Abstract
Two-component regulatory systems that utilize a multistep phosphorelay mechanism often involve a histidine-containing phosphotransfer (HPt) domain. These HPt domains serve an essential role as histidine-phosphorylated protein intermediates during phosphoryl transfer from one response regulator domain to another. In Saccharomyces cerevisiae, the YPD1 protein facilitates phosphoryl transfer from a hybrid sensor kinase, SLN1, to two distinct response regulator proteins, SSK1 and SKN7. Because the phosphorylation state largely determines the functional state of response regulator proteins, we have carried out a comparative study of the phosphorylated lifetimes of the three response regulator domains associated with SLN1, SSK1, and SKN7 (R1, R2, and R3, respectively). The isolated regulatory domains exhibited phosphorylated lifetimes within the range previously observed for other response regulator domains (i.e., several minutes to several hours). However, in the presence of YPD1, we found that the half-life of phosphorylated SSK1-R2 was dramatically extended (almost 200-fold longer than in the absence of YPD1). This stabilization effect was specific for SSK1-R2 and was not observed for SLN1-R1 or SKN7-R3. Our findings suggest a mechanism by which SSK1 is maintained in its phosphorylated state under normal physiological conditions and demonstrate an unprecedented regulatory role for an HPt domain in a phosphorelay signaling system.
Two-component signal transduction systems in prokaryotic and eukaryotic organisms regulate cellular responses to environmental changes (6, 11). In their simplest form, these regulatory pathways involve an autophosphorylating transmembrane histidine kinase and a cytoplasmic response regulator that is phosphorylated on an aspartic acid residue. Modified and expanded versions of two-component regulatory pathways requiring multiple phosphoryl transfer reactions between several phosphodonor and phosphoreceiver domains have also been identified (1, 6, 25, 27). Some of the more complex phosphorelay systems that have been described thus far include a hybrid sensor kinase (that also contains a phosphoaspartate receiver domain), a histidine-containing phosphotransfer (HPt) protein, and one or more response regulator proteins (4, 5, 7, 8, 14, 29, 34). HPt domains serve a dual purpose as a phosphoreceiver and phosphodonor in order to shuttle phosphoryl groups between two or more response regulator domains. It has also been suggested that the presence of HPt domains and use of multistep phosphorelay systems provide for additional points of regulation of signaling pathways (1, 11).
In most cases, response regulator proteins are activated upon phosphorylation. Hence, the intrinsic lifetime of the phosphorylated state of a response regulator is an important factor in determining the duration of the cellular response. Phosphorylated half-lives ranging from seconds for CheY and CheB (10, 37) to several hours for OmpR and Spo0F (13, 40) have been observed. In addition to the intrinsic phosphatase activity of the response regulator, several two-component systems utilize additional means of dephosphorylation. For example, in some cases the sensor histidine kinase exhibits phosphatase activity towards its cognate response regulator (12, 13, 21, 30, 31, 35). Alternatively, an auxiliary protein may act to accelerate or catalyze dephosphorylation of the response regulator (10, 24, 33). Another mechanism for signal decay has been identified in the Escherichia coli Arc system, whereby dephosphorylation of the cytoplasmic response regulator occurs through a “reverse” phosphorelay which is dependent on the C-terminal HPt domain of ArcB (9).
The osmoregulatory pathway in Saccharomyces cerevisiae consists of a multistep phosphorelay system involving the transmembrane hybrid histidine kinase SLN1, the HPt protein YPD1, and the response regulator SSK1 (36). In contrast to most two-component systems, these proteins are maintained in a phosphorylated state under normal environmental conditions. Under hyperosmotic stress conditions, SSK1 is rapidly dephosphorylated by a mechanism not well understood and in its dephosphorylated form activates a downstream mitogen-activated protein kinase cascade (28). The only other known response regulator in S. cerevisiae, SKN7, is also at least partially dependent on phosphorylation via SLN1 and YPD1 (18, 20). The multifunctional SKN7 protein has been implicated in maintenance of cell wall integrity (3), G1 cyclin expression (2, 23), and responses to oxidative and osmotic stress through a phosphorylation-dependent (SLN1-YPD1) as well as a phosphorylation-independent pathway (18–20, 22). Thus, YPD1 is required for phosphoryl group transfer between all three known response regulator domains in S. cerevisiae (i.e., SLN1-R1, SSK1-R2, and SKN7-R3).
We previously reported the half-lives of the phosphorylated response regulator domains associated with the yeast osmoregulatory proteins SLN1 and SSK1, which were determined, due to experimental constraints, in the presence of substoichiometric amounts of YPD1 (15). We have since developed a means for phosphorylating these response regulator domains that does not rely on the presence of YPD1. We were thus able to determine what effect the presence of YPD1 has on the phosphostability of the response regulator domains. Our results indicate that the HPt protein YPD1 has a dramatic stabilizing effect on phospho-SSK1-R2 but not phospho-SLN1-R1 or phospho-SKN7-R3. We believe that this effect is mediated, at least in part, by the formation of a stable HPt-response regulator domain complex.
