Abstract
Background
Readmission rates following ileostomy formation are high. Dehydration and consecutive renal failure are common causes of readmission, potentially pronounced by drugs affecting the homeostasis. The aim of the study was to assess the risk of dehydration after ileostomy formation in patients treated with angiotensin-converting enzyme inhibitors (ACEI), angiotensin II receptor blockers (ARB) or diuretics.
Method
This nationwide population-based cohort study used data derived from the Colorectal Cancer Data Base of several Swedish healthcare registers. The study included all patients operated on with elective anterior resection and temporary ileostomy for rectal cancer clinically staged I–III in Sweden in 2007–2016. Exposure was at least two dispensations of ACEI, ARB or diuretics within 1 year prior to surgery. Outcome was 90-day readmission due to dehydration including acute renal failure.
Results
In total, 3252 patients were included with 1173 (36.1%) exposed to ACEI, ARB or diuretics. The cumulative incidence for 90-day readmission due to dehydration was 29.0% (151 of 520) for exposed versus 13.8% (98 of 712) for unexposed. The proportion of readmissions due to any reason was 44.3% (520 of 1173) for exposed compared to 34.2% (712 of 2079) for unexposed. The incidence rate ratio for readmission due to dehydration was 2.83 (95% c.i. 2.21 to 3.63, P < 0.001). The hazard rate ratio was 2.45 (95% c.i. 1.83 to 3.27, P < 0.001) after adjusting for age, gender and comorbidity.
Conclusion
Medication with ACEI, ARB or diuretics defines a vulnerable patient group with increased risk of readmission due to dehydration after ileostomy formation.
This is the first population-based cohort study investigating the association between preoperative use of ACEI, ARB and diuretics on postoperative dehydration and acute renal failure in Swedish rectal cancer patients operated with anterior resection and temporary ileostomy. A two- to three-fold risk of 90-day readmission was observed among exposed participants compared to unexposed, with the risk being most pronounced for ACEI users.
Introduction
Protection of colorectal anastomoses, de-functioning of inflamed or obstructed colon and acute colectomy are common reasons to perform ileostomies. Absence of colonic passage may result in high stoma output with loss of fluids and electrolytes. Readmission rates of 20% within 30 days following ileostomy formation have been reported, with dehydration being the most common cause of readmission occurring in 6% of patients1. Consecutive negative impact on renal function has also been reported in the early postoperative phase and may persist after stoma closure, indicating risk of long-term renal impairment2–8. Being able to identify patients at risk of dehydration is therefore of great importance in order to prevent unplanned readmissions and protect long-term health after ileostomy formation9.
Medication with angiotensin-converting enzyme inhibitors (ACEI), angiotensin II receptor blockers (ARB) and diuretics are today commonly used in patients with hypertension or cardiovascular disease and are known to cause renal impairment in dehydrated patients. In Sweden, more than 2 million people aged 20 or above had at least one prescription of ARB, ACEI or diuretics during 202110. Usage of ACEI, ARB or diuretics in patients with ileostomy is therefore not uncommon. A few observational studies have previously reported ACEI, ARB and diuretics being risk factors for readmission due to dehydration and renal impairment following ileostomy formation2,11,12, but to our knowledge, larger population-based studies are lacking. Medication is usually interrupted the day of surgery, but the postoperative handling is less clear. At present, there are no official recommendations for treatment with ACEI, ARB and diuretics in patients with ileostomies.
The aim of this nationwide cohort study was therefore to investigate if preoperative use of ACEI, ARB or diuretics increases the risk of dehydration after elective ileostomy formation.
Methods
Study design
This population-based cohort study investigates the effect of preoperative use of ACEI, ARB or diuretics on the risk of readmission due to dehydration in rectal cancer patients treated with an ileostomy to protect the colorectal anastomosis after anterior resection. Data are derived from the Colorectal Cancer Data Base (CRCBaSe), a mega-linkage of several Swedish nationwide registers13. The study was approved by the Regional Ethical Review Board (Dnr 2014/71-31, 2018/328-32 and 2021-00342). This article was written according to the STROBE checklist for reporting of observational cohort studies14.
The Colorectal Cancer Data Base
The CRCBaSe is based on the Swedish Colorectal Cancer Register (SCRCR), a national quality register including nearly all diagnosed with rectal and colon cancers in Sweden since 1995–200715. The personal number, a unique identifier of all Swedish residents, enables linkage of data from several official registers held at the National Board of Health and Welfare (including the Prescribed Drug Register, the Inpatient Register, the Cause of Death Register) and Statistics Sweden13. At the time of data extraction for this study, CRCBaSe contained data of approximately 2.4 million individuals, including patients with colorectal cancer, comparators and relatives13.
