Abstract
In the past few years, nanotechnology has emerged as one of the most interesting and cutting-edge research areas across all disciplines. Nanotechnology allows progress in all science fields to make novel materials and industry-different devices. Generally, nanoparticle synthesis methods are chemical, physical, and biological. The chemical and physical techniques use potentially harmful compounds, and the expense of these processes renders them unsuitable for nanoparticle synthesis. In light of this, it needs development strategies that are sustainable, economical, and eco-friendly viable. Through, biosynthesis, nanoparticles can overcome these disadvantages. One of the biological strategies is the myco-synthesis method, which connects the fields of mycology and nanotechnology. In this study, magnetite (Fe3O4) NPs have been synthesized using a myco-synthesis method by selecting Aspergillus elegans as a fungal species. Two extracts were used, growth medium and an aqueous extract. A comparative analysis between nanoparticles synthesized through myco-synthesis and those produced using conventional chemical methods has been conducted to substantiate the significance of the biological approach. The results of this study unequivocally establish that myco-synthesized nanoparticles exhibit superior and enhanced characteristics compared to those synthesized through chemical means, as ascertained through a comprehensive array of characterization techniques employed throughout the investigation. This contrast is observable in terms of the aggregation state, the existence of capping and stabilizing agents enveloping the nanoparticles, their magnetic and thermal attributes, and the enduring stability of these nanoparticles. These results highlight the significant promise of employing phytochemicals extracted from Aspergillus elegans as a highly suitable option for the biofabrication of Fe3O4 nanoparticles.
Keywords: Myco-synthesis method, Fe3O4 NPs, Reducing agent, Capping agent, Stabilizing agent, Aspergillus elegans extract
1. Introduction
Nanotechnology has attracted exceptional attention due to its distinct physicochemical features and widespread application in several sectors in recent years. Several scientists and economists observed a dramatic growth in the use of nanomaterials to meet the rising demand in many application sectors, especially with the creation of novel nanomaterials Maurer-Jones et al., 2013 [1]. Nanoparticles (NP) are typically clumps of atoms that vary in size from one to one hundred nanometers. It is well known that a metal NP's dimensions, form, composition, crystallinity, and structure are the primary factors influencing its characteristics Sun and Xia, 2002 [2]. The unique crystallographic nature of NPs causes them to have a greater surface area, leading to a greater degree of reactivity. This is also true despite NPs having a very small physical structure [3]. NPs may be synthesized from various materials and formed into various forms. In the past few years, the subject of nanotechnology has emerged as a field of study that may use in optics in a wide variety of contexts [4], electronics [5], catalysis [6], biomedical sciences [7], energy science [8], agriculture [9], and environment among other industrial sectors [10].
Synthesis of nanomaterials may often be broken down into two primary categories: top-down and bottom-up. To get thin-layer crystals using a top-down strategy, it must frequently disrupt the van Der Waals forces holding the stacked bulk components together. The bottom-up synthesis technique for nanoparticles might entail the formation of ionic or covalent bonds. The top-down strategy often requires massive amounts of energy inputs and leaves a significant footprint on the environment. Manufacturing nanoparticles used to clean up the environment shouldn't result in any significant side effects [11]. Top-down approaches include laser ablation, ion sputtering, and mechanical milling [12]. Although top-down procedures are generally simpler to carry out, this method cannot be used to execute the process of performing on extremely tiny-sized particles. This method of producing nanoparticles comes with several significant challenges, the most significant of which is a change in the physicochemical characteristics of the nanoparticles and their surface chemistry. Bottom-up approaches by aggregating atoms, molecules, or smaller particles may accomplish the formation of nanoparticles. The production of the ultimate nanoparticle begins with the development of nanostructured building blocks of nanomaterials, which then proceed by integration to make the final stage nanoparticle. Bottom-up approaches include physical, chemical vapor deposition sol-gel, hydrothermal, spray, and laser pyrolysis [13]. In both physical and chemical techniques of fabricating nanoparticles, using chemicals and high temperatures and pressures negatively affects the surrounding environment. The biological method suited to the synthesis of nanoparticles involves the use of plant products and microorganisms. Biological techniques are among the most ideal for the creation of nanoparticles for the cleanup of contaminants because they are the most economically efficient, environmentally friendly, and adaptable approaches, and they also eliminate the use of harmful chemicals, high pressure, energy, and temperature [14].
As a direct consequence of this fact, scientists working in nanoparticle fabrication and construction have begun looking to biological systems for ideas. This should not come as a surprise, considering that it has been demonstrated that a variety of single-cell and multi-cell organisms, are capable of producing inorganic materials either within or outside of their cells. The synthesis of magnetite nanoparticles by magnetotactic bacteria is one well-known example of the production of inorganic materials by microorganisms [[15], [16], [17]]. S-layer bacteria are responsible for the production of gypsum and calcium carbonate layers. Diatoms are responsible for the synthesis of siliceous materials. The insights gained from studying nature have been essential in the creation of biomimetic strategies for the production of cutting-edge nanomaterials [[18], [19], [20], [21], [22]].
Fungal nanotechnology, also known as Myconanotechnology, is a made-up phrase that was first used in 2009 by an Indian researcher by the name of Rai M. (Myco is short for fungi, and nanotechnology refers to the manufacture and usage of materials in the 1–100 nm size range). Fungi produce nanoparticles used in various applications, most notably in the biomedical, environmental, and agricultural industries [[23], [24], [25]].
Mycogenic synthesis of nanoparticles is an important component of myco-nanotechnology. Due to the broad spectrum and variety of fungi, this process gives rise to an exciting new and practically applicable interdisciplinary science with considerable outcomes [26]. Myco-synthesized nanoparticles have seen significant usage in various applications, including the detection and elimination of pathogenic agents, the purification of wastewater, the preservation of food, the production of nematicides, and many more. Mycogenic nanoparticles, generated by a wide variety of fungal species, can be used in several agricultural applications to boost crop yield by enhancing growth and providing resistance to diseases. In addition, this will increase the toxicity of chemical pesticides, insecticides, and herbicides against plant ecosystems [27]. Pathogens responsible for infectious illnesses in humans have been demonstrated to be inhibited effectively by nanoparticles mediated by fungi, particularly when it comes to infections considered multi-resistant to conventional antibacterial drugs [28]. In the realm of heavy metal biosorption from wastewater contaminants, fungi-based nano sorbents are also a creative research direction that has been taken [29].
Iron oxides consist of anionic arrangements that commonly exhibit cubic or hexagonal symmetry. The spaces between particles are partially occupied by trivalent or divalent iron, with a prevalence of octahedral coordination in the form of FeO6. However, there have also been reports of tetrahedral coordination in the form of Fe3O4 [30]. Iron oxide nanoparticles have differentiated themselves from bulk materials as a direct consequence of the features of iron oxide that are inversely related to the size and morphology of their structure [31]. Several different types of iron oxides may be found in nature, including hematite (-Fe2O3), magnetite (Fe3O4), and maghemite (-Fe2O3) [32]. Because of its remarkably rigid framework and higher concentration of divalent iron ions, Fe3O4 has the highest level of magnetism compared to the other iron oxides [33]. While, Maghemite is a mixture of the structure and content of hematite and magnetite; it has a color that may be described as reddish-brown, and it is formed in the ground as a consequence of magnetite being exposed to the elements. Hematite is the oldest type of iron oxide discovered in scientific circles and is also referred to as ferric oxide [34].
Iron oxides are often considered to be the most explored magnetic nanoparticles due to their lack of toxicity and overall compatibility with living systems [35]. In addition to these characteristics, the capacity of iron oxide to be separated and its magnetic properties are also beneficial to the recycling process. This why the magnetic nanoparticles are chosen to be synthesized and used in different fields [36]. Some of the physical and magnetic properties of iron oxides are shown in Table 1.
Table 1.
Physical properties of iron oxides [37].
| Properties | Magnetite | Maghemite | Hematite |
|---|---|---|---|
| Molecular formula | Fe3O4 | γ-Fe2O3 | α-Fe2O3 |
| Crystallographic structure | Cubic | Cubic or tetrahedral | Rhombohedral or Hexagonal |
| Lattice Parameter (nm) | a = 0.8396 | a = 0.8347 | a = 0.5034 |
| Color | Black | Reddish-Brown | Red |
| Magnetic Status | Ferrimagnetic or superparamagnetic | Ferrimagnetic or superparamagnetic | Weakly Ferromagnetic |
| Hardness | 5.5 | 5 | 6.5 |
| Density (g/cm3) | 5.18 | 4.87 | 5.26 |
The synthesis of nanoparticulate magnetite (Fe3O4) and greigite (Fe3S4) by bacteria has been investigated deeply; nevertheless, the biochemical mechanism has not yet been completely understood. This study demonstrated that nanoparticulate magnetite may be generated extracellularly at room temperature by presenting the fungus Aspergillus elegans extract with combinations of the salts iron chloride (FeCl2) and iron chloride (FeCl3) [38,39]. Using fungal biomass and secreted enzymes as bioreducing and capping agents aligns with green chemistry principles. Fungi can mediate the nucleation and growth of nanoparticles, resulting in various shapes and sizes ranging from spheres and rods to triangles and hexagons [[40], [41], [42], [43], [44], [45]].
Microorganisms that survive in environments with high atmospheric pressure, acidity, and temperature are promising candidates for creating IONPs. Fungi have been reported as a novel and effective candidate for the environmentally friendly production of IONPs. This is because fungi are simple to handle, have a faster growth rate, and require low-cost maintenance, making them ideal organisms for the environmentally friendly synthesis of nanoparticles. They can survive agitation in bioreactors because of their filamentous form, and they can produce massive quantities of extracellular enzymes in a very short time when incubated [46].
Fungal cells possess transport mechanisms that allow them to remove metal ions from the surrounding environment. These ions are often present as metal salts dissolved in the growth medium. The uptake is driven by ion concentration gradients and facilitated by ion channels and transporters. Once inside the cells, metal ions are subjected to reduction processes. Fungi employ various intracellular reducing agents, such as NADH (nicotinamide adenine dinucleotide) and NADPH (nicotinamide adenine dinucleotide phosphate), which are cofactors involved in cellular metabolic reactions. These reducing agents donate electrons to the metal ions, converting them into neutral atoms or small clusters [47].
Myco-nanoparticles often exhibit excellent biocompatibility due to biomolecules on their surfaces. This characteristic enables functionalization through bioconjugation, enabling the attachment of ligands for targeted drug delivery or specific bio-recognition. The physical and chemical properties of myco-nanoparticles can be controlled by modulating the fungal growth conditions and synthesis parameters. This tunability facilitates the design of nanoparticles with desired properties, such as enhanced plasmonic resonance, improved magnetic behavior, or tailored catalytic activity [[48], [49], [50]].