MATERIALS AND METHODS
Materials.
All chemicals and biochemical reagents used were of ultrapure grade. NdeI was obtained from New England Biolabs. Pfu DNA polymerase was purchased from Stratagene. SmaI, DNA modifying enzymes, and oligonucleotides were from Life Technologies. Chromatography media were obtained from Pharmacia. [γ-32P]ATP (30 Ci/mmol) was purchased from Amersham. High-sensitivity Kodak BioMax MR film was used for autoradiography. The Immun-Star chemiluminescence protein detection kit was from BioRad. Antibodies against SSK1-R2 and YPD1 were raised in rabbits by Cocalico Biologicals, Inc. The expression vectors pGST-SLN1-HK, encoding the histidine kinase domain of SLN1 and pGST-SKN7, were kindly provided by R. Deschenes (University of Iowa).
Construction of SKN7-R3 expression vector.
For protein expression in bacterial cells, the gene fragment corresponding to the response regulator domain of the yeast SKN7 protein (designated R3) was amplified by PCR and subcloned into the pCYB2 vector of the IMPACT system (New England Biolabs). Specifically, a plasmid was constructed by subcloning an NdeI-SmaI fragment containing nucleotides 1,081 to 1,867 of the coding region of SKN7 (corresponding to amino acids 361 to 622) into pCYB2. An ATG start codon was included as part of the NdeI site at the 5′ end. Thus, a fusion protein was generated that consists of the SKN7-R3 domain located at the N terminus, followed by the yeast VMA1 protein-splicing intein domain and a chitin-binding domain. The entire coding region corresponding to the SKN7-R3-intein-chitin-binding domain fusion was further subcloned and placed under the control of the T7 RNA polymerase promoter in the pET11a expression plasmid (Novagen). This pET derivative was designated pFJS37.
Protein expression and purification.
Purification of YPD1, YPD1-H64Q, and the SLN1-R1 and SSK1-R2 response regulator domains has been described previously (15, 16, 38). The SLN1 histidine kinase domain (SLN1-HK) was expressed and purified as a glutathione-S-transferase (GST) fusion protein as described by Li et al. (20).
Expression and purification of the SKN7-R3 domain were performed similarly to that of the SSK1-R2 domain (15) with the following modifications. Cells were resuspended in lysis buffer (20 mM Tris [pH 7.5], 500 mM NaCl, 1 mM EDTA, 10% glycerol, 0.1% Triton X-100) and then lysed using a French press operated at 14,000 lb/in2. The cleavage buffer contained 20 mM Tris (pH 7.5), 50 mM NaCl, 0.1 mM EDTA, and 10% glycerol. A gel filtration step was not necessary. The protein was judged to be ≥95% homogeneous based on analysis by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis. Protein concentration was determined by absorbance at 280 nm using a calculated extinction coefficient of 6,580 M−1cm−1. Typical yields were 0.8 mg of pure protein/g (wet weight) of cells. Purified SKN7-R3 was stored in cleavage buffer at −20°C.
Preparation of phosphorylated response regulator domains.
Phosphorylation of response regulator domains was achieved by incubation with SLN1-HK and [γ-32P]ATP. GST-tagged SLN1-HK (3 μM) bound to glutathione-Sepharose 4B resin was incubated in the absence or presence of purified response regulator domain (12 μM) and 7 μM [γ-32P]ATP in 50 mM Tris-HCl (pH 8.0)–100 mM KCl–10 mM MgCl2–2 mM dithiothreitol–20% glycerol for 60 min at room temperature in a total reaction volume of 50 to 100 μl. The phosphorylated response regulator domain was recovered in the supernatant following a brief centrifugation step (1 min at 100 × g) to pellet the resin-bound GST-SLN1-HK.
Dephosphorylation of response regulator domains.
Isolated radiolabeled phosphorylated response regulator domains (5 μM) were incubated at room temperature in 50 mM Tris (pH 8.0)–10 mM MgCl2–1 mM dithiothreitol in a total volume of 50 μl. Wild-type YPD1 or YPD1-H64Q was added as indicated. Aliquots (4.5 μl) were removed at indicated time points, mixed with 4× stop buffer (0.25 M Tris-HCl [pH 6.8], 8% SDS, 60 mM EDTA, 40% glycerol, 0.008% bromophenol blue) to terminate the reaction, and kept at −20°C until gel analysis. Proteins were separated on an SDS–15% polyacrylamide gel and analyzed by autoradiography. Relative amounts of phosphorylated protein were determined by scanning densitometry of the autoradiograph using a BioRad GS-710 calibrated imaging densitometer. Rate constants for the dephosphorylation reaction and half-lives of the phosphorylated response regulator domains were determined by least-squares fitting of the natural logarithm of the data to a linear relationship assuming first-order kinetics.
