Abstract
Depression is associated with heart failure independent of traditional cardiovascular disease risk factors. Enhanced platelet activation has been suggested as a potential mechanism and has been associated with negative inotropic effects that can affect left ventricular ejection fraction (LVEF). We examined 131 consecutive acute coronary syndrome (ACS) patients to assess whether depression increased the risk for developing LV dysfunction, and to determine the effects of platelet serotonin signaling in this relationship. Major depression was assessed using the Structured Clinical Interview and depressive symptoms were measured using the Beck Depression Inventory (BDI), with BDI ≥ 10 defined as abnormal. LV dysfunction was defined as LVEF ≤ 45%. Platelet serotonin response was measured by serotonin augmented platelet aggregation and platelet serotonin receptor density. Mean age of ACS participants was 59 years, 78.6% male and 74.0% Caucasian. 34.4% of patients had a reduced LVEF ≤ 45% on presentation. Almost half (47.0%) of patients had BDI ≥ 10 and 18.0% had major depressive disorder. Platelet serotonin response was found to be augmented in depressed patients with low LVEF compared to depressed patients with normal LVEF (p < 0.020). However, the presence of LV dysfunction was found to be similar in both depressed (32.3%) and non-depressed (36.2%) patients (p = 0.714). This suggests alternative factors contribute to poor cardiovascular outcomes in depressed patients that are independent of LV function in post ACS patients.
Keywords: Acute coronary syndrome, Left ventricular dysfunction, Ejection fraction, Depression, Platelets, Serotonin
Introduction
Major depressive disorder (MDD) is highly prevalent in patients with acute coronary syndrome (ACS) and associated with increased risk of morbidity and mortality [1–5]. Recent studies have estimated MDD at 15–20% at the time of index ACS event, and up to 50% in the first 3 months post-event. In fact, even after controlling for age and traditional cardiovascular risk factors such as hypertension and diabetes, ACS patients who are depressed have a three to four-fold increase in mortality if they are diagnosed with depression at the time of index hospitalization [6–9].
Platelets are a major transporter of serotonin in the body and studies have shown increased platelet activation in depressed patients [10–13]. Zafar et al. showed increased platelet activation, a marker of increased thrombosis, specifically in post ACS patients with depression and anxiety [14]. Other studies have demonstrated that increased platelet function and other indices of platelet reactivity such as mean platelet volume, platelet distribution width are independently associated with decreased left ventricular (LV) contractility in ACS patients and has been proposed as a potential mechanism for the increased risk of death in individuals with heart disease and comorbid depression [13, 15–18].
Patients with greater reductions in LV dysfunction are well known to have increased risk for heart failure, hospitalizations, early all-cause mortality, and sudden cardiac death [19–21]. While well established as an independent risk factor for cardiovascular disease, the relationship between MDD and LV dysfunction is still not well understood. In the present study, we sought to establish whether the presence of clinical depression or depressive symptoms as an independent risk factor, increased the risk for patients to develop a subsequent reduction in their left ventricular ejection fraction (LVEF) after hospitalization for ACS. We also sought to understand the influence of the platelet serotonin-signaling pathway on this relationship.
Methods
Study participants
All consecutive patients with an ACS admitted to the inpatient cardiology service at a single urban academic medical center between February 2011 and May 2015, and who met inclusion criteria, were approached for participation in the Depression And Platelet Serotonin Study (DAPSS). Participants were enrolled within the first 72 h of hospitalization. ACS was defined as unstable angina or acute MI in accordance with World Health Organization definition [22]. We included consenting adults age ≥ 21 years with known stable coronary artery disease (CAD) or documented ACS due to thrombotic occlusion, and current aspirin use. Exclusion criteria included: age < 21 years, current use of antidepressants, current or previous (14 days) use of anti-platelet medications (glycoprotein IIb/IIIa inhibitor), active narcotic use by personal report or laboratory testing, baseline platelet count < 100,000/μl, and life expectancy of greater than 1 year. 145 patients met criteria for ACS at initial presentation, however for the purposes of our study which focuses on the effects of platelet function on MDD and LV dysfunction, we intentionally excluded 6 patients with ACS due to vasospastic coronary disease. Our final cohort consisted of 139 patients selected for this study. The study was approved by our Institutional Review Board and all patients provided informed consent.
Ventricular function assessment
Echocardiographic examinations were performed at a single clinical site using Phillips ie33 ultrasound machine (Phillips Healthcare, Andover, MA) with subjects in the left lateral decubitus position at the time of index ACS hospitalization. LVEF was calculated according to the modified Simpson’s rule using the apical four- and two-chamber views, with abnormal defined as EF ≤ 45% in accordance with the American College of Cardiology/American Heart Association guidelines [23, 24] for low and moderately reduced EF. As LVEF is frequently reported as a 5% range by convention, we used the lower range value for the purposes of our study. Patients with EF that was not documented during the initial encounter were not included in our analysis.
Depression assessment
The presence or absence of MDD was determined using the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID-IV-TR) [25] at initial assessment by a licensed clinical psychologist. The interviewer was blinded to all clinical factors including the results of all platelet measurements and LVEF. Depression severity was determined using the 21-item Beck Depression Inventory-II (BDI-II) [26]. The SCID-IV-TR interview assesses symptoms for a 1-month period prior to the assessment. The presence or absence of depression is categorical. In contrast, the BDI-II assesses depressive symptoms during the previous 2 weeks. Total BDI-II scores range from 0 to 63, and indicate different levels of depressive symptomatology, including: no (0), minimal (< 10), mild-to-moderate (10–18), moderate-to-severe (19–29), and severe (≥ 30) symptoms of depression [26]. Therefore, a score lower than 10 indicates a low probability of clinically significant depression and a total score of 10 or higher indicates at least mild-to-moderate symptoms of depression.