The colonies of A. elegans were bright yellow and prolific. Fully mature, septate, branching, hyaline mycelium. Each cell has several nuclei. The conidiophores were very elongated, frequently including a foot cell that was either straight or flexuous and swelled into a globular vesicle at the tip. Branches more or less clenched cover the vehicle's surface. The organism had globose, catenulate, dry, echinulate, or biseriate conidial heads, and its conidia were yellow to ochre in hue, while its sclerotia were a dark brown tint. A. elegans had the greatest mycelial development (95.33 mm) on the PDA medium. According to the results, A. elegans grows most rapidly at a temperature of 30 °C. Mycelial growth of A. elegans was greatest (88.25 mm) at pH 7, medium at pH 8 and pH 6, and least at pH 9 [51,52]. According to Kumla et al. [53], the following metabolites were recovered from A.elegans: clavatol, sitostanol, vioxanthin, xanthomegnin, viomellein, rubrosulphin, rubrosulphin diacetate, viopurpurin, ochratoxin A, ochratoxin A methyl ester, ochratoxin B, and ochratoxin.
The extract derived from A. elegans is endowed with phytochemical constituents, notably flavonoids. These compounds play a pivotal role in mitigating nanoparticle aggregation and facilitating the stabilization of the syntheszied nanoparticles. There is no study to create nanoparticles with this species of fungi. Hence, the novelty of this study was to create nanoparticles with A.elegans and compare the performance of nanoparticles using A.elegans extract with the NPs prepared from the conventional chemical method. This investigation aimed to synthesize nano magnetite, specifically Fe3O4 nanoparticles, employing extracts from A.elegans. These nanoparticles were subsequently subjected to a comparative analysis with magnetite nanoparticles prepared through the conventional chemical method, following the protocol outlined by Bharde et al. [54], albeit with certain modifications. This study aimed to enhance the quality of magnetic nanoparticles through a biologically mediated approach and evaluate their characteristics concerning conventionally synthesized counterparts.
2. Method and material
2.1. Materials
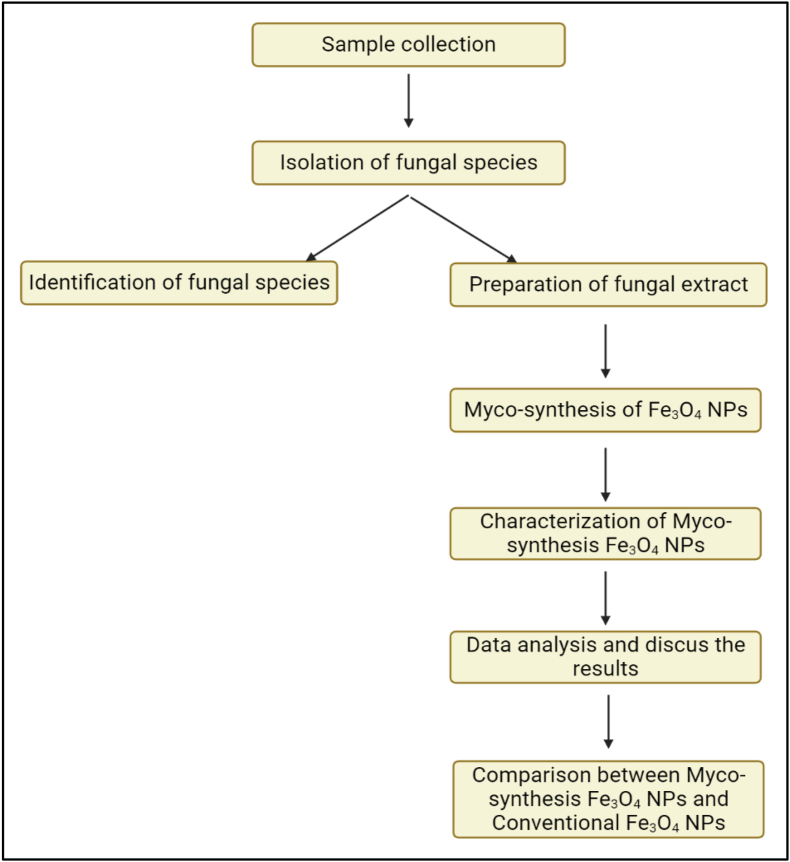
Iron chloride tetrahydrate (FeCl2·4H2O) and iron chloride hexahydrate (FeCl3·6H2O) were procured from Biochem Chemopharma Company, France, and utilized as precursors in the fabrication of magnetite nanoparticles. Additionally, sodium hydroxide (NaOH) was also acquired from Biochem Chemopharma Company. Specimens for the isolation of A.elegans were sourced from a landfill site in Soran city, located within the Kurdistan region of Iraq, at geographical coordinates (36.6246699 latitude and 44.5254009 longitude). Fig. 1 shows the flowchart of the study.
Fig. 1.
Flow chart of the experimental procedure of the study.
2.2. Isolation of A.elegans
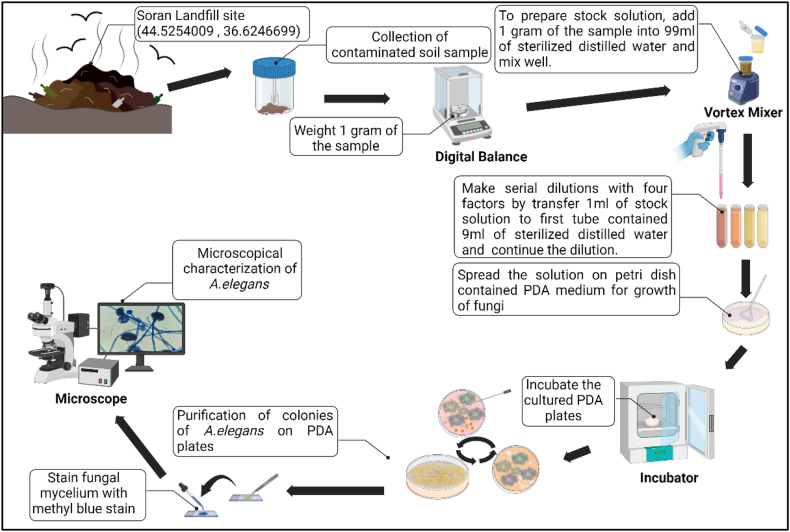
The isolation of fungal species from soil contaminated with municipal waste material was achieved using the dilution method, followed by transferring 100 μl from diluted samples onto Potato Dextrose Agar (PDA) medium. Afterthat, The culture plates were put into inucubator for 5–7 days at 30 °C. Next, individual colonies were subcultured onto pure PDA plates to obtain pure cultures [52]. The isolated fungi were identified through a process that involved morphological characterization of their colony growth patterns and microscopic examination of their fungal structures [[55], [56], [57], [58], [59],60]. The microscopic assessment of the isolated fungi was conducted using methylene blue staining techniques and light microscopy (model; Leica DM2700 P, Germany) (Fig. 2).
Fig. 2.
Isolation and identification of fungal species from landfill. The image was created with BioRender.com.
2.3. Preparation of fungal culture filtrate
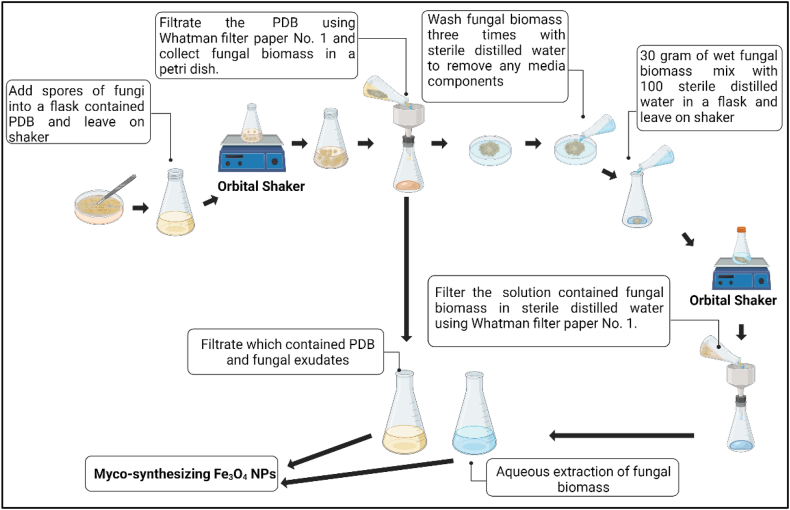
The fungal strain was cultivated in a Potato Dextrose Broth (PDB) medium consisting of 200 ml of potato extract and 20g of glucose dissolved in 1 L of distilled water, maintaining a pH of 7. The culture was incubated at 28 °C for 15 days. After this incubation period, the culture medium underwent filtration using Whatman filter paper no. 1. A volume of 100 ml of the filtered medium, referred to as the "growth medium," was aseptically transferred into a sterilized Erlenmeyer flask to synthesize Fe3O4 nanoparticles. Fifteen grams of the harvested fungal biomass were then separately re-suspended in 100 ml of sterile distilled water and subjected to further incubation at a temperature of 28 ± 2 °C while agitating at 150 rpm for 48 h. Subsequently, cell-free filtrates, also known as aqueous extracts, were obtained by separating the fungal biomass through filtration using Whatman filter paper No. 1. A volume of 100 ml of these aqueous extracts was employed for the biosynthesis of Fe3O4 nanoparticles as shown in Fig. 3.
Fig. 3.
Preparation of fungal extract for biosynthesis of Fe3O4-NPs. The image was created with BioRender.com.
2.4. Magnetite NPs synthesis using A. elegans extract
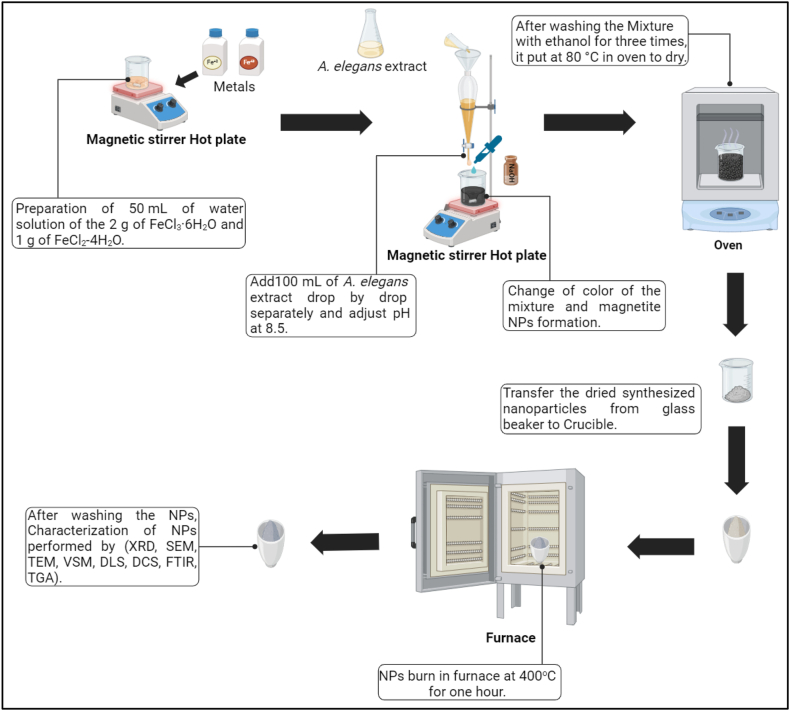
This study employed a reliable and consistent method to synthesize magnetite nanoparticles. To initiate the process, 100 ml of growth medium and 100 ml of aqueous extract were added separately drop by drop to a 50 ml water solution containing 2 g of FeCl3·6H2O and 1 gam of FeCl2·4H2O. The mixtures were continuously incubated at 50 °C with stirring for 30 min. The transformation of the color of the mixture to black signed the successful production of magnetite nanoparticles.
