Abstract
Objective
To reduce the frequency of insufficient overlap of intravenous (IV) and subcutaneous (SC) insulin during the treatment of diabetic ketoacidosis (DKA) as a quality improvement project.
Patients and Methods
Rates of insufficient IV and SC insulin overlap (< 2-hour overlap, SC insulin given after IV insulin discontinuation, or no SC insulin given after IV insulin discontinuation) were assessed in adults with DKA treated with IV insulin at a large tertiary care referral center in Rochester, Minnesota, from July 1, 2021, to March 15, 2023. After a preintervention analysis period, an electronic medical record–based best practice advisory was introduced to notify hospital providers discontinuing IV insulin if SC long-acting insulin had not been given in the previous 2-6 hours. Demographic characteristics and clinical outcomes before and after intervention were compared.
Results
A total of 352 patient encounters were included (251 in the preintervention phase and 101 in the postintervention phase). The rate of insufficient IV to SC insulin overlap decreased from (88 of 251) 35.1% before intervention to (20 of 101) 19.8% after intervention (P=.005). The rate of posttransition hypoglycemia (<70 mg/dL; to convert to mmol/L, multiply by 0.0259) decreased from (27 of 251) 10.7% to (4 of 101) 4% after intervention (P=.04). Rates of posttransition hyperglycemia (>250 mg/dL), rebound DKA, length of hospital stay, and duration of IV insulin therapy were similar before and after intervention.
Conclusion
Using quality improvement methodology, the rates of insufficient IV to SC insulin overlap during treatment of DKA in a large tertiary care referral center were measured and reduced through an electronic medical record–based best practice advisory targeting hospital providers.
Diabetic ketoacidosis (DKA) occurs in patients with diabetes during severe deficiency of insulin and/or during severe illness. Untreated, DKA can lead to progressive dehydration, acidosis, confusion, and even death.1 Treatment of DKA aims to reverse ketosis and hyperglycemia and typically requires hospitalization for intravenous (IV) fluid resuscitation and IV insulin therapy, followed by transitioning to subcutaneous (SC) insulin. However, the half-life of IV regular insulin is short at 20 minutes and SC long-acting insulin can take up to 2-4 hours to be absorbed from the skin, with peak effects seen after 4-6 hours.2,3 Therefore, the current American Diabetes Association guidelines recommend an overlap of at least 2-4 hours while transitioning from IV to SC insulin therapy to avoid gaps in insulin activity.4,5 Insufficient overlap time between IV and SC insulin while transitioning off IV insulin can lead to posttransition hyperglycemia or rebound DKA6 with increased use of hospital resources.7
The Mayo Clinic DKA management protocol and order set recommend at least 2 hours of IV and SC insulin overlap before stopping IV insulin infusion.4 However, endocrinology fellows (internal medicine physicians undergoing subspecialty endocrinology training supervised by attending endocrinologists) rotating on the hospital Diabetes Consult Service (DCS) had observed practice patterns during consults for post DKA management where there was less than 2 hours or sometimes no overlap between IV and SC insulin. The frequency and cause of insufficient overlap between IV and SC insulin and the associated clinical outcomes were unknown. This became the focus of the endocrine fellow quality improvement (QI) efforts.
Available Knowledge
The implementation of standardized protocols for DKA treatment6,7 and the earlier administration of long-acting SC insulin8, 9 have been reported in previous publications to reduce the inappropriate overlap time between IV and SC insulin during DKA management. For instance, in a retrospective study by Karajgikar et al6 the percentage of patients receiving an overlap of more than 1 hour between IV and SC insulin increased by 13% after implementing an electronic medical record (EMR)–based DKA admission protocol. In the same study the rate of rebound DKA, defined as a new elevation in anion gap after insulin cessation, decreased from 40% to 8.3%. Another retrospective study used a best practice advisory (BPA) to alert intensivists about DKA resolution, which led to improved clinical parameters like reduced duration of IV insulin therapy and lower rates of hyperglycemia, without affecting the duration of intensive care unit (ICU) stay or mortality.9
Rationale
All cases of DKA treated with IV insulin between July 2021 and October 2021 were retrospectively reviewed. In 26 out of 80 (32.5%) cases of DKA, IV to SC insulin overlaps were less than 2 hours. A root cause analysis with multidisciplinary stakeholders subsequently identified the following issues:
-
(a)
Overly lengthy and detailed DKA order set, which obscured the protocol’s recommendations for overlapping IV and SC insulin.
