoms and movement disorder |
| 2020 | Devamare et al. 26 | letter to the editor | 1 | India | 5–9 | m | behavioral or psychiatric symptoms, seizures, and movement disorder |
| 2019 | Garg et al. 27 | letter to the editor | 1 | India | 5–9 | m | altered mental status, seizures, and decreased level of consciousness |
| 2021 | Harsha et al. 28 | case report | 1 | India | 30–34 | m | subacute onset, behavioral or psychiatric symptoms, and movement disorder |
| 2022 | Poon et al. 29 | case report | 1 | USA | 30–34 | m | behavioral and psychiatric symptoms |
| 2020 | Rigoni et al. 30 | letter to the editor | 1 | Italy | 45–49 | m | working memory deficit and behavioral or psychiatric symptoms |
| 2018 | Wiels et al. 31 | case report | 1 | Belgium | 30–34 | m | behavioral and psychiatric symptoms |
| 2010 | Nagata et al. 32 | case report | 1 | Japan | 70–74 | m | behavioral and psychiatric symptoms |
| 2015 | M Khair et al. 33 | case report | 1 | Qatar | 0–4 | m | behavioral or psychiatric symptoms and seizures |
| 2017 | Serrano-Cardenas et al. 34 | letter to the editor | 1 | Spain | 60–64 | m | memory deficit and behavioral or psychiatric symptoms |
| 2011 | Blondin et al. 35 | case report | 1 | USA | 45–49 | f | subacute onset, memory deficit, and seizures |
| 2005 | Scheid et al. 36 | case report | 1 | Germany | 30–34 | m | seizures |
| 2009 | Deramecourt et al. 37 | case report | 1 | France | 40–44 | f | behavioral or psychiatric symptoms and memory deficit |
| 2014 | Piola et al. 38 | case report | 1 | Italy | 20–25 | f | memory deficit and behavioral or psychiatric symptoms |
| 2022 | Liao et al. 39 | case report | 1 | China | 60–65 | f | seizures and decreased level of consciousness |
| 2022 | Gajurel et al. 40 | case report | 1 | Nepal | 40–45 | f | movement disorder |
| 2021 | Kimura et al. 41 | case report | 1 | Japan | 20–25 | f | decreased level of consciousness |
| 2023 | Consoli et al. 42 | case report | 2 | Italy | 40–45 | m | seizure and behavioral or psychiatric symptoms |
| 35–40 | m | movement disorder and hearing loss |
m, male; f, female.
In all studies, different types of AIE were initially proposed as diagnoses. Later, after additional tests and follow-up, these patients were given different diagnoses. (except for 83 patients in a retrospective study in which the final diagnosis was not mentioned). 5
A total of 356 patients were included, with a slight female preponderance. The patient age ranged between 3 and 93 years (Table 1).
The main presentations were new onset of seizure, altered mental status, neuropsychiatric symptoms, memory deficit, and focal neurological signs (Table 1).
The main initial diagnosis in the studies was AIE, followed by paraneoplastic limbic encephalitis and anti-NMDA receptor encephalitis (Table 2).
Table 2.
Initial diagnosis, method of diagnosis, and types of mimics.
| Author | Initial diagnosis | Method of diagnosis | Mimic type |
|---|---|---|---|
| Diogo Costa et al. 5 | nm | nm | 31% autoimmune encephalitis,2% anti-NMDA receptor encephalitis66% non-autoimmune encephalitis |
| Flanagan et al. 12 | nm | 16% biopsy,2.8% genetic testing,1.8% infectious testing,84.1% laboratory testing and imaging | nm |
| Budhram et al. 13 | autoimmune encephalitis | VDRL test | autoimmune encephalitis |
| Thomas et al. 14 | anti-LGI1 limbic encephalitis | biopsy | limbic encephalitis |
| Zuhorn et al. 15 | autoimmune encephalitis | biopsy | autoimmune encephalitis with CASPR2 antibodies |
| Lu et al. 16 | anti-NMDA receptor encephalitis | surgical biopsy of the right parietal lesion | anti-NMDA receptor encephalitis |
| Van Steenhoven et al. 18 | nm | brain MRI suggestive of AIE, CSF pleocytosis, specific oligoclonal bands in CSF, repeated steroid responsiveness, or similar staining pattern on IHC in serum and CSF in the absence of a known neuronal autoantibody | nm |
| AbdeleRahman et al. 19 | mesial temporal encephalitis | VDRL | mesial temporal encephalitis |
| Vogrig et al. 20 | antibody-negative autoimmune encephalitis | brain biopsy | antibody-negative autoimmune encephalitis |
| autoimmune encephalitis | brain biopsy | autoimmune encephalitis | |
| HSV and autoimmune encephalitis | brain biopsy | autoimmune encephalitis | |
| autoimmune encephalitis | brain biopsy | autoimmune encephalitis | |
| nm | brain biopsy | nm | |
| nm | brain biopsy | nm | |
| Macchia et al. 21 | autoimmune limbic encephalitis | MRI | autoimmune limbic encephalitis |
| Badruddin et al. 25 | their DDX: HSV encephalitis – limbic encephalitis – Morvan syndrome | clinical history | autoimmune-mediated limbic encephalitis |
| Devamare et al. 26 | viral encephalitis and anti-NMDA receptor encephalitis | nm | anti-NMDA receptor encephalitis |
| Garg et al. 27 | autoimmune encephalitis | nm | autoimmune encephalitis |
| Harsha et al. 28 | autoimmune encephalitis and paroxysmal kinesiogenic dyskinesia | clinical history | autoimmune encephalitis |
| Poon et al. 29 | autoimmune, infectious encephalitis | morning cortisol | autoimmune encephalitis or infectious encephalitis |
| Rigoni et al. 30 | autoimmune encephalitis | biopsy | autoimmune encephalitis |