Additionally, 1 N NaOH solution was incorporated into the mixture above. Furthermore, the solution was consistently heated to 80 °C until the water had evaporated, resulting in the participation of nanoparticles at the bottom of the container.
Subsequently, the participating particles were subjected to three rounds of washing, two rounds with double-distilled water and then with ethanol, in order to purify the synthesized nanoparticle from all contaminants. The synthesized nanoparticles were then subjected to a heat treatment in a furnace at 400 °C for 1 h, as illustrated in Fig. 4.
Fig. 4.
Myco-synthesized Fe3O4 NPs using growth medium and aqueous extract of A.elegans.
2.5. Characterization of myco-synthesized NPs
A variety of distinct techniques and instruments were employed in the comprehensive characterization of the Fe3O4 nanoparticles produced through myco-synthesis. X-ray diffraction (XRD) analysis was executed using the Panalytical X'Pert3 Powder instrument, which featured a diffractometer system (XPERT-PRO) and employed Cu ka radiation. The diffraction pattern was recorded over a 2θ range spanning from 5° to 70°, utilizing a 2θ step scan of 0.010, with each step counted for 0.5s. The generator operated at 45 kV and 40 mA [61,62]. The resulting micrograph was cross-referenced with the Joint Committee on Power Diffraction Standards Library to ascertain the crystalline structure of the nanoparticles. Moreover, the nanoparticle sample underwent lyophilization and underwent further scrutiny. Morphological attributes were examined through scanning electron microscopy, specifically utilizing the FEI Model QUANTA 450. Zeta potential measurements were performed using a Nano ZS90 Zeta sizer from Malvern Instruments, incorporating a He–Ne laser (633 nm, 5 Mw). Dynamic light scattering (DLS) analysis was conducted utilizing the same Nano ZS90 Zeta sizer. Additionally, the analytical repertoire encompassed Differential Scanning Calorimetry (DSC), Vibrating-Sample Magnetometry (VSM), Thermogravimetric Analysis (TGA) with the TGAQ500 V20.13 Build 39 instrument sourced from TA Instruments Co., USA, Transmission Electron Microscopy (TEM), and Fourier Transform Infrared (FTIR) spectroscopy.
3. Results and discussion
3.1. Isolation and identification of Aspergillus elegans
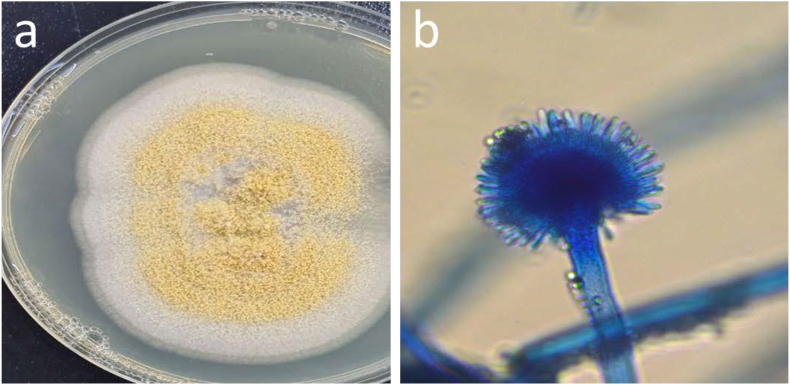
The isolated fungi, Aspergillus elegans were characterized based on the morphology of the colonies (Fig. 5a) and microscopic characterization of the mycelium, sporangium, and conidia (Fig. 5b). The isolated fungus conformed to belong Aspergillus genus; it was correctly Aspergillus elegans.
Fig. 5.
Macroscopic and microscopic characterization of fungal species a) Colony of A.elegans on PDA medium b) Sporangium and sporangiophore of A.elegans under a light microscope.
3.2. XRD analysis of myco-synthesized NPs
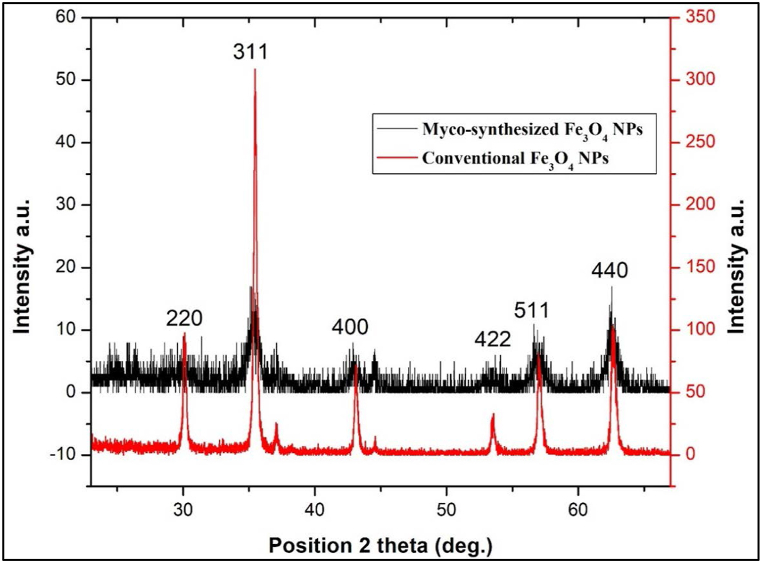
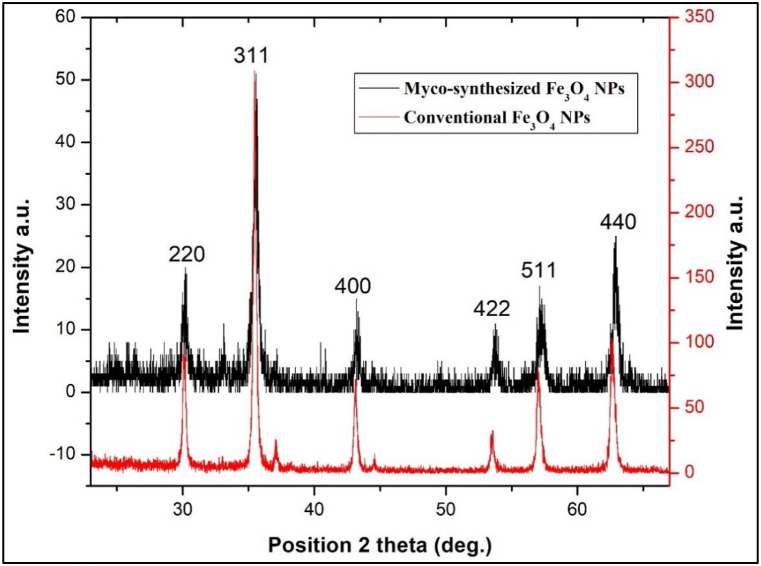
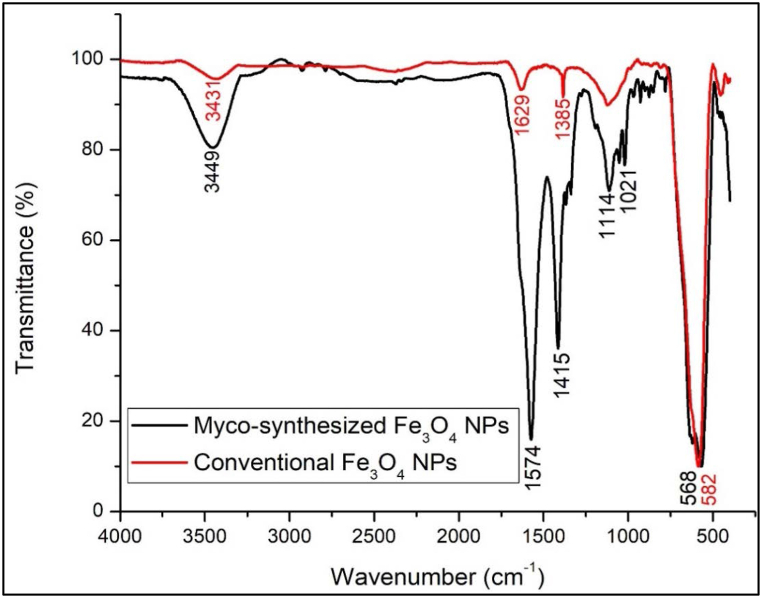
X-ray diffraction (XRD) stands out as one of the most commonly employed techniques for nanoparticle (NP) characterization. The device used in this study featured a Cu anode, a wavelength of 0.154 nm, a maximum power of 2.2 kW, and a long, fine-focus ceramic tube. Fig. 6, Fig. 7 present the XRD powder diffraction patterns of Fe3O4 NPs, synthesized via both biosyntheses using A.elegans and the conventional chemical method. Notably, the diffraction peaks observed for the myco-synthesized Fe3O4 NPs exhibit broadening, which can be attributed to the small crystallite size. This phenomenon indicates a higher level of phase purity in the product. The cubic structure of Fe3O4 served as an index to all the identified diffraction peaks, which are all connected to Fe3O4 (JCPDS no. 19–629) Fig. 6, Fig. 7. The XRD pattern revealed the peaks at 30.17°, 35.62°, 43.21°, 53.75°, 57.27°, and 62.92° for my-synthesized Fe3O4 NPs from aqueous extract of fungi and 35.28°, 43.08°, 44.51°, 56.72°, and 62.46° for myco-synthesized Fe3O4 NPs from the growth medium solution of fungi and 30.08°, 35.46°, 43.1°, 53.53°, 57.02°, and 62.7° for the magnetite NPs prepared from the conventional chemical method. The identification of the particles as Fe3O4 with a cubic inverse-spinel structure is substantiated by the observed 2θ values corresponding to specific Bragg's reflections for FCC structures, namely (220), (311), (400), (422), (511), and (440) planes. Moreover, the average particle sizes were determined to be 21.18 nm and 13.96 nm for Fe3O4 nanoparticles synthesized through myco-synthesis using the growth medium and aqueous extract, respectively. In contrast, magnetite nanoparticles prepared via the traditional chemical method exhibited an average size of 27.9 nm, as calculated employing the Debye–Scherrer formula.
Fig. 6.