Participants
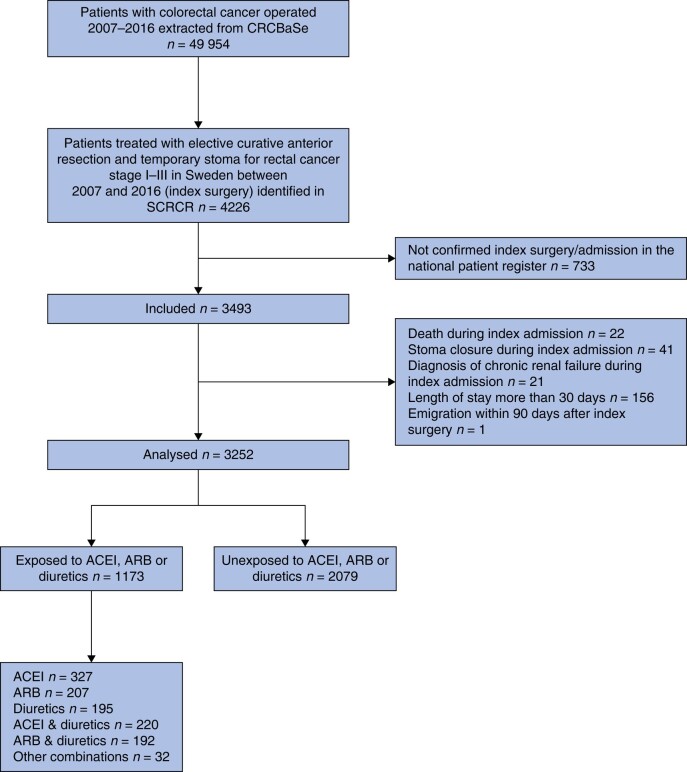
All patients 18 years and older at the time of diagnosis treated with elective anterior resection and temporary ileostomy with curative intent for rectal cancer clinically staged I–III (index surgery) in Sweden between 1 January 2007 and 31 December 2016 were considered eligible. In the event of synchronous tumours, the one with the highest clinical tumour stage was included. Index admission started the day of admission prior to index surgery and was the sum of uninterrupted admissions generating posts in the Inpatient Register. Index admissions without confirmed procedure codes for anterior resection and ileostomy in the Inpatient Register were excluded. Other exclusion criteria included systemic disease or ileostomy prior to index surgery, diagnosis of chronic renal failure or stoma closure or death during index admission and length of index admission exceeding 30 days. Emigration within 90 days from index surgery was also an exclusion criterion due to loss of follow-up (Fig. 1 and Supplementary Appendix S1).
Fig. 1.
Flowchart illustrating selection of study participants
ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor blockers; CRCBaSe, Colorectal Cancer Data Base.
Pilot study
The present nationwide cohort study is based on a pilot study investigating the risk of readmission due to dehydration or acute renal failure in patients treated with ACEI or ARB. Medical records of 749 patients with rectal cancer operated with anterior resection and temporary ileostomy in the Stockholm Region 2007–2015 were reviewed with similar inclusion and exclusion criteria. Patients treated with diuretics, no ACEI/ARB, were excluded in the analysis (n = 31). Exposure to ACEI/ARB were based on the same ATC codes as the main study but defined as regular prescription at the day of discharge after index operation. Outcome was death or readmission due to dehydration or acute renal failure within 90 days after index operation, based on the same ICD-10 codes as the main study or predefined notes identified in medical records at readmission. Comparison on an individual level between data derived from CRCBaSe and medical records was not possible as CRCBaSe data are pseudo-anonymized; however the results of the pilot study were used to compare effect sizes.
Exposure: dispensation of medications
Participants with at least two dispensations of ACEI, ARB or diuretics at a Swedish pharmacy within a time period of 365 days prior to index surgery were considered exposed. This information was obtained from the Prescribed Drug Register based on Anatomical Therapeutical Chemical (ATC) codes. Exposed participants were classified as either ACEI (only), ARB (only), diuretics (only), ACEI and diuretics (combined), ARB and diuretics (combined) or other combinations (Supplementary Appendix S2). To assess differences in the risk of dehydration between substances, a subgroup analysis was performed comparing the exposed subgroups with the unexposed group separately. Participants in the other combinations group were not included in the subgroup analysis.
Outcome of interest: readmission due to dehydration
Readmission to hospital within 90 days after index surgery with diagnosis of dehydration was the measured outcome, with time at risk starting the day after discharge from index admission. Study participants were censored in case of readmission due to other reasons, death or at end of follow-up 90 days after index surgery. Diagnosis of dehydration was based on the International Classification of Disease codes 10th edition (ICD-10) for volume depletion, electrolyte imbalance or acute renal failure. ICD-10 codes and admission and discharge dates of inpatient care were collected from the Inpatient Register and dates of death from the Cause of Death Register (Supplementary Appendix S3).