| Wiels et al. 31 | progressive encephalomyelitis with rigidity and myoclonus with anti-glycine receptor antibodies | biopsy | progressive encephalomyelitis with rigidity and myoclonus |
| Nagata et al. 32 | limbic encephalitis | neuropathological diagnosis | limbic encephalitis |
| M Khair et al. 33 | autoimmune encephalitis | by the diagnostic criteria of common variable immunodeficiency | autoimmune encephalitis |
| Serrano-Cardenas et al. 34 | mesial encephalitis | microbiological studies | mesial encephalitis |
| Blondin et al. 35 | limbic encephalitis | CT, MRI, histological analysis | limbic encephalitis |
| Scheid et al. 36 | paraneoplastic limbic encephalitis | VDRL test | paraneoplastic limbic encephalitis |
| Deramecourt et al. 37 | paraneoplastic limbic encephalitis with pure cognitive impairment | MRI and stereotactic biopsies | paraneoplastic limbic encephalitis |
| Piola et al. 38 | anti-NMDA receptor encephalitis | CT scan, surgical monitoring of intracranial pressure, brain angiography, and autopsy | anti-NMDA receptor encephalitis |
| Liao et al. 39 | anti-LGI1 encephalitis | rapid plasma reagin and Treponema pallidum particle agglutination | anti-LGI1 encephalitis |
| Gajurel et al. 40 | autoimmune encephalitis | lip biopsy for mucosal lymphocytic infiltrates | autoimmune encephalitis |
| Kimura et al. 41 | anti-NMDA receptor encephalitis | clinically | anti-NMDA receptor encephalitis |
| Consoli et al. 42 | acute disseminated encephalomyelitis | brain biopsy | acute disseminated encephalomyelitis |
| rhombencephalitis | brain biopsy | rhombencephalitis |
DDX, differential diagnosis; HSV, herpes simplex virus; VDRL, venereal disease research laboratory; NMDA, N-methyl-D-aspartate; nm, not mentioned; MRI, magnetic resonance imaging; CT, computed tomography; AIE, autoimmune encephalitis; CSF, cerebrospinal fluid; IHC, immunohistochemistry.
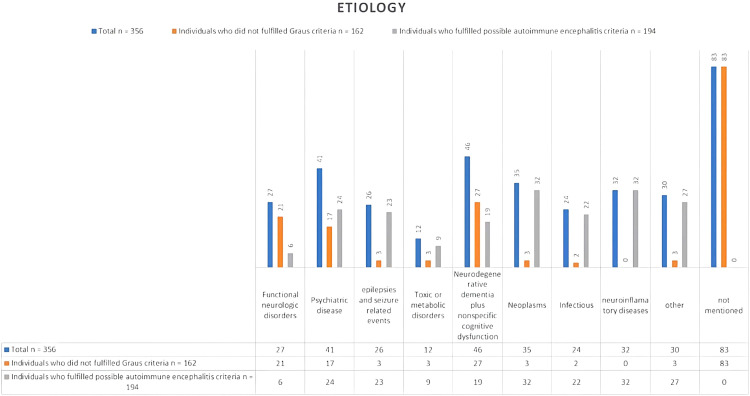
The final etiologies for the total population and individuals who fulfilled the possible AIE criteria are listed in Figure 2. The total number of etiologies mentioned was 79 for those not meeting the Graus criteria for possible diagnosis and 194 for those meeting this criteria (Figure 2).
Figure 2.
Bar chart showing the different etiologies that mimic autoimmune encephalitis.
The most common differential diagnosis lists differed between the two groups. Dementia and other cognitive disorders were most common for patients who did not meet the Graus criteria, followed by psychiatric diseases, especially conversion disorder. This finding differs from that of patients who met the possible Graus criteria, where neuroinflammatory disorders such as multiple sclerosis, along with neoplasms, were the primary considerations. Psychiatric diseases and convulsive disorders followed. The third position included infectious diseases, especially neurosyphilis. The final diagnosis was most frequently established by a brain biopsy; laboratory testing; imaging including electroencephalography (EEG), MRI, and computed tomography (CT); antibody tests; and CSF tests (Table 3).
Table 3.
Final diagnosis, findings, and management of autoimmune encephalitis mimics.
| Author | EEG | Findings | MRI | Findings | Antibody | CSF | Findings | Initial management | Final management |
|---|---|---|---|---|---|---|---|---|---|
| Diogo Costa et al. 5 | 0 | 0 | 0 | 0 | anti-CASPR2 antibodies in 1 patient | 0 | specific oligoclonal bands in 23 patients | 0 | 0 |
| Flanagan et al. 12 | 0 | 0 | 1 | MRI showed encephalitis features in 18% with either features of limbic encephalitis in 9.3% or multifocal abnormalities compatible with demyelination or inflammation in 8.4% | thyroid peroxidase antibodies in 24 patients, neural autoantibody positivity in 48 patients, antibodies to GAD65 in 14 patients, voltage-gated potassium channel complex (LGI1 and CASPR2 negative) in 10 patients, NMDA receptor (cell-based assay) in 10 patients | 1 | pleocytosis in 16 patients, neural autoantibodies in 7 patients | 0 | 0 |
| Budhram et al. 13 | 1 | left posterior temporal slowing, a repeat electroencephalogram after 3 months showed intermittent seizure activity | 1 | T2 hyperintensity of the left thalamus and medial temporal lobe; repeat brain MRI after 3 months showed left hippocampal atrophy without signal abnormality | anti-GAD antibodies | 1 | CSF immunoglobulin G and oligoclonal bands | intravenous immunoglobulin | intravenous aqueous crystalline penicillin G |
| Thomas et al. 14 | 1 | intermittent polymorph slowing in the delta band (1–2/s) together with intermittent sharp waves and sharp-slow-waves over the right anterior temporal lobe | 1 | Cerebral MRI showed volume and signal increase within right medial temporal lobe with focal extension to neocortical areas on T2/FLAIR images without contrast enhancement | anti-LGI1 IgG antibodies in serum | 1 | slight lymphocytic pleocytosis (8/μL) and dysfunction of the blood-CSF barrier (albumin ratio 8.3 × 10−3) | tryptophan-immunoadsorption followed by methylprednisolone pulse therapy as well as levetiracetam | high-dose methotrexate-based chemotherapy followed by whole brain irradiation |