XRD analysis for Myco-synthesized Fe3O4 NPs from the growth medium and the magnetite NPs prepared from the conventional chemical method.
Fig. 7.
XRD analysis for Myco-synthesized Fe3O4 NPs from aqueous extract and the magnetite NPs prepared from conventional chemical method.
3.3. Scanning electron microscope (SEM)
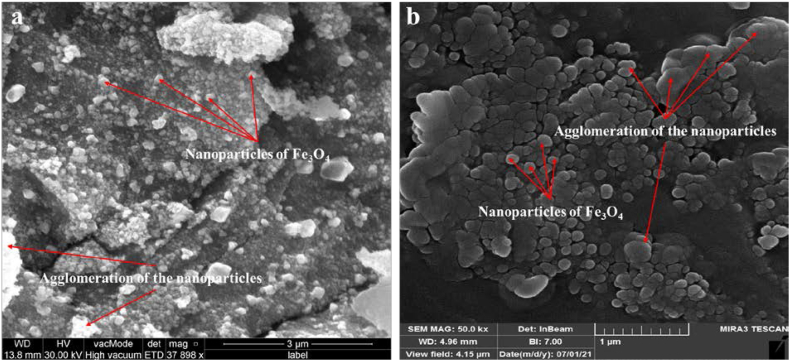
To describe the surface morphology of the biosynthesized Fe3O4 NPs, SEM examination was utilized [[63], [64], [65], [66], [67], [68]]. Fig. 8, Fig. 9 display the photos of the magnetite nanoparticles produced by myco-synthesized and conventional chemical method. As can be observed, most of the Fe3O4 NPs produced via myco-synthesized are on the nanoscale and have a uniformly hexagonal shape (Fig. 8, Fig. 9a). In addition, the Fe3O4 NPs have a somewhat agglomerated structure due to synthesized nanoparticles by biological methods. Agglomeration or aggregation occurs due to the high attraction that biosynthetic NPs have for one another, which contributes to the increased surface area that the myco-synthesized NPs possess. The magnetite nanoparticles produced by the conventional chemical process do not have a uniform distribution, and their diameters are enormous (Fig. 8, Fig. 9b). In addition, the magnetite nanoparticles produced using conventional chemical processes resulted in a highly agglomerated product [63,66,69].
Fig. 8.
SEM image a) Myco-synthesized Fe3O4 NPs from growth medium b) the magnetite NPs prepared from conventional chemical method.
Fig. 9.
SEM image a) Myco-synthesized Fe3O4 NPs from aqueous extract b) the magnetite NPs prepared from conventional chemical method.
The formation of Fe3O4 NPs nanoparticles relies on several growth parameters. These growth factors include the biomass of fungal species, concentration of fungal extract, type of growth media, concentration of salt, amount of time spent growing or reacting, temperature, and pH of the solution. Consequently, proper optimization of these development components is essential for NPs to attain the smaller size and uniform shapes needed for maximal manipulation and demand. In this study, the growth parameter used are the optimal growth parameters [[70], [71], [72]].
3.4. Fourier transform infrared (FTIR)
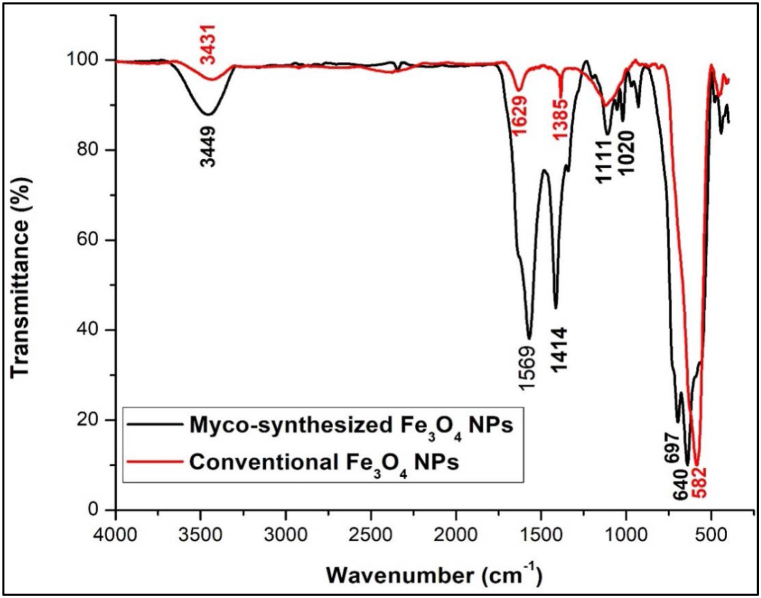
One of the accurate methods for investigation of the transmission bands of bimetallic magnetic systems is analyzed by FTIR spectroscopy, which is a method that can differentiate between the primary phases of iron oxide with a high degree of precision [[73], [74], [75]]. Fig. 10, Fig. 11 present the FT-IR spectra of Fe3O4 nanoparticles that were produced using the myco-synthesized and conventional chemical methods. An OH-stretching vibration can be seen at 3449 cm−1 from both myco-synthesized Fe3O4 NPs. This vibration receives contributions from symmetrical and asymmetrical modes of the O–H bonds related to the surface iron atoms. The identical peak was observed again within the magnetite NPs that were made using the conventional chemical process that was noticeably narrower. The large surface area of the myco-synthesized Fe3O4 NPs is most likely to blame for the broadness of the OH group found on the surface of the NPs. As a result, there are more sites on the surface of the NPs. The findings of this inquiry from those studies were not like others in other investigations, which may be because a different fungal species (A.elegans) was used in this investigation [[75], [76], [77]]. The second peak, which indicated the OH bending vibration, was 1574, 1569, and 1629 cm−1 from myco-synthesized Fe3O4 NPs from the growth medium, aqueous extract, and conventional chemical methods, respectively. These values were compatible with previously reported FTIR spectra for spinel Fe3O4 [78,79]. Also, the peak at 1629 cm−1 may indicate the presence of Fe–O. It may be deduced from the absorption bands at 1415 cm−1, 1414 cm−1, 1385 cm−1 from myco-synthesized Fe3O4 NPs from the growth medium solution, aqueous extract and conventional chemical methods that asymmetric stretching of CO originating from the acid group was present, respectively. For both myco-synthesized Fe3O4 NPs and conventional chemical methods, the stretching vibration mode associated with the metal-oxygen Fe–O bonds in Fe's crystalline lattice is thought to be responsible for the strong peak observed at 568 cm−1, 640 cm−1, and 582 cm−1, respectively. They are quite noticeable in the structures of all spinel minerals, especially ferrites [[80], [81], [82]].
Fig. 10.
FTIR spectra of Myco-synthesized Fe3O4 NPs from the growth medium, and the magnetite NPs prepared from the conventional chemical method.
Fig. 11.
FTIR spectra of Myco-synthesized Fe3O4 NPs from aqueous extract and the magnetite NPs prepared from the conventional chemical method.
3.5. Transmission electron microscope (TEM)
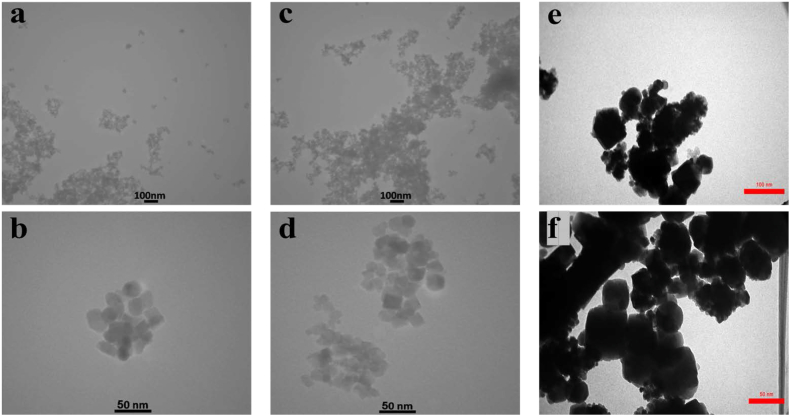
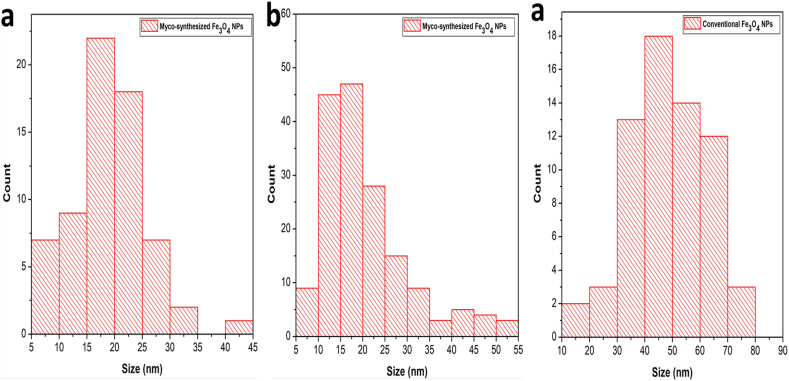
An investigation of the shape and distribution of myco-synthesized Fe3O4 NPs and conventional Fe3O4 NPs was carried out using transmission electron microscopy (TEM). Both myco-synthesized Fe3O4 NPs (Fig. 12a–d), and conventional Fe3O4 NPs (Fig. 12e and f) seemed to be composed of hexagonal particles when seen using typical transmission electron micrographs. The particle size histogram of myco-synthesized Fe3O4 NPs from the growth medium (Fig. 13a) demonstrated particle diameters ranging from 5 to 55 nm, with an average size of 19.2 nm. The particle size histogram of myco-synthesized Fe3O4 NPs from aqueous extract (Fig. 13b) demonstrated particle diameters ranging from 5 to 45 nm, with an average size of 22.2 nm. While the particle size histogram of conventional Fe3O4 NPs (Fig. 13c) demonstrated particle diameters ranging from 10 to 80 nm, mostly about 60 nm). The findings of this study agree with previous studies [77,83]. It has been shown that the Fe3O4-NPs produced by the myco-synthesized approach are considerably more stable and smaller than those produced by the conventional chemical process. When the size of the Fe3O4-NPs is reduced, the surface area increases, which results in an increased number of capping agents around the myco-synthesized Fe3O4-NPs. The presence of additional capping agents surrounding myco-synthesized Fe3O4 nanoparticles enhances both their stability and zeta potential value. Furthermore, the substantial surface area of Fe3O4 nanoparticles offers several advantages, including the ability to efficiently detect molecular interactions and facilitate targeted biomolecule interactions. In essence, myco-synthesized Fe3O4 nanoparticles exhibit excellent stability and hold significant promise for a wide range of applications, including but not limited to biosensors, drug delivery, magnetic resonance imaging (MRI), cancer diagnostics, electrical devices, physical instruments, and sensor systems for monitoring pollutant gases [84].
Fig. 12.
