Abstract
Chest pain, a common symptom in cardiovascular care, often leads to the investigation of obstructive coronary artery disease (CAD). However, many patients experience chest pain without obstructive CAD, termed INOCA (Ischemia with Non-Obstructive Coronary Arteries) or CMD (Coronary Microvascular Dysfunction). INOCA can be attributed to endothelial dysfunction, vascular smooth muscle dysfunction, or both, affecting about 20–30 % of patients with nonobstructive CAD. The diagnostic approach for INOCA includes both invasive and non-invasive methods, with cardiac PET (Positron Emission Tomography) playing a significant role in risk stratification and management. PET evaluates various parameters like myocardial blood flow under stress and rest, myocardial flow reserve, and myocardial ischemia. Such comprehensive assessment is essential in accurately diagnosing and managing INOCA, considering the complexity of this condition.
Keywords: Coronary microvascular dysfunction, Ischemia with no obstructive coronary arteries, Ischemia, Cardiac PET
1. Introduction
Chest pain continues to be a common presenting symptom in cardiovascular care. [1] While obstructive coronary artery disease (CAD) is the primary cardiac concern, many patients continue to experience chest pain despite no obstructive epicardial coronary disease. This condition was initially labeled as “syndrome X" and most recently referred to as Ischemia with Non-Obstructive Coronary Arteries (INOCA) or Coronary Microvascular Dysfunction (CMD) [2]. INOCA can be due to endothelial dysfunction, vascular smooth muscle dysfunction, or a combination of both [3]. It is estimated that approximately 20 % to 30 % of patients with nonobstructive CAD demonstrate ischemia (Figure). Increased coronary vasoreactivity (vasospasm), typically occurring in approximately 20–25 % of cases, affects primarily the epicardial arteries and to a lesser extent the microvasculature. [4] Intracoronary acetylcholine provocation testing primarily assesses epicardial vasospasm but cannot rule out coexisting microvascular spasm. However, there is new evidence suggesting that the introduction of a second acetylcholine challenge following intracoronary nitroglycerin administration can diagnose underlying microvascular spasm. [5,6] Mechanistically, there is an overlap between increased microvascular coronary vasoreactivity and decreased epicardial vascular smooth muscle relaxation, contributing to the complexity of CMD [5] (Fig. 1, Fig. 2).
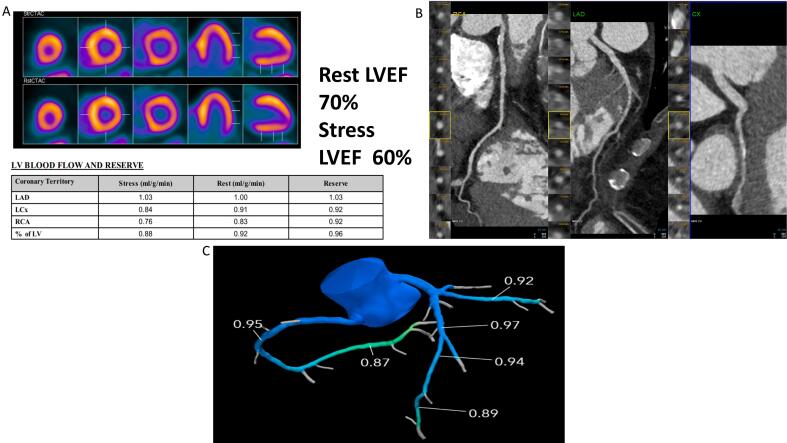
Fig. 1.
58-year-old female patient with obesity, hypercholesterolemia and hypertension presenting with chest pain. Nonspecific resting EKG changes. The patient underwent PET myocardial perfusion imaging which was normal on the relative perfusion imaging (A). There was a 10 % drop in ejection fraction and severely reduced myocardial blood flow reserve as well as hyperemic myocardial blood flow. Coronary CT angiography (B) and FFR CT (C) confirmed no evidence of epicardial coronary artery disease. Patient was started on nitrates as well as high intensity statin with significant improvement in her symptoms.