| Zuhorn et al. 15 | 1 | generalized periodic pattern with triphasic waves | 1 | microangiopathic lesions: left-sided lesions in the thalamus and parietooccipital, temporo-mesial, thalamic, frontal and parietal cortices, as well as right-sided lesions in the basal ganglia | CASPR2-antibodies | 1 | pleocytosis of 7 leukocytes/μL (<5 leukocytes/μL) with a total protein of 701 mg/L (<450 mg/L) and 2.31 mmol/L lactate (1.2–2.1 mmol/L) | high-dosage intravenous methylprednisolone | nm |
| Lu et al. 16 | 1 | video-EEG showed slight abnormality | 1 | bilateral frontal parietal lesions enlarged slightly; the enhancement became more obvious than before | anti- NMDA | 1 | anti-NMDA receptor antibodies were detected in CSF and serum | corticosteroids and gamma globulin | radiotherapy and chemotherapy |
| Van Steenhoven et al. 18 | 1 | epileptic abnormalities on EEG | 1 | bilateral mesiotemporal hyperintensities | false-positive antibodies in serum (12%) | 0 | white blood cell count > 5/μL n = 43/96 oligoclonal bands n = 18/56 | nm | nm |
| AbdeleRahman et al. 19 | 1 | focal left anterior temporal lobe slow waves | 1 | abnormal FLAIR and T2 signal within the bilateral frontal and mesial temporal lobes | 0 | 1 | CSF analysis showed the following: glucose 48 mg/dL, erythrocytes 3/mL, leukocytes 220/mL (lymphocytes 69%, neutrophils 11%, monocytes 20%) | intravenous acyclovir | penicillin G for 21 days |
| Vogrig et al. 20 | 1 | confirmed focal status epilepticus | 1 | bitemporal — mostly left side — hypersignal on T2-weighted and FLAIR images; a control brain MRI —performed 1 month after the first admission — showed the unprecedented appearance of left temporal contrast enhancement | 0 | 1 | CSF analysis revealed 10 white cells/mm3 (83% lymphocytes), slightly elevated protein content (50 mg/dL) and presence of CSF-exclusive oligoclonal bands | nm | standard radio-chemotherapy |
| 0 | 0 | 1 | right temporal lesion, hyperintense on T2-weighted and FLAIR images | 0 | 1 | white cell count of 5/mm3, normal glucose level, and a protein level of 38 mg/dL | immunoglobulin | nm | |
| 0 | 0 | 1 | left-sided mesial temporal lesion with slight patchy enhancement after gadolinium administration | 0 | 1 | negative | intravenous acyclovir | nm | |
| 1 | lateralized periodic discharges | 1 | left temporo-insular abnormality on FLAIR sequences | 0 | 1 | negative | nm | nm | |
| 0 | 0 | 1 | T2 left limbic hypersignal; control MRI study revealed marked extension of the lesion over the parietal lobe | 0 | 0 | 0 | nm | nm | |
| 1 | abnormal for the presence of lateralized periodic discharges | 1 | T2-hyperintense lesion on right temporo-insular cortex with slight patchy contrast-enhancement | 0 | 0 | 0 | steroid bolus | nm | |
| Macchia et al. 21 | 0 | 0 | 1 | non-enhancing, bilateral hippocampal lesions | negative | 1 | normal | intravenous methylprednisolone | temozolomide and radiation |
| Badruddin et al. 25 | 1 | frequent diffuse polyspike and spike-wave discharges | 1 | hyperintensity on T2 imaging | 0 | 1 | CSF oligoclonal bands | nm | nm |
| Devamare et al. 26 | 1 | diffuse slow background activity; repeated EEG showed pseudoperiodic complexes | 1 | altered signal intensity in subcortical region of right parietal lobe | negative | 1 | measles antibody (1:4) | intravenous methylprednisolone | soprinosine, clonazepam, and valparin |
| Garg et al. 27 | 1 | periodic discharges | 1 | signal changes in left hippocampus, parahippocampal gyrus, and medial temporal lobe | anti-measles antibodies | 1 | anti-measles antibodies | nm | nm |
| Harsha et al. 28 | 1 | normal | 1 | subtle T2/FLAIR hyperintensities of right cerebellar white matter, right cingulate gyrus, left posterior limb of internal capsule; repeated MRI: T2/FLAIR showed subtle hyperintensity involving the left cerebellar hemisphere white matter, left middle cerebellar peduncle, left parieto-occipital white matter, and left thalamus | negative | 1 | normal | methylprednisolone and sodium valproate | stop pregabalin use |
| Poon et al. 29 | 1 | high amplitude waves in the left hemisphere and suppression in the right hemisphere, without interictal epileptiform activity | 1 | normal | low serum IgG | 1 | 67 white blood cells/µL (normal: 0 to 5 cells/µL) with 72% polymorphonuclear cells, and protein 66 mg/dL (normal: 14 to 45 mg/dL) | intravenous fluids | hydrocortisone and fludrocortisone and further intravenous immunoglobulin |
| Rigoni et al. 30 | 1 | normal | 1 | multiple T2 hyperintense confluent lesions involving mainly the diencephalic area, basal nuclei, thalami, and left temporal lobe, but also the brainstem, periventricular regions, right temporal lobe, and left fronto-insular cortex, with slight contrast enhancement; cerebral positron emission tomography revealed bilateral hippocampal hypermetabolism | negative | 1 | lymphocytic pleocytosis (7 cells/mm3) and mild blood-CSF barrier damage without oligoclonal bands | chemotherapy with rituximab-cyclophosphamide-doxorubicin-vincristine | |
| Wiels et al. 31 | 1 | Normal | 1 | normal | glycine receptor antibodies | 1 | elevated protein level | corticosteroids, plasmapheresis, and cyclophosphamide | nm |
| Nagata et al. 32 | 0 | 0 | 1 | no remarkable changes | 0 | 1 | mild pleocytosis of 11–20 cells/mm3, increased protein levels of 74–84 mg/dL | dexamethasone and glycerol | urgent neurosurgery |