Gel mobility shift assay.
Phosphorylated response regulator domains were prepared as described above, except that nonradiolabeled ATP was used in the incubation reaction. Parallel mock reaction mixtures contained the same components, except that ATP was omitted. Isolated phosphorylated and mock-treated response regulator domains (16 μM) were incubated with either 1.6 μM wild-type YPD1 or 1.6 μM YPD1-H64Q in phosphorylation reaction buffer in a total volume of 12 μl for 5 min at room temperature. Sample buffer (4 μl) containing 0.15 M Tris (pH 8.8)–40% glycerol was added, and the samples were loaded onto a native 15% polyacrylamide gel and electrophoresed at 250 V for 40 min. Following gel electrophoresis, proteins were electroblotted to polyvinylidene difluoride membranes in transfer buffer containing 25 mM Tris (pH 8.3), 192 mM glycine, 20% MeOH, and 0.1% SDS. Duplicate membranes were probed with antisera against SSK1-R2 and YPD1 and developed using the Immun-Star chemiluminescence detection system (BioRad).
RESULTS
Phosphorylation of response regulator domains via SLN1-HK.
Glutathione-Sepharose-bound GST-SLN1-HK was autophosphorylated in the presence of [γ-32P]ATP (Fig. 1, lane 1). Upon addition of purified response regulator domains, phosphoryl transfer from the SLN1-HK domain to each of the three response regulator domains was observed (Fig. 1, lanes 2 through 4). The differences in the amount of phosphoprotein generated may be attributed to differences in transfer rate between SLN1-HK and each response regulator domain. In addition, phosphate hydrolysis of the response regulator domain may contribute to the overall level of radiolabeled protein observable after the 60-min incubation time. Nonetheless, we have demonstrated that in vitro SLN1-HK can serve as a direct phosphoryl group donor to all three response regulator domains used in this study. Furthermore, because we were able to remove the resin-bound GST-SLN1-HK domain from the reaction mixture by centrifugation, dephosphorylation rates for the SLN1, SSK1, and SKN7 response regulator domains could accurately be determined in the absence of the normal protein phosphodonor.
FIG. 1.
Phosphorylation of response regulator domains. Phosphorylation reaction mixtures (30 μl) contained 4 μM GST-SLN1-HK and 11 μM [γ-32P]ATP in the absence and presence of 8 μM of the response regulator domains as indicated. Reaction mixtures were incubated for 60 min at room temperature, and reactions were stopped by the addition of 10 μl of 4× stop buffer. Reaction products were separated on an SDS-15% polyacrylamide gel. Immediately following SDS-polyacrylamide gel electrophoresis, the wet gel was wrapped in plastic film and subjected to autoradiography at −80°C.
Half-life of phospho-SSK1-R2 in the presence of YPD1.
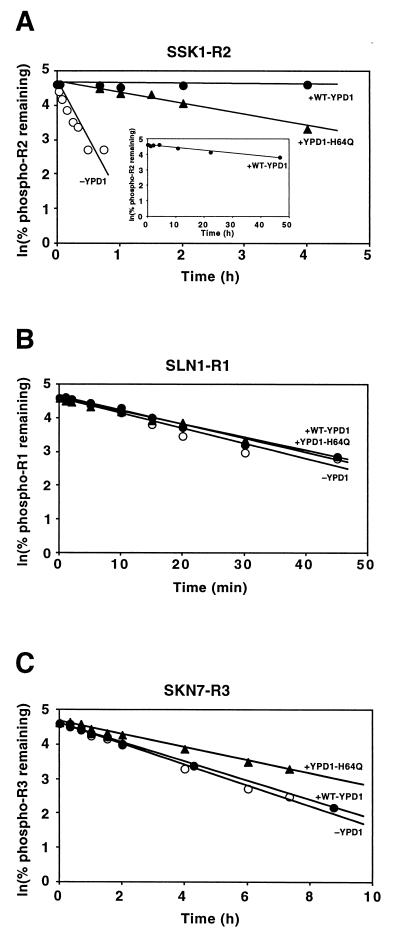
We previously reported a half-life of approximately 40 h for phospho-SSK1-R2 that was measured in the presence of substoichiometric amounts of YPD1 (15). By utilizing resin-bound GST-SLN1-HK to phosphorylate SSK1-R2, we were able to examine the half-life of phospho-SSK1-R2 in the absence of any upstream phosphodonor. Strikingly, the half-life of the isolated phospho-SSK1-R2 domain was only 13 min (Fig. 2A and Table 1). However, when YPD1 was included in the incubation reaction containing phospho-SSK1-R2, the previously determined half-life of about 40 h was reproduced (Fig. 2A and Table 1). This extended half-life was observed when YPD1 was present at one-tenth the concentration of SSK1-R2 as well as with equimolar amounts. During the time course of the dephosphorylation experiment, “reverse” phosphoryl transfer from phospho-SSK1-R2 to YPD1 was not observed. We also examined whether this stabilization effect by wild-type YPD1 was dependent on the phosphorylatable histidine residue H64 of YPD1. In the presence of the YPD1-H64Q mutant, the half-life of phospho-SSK1-R2 was increased only 10-fold, to approximately 2 h (Fig. 2A and Table 1).