-
(b)
Locating and accessing the institution’s DKA protocol was challenging.
-
(c)
The DKA order set generated specific wording in the EMR orders to stop IV insulin 2 hours after the SQ insulin order was placed, inadvertently leading to insulin overlap less than 2 hours when SQ insulin was not immediately available at the bedside and had to be transported through the hospital.
To enhance the root cause analysis, an email-based survey was distributed in February 2022 to nurses who care for patients with DKA. The survey received 45 responses. The primary reason cited for insufficient overlap between IV and SQ insulin during DKA management was unfamiliarity with this aspect of the protocol (31%). In addition, the most suggested intervention was to implement an EMR-based reminder to overlap insulin according to the protocol (38%) (see Supplemental Material, available online at http://www.mcpiqojournal.org).
On the basis of these data and with input from various disciplines, a BPA intervention was selected. The intervention was designed to alert the health care providers (physicians, nurse practitioners, and physician assistants) regarding the recommended duration of overlap between IV and SC insulin during DKA management.
Specific Aims
At the end of the preintervention phase from July 1, 2021, to September 14, 2022, the rate of IV to SC insulin overlap less than 2 hours during DKA was 35.1%. Therefore, the aim of this project was to decrease the percentage of insufficient IV and SC insulin overlap after 6 months of the BPA intervention without negatively impacting the rate of posttransition hypoglycemia. Simultaneously, the effect of the BPA on the frequency of other treatment-related adverse outcomes, including posttransition hyperglycemia, rebound DKA, length of IV insulin time, and length of hospital stay, was assessed.
Patients and Methods
Context
This QI project was conducted at Mayo Clinic Rochester, a tertiary care referral center located in Rochester, Minnesota. It was led by a team of 10 endocrinology fellows. The preintervention period spanned from July 1, 2021, to September 14, 2022, whereas the postintervention period extended from September 15, 2022, to March 15, 2023. The 6-month postintervention period was specifically chosen to allow for sufficient time to gather and analyze the data before some fellows graduated from the program by June 30, 2023. The QI paradigm of define, measure, analyze, improve, and control was used.
At the center, hospital-based diabetes care is assisted by the DCS, which is composed of a multidisciplinary team of supervising physicians, fellows, physician assistants, nurse practitioners, and certified diabetes care and education specialists. When a patient presents with DKA, the primary admitting service oversees the initial diagnosis and management, including placing orders in the EMR. The DCS might be engaged as a consulting service at various parts of DKA management at the discretion of the primary admitting service.
Intervention
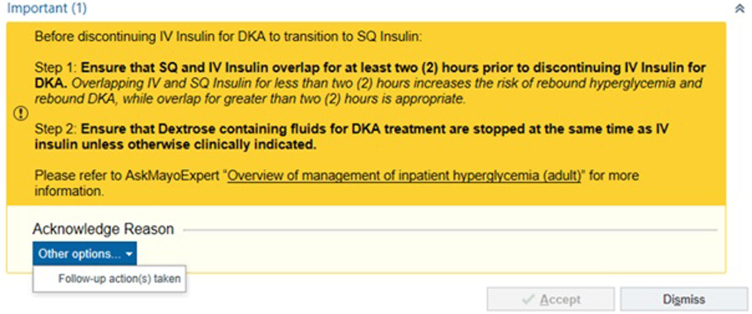
A BPA was developed collaboratively with a team of nursing informatics specialists and was implemented on September 15, 2022 (Figure 1). The BPA was designed to be triggered within the EMR when the IV insulin order for DKA was discontinued without administration of long-acting insulin within the preceding 2-6 hours. On activation, it displayed a 2-step checklist. Step 1 included a reminder to overlap IV and SC insulin for at least 2 hours, and Step 2 provided guidance on appropriately discontinuing IV dextrose-containing fluids to prevent posttransition hyperglycemia or hypoglycemia (see Supplemental Material, available online at http://www.mcpiqojournal.org). To close the BPA, acknowledgment of “Follow-up action(s) taken” was required. The BPA also included a link to the institutional DKA management protocol.