| M Khair et al. 33 | 1 | continuous spike and slow wave | 1 | bilateral basal ganglia high signal followed by brain atrophy | negative | 1 | negative | steroids, intravenous immunoglobulins, and plasmapheresis; a tracheostomy and a PEG tube was inserted | immunoglobulins |
| Serrano-Cardenas et al. 34 | 1 | normal | 1 | FLAIR and T2-weighted MRI sequences demonstrated a hyperintense signal with mild-moderate expansiveness located in both the medial and anterior temporal lobes as well as a mild subcortical-predominant cerebral atrophy | 0 | 1 | intrathecal IgG synthesis and oligoclonal bands were detected in CSF | nm | penicillin |
| Blondin et al. 35 | 1 | mild generalized slowing, no epileptiform discharges, and no pathological response to photic stimulation | 1 | normal | 0 | 1 | normal | methylprednisolone | tumor resection, local radiation therapy followed by systemic chemotherapy with etoposide and carboplatin |
| Scheid et al. 36 | 1 | generalized slowing and epileptiform discharges | 1 | contrast-enhancing (T1) hyperintense signal alteration in the left medial temporal lobe on FLAIR and T2-weighted images | Treponema pallidum IgG-western blot | 1 | oligoclonal bands; VDRL titer 1:8, in CSF 1:4; Treponema pallidum IgG-western blot | nm | penicillin |
| Deramecourt et al. 37 | 1 | normal | 1 | cystic lesion in the left hippocampus with enhancement after contrast administration | negative | 0 | 0 | nm | resection of the left temporal lobe was performed |
| Piola et al. 38 | 1 | diffuse background slowing and delta activity with superimposed bursts of rhythmic beta frequency activity on frontal and temporal regions, a pattern known as extreme delta brush | 1 | mild leptomeningeal enhancement without brain lesion | 0 | 1 | negative | methylprednisolone | nm |
| Liao et al. 39 | 1 | irregular slow waves with medium to high amplitudes in the right temporal lobe, which spread to the other lobes and showed sharp waves | 1 | mild to moderate cord enhancement in the right temporal lobe | negative serum LGI1 antibody | 1 | positive RPR and TPPA tests | sodium valproate, diazepam, and levetiracetam | penicillin G sodium |
| Gajurel et al. 40 | 0 | 0 | signal intensity in the bilateral medial temporal lobe and midbrain | 0 | 0 | 0 | methylprednisolone | steroids | |
| Kimura et al. 41 | 0 | 0 | 0 | 0 | IgG anti-GQ1b antibodies | 0 | 0 | immunoglobulin and methylprednisolone | 0 |
| Consoli et al. 42 | 1 | sporadic diphasic high-amplitude sharp waves in the left anterior temporal lobe regions | 1 | diffuse cortico-subcortical T2 and FLAIR images; hyperintense lesions involving the bilateral hippocampus, fusiform gyri, right frontoparietal cortex, left thalamus, and right pulvinar nuclei | anti-recoverin antibodies | 0 | 0 | corticosteroids | radiotherapy and chemotherapy |
EEG, electroencephalography; MRI, magnetic resonance imaging; CSF, cerebrospinal fluid; nm, not mentioned; FLAIR, fluid-attenuated inversion recovery; PEG, percutaneous endoscopic gastrostomy; NMDA, N-methyl-D-aspartate; VDRL, venereal disease research laboratory; RPR, rapid plasma reagin; TPPA, Treponema pallidum particle agglutination.
EEG was performed for 24 of the most frequent abnormalities including waves with slowing and discharges (Table 3).
All patients had undergone MRI, and the most frequent findings were suggestive of a brain lesion (8 of 25 patients). CT scans were performed on 9 of 25 patients; 5 scans were unremarkable or normal, and 1 thoracic CT scan showed a hyperdense lesion of 1.6 cm in the left superior pulmonary lobe that was suspected to be carcinoma.
The other patients showed non-specific mild global atrophy, obscure cerebral gyri in the left frontal and temporal regions, or hypometabolism in the frontoparietal and parietooccipital cortices (Table 3).
In total, 117 of 356 patients showed positivity for antibodies including anti-GAD antibodies and anti-measles antibodies, low titers of glycine receptor antibodies, anti-LGI1 IgG antibodies, CASPR2-antibodies, anti-NMDA and Treponema pallidum antibodies (IgG-western blot). Thyroid peroxidase antibodies were present in 24 patients, neural autoantibodies were found in 48 patients, GAD65 antibodies were present in 14 patients, voltage-gated potassium channel complex antibodies (LGI1 and CASPR2 negative) were present in 10 patients, and NMDA receptor antibodies (cell-based assay) were present in 10 patients. In addition to IgG, anti-GQ1b antibody positivity, anti-recoverin antibodies, and anti-GluR3 antibodies were found in some cases.
CSF analysis was performed for 25 patients, and only eight tests were normal. The most common abnormalities were lymphocytic pleocytosis, elevated protein levels, and neural autoantibodies. Other abnormalities included blood-CSF barrier damage, positive oligoclonal bands, and high titers of anti-measles antibodies. In addition, positive rapid plasma reagin and Treponema pallidum particle agglutination tests and anti-GluR3 antibodies were found.
Of the 25 patients, only 2 patients had undergone a Mini Mental State Examination (raw scores 13/30 and 25/30). Other patients underwent a neuropsychiatric evaluation, which revealed profound memory loss (Table 3).