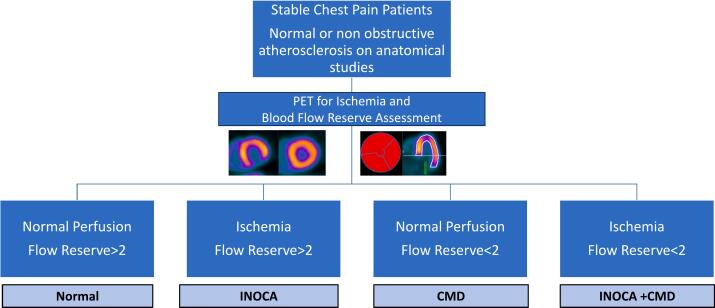
Fig. 2.
Diagnositic Algorthim for INOCA using PET in patients with stable chest pain and no obstructive disease on anatomical studies. Abbreviations: PET: positron emission tomography, INOCA: ischemia without obstructive coronary artery disease. CMD: coronary microvascular dysfunction.
Multiple invasive and non-invasive tools are available for the assessment and workup of patients with suspected INOCA. Invasive coronary reactivity testing assesses vasospasm, as well as both non-endothelial-dependent, and endothelium-dependent microvascular reactivity. [4] Apart from diagnosis, these parameters also have prognostic implications. For instance, the Women's Ischemia Syndrome Evaluation (WISE) study, sponsored by the National Institutes of Health-NHLBI, discovered that impaired coronary flow reserve (defined as <2.32) in women without obstructive CAD was associated with an elevated risk of major CAD events during a 10-year follow-up period. [7] Furthermore, among women without obstructive CAD, epicardial vasoconstriction was significantly linked to a higher rate of hospitalization for angina. [8]
Invasive coronary assessment carries with it inherent risks and there is emerging evidence that non-invasive assessment is also useful. The current ACC AHA guidelines notes that there is compelling prognostic evidence supporting the valuable contribution of Positron Emission Tomography Myocardial Blood Flow Reserve (PET MFR) techniques in the assessment and management of INOCA. [9] Cardiac PET plays a pivotal role in the non-invasive evaluation and management of patients with INOCA. Despite experiencing symptoms indicative of ischemia, conventional angiography reveals no apparent stenosis in the coronary arteries, leaving healthcare professionals and patients uncertain of the diagnosis. In this context, positron emission tomography emerges as a powerful tool, offering invaluable insights into myocardial blood flow (MBF) and myocardial flow reserve (MFR), shedding light on both the underlying pathophysiology, and potentially guiding crucial treatment decisions.
In recent decades, there has been a notable surge in the utilization of PET myocardial perfusion imaging (PET MPI). [1,10] When performing PET MPI, various aspects can be evaluated, including relative perfusion, absolute MBF under both stress and rest conditions, MFR, left ventricular ejection fraction, transient ischemic dilatation, and the presence/burden of coronary artery calcium. [11,12] This comprehensive assessment is typically completed within a concise timeframe of approximately 20 min. Each of these parameters offer valuable insights that enhance the precision to evaluate and manage patients with suspected INOCA. [13,14] It is also important to note that PET is associated with very low radiation dose exposure (1–3 mSiv).