Platelet aggregation
Platelet aggregation studies were conducted in platelet rich plasma (PRP) and assays performed within 2–4 h of blood draw. Serotonin (5-HT)-hydrochloride (at various concentrations) augmented with epinephrine (1 μM) was added to PRP. Platelet aggregation was assessed using standard light transmission in PRP with a CHRONO-LOG® Aggregometer (Havertown, PA). Platelet aggregation results are expressed as area under the curve (AUC). The full methodology has previously been described by Williams et al. [15].
Serotonin receptor density collection and quantification
Similarly, serotonin receptor density collection and quantification methodology has been previously reported [15, 27].
Statistical analysis
We divided the sample by their BDI-II score as minimal depressive symptoms (BDI < 10) and depressive symptoms (BDI ≥ 10) and SCID-IV as absence versus presence of MDD. We tabulated demographic and cardiovascular risk factor characteristics, overall and stratified either by depression status of patients or presence of left ventricular dysfunction. We presented means and standard deviations of continuous variables (unless specified otherwise) and percentages for categorical variables. Differences between groups were examined using t-tests for continuous variables and Chi square tests for categorical variables. Statistical significance is defined as p value < 0.05. The data was analyzed using Stata (Version 15.1, StataCorp, College Station, TX).
Results
Study population
Of the 300 participants recruited, 139 patients met criteria for ACS and 131 had a documented LVEF by 2D echocardiography at initial presentation. The mean age of participants was 59 ± 12 years, with a majority being males (78.6%) and Caucasian (74.0%), as shown in Table 1. 45 patients (34.4%) had a recorded EF ≤ 45%. 62 patients (47.1%) had at least mild depressive symptoms (BDI ≥ 10) while 18.0% had MDD by SCID-IV.
Table 1.
Baseline demographic and clinic characteristics of study participants
| Left ventricular ejection fraction | Beck-depression inventory | Structured clinical interview | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| EF ≤ 45 (N = 45) | EF > 45 (N = 86) | p value | BDI < 10 (N = 69) | BDI ≥ 10 (N = 62) | p value | No MDD (N = 100) | MDD (N = 22) | p value | |
|
| |||||||||
| Age, mean, years (SD) | 61 (10) | 59 (13) | 0.410 | 61 (13) | 58 (11) | 0.110 | 60 (12) | 57 (13) | 0.480 |
| BMI, mean (SD) | 31.4 (6.1) | 30.9 (7.4) | 0.820 | 29.5 (6.2) | 32.3 (7.8) | 0.025 | 30.5 (6.4) | 31.2(8.0) | 0.650 |
| Gender, n (%) | |||||||||
| Female | 8 (17.7) | 19 (22.1) | 0.540 | 14 (20.3) | 13 (21.0) | 0.890 | 22 (22.0) | 4 (18.2) | 1.000 |
| Race, n (%) | |||||||||
| African American | 8 (17.8) | 18 (20.9) | 0.220 | 12 (17.4) | 14 (22.6) | 0.590 | 20 (20.0) | 5 (22.7) | 0.960 |
| White | 37 (82.2) | 60 (70.0) | 54 (78.3) | 43 (69.4) | 75 (75.0) | 16 (72.7) | |||
| Asian | 0 (0.0) | 1 (1.0) | 0 (0.0) | 1 (1.6) | 0 | 0 | |||
| Other | 0 (0.0) | 7 (8.1) | 3 (4.3) | 3 (4.8) | 5 (5.0) | 1 (4.5) | |||
| Ethnicity, n (%) | |||||||||
| Hispanic | 0 (0.0) | 4 (4.7) | 0.140 | 2 (2.9) | 2 (3.2) | 0.900 | 2 (2.0%) | 2 (9.1%) | 0.091 |
| Non-Hispanic | 45 (100.0) | 82 (95.3) | 67 (97.1) | 60 (96.8) | 98 (98.0%) | 20 (90.9%) | |||
| Education, n (%) | |||||||||
| Advance education | 0 (0.0) | 7 (8.1) | 0.035 | 3 (4.3) | 4 (6.5) | 0.560 | 4 (4%) | 1 (5%) | 0.760 |
| College graduate | 10 (22.2) | 12 (14.0) | 14 (20.3) | 8 (12.9) | 18 (18%) | 4 (18%) | |||
| College non-graduate | 7 (15.6) | 23 (26.7) | 15 (21.7) | 14 (22.6) | 24 (24%) | 3 (14%) | |||