Steroids (methylprednisolone or dexamethasone) were most commonly used as initial management (11 of 25 cases). Other medications and procedures included intravenous immunoglobulins (4 cases), intravenous acyclovir (2 cases), intravenous fluids, sodium valproate, cyclophosphamide, tryptophan immunoadsorption, levetiracetam, and plasmapheresis.
The final management in most cases targeted controlling the underlying pathology that mimicked AIE.
The most frequent management strategy was chemotherapy and/or radiation (5 cases). The other strategies included penicillin G (4 cases), neurosurgery (3 cases), intravenous immunoglobulins (2 cases), isoprinosine, clonazepam, valparin, stopping pregabalin use, hydrocortisone, and fludrocortisone (Table 3).
Discussion
According to the Brighton Collaboration Encephalitis Working Group, the term encephalitis refers to encephalopathy or any other neurological symptom emerging from brain parenchymal inflammation. 10 This inflammation is initially caused by infections, followed by acute disseminated encephalomyelitis. 1 The immune basis of this inflammation was first mentioned by Corsellis et al. in 1968, when they referred to cases of paraneoplastic limbic encephalitis. 11 Later, this term expanded to be a part of AIE. 5
In this context, AIE consists of a set of neuropathies caused by an immune-mediated process and shares the presence of gray matter inflammation in the brain, which may extend to involve the white matter, meninges, and spinal cord. 2
According to the definition, AIE can be classified anatomically depending on the location (limbic, cortical/subcortical, striatal, diencephalic, brainstem, cerebellar, encephalomyelitis, meningoencephalitis, or combined) 2 or pathologically depending on the antibody type and the immune process involved 1 (cytotoxic T-cell-mediated antibodies against intracellular antigens vs antibodies against surface antigens that involve humoral immunity). 12 Seronegative AIE has also been described and may represent antibodies that have not been reported. 2
AIE symptoms vary according to the affected area of the brain and usually overlap. 2 Therefore, the diagnosis is difficult and depends on a high clinical suspicion because, first, the progression of symptoms is gradual and sometimes it takes weeks before the clinical picture becomes clear. 5 Second, MRI results demonstrate non-specific inflammatory findings or appear normal in most cases. 1
AIE is a rare disease (the incidence is approximately 3 to 9 per million) 5 but encompasses a high rate of misdiagnosed cases, 5 which may lead to medical errors because of unnecessary application of steroids and immunosuppressive therapy. Despite the publication of the Graus criteria in 2016, in which the diagnosis is classified according to its reliability as possible or definite, 5 cases mimicking AIE are still being published. This phenomenon can be understood in the following context. In two later studies4,5 the sensitivity of Graus’ criteria did not exceed 80%, and the specificity was approximately 84% to 94%, with an established linear relationship between sensitivity and AIE progression. 4 In another two retrospective studies that were conducted in leading clinics in the USA and specialist centers in Portugal, the numbers of mimicking conditions were 107 of 393 in the study by Flanagan et al 12 and 26 of 39 in the study by Costa et al., 5 and similar studies in other countries were lacking. Additionally, the prevalence of mimicking conditions greatly surpassed that of AIE. 12 Finally, the rush to order neuronal antibodies and overinterpretation of their existence was mentioned by Flanagan et al. 12 as an affecting factor because positivity for neuronal antibodies may be seen in up to 5% of patients. 12 This argument was supported by our review regarding misdiagnosis of mimicker cases, which mentioned the positivity of these antibodies, especially antibodies against GAD, CASPER2, LG-1, and NMDA.13–16
Many neurological diseases are considered in the differential diagnosis of AIE. Fortunately, many can be ruled out through a detailed history, neurological examination, laboratory analyses, and other investigations such as CSF analysis and brain MRI. 2 According to our review, neurodegenerative dementia (such as Alzheimer’s disease, dementia with Lewy bodies, Creutzfeldt-Jakob disease, behavioral variant frontotemporal dementia, vascular cognitive impairment, and normal pressure hydrocephalus) and nonspecific cognitive syndrome were the most common conditions, followed by psychiatric disorders, especially functional neurological disorders or conversion syndrome. Other diseases included neoplasms such as gliomas (glioblastoma, astrocytoma, or those not otherwise specified), lymphomas, cerebellar medulloblastoma with cerebellar cognitive syndrome, infectious encephalitis (especially viral encephalitis and neurosyphilis, even without recognizing the primary infection, human immunodeficiency virus, and residual prior viral encephalitis), seizures, and psychological reasons such as depression, anxiety, schizophrenia, and bipolar syndrome. Other causes included multiple sclerosis, small vessel vasculitis, neurosarcoidosis, toxic-metabolic causes including medication, delayed neuropsychiatric syndrome of carbon monoxide poisoning, non-immunotherapy responsive progressive cerebellar degeneration with cerebellar cognitive syndrome, Kleine–Levin syndrome, mitochondrial encephalomyopathy, lactic acidosis, stroke-like episodes, adrenal insufficiency, and Wernicke encephalopathy. Four other cases were also mentioned, including common variable immunodeficiency, neuroendocrine tumor with a merkel cell carcinoma immunophenotype, pregabalin abuse, and 4-aminopyridine toxicity. It should be noted that the last case did not meet the Graus 2016 criteria because of the presence of a clear cause of encephalopathy, as the patient had mentioned taking medication and had hyperacute development of symptoms. This result is different from that of the retrospective study by Flangan et al., when considering cases that did not meet the probable Graus criteria. 12 That study focused on AIE mimickers in the adult population and functional neurological disorders followed by dementia. This discrepancy is possibly attributable to the larger sample size of our study. It is important to note that these findings should be interpreted with respect to the patient's age. 17 Functional neurological disorders were predominant in the pediatric group, followed by epilepsy. 18 Genetic diseases such as mitochondrial diseases and leukodystrophies are also important differential diagnoses. Additional conditions to consider, as per Dalmau and Graus, include new-onset refractory status epilepticus, pediatric autoimmune neuropsychiatric disorder associated with streptococcal infection, and the closely linked pediatric acute-onset neuropsychiatric syndrome. 17
Some differential diagnoses complied with the possible Graus criteria, and some even included concurrent antineuronal antibodies. These were considered to be true mimickers. Unfortunately, because of the significant heterogeneity among the study designs, we could not perform a major review and only attempted to highlight the mimickers.
