Summary
Background
Understanding the impact of CYP2D6 metabolism on paroxetine, a widely used antidepressant, is essential for precision dosing.
Methods
We conducted an 8-week, multi-center, single-drug, 2-week wash period prospective cohort study in 921 Chinese Han patients with depressive or anxiety disorders (ChiCTR2000038462). We performed CYP2D6 genotyping (single nucleotide variant and copy number variant) to derive the CYP2D6 activity score and evaluated paroxetine treatment outcomes including steady-state concentration, treatment efficacy, and adverse reaction. CYP2D6 metabolizer status was categorized into poor metabolizers (PMs), intermediate metabolizers (IMs), extensive metabolizers (EMs), and ultrarapid metabolizers (UMs). The influence of CYP2D6 metabolic phenotype on paroxetine treatment outcomes was examined using multiple regression analysis and cross-ethnic meta-analysis. The therapeutic reference range of paroxetine was estimated by receiver operating characteristic (ROC) analyses.
Findings
After adjusting for demographic factors, the steady-state concentrations of paroxetine in PMs, IMs, and UMs were 2.50, 1.12, and 0.39 times that of EMs, with PM and UM effects being statistically significant (multiple linear regression, P = 0.03 and P = 0.04). Sex and ethnicity influenced the comparison between IMs and EMs. Moreover, poor efficacy of paroxetine was associated with UM, and a higher risk of developing adverse reactions was associated with lower CYP2D6 activity score. Lastly, cross-ethnic meta-analysis suggested dose adjustments for PMs, IMs, EMs, and UMs in the East Asian population to be 35%, 40%, 143%, and 241% of the manufacturer's recommended dose, and 62%, 68%, 131%, and 159% in the non-East Asian population.
Interpretation
Our findings advocate for precision dosing based on the CYP2D6 metabolic phenotype, with sex and ethnicity being crucial considerations in this approach.
Funding
National Natural Science Foundation of China; Academy of Medical Sciences Research Unit.
Keywords: CYP2D6, Copy number variant, Metabolizer status, Paroxetine, Dose adjustment, Precision medicine
Research in context.
Evidence before this study
We searched the PubMed database using the terms “(cytochrome P450 2D6 or CYP2D6)” AND “paroxetine” and found 40 studies related to paroxetine out of 359 articles. These studies showed that: 1) the activity scoring system provided a more detailed classification for CYP2D6 metabolizer status compared to the traditional standard; 2) CYP2D6 metabolizer status or CYP2D6 genotype have impacts on pharmacokinetic parameters (e.g., steady-state concentration, AUC, clearance, and half-life) of paroxetine, different CYP2D6 metabolizer statuses may require different dose adjustments for paroxetine; 3) CYP2D6 metabolizer status may phenoconvert after administered paroxetine in a subset of individuals; 4) The relationship between CYP2D6 metabolizer status and treatment efficacy or adverse reaction of paroxetine remains unclear. In particular, CYP2D6 ultrarapid metabolizers may not respond to paroxetine. Two studies found a higher incidence of paroxetine-induced sexual dysfunction in female CYP2D6 poor metabolizers, compared to female CYP2D6 extensive metabolizers. However, other studies showed no significant difference of adverse reaction across CYP2D6 metabolizer statuses. The results of the search indicate a lack of relevant discussions on paroxetine metabolism under different CYP2D6 metabolizer status in the Chinese Han population.
Added value of this study
To our knowledge, this study represents the largest, single-drug, prospective cohort study on precision dosing of paroxetine in patients with depressive or anxiety disorders. The main finding was the association of CYP2D6 metabolic phenotype with paroxetine exposure, efficacy, and adverse reaction, while the CYP2D6 copy number variant was associated with paroxetine Css and efficacy. Moreover, we found that ethnicity and sex influenced the comparison between CYP2D6 intermediate metabolizers and extensive metabolizers, which lack exploration in previous studies and meta-analysis. Compared to other ethnicities, greater dose adjustment was recommended for East Asians based on CYP2D6 metabolizer status. Lastly, the therapeutic reference range for paroxetine was determined to be 31.95–79.65 ng/ml by considering both its efficacy and adverse reactions.
Implications of all the available evidence
Our findings and previous research suggest that it is practical to infer metabolic phenotype from the CYP2D6 genotype. CYP2D6 metabolizer phenotype affects paroxetine exposure and clinical outcomes, influenced by sex and ethnicity. Dose adjustment is recommended based on CYP2D6 metabolizer status to guide precision dosing of paroxetine, with sex and ethnicity being crucial considerations in this approach.
Introduction
Antidepressants are the critical treatment for depressive and anxiety disorders, which affect social function and quality of life.1,2 However, patients considerably vary in their response to antidepressants,3,4 and require multiple medication changes to find the optimal prescription, which is a notable clinical challenge. Precision medicine may optimize antidepressant selection and dosage based on patient features, such as genotype,5 to enhance outcomes and reduce adverse drug reactions (ADRs).
Paroxetine, a selective serotonin reuptake inhibitor (SSRI) antidepressant, has good efficacy but moderate tolerability.6 Paroxetine is mainly metabolized by the CYP2D6 enzyme into inactive metabolites. Low paroxetine concentrations reduce the likelihood of benefit, while high paroxetine concentrations raise the possibility of ADRs.7 Monitoring the paroxetine concentration and using its therapeutic reference range (TRR) may help adjust dosing and increase patient compliance and satisfaction.8
The CYP2D6 gene has extensive single nucleotide variants (star alleles) and copy number variants (CNVs), causing large variations in CYP2D6 enzyme activity. Therefore, deriving activity score (AS) based on CYP2D6 genotype is a validated method for predicting metabolizer status. The Clinical Pharmacogenetics Implementation Consortium (CPIC) and the Dutch Pharmacogenetics Working Group (DPWG) developed a consensus AS-inferred standard.9 However, more clinical research is needed to validate these standards. Also, previous studies mainly studied star alleles,10 ignoring CNV, which affects CYP2D6 enzyme activity.
Paroxetine concentration depends on many factors, especially CYP2D6 metabolizer status.11 Therefore, the CPIC guideline suggested dose adjustment of paroxetine according to CYP2D6 metabolizer status.7 It indicated that ultrarapid metabolizers (UMs) should choose antidepressants that CYP2D6 does not mainly metabolize and that intermediate metabolizers (IMs) and poor metabolizers (PMs) should consider a lower starting dose and slower titration schedule than extensive metabolizers (EMs). However, the DPWG guideline recommended no action for these gene–drug interactions (IMs and PMs) due to a lack of clinical effects.12 However, these guidelines focus mainly on European populations, while East Asians tend to have a higher prevalence of CYP2D6 genetic variants associated with reduced enzyme activity.13 Further clinical research is needed to support these recommendations, especially for East Asians.
Therefore, this study aims to improve precision dosing of paroxetine, especially in the Chinese Han population. We conducted a prospective cohort study with objectives: (1) investigating the effect of CYP2D6 metabolic phenotype, (2) and the effect of CYP2D6-CNV on paroxetine concentration and treatment efficacy and adverse reaction; (3) determining the TRR of paroxetine. We conducted meta-analyses to validate and expand our findings and performed pooled analyses to recommend dose adjustment for different CYP2D6 metabolizer statuses.
Methods
Study design and participants of the PMEDA study
This trial was an 8-week, multi-centre, single-drug prospective cohort study evaluating the effects of CYP2D6 genotype and phenotype on paroxetine outcomes in patients with major depressive disorder (MDD), generalized anxiety disorder (GAD), or panic disorder (PD) from the Precision Medicine to Enhance Depression and Anxiety Outcome (PMEDA) consortium. The consortium was established in 2021 with members from seventeen Chinese hospitals. Supplement 1 detailed the setting, variables, and bias of the PMEDA study. Supplement 2 provides the protocol of the PMEDA study.
A priori sample size calculation was based on expected small effect size (f2 = 0.015) for increase of explained variance of three additional predictors (dummy variables for CYP2D6 metabolizer status) in the multiple linear regression model, which includes five predefined demographic covariates. A target sample size of 822 was able to achieve an 85% power at a significance (alpha) level of 0.05. Adjusting for approximately 20–30% dropout results in a target sample size of about 1100 participants.
Psychiatric clinicians diagnosed patients using the Structured Clinical Interview of Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Included patients had a diagnosis of MDD, GAD, or PD, were aged 18–65 years, were of Han Chinese ancestry, had no systemic antidepressant treatment or use of CYP2D6-inducing or -inhibiting drugs within two weeks before enrollment, had no language barriers, and could cooperate with assessment and treatment. Patients with MDD scored ≥17 on 17-item Hamilton Depression Scale (HAMD-17) and ≤13 on Hypomania Checklist-32 (HCL-32). Patients with GAD scored ≥14 on Hamilton Anxiety Scale (HAMA). Patients with PD scored ≥7 on Panic Disorder Severity Scale (PDSS).14 Patients who were either first-episode or relapsed with discontinuing antidepressant treatment for over two weeks were enrolled.
We excluded patients with other mental disorders, pregnancy or lactation, severe suicidal tendencies or harm to others, severe or unstable physical illnesses, secondary depressive and anxiety disorders due to endocrine disease, epilepsy, Parkinson's disease, Huntington's disease and traumatic brain injury, participation in another trial, or unwillingness or inability to complete this trial. Moreover, patients who took less than 80% of the prescribed medication were considered to have poor adherence and excluded.
Patients who met any exclusion criteria, had intolerable ADRs, or requested to withdraw were withdrawn from the study. Doctors from the PMEDA consortium provided a final assessment and developed alternative treatment plans. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Intervention and procedure
After baseline assessments and laboratory tests, patients received paroxetine hydrochloride tablets (Seroxat, immediate release formulation) at 10 mg/d monotherapy in the first week and 20–40 mg/d after one week. Adjunctive medicine that does not undergo CYP2D6 metabolism could be used if necessary. Doctors followed up on patients at four and eight weeks, including clinical scale assessments and laboratory tests. Based on DrugBank V5.0 (https://go.drugbank.com/),15 drugs that inhibit or increase the biosynthesis or actions of the CYP2D6 enzyme, referred to as CYP2D6-inhibiting or inducing drugs, were prohibited for two-weeks before enrollment and during the study. Physical therapy was restricted during the study.