2. Radiotracers
In the USA, two primary radiotracers are used, 13 N-ammonia and 82Rubidium in PET MPI. It is worth noting that 15O-water, while validated against radioactive microspheres in animal models, remains unapproved by the FDA for clinical use in the US, but is clinically used in Europe for assessment of MBF. 15O-water stands out due to its inert and freely diffusible nature, resulting in a linear correlation with MBF and near-perfect initial tissue extraction, establishing it as the gold standard for MBF evaluation against which novel radiotracers are validated. [15] Nevertheless, its practicality is limited due to dependence on an onsite cyclotron, and a short half-life of 2.09 min. In contrast, 13 N-ammonia and 82Rubidium exhibit non-linear MBF profiles with varying retention fractions. While 13 N-ammonia offers excellent image quality and allows for comprehensive ischemia evaluation, its reliance on cyclotron availability remains a barrier for widespread use. On the other hand, 82Rubidium, a generator-produced tracer, mirrors the diagnostic accuracy of 13 N-ammonia and allows for a short turnaround time between rest and stress due to its ultra-short half-life of 76 s. However, it sacrifices some spatial resolution compared to ammonia due to its longer positron range. [11,13]
Flurpiridaz is a new PET radiotracer which is currently being reviewed by the FDA. It is specifically designed to enhance myocardial perfusion imaging. Compared to traditional SPECT imaging, Flurpiridaz PET offers several advantages. These include higher spatial resolution, shorter imaging durations, and the ability to accurately quantify myocardial blood flow, leading to potentially more accurate diagnoses and better patient management. The Phase-III trial was a large-scale, multicenter study conducted across the US, Canada, and Finland. It involved 795 participants and aimed to compare the diagnostic efficacy of Flurpiridaz PET with technetium-99 m-labeled SPECT imaging. [16,17] Flurpiridaz PET demonstrated a higher sensitivity in detecting coronary artery disease, particularly in demographics traditionally considered challenging for cardiac imaging, such as women, patients with obesity, and those undergoing pharmacological stress testing. The trial also highlighted the superior image quality of Flurpiridaz PET, which translated into greater diagnostic certainty. This aspect is particularly crucial in complex clinical scenarios where traditional imaging modalities might yield inconclusive results, such as the case with INOCA. [18,19] The introduction of Flurpiridaz PET into clinical practice could significantly enhance access to PET as the long half-life of Flurpiridaz (109.8 min) allows for shipment and transportation to various PET centers akin to technetium-99 m SPECT. [20]
3. Different roles of PET in INOCA
3.1. Detection of myocardial ischemia
Since the hallmark of INOCA is the presence of ischemia, an accurate diagnosis is essential as a first step. Cardiac PET can assess relative myocardial perfusion and blood flow. Multiple studies have shown that cardiac PET has the highest diagnostic accuracy in the assessment of ischemia compared to other modalities. [11,21,22]. Overall, PET MPI has an average 90 % sensitivity for detecting at least one coronary artery with >50 % stenosis, with an average specificity of 89 %. This yields a positive and negative predictive value of 94 % and 73 %, respectively, with a diagnostic accuracy of 90 %. Importantly, the sensitivity of PET MPI to diagnose obstructive CAD detection remains high in single versus multi-vessel disease, in obese individuals, and in both men and women. [23] It is worth noting, however, that most of the available accuracy data pertains to older generation PET scanners equipped with radionuclide attenuation correction and using pharmacologic (vasodilator) stress rather than exercise.
3.2. Measurement of myocardial blood flow reserve (MFR)
Cardiac PET can also measure MFR, which is an important parameter in INOCA evaluation. Reduced MFR is often seen in INOCA patients, indicating impaired microvascular function [24]. Depending on the stress protocol used, MFR measurement can assess endothelial-dependent and endothelial-independent function. Cardiac PET can quantify MFR and help diagnose microvascular dysfunction. This is a routine part of the cardiac PET perfusion test and does not require additional radiation exposure. Individuals at the highest risk of experiencing coronary microvascular dysfunction include women and those with conditions such as hypertension, diabetes, obesity and insulin resistance [25] as well as patients with long COVID [[26], [27], [28]]. Studies utilizing cardiac PET show that PET-derived MFR can aid in identifying cases of microvascular angina by identifying low MFR. Demonstration of normal or non-obstructive atherosclerosis with anatomic imaging is often needed to confirm the diagnosis (Fig. 1).
It is also important to note that the hallmark of INOCA on cardiac PET that the inability to vasodilate with reduced hyperemic myocardial blood flow. These patients tend to have reduced stress MBF irrespective of the resting blood flow.
3.3. Differentiation from other causes
Cardiac PET can help differentiate INOCA from other cardiac or non-cardiac conditions that may mimic anginal symptoms. It can provide valuable information to rule out alternative causes of chest pain. With the use of CT attenuation correction, cardiac PET allows for diagnosis of important incidental findings in the lungs and mediastinum, such as hiatal hernias or lung malignancies. [29]
3.4. When to refer patients for PET for INOCA assessment
The guidelines from the American College of Cardiology (ACC) and the American Heart Association (AHA) (9) recommend considering PET with myocardial blood flow assessment for patients with stable chest pain who exhibit multiple cardiovascular risk factors such as diabetes, hypertension, left ventricular hypertrophy, slow coronary flow on angiography, or infiltrative heart diseases in whom INOCA is suspected. Cardiac PET is especially useful for evaluating these patients after obstructive epicardial coronary disease has been ruled out through anatomical testing. (figure) The current guidelines suggest that non invasive approach can be utilized in lie of invasive assessment of microvascular dysfunction. Currently, in the United States, only few centers perform routine invasive assessment of microvascular dysfunction and the assessment of INCOA and CMD is mostly based on non invasive testing by PET myocardial perfusion imaging and blood flow assessment. Non invasive imaging does not adequately assess angina due to coronary spasm and most often this is diagnosed via a therapeutic trial of calcium channel blockers or nitrates.