Figure 1.
Best practice advisory screenshot. The figure displays a screenshot of the electronic medical record–based best practice advisory. The best practice advisory triggered when providers attempted to discontinue intravenous (IV) insulin without administering subcutaneous long-acting insulin in the prior 2-6 hours. See Supplemental Material (available online at http://www.mcpiqojournal.org) for further discussion about dextrose-containing fluid. DKA, diabetic ketoacidosis; SQ, subcutaneous.
The possibility of creating a similar BPA that would trigger for bedside nurses after comparable circumstances was considered. However, this was not pursued after stakeholder input regarding potential alarm fatigue for nurses and unproven benefits of this type of BPA.
Measures
During the preintervention and postintervention periods, Mayo Data Explorer, a self-service EMR data exploration and retrieval tool, was used to identify consecutive episodes of DKA. Monthly screenings identified inpatient progress notes, including the terms “DKA” or “Diabetic Ketoacidosis” in the assessment and plan sections. The inclusion criteria comprised patients aged 18 years or older who were treated for DKA with IV insulin. Patients with β-hydroxybutyrate levels less than 3.0 mmol/L on admission, a commonly accepted cutoff to define ketosis in DKA,10 were excluded.
Participant records underwent data extraction using a standardized online data collection form. Demographic details, including gender orientation and age, were extracted from the patient identification section in the EMR. Type of diabetes was determined from the problem list in the assessment and plan section of the DCS, or primary team notes. If the type of diabetes was unclear (ie, new diagnosis with features of potential type 1 or type 2 diabetes) or conditions other than type 1 or type 2 diabetes were present (ie, such as cystic fibrosis), diabetes was categorized as other. The primary team was identified on the basis of the hospital team that authored the admission history and physical. Data regarding insulin administration timing (IV insulin initiation and discontinuation and overlap between SC and IV insulin) were extracted from the EMR’s medication administration record time stamps.
Insufficient IV to SC insulin overlap was defined as any instance when the overlap of IV and SC insulin duration was less than 2 hours, including when SC insulin was given after IV insulin at any point in the hospitalization or when no SC insulin was given after IV insulin was discontinued. Posttransition hyperglycemia was defined as any glucose value greater than or equal to 250 mg/dL (to convert to mmol/L, multiply by 0.0259) in the 12 hours after IV insulin discontinuation. Posttransition hypoglycemia was identified as any glucose value less than 70 mg/dL in the same 12-hour timeframe. Rebound DKA was defined as an elevated anion gap greater than 15 in the 12 hours after stopping IV insulin, on the basis of previous definitions of rebound DKA.6
Although there were initial discussions about stratifying the results by minority status (on the basis of race, ethnicity, or language), this consideration was excluded from the measurement phase because of the low rate of minority patients within the sample.
To ensure data accuracy and internal quality control, a 2-step verification process was implemented. After initial data collection, the timing of insulin administration and discontinuation underwent verification by an independent reviewer for 26 random participants. The concordance rate for this process was 100%.
Analysis
Continuous variables were summarized with means or medians, as appropriate, and categorical variables were described with counts and percentages. Comparisons between the preintervention and postintervention periods were established with 2-tailed T-tests or Wilcoxon rank sum tests for the continuous variables, and chi-squared tests for the categorical variables. A P value less than .05 was used as the cutoff for statistical significance. Statistical analysis was performed using BlueSky (v10.3.1-pro).