Additional variables
Patient, tumour and treatment characteristics were extracted from SCRCR and Statistics Sweden including gender, age at cancer diagnosis and ASA classification (Supplementary Appendix S4). BMI was calculated according to the formula [weight (kg)]/[length (m)]216 and tumours classified according to TNM stage. Length of hospital stay was calculated based on number of days between index surgery and discharge of index admission. Dispensations of calcium channel blockers or beta blockers (at least two within a time period of 365 days prior to index surgery), as well as dispensation of loperamide, opioids or oxycodone/naloxone (at least one after discharge from index surgery but before readmission/end of follow-up 90 days), were collected based on ATC codes from the Prescribed Drug Register. The Charlson Comorbidity Index (CCI) score (weighted) was calculated based on ICD-10 codes from the Inpatient Register (5 years prior to colorectal cancer diagnosis) and Swedish Cancer Registry (10 years prior to colorectal cancer diagnosis)17. No point was given for colorectal cancer. Study participants with ICD-10 codes I00–99 during index admission were regarded as having cardiovascular comorbidity and those with ICD-10 codes E10–14 as having diabetes mellitus18.
Statistical methods
Statistical analyses were performed with Stata version 15 (StataCorp LP, College Station, TX). Wilcoxon rank sum or Fisher exact tests were used for group comparisons with a two-sided 0.05 level of statistical significance. The time to readmission was compared between groups by incidence rate ratios (IRR) and the Kaplan–Meier method including log rank test. After graphical assessment of the proportional hazard assumption by ‘log-log’ plots and Schoenfeld residuals, Cox regression models were used to adjust analyses for age, gender, ASA score and CCI score (weighted). Further covariates such as country of birth, BMI, preoperative radiotherapy, duration of index surgery, perioperative bleeding, length of stay during index admission, dispensation of loperamide, opioids or oxycodone/naloxone and pathological TNM stage were considered for inclusion in final models if they changed point estimates by more than 10% or if the interaction term with the group variable indicated significant effect modification. Participants with no dispensations registered during the studied time period were considered healthy/unmedicated and were allocated to the unexposed group. Readmissions with no ICD-10 codes registered were included in the any reason group because the reason for readmission was unknown.
Results
Participants
Between 2007 and 2016, 4226 elective anterior resections with temporary stoma for rectal cancer clinically staged I–III were performed in Sweden. Seven hundred and thirty-three patients were excluded due to admissions without confirmed procedure codes for anterior resection and ileostomy in the Inpatient Register; 3252 patients were included in the analysis (Fig. 1), including 9 patients with synchronous tumours. In total, 36.1% (1173 of 3252) had at least two dispensations of ACEI, ARB or diuretics within 365 days prior to index surgery. Overall, 2079 participants were defined as unexposed.
Patient characteristics at baseline and information regarding oncological treatment are presented in Table 1. The proportion of men and age at diagnosis was higher in the exposed group compared to the unexposed group (66.2% versus 57.8% and 70 years versus 65 years respectively). The exposed group also had higher ASA and CCI scores, with a diagnosis of cardiovascular disease, diabetes and obesity (BMI > 30) being more common. Preoperative radiotherapy and postoperative chemotherapy were more often used in the unexposed group (68.9% versus 62.8% and 27.9% versus 20.8% respectively). The observed differences in median perioperative bleeding and postoperative time during index admission were of limited clinical significance.
Table 1.