Primary outcome measurement
The primary outcome was the steady-state concentration (Css) of paroxetine in plasma. Blood samples were collected from patients at the 4-week treatment endpoint, ensuring stable daily dosing for at least ten days. Samples were collected immediately before ingesting the morning dose, 20–24 h after the last medication. The dose of paroxetine at blood collection was recorded. Dried blood spots of plasma and whole blood were made and transported to the centralized testing laboratory. We detected paroxetine concentrations in plasma using liquid chromatography-tandem mass spectrometry (LC-MS/MS, AB5500, AB Sciex).16 The lower limit of quantitation (LLOQ) for this method was 0.1 ng/ml, while the limit of detection (LOD) was 2 ng/ml. Detailed laboratory methods are in Supplement 1.
Secondary outcome measurement
The secondary outcomes were paroxetine efficacy and ADR status. Treatment efficacy was quantified as the percentage improvement in symptom severity from baseline at 4-week and 8-week follow-up endpoints. Symptom severity was measured using different scales: HAMD for patients with MDD, HAMA for those with GAD, and PDSS for those with PD.17, 18, 19 The formula for percentage improvement is:
ADR was evaluated by the Treatment Emergent Symptom Scale (TESS)20 with laboratory tests. Patients with ADR had at least one item on the TESS with a score of more than two points, which indicated that ADR could be directly observed in these patients, with some functional impact. The patients felt uncomfortable due to the ADR, but it did not seriously impact their lives (three points) or severely affect their daily lives (four points). Efficacy and ADR rater were blinded to the results of CYP2D6 metabolic phenotype and paroxetine concentration.
CYP2D6 genotyping
Genomic DNA was extracted from dried blood spots using the Mag-MK Blood Spot DNA Extraction Kit (QIAGEN, Hilden, Germany). The rationale of CYP2D6 variant genotyping was based on previous pharmacogenomics studies21,22 and tailored by excluding ∗2 and ∗2A due to their lack of functional impact on CYP2D6 enzyme, and ∗9 due to its rarity in the Chinese population.23, 24, 25 Meanwhile, we included ∗14A, which is classified as a no function variant.26 Therefore, we genotyped CYP2D6 ∗3, ∗4, ∗5, ∗6, ∗7, ∗8, ∗10, ∗11, ∗12, ∗14A, ∗14B, ∗15, ∗17, and ∗41 alleles using a nucleotide Matrix-Assisted Laser Desorption/Ionization-Time of Flight Mass Spectrometry (MALDI-TOF MS) assay (Shanghai Conlight Medical Laboratory Co., Ltd.). Patients without the above variation were assumed to carry CYP2D6∗1.
We used the TaqMan real-time qPCR reaction to identify the copy number of the CYP2D6 gene. We used an Applied Biosystems 7500 instrument (Thermo Fisher, Waltham, USA) under the standard conditions: 50 °C for 2 min, 95 °C for 10 min, followed by 40 cycles at 95 °C for 15s and 60 °C for 60s. The copy number of CYP2D6 was calculated using ΔΔCt relative quantitative method with CopyCaller V2.3.1 software (Thermo Fisher, Waltham, USA).27 Then, we combined the data for zero and one copy of CYP2D6 as CYP2D6-CNV-deletion and more than two copies as CYP2D6-CNV-duplication. Two copies were in the subgroup without CYP2D6-CNV. Detailed laboratory methods are in Supplement 1.
Conversion from CYP2D6 genotype to phenotype
We identified alleles and CNVs for CYP2D6 gene using the above molecular platforms, combined allele sequencing and copy number results to assemble haplotypes, and then empirically assigned diplotypes. Each allele was assigned an AS value, and the final AS was the sum of the individual values for each subject. The CYP2D6 AS was used to infer CYP2D6 metabolizer status according to the latest standard.9 Patients were categorized as UMs (AS >2.25), EMs (1.25 ≤ AS ≤2.25), IMs (0 < AS<1.25), or PMs (AS = 0). We used numerical (AS) and categorical (metabolizer status) variables to assess the CYP2D6 metabolic phenotype, which reflects CYP2D6 enzyme activity.
Statistics
To handle the missing data in our multivariable analysis, we employed a complete-case analysis approach. Due to the skewed distribution of paroxetine Css, we performed a natural log-transformation for further parameter testing.28 In descriptive analysis, we performed a one-way ANOVA to test for significance among the CYP2D6 metabolizer statuses.
For the primary analysis, referred to as adjusted model one, we employed multiple linear regression to explore the independent effect of CYP2D6 metabolic phenotype on paroxetine Css and efficacy, and multiple logistic regression to investigate its independent effect on paroxetine ADR status, both controlling for predefined covariates. Predefined covariates for paroxetine Css are age, sex, BMI, smoking habit and drinking habit, and predefined covariates for paroxetine efficacy and ADR are age and sex.
For the post-hoc sensitivity analysis, referred to as adjusted model two, we incorporated additional clinical covariates that have been reported to be associated with the outcome. Daily dose was included as covariates in the analysis of paroxetine Css, efficacy, and ADR status, since previous studies indicated its association with these outcomes.29, 30, 31 Current episode duration, baseline symptom severity, and adjunctive medication status were included as covariates in the analysis of paroxetine efficacy.31,32 Furthermore, first-episode or not was taken into account in the analysis of paroxetine ADR status.33
Given that same measurement for paroxetine Css and ADR status was performed across different disease groups, data from three psychiatric disorders was combined in their analysis. Since efficacy was measured by different scales, we first conducted the linear regression analysis for each disease group. Then the effect of the CYP2D6 metabolic phenotype on paroxetine efficacy across different disease groups was represented by pooled standardized coefficients, calculated using random-effect meta-analyses.
In the exploratory analysis, we flexibly modelled and visualized the associations of CYP2D6 AS with paroxetine Css and efficacy using restricted cubic spline (RCS) linear regression, and the association between CYP2D6 AS and ADR status using RCS logistic regression, implemented with R package “rms”. To balance best fit and overfitting of splines, we selected the number of knots (between three and seven) based on the lowest Akaike information criterion,34 and assessed nonlinearity using the Wald test. Since the association of CYP2D6 AS and ADR status appeared approximately linear below and above the value of CYP2D6 AS associated with the lowest ADR risk (corresponding to the value with the lowest odds of developing ADR on the spline curve34), we additionally used a multiple linear model to calculate odds ratios per standard deviation increase in CYP2D6 AS.35 Covariates were the same as those involved in the main analysis.
All statistical analyses were performed using R software version 4.2.3, the significance level was set at a two-side P-value of <0.05.
Therapeutic reference range
The TRR is defined as the range of paroxetine Css in plasma associated with therapeutic outcome, with a lower limit below which response is unlikely and an upper limit above which tolerability decreases. Treatment response was defined as a binary variable, responder and non-responder. MDD patients with ≥50% improvement in HAMD-17 from baseline at follow-up to the end of follow-up period was defined as responder, other was defined as non-responder.19 Similarly, GAD patients with ≥50% improvement in HAMA was responder,18 PD patients with ≥50% improvement in PDSS was responder.17,36 To estimate the TRR, we conducted receiver operating characteristic (ROC) analyses to identify the cut-off value that separated responders from non-responders or patients with or without ADR. We used the Youden index to determine the best cut-off value. To correspond the measured time of paroxetine concentration and therapeutic outcome, we used 4-weekend measurement to perform ROC analysis. When we subset patients using the best cut-off, and the subgroups have significantly different response rates or ADR frequency, we suggest the cut-off could be the limit of TRR.
Meta-analysis and dose adjustment
To validate and expand the findings in our cohort and compute quantitative dose adjustments for paroxetine, we followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to perform a systematic review and meta-analysis with our cohort, registered at PROSPERO (CRD42023430543). We searched PubMed, the Cochrane Library, Embase, and the Web of Science databases using free text and controlled terms to identify relevant literature. The search strategy and selection criteria were listed in Supplement 1 (Supplemental Methods and Table S1). Two authors screened literature and extracted data, conducting a quality assessment using strengthening the Reporting of Genetic Association Studies (STREGA) guidelines37 and Cochrane's risk of bias tool.
Considering the influence of dose on Css, we used the ratio of means (RoM) to compare paroxetine Css of different CYP2D6 metabolizer statuses, which made it possible to compare results from studies with different doses.38 To enrich pharmacokinetic parameters, we also compared the area under the concentration–time curve (AUC) and peak concentration (Cmax) among metabolizer statuses. We conducted a random-effects meta-analysis for studies with at least three participants in a metabolizer status subgroup and assessed heterogeneity using I2 statistics. Pre-planned subgroup analyses stratified by ethnicity were conducted. Potential bias was evaluated using funnel plots since the number of included studies was insufficient. Furthermore, we determined the metabolizer status-related Css range by combining Css of each metabolizer status from the included studies. All analyses were conducted with RevMan 5.4, and two-sided P-values <0.05 were considered statistically significant.
Dose adjusted ratios were calculated according to previous studies39,40 and Supplement 1 offers details on the calculation process. This approach was based on differences in pharmacokinetic data (i.e., Css, Cmax, and AUC) between EMs and the other three metabolizers. The pooled estimate of dose-adjusted ratios for paroxetine was computed as the mean of study that exhibited statistically significant differences in pharmacokinetic data between EMs and other three metabolizers.