| High school graduate | 12 (26.6) | 28 (32.6) | 17 (24.6) | 23 (37.0) | 28 (29%) | 9 (41%) | |||
| Lower level | 16 (35.6) | 16 (18.6) | 18 (26.1) | 13 (21.0) | 24 (24%) | 5 (23%) | |||
| Marital status, n (%) | |||||||||
| Married | 23 (51.1) | 41 (47.7) | 0.380 | 37 (53.6) | 29 (46.8) | 0.550 | 53 (54%) | 9 (41%) | 0.140 |
| Single | 11 (24.4) | 29 (33.7) | 22 (31.9) | 18 (29.0) | 33 (33%) | 6 (27%) | |||
| Widowed | 7 (15.6) | 6 (7.0) | 4 (5.8) | 7 (11.3) | 5 (5%) | 4 (18%) | |||
| Divorced | 4 (8.9) | 10 (11.6) | 6 (8.7) | 8 (12.9) | 8 (8%) | 3 (14%) | |||
| Comorbidities, n (%) | |||||||||
| History MI | 24 (53.3) | 24 (27.9) | 0.003 | 20 (29.0) | 28 (45.2) | 0.053 | 36 (36%) | 9 (41%) | 0.690 |
| History ACS | 23 (51.1) | 25 (29.1) | 0.017 | 23 (33.3) | 26 (42.0) | 0.330 | 37 (37%) | 7 (32%) | 0.620 |
| History CAD | 24 (53.3) | 35 (40.7) | 0.190 | 29 (42.0) | 31 (50.0) | 0.410 | 48 (48.0%) | 8 (36.4%) | 0.320 |
| History depression | 7 (15.5) | 12 (14.0) | 0.820 | 9 (13.1) | 10 (16.1) | 0.048 | 14 (14%) | 5 (23%) | <0.001 |
| Hypertension | 36 (80.0) | 60 (70.0) | 0.250 | 46 (66.7) | 51 (82.3) | 0.048 | 73 (73.0%) | 17 (77.3%) | 0.680 |
| Hyperlipidemia | 33 (73.3) | 60 (70.0) | 0.740 | 46 (66.7) | 48 (77.4) | 0.190 | 71 (71.0%) | 16 (72.7%) | 0.870 |
| Current smoking | 15 (33.3) | 34 (39.5) | 0.460 | 22 (31.9) | 27 (43.5) | 0.150 | 36 (36.0%) | 8 (36.4%) | 0.970 |
| Diabetes | 19 (42.2) | 24 (28.0) | 0.110 | 15 (21.7) | 28 (45.2) | 0.003 | 31 (31.0%) | 9 (40.9%) | 0.370 |
| CABG | 8 (17.8) | 7 (8.1) | 0.110 | 9 (13.0) | 6 (9.7) | 0.570 | 12 (12.0%) | 2 (9.1%) | 0.700 |
| Medications, n (%) | |||||||||
| Aspirin | 44 (97.8) | 82 (95.3) | 0.300 | 68 (98.6) | 61 (98.4) | 0.930 | 97 (99%) | 21 (95%) | 0.240 |
| Plavix | 39 (86.7) | 77 (89.5) | 0.580 | 63 (91.3) | 56 (90.3) | 0.820 | 89 (91%) | 19 (86%) | 0.530 |
| Coumadin | 4 (8.9) | 1 (1.2) | 0.030 | 5 (7.2) | 0 (0.0) | 0.031 | 4 (4%) | 1 (5%) | 0.930 |
| Heparin | 29 (64.4) | 61 (70.9) | 0.480 | 52 (75.4) | 43 (69.4) | 0.460 | 68 (71%) | 14 (64%) | 0.510 |
| ACE-I | 25 (55.6) | 34 (39.5) | 0.078 | 30 (43.5) | 29 (46.7) | 0.630 | 49 (50%) | 7 (32%) | 0.120 |
| α-Blockers | 0 (0.0) | 1 (1.1) | 0.470 | 1 (1.4) | 0 (0.0) | 0.350 | 1 (1%) | 0 (0%) | 0.630 |
| ARB | 2 (4.4) | 7 (8.1) | 0.430 | 5 (7.2) | 4 (6.5) | 0.880 | 7 (7%) | 1 (5%) | 0.660 |
| β-Blockers | 39 (86.7) | 69 (80.2) | 0.340 | 59 (85.5) | 52 (83.9) | 0.760 | 85 (87%) | 16 (73%) | 0.100 |
| CCB | 4 (8.9) | 20 (23.3) | 0.043 | 13 (18.8) | 11 (17.7) | 0.910 | 19 (19%) | 3 (14%) | 0.530 |
| Diuretics | 14 (31.1) | 12 (14.0) | 0.019 | 15 (21.7) | 11 (17.7) | 0.600 | 21 (21%) | 3 (14%) | 0.410 |
| Other anti-HTN | 3 (6.7) | 6 (7.0) | 0.950 | 2 (2.9) | 7 (11.3) | 0.054 | 6 (6%) | 2 (9%) | 0.610 |
| Antianginal | 20 (44.4) | 42 (48.9) | 0.320 | 33 (47.8) | 29 (46.8) | 0.980 | 45 (46%) | 10 (45%) | 0.970 |
| Antiarrhythmic | 2 (4.4) | 0 (0.0) | 0.049 | 2 (2.9) | 0 (0.0) | 0.180 | 2 (2%) | 0 (0%) | 0.500 |
| Antilipemic | 40 (88.9) | 79 (91.6) | 0.510 | 65 (94.2) | 57 (91.9) | 0.590 | 91 (93%) | 20 (91%) | 0.750 |
| Anti-glycemic | 16 (35.6) | 17 (19.8) | 0.048 | 12 (17.4) | 21 (33.9) | 0.025 | 25 (26.0) | 6 (27.0) | |
| Antidepressant | 1 (2.2) | 0 (1.0) | 0.170 | 0 (0.0) | 1 (1.6) | 0.290 | 1 (1.0) | 0 (0.0) | 0.630 |
| Laboratory values, n (%) | |||||||||
| Troponin ng/dl, mean (SD) | 15.3 (31.0) | 5.1 (9.4) | 0.007 | 12.2 (26.3) | 4.8 (8.9) | 0.043 | 8.0 (11.7) | 12.2 (41.4) | 0.400 |