Patient and treatment characteristics of 3252 Swedish patients treated with anterior resection and ileostomy for rectal cancer between 2007 and 2016
| Exposed N = 1173 |
Unexposed N = 2079 |
P | |
|---|---|---|---|
| Sex | <0.001 | ||
| Male | 776 (66.2) | 1201 (57.8) | |
| Female | 397 (33.8) | 878 (42.2) | |
| Country of birth | 0.801 | ||
| Sweden | 993 (84.7) | 1751(84.2) | |
| Other | 180 (15.4) | 327 (15.7) | |
| Missing | 0 (0.0) | 1 (0.1) | |
| Age at cancer diagnosis (years) | 70 (64–75) | 65 (57–71) | <0.001 |
| Age (years) | <0.001 | ||
| <55 | 58 (4.9) | 381 (18.3) | |
| 55–65 | 249 (21.2) | 609 (29.3) | |
| 65–75 | 549 (46.8) | 799 (38.4) | |
| >75 | 317 (27.0) | 290 (14.0) | |
| BMI (kg/m2) | 26.3 (24.2–29.2) | 25.2 (23.0–27.7) | <0.001 |
| BMI (kg/m2) | <0.001 | ||
| <18.5 | 11 (0.9) | 30 (1.4) | |
| 18.5–24.9 | 369 (31.5) | 914 (44.0) | |
| 25.0–29.9 | 508 (43.3) | 811 (39.0) | |
| >30 | 237 (20.2) | 248 (11.9) | |
| Missing | 48 (4.1) | 76 (3.7) | |
| ASA classification | <0.001 | ||
| ASA 1 | 39 (3.3) | 803 (38.6) | |
| ASA 2 | 826 (70.4) | 1062 (51.1) | |
| ASA 3 + 4 | 296 (25.2) | 179 (8.6) | |
| Missing | 12 (1.0) | 35 (1.7) | |
| CCI score | <0.001 | ||
| 0 | 738 (62.9) | 1670 (80.3) | |
| 1 | 164 (14.0) | 113 (5.4) | |
| 2 | 169 (14.4) | 234 (11.3) | |
| >=3 | 92 (7.8) | 45 (2.2) | |
| Missing | 10 (0.9) | 17 (0.8) | |
| Diagnosis of cardiovascular disease | <0.001 | ||
| No | 382 (32.6) | 1796 (86.4) | |
| Yes | 791 (67.4) | 283 (13.6) | |
| Diagnosis of diabetes mellitus | <0.001 | ||
| No | 976 (83.2) | 1985 (95.5) | |
| Yes | 197 (16.8) | 94 (4.5) | |
| Preoperative radiotherapy | <0.001 | ||
| No | 437 (37.3) | 646 (31.1) | |
| Yes | 736 (62.8) | 1433 (68.9) | |
| Length of index surgery (min) | 257 (206–240) | 254 (200–326) | 0.057 |
| Perioperative bleeding index surgery (ml) | 380 (200–650) | 300 (150–600) | <0.001 |
| Time from index surgery to discharge of index admission (days) | 10 (7–15) | 9 (7–14) | <0.001 |
| Pathological TNM stage | 0.466 | ||
| 1 | 398 (33.9) | 682 (32.8) | |
| 2 | 347 (29.6) | 584 (28.1) | |
| 3 | 420 (35.8) | 801 (38.5) | |
| 4 | 6 (0.5) | 9 (0.4) | |
| Missing | 2 (0.2) | 3 (0.1) | |
| Postoperative chemotherapy | <0.001 | ||
| No | 929 (79.2) | 1499 (72.1) | |
| Yes | 244 (20.8) | 580 (27.9) |
Continuous variables reported as median (i.q.r.) and categorical variables as frequency (proportion). Exposed defined as having at least two dispensations of ACEI, ARB or diuretics within a time period of 365 days prior to index surgery. P calculated using Fisher’s exact test or Wilcoxon rank sum-test. ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor blockers; CCI, Charlson Comorbidity Index (weighted).
Dispensation of medications
One hundred and forty-two patients had no dispensation registered. Among the exposed (1173), the distribution of dispensations was 27.9% ACEI only, 17.7% ARB only, 16.6% diuretics only, 18.8% ACEI and diuretics combined, 16.3% ARB and diuretics combined and 2.7% other combinations (Fig. 1). Dispensation of calcium channel blockers and beta blockers was higher in the exposed group (31.0% versus 8.2% and 45.2% versus 12.2% respectively), whereas dispensation of opioids was higher in the unexposed group (51.3% versus 45.4%). Dispensation of loperamide and oxycodone/naloxone was similar in both groups.
Readmissions within 90 days after index surgery
In total, 37.9% (1232 of 3252) were readmitted at least once within 90 days after index surgery. In 15 cases no ICD-10 codes were registered for readmissions. Readmissions due to any reason were higher in the exposed group, occurring in 44.3% (520 of 1173) of the exposed compared to 34.2% (712 of 2079) of the unexposed participants, with median time to readmission being shorter (12 and 20.5 days respectively) in the exposed group (Table 2). Readmission due to dehydration occurred in 7.7% of all participants (249 of 3252) and 20.2% of all readmissions (249 of 1232), accounting for 29.0% (151 of 520) of the readmissions in the exposed group compared to 13.8% (98 of 712) of the readmissions in the unexposed group, with comparable median time to readmission (10 versus 9 days). Total person time of the exposed group was 64 757 days and 130 907 days for the unexposed group, resulting in an incidence rate of 2.3 events/1000 days among exposed versus 0.7 events/1000 days among unexposed, yielding an IRR of 3.11 (95% c.i. 2.40 to 4.06).
Table 2.