Ethical Considerations
This project was approved by the Mayo Clinic institutional review board and a Mayo Clinic QI oversight committee. Informed consent was not required but all participants had previously provided authorization to use their retrospective EMR data through the Minnesota Research Authorization.
Results
This study included 352 consecutive patient encounters for DKA treated with IV insulin. Of these, 251 patients were in the 14.5-month preintervention group and 101 were in the 6-month postintervention group (Table 1). The mean age was lower in the preintervention group (47.5±17.8 years vs 53±20.1; P=.01), whereas gender identity was balanced. Type 1 diabetes (61.0% and 58.4% in the preintervention and postintervention groups, respectively) was the most common type of diabetes, followed by type 2 diabetes (33.1% and 37.6% in preintervention and postintervention groups, respectively; P=.59). Most patients were cared for by medical ICU teams (53.8% and 51.4% in preintervention and postintervention groups, respectively), followed by hospital internal medicine teams (22.2% and 23.8% in preintervention and postintervention groups, respectively; P=.63). The severity of DKA was similar in the 2 groups as determined by mean admission β-hydroxybutyrate (6±3 mmol/L vs 6.6±3.3 mmol/L; P=.15).
Table 1.
Demographic Characteristics
| Characteristic | Preintervention (n=251) | Postintervention (n=101) | P value |
|---|---|---|---|
| Age (y), mean ± SD | 47.5±17.8 | 53±20.1 | .01 |
| Gender, n (%) | |||
| Women | 126 (50.2) | 48 (47.5) | .11 |
| Men | 123 (49) | 51 (50.5) | |
| Transgender women | 0 (0.0) | 0 (0.0) | |
| Transgender Men | 0 (0.0) | 0 (0.0) | |
| Nonbinary/nonconforming | 0 (0.0) | 2 (2.0) | |
| Other | 2 (0.8) | 0 (0.0) | |
| Type of diabetes, n (%) | |||
| Type 1 | 153 (61) | 59 (58.4) | .59 |
| Type 2 | 83 (33) | 38 (37.6) | |
| Other | 15 (6.0) | 4 (4.0) | |
| Primary treating service, n (%) | |||
| Hospital internal medicine team | 56 (22.2) | 24 (23.8) | .63 |
| Subspecialty medicine team | 25 (10.0) | 8 (7.9) | |
| Medical ICU team | 135 (53.8) | 52 (51.4) | |
| General surgical team | 3 (1.2) | 4 (4.0) | |
| Surgical ICU team | 12 (4.8) | 4 (4.0) | |
| Other | 20 (8.0) | 9 (8.9) | |
| Admission β-hydroxybutyrate (mmol/L), mean ± SD | 6±3.0 | 6.6±3.3 | .15 |
ICU, intensive care unit.
After the intervention, overall insufficient IV and SC insulin overlap rates decreased from 35.1% to 19.8% after intervention (P=.005) (Table 2). Episodes of insufficient overlap were classified by subgroups based on when SQ insulin was given. Those receiving IV and SC insulin overlap between 0 to less than 2 hours decreased from 23.5% to 13.7% (P=.04). The frequency of patients encounters with SC insulin given after IV insulin discontinuation decreased from 10.3% to 3% (P=.02). There was no statistical difference in frequency of patient encounters when no SC insulin was given in preintervention and postintervention groups (1.2% and 3%, respectively; P=.24). The rare episodes when no SC insulin was given included patients with type 2 diabetes and DKA not previously on insulin, those with euglycemic DKA not previously on insulin, and those who left against medical advice before SC insulin could be administered.
Table 2.