Ethics
The study was approved by the ethics committee of the Peking University Sixth Hospital and each participating site. The reference number was 2020-LUNSHEN-49 and approval date was September 15, 2020. This study was registered at the Chinese Clinical Trial Registry (ChiCTR2000038462). The patient enrollment period was between March 2021 and April 2023 and informed written consent was obtained for all included subjects in accordance with the Declaration of Helsinki.
Role of funders
All funders had no role in the study design, data collection, analysis, interpretation, decision to submit the paper for publication, or report writing.
Results
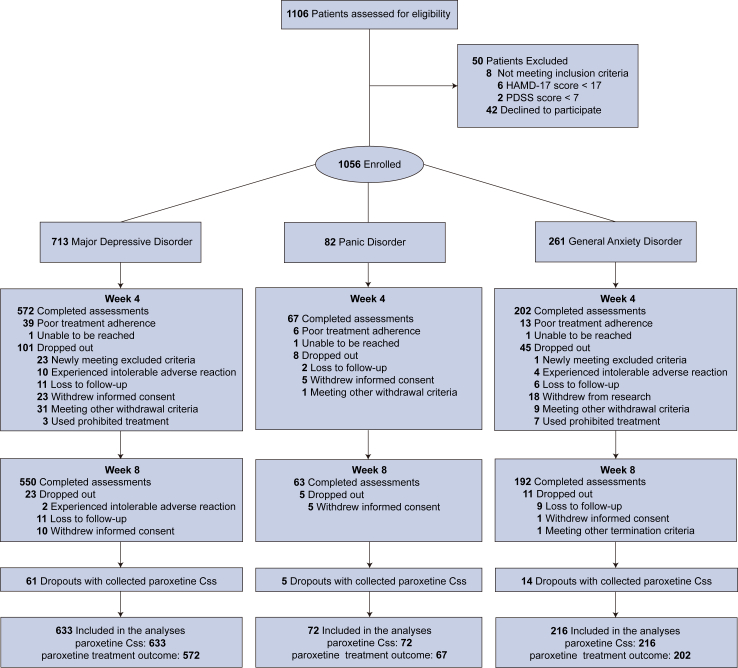
In the PMEDA study, 1056 patients were enrolled. Of these, 58 patients with poor adherence were excluded. Before the 4-week follow-up, 154 (14.58%) dropped out. The average follow-up time was 7.5 weeks. Among those who met the withdrawal criteria, 80 patients without prohibited treatment agreed to have blood samples collected at termination (>10 days follow-up), including four patients with intolerable ADR. Their paroxetine Css and CYP2D6 genotype data were analysed. However, since they discontinued the treatment before the completion of the 4-week period, their efficacy and ADR data for the 4-week and 8-week follow-up periods were not available.
For the primary outcome, we combined data from three psychiatric disorders, yielding a total of 921 patients that were available for the primary outcome (Fig. 1). The four CYP2D6 metabolizer statuses were comparable regarding baseline characteristics except for drinking and smoking habits (Table 1), which were used as covariates. The frequency of CYP2D6 diplotype, the distribution of raw-form and log-transform paroxetine Css are in Supplemental Figure S1. For the secondary outcome, we analysed data from 841 patients at the 4-week follow-up. Of these, 805 also had available data at the 8-week follow-up (Fig. 1). Efficacy data was shown in Table 2.
Fig. 1.
Flow chart of the prospective cohort study from the precision medicine to enhance depression and anxiety outcome (PMEDA) consortium.
Table 1.
Demographic and clinical characteristics of the prospective cohort.
| PM |
IM |
EM |
UM |
Test statistic | |
|---|---|---|---|---|---|
| (N = 6) | (N = 379) | (N = 531) | (N = 5) | ||
| Age, year, mean (sd) | 41.8 (19.2) | 40.9 (15.5) | 41.8 (15.3) | 34.4 (14.0) | F3917 = 0.63, P = 0.59a |
| Female, n (%) | 4 (66.7) | 254 (67.0) | 380 (71.6) | 3 (60.0) | = 2.40, P = 0.49b |
| Weight, kg, mean (sd) | 64.5 (7.1) | 62.0 (9.5) | 61.6 (8.7) | 56.6 (3.8) | F3910 = 0.89, P = 0.45a |
| BMI, kg/m2, mean (sd) | 22.9 (2.1) | 22.5 (3.0) | 22.4 (2.8) | 20.6 (2.1) | F3910 = 0.80, P = 0.49a |
| Marriage, n (%) | = 6.37, P = 0.70b | ||||
| Married | 4 (66.7%) | 252 (67.0%) | 378 (71.7%) | 2 (40.0%) | |
| Unmarried | 2 (33.3%) | 111 (29.5%) | 128 (24.3%) | 3 (60.0%) | |
| Divorced/separated | 0 (0.0%) | 7 (1.9%) | 11 (2.1%) | 0 (0.0%) | |
| Widowed | 0 (0.0%) | 6 (1.6%) | 10 (1.9%) | 0 (0.0%) | |
| Smoker, n (%) | 1 (16.7%) | 33 (8.8%) | 35 (6.6%) | 2 (40.0%) | = 9.38, P = 0.02b |
| Drinker, n (%) | 0 (0.0%) | 40 (10.6%) | 41 (7.8%) | 2 (40.0%) | = 8.57, P = 0.04b |
| Have family history of psychiatric condition (%) | 1 (16.7%) | 26 (6.9%) | 30 (5.7%) | 0 (0.0%) | = 2.01, P = 0.57b |
| First-episode, n (%) | 4 (66.7%) | 243 (64.8%) | 329 (62.7%) | 4 (80.0%) | = 1.04, P = 0.79b |
| Age of first-episode, year, mean (sd) | 38.5 (17.1) | 37.8 (14.6) | 38.2 (13.9) | 25.0 (19.0) | F3904 = 1.18, P = 0.32a |
| Current episode duration, month, median (IQR) | 1.5 (1.75) | 1.0 (1.0) | 1.0 (1.0) | 1.0 (1.0) | = 4.58, P = 0.21c |
| Drug naïve, n (%) | 4 (66.7%) | 242 (64.4%) | 354 (67.0%) | 5 (100.0%) | = 3.28, P = 0.35b |
| Paroxetine daily dose, mg/d, mean (sd) | 23.3 (5.2) | 22.8 (6.6) | 22.7 (6.7) | 20.0 (0.0) | F3917 = 0.32, P = 0.81a |
| Receiving adjunctive medicine, n (%) | 3 (50.0%) | 267 (70.4%) | 394 (74.2%) | 3 (60.0%) | = 3.48, P = 0.32b |
| Baseline HAMD scored, mean (sd) | 26.3 (9.5) | 22.8 (5.1) | 23.7 (5.6) | 21.5 (4.1) | F3629 = 1.83, P = 0.14a |
| Baseline HAMA scoree, mean (sd) | 18.7 (5.0) | 23.2 (6.0) | 23.6 (6.0) | 17.0 (NA) | F3209 = 1.05, P = 0.37a |
| Baseline PDSS scoref, mean (sd) | NA | 15.1 (3.3) | 15.6 (4.2) | NA | F368 = 0.29, P = 0.59a |
| CYP2D6 activity score, mean (sd) | 0.0 (0.0) | 0.6 (0.2) | 1.5 (0.3) | 4.3 (2.9) | F3917 = 634.08, P < 0.01a |
| CYP2D6 CNV, n (%) | 566.90, P < 0.01b | ||||
| None | 2 (33.3%) | 252 (66.5%) | 524 (98.7%) | 0 (0.0%) | |
| Deletion | 4 (66.7%) | 126 (33.2%) | 0 (0.0%) | 0 (0.0%) | |
| Duplication | 0 (0.0%) | 1 (0.3%) | 7 (1.3%) | 5 (100.0%) | |
| 4-week paroxetine Css, ng/ml, geometric mean (95% CI) | 82.5 (38.5–177.0) | 37.4 (33.8–41.4) | 34.4 (31.3–37.7) | 12.4 (3.32–46.2) | = 9.69, P = 0.02c |
| 4-weekend TESS score, mean (sd) | 2.8 (2.8) | 1.9 (3.0) | 1.7 (2.6) | 3.6 (3.0) | F3801 = 1.35, P = 0.26a |
| 8-weekend TESS score, mean (sd) | 0.8 (1.0) | 0.8 (2.1) | 0.7 (2.0) | 1.0 (2.0) | F3795 = 0.18, P = 0.91a |
| Incidence of ADR, n (%) | 1 (16.7%) | 35 (10.7%) | 33 (7.1%) | 0 (0.0%) | = 4.20, P = 0.24b |
ANOVA Test.
Chi-square Test.
Kruskal–Wallis Test.
Measured in the patients with major depressive disorder.
Measured in the patients with general anxiety disorder.
Measured in the patients with panic disorder. Note: N is the number of non-missing values. Abbreviation: UM, ultrarapid metabolizer; EM, extensive metabolizer; IM, intermediate metabolizer, PM, poor metabolizer; HAMD, 17-item Hamilton Depression Scale; HAMA, Hamilton Anxiety Scale; PDSS, Panic Disorder Severity Scale; Css, steady-state concentration; TESS, Treatment Emergent Symptom Scale; ADR, adverse drug reaction.
Table 2.
Paroxetine treatment efficacy of patients in the PMEDA study.
| MDD | GAD | PD | |
|---|---|---|---|
| Percentage improvement in symptom severity at 4-week, %, mean (sd) | 50.9 (14.4) | 45.4 (18.5) | 45.6 (17.0) |
| Responder at 4-week, n (%) | 350 (61.2%) | 82 (40.6%) | 24 (35.8%) |
| Percentage improvement in symptom severity at 8-week, % | 72.7 (18.1) | 71.9 (21.1) | 70.9 (12.3) |
| Responder at 8-week, n (%) | 487 (88.5%) | 173 (90.1%) | 60 (95.2%) |
Note: Data presents in mean (sd) or sample size (frequency). MDD, major depressive disorder; GAD, general anxiety disorder; PD, panic disorder.