Number of events (at least one readmission due to any reason and dehydration or death respectively) within 90 days after index surgery among the 3252 analysed participants
| Total N = 3252 |
Exposed N = 1173 |
Unexposed N = 2079 |
P | |
|---|---|---|---|---|
| Readmission due to any reason | 1232 of 3252 (37.9) | 520 of 1173 (44.3) | 712 of 2079 (34.2) | <0.001 |
| Readmission due to dehydration | 249 of 3252 (7.7) | 151 of 1173 (12.9) | 98 of 2079 (4.7) | <0.001 |
| Diagnosis of dehydration at readmission | 249 of 1232 (20.2) | 151 of 520 (29.0) | 98 of 712 (13.8) | <0.001 |
| ICD-10 code for volume depletion or electrolyte imbalance | 217 of 249 (87.1) | 126 of 151 (83.4) | 91 of 98 (92.9) | <0.001 |
| ICD10 code for acute renal failure | 68 of 249 (27.3) | 58 of 151 (38.4) | 10 of 98 (10.2) | <0.001 |
| 90-day mortality | 19 of 3253 (0.6) | 6 of 1173 (0.5) | 13 of 2079 (0.6) | 0.813 |
Variables are reported as frequency (proportion). Exposed was defined as at least two dispensations of ACEI, ARB or diuretics within 365 days prior to index surgery. ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor blockers.
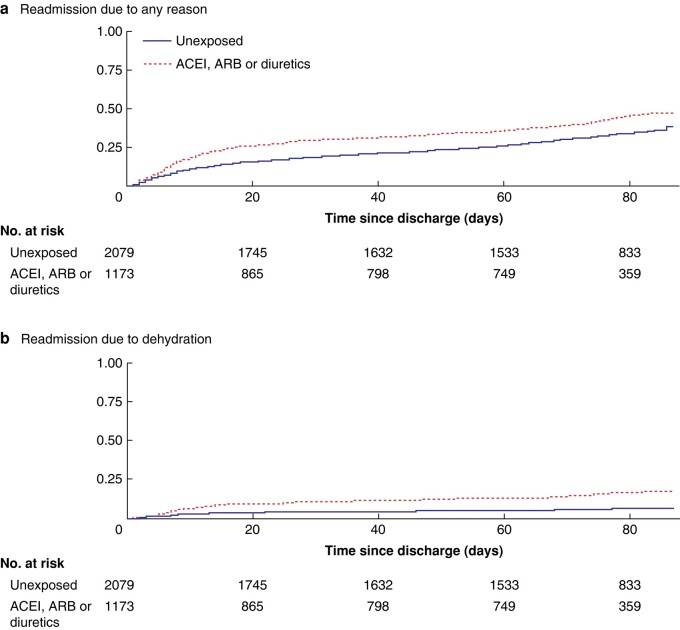
Kaplan–Meier graphs and Cox regression models
The cumulative incidence proportion shows significant differences for exposed and unexposed groups regarding readmission due to dehydration as well as due to any reason (P < 0.001; Fig. 2). The HR ratio for readmission with diagnosis of dehydration, adjusted for age, gender, ASA score and CCI score, was 2.45 (95% c.i. 1.83 to 3.27; Table 3). Addition of treatment-related variables such as TNM stage, preoperative radiotherapy, perioperative bleeding, duration of index surgery and time between surgery and discharge during index admission changed the HR by less than 3.4% and therefore they were not included in the final model. The effect of dispensation of loperamide, opioids or oxycodone/naloxone was minimal and was not adjusted for. Diagnosis of cardiovascular disease or diabetes mellitus, and dispensation of calcium channel or beta blockers, were not included in the final model due to collinearity with the exposure to ACEI, ARB and diuretics. No significant effect modification was detected. In a subanalysis comparing different dispensations with unexposed, the adjusted HR was 3.51 for ACEI only, 3.34 for ACEI and diuretics combined, 2.04 for ARB and diuretics combined, 1.90 for ARB only and 1.22 for diuretics only (Table 3).
Fig. 2.
Kaplan–Meier curves on time to readmission due to any reason and dehydration respectively
ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor blockers.
Table 3.
Hazard rate ratios for exposure to preoperative use of ACEI, ARB or diuretics on the risk of 90-day readmission due to dehydration among the 3252 analysed participants
| Unadjusted | Adjusted* | |||||
|---|---|---|---|---|---|---|
| HR | 95% c.i. | P | HR | 95% c.i. | P | |
| Unexposed ACEI, ARB or diuretics |
Ref 3.00 |
2.33,3.87 | <0.001 | Ref 2.45 |
1.83,3.27 | <0.001 |
| Unexposed ACEI only |
Ref 3.92 |
2.81,5.46 | <0.001 | Ref 3.51 |
2.45,5.03 | <0.001 |
| Unexposed ACEI and diuretics combined |
Ref 4.27 |
2.94,6.18 | <0.001 | Ref 3.34 |
2.22,5.03 | <0.001 |
| Unexposed ARB and diuretics combined |
Ref 2.48 |
1.53,4.02 | <0.001 | Ref 2.04 |
1.24,3.37 | 0.005 |
| Unexposed ARB only |
Ref 2.04 |
1.25,3.33 | 0.005 | Ref 1.90 |
1.14,3.17 | 0.013 |
| Unexposed Diuretics only |
Ref 1.86 |
1.10,3.16 | 0.021 | Ref 1.22 |
0.70,2.15 | 0.481 |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; Ref, Reference value 1.0. *Adjusted for categorical variables age, gender, score and Charlson Comorbidity Index score (weighted).