FIG. 2.
Dephosphorylation rates of response regulator domains. Isolated phosphorylated SSK1-R2 (A), SLN1-R1 (B), or SKN7-R3 (C) was incubated in the absence (○) or presence of either a one-tenth molar concentration of wild-type YPD1 (●) or an equimolar concentration of YPD1-H64Q (▴). Aliquots were removed at indicated time points and analyzed as described in Materials and Methods. The autoradiographs were analyzed by scanning densitometry to determine the fraction of phosphorylated response regulator remaining. Dephosphorylation of the response regulator domains followed first-order rate kinetics, and the rate constants and half-lives were determined accordingly. The lines represent computer-generated least-squares fitting to a linear relationship. Data shown are from representative experiments that were performed multiple times. The inset in panel A shows the expanded time scale of the dephosphorylation reaction of phospho-SSK1-R2 in the presence of wild-type YPD1.
TABLE 1.
Half-lives of phosphorylated response regulator domains in the absence and presence of YPD1
| Response regulator domain | Half-life (min) ± SEM
|
||
|---|---|---|---|
| − YPD1 | + WT-YPD1a | + YPD1-H64Qb | |
| SLN1-R1 | 13 ± 2 | 17 ± 1c | 18 ± 1 |
| SSK1-R2 | 13 ± 3 | 2,280 ± 240c | 138 ± 6 |
| SKN7-R3 | 144 ± 6 | 156 ± 12 | 258 ± 24 |
The concentration of WT-YPD1 in the incubation was one-tenth of the concentration of the phosphorylated response regulator domain.
The concentration of YPD1-H64Q in the incubation was equimolar to the concentration of the phosphorylated response regulator domain.
See also Janiak-Spens et al. (15).
Half-lives of phospho-SLN1-R1 and phospho-SKN7-R3 in the presence of YPD1.
The half-lives of isolated phospho-SLN1-R1 in the absence and presence of a one-tenth molar concentration of wild-type YPD1 did not differ from one another and were the same as the previously reported value of 13 min (15) (Fig. 2B and Table 1). We then examined whether the presence of increased concentrations of YPD1 had an effect on the half-life of phospho-SLN1-R1. When wild-type YPD1 was present in equimolar concentrations (or in 10-fold excess), the half-life of phospho-SLN1-R1 alone could not be determined, since more than 70% of the radiolabel had transferred from SLN1-R1 to YPD1 within seconds after the addition of YPD1 (data not shown). However, when the YPD1-H64Q mutant was added to the incubation reaction containing phospho-SLN1-R1, radiolabel in YPD1 was not observed (data not shown). In the presence of an equimolar amount of YPD1-H64Q, the half-life of phospho-SLN1-R1 was approximately 18 min (Table 1).
The half-life determined for isolated phosphorylated SKN7-R3 was 2.4 h, about 10-fold longer than that of the other two isolated phosphorylated response regulator domains (Fig. 2C and Table 1). Similar to observations made for phospho-SLN1-R1, the presence of a one-tenth concentration of wild-type YPD1 had no stabilizing effect on phospho-SKN7-R3 (Fig. 2C and Table 1). When phospho-SKN7-R3 was incubated with an equimolar concentration of wild-type YPD1, approximately 50% of the radiolabel was found associated with YPD1, indicating that the reverse reaction competes with phosphate hydrolysis (data not shown). In the presence of an equimolar concentration of the YPD1-H64Q mutant in the incubation reaction, a twofold increase in the lifetime was observed (Fig. 2C and Table 1).
Complex formation between phospho-SSK1-R2 and YPD1.
To further characterize the stabilizing effect of YPD1 on phospho-SSK1-R2, we looked for evidence of a stable complex between the two proteins by using a gel mobility shift assay. Unphosphorylated and phosphorylated SSK1-R2 were incubated in the absence and presence of YPD1 or YPD1-H64Q. The proteins were separated on a native polyacrylamide gel and then analyzed by Western blotting. The membrane was probed with either anti-SSK1-R2 (Fig. 3A) or anti-YPD1 (Fig. 3B) antisera. Only in the presence of phospho-SSK1-R2 and either wild-type YPD1 or YPD1-H64Q (Fig. 3A and B, lanes 8 and 9) was a stable complex observed, as indicated by a band that migrated more slowly than YPD1 alone and contained both SSK1-R2 (Fig. 3A) and YPD1 (Fig. 3B). In contrast, the absence of a mobility-shifted band when unphosphorylated SSK1-R2 and YPD1 were incubated together indicates that these two proteins do not form a stable complex (Fig. 3A and B, lanes 5 and 6). The absence of a band for isolated SSK1-R2 (Fig. 3A, lane 1) is due to the net positive charge of SSK1-R2 (calculated pI, 10.5) that results in the protein migrating towards the cathode. In similar assays, incubation of YPD1 or the YPD1-H64Q mutant with either phospho-SLN1-R1 or phospho-SKN7-R3 or the unphosphorylated domains did not result in a mobility-shifted band in native gels (data not shown).