CYP2D6 metabolic phenotype and paroxetine Css in the PMEDA study
We began by examining the effect of CYP2D6 metabolizer status on paroxetine Css (Table 3). In the primary analysis (adjusted model one), after adjusting for age, sex, smoking habit, drinking habit and BMI, we found that the paroxetine Css of PMs, IMs, and UMs were 2.50, 1.12, and 0.39 times that of EMs, respectively, with PM and UM effects being statistically significant (multiple linear regression, exponentiated β = 2.50, 95% CI: 1.08–5.76, P = 0.03; exponentiated β = 0.39, 95% CI: 0.15–0.97, P = 0.04, respectively).
Table 3.
The impact of CYP2D6 metabolic phenotype on log-transformed steady-state concentration.
| Unadjusted model |
Adjusted model onea |
Adjusted model twob |
||||
|---|---|---|---|---|---|---|
| Exponentiated β (95% CI) | P | Exponentiated β (95% CI) | P | Exponentiated β (95% CI) | P | |
| Whole sample | ||||||
| CYP2D6 PM | 2.40 (1.03, 5.59) | 0.04 | 2.50 (1.08, 5.76) | 0.03 | 2.40 (1.09, 5.29) | 0.03 |
| CYP2D6 IM | 1.09 (0.95, 1.25) | 0.23 | 1.12 (0.98, 1.28) | 0.11 | 1.12 (0.98, 1.27) | 0.10 |
| CYP2D6 UM | 0.36 (0.14, 0.91) | 0.03 | 0.39 (0.15, 0.97) | 0.04 | 0.43 (0.18, 1.02) | 0.06 |
| Activity score | 0.91 (0.82, 1.02) | 0.10 | 0.90 (0.81, 0.999) | 0.048 | 0.91 (0.82, 1.01) | 0.07 |
| Female | ||||||
| CYP2D6 PM | 2.07 (0.75, 5.68) | 0.16 | 2.14 (0.78, 5.89) | 0.14 | 1.85 (0.72, 4.78) | 0.21 |
| CYP2D6 IM | 1.23 (1.05, 1.45) | 0.01 | 1.23 (1.05, 1.45) | 0.01 | 1.23 (1.06, 1.44) | 0.01 |
| CYP2D6 UM | 0.25 (0.08, 0.79) | 0.02 | 0.24 (0.08, 0.80) | 0.02 | 0.30 (0.10, 0.90) | 0.03 |
| Activity score | 0.82 (0.72, 0.93) | 0.002 | 0.82 (0.72, 0.93) | 0.002 | 0.83 (0.73, 0.93) | 0.002 |
| Male | ||||||
| CYP2D6 PM | 3.32 (0.76, 14.61) | 0.11 | 3.70 (0.84, 16.22) | 0.08 | 4.00 (0.96, 16.59) | 0.06 |
| CYP2D6 IM | 0.87 (0.68, 1.12) | 0.28 | 0.89 (0.70, 1.15) | 0.38 | 0.90 (0.71, 1.14) | 0.38 |
| CYP2D6 UM | 0.68 (0.16, 3.00) | 0.61 | 0.79 (0.18, 3.45) | 0.75 | 0.83 (0.20, 3.45) | 0.80 |
| Activity score | 1.18 (0.96, 1.45) | 0.12 | 1.15 (0.94, 1.42) | 0.18 | 1.17 (0.96, 1.43) | 0.12 |
Sex, age, body mass index, smoking and drinking habit were used as covariates.
Sex, age, body mass index, smoking and drinking habit, and paroxetine daily dose were used as covariates. Note: For CYP2D6 metabolizer status, β represents the effect size of each CYP2D6 metabolizer status compared to CYP2D6 EMs. For the CYP2D6 activity score, β represents the outcome change for each additional activity score unit. Css, steady state concentration; UM, ultrarapid metabolizer; EM, extensive metabolizer; IM, intermediate metabolizer, PM, poor metabolizer.
In the post-hoc sensitivity analysis (adjusted model two), further adjustments for daily dose yielded similar results, with a significant effect remaining for PMs (exponentiated β = 2.40, 95% CI: 1.09–5.29, P = 0.03), and when we add interaction terms (IM∗sex, PM∗sex, UM∗sex) in the model, we found significant interaction between sex and IM on paroxetine Css (t = 2.13, P = 0.03).
Stratified analysis revealed sex differences in the association between metabolizer status and paroxetine Css (Table 3). In female patients, after adjusting the same demographic covariates, the paroxetine Css of PMs, IMs, and UMs were 2.14, 1.23, and 0.24 times that of EMs, respectively, with IM and UM effects remaining significant (exponentiated β = 1.23, 95% CI: 1.05–1.45, P = 0.01; exponentiated β = 0.24, 95% CI: 0.08–0.80, P = 0.02, respectively). Similar results were found even after additionally adjusting daily dose. However, no significant association was observed in males in our predefined model and after additionally adjusting for daily dose.
Lastly, we explored the effect of CYP2D6 AS on paroxetine Css (Table 3). In our predefined model, after adjusting for the same demographic covariates, a marginally statistically significant negative effect of AS on paroxetine Css was observed (exponentiated β = 0.90, 95% CI: 0.81–0.999, P = 0.048), which was not significant when further adjusting for daily dose. A significant interaction was found between sex and AS on paroxetine Css after adjusting the same demographic covariates and daily dose (t = −3.01, P = 0.003), with a strong negative association in females (exponentiated β = 0.83, 95% CI: 0.73–0.93, P = 0.002) but not in males (exponentiated β = 1.17, 95% CI: 0.96–1.43, P = 0.12). No non-linear relationship was observed between paroxetine Css and AS, supporting the use of linear models (Supplemental Figure S2).
CYP2D6 metabolic phenotype and paroxetine efficacy in the PMEDA study
At the 4-week treatment endpoint (Supplementary Table S2), we observed a trend of lower percentage improvement in symptom severity for UMs compared to EMs in the MDD group, after adjusting for predefined demographic covariates (age and sex), which was not statistically significant (multiple linear regression, standardized β = −0.93, SE = 0.50, P = 0.07). However, in post-hoc analysis, when further adjusting for current episode duration, baseline symptom severity, daily dose, and adjunctive medication status, the effect of UM became marginally statistically significant (standardized β = −0.98, SE = 0.50, P = 0.049). As for GAD and PD groups, the effect of metabolizer status on the percentage improvement in symptom severity was not significant in our predefined model and post-hoc analysis.
At the 8-week treatment endpoint (Supplementary Table S2), no significant association was found between CYP2D6 metabolizer status and the percentage improvement in symptom severity across all disease groups. The pooled standardized coefficients of CYP2D6 metabolizer status across different disease groups are presented in the Supplemental Table S3.
Regarding the AS, we found no significant association between AS and the percentage improvement in symptom severity in multiple linear regression (Supplementary Table S2) and RCS analysis (Supplemental Figure S2).
CYP2D6 metabolic phenotype and paroxetine ADR in the PMEDA study
When applied multiple logistic regression model and adjusted predefined covariates (sex, age) and additional clinical covariates (first-episode status, daily dose), we found a negative association between the AS and the risk of developing ADR (Supplementary Table S4).
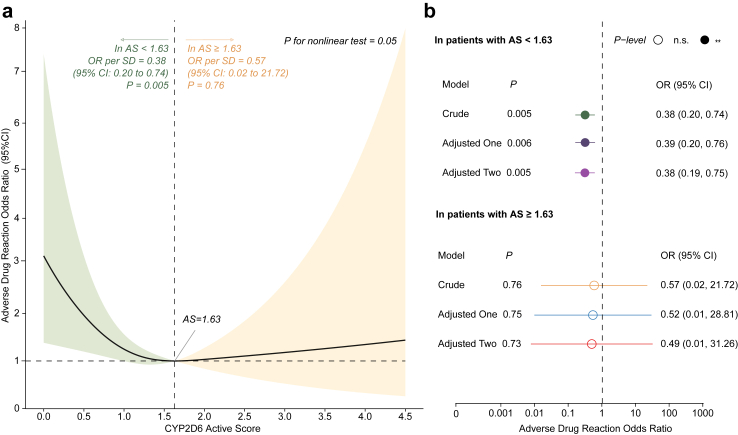
Interestingly, our exploratory analysis revealed a ‘U-shaped’ relationship between AS and ADR status (Fig. 2a), which was observed in the RCS logistic regression analysis without covariates. In this analysis, ADR status served as the dependent variable, while AS was the independent variable. The reference point was set at the median value of AS, with three knots placed at the 10th, 50th, and 90th percentiles of AS. Consequently, the value of AS associated with the lowest ADR risk was 1.63, which corresponded to the value with the lowest odds of developing ADR on the spline curve (Fig. 2a). The association between AS and ADR status appeared to be approximately linear below and above an AS of 1.63.
Fig. 2.
Association of CYP2D6 Activity score and incidence of Adverse Drug Reaction (ADR) of Paroxetine. Panel a displays the restricted cubic spline curves of the CYP2D6 activity score and paroxetine ADR. For ease of presentation, the reference point is set at the activity score value associated with the lowest ADR risk, with three knots positioned at the 10th, 50th, and 90th percentiles. The odds ratios, represented by solid lines, are crude, and the 95% confidence intervals are indicated by shaded areas. Panel b shows multiple linear models which calculate odds ratios of developing ADR per standard deviation increase in activity score, separately within subgroups with activity score below or above 1.63. Adjustments were made for sex and age in adjusted model one, and for sex, age, first-episode status, and daily dose in adjusted model two.
Therefore, we additionally employed a multiple linear model to calculate odds ratios per standard deviation increase in AS, separately within subgroups with AS below or above 1.63 (Fig. 2b). Among patients with an AS less than 1.63, the risk of ADR increased by 127% for every unit decrease in AS (crude OR = 0.38, 95% CI: 0.20–0.74, P = 0.005). The independent effect size of AS remained consistent after adjusting for age and sex, and even after additionally adjusting for first-episode status and daily dose, as depicted in Fig. 2b. However, we found no significant association among patients with an AS of 1.63 or higher in both crude and adjusted models, which had wide confidence intervals.