Pilot study
In the record-based pilot study, exposure to ACEI or ARB for patients in the Stockholm region was 24.9% (179 of 718). In total, 30.2% (217 of 718) were readmitted at least once within 90 days after index surgery. Readmission due to any reason was higher in the exposed group, occurring in 38.5% (69 of 179) of the exposed compared to 27.5% (148 of 539) of the unexposed participants. Among all readmitted patients, 44.2% (96 of 217) had signs of dehydration or acute renal failure (based on ICD-10 codes or notes in medical records). Readmission due to dehydration accounted for 59.4% (41 of 69) of all readmissions in the exposed group, compared to 37.2% (55 of 148) in the unexposed group. Total person time was 9875 days for the exposed group and 33 937.5 days for the unexposed group, resulting in an incidence rate of 4.2 events/1000 days among exposed versus 1.6 events/1000 days among unexposed, yielding an IRR of 2.5 (95% c.i. 1.7 to 3.9).
Discussion
This nationwide cohort study shows that patients with rectal cancer with preoperative use of ACEI, ARB and diuretics have a two- to three-fold risk of being readmitted due to dehydration within 90 days after elective anterior resection and ileostomy formation compared to unexposed participants.
In this study, 37.9% of the participants were readmitted within 90 days after index surgery with 20.2% of the remissions being due to dehydration. These findings give an incidence of dehydration-related readmissions after ileostomy formation of 7.7%, similar to the previously described 5.0%–10.3%1,5,9. Median time to readmission was 10 days, which also is in line with previous findings varying from 7 to 13 days11,19–21. Others have previously reported that postoperative use of diuretics is a significant risk factor for readmission due to dehydration after ileostomy formation (OR 2.44, 95% c.i. 1.5 to 3.8; P = 0.0001)11. Similar findings were observed by others; however, they were not statistically significant (P = 0.08)22,23. Another group previously reported usage of ACEI or ARB being associated with increased risk of readmission for dehydration following ileostomy formation (OR 13.56, 95% c.i. 3.54 to 51.92; P < 0.001)12. A similar experience also concluded that usage of ACEI and ARB was associated with postoperative renal decline 6 months after stoma closure2. However, these are all limited to retrospective single-site cohort studies with different study populations and varying definitions of exposure, outcome and follow-up time.
The strengths of this study include its population-based design with usage of register data with almost complete national coverage, minimizing the risk of selection bias as well as increasing the generalizability. Besides detailed information on patient-, tumour- and treatment-related characteristics in the SCRCR, additional information was retrieved from other high-quality national registers from the National Board of Welfare and Statistics Sweden due to the unique Personal Identification Number assigned to all Swedish residents. As a result, a large cohort was obtained with complete follow-up. The pilot study also made it possible to validate the results of the main study.
Exposure to ACEI, ARB or diuretics resulted as expected in an exposed group characterized by increased age and comorbidity. The study is limited by exposure and outcome being restricted to only register-based variables, in contrast to the pilot study where information in medical records was obtained manually. Classification of exposure was defined as two dispensations of ACEI, ARB or diuretics within a defined time period. However, it was not possible to track compliance and potential interruptions of these medications in the Prescribed Drug Register, especially in the early postoperative phase. We therefore make the assumption that participants exposed preoperatively also were exposed post-surgery, which is confirmed by the record-based pilot study where exposure status was identified in the discharge summary of the index surgery. A postoperative disruption of medication would result in a misclassification of exposure diluting the result. In addition, outcome was defined by ICD-10 codes set by discharging doctors. Diagnosis of dehydration can be difficult due to variations in diagnosis criteria (for example, clinical signs, laboratory findings, stoma output, urine production, etc.) and were potentially missed or misclassified. Dehydration may also lead to other diagnoses such as hypotension, syncope, fall injuries, unconsciousness, arrhythmias, etc. that we did not measure. Therefore, using ICD-10 codes from the Inpatient Register most likely limits the estimation of the true incidence of dehydration-related readmissions. Compared to the pilot study, the proportion of exposure was higher and readmission due to dehydration was lower in the register-based study. However, despite differences in methodology, the overestimation of exposure and underestimation of outcome do not seem to severely bias the study findings as the incidence rate ratios were similar. Adjustment for age, gender, ASA and CCI score reduced expected confounding and further treatment- or cancer-related factors did not change estimates substantially, indicating a low level of residual confounding. It is not surprising that preoperative use of ACEI, ARB or diuretics increases the risk of postoperative dehydration after ileostomy formation because it results in a physiological condition associated with loss of electrolytes and water in combination with drugs affecting the kidney’s ability to regulate the homeostasis. The negative effect of ACEI and ARB seems to be even more pronounced than the effect of diuretics and pathophysiologically similar effects can be expected after ileostomy formation in an emergency setting. The absolute risk of dehydration is most likely underestimated in the present study, which should be accounted for when generalizing the findings. Many patients experience high outputs from the ileostomy early in the postoperative phase with a spontaneous gradual decrease in output, but this may also be a clinical sign for dehydration. This implies a risk for delayed discovery of renal failure, especially in the case of limited adaptive capacity of the kidneys. In fact, the proportion of readmissions due to acute renal failure was 10 times higher among the exposed compared to unexposed, compared to twice as high for dehydration.