FIG. 3.
Gel mobility shift assay as evidence for an SSK1-R2 · YPD1 complex. Isolated phosphorylated and mock-reacted SSK1-R2 was incubated with one-tenth molar concentrations of either wild-type YPD1 or YPD1-H64Q as described in Materials and Methods. Reaction mixtures were separated on a native 15% polyacrylamide gel and then analyzed by Western blotting. Duplicate membranes were probed with anti-SSK1-R2 (A) or anti-YPD1 (B) antisera and visualized using chemiluminescence. Lanes 1, purified SSK1-R2; lanes 2, purified wild-type YPD1; lanes 3, purified YPD1-H64Q; lanes 4 through 6, mock-reacted SSK1-R2 in the absence or presence of YPD1 as indicated; lanes 7 through 9, phosphorylated SSK1-R2 in the absence or presence of YPD1 as indicated. The origins of the gel lanes for both panels are indicated on the right. The direction of migration was toward the anode as indicated on the left.
DISCUSSION
The primary role of HPt domains in phosphorelay signal transduction pathways is to transfer a phosphoryl group from one response regulator domain to another. However, additional functions have been reported for HPt domains, such as aiding in signal attenuation via dephosphorylation of the response regulator (9) and providing a means for specificity within a signaling pathway (26). To further investigate the unusually long half-life of the phosphorylated form of SSK1-R2 (15), we examined what effect YPD1 might have on the lifetime of all three phosphorylated response regulator domains (SLN1-R1, SSK1-R2, and SKN7-R3) from S. cerevisiae. Our results indicate a novel role for an HPt domain in stabilizing the phosphorylated state of a response regulator domain.
The half-life of phospho-SSK1-R2 is specifically affected by YPD1.
To our initial surprise, using the isolated phosphorylated SSK1-R2 domain, we determined a half-life of 13 min. This was in contrast to our previous result, which indicated a half-life of 42 h for phospho-SSK1-R2 in the presence of substoichiometric amounts of YPD1 (15). However, when we added YPD1 to the incubation reaction mixture containing isolated phospho-SSK1-R2, we again observed a half-life of approximately 40 h, which represents nearly a 200-fold stabilization effect. In the initial reaction mixture, we estimated that approximately 10% of the isolated SSK1-R2 is actually present in the phosphorylated form. Thus, the presence of a one-tenth molar concentration of YPD1 is sufficient to stabilize the response regulator domain and suggests the formation of a stable 1:1 complex between the two proteins. In contrast to the observations made for phospho-SSK1-R2, the stability of both phospho-SLN1-R1 and phospho-SKN7-R3 was far less affected by the presence of YPD1.
It is interesting that phospho-SKN7-R3 has a 10-fold longer intrinsic half-life in the absence of YPD1 than both of the other response regulator domains. It is tempting to speculate that because of the role of SKN7 as a transcription factor (20, 22) and therefore the necessity of translocating SKN7 in its phosphorylated form to the nucleus, a longer lifetime of phospho-SKN7 may be desirable in order to ensure signaling via this route.
Based on our observations using the gel mobility shift assays, YPD1 appears to form a more stable complex with phosphorylated SSK1-R2 than with the unphosphorylated form. This suggests a relatively strong interaction between phospho-SSK1-R2 and YPD1, which may account for the observed stabilization effect exerted by YPD1. No complex was observed between YPD1 and either phospho-SLN1-R1 or phospho-SKN7-R3 (data not shown).
We also investigated the requirement of the phosphorylatable histidine residue of YPD1 by using the mutant, YPD1-H64Q, which is defective in phosphoryl transfer. When YPD1-H64Q was added to the incubation reaction mixture containing phospho-SSK1-R2, the observed half-life was 10-fold longer than in the absence of YPD1 but not nearly as long as in the presence of wild-type YPD1. Based on the known three-dimensional structure of YPD1 (32, 39), we speculate that one reason for the difference observed with the H64Q mutant may be that the His64 side chain is required for forming a productive interaction surface between YPD1 and SSK1-R2. When His64 is replaced by a glutamine side chain, the molecular interface thus formed might be altered in a manner that would allow access to the phosphoryl group by a hydrolytic water molecule. Another possible explanation is that the binding affinity is reduced in the case of the mutant YPD1. However, in our gel mobility shift assay, we observe approximately equal amounts of the phospho-SSK1-R2 · YPD1 complex formed in the presence of wild-type or mutant YPD1. This suggests that the affinity of the YPD1-H64Q mutant for the SSK1-R2 domain is very similar to that of the wild-type protein. Determination of the actual binding constants for these two protein domains will be necessary to further characterize this interaction.