TEM image for (a and b) Myco-synthesized Fe3O4 NPs from the growth medium, (c and d) Myco-synthesized Fe3O4 NPs from aqueous extract, and (e and f) the magnetite NPs prepared from conventional chemical method.
Fig. 13.
A particle size distribution histogram determined from the TEM images a) Particle size distribution of Myco-synthesized Fe3O4 NPs from the growth medium b) Particle size distribution of Myco-synthesized Fe3O4 NPs from aqueous extract c) Particle size distribution of the magnetite NPs prepared from conventional chemical method.
3.6. Vibrating-sample magnetometer (VSM)
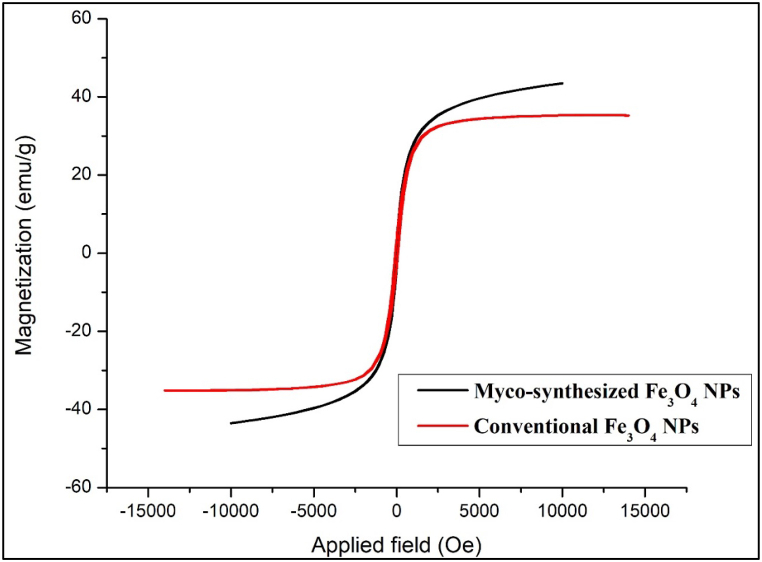
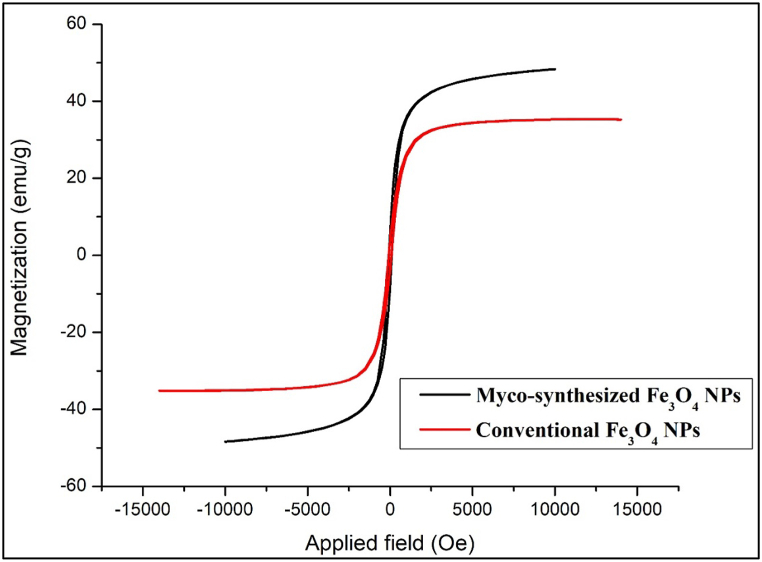
To study synthesized Fe3O4 magnetic behavior, the magnetic properties of the NPs were measured at room temperature with a vibrating sample magnetometer (VSM) [85]. Fig. 14, Fig. 15 show the hysteresis loops of the samples. The saturation magnetization was 43.5 emu/g and 48.3 emu/g for myco-synthesized Fe3O4 NPs from the growth medium and aqueous extract, respectively. While, the saturation magnetization of the magnetite NPs prepared from the conventional chemical method was 35.4 emu/g as shown in Fig. 14, Fig. 15. From both myco-synthesized Fe3O4 NPs, no reduced remanence and coercivity were detected, indicating that myco-synthesized Fe3O4 NPs are superparamagnetic. The findings obtained from this study about saturation magnetization of the nanoparticle were more than green synthesized Fe3O4 NPs from Rhus coriaria extract that was confirmed in a previous study[86], which states that the magnetization value is 41 emu/g.
Fig. 14.
VSM analysis for Myco-synthesized Fe3O4 NPs from the growth medium and the magnetite NPs prepared from the conventional chemical method.
Fig. 15.
VSM analysis for Myco-synthesized Fe3O4 NPs from aqueous extract and the magnetite NPs prepared from the conventional chemical method.
3.7. Thermogravimetric analysis
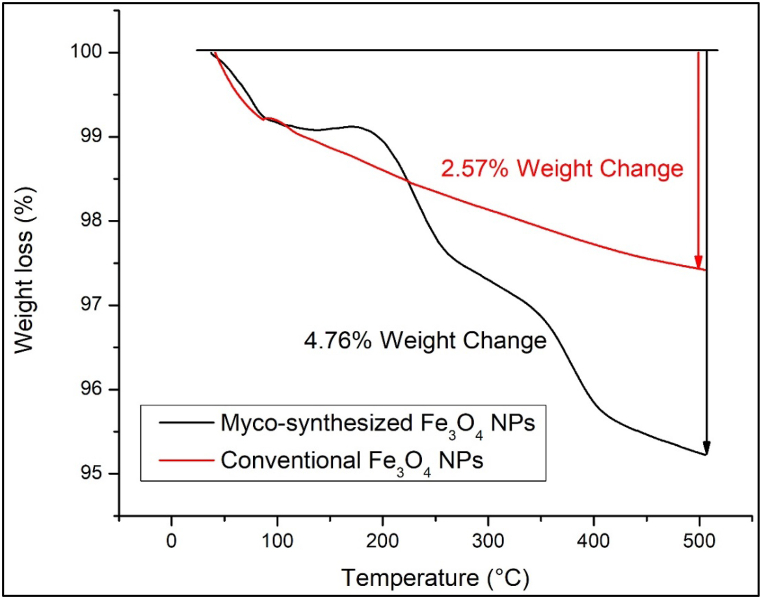
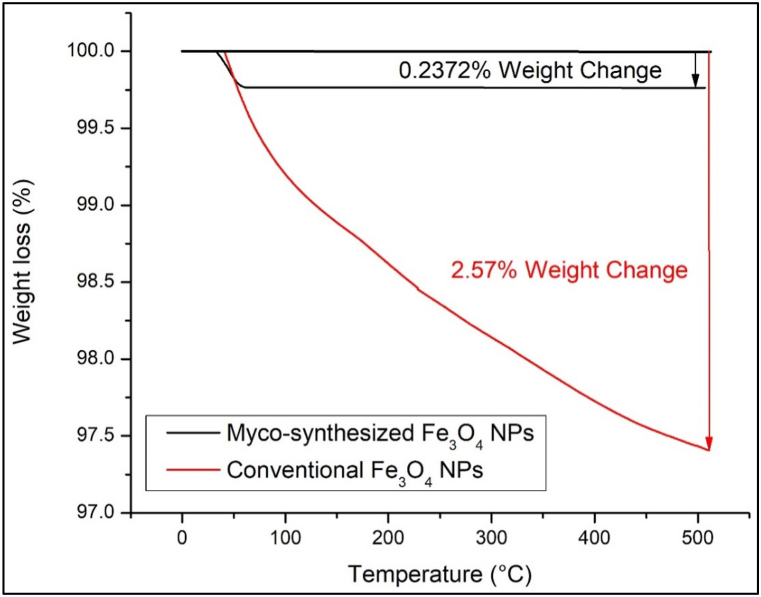
Thermogravimetric analysis is a technique used to track alterations in the physical and chemical properties of materials as a function of temperature [[87], [88], [89], [90]]. In the case of myco-synthesized Fe3O4 nanoparticles and magnetite nanoparticles prepared through a conventional chemical approach, TGA was conducted over a temperature range of 0–500 °C, as depicted in Fig. 16, Fig. 17. The initial weight loss observed for the magnetite nanoparticles under 100 °C, is attributed to moisture content. Subsequently, decomposition occurs around 318 °C, attributed to the breakdown of protein chains, with capping agent decomposition occurring in the range of 150–400 °C, in accordance with previous findings [91]. In Fig. 16 shows myco-synthesized Fe3O4 nanoparticles experienced a weight loss of 4.76 % when subjected to a temperature of 500 °C, while magnetite nanoparticles prepared through the conventional chemical method exhibited a 2.57 % reduction in weight under the same temperature conditions. The large weight loss of myco-synthesized Fe3O4 NPs from the growth medium after being heated to 250 °C occurred due to capping agent decomposition [86,91]. However, the weight of myco-synthesized Fe3O4 NPs from aqueous extract was decreased by 0.24 % at 500 °C because of the evaporation of physical water inside the nanoparticles [92]. Based on the TGA analysis, myco-synthesized Fe3O4 NPs from the aqueous extract were stronger when exposed to high temperatures than the magnetite NPs prepared from the conventional chemical method at the same temperature.
Fig. 16.
TGA analysis for Myco-synthesized Fe3O4 NPs from the growth medium and the magnetite NPs prepared from the conventional chemical method.
Fig. 17.
TGA analysis for Myco-synthesized Fe3O4 NPs from aqueous extract and the magnetite NPs prepared from the conventional chemical method.
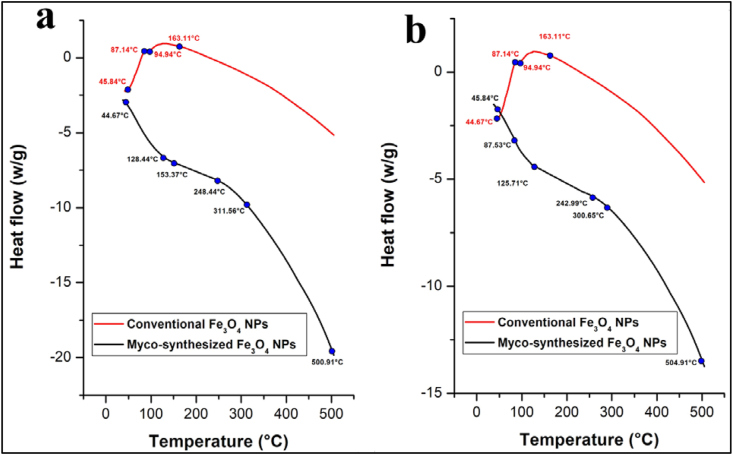
3.8. Differential Scanning Calorimetry (DSC)
Differential Scanning Calorimetry, abbreviated as DSC, is a thermal analysis method that quantifies the heat exchange within a sample while subjecting it to a precisely controlled temperature program. This technique serves as a robust tool for assessing various material characteristics, including critical factors such as the glass transition temperature (Tg), melting and crystallization phenomena, specific heat capacity, curing mechanisms, purity levels, susceptibility to oxidation, and thermal stability [66,86]. DSC analysis for both myco-synthesized Fe3O4 NPs shown that the glass Transition temperature of myco-synthesized Fe3O4 NPs was about 300 °C it was very high compared to the glass Transition temperature of the magnetite NPs prepared from the conventional chemical which is 163 °C (Fig. 18ab). Additionally, increasing (Tg) improves handling characters, solubility, and reproducibility in the dissolution of solids and also increases entropy that led myco-synthesized Fe3O4 NPs to be amorphous nanoparticle [93,94].