The finding of an overall readmission rate of almost 40% also confirms the problem with high rates of unplanned readmissions following rectal cancer surgery and ileostomy formation. Therefore, the ability to identify patients at risk for readmission is urgent in order to improve postoperative care and usage of healthcare resources. Swedish package leaflets do not mention specifically cautious use of ACEI, ARB or diuretics in the case of presence of an ileostomy. Length of hospital stay has also diminished after introduction of minimally invasive surgery and protocols for enhanced recovery. In consequence, adaptation to ileostomy occurs to a greater extent outside hospital care with restricted possibilities to monitor signs of dehydration. Enhanced awareness and improved postoperative follow-up may prevent severe dehydration, unnecessary readmissions as well as potentially prevent long-term effects on renal function.
Overall, preoperative use of ACEI, ARB or diuretics is associated with an increased risk for readmission due to dehydration in patients treated with anterior resection and ileostomy. In the majority of patients, readmission occurred within the first 2–3 weeks after discharge and might be avoided by enhanced awareness and closer postoperative follow-up.
Supplementary Material
Acknowledgements
This project was made possible by the continuous work of the steering groups of the Swedish Colorectal Cancer Register (SCRCR) and the Colorectal Cancer Base Sweden (CRCBaSe). Special thanks to Ida Hed Myrberg for statistical advice and support regarding CRCBaSe. All authors have approved the final version of the article.
Contributor Information
Louise de la Motte, Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden; Department of Pelvic Cancer, Colorectal Surgery Unit, Karolinska University Hospital, Stockholm, Sweden.
Caroline Nordenvall, Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden; Department of Pelvic Cancer, Colorectal Surgery Unit, Karolinska University Hospital, Stockholm, Sweden.
Anna Martling, Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden; Department of Pelvic Cancer, Colorectal Surgery Unit, Karolinska University Hospital, Stockholm, Sweden.
Christian Buchli, Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden; Department of Pelvic Cancer, Colorectal Surgery Unit, Karolinska University Hospital, Stockholm, Sweden.
Author contributions
Louise de la Motte (Conceptualization and study design, Data management, Analysis and interpretation of data, Writing—original draft, Writing—review & editing), Caroline Nordenvall (Conceptualization and study design, involved in the megalinkage of registers/CRCBaSe, Analysis and interpretation of data, Writing—review & editing), Anna Martling (Conceptualization and study design, involved in the megalinkage of registers/CRCBaSe, Writing—review & editing) and Christian Buchli (Conceptualization and study design, Analysis and interpretation of data, Writing—original article, Writing—review & editing)
Funding
The study was supported financially by the Swedish Cancer Society, the Cancer Society in Stockholm, the Swedish Cancer and Allergy Foundation (Cancer- och allergifonden), and the Regional Agreement on Medical Training and Clinical Research between Region Stockholm and Karolinska Institutet (ALF project).
Disclosure
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS Open online.
Data availability
The study is based on depersonalized data which cannot be shared by the authors due to the instructions of the ethical permission. For further questions regarding data availability, please contact the board of CRCBaSe (email: crcbase@mmk.ki.se).