For patients who show no signs of ischemia and who have a normal myocardial blood flow reserve as measured by PET, the risk of cardiovascular events is generally considered low. These findings suggest absence of significant microvascular dysfunction. In addition, patients with ischemia and low flow reserve meet the diagnostic criteria for CMD and INOCA.
Conversely, patients who do not have ischemia but have reduced myocardial blood flow reserve, there is a strong indication of coronary microvascular dysfunction. This condition necessitates specific and targeted treatment approach, often includes medical therapy tailored to improve microvascular health and function. Managing CMD is critical as it has been linked to an increased risk of major adverse cardiovascular events and can significantly impair quality of life.
Incorporating cardiac PET into the diagnostic pathway for selected patient populations enriches clinical decision-making by providing insights into coronary and microvascular circulation. This is particularly crucial for patients who continue to experience symptoms despite having no significant epicardial coronary stenosis on traditional imaging modalities. It guides the implementation of personalized medical interventions that are more likely to improve patient outcomes.
Thus, cardiac PET is not only a tool for diagnosing ischemia but also an essential tool for assessing coronary microvascular health, making it an invaluable asset in the management of complex cardiac cases. Clinicians should consider cardiac PET for patients fitting the described profiles (stable chest patients, multiple co morbidities and no obstructive CAD) to ensure a thorough evaluation and to guide subsequent management strategies effectively. This approach aligns with current guidelines and best practices, aiming to optimize care for patients with potential microvascular involvement in their cardiac symptoms (Fig. 2).
3.5. Risk stratification of INOCA patients
Cardiac PET can help risk-stratify INOCA patients by identifying those at higher risk for adverse cardiovascular events. Cardiac PET can also guide treatment decisions and help optimize patient care.
While conventional PET MPI is a cornerstone in CAD management, its potential extends beyond simply separating low- and high-risk patients. In INOCA, cardiac PET unveils areas of the heart with inadequate blood flow despite patent epicardial vessels. MFR, with its remarkable consistency across diverse analytical methods, makes it a reliable prognostic tool. In addition to MFR, hyperemic MBF may be of clinical value, but needs further testing, as its sensitivity to methodological choices necessitates stricter standardization within tracers and systems. This consistency is crucial in INOCA, where subtle variations in blood flow can hold significant prognostic value [30].
Multiple studies have confirmed the prognostic value of PET MFR. The results are consistent across radiotracer and different PET systems. There is a clear association between impaired MFR (<2) and risk of adverse cardiac events, independent of traditional risk factors and PET perfusion parameters. [31] This underscores the incremental prognostic value of PET MFR, especially in guiding management decisions in INOCA patients. Furthermore, a recent meta-analysis focused on the role of PET MPI (particularly MFR) and risk of adverse events. Examining over 46,000 patients with known or suspected CAD, the study revealed a strong association between impaired MFR (of which INOCA represents an important subset) and an increased risk of future cardiovascular events. [24] This finding provides robust evidence for the potential of PET MPI to not only diagnose INOCA but also stratify risk and potentially guide treatment decisions.
Navigating the INOCA treatment landscape requires a multifaceted approach that recognizes the limitations of each measure, incorporates standardized methodologies, and embraces cutting-edge technology like digital PET. While MBF and MFR offer valuable insights, it is crucial to consider them in conjunction with other clinical factors and imaging findings.
3.6. Monitoring treatment response
INOCA management remains a clinical challenge. While traditional therapies targeting large arteries have shown limited efficacy in addressing INOCA, exciting new avenues are emerging, offering a glimmer of hope for individuals affected by this condition. Traditionally, treatment for INCOA is focused on managing major risk factors like obesity, hypertension, high cholesterol, and diabetes. However, these approaches often fall short in directly addressing microvascular abnormalities. Furthermore, medications like nitrates and beta-blockers, while beneficial in some cases, can sometimes worsen MVD due to their vasodilatory effects.