The most common mimickers in this category were neuroinflammatory diseases, which included the same number of patients as those with neoplasms. This was followed by seizures and epileptic disorders, along with psychiatric disorders and infectious diseases, and finally dementia and other cognitive disorders. One study also noted that Susac’s syndrome is a significant differential diagnosis for individuals meeting Graus' possible criteria, 8 with no published cases yet.
AIE and its mimics can affect both sexes equally. There is a negligible difference in sex preponderance according to our study and studies of Diogo Costa et al. 5 and Eoin P. Flanagan et al. 12 The median age for this disease is 61.7 ± 15.5 years. 2 However, it affected individuals aged from 3 to 93 years.
Symptoms of AIE and its mimics are similar, depending on the area that they affect. In general, the most common symptoms reported in the medical literature and in our study were altered mental status (most common), seizures, movement disorders, neuropsychiatric symptoms, working memory deficits, focal neurological signs, and other symptoms such as ataxia, autonomic dysfunction, paraparesis or tetraparesis, visual loss, and generalized pain. 2
In two literature reviews1,10 concerned with syphilis and glioblastoma and their potential to mimic AIE, the symptoms were not different from the symptoms reported above. Nevertheless, in two cases, the presence of speech disorders, progressive forgetfulness, and apathy was noted in patients with syphilis.13,19 This was also true for glioblastoma, in which in addition to language dysfunction, right-side hypoesthesia and homonymous lateral hemianopia were reported. 20
Suspicion of an AIE diagnosis in a patient relies on the clinical presentation. 5 However, reaching a definitive diagnosis requires a variety of diagnostic tools, primarily MRI. It should be noted that brain biopsy, despite its morbidity, remains the gold standard for these diseases. 10 Fortunately, it is not usually indicated, 2 but in most of our reviewed cases, it was used to confirm the diagnosis. Therefore, we suggest that it should be performed in complicated cases to prevent a delay in treatment.
MRI remains the most critical component in the diagnosis of neurological disorders and was used in all cases reviewed thus far because of its importance. Graus noted that detecting bilateral limbic encephalitis is the only definitive method for diagnosing AIE, as other types of AIE present with nonspecific inflammatory changes. 8 AIE appears as a bilateral hyperintense lesion on T2/fluid attenuated inversion recovery (FLAIR) sequences. This pattern has been observed in many other diseases, some of which only imitate AIE radiologically (such as herpes simplex encephalitis, status epilepticus, mesial temporal sclerosis, and posterior cerebral artery infarct). 12 Other diseases are real mimickers. For example, according to our results, neurosyphilis and AIE have the same MRI findings. Another challenging example is Creutzfeldt–Jakob disease, which according to Macchi’s review, 21 tends to exhibit the same radiological picture as AIE. One exception was observed by Zuhorn et al., 15 in which no MRI abnormality was detected.
EEG is essential to examine AIE and its mimics. A normal pattern, focal or diffuse slowing, periodic discharges, and extreme delta brush are all common in both AIE and its mimics. However, in our view, EEG was not very useful in differentiating among these diseases, except for Creutzfeldt–Jakob disease, where EEG (disease-typical periodic sharp wave complexes, which are different from those observed in AIE) is considered crucial based on the diagnostic calcification established by the World Health Organization. The main problem is that this pattern usually emerges in later stages of the disease. 22
As shown by the Graus criteria, the CSF plays a pivotal role in the diagnosis of AIE. Detection of antineuronal antibodies and moderate lymphocytic pleocytosis, with a count of less than 100 cells, are the typical findings. CSF analysis was performed in most cases and revealed nonspecific abnormalities. However, it can be useful in detection of Treponema pallidum and other infections. 23 It is important to note that the absence of pleocytosis does not exclude the possibility of an AIE diagnosis. 1 In our review of mimickers, the most common abnormalities were lymphocytic pleocytosis, elevated protein levels, and even a concurrent presence of neural autoantibodies, which led to misdiagnosis.13–16
Brain CT was used in nine of the studied cases. Five of those nine cases had normal results. This finding demonstrates the small role of CT in disease diagnosis. Nonetheless, CT could be helpful when considering paraneoplastic cases or extra-neurological syndromes. Otherwise, it has a low priority.
In conclusion, accurate and rapid diagnosis of AIE and administration of immunosuppressants, especially methylprednisolone, is essential to prevent death. According to our study, we suggest that a rapid screen for these mimickers, especially those that meet the Graus criteria, such as glioma, seizures, and syphilis, is essential and should be included in the Graus criteria and within the primary work-up. Additionally, risk is involved in the hurried use of immunosuppressive drugs. Unfortunately, the lack of adequate studies and the heterogeneity in the present cases prevented us from focusing on studies that met the Graus criteria. We also recommend that larger studies should be carried out in the future focusing on these mimickers in particular.
Limitations
In our study, we focused on mimickers of limbic AIE in particular because of the absence of clear boundaries between diseases considered an AIE subtype and those deemed an independent entity and the presence of many other diseases with immunological backgrounds, such as acute disseminated encephalomyelitis, Bickerstaff’s brainstem encephalitis, Hashimoto encephalopathy, and Rasmussen's disease. Another important point is that the criteria focus on syndromes with a subacute presentation, leading to the exclusion of chronic syndromes such as stiff person syndrome and Morvan syndrome. 24 The latter can be caused by both immunological and non-immunological pathologies.
Low numbers of studies with some heterogeneity among them (28 cases and three retrospective studies).