CYP2D6-CNV associated with paroxetine treatment outcome in the PMEDA study
In the PMEDA study, four patients (0.43%) had zero copies of the CYP2D6 gene, 126 (13.68%) had one copy deletion of the CYP2D6 gene, and 13 (1.41%) carried at least one copy duplication of the CYP2D6 gene. Overall, CYP2D6-CNV accounts for 19.1% of the variance in AS.
After adjusting for age, sex, smoking status, drinking status and BMI, the paroxetine Css of CYP2D6-CNV-deletion carrier and CYP2D6-CNV-duplication carrier were 1.53 and 0.64 times that of CYP2D6-CNV non-carriers (multiple linear regression, exponentiated β = 1.53, 95% CI: 1.26–1.85, P < 0.001; exponentiated β = 0.64, 95% CI: 0.36–1.12, P = 0.12, respectively), which were consistent in the sensitivity analysis (Supplementary Table S5). Stratified analysis revealed no sex difference in the association between CYP2D6-CNV-deletion and paroxetine (Supplementary Table S5). However, CYP2D6-CNV-duplication was only associated with lower paroxetine Css in the unadjusted model of the female subgroup (exponentiated β = 0.52, 95% CI: 0.27–0.98, P = 0.04), but this association became non-significant in adjusted models one and two (exponentiated β = 0.53, 95% CI: 0.28–0.999, P = 0.05; exponentiated β = 0.61, 95% CI: 0.34–1.12, P = 0.11, respectively).
Regarding efficacy, we calculated the standardized coefficients of CYP2D6-CNV for each disease group, adjusting for age, sex, current episode duration, baseline symptom severity, daily dose, and adjunctive medication status (Supplementary Table S2), which resulted in no significant association in single disease group. However, pooled analysis showed that CYP2D6-CNV-deletion carriers had significantly greater symptom improvement after 8-week paroxetine treatment compared to non-carriers (pooled standardized β = 0.21, 95% CI: 0.01–0.41, P = 0.04, I2 < 0.01).
No significant association was observed between CYP2D6-CNV and ADRs (Supplementary Table S4).
Therapeutic reference range for paroxetine in the PMEDA study
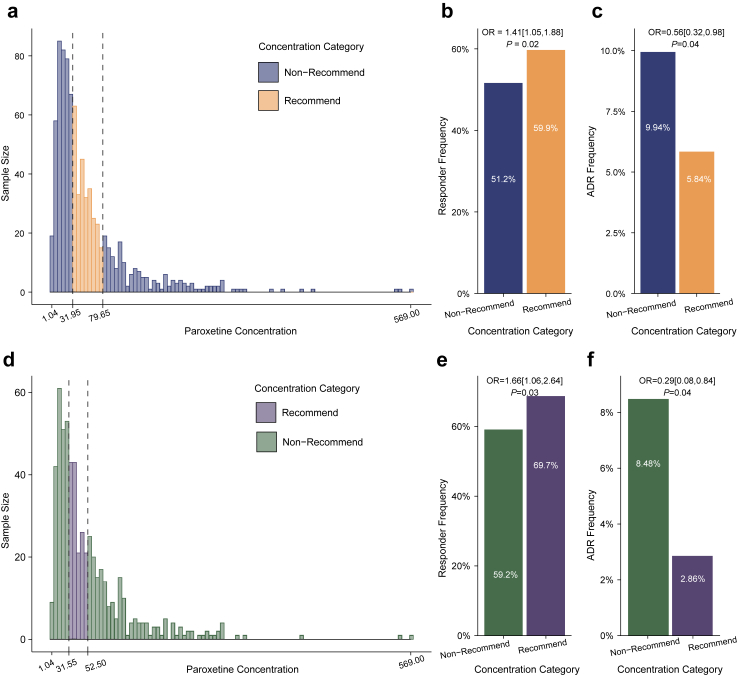
In the entire study population, ROC analysis revealed that the best cut-off to distinguish responders from non-responders was 91.4 ng/ml, the upper limit of paroxetine Css for achieving treatment response. Then, 31.95 ng/ml was the second cut-off, which was the low limit and identified among patients with Css below the upper limits. After adjusting sex and age, patients with Css within 31.95–91.4 ng/ml had a significantly higher responder frequency (OR = 1.47, 95% CI: 1.11–1.95, P = 0.01) compared to those with Css levels outside of this range. Furthermore, ROC analysis showed that the best cut-off to distinguish patients with ADR and without ADR was 79.65 ng/ml, and patients with Css below it had a significantly lower ADR frequency after adjusting sex and age (OR = 0.44, 95% CI: 0.26–0.76, P = 0.003). Therefore, paroxetine Css from 31.95 to 79.65 ng/ml was determined as the TRR for patients with depressive or anxiety disorders (Fig. 3a, Supplemental Figure S3). Compared to patients without TRR, patients within TRR had higher responder frequency (Fig. 3b) and lower ADR frequency (Fig. 3c).
Fig. 3.
Therapeutic reference range for patients with depressive and anxiety disorder in Chinese Han population. Abbreviation: ADR, adverse drug reaction. The histograms illustrate the distribution of paroxetine steady-state concentration (raw form) in the entire sample (Panel a) and the major depressive disorder (MDD) subgroup (Panel d), respectively. Black dashed line represents the boundary of the therapeutic reference range. Steady-state concentrations of paroxetine within the range are categorized as the “recommend category”, while those out of the range are classified as the “non-recommend category”. Panels b and e depict the proportion of patients identified as responders in the entire sample and the MDD subgroup, respectively. The numbers on the columns represent the responder frequencies of corresponding groups. Panels c and f present bar charts of ADR frequencies for the entire sample and the MDD subgroup, respectively. The numbers on the columns represent the ADR frequencies of corresponding groups. The presented odds ratios, calculated using multivariable logistic regression and adjusted for age and sex, represent the odds of being a responder or developing an ADR in the recommended category compared to the non-recommended category.
In patients with MDD, we performed the same ROC analysis, which revealed that patients with Css within 31.55–97.6 ng/ml had a significantly higher responder frequency after adjusting sex and age (OR = 1.74, 95% CI: 1.22–2.49, P = 0.002) compared to those with Css levels outside of this range. Furthermore, the best cutoff to distinguish patients with ADR and without ADR was 52.5 ng/ml, and patients with Css below it had a significantly lower ADR frequency after adjusting sex and age (OR = 0.38, 95% CI: 0.19–0.72, P = 0.004). Therefore, paroxetine Css from 31.55 to 52.5 ng/ml was determined as the TRR for patients with MDD (Fig. 3d, Supplemental Figure S3). Compared to patients without TRR, patients within TRR had higher responder frequency (Fig. 3e) and lower ADR frequency (Fig. 3f).
Cross-ethnic meta-analysis on CYP2D6 metabolic phenotype and paroxetine exposure
We screened 6187 eligible published articles and included 11 studies that met the criteria (Supplemental Figure S4). Supplemental Tables S6 and S7 list the basic information and quality evaluation results of each study. We inferred the CYP2D6 metabolizer status for all studies using the AS-inferred standard. Along with our prospective cohort, the final meta-analysis included 12 studies with 1570 participants.
The meta-analysis replicated and extrapolated our findings in the PMEDA study (Supplemental Table S8 and Figures S5 and S6). We found that PMs had 2.46 times more Css than EMs (RoM = 2.46; 95% CI: 1.24–4.89, n = 2, P = 0.01). Furthermore, IMs had 2.89 times more AUC and 2.76 times more Cmax than those of EMs (AUC: RoM = 2.89, 95% CI: 1.54–5.44, n = 4, P = 0.001; Cmax: RoM = 2.76, 95% CI: 1.49–5.12, n = 3, P = 0.001). In the subgroup analysis, we noticed a trend that IMs had higher Css than EMs, which was significant among non-East-Asian population but not East Asian population (RoM = 1.36, 95% CI: 1.02–1.82, n = 3, P = 0.04; RoM = 1.48; 95% CI: 0.56–3.94, n = 2, P = 0.43, respectively). This suggests that the comparison between IMs and EMs of Css may vary by ethnicity.
Sub-combinations for data under different paroxetine doses (Supplemental Figure S7) indicate that the weighted-average paroxetine Css for IMs was 48.93 ng/ml (95% CI: 30.62–67.23) and for EMs is 41.82 ng/ml (95% CI: 23.86–59.78) when the dose range is 10–60 mg/d. The funnel plot of included studies was essentially symmetrical (Supplemental Figure S8).
Dose adjustment of paroxetine
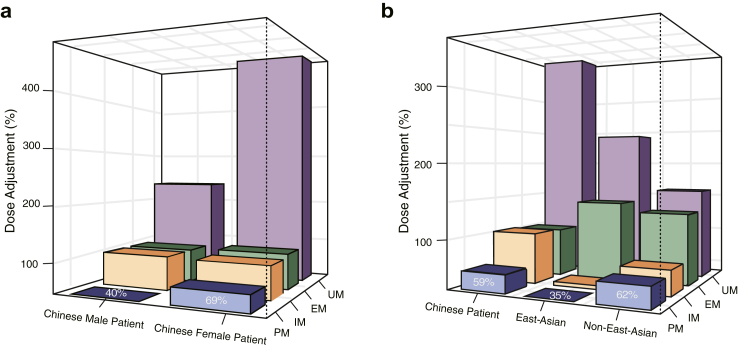
Based on paroxetine Css differences among CYP2D6 metabolizer statuses, we calculated dose-adjusted ratios of paroxetine for Chinese Han patients with depressive or anxiety disorders (Fig. 4a). The results suggested that PMs, IMs, EMs, and UMs should receive approximately 59%, 103%, 101%, and 364% of the manufacturer's recommended dose, respectively. For female PMs, IMs, EMs, and UMs, the dose adjustment ratio was 69%, 98%, 104%, and 479%, respectively. For male PMs, IMs, EMs, and UMs, the dose adjustment ratio was 40%, 114%, 94%, and 225%, respectively. We advise cautiously adjusting dose for PMs due to the relatively wide confidence interval observed in our multiple linear regression analysis. As for male IMs and male UMs, we do not recommend dose adjustment, since our multiple linear regression analysis did not reveal a significant difference between these metabolizer statuses and EM in male patients.