New novel therapeutics are currently being studied in clinical trials and include Nitric oxide (NO) donors, mitochondrial targeting therapies, anti-inflammatory agents, stem cell therapy and targeted gene therapy. Myocardial blood flow reserve (MFR) can indeed be targeted therapeutically in managing patients with INOCA. Studies are currently exploring how coronary microvascular function can be influenced before and after the initiation of newer therapies, such as advanced lipid-lowering agents, and in patients undergoing weight reduction surgeries. It is hypothesized that improving the risk factor profile could enhance microvascular function. PET MFR is being used to assess the effectiveness of these treatment strategies aimed at improving microvascular function in INOCA patients. It allows for serial evaluations to track changes in MFR and myocardial perfusion over time and correlate that with symptom improvement. The outcomes of these studies are expected to provide deeper insights into this crucial aspect of cardiovascular health.
These interventions primarily focus on addressing the underlying endothelial dysfunction, a pivotal factor in the development of CMD. This approach not only underscores the importance of precise and individualized patient care but also highlights the potential of targeted therapies to significantly impact clinical outcomes in patients with CMD.
Thus, targeting MFR involves using specific pharmacological therapies that can modify endothelial health and function, thereby potentially improving MFR and patient outcomes. This approach is in line with the broader goals of precision medicine, aiming to tailor therapy based on individual physiological characteristics and needs.
3.7. Words of caution
When assessing possible INOCA using cardiac PET, it is imperative to adhere to well-established protocols to ensure accuracy and reproducibility of the results. Several key considerations should be meticulously addressed to guarantee the quality of the assessment.
First, the proper timing of radiotracer injection is paramount. Precise adherence to timing protocols ensures that the assessment captures the desired physiological responses accurately. Any deviation from these protocols may compromise the validity of the findings [32].
Furthermore, technical nuances must be considered, as they can lead to abnormal measurements of MBF. For instance, the ingestion of caffeine prior to the study can significantly affect MBF and should not be misinterpreted as indicative of INOCA. It is essential to be aware of such confounding factors to avoid misdiagnosis. Splenic switch-off has emerged as a potential tool for confirming an adequate response to vasodilator stress, further enhancing the reliability of the assessment. [33]
Additionally, elevated resting blood flow, often associated with high resting blood pressure, can obscure the detection of reduced flow reserve. While this has prognostic significance, it can affect the diagnostic accuracy of PET myocardial blood flow. [32] Therefore, a comprehensive evaluation, including both hyperemic flow and flow reserve, is essential for precise INOCA assessment.
Finally, motion artifacts can also make blood flow measurements inaccurate and appropriate motion correction is often needed to ensure that the MFR measurement is accurate. [34,35]
However, it is also crucial to keep in mind that reduced MFR is not exclusive to INOCA and can also occur in patients with diffuse obstructive epicardial disease. [36] Consequently, ruling out obstructive epicardial coronary artery disease is imperative before establishing a definitive INOCA diagnosis. [14]
4. Conclusion
In summary, cardiac PET plays a significant role in risk stratification and management of INOCA patients. PET evaluates various parameters like myocardial blood flow under stress and rest, myocardial flow reserve, and myocardial ischemia. Such comprehensive assessment is essential in accurately diagnosing and managing INOCA, predicting outcomes and following responses to therapy considering the complexity of this condition. By shedding light on the underlying pathophysiology, stratifying risk, and potentially guiding treatment decisions, PET MPI has the potential to significantly improve patient outcomes and quality of life for this unique and often perplexing population. However, further research and ongoing dialogue are crucial to optimize the role of PET imaging in INOCA management and ensure accurate diagnosis, effective risk stratification, and ultimately, improved clinical outcomes for these patients.
Source of funding
None.
Disclosure
Dr. Al-mallah receives research support from Siemens, unrelated to this work. He is also a consultant to Genral Electric and Jublant. All other authors declare no conflicts of interest.