SD standard deviation, CAD coronary artery disease, ACS acute coronary syndrome, CABG coronary artery bypass surgery, BDI-II Beck Depression Inventory II, SCID structured clinical interview, BMI body mass index, EF ejection fraction, MDD major depressive disorder, ACEI angiotensin converting enzyme inhibitor, ARB angiotensin receptor blocker, CCB calcium channel blocker, anti-HTN antihypertensive
Demographic and clinical characteristics of patients with reduced ejection fraction
Demographic and clinical characteristics of patients with reduced LVEF are summarized in Table 1. The mean age of presentation of patients with reduced LVEF was 61 ± 10 years compared to 59 ± 13 years in patients with preserved LVEF, p < 0.410. Patients with reduced LVEF were less educated (P < 0.035), had a prior history of MI (p < 0.003) or ACS (p < 0.017), were more likely to be on diuretics (p < 0.019), Coumadin (p < 0.030), calcium channel blockers (p < 0.043), anti-glycemic drugs (p < 0.048), and had a significantly higher troponin at presentation (mean ± SD; 15.3 ± 31 vs 5.1 ± 9.4 ng/ml, p < 0.007). There were no significant differences in gender, race, marital status, smoking habits or BMI.
Demographic and clinical characteristics of patients with depression
The mean BDI-II score for participants was 10.0 ± 8.2. The depressed group (BDI ≥ 10) had a higher BMI (32.3 vs 29.5 units, p < 0.025) and were more likely to have a history of prior MI (46.0% vs 29%, p < 0.043), hypertension (82% vs 67%, p < 0.048) and diabetes (46% vs 22%, p < 0.003). There were no age, gender, racial or education level differences noted between groups.
Platelet aggregation, ejection fraction and depression
Amongst patients with depression (BDI ≥ 10), platelet response to 0.3 μM augmented serotonin showed significantly more platelet aggregation (measured as area under the curve) in patients with LVEF ≤ 45% compared to patients with LVEF > 45% [median (Interquartile range): 246 (137–312) vs 158 (105–221), n = 62, p < 0.02] as seen in Fig. 1. Amongst patients without depressive symptoms (BDI < 10), no such difference was noted, LVEF ≤ 45% compared to patients with LVEF > 45% [median (Interquartile range): 188 (89–202) vs 173 (113–226), n = 62, p = NS].
Fig. 1.
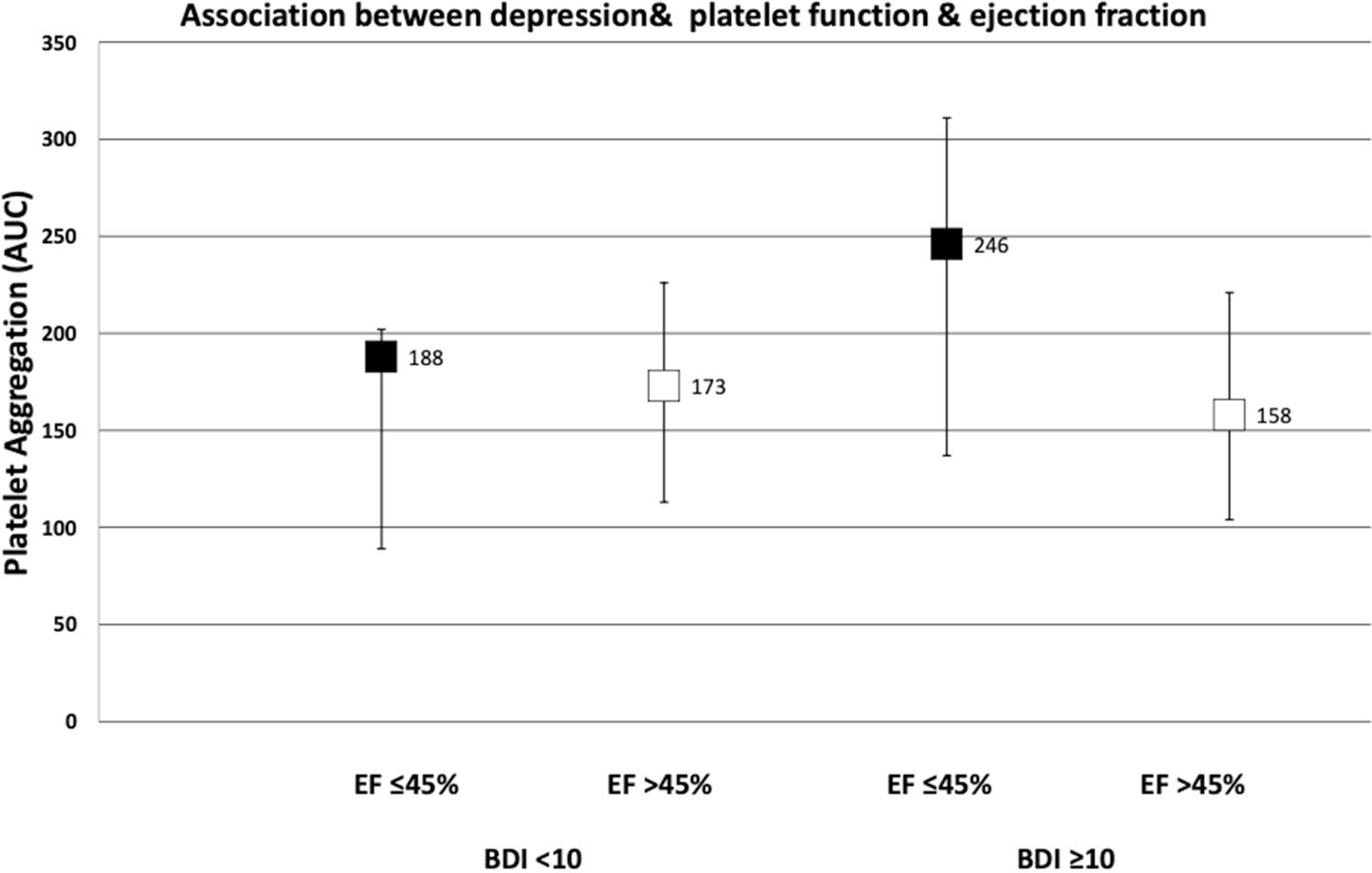
Association between depression, platelet function and ejection fraction. Results expressed as Area Under the Curve (AUC) with median and IQR. BDI Beck Depression Inventory, EF ejection fraction, 5HT serotonin, IQR interquartile range
Platelet serotonin receptor density, ejection fraction and depression category
In patients with depressive symptoms (BDI ≥ 10), platelet serotonin density was not significantly different in patients with low LVEF ≤ 45% [median (interquartile ranges): 741 fmol/μg (364 to 1091)] compared to those with moderate to preserved ventricular function [486 fmol/μg (233 to 862)] rank sum p = 0.44]. The relationship between receptor density and ejection fraction using continuous LVEF values was also assessed with similar results above.
Depression and LV ejection fraction
Our study found that the percentage of patients with EF ≤ 45% in depressed (BDI ≥ 10) and MDD groups compared to nondepressed groups were not statistically different [BDI ≥ 10: 32.3%), BDI < 10: 36.2%; p = 0.717) and MDD: 22.7% vs non-MDD 38.0%; p = 0.222], as shown in Table 2.
Table 2.
Incidence of left ventricular depression in patients with and without depression
| Depressed LVEF ≤ 45 | |
|---|---|
|
| |
| BDI < 10 n (%) | 25 (36.2%) |
| BDI ≥ 10 n (%) | 20 (32.3%) |
| p | 0.714 |
| MDD-no n (%) | 38 (38.0%) |
| MDD-yes n (%) | 5 (22.7%) |
| p | 0.222 |
BDI Beck Depression Inventory, MDD major depressive disorder, LVEF left ventricular ejection fraction
Discussion