Fig. 4.
Dose adjustments based on difference in pharmacokinetic parameters of paroxetine across CYP2D6 metabolizer statuses. Abbreviation: UM, ultrarapid metabolizer; EM, extensive metabolizer; IM, intermediate metabolizer; PM, poor metabolizer. The average dose (DAv) recommended by the manufacturer was considered the pragmatic results of large-scale studies performed within genetically mixed populations consisting of CYP2D6 PMs, IMs, EMs, and UMs. Dose-adjusted ratios for CYP2D6 PMs, IMs, EMs, and UMs were estimated based on differences in paroxetine pharmacokinetic parameters (i.e., peak concentration, area under the concentration–time curve, and concentration at steady state) among different CYP2D6 metabolizer statuses. The figure shows the specific values of dose-adjusted ratio, with DAv equal to 100%. Panel a displays the dose-adjusted ratios for the sex subgroup of Chinese Han patients, and panel b shows the dose-adjusted ratios for Chinese Han patients, East Asian population, and non-East-Asian population.
Given that integrating evidence from multiple sources can improve proposing dose adjustment, we conducted pooled analyses to calculate quantitative dose-adjusted ratios using data from the studies included in our meta-analysis, which based on differences in the paroxetine pharmacokinetic parameters (i.e., Cmax, AUC, and Css) across metabolizer statuses (Fig. 4b, Supplemental Table S9). Briefly, pooled analyses suggested that for East Asian population, PMs, IMs, EMs, and UMs were recommended to receive approximately 35%, 40%, 143%, and 241% of the manufacturer's recommended dose. For non-East-Asian population, the recommended dose adjustment for PMs, IMs, EMs, and UMs were 62%, 68%, 131%, and 159% of the manufacturer's recommended dose, respectively (Supplemental Table S10).
Discussion
This study presents several key findings: (1) The CYP2D6 metabolic phenotype was associated with paroxetine exposure, efficacy, and adverse reaction, with sex and ethnicity exerting influences. These findings highlight the significance of considering the CYP2D6 metabolic phenotype in paroxetine treatment. (2) CYP2D6-CNV-deletion carriers had significantly greater symptom improvement after 8-week paroxetine treatment compared to non-carriers. (3) The paroxetine TRR was found to be 31.95–79.65 ng/ml for depressive or anxiety disorder, and 31.55–52.5 ng/ml for major depressive disorder. (4) Compared to non-East-Asian population, we recommend larger dose adjustments for different CYP2D6 metabolizer statuses in East Asians and cautious adjustments for Chinese Han male patients.
We found that sex modulates the effects of CYP2D6 metabolic phenotype and CYP2D6-CNV on paroxetine Css. Females with increased CYP2D6 enzyme activity (due to CYP2D6 gene duplication or being UMs) had a more pronounced difference in paroxetine Css from those with normal activity (CYP2D6-CNV non-carrier or being EMs) than males. Therefore, these findings underscore the necessity of integrating sex and CYP2D6 metabolizer status in paroxetine dosing. While we acknowledge that these observed sex differences may be influenced by various factors such as the reduced statistical power when splitting the dataset by sex, sex differences in the pharmacodynamics of paroxetine (higher CYP2D6 enzyme activity in females41), and inherent sex difference in other factors that affect plasma drug levels (body weight and fat distribution, blood volume, and renal excretion rates42). However, it does not diminish the importance of considering both sex and CYP2D6 metabolizer status in paroxetine dosing.30
In the MDD group, we observed a trend of lower symptom improvement in UMs compared to EMs, which was marginally significant after adjusting for demographic and clinical covariates. However, the scarcity of UMs in the GAD and PD groups limits our ability to extend this analysis to anxiety disorders. Regarding the entire study population, CYP2D6-CNV deletion carriers showed significantly greater symptom improvement after 8-week paroxetine treatment compared to non-carriers. Despite potential heterogeneity introduced by assessing efficacy using various scales for different diagnoses and pooling standardized coefficients, our results showed minimal heterogeneity, suggesting it did not significantly impact our findings. Our findings enhance the understanding of the impact of CYP2D6-CNV on paroxetine efficacy. This extends the previously studied effect of CYP2D6-CNV deletion, which leads to higher paroxetine exposure.10
Interestingly, the relationship between the CYP2D6 AS and ADRs exhibited a non-linear pattern. In patients with lower AS (i.e., AS <1.63: including PMs, IMs, and some EMs), AS was negatively correlated with ADRs. However, in patients with higher AS (i.e., AS ≥1.63: including UMs and some EMs), the association was not significant and with wide confidence interval, which indicated a large level of variation. These results were consistent with a previous study that reported that patients experiencing ADRs did not include UMs, but included PMs.43
The paroxetine TRR in this study, determined using dried blood spot measurements, enhanced improving treatment outcome of paroxetine, as we considered both paroxetine efficacy and ADR, and included subjects who withdrew to provide more comprehensive and clinically significant data. The TRR is similar to a previous guideline that recommended 20–65 ng/ml for paroxetine concentration based only on treatment efficacy data.8 Moreover, the TRR is supported by the pharmacological mechanism of paroxetine. When the blood concentration of paroxetine reached 30 ng/ml, the occupancy rate of the 5-HT transporter reached 85% and could cause therapeutic effect. Furthermore, when the concentration increases to 80 ng/ml, the occupancy rate of the 5-HT transporter stabilized at around 90%.44 For future studies, a preferable design would be a double-blind, randomized controlled trial, where patients are treated with doses that result in the predefined TRR.8
Along with findings in the PMEDA study, our cross-ethnic meta-analysis suggested that higher paroxetine exposure among PMs or IMs than EMs consistent with the previous meta-analysis,45 and align with the associations catalogued by the U.S. Food and Drug Administration (FDA), which indicated the CYP2D6 metabolizer status may alter systemic concentration of paroxetine. Furthermore, these findings support implementing precision dosing based on CYP2D6 metabolic phenotype, as the CPIC guideline suggested.7 Nevertheless, our analysis of a large sample size challenges the conclusion of the latest DPWG guideline, which was based on studies with a small sample size and indicated that the influence of CYP2D6 metabolizer status on pharmacokinetic parameters may lack clinical significance.12 Our discovery underscores the need for additional research and validation.
Notably, our meta-analysis suggested that ethnicity influenced the comparison between IMs and EMs. In particular, IMs had a significantly higher paroxetine Css than EMs only among non-East-Asian populations. Whereas significant differences in AUC and Cmax were found between IMs and EMs only among East Asian populations. These findings underscore the importance of ethnicity in paroxetine metabolism and highlight the risk of directly applying guidelines based on findings from other ethnicities to East Asians. Consequently, we provided dose recommendations tailored to various CYP2D6 metabolizer statuses and ethnicities.
While a previous systematic review suggested a 49% and 19% reduction in paroxetine dose for PMs and IMs, respectively, and a 69% increase for UMs,40 our meta-analytic data indicated a more nuanced approach. For the non-East-Asian population, we recommend a 38% and 32% reduction in paroxetine dose for PMs and IMs, respectively, and a 59% increase for UMs. For East Asian population, we propose a 65% reduction in the recommended dose for PMs and a 60% reduction for IMs, while suggesting an increase of 141% for UMs. This approach emphasizes the necessity of careful monitoring and individualized dosing of paroxetine for East Asian population. Notably, we assumed a linear relationship between relative adjustments and changes in pharmacokinetic parameters across metabolizer statuses, which is specific to the doses in the meta-analysis due to paroxetine's non-linear pharmacokinetics.39 Our recommendations for UMs in the non-Chinese population were extrapolated, and sex-specific dose recommendations were not provided due to the lack of sex-subgroup results in the included studies. Currently, paroxetine is available in tablet form, and the range of dosages may not allow for precise dose adjustment based on CYP2D6 metabolizer status. This presents an exciting opportunity for future pharmaceutical research and development. We anticipate the creation of new formulations or dosage regimens that can cater to the needs of patients with different CYP2D6 metabolizer statuses. Such advancements could significantly enhance the efficacy and safety of paroxetine pharmacotherapy.
Our study has several strengths and clinical implications. To our knowledge, this is the largest prospective cohort study to develop precision dosing of paroxetine for patients with depressive or anxiety disorders to date. First, the PMEDA study had a rigorous scientific design to avoid potential confounders. The enrolled patients were either drug-naïve or with a 2-week washout period before enrollment. This increased the validity of our findings. Second, we determined the TRR for paroxetine-treated patients by considering efficacy and ADRs, which could help clinicians monitor paroxetine treatment. Third, we predicted the CYP2D6 metabolizer status using the latest AS-inferred standard, influenced by CYP2D6-CNV, and expected to be more accurate and reliable than the traditional standard. We also found that CYP2D6-CNV-deletion was associated with paroxetine exposure and efficacy, suggesting that the CNV test should be included in clinical CYP2D6 genotyping. Fourth, we explored how ethnicity and sex modulate the effect of CYP2D6 metabolizer status on paroxetine exposure. This is important for precision medicine, as different populations may respond differently to paroxetine and require different doses. Finally, we performed a meta-analysis of 12 studies with 1570 participants. The pooled analyses provided dose recommendations for different CYP2D6 metabolizer statuses, which could help clinicians optimize the paroxetine dosage for appropriate drug exposure. Based on these strengths and implications, our study provides valuable insights into precision dosing of paroxetine.