Phosphoryl transfer reactions in yeast two-component signaling pathways.
We have demonstrated that the SLN1-HK domain can readily serve as a phosphodonor to all three yeast response regulator domains in vitro. Phosphoryl transfer between SLN1-HK and SLN1-R1 favors phosphorylation of the SLN1-R1 domain since no reverse transfer from phospho-SLN1-R1 to SLN1-HK has been observed. However, the subsequent phosphoryl transfer reactions between SLN1-R1, YPD1, and SKN7-R3 are readily reversible (data not shown). In contrast, the reaction between phospho-YPD1 and SSK1-R2 strongly favors the forward reaction, i.e., formation of phospho-SSK1-R2. Unlike observations made with the ArcB/ArcA phosphorelay system, addition of SLN1-R1 and YPD1 together to an incubation mixture containing phospho-SSK1-R2 did not result in dephosphorylation of SSK1-R2. Furthermore, SLN1-HK does not appear to possess any phosphatase activity towards SSK1-R2 (F. Janiak-Spens and A. West, unpublished data).
Role of HPt domains in phosphorelay systems.
Multistep phosphorelay systems that include the use of HPt domains appear to be more common than initially thought (1, 27). HPt domains can be found as subdomains of sensor kinases, for example as part of a tripartite structure as in the BvgS, EvgS, ArcB, BarA, and TorS proteins (14, 17, 34), or as distinct proteins like YPD1, Spo0B, and LuxU (5, 8, 29). Apart from transferring a phosphoryl group between two response regulators, it has been demonstrated that several HPt domains possess additional functions. For example, the HPt domains of the BvgS/EvgS systems have been shown to confer signaling specificity between the hybrid kinases and their respective response regulator in vitro (26), whereas the HPt domain of the ArcB sensor kinase also promotes signal decay of its downstream response regulator ArcA (9).
The lifetime of a phosphorylated response regulator, aside from the intrinsic phosphate hydrolysis rate, can be modulated by either the phosphatase activity of the corresponding sensor kinase (12, 13, 21, 30, 31, 35) or by an independent protein phosphatase (10, 24, 33). The reported signal decay activity of the ArcB HPt domain provides an additional means of regulation (9). However, these functions can all be categorized, in general, as dephosphorylation activities. In this study, we have demonstrated the converse effect; that is, the yeast HPt protein YPD1 extends the phosphorylated lifetime of a response regulator domain. Our findings provide one possible mechanism whereby SSK1 can be maintained in a phosphorylated inactive state under normal osmotic conditions. A major question still remains—how is SSK1 rapidly dephosphorylated under hyperosmotic shock conditions? Perhaps disruption of a relatively stable SSK1-R2 · YPD1 complex is the first step in the process of SSK1-dependent activation of the downstream HOG1 mitogen-activated protein kinase cascade. Studies are underway to further characterize the nature of the interaction between SSK1 and YPD1.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant GM59311 to A.H.W. from the National Institutes of Health. D.P.S. was supported, in part, by a National Science Foundation Summer Undergraduate Research Fellowship (CHE-9531538). A.H.W. is a Cottrell Scholar of Research Corporation.
The authors gratefully acknowledge R. J. Deschenes for providing expression plasmids for GST fusion proteins. We also thank members of the West laboratory for helpful discussions.
REFERENCES
- 1.Appleby J L, Parkinson J S, Bourret R B. Signal transduction via the multi-step phosphorelay: not necessarily a road less traveled. Cell. 1996;86:845–848. doi: 10.1016/s0092-8674(00)80158-0. [DOI] [PubMed] [Google Scholar]
- 2.Bouquin N, Johnson A L, Morgan B A, Johnston L H. Association of the cell cycle transcription factor Mbp1 with the Skn7 response regulator in budding yeast. Mol Biol Cell. 1999;10:3389–3400. doi: 10.1091/mbc.10.10.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown J L, North S, Bussey H. SKN7, a yeast multicopy suppressor of a mutation affecting cell wall β-glucan assembly, encodes a product with domains homologous to prokaryotic two-component regulators and to heat shock transcription factors. J Bacteriol. 1993;175:6908–6915. doi: 10.1128/jb.175.21.6908-6915.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown J M, Firtel R A. Phosphorelay signalling: new tricks for an ancient pathway. Curr Biol. 1998;8:662–665. doi: 10.1016/s0960-9822(07)00417-4. [DOI] [PubMed] [Google Scholar]