Depression is associated with an increase in platelet reactivity in ACS patients, and serotonin, a platelet activator, has been hypothesized to play a role in this relationship [11, 12]. Despite this known association between MDD and ACS, few studies have investigated whether LV function is similarly affected by depression and the platelet serotonin pathway in post ACS patients.
In this prospective subgroup analysis of the DAPSS database, we assessed LV function in ACS patients with and without depression and also assessed their relationship with the platelet serotonin pathway. There were several notable findings. The prevalence of clinically significant depressive symptoms was 47.1% while that for major depressive disorder in our cohort was 18.2%, similar to what has been reported in prior studies (BDI > 10 ~ 30 to 50%; MDD ~ 15 to 20%) [6–9]. Secondly, amongst patients with depressive symptoms, platelet aggregation to serotonin augmented with epinephrine was significantly increased in patients with reduced LVEF compared to patients with normal ventricular function. Finally, despite the increase in platelet aggregation, we found no association between the presence of MDD or depressive symptoms with the presentation of LV dysfunction in ACS patients at time of index hospitalization.
Platelets are the major transporter of serum serotonin in the body [15, 28]. Serotonin is taken up into the platelet via the serotonin transporter and then stored in dense granules with calcium and adenosine triphosphate [29]. Once released from the platelet, serotonin induces a number of biological processes by interacting with various membrane receptors. One such receptor, the serotonin 2A receptor leads to further platelet activation and vasoconstriction, which has been shown to have several endovascular and cardiovascular effects. Increased serotonin mediated platelet reactivity has been postulated to be a major mechanism linking depression to ACS [30]. We previously have demonstrated that depressed cardiovascular patients had higher serotonin receptor density, which correlated with significantly higher incidence of major and minor cardiac adverse events [15]. Several studies have similarly reported increased serotonin receptor density, activation and activity in patients with MDD with and without cardiovascular disease, as well as poor outcomes in post-acute myocardial infarction [31–34].
Given that increased platelet activity in depressed patients with ACS has been previously demonstrated, we wanted to investigate whether this increased activity contributed to decreased left ventricular ejection fraction [14, 15]. Our results showed a significant increase in platelet aggregation in depressed patients with LVEF ≤ 45%. We did not find any studies that have specifically looked at this relationship previously. Berger et al. used a similar methodology to identify a population of healthy young volunteers without CAD who demonstrated exaggerated platelet aggregation and hyperreactivity to epinephrine augmented serotonin, and theorized this population may be at increased risk for atherosclerotic disease [35]. Our study demonstrated increased platelet reactivity amongst post ACS depressed patients with LV dysfunction, a finding not previously reported and identifies a high-risk population that can be targeted for medical interventions. This finding could explain why previous studies have reported poor outcomes and increased incidence of adverse cardiac events in post ACS patients with depression, as LV dysfunction is well known as an independent risk factor for increased morbidity and mortality within this patient population [15, 20, 21].