Caveats and limitations
We acknowledge limitations of our study. Firstly, there may be uncertainties in inferring the CYP2D6 metabolic phenotype, as CYP2D6 gene has many rare variants46 and we did not detect all CYP2D6 variants. Enzyme function may be overestimated in some patients who have rare variants that would render them IMs or PMs. Specially, without CYP2D6∗2 (AS = 1), ∗9 (AS = 0.5), ∗29(AS = 0.5) detection, our testing had a slightly lower capacity to detect known non-wild-type CYP2D6 variations than the Association of Molecular Pathology (AMP) recommendation47 (Supplemental Method). However, <0.1% of patients of East Asian ancestry carry ∗9 and ∗29. Therefore, practically, due to the low prevalence of these variants or lack of functional impact on CYP2D6 enzyme activity (∗2), we still could reliably predict phenotypes. We advise future studies to follow the AMP recommendations. Secondly, despite our large sample size, the limited numbers of UMs, PMs, and studies included in the meta-analysis may have hindered our ability to detect significant associations. Our findings may not be fully generalizable as some patients did not consent to measure paroxetine Css after experiencing intolerable adverse reactions. Our secondary findings are preliminary due to unadjusted multiple testing, and may not extend to treatment-resistant depression given our study's relatively high response rate. Future studies are needed to confirm our findings, and they should be applied clinically with caution. Finally, several factors can influence paroxetine concentration. We have adjusted variables such as sex, ethnicity, BMI, and daily dose. However, given the variety of antidepressants and antipsychotics that undergo metabolism by the CYP2D6 enzyme, further research is necessary to explore the effects of drug–drug interactions and gene–environment interactions.
Conclusion
In conclusion, this study provided a comprehensive perspective on precision dosing of paroxetine. CYP2D6 metabolic phenotype and CYP2D6-CNV were associated with paroxetine exposure and clinical outcomes, which was influenced by sex. Ethnicity influenced the effect of CYP2D6 metabolizer status on paroxetine exposure. The paroxetine therapeutic reference range was found to be 31.95–79.65 ng/ml for depressive or anxiety disorders, and 31.55–52.5 ng/ml for major depressive disorder. We recommend greater dose adjustment for East Asians based on CYP2D6 metabolizer status and cautious adjustments for Chinese Han male patients.
Contributors
The PMEDA consortium, led by WY, collaborated on this study. WY, ZL, Yun Z, LK, and YL conceived and designed the project. WY, ZL, Yun Z, LK, Yu S, Yong Z, LS, CC, AW, SW, AN, JP, KW, ZC, HW, JW, Jian-min Z led the data collection in their respective centres, with specific members of the PMEDA consortium (HB, MH, BL, JH, JX, RJ, JZ, YH, HY, GL, LP, Hui Y, XC, WF, RZ, RL, XZ, RT, FD, CZ, TZ, YY, Ji-ting G, DW, YC, Yifan S, Yong-can Z, and WW) also involved. JH, CH, and XS performed genotyping and concentration detection. WY, YL, Yu S, and JG have directly accessed and verified the underlying data reported in the manuscript. YL and JG did the statistical analyses, prepared the tables and figures. YL, Yu S, and JG wrote the first draft of the study. YL, JG, ZK, YZ, and YS made the further interpretation of data. WY, ZL, Yun Z, and LK edited the manuscript and provided supervision. All authors contributed to the drafting and critical revision of the work and made substantial contributions to the concept and design of the study and data acquisition, analysis, and interpretation. All authors read and approved the final manuscript.
Data sharing statement
The dataset presented in this study are available from the corresponding authors on reasonable request with research proposal. All requests must be approved by the relevant ethics boards and data custodians.
Declaration of interests
The PMEDA consortium is funded by the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2021-I2M-C&T-B-099) and “the Fundamental Research Funds for the Central Universities” (Peking University Medicine Fund for world's leading discipline or discipline cluster development, BMU2022DJXK007). WY, YL, Yu S, JG, ZK, and YZ disclose that there is a planned and pending patent related to a device for predicting the treatment outcome of paroxetine, which is relevant to the content of this manuscript. Other authors declare that they have no competing interests.
Acknowledgements
This study was supported by the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2019-I2M-5–006 and 2021-I2M-C&T-B-099 to Weihua Yue); “the Fundamental Research Funds for the Central Universities” (Peking University Medicine Fund for world's leading discipline or discipline cluster development, BMU2022DJXK007 to Weihua Yue); National Natural Science Foundation of China (82330042 to Weihua Yue, 82301687 to Yuyanan Zhang); National Key R&D Program of China (2021YFF1201103 and 2023YFE0119400 to Weihua Yue); STI2030-Major Projects-2021ZD0200702 (to Weihua Yue); Beijing Municipal Health Commission Research Ward Programme (3rd batch to Weihua Yue); Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2023-PT320-08 to Weihua Yue); China Postdoctoral Science Foundation (2022M720302 to Yaoyao Sun). The funders did not have any role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2024.105165.
Contributor Information
Li Kuang, Email: kuangli0308@163.com.
Yunshu Zhang, Email: yunshucoffee@sina.com.
Zhongchun Liu, Email: zcliu6@whu.edu.cn.
Weihua Yue, Email: dryue@bjmu.edu.cn.
Precision Medicine to Enhance Depression and Anxiety Outcome Consortium:
Yundan Liao, Yutao Sun, Jing Guo, Zhewei Kang, Yaoyao Sun, Yuyanan Zhang, Hanping Bai, Maolin Hu, Bing Li, Jingshan Han, Jiaojiao Xiang, Ruhong Jiang, Jian Zhang, Yuxiang He, Huailiang Yang, Guifang Liu, Lili Peng, Hui Yu, Xialong Cheng, Wenmei Fang, Rongyan Zheng, Ruiqian Lin, Xiao-yan Zhai, Rui Tang, Fangyi Deng, Chunyan Zhu, Ting Zhang, Yan Yang, Ji-ting Geng, Di Wu, Yi-huan Chen, Yifan Sun, Yong-can Zhou, Wei-xin Wang, Jian-min Zhang, Jun Wang, Hua-ning Wang, Zhi-yu Chen, Kai Wang, Jiyang Pan, Ai-hua Ni, Saizheng Weng, Anzhen Wang, Changbin Cao, Lidong Sun, Yong Zhang, Li Kuang, Yunshu Zhang, Zhongchun Liu, and Weihua Yue
Appendix A. Supplementary data
References
- 1.Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Psychiatry. 2022;9:137–150. doi: 10.1016/S2215-0366(21)00395-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saris I.M.J., Aghajani M., van der Werff S.J.A., van der Wee N.J.A., Penninx B.W.J.H. Social functioning in patients with depressive and anxiety disorders. Acta Psychiatr Scand. 2017;136:352–361. doi: 10.1111/acps.12774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warden D., Rush A.J., Trivedi M.H., Fava M., Wisniewski S.R. The STAR∗D Project results: a comprehensive review of findings. Curr Psychiatry Rep. 2007;9:449–459. doi: 10.1007/s11920-007-0061-3. [DOI] [PubMed] [Google Scholar]
- 4.Undurraga J., Baldessarini R.J. Randomized, placebo-controlled trials of antidepressants for acute major depression: thirty-year meta-analytic review. Neuropsychopharmacology. 2012;37:851–864. doi: 10.1038/npp.2011.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jukic M., Milosavljević F., Molden E., Ingelman-Sundberg M. Pharmacogenomics in treatment of depression and psychosis: an update. Trends Pharmacol Sci. 2022;43:1055–1069. doi: 10.1016/j.tips.2022.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Cipriani A., Furukawa T.A., Salanti G., et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391:1357–1366. doi: 10.1016/S0140-6736(17)32802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bousman C.A., Stevenson J.M., Ramsey L.B., et al. Clinical pharmacogenetics implementation consortium (CPIC) guideline for CYP2D6, CYP2C19, CYP2B6, SLC6A4, and HTR2A genotypes and serotonin reuptake inhibitor antidepressants. Clin Pharmacol Ther. 2023;114:51–68. doi: 10.1002/cpt.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hiemke C., Bergemann N., Clement H.W., et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51:9–62. doi: 10.1055/s-0043-116492. [DOI] [PubMed] [Google Scholar]
- 9.Caudle K.E., Sangkuhl K., Whirl-Carrillo M., et al. Standardizing CYP2D6 genotype to phenotype translation: consensus recommendations from the clinical pharmacogenetics implementation consortium and Dutch pharmacogenetics working group. Clin Transl Sci. 2020;13:116–124. doi: 10.1111/cts.12692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sawamura K., Suzuki Y., Someya T. Effects of dosage and CYP2D6-mutated allele on plasma concentration of paroxetine. Eur J Clin Pharmacol. 2004;60:553–557. doi: 10.1007/s00228-004-0792-6. [DOI] [PubMed] [Google Scholar]
- 11.Findling R.L., Nucci G., Piergies A.A., et al. Multiple dose pharmacokinetics of paroxetine in children and adolescents with major depressive disorder or obsessive-compulsive disorder. Neuropsychopharmacology. 2006;31:1274–1285. doi: 10.1038/sj.npp.1300960. [DOI] [PubMed] [Google Scholar]