- 5.Burbulys D, Trach K A, Hoch J A. Initiation of sporulation in B. subtilis is controlled by a multicomponent phosphorelay. Cell. 1991;64:545–552. doi: 10.1016/0092-8674(91)90238-t. [DOI] [PubMed] [Google Scholar]
- 6.Chang C, Stewart R C. The two-component system: regulation of diverse signaling pathways in prokaryotes and eukaryotes. Plant Physiol. 1998;117:723–731. doi: 10.1104/pp.117.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Agostino I B, Kieber J J. Phosphorelay signal transduction: the emerging family of plant response regulators. Trends Biochem Sci. 1999;24:452–456. doi: 10.1016/s0968-0004(99)01465-6. [DOI] [PubMed] [Google Scholar]
- 8.Freeman J A, Bassler B L. Sequence and function of LuxU: a two-component phosphorelay protein that regulates quorum sensing in Vibrio harveyi. J Bacteriol. 1999;181:899–906. doi: 10.1128/jb.181.3.899-906.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Georgellis D, Kwon O, De Wulf P, Lin E C C. Signal decay through a reverse phosphorelay in the Arc two-component signal transduction system. J Biol Chem. 1998;273:32864–32869. doi: 10.1074/jbc.273.49.32864. [DOI] [PubMed] [Google Scholar]
- 10.Hess J F, Oosawa K, Kaplan N, Simon M I. Phosphorylation of three proteins in the signaling pathway of bacterial chemotaxis. Cell. 1988;53:79–87. doi: 10.1016/0092-8674(88)90489-8. [DOI] [PubMed] [Google Scholar]
- 11.Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C.: ASM Press; 1995. [Google Scholar]
- 12.Hsing W, Silhavy T J. Function of conserved histidine-243 in phosphatase activity of EnvZ, the sensor of porin osmoregulation in Escherichia coli. J Bacteriol. 1997;179:3729–3735. doi: 10.1128/jb.179.11.3729-3735.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Igo M M, Ninfa A J, Stock J B, Silhavy T J. Phosphorylation and dephosphorylation of a bacterial transcriptional activator by a transmembrane receptor. Genes Dev. 1989;3:1725–1734. doi: 10.1101/gad.3.11.1725. [DOI] [PubMed] [Google Scholar]
- 14.Ishige K, Nagasawa S, Tokishita S, Mizuno T. A novel device of bacterial signal transducers. EMBO J. 1994;13:5195–5202. doi: 10.1002/j.1460-2075.1994.tb06850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janiak-Spens F, Sparling J M, Gurfinkel M, West A H. Differential stabilities of phosphorylated response regulator domains reflect functional roles of the yeast osmoregulatory SLN1 and SSK1 proteins. J Bacteriol. 1999;181:411–417. doi: 10.1128/jb.181.2.411-417.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janiak-Spens F, West A H. Functional roles of conserved amino acid residues surrounding the phosphorylatable histidine of the yeast phosphorelay protein YPD1. Mol Microbiol. 2000;37:136–144. doi: 10.1046/j.1365-2958.2000.01973.x. [DOI] [PubMed] [Google Scholar]
- 17.Jourlin C, Ansaldi M, Méjean V. Transphosphorylation of the TorR response regulator requires the three phosphorylation sites of the TorS unorthodox sensor in Escherichia coli. J Mol Biol. 1997;267:770–777. doi: 10.1006/jmbi.1997.0919. [DOI] [PubMed] [Google Scholar]
- 18.Ketela T, Brown J L, Stewart R C, Bussey H. Yeast Skn7p activity is modulated by the Sln1p-Ypd1p osmosensor and contributes to regulation of the HOG1 pathway. Mol Gen Genet. 1998;259:372–378. doi: 10.1007/s004380050824. [DOI] [PubMed] [Google Scholar]
- 19.Krems B, Charizanis C, Entian K-D. The response regulator-like protein Pos9/Skn7 of Saccharomyces cerevisiae is involved in oxidative stress resistance. Curr Genet. 1996;29:327–334. doi: 10.1007/BF02208613. [DOI] [PubMed] [Google Scholar]
- 20.Li S, Ault A, Malone C L, Raitt D, Dean S, Johnston L H, Deschenes R J, Fassler J S. The yeast histidine protein kinase, Sln1p, mediates phosphotransfer to two response regulators, Ssk1p and Skn7p. EMBO J. 1998;17:6952–6962. doi: 10.1093/emboj/17.23.6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu W, Hulett F M. Bacillus subtilis PhoP binds to the phoB tandem promoter exclusively within the phosphate starvation-inducible promoter. J Bacteriol. 1997;179:6302–6310. doi: 10.1128/jb.179.20.6302-6310.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morgan B A, Banks G R, Toone W M, Raitt D, Kuge S, Johnston L H. The Skn7 response regulator controls gene expression in the oxidative stress response of the budding yeast Saccharomyces cerevisiae. EMBO J. 1997;16:1035–1044. doi: 10.1093/emboj/16.5.