CRediT authorship contribution statement
Mouaz H. Al-Mallah: Writing – original draft, Supervision, Conceptualization. Malek Nayfeh: Writing – review & editing. Mahmoud Alrifai: Writing – review & editing.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Mouaz Al-Mallah reports a relationship with Siemens that includes: research grants. He is a consultant to General Electric and Jubilant. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
None.
References
- 1.Sanghani R., Al-Mallah M.H., Thompson R. Challenges and strategies to enable access to cardiac positron emission tomography in different parts of the world: the north american perspective. J. Nucl. Cardiol. 2024;31 doi: 10.1016/j.nuclcard.2023.101790. [DOI] [PubMed] [Google Scholar]
- 2.Herscovici R., Sedlak T., Wei J., Pepine C.J., Handberg E., Merz C.N.B. Ischemia and no obstructive coronary artery disease (inoca): what is the risk? J. Am. Heart Assoc. 2018;7 doi: 10.1161/JAHA.118.008868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bairey Merz C.N., Pepine C.J., Walsh M.N., Fleg J.L., Camici P.G., Chilian W.M., et al. Ischemia and no obstructive coronary artery disease (inoca) developing evidence-based therapies and research agenda for the next decade. Circulation. 2017;135:1075–1092. doi: 10.1161/CIRCULATIONAHA.116.024534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kunadian V., Chieffo A., Camici P.G., Berry C., Escaned J., Maas A., et al. An eapci expert consensus document on ischaemia with non-obstructive coronary arteries in collaboration with european society of cardiology working group on coronary pathophysiology & microcirculation endorsed by coronary vasomotor disorders international study group. EuroIntervention. 2021;16:1049–1069. doi: 10.4244/EIJY20M07_01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pepine C.J. Anoca/inoca/minoca: open artery ischemia. Am. Heart J. Plus. 2023:26. doi: 10.1016/j.ahjo.2023.100260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seitz A., Feenstra R., Konst R.E., Martínez Pereyra V., Beck S., Beijk M., et al. Acetylcholine rechallenge: a first step toward tailored treatment in patients with coronary artery spasm. JACC Cardiovasc. Interv. 2022;15:65–75. doi: 10.1016/j.jcin.2021.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Pepine C.J., Anderson R.D., Sharaf B.L., Reis S.E., Smith K.M., Handberg E.M., et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the national heart, lung and blood institute wise (women’s ischemia syndrome evaluation) study. J. Am. Coll. Cardiol. 2010;55:2825–2832. doi: 10.1016/j.jacc.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aldiwani H., Zaya M., Suppogu N., Quesada O., Johnson B.D., Mehta P.K., et al. Angina hospitalization rates in women with signs and symptoms of ischemia but no obstructive coronary artery disease: a report from the wise (women’s ischemia syndrome evaluation) study. J. Am. Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.013168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gulati M., Levy P.D., Mukherjee D., Amsterdam E., Bhatt D.L., Birtcher K.K., et al. 2021 aha/acc/ase/chest/saem/scct/scmr guideline for the evaluation and diagnosis of chest pain: a report of the american college of cardiology/american heart association joint committee on clinical practice guidelines. Circulation. 2021;144:e368–e454. doi: 10.1161/CIR.0000000000001029. [DOI] [PubMed] [Google Scholar]