Preintervention and Postintervention Insufficient Intravenous and Subcutaneous Insulin Overlapa
| Characteristic | Preintervention (n=251) | Postintervention (n=101) | P value |
|---|---|---|---|
| Insufficient IV and SC insulin overlap,b n (%) | |||
| Yes | 88 (35.1) | 20 (19.8) | .005 |
| No | 163 (64.9) | 81 (80.2) |
IV, intravenous; SC, subcutaneous
Including: no SC insulin given, SC insulin given after IV insulin drip discontinuation, and SC given less than 2 hours before IV drip discontinuation
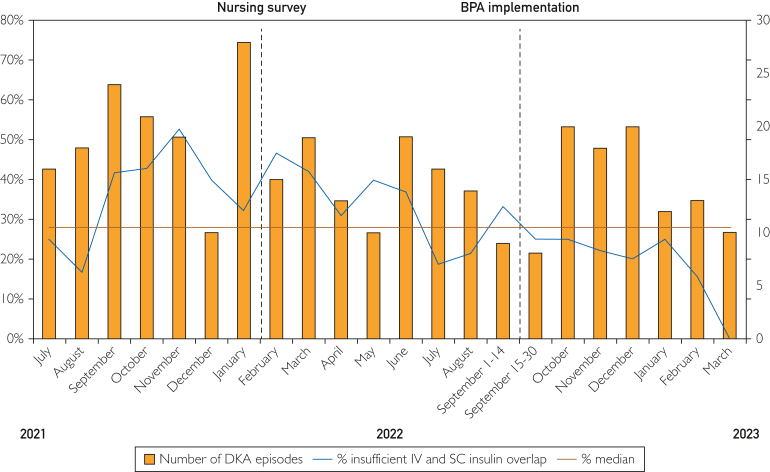
Monthly changes in the primary outcome were tracked using a run chart (Figure 2). This revealed multiple cause variations, such as too few crossings of the median during measurements (4 crossings per 22 data points) and 2 shifts (7 points above or below median) first from September 2021 to July 2022 and later after BPA implementation from September 2022 to March 2023.
Figure 2.
Monthly insufficient intravenous (IV) and subcutaneous (SC) insulin overlap. The run chart depicts the monthly presentation of insufficient IV and SC insulin overlap. The X axis represents the months. The left Y axis represents the percentage of insufficient IV and SC insulin overlap. The right Y axis represents the number of diabetic ketoacidosis (DKA) episodes. BPA, best practice advisory.
In a subgroup analysis looking at the rate of insufficient IV and SC insulin overlap by the primary service, no statistical difference was seen in the postintervention group for hospital internal medicine and subspecialty medicine teams (P>.05). Statistically significant improvement was noted in the postintervention groups for medical ICU (35.8%-23.1%; P=.04) and general surgical teams (66.7%-0%; P=.05) (Table 3).
Table 3.
Preintervention and Postintervention Insufficient Intravenous and Subcutaneous Insulin Overlap Stratified by Type of Primary Service
| Primary service | Insufficient IV and SC insulin overlap | Preintervention (n=251), n (%) | Postintervention (n=101), n (%) | P value |
|---|---|---|---|---|
| Hospital internal medicine | Yes | 12 (21.4) | 6 (25.0) | .72 |
| No | 44 (78.6) | 18 (75.0) | ||
| Subspecialty medicine | Yes | 10 (40.0) | 1 (12.5) | .15 |
| No | 15 (60.0) | 7 (87.5) | ||
| Medical ICU | Yes | 52 (38.5) | 12 (23.1) | .04 |
| No | 83 (61.5) | 40 (76.9) | ||
| General surgical | Yes | 2 (66.7) | 0 (0.0) | .05 |
| No | 1 (33.3) | 4 (100.0) | ||
| Surgical ICU | Yes | 8 (66.7) | 1 (25.0) | .14 |
| No | 4 (33.3) | 3 (75.0) | ||
| Other | Yes | 4 (20.0) | 0 (0.0) | .14 |
| No | 16 (80.0) | 9 (100.0) |
ICU, intensive care unit; IV, intravenous; SC, subcutaneous.
The median duration of hospitalization and duration of IV insulin therapy were similar in the preintervention and postintervention groups (P>.05) (Table 4). The rate of posttransition hypoglycemia decreased from 10.7%-4% (P=.04) after the intervention. The rates of posttransition hyperglycemia, rebound DKA, and death during hospital stay were not statistically different in the postintervention group (P>.05) (Table 4).