We considered that all of the 25 included cases had met the Graus criteria theoretically by having a logical reason to deny other differential diagnoses, especially in the cases of acute medication toxicity (4-aminopyridine toxicity).
Footnotes
Author contributions: MSA: Conceptualization, Methodology, Writing – Original Draft; MM: Conceptualization, Software, Writing – Original Draft; MT: Investigation, Writing – Original Draft; NA: Investigation, Writing – Original Draft; TB: Writing – Original Draft; MZBA: Conceptualization, Methodology, Writing – Review & Editing.
The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Muhammad Mazketly https://orcid.org/0000-0003-1600-8456
References
- 1.Dalmau J, Rosenfeld MR, DeAngelis LM, et al. “Paraneoplastic and autoimmune encephalitis,” UpToDate. UpToDate, Waltham https//www.uptodate.com/contents/paraneoplastic-and-autoimmune-encephalitis, 2017.
- 2.Abboud H, Probasco JC, Irani S, Autoimmune Encephalitis Alliance Clinicians Network et al. Autoimmune encephalitis: proposed best practice recommendations for diagnosis and acute management. J Neurol Neurosurg Psychiatry 2021; 92: 757–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dutra LA, Abrantes F, Toso FF, et al. Autoimmune encephalitis: a review of diagnosis and treatment. Arq Neuropsiquiatr 2018; 76: 41–49. [DOI] [PubMed] [Google Scholar]
- 4.Li L, Sun L, Du R, et al. Application of the 2016 diagnostic approach for autoimmune encephalitis from Lancet Neurology to Chinese patients. BMC Neurol 2017; 17: 195. doi: 10.1186/s12883-017-0974-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costa D, Sardoeira A, Carneiro P, et al. Autoimmune encephalitis: suspicion in clinical practice and mimics. J Neuroimmunol 2022; 365: 577824. [DOI] [PubMed] [Google Scholar]
- 6.Baumgartner A, Rauer S, Hottenrott T, et al. Admission diagnoses of patients later diagnosed with autoimmune encephalitis. J Neurol 2019; 266: 124–132. doi: 10.1007/s00415-018-9105-3. [DOI] [PubMed] [Google Scholar]
- 7.Giordano A, Fazio R, Gelibter S, et al. Diagnosing autoimmune encephalitis in a real-world single-centre setting. J Neurol 2020; 267: 449–460. doi: 10.1007/s00415-019-09607-3. [DOI] [PubMed] [Google Scholar]
- 8.Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 2016; 15: 391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abboud H, Rossman I, Mealy MA, et al. Neuronal autoantibodies: differentiating clinically relevant and clinically irrelevant results. J Neurol 2017; 264: 2284–2292. doi: 10.1007/s00415-017-8627-4. [DOI] [PubMed] [Google Scholar]
- 10.Venkatesan A, Tunkel AR, Bloch KC, International Encephalitis Consortium et al. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clin Infect Dis 2013; 57: 1114–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corsellis JA, Goldberg GJ, Norton AR. “ Limbic encephalitis” and its association with carcinoma. Brain 1968; 91: 481–496. doi: 10.1093/brain/91.3.481. [DOI] [PubMed] [Google Scholar]
- 12.Flanagan EP, Geschwind MD, Lopez-Chiriboga AS, et al. Autoimmune encephalitis misdiagnosis in adults. JAMA Neurol 2023; 80: 30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Budhram A, Silverman M, Burneo JG. Neurosyphilis mimicking autoimmune encephalitis in a 52-year-old man. CMAJ 2017; 189: E962–E965. doi: 10.1503/cmaj170190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas C, Lehrich C, Gross CC, et al. Primary B cell lymphoma of the CNS mimicking anti-LGI1 limbic encephalitis. Front Neurol 2018; 9: 658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zuhorn F, Hübenthal A, Rogalewski A, et al. Creutzfeldt-Jakob disease mimicking autoimmune encephalitis with CASPR2 antibodies. BMC Neurol 2014; 14: 227–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu J, Zhang JH, Miao AL, et al. Brain astrocytoma misdiagnosed as anti-NMDAR encephalitis: a case report. BMC Neurol 2019; 19: 210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalmau J, Graus F. Diagnostic criteria for autoimmune encephalitis: utility and pitfalls for antibody-negative disease. Lancet Neurol 2023; 22: 529–540. doi: 10.1016/S1474-4422(23)00083-2. PMID: 37210100. [DOI] [PubMed] [Google Scholar]
- 18.Van Steenhoven RW, De Vries JM, Bruijstens AL, et al. Mimics of autoimmune encephalitis: validation of the 2016 Clinical Autoimmune Encephalitis Criteria. Neurol Neuroimmunol Neuroinflamm 2023; 10: e200148. doi: 10.1212/NXI.0000000000200148. PMID: 37582614; PMCID: PMC10427145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.AbdeleRahman KT, Santamaria DD, Rakocevic G. Pearls & oysters: neurosyphilis presenting as mesial temporal encephalitis. Neurology 2012; 79: e206–e208. [DOI] [PubMed] [Google Scholar]
- 20.Vogrig A, Joubert B, Ducray F, et al. Glioblastoma as differential diagnosis of autoimmune encephalitis. J Neurol 2018; 265: 669–677. doi: 10.1007/s00415-018-8767-1. [DOI] [PubMed] [Google Scholar]
- 21.Macchi ZA, Kleinschmidt-DeMasters BK, Orjuela KD, et al. Glioblastoma as an autoimmune limbic encephalitis mimic: A case and review of the literature. J Neuroimmunol 2020; 342: 577214. [DOI] [PubMed] [Google Scholar]