Despite the increased platelet reactivity in patients with impaired LVEF, our results did not find any statistically significant differences when we compared patients with depression and LVEF ≤ 45% at time of event. Other investigators have reported similar findings, although not always in the context of post-ACS at index hospitalization. Lett et al. studied 1020 stable CAD patients and found no evidence that MDD was associated with systolic dysfunction, diastolic dysfunction, inducible ischemia or LV regional wall abnormalities. They concluded that baseline cardiac disease severity was unlikely to be responsible for the increase risk of CAD in patients with depression [36]. Similarly, Spijkjer-man et al., Lesperance et al., and Strik et al. in their analysis of post MI patients with depressive symptoms also found no association between depression and low LVEF [37–39]. There are several potential reasons why there is limited association between depressive symptoms and LV dysfunction. Bush et al. showed that the presence of only minimal symptoms of depression (BDI 4–9) increased mortality risk after myocardial infarction [40] suggesting mood changes required to cause clinically significant outcomes may not be significant enough to elicit structural changes in the myocardium during the index event. The long-term effects of MDD on the myocardium in these patients however is unknown. In another study by Kim et al., early changes in LV diastolic function parameters such as transmittal A wave velocity, E/A ratio, TDI early diastolic velocity (Ea) were progressively altered across levels of depression in healthy adults without known cardiovascular disease (p < 0.001) [41]. Diastolic abnormalities were even more pronounced in patients with moderate or severe MDD. These findings of diastolic dysfunction in healthy patients with depression suggest abnormalities in LV relaxation that may signify myocardial involvement that precedes the development of cardiovascular disease [41]. Another potential hypothesis for why MDD does not have a direct association with LV dysfunction is that while MDD does not lead to a direct suppression of LV contractility, there is an increased risk in subsequent cardiovascular outcomes via other mechanisms that are still unknown. Several proposed mechanisms include high plasma levels of inflammatory and interleukin 6, tumor necrosis factor, C-reactive protein, d-dimers, fibrinogen, beta thromboglobulin, endothelial dysfunction, low heart rate variability and lifestyle factors such as diet, alcohol use, tobacco use [42–45]. Finally, it is possible a relationship between depression and LVEF exists but our relatively small sample size was not powered enough to demonstrate this relationship.
An important limitation to our study is that there were only 131 patients that met criteria for inclusion for the study, with 28 females and 34 non-Caucasians. The small sample size greatly limits the power for elucidating statistical significance and the unequal representation limits the generalizability of the results to women and non-white populations. Another limitation included the inability to account for patients who presented with MI with previously reduced ejection fractions prior to the ACS event as most patients had no prior echocardiography data documented. Therefore, a major assumption during our data analysis was that all reduction in myocardial infarction was developed from the infarct at index hospitalization. One of the strengths of our study is that all clinical data was collected when patients were actively hospitalized, an aspect that has not been widely examined in this clinical population, as many studies are performed several months after the index event. This strongly suggests that most of the clinical symptoms reported are a reflection of prehospitalization states and avoids potential disease related causes of depression, such as psychological and social consequences, feelings of loss, denial or defeat that can exacerbate depressive symptoms.
Conclusion
There is still considerable debate whether the association of depression with adverse cardiovascular outcomes is confounded by worse baseline cardiac disease severity in depressed patients. Although the presence of depression is known to increase mortality and increase number of adverse events in post myocardial infarction patients, that relationship may be independent of any direct myocardial effects, specifically LV dysfunction. The lack of association between major depression or depressive symptoms and depressed left ventricular function may suggest that the greater underlying cardiac disease severity may be explained by alternative mechanisms leading to increased risk of cardiovascular disease events associated with depression. Finally, the presence of increased platelet reactivity in depressed patients with low LVEF also suggests that there are other factors contributing to poor cardiovascular outcomes in depressed patients that are independent of LV function or cardiac disease severity. Future studies will continue to aim at elucidating the mechanisms of cardiac dysfunction in depressed patients that will enable us to better understand and develop improved medical therapies.
Highlights.
Depression is an independent risk factor for cardiovascular disease
LV dysfunction increases mortality and morbidity in post ACS patients
Platelet-serotonin pathway plays an important role in pathogenesis of depression and ACS
Platelet serotonin response is augmented in depressed patients with low LVEF
LV dysfunction is similar in both depressed and nondepressed patients at index hospitalization for ACS.
Funding
MW was funded by Grant RO1 HL096694 from the United States National Heart, Lung, and Blood Institute (Bethesda, MD). D.V. was supported by the Johns Hopkins Biostatistics, Epidemiology and Data Management Core.
Abbreviations
- ACS
Acute coronary syndrome
- LVEF
Left ventricular ejection fraction
- MDD
Major depressive disorder
- BDI
Beck Depression Inventory
- SCID
Structured clinical inventory for depression
- IQR
Interquartile range
- CAD
Coronary artery disease
- CVD
Cardiovascular disease
Footnotes
Conflict of interest MW serves on the speaker’s bureau of Maryland State Medical Society and Rockpointe Corporation that is supported by an unrestricted educational grant from AstraZeneca. The other authors report no conflicts of interest.