Fig. 18.
DCS curve for a) Myco-synthesized Fe3O4 NPs from the growth medium b) Myco-synthesized Fe3O4 NPs from aqueous extract and the magnetite NPs prepared from conventional chemical method.
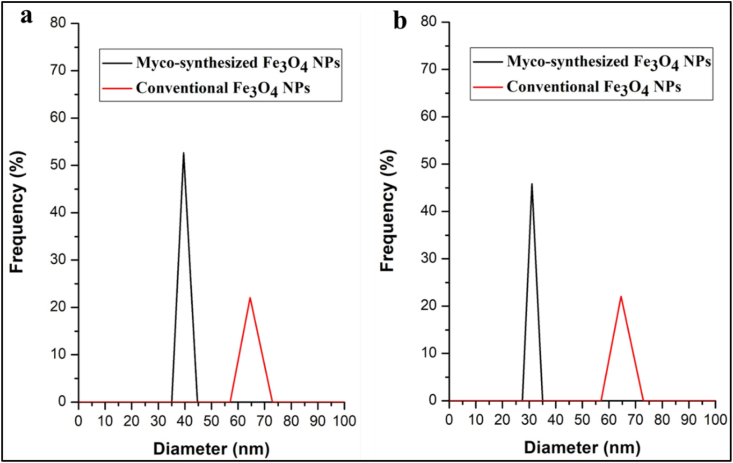
3.9. Dynamic light scattering (DLS)
The DLS measurements reveal distinct nanoparticle sizes for different synthesis methods. Myco-synthesized Fe3O4 nanoparticles from the growth medium exhibit an average size of 39.5 nm (Fig. 19a), while those from the aqueous extract are smaller, measuring around 31 nm (Fig. 19b). In contrast, nanoparticles produced using the conventional chemical method are considerably larger, with an average size of 60 nm (Fig. 19ab). These variations in nanoparticle size can be attributed to the unique conditions and mechanisms involved in each synthesis approach. The growth medium may have facilitated the formation of smaller particles due to specific biochemical factors. In contrast, the chemical method likely led to larger nanoparticles through controlled nucleation and growth processes. The results agree with previous studies [77,83]. The nanoparticle sizes obtained from DLS measurements appear larger than those determined via TEM analysis. This dissimilarity arises from the influence of phytochemical molecules, which effectively augment the particle size. Additionally, the variation in conditions during the two analysis methods contributes to the contrast in size measurements. Specifically, the TEM analysis involves dried particles, whereas DLS analyzes particles suspended in aqueous solutions. Consequently, the hydrodynamic size of nanoparticles in colloidal suspension consistently exceeds the sizes determined by TEM, primarily due to the presence of adsorbed aqueous molecules [83,95].
Fig. 19.
DLS analysis for Myco-synthesized Fe3O4 NPs a) Myco-synthesized Fe3O4 NPs from the growth medium b) Myco-synthesized Fe3O4 NPs from the aqueous extract and compared to the magnetite NPs prepared from conventional chemical method.
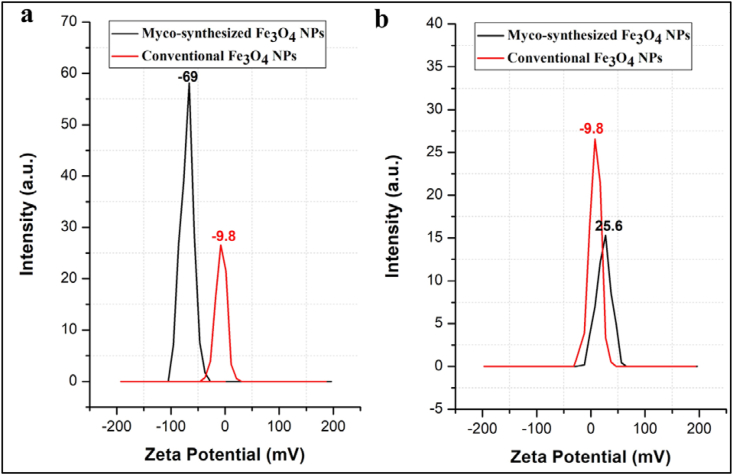
3.10. Zeta potential
Zeta potential analysis is a technique used to measure the surface charge or electrokinetic potential of particles suspended in a liquid medium, typically in colloidal systems. It provides insights into the stability and behavior of colloidal dispersions, such as nanoparticles, colloidal particles, and macromolecules. Nanoparticles possessing zeta potentials ranging from >-10 mV to <+10 mV have a neutral charge. Conversely, Nanoparticles possessing zeta potentials ranging from >-30 mV to <+30 mV are categorized as strongly cationic and strongly anionic, respectively [96]. The zeta potential measurement for Fe3O4 nanoparticles produced in this work from the growing media by myco-synthesis was found to be -69 mV (Fig. 20a). The surface of the nanoparticles had a negative charge, which helped keep them stable and prevented the aggregation of iron particles. In comparison, the magnetite nanoparticles made using the traditional chemical approach had a zeta potential value of -9.8 mV (Fig. 20ab). Therefore, it can be inferred from these findings that the myco-synthesized Fe3O4 nanoparticles are more stable than the magnetite nanoparticles made by a traditional chemical method.
Fig. 20.
Zeta Potential analysis. a) Myco-synthesized Fe3O4 NPs from the growth medium b) Myco-synthesized Fe3O4 NPs from aqueous extract, both Myco-synthesized Fe3O4 NPs are compared to the magnetite NPs prepared from conventional chemical method.
4. Conclusions
In conclusion, this study employed a myco-synthesized approach to fabricate Fe3O4 nanoparticles and systematically compared them with Fe3O4 nanoparticles produced through a conventional chemical method. Generally, Myco-synthesized Fe3O4 NPs from the growth medium showed a quite better characterizations compared Myco-synthesized Fe3O4 NPs from the aqueous solution medium due to the reducing agents were screated into the medium to obtained the nutrients. Several key findings emerged from the microstructure tests, shedding light on the distinct characteristics and advantages of myco-synthesized Fe3O4 nanoparticles, which were smaller in size, hexagonal in shape, more stable, and also have higher magnetization values compared to Fe3O4 nanoparticles produced through a conventional chemical method.
Data availability statement
Available in the manuscript.
CRediT authorship contribution statement
Renjbar Muksy Mhammedsharif: Visualization, Validation, Supervision, Software. Parwin Jalal Jalil: Conceptualization, Validation, Visualization. Nzar Piro: Project administration, Resources. Ahmed Mohammed: Funding acquisition, Data curation, Conceptualization. Peyman K. Aspoukeh: Conceptualization, Investigation, Methodology.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Maurer-Jones M.A., Gunsolus I.L., Murphy C.J., Haynes C.L. Toxicity of engineered nanoparticles in the environment. Anal. Chem. 2013;85:3036–3049. doi: 10.1021/ac303636s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun Y., Xia Y. Shape-controlled synthesis of gold and silver nanoparticles. Science. 2002;298:2176–2179. doi: 10.1126/science.1077229. [DOI] [PubMed] [Google Scholar]
- 3.Osaka T., Matsunaga T., Nakanishi T., Arakaki A., Niwa D., Iida H. Synthesis of magnetic nanoparticles and their application to bioassays. Anal. Bioanal. Chem. 2006;384:593–600. doi: 10.1007/s00216-005-0255-7. [DOI] [PubMed] [Google Scholar]
- 4.Yu H., Li P., Zhang L., Zhu Y., Al-Zahrani F.A., Ahmed K. Application of optical fiber nanotechnology in power communication transmission. Alex. Eng. J. 2020;59:5019–5030. [Google Scholar]
- 5.Tawade B.V., Apata I.E., Singh M., Das P., Pradhan N., Al-Enizi A.M., Karim A., Raghavan D. Recent developments in the synthesis of chemically modified nanomaterials for use in dielectric and electronics applications. Nanotechnology. 2021;32 doi: 10.1088/1361-6528/abcf6c. [DOI] [PubMed] [Google Scholar]
- 6.Tang J., Daiyan R., Ghasemian M.B., Idrus-Saidi S.A., Zavabeti A., Daeneke T., Yang J., Koshy P., Cheong S., Tilley R.D. Advantages of eutectic alloys for creating catalysts in the realm of nanotechnology-enabled metallurgy. Nat. Commun. 2019;10:4645. doi: 10.1038/s41467-019-12615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dolai J., Mandal K., Jana N.R. Nanoparticle size effects in biomedical applications. ACS Appl. Nano Mater. 2021;4:6471–6496. [Google Scholar]
- 8.Sharma V.K. Optimal design for digital comparator using QCA nanotechnology with energy estimation. Int. J. Numer. Model. Electron. Network. Dev. Field. 2021;34 [Google Scholar]
- 9.Neme K., Nafady A., Uddin S., Tola Y.B. Application of nanotechnology in agriculture, postharvest loss reduction and food processing: food security implication and challenges. Heliyon. 2021;7 doi: 10.1016/j.heliyon.2021.e08539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das P.K., Mohanty C., Purohit G.K., Mishra S., Palo S. Nanoparticle assisted environmental remediation: applications, toxicological implications and recommendations for a sustainable environment. Environ. Nanotechnol. Monit. Manag. 2022;18 [Google Scholar]
- 11.Song Y., Kirkwood N., Maksimović Č., Zheng X., O'Connor D., Jin Y., Hou D. Nature based solutions for contaminated land remediation and brownfield redevelopment in cities: a review. Sci. Total Environ. 2019;663:568–579. doi: 10.1016/j.scitotenv.2019.01.347. [DOI] [PubMed] [Google Scholar]
- 12.Rahimzadeh C.Y., Barzinjy A.A., Mohammed A.S., Hamad S.M. Green synthesis of SiO2 nanoparticles from Rhus coriaria L. extract: Comparison with chemically synthesized SiO2 nanoparticles. PLoS One. 2022;17(8) doi: 10.1371/journal.pone.0268184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jamkhande P.G., Ghule N.W., Bamer A.H., Kalaskar M.G. Metal nanoparticles synthesis: an overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019;53 [Google Scholar]
- 14.Saravanan A., Kumar P.S., Karishma S., Vo D.-V.N., Jeevanantham S., Yaashikaa P., George C.S. A review on biosynthesis of metal nanoparticles and its environmental applications. Chemosphere. 2021;264 doi: 10.1016/j.chemosphere.2020.128580. [DOI] [PubMed] [Google Scholar]
- 15.Lovley D.R., Stolz J.F., Nord Jr G.L., Phillips E.J. Anaerobic production of magnetite by a dissimilatory iron-reducing microorganism. Nature. 1987;330:252–254. [Google Scholar]
- 16.Spring S., Schleifer K.-H. Diversity of magnetotactic bacteria. Syst. Appl. Microbiol. 1995;18:147–153. [Google Scholar]
- 17.Dickson D.P. Nanostructured magnetism in living systems. J. Magn. Magn Mater. 1999;203:46–49. [Google Scholar]
- 18.Mann S. Molecular tectonics in biomineralization and biomimetic materials chemistry. Nature. 1993;365:499–505. [Google Scholar]
- 19.Oliver S., Kuperman A., Coombs N., Lough A., Ozin G.A. Lamellar aluminophosphates with surface patterns that mimic diatom and radiolarian microskeletons. Nature. 1995;378:47–50. [Google Scholar]