- 12.Brouwer J.M.J.L., Nijenhuis M., Soree B., et al. Dutch Pharmacogenetics Working Group (DPWG) guideline for the gene-drug interaction between CYP2C19 and CYP2D6 and SSRIs. Eur J Hum Genet. 2022;30:1114–1120. doi: 10.1038/s41431-021-01004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koopmans A.B., Braakman M.H., Vinkers D.J., Hoek H.W., van Harten P.N. Meta-analysis of probability estimates of worldwide variation of CYP2D6 and CYP2C19. Transl Psychiatry. 2021;11:141. doi: 10.1038/s41398-020-01129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rollman B.L., Herbeck Belnap B., Reynolds C.F., Schulberg H.C., Shear M.K. A contemporary protocol to assist primary care physicians in the treatment of panic and generalized anxiety disorders. Gen Hosp Psychiatry. 2003;25:74–82. doi: 10.1016/s0163-8343(03)00004-5. [DOI] [PubMed] [Google Scholar]
- 15.Wishart D.S., Feunang Y.D., Guo A.C., et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018;46:D1074–D1082. doi: 10.1093/nar/gkx1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saint-Marcoux F., Sauvage F.-L., Marquet P. Current role of LC-MS in therapeutic drug monitoring. Anal Bioanal Chem. 2007;388:1327–1349. doi: 10.1007/s00216-007-1320-1. [DOI] [PubMed] [Google Scholar]
- 17.Goddard A.W., Mahmud W., Medlock C., Shin Y.-W., Shekhar A. A controlled trial of quetiapine XR coadministration treatment of SSRI-resistant panic disorder. Ann Gen Psychiatry. 2015;14:26. doi: 10.1186/s12991-015-0064-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lenze E.J., Mulsant B.H., Shear M.K., et al. Efficacy and tolerability of citalopram in the treatment of late-life anxiety disorders: results from an 8-week randomized, placebo-controlled trial. Am J Psychiatry. 2005;162:146–150. doi: 10.1176/appi.ajp.162.1.146. [DOI] [PubMed] [Google Scholar]
- 19.Rush A.J., Fava M., Wisniewski S.R., et al. Sequenced treatment alternatives to relieve depression (STAR∗D): rationale and design. Control Clin Trials. 2004;25:119–142. doi: 10.1016/s0197-2456(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 20.National Institute of Mental Health TESS (treatment emergent symptom scale-write-in) Psychopharmacol Bull. 1985;21:1069–1072. [Google Scholar]
- 21.Greden J.F., Parikh S.V., Rothschild A.J., et al. Impact of pharmacogenomics on clinical outcomes in major depressive disorder in the GUIDED trial: a large, patient- and rater-blinded, randomized, controlled study. J Psychiatr Res. 2019;111:59–67. doi: 10.1016/j.jpsychires.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Tanner J.-A., Davies P.E., Voudouris N.C., et al. Combinatorial pharmacogenomics and improved patient outcomes in depression: treatment by primary care physicians or psychiatrists. J Psychiatr Res. 2018;104:157–162. doi: 10.1016/j.jpsychires.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Johansson I., Lundqvist E., Bertilsson L., Dahl M.L., Sjöqvist F., Ingelman-Sundberg M. Inherited amplification of an active gene in the cytochrome P450 CYP2D locus as a cause of ultrarapid metabolism of debrisoquine. Proc Natl Acad Sci U S A. 1993;90:11825–11829. doi: 10.1073/pnas.90.24.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dahl M.L., Yue Q.Y., Roh H.K., et al. Genetic analysis of the CYP2D locus in relation to debrisoquine hydroxylation capacity in Korean, Japanese and Chinese subjects. Pharmacogenetics. 1995;5:159–164. doi: 10.1097/00008571-199506000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Saito T., Gutiérrez Rico E.M., Kikuchi A., et al. Functional characterization of 50 CYP2D6 allelic variants by assessing primaquine 5-hydroxylation. Drug Metab Pharmacokinet. 2018;33:250–257. doi: 10.1016/j.dmpk.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Chan W., Li M.S., Sundaram S.K., Tomlinson B., Cheung P.Y., Tzang C.H. CYP2D6 allele frequencies, copy number variants, and tandems in the population of Hong Kong. J Clin Lab Anal. 2019;33 doi: 10.1002/jcla.22634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hosono N., Kato M., Kiyotani K., et al. CYP2D6 genotyping for functional-gene dosage analysis by allele copy number detection. Clin Chem. 2009;55:1546–1554. doi: 10.1373/clinchem.2009.123620. [DOI] [PubMed] [Google Scholar]
- 28.de Graan A.-J.M., Teunissen S.F., de Vos F.Y.F.L., et al. Dextromethorphan as a phenotyping test to predict endoxifen exposure in patients on tamoxifen treatment. J Clin Oncol. 2011;29:3240–3246. doi: 10.1200/JCO.2010.32.9839. [DOI] [PubMed] [Google Scholar]
- 29.Kim J.-R., Woo H.I., Chun M.-R., et al. Exposure-outcome analysis in depressed patients treated with paroxetine using population pharmacokinetics. Drug Des Devel Ther. 2015;9:5247–5254. doi: 10.2147/DDDT.S84718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X.-L., Huang S.-Q., Xiao T., et al. Pharmacokinetics of immediate and sustained-release formulations of paroxetine: population pharmacokinetic approach to guide paroxetine personalized therapy in Chinese psychotic patients. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.966622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gex-Fabry M., Eap C.B., Oneda B., et al. CYP2D6 and ABCB1 genetic variability: influence on paroxetine plasma level and therapeutic response. Ther Drug Monit. 2008;30:474–482. doi: 10.1097/FTD.0b013e31817d6f5d. [DOI] [PubMed] [Google Scholar]
- 32.Serretti A., Gibiino S., Drago A. Specificity profile of paroxetine in major depressive disorder: meta-regression of double-blind, randomized clinical trials. J Affect Disord. 2011;132:14–25. doi: 10.1016/j.jad.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 33.Demyttenaere K., Albert A., Mesters P., Dewé W., De Bruyckere K., Sangeleer M. What happens with adverse events during 6 months of treatment with selective serotonin reuptake inhibitors? J Clin Psychiatry. 2005;66:859–863. doi: 10.4088/jcp.v66n0708. [DOI] [PubMed] [Google Scholar]
- 34.Johannesen C.D.L., Langsted A., Mortensen M.B., Nordestgaard B.G. Association between low density lipoprotein and all cause and cause specific mortality in Denmark: prospective cohort study. BMJ. 2020;371 doi: 10.1136/bmj.m4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee D.H., Keum N., Hu F.B., et al. Predicted lean body mass, fat mass, and all cause and cause specific mortality in men: prospective US cohort study. BMJ. 2018;362 doi: 10.1136/bmj.k2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.An J.H., Jang E.H., Kim A.Y., et al. Ratio of plasma BDNF to leptin levels are associated with treatment response in major depressive disorder but not in panic disorder: a 12-week follow-up study. J Affect Disord. 2019;259:349–354. doi: 10.1016/j.jad.2019.08.021. [DOI] [PubMed] [Google Scholar]
- 37.Little J., Higgins J.P.T., Ioannidis J.P.A., et al. STrengthening the REporting of Genetic Association studies (STREGA)--an extension of the STROBE statement. Eur J Clin Invest. 2009;39:247–266. doi: 10.1111/j.1365-2362.2009.02125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Friedrich J.O., Adhikari N.K., Beyene J. The ratio of means method as an alternative to mean differences for analyzing continuous outcome variables in meta-analysis: a simulation study. BMC Med Res Methodol. 2008;8:32. doi: 10.1186/1471-2288-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kirchheiner J., Brøsen K., Dahl M.L., et al. CYP2D6 and CYP2C19 genotype-based dose recommendations for antidepressants: a first step towards subpopulation-specific dosages. Acta Psychiatr Scand. 2001;104:173–192. doi: 10.1034/j.1600-0447.2001.00299.x. [DOI] [PubMed] [Google Scholar]
- 40.Stingl J.C., Brockmöller J., Viviani R. Genetic variability of drug-metabolizing enzymes: the dual impact on psychiatric therapy and regulation of brain function. Mol Psychiatry. 2013;18:273–287. doi: 10.1038/mp.2012.42. [DOI] [PubMed] [Google Scholar]
- 41.Labbé L., Sirois C., Pilote S., et al. Effect of gender, sex hormones, time variables and physiological urinary pH on apparent CYP2D6 activity as assessed by metabolic ratios of marker substrates. Pharmacogenetics Genom. 2000;10:425. doi: 10.1097/00008571-200007000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Seeman M.V. The pharmacodynamics of antipsychotic drugs in women and men. Front Psychiatry. 2021;12 doi: 10.3389/fpsyt.2021.650904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johansson I., Ingelman-Sundberg M. CNVs of human genes and their implication in pharmacogenetics. Cytogenet Genome Res. 2008;123:195–204. doi: 10.1159/000184709. [DOI] [PubMed] [Google Scholar]
- 44.Meyer J.H., Wilson A.A., Sagrati S., et al. Serotonin transporter occupancy of five selective serotonin reuptake inhibitors at different doses: an [11C]DASB positron emission tomography study. Am J Psychiatry. 2004;161:826–835. doi: 10.1176/appi.ajp.161.5.826. [DOI] [PubMed] [Google Scholar]
- 45.Milosavljevic F., Bukvic N., Pavlovic Z., et al. Association of CYP2C19 and CYP2D6 poor and intermediate metabolizer status with antidepressant and antipsychotic exposure: a systematic review and meta-analysis. JAMA Psychiatry. 2021;78:270–280. doi: 10.1001/jamapsychiatry.2020.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mizzi C., Peters B., Mitropoulou C., et al. Personalized pharmacogenomics profiling using whole-genome sequencing. Pharmacogenomics. 2014;15:1223–1234. doi: 10.2217/pgs.14.102. [DOI] [PubMed] [Google Scholar]
- 47.Pratt V.M., Cavallari L.H., Del Tredici A.L., et al. Recommendations for clinical CYP2D6 genotyping allele selection: a joint consensus recommendation of the association for molecular pathology, college of American pathologists, Dutch pharmacogenetics working group of the royal Dutch pharmacists association, and the European society for pharmacogenomics and personalized therapy. J Mol Diagn. 2021;23:1047–1064. doi: 10.1016/j.jmoldx.2021.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.