References
- 1. Vogel I, Shinkwin M, van der Storm SL, Torkington J, Cornish JA, Tanis PJ et al. Overall readmissions and readmissions related to dehydration after creation of an ileostomy: a systematic review and meta-analysis. Tech Coloproctol 2022;26:333–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fielding A, Woods R, Moosvi SR, Wharton RQ, Speakman CTM, Kapur S et al. Renal impairment after ileostomy formation: a frequent event with long-term consequences. Colorectal Dis 2020;22:269–278 [DOI] [PubMed] [Google Scholar]
- 3. Li L, Lau KS, Ramanathan V, Orcutt ST, Sansgiry S, Albo D et al. Ileostomy creation in colorectal cancer surgery: risk of acute kidney injury and chronic kidney disease. J Surg Res 2017;210:204–212 [DOI] [PubMed] [Google Scholar]
- 4. Rutegard M, Haggstrom J, Back E, Holmgren K, Wixner J, Rutegard J et al. Defunctioning loop ileostomy in anterior resection for rectal cancer and subsequent renal failure: nationwide population-based study. BJS Open 2023;7:zrad010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Borucki JP, Schlaeger S, Crane J, Hernon JM, Stearns AT. Risk and consequences of dehydration following colorectal cancer resection with diverting ileostomy. A systematic review and meta-analysis. Colorectal Dis 2021;23:1721–1732 [DOI] [PubMed] [Google Scholar]
- 6. Gessler B, Haglind E, Angenete E. A temporary loop ileostomy affects renal function. Int J Colorectal Dis 2014;29:1131–1135 [DOI] [PubMed] [Google Scholar]
- 7. Yaegashi M, Otsuka K, Kimura T, Matsuo T, Fujii H, Sato K et al. Early renal dysfunction after temporary ileostomy construction. Surg Today 2020;50:703–710 [DOI] [PubMed] [Google Scholar]
- 8. Van Butsele J, Bislenghi G, D'Hoore A, Wolthuis AM. Readmission after rectal resection in the ERAS-era: is a loop ileostomy the Achilles heel? BMC Surg 2021;21:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu C, Bhat S, Sharma P, Yuan L, O'Grady G, Bissett I. Risk factors for readmission with dehydration after ileostomy formation: a systematic review and meta-analysis. Colorectal Dis 2021;23:1071–1082 [DOI] [PubMed] [Google Scholar]
- 10. The National Board of Health and Welfare . Statistics and Data. Pharmaceuticals. Sweden: National Board of Health and Welfare, 2021 [Google Scholar]
- 11. Messaris E, Sehgal R, Deiling S, Koltun WA, Stewart D, McKenna K et al. Dehydration is the most common indication for readmission after diverting ileostomy creation. Dis Colon Rectum 2012;55:175–180 [DOI] [PubMed] [Google Scholar]
- 12. Charak G, Kuritzkes BA, Al-Mazrou A, Suradkar K, Valizadeh N, Lee-Kong SA et al. Use of an ACE inhibitor or angiotensin receptor blocker is a major risk factor for dehydration requiring readmission in the setting of a new ileostomy. Int J Colorectal Dis 2018;33:311–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weibull CE, Boman SE, Glimelius B, Syk I, Matthiessen P, Smedby KE et al. CRCBase: a Swedish register-based resource for colorectal adenocarcinoma research. Acta Oncol 2023;62:342–349 [DOI] [PubMed] [Google Scholar]
- 14. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cancercentrum . Svenska Kolorektalcancerregistret. Sweden: Cancercentrum, 2022 [Google Scholar]
- 16. World Health Organization . Obesity and Overweight [Internet]. Geneva: World Health Organization, 2024. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight [Google Scholar]
- 17. Ludvigsson JF, Appelros P, Askling J, Byberg L, Carrero JJ, Ekstrom AM et al. Adaptation of the Charlson Comorbidity Index for register-based research in Sweden. Clin Epidemiol 2021;13:21–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. World Health Organization . ICD-10 Version: 2019. Geneva: World Health Organization, 2019 [Google Scholar]
- 19. Hayden DM, Pinzon MC, Francescatti AB, Edquist SC, Malczewski MR, Jolley JM et al. Hospital readmission for fluid and electrolyte abnormalities following ileostomy construction: preventable or unpredictable? J Gastrointest Surg 2013;17:298–303 [DOI] [PubMed] [Google Scholar]
- 20. Fish DR, Mancuso CA, Garcia-Aguilar JE, Lee SW, Nash GM, Sonoda T et al. Readmission after ileostomy creation: retrospective review of a common and significant event. Ann Surg 2017;265:379–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Paquette IM, Solan P, Rafferty JF, Ferguson MA, Davis BR. Readmission for dehydration or renal failure after ileostomy creation. Dis Colon Rectum 2013;56:974–979 [DOI] [PubMed] [Google Scholar]
- 22. Justiniano CF, Temple LK, Swanger AA, Xu Z, Speranza JR, Cellini C et al. Readmissions with dehydration after ileostomy creation: rethinking risk factors. Dis Colon Rectum 2018;61:1297–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gonella F, Valenti A, Massucco P, Russolillo N, Mineccia M, Fontana AP et al. A novel patient-centered protocol to reduce hospital readmissions for dehydration after ileostomy. Updates Surg 2019;71:515–521 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study is based on depersonalized data which cannot be shared by the authors due to the instructions of the ethical permission. For further questions regarding data availability, please contact the board of CRCBaSe (email: crcbase@mmk.ki.se).