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morgan B A, Bouquin N, Merrill G F, Johnston L H. A yeast transcription factor bypassing the requirement for SBF and DSC1/MBF in budding yeast has homology to bacterial signal transduction proteins. EMBO J. 1995;14:5679–5689. doi: 10.1002/j.1460-2075.1995.tb00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohlsen K L, Grimsley J K, Hoch J A. Deactivation of the sporulation transcription factor Spo0A by the Spo0E protein phosphatase. Proc Natl Acad Sci USA. 1994;91:1756–1760. doi: 10.1073/pnas.91.5.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parkinson J S, Kofoid E C. Communication modules in bacterial signaling proteins. Annu Rev Genet. 1992;26:71–112. doi: 10.1146/annurev.ge.26.120192.000443. [DOI] [PubMed] [Google Scholar]
- 26.Perraud A-L, Kimmel B, Weiss V, Gross R. Specificity of the BvgAS and EvgAS phosphorelay is mediated by the C-terminal HPt domains of the sensor proteins. Mol Microbiol. 1998;27:875–887. doi: 10.1046/j.1365-2958.1998.00716.x. [DOI] [PubMed] [Google Scholar]
- 27.Perraud A-L, Weiss V, Gross R. Signalling pathways in two-component phosphorelay systems. Trends Microbiol. 1999;7:115–120. doi: 10.1016/s0966-842x(99)01458-4. [DOI] [PubMed] [Google Scholar]
- 28.Posas F, Saito H. Activation of the yeast SSK2 MAP kinase kinase kinase by the SSK1 two-component response regulator. EMBO J. 1998;17:1385–1394. doi: 10.1093/emboj/17.5.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Posas F, Wurgler-Murphy S M, Maeda T, Witten E A, Thai T C, Saito H. Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 “two-component” osmosensor. Cell. 1996;86:865–875. doi: 10.1016/s0092-8674(00)80162-2. [DOI] [PubMed] [Google Scholar]
- 30.Schröder I, Wolin C D, Cavicchioli R, Gunsalus R P. Phosphorylation and dephosphorylation of the NarQ, NarX, and NarL proteins of the nitrate-dependent two-component regulatory system of Escherichia coli. J Bacteriol. 1994;176:4985–4992. doi: 10.1128/jb.176.16.4985-4992.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skarphol K, Waukau J, Forst S A. Role of His243 in the phosphatase activity of EnvZ in Escherichia coli. J Bacteriol. 1997;179:1413–1416. doi: 10.1128/jb.179.4.1413-1416.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song H K, Lee J Y, Lee M G, Min J M K, Yang J K, Suh S W. Insights into eukaryotic multistep phosphorelay signal transduction revealed by the crystal structure of Ypd1p from Saccharomyces cerevisiae. J Mol Biol. 1999;293:753–761. doi: 10.1006/jmbi.1999.3215. [DOI] [PubMed] [Google Scholar]
- 33.Sourjik V, Schmitt R. Phosphotransfer between CheA, CheY1, and CheY2 in the chemotaxis signal transduction chain of Rhizobium meliloti. Biochemistry. 1998;37:2327–2335. doi: 10.1021/bi972330a. [DOI] [PubMed] [Google Scholar]
- 34.Uhl M A, Miller J F. Integration of multiple domains in a two-component sensor protein: the Bordetella pertussis BvgAS phosphorelay. EMBO J. 1996;15:1028–1036. [PMC free article] [PubMed] [Google Scholar]
- 35.Walker M S, DeMoss J A. Phosphorylation and dephosphorylation catalyzed in vitro by purified components of the nitrate sensing system, NarX and NarL. J Biol Chem. 1993;268:8391–8393. [PubMed] [Google Scholar]
- 36.Wurgler-Murphy S M, Saito H. Two-component signal transducers and MAPK cascades. Trends Biochem Sci. 1997;22:172–176. doi: 10.1016/s0968-0004(97)01036-0. [DOI] [PubMed] [Google Scholar]
- 37.Wylie D, Stock A, Wong C-Y, Stock J. Sensory transduction in bacterial chemotaxis involves phosphotransfer between Che proteins. Biochem Biophys Res Commun. 1988;151:891–896. doi: 10.1016/s0006-291x(88)80365-6. [DOI] [PubMed] [Google Scholar]
- 38.Xu Q, Nguyen V, West A H. Purification, crystallization, and preliminary X-ray diffraction analysis of the yeast phosphorelay protein YPD1. Acta Cryst D. 1999;55:291–293. doi: 10.1107/S090744499800866X. [DOI] [PubMed] [Google Scholar]
- 39.Xu Q, West A H. Conservation of structure and function among histidine-containing phosphotransfer (HPt) domains as revealed by the crystal structure of YPD1. J Mol Biol. 1999;292:1039–1050. doi: 10.1006/jmbi.1999.3143. [DOI] [PubMed] [Google Scholar]
- 40.Zapf J, Grimshaw C E, Hoch J A, Varughese K I, Whiteley J M. A source of response regulator autophosphatase activity: the critical role of a residue adjacent to the Spo0F autophosphorylation active site. Biochemistry. 1998;37:7725–7732. doi: 10.1021/bi9729615. [DOI] [PubMed] [Google Scholar]