- 20.Kroger N., Deutzmann R., Sumper M. Polycationic peptides from diatom biosilica that direct silica nanosphere formation. Science. 1999;286:1129–1132. doi: 10.1126/science.286.5442.1129. [DOI] [PubMed] [Google Scholar]
- 21.Pum D., Sleytr U.B. The application of bacterial S-layers in molecular nanotechnology. Trends Biotechnol. 1999;17:8–12. [Google Scholar]
- 22.Sleytr U.B., Messner P., Pum D., Sára M. Crystalline bacterial cell surface layers (S layers): from supramolecular cell structure to biomimetics and nanotechnology. Angew. Chem. Int. Ed. 1999;38:1034–1054. doi: 10.1002/(SICI)1521-3773(19990419)38:8<1034::AID-ANIE1034>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 23.Mahendra Rai M.R., Alka Yadav A.Y., Bridge P., Aniket Gade A.G. Myconanotechnology: a new and emerging science. Applied mycology. 2009:258–267. [Google Scholar]
- 24.Jagtap P., Nath H., Kumari P.B., Dave S., Mohanty P., Das J., Dave S. Fungi Bio-Prospects in Sustainable Agriculture, Environment and Nano-Technology. 2021. Mycogenic fabrication of nanoparticles and their applications in modern agricultural practices & food industries; pp. 475–488. [Google Scholar]
- 25.Muksy R., Kolo K., Abdullah S.M. Bacterial and fungal-mineral interactions and their application in bioremediation–A review. Journal of Ecological Engineering. 2023;24 [Google Scholar]
- 26.Rai M., Gade A., Yadav A. Biogenic nanoparticles: an introduction to what they are, how they are synthesized and their applications. Metal nanoparticles in microbiology. 2011:1–14. [Google Scholar]
- 27.Deka D., Rabha J., Jha D.K. Application of Myconanotechnology in the sustainable management of crop production system. Mycoremediation and Environmental Sustainability. 2018;2:273–305. [Google Scholar]
- 28.Castro-Longoria E., Garibo-Ruiz D., Martínez-Castro S. Myconanotechnology to treat infectious diseases: a perspective. Fungal Nanotechnology: Applications in Agriculture, Industry, and Medicine. 2017:235–261. [Google Scholar]
- 29.Shakya M., Rene E.R., Nancharaiah Y.V., Lens P.N. Fungal-based nanotechnology for heavy metal removal. Nanotechnology, Food Security and Water Treatment. 2018:229–253. [Google Scholar]
- 30.Otero-González L., Mikhalovsky S.V., Václavíková M., Trenikhin M.V., Cundy A.B., Savina I.N. Novel nanostructured iron oxide cryogels for arsenic (As (III)) removal. J. Hazard Mater. 2020;381 doi: 10.1016/j.jhazmat.2019.120996. [DOI] [PubMed] [Google Scholar]
- 31.Lassoued A., Dkhil B., Gadri A., Ammar S. Control of the shape and size of iron oxide (α-Fe2O3) nanoparticles synthesized through the chemical precipitation method. Results Phys. 2017;7:3007–3015. [Google Scholar]
- 32.Campos E.A., Pinto D.V.B.S., Oliveira J.I. S.d., Mattos E.d.C., Dutra R.d.C.L. Synthesis, characterization and applications of iron oxide nanoparticles-a short review. J. Aero. Technol. Manag. 2015;7:267–276. [Google Scholar]
- 33.Arun T., Prabakaran K., Udayabhaskar R., Mangalaraja R., Akbari-Fakhrabadi A. Carbon decorated octahedral shaped Fe3O4 and α-Fe2O3 magnetic hybrid nanomaterials for next generation supercapacitor applications. Appl. Surf. Sci. 2019;485:147–157. [Google Scholar]
- 34.Wang W., Howe J.Y., Gu B. Structure and morphology evolution of hematite (α-Fe2O3) nanoparticles in forced hydrolysis of ferric chloride. J. Phys. Chem. C. 2008;112:9203–9208. [Google Scholar]
- 35.Guo L., He N., Zhao Y., Liu T., Deng Y. Autophagy modulated by inorganic nanomaterials. Theranostics. 2020;10:3206. doi: 10.7150/thno.40414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fraga-García P., Kubbutat P., Brammen M., Schwaminger S., Berensmeier S. Bare iron oxide nanoparticles for magnetic harvesting of microalgae: from interaction behavior to process realization. Nanomaterials. 2018;8:292. doi: 10.3390/nano8050292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lam U.T., Mammucari R., Suzuki K., Foster N.R. Processing of iron oxide nanoparticles by supercritical fluids. Ind. Eng. Chem. Res. 2008;47:599–614. [Google Scholar]
- 38.Matsunaga T., Sakaguchi T. Molecular mechanism of magnet formation in bacteria. J. Biosci. Bioeng. 2000;90:1–13. doi: 10.1016/s1389-1723(00)80001-8. [DOI] [PubMed] [Google Scholar]
- 39.Schüler D. The biomineralization of magnetosomes in Magnetospirillum gryphiswaldense. Int. Microbiol. 2002;5:209–214. doi: 10.1007/s10123-002-0086-8. [DOI] [PubMed] [Google Scholar]
- 40.Banerjee K., Ravishankar Rai V. A review on mycosynthesis, mechanism, and characterization of silver and gold nanoparticles. BioNanoScience. 2018;8:17–31. [Google Scholar]
- 41.Husen A., Siddiqi K.S. Plants and microbes assisted selenium nanoparticles: characterization and application. J. Nanobiotechnol. 2014;12:1–10. doi: 10.1186/s12951-014-0028-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maliszewska I., Juraszek A., Bielska K. Green synthesis and characterization of silver nanoparticles using ascomycota fungi Penicillium nalgiovense AJ12. J. Cluster Sci. 2014;25:989–1004. [Google Scholar]
- 43.Vágó A., Szakacs G., Sáfrán G., Horvath R., Pécz B., Lagzi I. One-step green synthesis of gold nanoparticles by mesophilic filamentous fungi. Chem. Phys. Lett. 2016;645:1–4. [Google Scholar]
- 44.Kitching M., Ramani M., Marsili E. Fungal biosynthesis of gold nanoparticles: mechanism and scale up. Microb. Biotechnol. 2015;8:904–917. doi: 10.1111/1751-7915.12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanghi R., Verma P. Biomimetic synthesis and characterisation of protein capped silver nanoparticles. Bioresour. Technol. 2009;100:501–504. doi: 10.1016/j.biortech.2008.05.048. [DOI] [PubMed] [Google Scholar]
- 46.Gade A., Ingle A., Whiteley C., Rai M. Mycogenic metal nanoparticles: progress and applications. Biotechnol. Lett. 2010;32:593–600. doi: 10.1007/s10529-009-0197-9. [DOI] [PubMed] [Google Scholar]
- 47.Narayanan K.B., Sakthivel N. Biological synthesis of metal nanoparticles by microbes. Adv. Colloid Interface Sci. 2010;156:1–13. doi: 10.1016/j.cis.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 48.Banerjee K., Ravishankar Rai V. Study on green synthesis of gold nanoparticles and their potential applications as catalysts. J. Cluster Sci. 2016;27:1307–1315. [Google Scholar]
- 49.Du L., Xu Q., Huang M., Xian L., Feng J.-X. Synthesis of small silver nanoparticles under light radiation by fungus Penicillium oxalicum and its application for the catalytic reduction of methylene blue. Mater. Chem. Phys. 2015;160:40–47. [Google Scholar]
- 50.Sandhu S.S., Shukla H., Shukla S. Biosynthesis of silver nanoparticles by endophytic fungi: its mechanism, characterization techniques and antimicrobial potential. Afr. J. Biotechnol. 2017;16:683–698. [Google Scholar]
- 51.Chanda I.J., Ara I., Sultana R., Alam N. Morphological and molecular characterization of Aspergillus elegans from small indigenous dry fish ‘Jat Puti'Puntius sophore (Hamilton 1822) in Bangladesh. Jahangirnagar University Journal of Biological Sciences. 2021;10:39–47. [Google Scholar]
- 52.Muksy R., Kolo K. Experimental observations and assessment of landfill leachate bioremediation by autochthonous fungi species and their effective geoactivities. Journal of Ecological Engineering. 2023;24:312–327. [Google Scholar]
- 53.Kumla D., Sousa E., Marengo A., Dethoup T., Pereira J.A., Gales L., Freitas-Silva J., Costa P.M., Mistry S., Silva A.M. 1, 3-Dioxepine and spiropyran derivatives of viomellein and other dimeric naphthopyranones from cultures of Aspergillus elegans KUFA0015 and their antibacterial activity. Phytochemistry. 2021;181 doi: 10.1016/j.phytochem.2020.112575. [DOI] [PubMed] [Google Scholar]
- 54.Bharde A., Rautaray D., Bansal V., Ahmad A., Sarkar I., Yusuf S.M., Sanyal M., Sastry M. Extracellular biosynthesis of magnetite using fungi. Small. 2006;2:135–141. doi: 10.1002/smll.200500180. [DOI] [PubMed] [Google Scholar]
- 55.Watanabe T. CRC press; 2002. Pictorial Atlas of Soil and Seed Fungi: Morphologies of Cultured Fungi and Key to Species. [Google Scholar]
- 56.Frisvad J.C., Frank J.M., Houbraken J., Kuijpers A.F., Samson R.A. New ochratoxin A producing species of Aspergillus section Circumdati. Stud. Mycol. 2004;50:23–44. [Google Scholar]
- 57.Visagie C., Houbraken J., Rodriques C., Pereira C.S., Dijksterhuis J., Seifert K., Jacobs K., Samson R. Five new Penicillium species in section Sclerotiora: a tribute to the Dutch Royal family. Persoonia-Molecular Phylogeny and Evolution of Fungi. 2013;31:42–62. doi: 10.3767/003158513X667410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sciortino Jr.C.V. John Wiley & Sons; 2017. Atlas of Clinically Important Fungi. [Google Scholar]
- 59.Alsohaili S.A., Bani-Hasan B.M. Vol. 11. Jordan Journal of Biological Sciences; 2018. (Morphological and Molecular Identification of Fungi Isolated from Different Environmental Sources in the Northern Eastern Desert of Jordan). [Google Scholar]