- 22.Wieser HG, Schindler K, Zumsteg D. EEG in Creutzfeldt–Jakob disease. Clin Neurophysiol 2006; 117: 935–951. doi: 10.1016/j.clinph.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Daey Ouwens IM, Fiolet ATL, Thijs RD, et al. Neurosyphilis mimicking autoimmune encephalitis: a case report and review of the literature. Clin Neuropsychiatry 2020; 17: 175–180. Jun. doi: 10.36131/cnfioritieditore20200305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masood W, Sitammagari KK. Morvan Syndrome. [Updated 2022 Jun 21]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. Jan. [PubMed]
- 25.Badruddin A, Menon RS, Reder AT. 4-Aminopyridine toxicity mimics autoimmune-mediated limbic encephalitis. Neurology 2009; 72: 1100–1101. doi: 10.1212/01.wnl.0000345063.17185.13. [DOI] [PubMed] [Google Scholar]
- 26.Devamare S, Ihtisham K, Bhasin H, et al. Subacute sclerosing panencephalits mimicking anti-NMDA receptor encephalitis. Ann Indian Acad Neurol 2020; 23: 411–413. doi: 10.4103/aian.AIAN_184_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garg RK, Kumar N, Rizvi I, et al. Fulminant subacute sclerosing panencephalitis mimicking autoimmune encephalitis. Pediatr. Infect. Dis. J 2019; 38: e64–e64. Available: https://journals.lww.com/pidj/Fulltext/2019/03000/Fulminant_Subacute_Sclerosing_Panencephalitis.34.aspx. [DOI] [PubMed] [Google Scholar]
- 28.Harsha K, Joshy E, Aravinda R, et al. Chronic pregabalin abuse with subacute encephalopathy mimicking autoimmune encephalitis. Neurol India 2021; 69: 1785–1788. doi: 10.4103/0028-3886.333486. [DOI] [PubMed] [Google Scholar]
- 29.Poon JT, Salzman K, Clardy SL, et al. Adrenal crisis presenting as recurrent encephalopathy mimicking autoimmune, infectious encephalitis, and common variable immune deficiency: a case report. Neurologist 2022; 27: 206–210. Available: https://journals.lww.com/theneurologist/Fulltext/2022/07000/Adrenal_Crisis_Presenting_as_Recurrent.9.aspx. [DOI] [PubMed] [Google Scholar]
- 30.Rigoni E, Farina L, Bini P, et al. Primary central nervous system lymphoma mimicking autoimmune encephalitis: the role of autoantibody testing. Neurol Sci 2021; 42: 1193–1196. doi: 10.1007/s10072-020-04739-1. [DOI] [PubMed] [Google Scholar]
- 31.Wiels WA, Du Four S, Seynaeve L, et al. Early-onset Creutzfeldt-Jakob disease mimicking immune-mediated encephalitis. Front Neurol 2018; 9: 242. Available: https://www.frontiersin.org/articles/10.3389/fneur.2018.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagata R, Ikeda K, Nakamura Y, et al. A case of gliomatosis cerebri mimicking limbic encephalitis: malignant transformation to glioblastoma. Intern Med 2010; 49: 1307–1310. [DOI] [PubMed] [Google Scholar]
- 33.Khair AM, Ehlayel M, Alshami R, et al. Autoimmune encephalitis as the sole presentation of common variable immunodeficiency: First report in a child. J Clin Case Rep 2015; 5: 2. [Google Scholar]
- 34.Serrano-Cardenas KM, Sánchez-Rodriguez A, Pozueta A, et al. Mesial encephalitis: an uncommon presentation of neurosyphilis: a case report and review of the literature. Neurol Sci 2018; 39: 173–176. [DOI] [PubMed] [Google Scholar]
- 35.Blondin NA, Vortmeyer AO, Harel NY. Paraneoplastic syndrome of inappropriate antidiuretic hormone mimicking limbic encephalitis. Arch Neurol 2011; 68: 1591–1594. [DOI] [PubMed] [Google Scholar]
- 36.Scheid R, Voltz R, Vetter T, et al. Neurosyphilis and paraneoplastic limbic encephalitis: important differential diagnoses. J Neurol 2005; 252: 1129–1132. [DOI] [PubMed] [Google Scholar]
- 37.Deramecourt V, Bombois S, Debette S, et al. Bilateral temporal glioma presenting as a paraneoplastic limbic encephalitis with pure cognitive impairment. Neurologist 2009; 15: 208–211. [DOI] [PubMed] [Google Scholar]
- 38.Piola M, Mascoli N, Barca S, et al. Cryptococcal encephalitis with fulminant intracranial hypertension mimicking anti-NMDA receptor encephalitis. Neurol Sci 2015; 36: 1067–1069. doi: 10.1007/s10072-014-1984-1. Epub 2014 Oct 21. PMID: 25331862. [DOI] [PubMed] [Google Scholar]
- 39.Liao H, Zhang Y, Yue W. Case report: a case report of neurosyphilis mimicking limbic encephalitis. Front Neurol 2022; 13: 862175. doi: 10.3389/fneur.2022.862175. PMID: 35645969; PMCID: PMC9133385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gajurel BP, Giri S, Poudel N, et al. MRI of the brain mimicking autoimmune encephalitis in Sjögren syndrome with chorea: a case report. Ann Med Surg (Lond) 2023; 85: 922–925. doi: 10.1097/MS9.0000000000000101. PMID: 37113894; PMCID: PMC10129085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kimura M, Yoshimura H, Kohara N. Abnormal movements in Bickerstaff brainstem encephalitis mimicking anti-N-methyl-D-aspartate receptor encephalitis. JAMA Neurol 2021; 78: 1149. doi: 10.1001/jamaneurol.2021.1698. PMID: 34125157. [DOI] [PubMed] [Google Scholar]
- 42.Consoli S, Dono F, Evangelista G, et al. Case report: brain tumor's pitfalls: two cases of high-grade brain tumors mimicking autoimmune encephalitis with positive onconeuronal antibodies. Front Oncol 2023; 13: 1254674. doi: 10.3389/fonc.2023.1254674. PMID: 37692853; PMCID: PMC10484219. [DOI] [PMC free article] [PubMed] [Google Scholar]