- 10.Reeves R.A., Halpern E.J., Rao V.M. Cardiac imaging trends from 2010 to 2019 in the medicare population. Radiology: Cardiothoracic Imaging. 2021;3 doi: 10.1148/ryct.2021210156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Mallah M.H., Sitek A., Moore S.C., Di Carli M., Dorbala S. Assessment of myocardial perfusion and function with pet and pet/ct. J. Nucl. Cardiol. 2010;17:498–513. doi: 10.1007/s12350-010-9223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chareonthaitawee P., Bateman T.M., Beanlands R.S., Berman D.S., Calnon D.A., Di Carli M.F., et al. Atlas for reporting pet myocardial perfusion imaging and myocardial blood flow in clinical practice: an information statement from the american society of nuclear cardiology. J. Nucl. Cardiol. 2023;30:2850–2906. doi: 10.1007/s12350-023-03378-1. [DOI] [PubMed] [Google Scholar]
- 13.El-Tallawi K.C., Aljizeeri A., Nabi F., Al-Mallah M.H. Myocardial perfusion imaging using positron emission tomography. Methodist Debakey Cardiovasc. J. 2020;16:114–121. doi: 10.14797/mdcj-16-2-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aljizeeri A., Ahmed A.I., Alfaris M.A., Ahmed D., Farea J., Elneama A., et al. Myocardial flow reserve and coronary calcification in prognosis of patients with suspected coronary artery disease. JACC Cardiovasc. Imaging. 2021;14:2443–2452. doi: 10.1016/j.jcmg.2021.01.024. [DOI] [PubMed] [Google Scholar]
- 15.Maaniitty T., Knuuti J., Saraste A. 15o-water pet mpi: current status and future perspectives. Semin. Nucl. Med. 2020;50:238–247. doi: 10.1053/j.semnuclmed.2020.02.011. [DOI] [PubMed] [Google Scholar]
- 16.Maddahi J., Lazewatsky J., Udelson J.E., Berman D.S., Beanlands R.S.B., Heller G.V., et al. Phase-iii clinical trial of fluorine-18 flurpiridaz positron emission tomography for evaluation of coronary artery disease. J. Am. Coll. Cardiol. 2020;76:391–401. doi: 10.1016/j.jacc.2020.05.063. [DOI] [PubMed] [Google Scholar]
- 17.Bourque J.M., Hanson C.A., Agostini D., Bateman T.M., Bax J.J., Beanlands R.S.B., et al. Assessing myocardial perfusion in suspected coronary artery disease: rationale and design of the second phase 3, open-label multi-center study of flurpiridaz (f-18) injection for positron emission tomography (pet) imaging. J. Nucl. Cardiol. 2021;28:1105–1116. doi: 10.1007/s12350-021-02527-8. [DOI] [PubMed] [Google Scholar]
- 18.Packard R.R.S., Cooke C.D., Van Train K.F., Votaw J.R., Sayre J.W., Lazewatsky J.L., et al. Development, diagnostic performance, and interobserver agreement of a (18)f-flurpiridaz pet automated perfusion quantitation system. J. Nucl. Cardiol. 2022;29:698–708. doi: 10.1007/s12350-020-02335-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aljizeeri A., Badarin F.A., Al-Mallah M.H. Automation in nuclear cardiology: time for flurpiridaz to join the club. J. Nucl. Cardiol. 2022;29:709–711. doi: 10.1007/s12350-020-02421-9. [DOI] [PubMed] [Google Scholar]
- 20.Poitrasson-Rivière A., Moody J.B., Renaud J.M., Hagio T., Arida-Moody L., Buckley C., et al. Impact of residual subtraction on myocardial blood flow and reserve estimates from rapid dynamic pet protocols. J. Nucl. Cardiol. 2022;29:2262–2270. doi: 10.1007/s12350-021-02837-x. [DOI] [PubMed] [Google Scholar]
- 21.Danad I., Raijmakers P.G., Driessen R.S., Leipsic J., Raju R., Naoum C., et al. Comparison of coronary ct angiography, spect, pet, and hybrid imaging for diagnosis of ischemic heart disease determined by fractional flow reserve. JAMA Cardiol. 2017;2:1100–1107. doi: 10.1001/jamacardio.2017.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dewey M., Siebes M., Kachelrieß M., Kofoed K.F., Maurovich-Horvat P., Nikolaou K., et al. Clinical quantitative cardiac imaging for the assessment of myocardial ischaemia. Nat. Rev. Cardiol. 2020;17:427–450. doi: 10.1038/s41569-020-0341-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sampson U.K., Dorbala S., Limaye A., Kwong R., Di Carli M.F. Diagnostic accuracy of rubidium-82 myocardial perfusion imaging with hybrid positron emission tomography/computed tomography in the detection of coronary artery disease. J. Am. Coll. Cardiol. 2007;49:1052–1058. doi: 10.1016/j.jacc.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed A.I., Saad J.M., Alahdab F., Han Y., Nayfeh M., Alfawara M.S., et al. Prognostic value of positron emission tomography derived myocardial flow reserve: a systematic review and meta-analysis. Atherosclerosis. 2023;382 doi: 10.1016/j.atherosclerosis.2023.117280. [DOI] [PubMed] [Google Scholar]