Consent to participate Informed consent was obtained from all individual participants included in the study.
Ethics approval This study was approved by the Johns Hopkins institutional review board
Availability of data and material
Raw data available on open science framework https://osf.io/hyj69/?view_only=35a1d31edd8740309b24ae5d96502e7e.
References
- 1.Carney RM, Blumenthal JA, Catellier D et al. (2003) Depression as a risk factor for mortality after acute myocardial infarction. Am J Cardiol 92(11):1277–1281 [DOI] [PubMed] [Google Scholar]
- 2.Carney RM, Rich MW, Freedland KE et al. (1988) Major depressive disorder predicts cardiac events in patients with coronary artery disease. Psychosom Med 50:627–733 [DOI] [PubMed] [Google Scholar]
- 3.Frasure-Smith N, Lespérance F, Juneau M, Talajic M, Bourassa MG (1999) Gender, depression, and one-year prognosis after myocardial infarction. Psychosom Med 61(1):26–37 [DOI] [PubMed] [Google Scholar]
- 4.Musselman DL, Evans DL, Nemeroff CB (2003) The relationship of depression to cardiovascular disease. Arch Gen Psychiatry 55:580–592 [DOI] [PubMed] [Google Scholar]
- 5.Frasure-Smith N, Lespérance F, Talajic M (1995) Depression and 18-month prognosis after myocardial infarction. Circulation 91:99–1005 [DOI] [PubMed] [Google Scholar]
- 6.Ziegelstein RC (2001) Depression after myocardial infarction. Cardiol Rev 9(1):45–51 [DOI] [PubMed] [Google Scholar]
- 7.Carney RM, Freedland KE (2008) Depression in patients with coronary heart disease. Am J Med 121(11):S20–S27 [DOI] [PubMed] [Google Scholar]
- 8.Thombs BD, De Jonge P, Coyne JC et al. (2008) Depression screening and patient outcomes in cardiovascular care: a systematic review. JAMA 300:2161–2171 [DOI] [PubMed] [Google Scholar]
- 9.Lespérance F, Frasure-Smith N, Juneau M, Théroux P (2000) Depression and 1-year prognosis in unstable angina. Arch Intern Med 160:1354–1360 [DOI] [PubMed] [Google Scholar]
- 10.Shimbo D, Child J, Davidson K et al. (2002) Exaggerated serotonin-mediated platelet reactivity as a possible link in depression and acute coronary syndromes. Am J Cardiol 89(3):331–333 [DOI] [PubMed] [Google Scholar]
- 11.Morel-Kopp M, Mclean L, Chen Q et al. (2009) The association of depression with platelet activation: evidence for a treatment effect. J Thromb Haemost 7(4):573–581 [DOI] [PubMed] [Google Scholar]
- 12.Musselman DL, Tomer A, Manatunga AK et al. (1996) Exaggerated platelet reactivity in major depression. Am J Psychiatry 153(10):1313–1317 [DOI] [PubMed] [Google Scholar]
- 13.Ziegelstein RC, Parakh K, Sakhuja A, Bhat U (2009) Platelet function in patients with major depression. Intern Med J 39(1):38–43 [DOI] [PubMed] [Google Scholar]
- 14.Zafar MU, Paz-Yepes M, Shimbo D et al. (2010) Anxiety is a better predictor of platelet reactivity in coronary artery disease patients than depression. Eur Heart J 31:1573–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams MS, Ziegelstein RC, McCann UD, Gould NF, Ashvetiya T, Vaidya D (2019) Platelet serotonin signaling in patients with cardiovascular disease and comorbid depression. Psychosom Med 81:352–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujita S, Takeda Y, Kizawa S et al. (2015) Platelet volume indices are associated with systolic and diastolic cardiac dysfunction, and left ventricular hypertrophy. BMC Cardiovasc Disord 15:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yazici HU, Poyraz F, Sen N et al. (2011) Relationship between mean platelet volume and left ventricular systolic function in patients with metabolic syndrome and ST-elevation myocardial infarction. Clin Investig Med 34:E330. [DOI] [PubMed] [Google Scholar]
- 18.Jiang W, Boyle SH, Ortel TL et al. (2015) Platelet aggregation and mental stress induced myocardial ischemia: results from the Responses of Myocardial Ischemia to Escitalopram Treatment (REMIT) study. Am Heart J 169:496–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Angaran P, Dorian P, Ha ACT et al. (2020) Association of left ventricular ejection fraction with mortality and hospitalizations. J Am Soc Echocardiogr. 10.1016/j.echo.2019.12.016 [DOI] [PubMed] [Google Scholar]
- 20.Miller AL, Dib C, Li L et al. (2012) Left ventricular ejection fraction assessment among patients with acute myocardial infarction and its association with hospital quality of care and evidencebased therapy use. Circ Cardiovasc Qual Outcomes 5:662–667 [DOI] [PubMed] [Google Scholar]
- 21.Parodi G, Antoniucci D (2010) Left ventricular remodeling after primary percutaneous coronary intervention. Am Heart J 160:S11–S15 [DOI] [PubMed] [Google Scholar]
- 22.Mendis S, Thygesen K, Kuulasmaa K et al. (2011) World Health Organization definition of myocardial infarction: 2008–09 revision. Int J Epidemiol 40:139–146 [DOI] [PubMed] [Google Scholar]