- 60.Ahmed J., Singh G., Xess I., Pandey M., Mohan A., Sachdev J., Mani P., Rana B. Emerging Aspergillus lentulus infections in India. Indian J. Med. Microbiol. 2022;40:160–162. doi: 10.1016/j.ijmmb.2021.10.011. [DOI] [PubMed] [Google Scholar]
- 61.Pirouei M., Kolo K., Kalaitzidis S.P., Abdullah S.M. Newly discovered gossanite-like and sulfide ore bodies associated with microbial activity in the Zagros ophiolites from the Rayat area of NE Iraq. Ore Geol. Rev. 2021;135 [Google Scholar]
- 62.Mhammedsharif R.M., Kolo K.Y. A case study of environmental pollution by pathogenic bacteria and metal (oid) s at Soran Landfill Site, Erbil, Iraqi Kurdistan Region. Environ. Monit. Assess. 2023;195:811. doi: 10.1007/s10661-023-11352-0. [DOI] [PubMed] [Google Scholar]
- 63.Mahdavi M., Ahmad M.B., Haron M.J., Namvar F., Nadi B., Rahman M.Z.A., Amin J. Synthesis, surface modification and characterisation of biocompatible magnetic iron oxide nanoparticles for biomedical applications. Molecules. 2013;18:7533–7548. doi: 10.3390/molecules18077533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou C., Zhu H., Wang Q., Wang J., Cheng J., Guo Y., Zhou X., Bai R. Adsorption of mercury (II) with an Fe 3 O 4 magnetic polypyrrole–graphene oxide nanocomposite. RSC Adv. 2017;7:18466–18479. [Google Scholar]
- 65.Vg V.K., Prem A.A. Green synthesis and characterization of iron oxide nanoparticles using Phyllanthus niruri extract. Orient. J. Chem. 2018;34 [Google Scholar]
- 66.Barzinjy A.A., Azeez H.H. Green synthesis and characterization of zinc oxide nanoparticles using Eucalyptus globulus Labill. leaf extract and zinc nitrate hexahydrate salt. SN Appl. Sci. 2020;2:991. [Google Scholar]
- 67.Bouafia A., Laouini S.E., Khelef A., Tedjani M.L., Guemari F. Effect of ferric chloride concentration on the type of magnetite (Fe3O4) nanoparticles biosynthesized by aqueous leaves extract of artemisia and assessment of their antioxidant activities. J. Cluster Sci. 2021;32:1033–1041. [Google Scholar]
- 68.Shnawa B.H., Mhammedsharif R.M., Jalil P.J., Hamadamin S.I., Ahmad S.F., Abdulrahman K.M., Ahmed M.H. Antimicrobial activity of plant-extract-mediated synthesis of Silver-Zinc Oxide nanocomposites and their acaricidal efficacy on Hyalomma marginatum ticks. Biocatal. Agric. Biotechnol. 2023 [Google Scholar]
- 69.Sundrarajan M., Ambika S., Bharathi K. Plant-extract mediated synthesis of ZnO nanoparticles using Pongamia pinnata and their activity against pathogenic bacteria. Adv. Powder Technol. 2015;26:1294–1299. [Google Scholar]
- 70.Pantidos N., Horsfall L.E. Biological synthesis of metallic nanoparticles by bacteria, fungi and plants. J. Nanomed. Nanotechnol. 2014;5:1. [Google Scholar]
- 71.Balakumaran M., Ramachandran R., Balashanmugam P., Mukeshkumar D., Kalaichelvan P. Mycosynthesis of silver and gold nanoparticles: optimization, characterization and antimicrobial activity against human pathogens. Microbiol. Res. 2016;182:8–20. doi: 10.1016/j.micres.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 72.Elamawi R.M., Al-Harbi R.E., Hendi A.A. Biosynthesis and characterization of silver nanoparticles using Trichoderma longibrachiatum and their effect on phytopathogenic fungi. Egyptian journal of biological pest control. 2018;28:1–11. [Google Scholar]
- 73.Bertolucci E., Galletti A.M.R., Antonetti C., Marracci M., Tellini B., Piccinelli F., Visone C. 2015 IEEE International Instrumentation and Measurement Technology Conference (I2MTC) Proceedings. IEEE; 2015. Chemical and magnetic properties characterization of magnetic nanoparticles; pp. 1492–1496. [Google Scholar]
- 74.Yew Y.P., Shameli K., Miyake M., Kuwano N., Bt Ahmad Khairudin N.B., Bt Mohamad S.E., Lee K.X. Green synthesis of magnetite (Fe 3 O 4) nanoparticles using seaweed (Kappaphycus alvarezii) extract. Nanoscale Res. Lett. 2016;11:1–7. doi: 10.1186/s11671-016-1498-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ebrahiminezhad A., Zare-Hoseinabadi A., Berenjian A., Ghasemi Y. Green synthesis and characterization of zero-valent iron nanoparticles using stinging nettle (Urtica dioica) leaf extract. Green Process. Synth. 2017;6:469–475. [Google Scholar]
- 76.Ebrahiminezhad A., Najafipour S., Kouhpayeh A., Berenjian A., Rasoul-Amini S., Ghasemi Y. Facile fabrication of uniform hollow silica microspheres using a novel biological template. Colloids Surf. B Biointerfaces. 2014;118:249–253. doi: 10.1016/j.colsurfb.2014.03.052. [DOI] [PubMed] [Google Scholar]
- 77.Gadgeel A., Mhaske S., Duerr C., Liu K. In-situ preparation and characterization of aconitic acid capped Fe 3 O 4 nanoparticle by using citric acid as a reducing agent. J. Inorg. Organomet. Polym. Mater. 2019;29:1688–1700. [Google Scholar]
- 78.Karami B., Hoseini S.J., Eskandari K., Ghasemi A., Nasrabadi H. Synthesis of xanthene derivatives by employing Fe3O4 nanoparticles as an effective and magnetically recoverable catalyst in water. Catal. Sci. Technol. 2012;2:331–338. [Google Scholar]
- 79.Akbarzadeh P., Koukabi N., Kolvari E. Three-component solvent-free synthesis of 5-substituted-1 H-tetrazoles catalyzed by unmodified nanomagnetite with microwave irradiation or conventional heating. Res. Chem. Intermed. 2019;45:1009–1024. [Google Scholar]
- 80.Waldron R. Infrared spectra of ferrites. Physical review. 1955;99:1727. [Google Scholar]
- 81.Lu W., Shen Y., Xie A., Zhang W. Green synthesis and characterization of superparamagnetic Fe3O4 nanoparticles. J. Magn. Magn Mater. 2010;322:1828–1833. [Google Scholar]
- 82.Bordbar A., Rastegari A., Amiri R., Ranjbakhsh E., Abbasi M., Khosropour A. Characterization of modified magnetite nanoparticles for albumin immobilization. Biotechnology research international. 2014;2014 doi: 10.1155/2014/705068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sagar V., Atluri V.S.R., Tomitaka A., Shah P., Nagasetti A., Pilakka-Kanthikeel S., El-Hage N., McGoron A., Takemura Y., Nair M. Coupling of transient near infrared photonic with magnetic nanoparticle for potential dissipation-free biomedical application in brain. Sci. Rep. 2016;6 doi: 10.1038/srep29792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nguyen M.D., Tran H.-V., Xu S., Lee T.R. Fe3O4 Nanoparticles: structures, synthesis, magnetic properties, surface functionalization, and emerging applications. Appl. Sci. 2021;11 doi: 10.3390/app112311301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sureshkumar V., Kiruba Daniel S., Ruckmani K., Sivakumar M. Fabrication of chitosan–magnetite nanocomposite strip for chromium removal. Appl. Nanosci. 2016;6:277–285. [Google Scholar]
- 86.Piro N.S., Hamad S.M., Mohammed A.S., Barzinjy A.A. Green synthesis magnetite (Fe₃O₄) nanoparticles from Rhus coriaria extract: a characteristic comparison with a conventional chemical method. IEEE Trans. NanoBioscience. 2022;22:308–317. doi: 10.1109/TNB.2022.3187344. [DOI] [PubMed] [Google Scholar]
- 87.Ebnesajjad S. Handbook of Adhesives and Surface Preparation. Elsevier; 2011. Surface and material characterization techniques. [Google Scholar]
- 88.Inan T. Recent Developments in Polymer Macro, Micro and Nano Blends. Elsevier. 2017. Thermoplastic-based nanoblends: preparation and characterizations. [Google Scholar]
- 89.Parameshwaran R., Sarı A., Jalaiah N., Karunakaran R. Handbook of Thermal Analysis and Calorimetry. Elsevier; 2018. Applications of thermal analysis to the study of phase-change materials. [Google Scholar]
- 90.Sarfraz A., Raza A.H., Mirzaeian M., Abbas Q., Raza R. Reference Module in Materials Science and Materials Engineering. Elsevier BV; 2020. Electrode materials for fuel cells. [Google Scholar]
- 91.Awwad A.M., Salem N.M. A green and facile approach for synthesis of magnetite nanoparticles. Nanosci. Nanotechnol. 2012;2:208–213. [Google Scholar]
- 92.Ozmen M., Can K., Arslan G., Tor A., Cengeloglu Y., Ersoz M. Adsorption of Cu (II) from aqueous solution by using modified Fe3O4 magnetic nanoparticles. Desalination. 2010;254:162–169. [Google Scholar]
- 93.Mahdavian A.R., Mirrahimi M.A.-S. Efficient separation of heavy metal cations by anchoring polyacrylic acid on superparamagnetic magnetite nanoparticles through surface modification. Chem. Eng. J. 2010;159:264–271. [Google Scholar]
- 94.Kumar S.S., Venkateswarlu P., Rao V.R., Rao G.N. Synthesis, characterization and optical properties of zinc oxide nanoparticles. Int. Nano Lett. 2013;3:1–6. [Google Scholar]
- 95.Maity D., Kale S., Kaul-Ghanekar R., Xue J.-M., Ding J. Studies of magnetite nanoparticles synthesized by thermal decomposition of iron (III) acetylacetonate in tri (ethylene glycol) J. Magn. Magn Mater. 2009;321:3093–3098. [Google Scholar]
- 96.Clogston J.D., Patri A.K. Zeta potential measurement. Characterization of nanoparticles intended for drug delivery. 2011:63–70. doi: 10.1007/978-1-60327-198-1_6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Available in the manuscript.