- 25.Aljizeeri A., Ahmed A.I., Suliman I., Alfaris M.A., Elneama A., Al-Mallah M.H. Incremental prognostic value of positron emission tomography-derived myocardial flow reserve in patients with and without diabetes mellitus. Eur. Heart J. Cardiovasc. Imaging. 2023;24:563–571. doi: 10.1093/ehjci/jead023. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed A.I., Saad J.M., Han Y., Alahdab F., Malahfji M., Nabi F., et al. Coronary microvascular health in patients with prior covid-19 infection. JACC Cardiovasc. Imaging. 2022;15:2153–2155. doi: 10.1016/j.jcmg.2022.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmed A.I., Al Rifai M., Alahdab F., Saad J.M., Han Y., Alfawara M.S., et al. Coronary microvascular health in symptomatic patients with prior covid-19 infection: an updated analysis. Eur. Heart J. Cardiovasc. Imaging. 2023;24:1544–1554. doi: 10.1093/ehjci/jead118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malahfji M., Crudo V., Ahmed A.I., Saeed M., Saad J.M., Zoghbi W.A., et al. Coronary microvascular dysfunction and covid-19: implications for long covid patients. J. Nucl. Cardiol. 2023;30:2204–2206. doi: 10.1007/s12350-022-03073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Mallah M.H., Bateman T.M., Branch K.R., Crean A., Gingold E.L., Thompson R.C., et al. 2022 asnc/aapm/scct/snmmi guideline for the use of ct in hybrid nuclear/ct cardiac imaging. J. Nucl. Cardiol. 2022;29:3491–3535. doi: 10.1007/s12350-022-03089-z. [DOI] [PubMed] [Google Scholar]
- 30.Murthy V.L., Naya M., Taqueti V.R., Foster C.R., Gaber M., Hainer J., et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation. 2014;129:2518–2527. doi: 10.1161/CIRCULATIONAHA.113.008507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schindler T.H., Fearon W.F., Pelletier-Galarneau M., Ambrosio G., Sechtem U., Ruddy T.D., et al. Myocardial perfusion pet for the detection and reporting of coronary microvascular dysfunction. JACC Cardiovasc. Imaging. 2023;16:536–548. doi: 10.1016/j.jcmg.2022.12.015. [DOI] [PubMed] [Google Scholar]
- 32.Alnabelsi T., Thakkar A., Ahmed A.I., Han Y., Al-Mallah M.H. Pet/ct myocardial perfusion imaging acquisition and processing: ten tips and tricks to help you succeed. Curr. Cardiol. Rep. 2021;23:39. doi: 10.1007/s11886-021-01476-5. [DOI] [PubMed] [Google Scholar]
- 33.Saad J.M., Ahmed A.I., Han Y., El Nihum L.I., Alahdab F., Nabi F., et al. Splenic switch-off in regadenoson (82)rb-pet myocardial perfusion imaging: assessment of clinical utility. J. Nucl. Cardiol. 2023;30:1484–1496. doi: 10.1007/s12350-022-03158-3. [DOI] [PubMed] [Google Scholar]
- 34.Han Y., Ahmed A.I., Hayden C., Jung A.K., Saad J.M., Spottiswoode B., et al. Change in positron emission tomography perfusion imaging quality with a data-driven motion correction algorithm. J. Nucl. Cardiol. 2022;29:3426–3431. doi: 10.1007/s12350-021-02902-5. [DOI] [PubMed] [Google Scholar]
- 35.Han Y., Ahmed A.I., Saad J.M., Alahdab F., Al Rifai M.S., Murthy V.L., et al. Ejection fraction and ventricular volumes on rubidium positron emission tomography: prospective validation against cardiovascular magnetic resonance. J. Nucl. Cardiol. 2024;101810 doi: 10.1016/j.nuclcard.2024.101810. [DOI] [PubMed] [Google Scholar]
- 36.Alahdab F., Al Rifai M., Ahmed A.I., Al-Mallah M.H. Advances in digital pet technology and its potential impact on myocardial perfusion and blood flow quantification. Curr. Cardiol. Rep. 2023;25:261–268. doi: 10.1007/s11886-023-01850-5. [DOI] [PubMed] [Google Scholar]