- 23.Yancy CW, Jessup M, Bozkurt B et al. (2013) 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American college of cardiology foundation/American Heart Association task force on practice guidelines. Circulation 128:1810–1852 [DOI] [PubMed] [Google Scholar]
- 24.Hsu JJ, Ziaeian B, Fonarow GC (2017) Heart failure with midrange (borderline) ejection fraction. JACC Heart Fail 5:763–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.First MB, Spitzer RL, Gibbon M, Williams JBW (2002) Structured clinical interview for DSM-IV axis I disorders, research version, patient edition (SCID-I/P). Biometrics Research, New York State Psychiatric Institude, New York [Google Scholar]
- 26.Beck AT, Steer RA, Brown GK (1996) Manual for the Beck Depression Inventory-II. TX Psychol Corp, San Antonio [Google Scholar]
- 27.Arranz B, Rosel P, Sarró S et al. (2003) Altered platelet serotonin 5-HT2A receptor density but not second messenger inositol trisphosphate levels in drug-free schizophrenic patients. Psychiatry Res 118:165–174 [DOI] [PubMed] [Google Scholar]
- 28.Lv J, Liu F (2017) The role of serotonin beyond the central nervous system during embryogenesis. Front Cell Neurosci 11:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ayme-Dietrich E, Aubertin-Kirch G, Maroteaux L, Monassier L (2017) Cardiovascular remodeling and the peripheral serotonergic system. Arch Cardiovasc Dis 110(1):51–59 [DOI] [PubMed] [Google Scholar]
- 30.Williams MS (2012) Platelets and depression in cardiovascular disease: a brief review of the current literature. World J Psychiatry 2:114–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mendelson SD (2000) The current status of the platelet 5-HT(2A) receptor in depression. J Affect Disord 57:13–24 [DOI] [PubMed] [Google Scholar]
- 32.Hrdina PD, Bakish D, Chudzik J, Ravindran A, Lapierre YD (1995) Serotonergic markers in platelets of patients with major depression: upregulation of 5-HT2 receptors. J Psychiatry Neurosci 20:11–19 [PMC free article] [PubMed] [Google Scholar]
- 33.Sheline YI, Bardgett ME, Jackson JL, Newcomer JW, Csernansky JG (1995) Platelet serotonin markers and depressive symptomatology. Biol Psychiatry 37:442–447 [DOI] [PubMed] [Google Scholar]
- 34.Rao ML, Hawellek B, Papassotiropoulos A, Deister A, Frahnert C (1998) Upregulation of the platelet serotonin(2A) receptor and low blood serotonin in suicidal psychiatric patients. Neuropsychobiology 38:84–89 [DOI] [PubMed] [Google Scholar]
- 35.Berger JS, Becker RC, Kuhn C, Helms MJ, Ortel TL, Williams R (2013) Hyperreactive platelet phenotypes: relationship to altered serotonin transporter number, transport kinetics and intrinsic response to adrenergic co-stimulation. Thromb Haemost 109:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lett H, Ali S, Whooley M (2008) Depression and cardiac function in patients with stable coronary heart disease: findings from the heart and soul study. Psychosom Med 70:444–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spijkerman TA, Van Den Brink RHS, Jansen JHC, Crijns HJGM, Ormel J (2005) Who is at risk of post-MI depressive symptoms? J Psychosom Res 58(5):425–432 [DOI] [PubMed] [Google Scholar]
- 38.Lespérance F, Frasure-Smith N, Talajic M (1996) Major depression before and after myocardial infarction: its nature and consequences. Psychosom Med 58(2):99–110 [DOI] [PubMed] [Google Scholar]
- 39.Strik JJMH, Lousberg R, Cheriex EC, Honig A (2004) One year cumulative incidence of depression following myocardial infarction and impact on cardiac outcome. J Psychosom Res 56:59–66 [DOI] [PubMed] [Google Scholar]
- 40.Bush DE, Ziegelstein RC, Tayback M et al. (2001) Even minimal symptoms of depression increase mortality risk after acute myocardial infarction. Am J Cardiol 88:337–341 [DOI] [PubMed] [Google Scholar]
- 41.Kim Y-H, Kim SH, Lim SY et al. (2012) Relationship between depression and subclinical left ventricular changes in the general population. Heart 98(18):1378–1383 [DOI] [PubMed] [Google Scholar]
- 42.Carney RM, Freedland KE, Veith RC (2005) Depression, the autonomic nervous system, and coronary heart disease. Psychosom Med 67(SUPPL. 1):S29–S33 [DOI] [PubMed] [Google Scholar]
- 43.Kent LK, Shapiro PA (2009) Depression and related psychological factors in heart disease. Harv Rev Psychiatry 17(6):377–388 [DOI] [PubMed] [Google Scholar]
- 44.Denollet J, de Jonge P, Kuyper A et al. (2009) Depression and type D personality represent different forms of distress in the Myocardial INfarction and Depression—Intervention Trial (MIND-IT). Psychol Med 39(05):749–756 [DOI] [PubMed] [Google Scholar]
- 45.Welin C, Lappas G, Wilhelmsen L (2000) Independent importance of psychosocial factors for prognosis after myocardial infarction. J Intern Med 247:629–639 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data available on open science framework https://osf.io/hyj69/?view_only=35a1d31edd8740309b24ae5d96502e7e.