Table 4.
Resource Utilization and Safety Metricsa
| Outcome | Preintervention (n=251) | Postintervention (n=101) | P value |
|---|---|---|---|
| Hospital stay (d), median (IQR) | 4 (2-8) | 4 (3-9.5) | .07 |
| IV insulin therapy duration (h), median (IQR) | 19 (15-27.5) | 20 (15-28) | .55 |
| Posttransition hyperglycemia (>250 mg/dL)b within the 12 h following IV to SC insulin transition, n (%) | 115 (45.8) | 39 (38.6) | .21 |
| Posttransition hypoglycemia, n (%) | 27 (10.7) | 4 (4.0) | .04 |
| Rebound DKA, n (%) | 17 (6.8) | 7 (6.9) | .97 |
| Death during hospital stay, n (%) | 6 (2.4) | 2 (2.0) | .82 |
DKA, diabetic ketoacidosis; IQR, interquartile range; IV, intravenous; SC, subcutaneous.
SI conversion factors: To convert posttransition hyperglycemia values to mmol/L, multiply by 0.0259.
Discussion
Summary
Using principles of QI, an EMR-based BPA intervention alerting hospital providers to reduce insufficient IV and SC insulin overlap time during treatment of DKA in adults was designed and implemented.
Interpretation
Our study found a 15% decrease in the incidence of inadequate overlap between IV and SC insulin of less than 2 hours after implementation of a BPA during the management of patients with DKA. These findings align with a previous study that reported a similar reduction in rate of insufficient overlap of less than 1 hour by 13% using a standardized EMR-based DKA order set.6 In contrast to this previous study, our project focused on improving compliance to an existing EMR-based protocol. Also, insufficient IV and SC insulin overlap was defined as less than 2 hours in concordance with the current American Diabetes Association guidelines,4 and institutional protocol. Increased nursing awareness of the adequate overlap recommendations due to the root cause analysis from the hospital leadership and the distribution of nursing surveys may also have influenced some practices outside the BPA intervention. A slight decrease in the rates of insufficient IV to SC overlap was noted in February 2022, corresponding to the time of nursing survey distribution. However, the most robust improvement was only noted in the postintervention period after implementation of the BPA (Figure 2). In general, the rate of insufficient IV to SC insulin overlap appeared to decrease during the entire study. This could also be related to increased availability of hospital resources as waves of coronavirus disease 2019 infections subsided through 2022 and 2023.
The rates of rebound DKA did not change significantly after intervention in our study (P>.05). It is also important to highlight that the baseline rates of rebound DKA in our study were much lower than previously noted in literature6 (6.8% vs 40%).
A similar reduction in rates of posttransition hypoglycemia (6.4%) compared with a previous study using an EMR-based BPA to alert ICU providers that DKA had resolved (7%) was observed.9 This can be attributed to increased focus on the timing of IV and SC insulin overlap and enhanced accessibility to the steps outlined in the DKA protocol. Unlike previous studies, no changes in the duration of IV insulin therapy were observed before and after BPA implementation.9 This could be explained by the fact that the BPA only appeared at the end of the IV insulin therapy in our intervention.
Our study has several strengths and limitations. The strengths of this QI project include the extended period of define, measure, and analyze, which led to a targeted intervention on the basis of identified root causes. The data set excludes cases of hyperglycemia without the presence of ketosis that are mislabeled or treated as DKA. The intervention in the form of a hospital wide BPA, was able to capture all patient encounters with DKA, and was not limited to patients who were critically ill or in the ICU. Moreover, since this intervention was EMR-based, it required minimal maintenance, and will function independently of provider and nurse turnover. The BPA triggered 190 times during the 6-month intervention phase (approximately once per day), indicating successful engagement with the health care providers.
Our study also has several limitations. These include potential referral bias as the patients were exclusively from a large tertiary academic center, which may not fully represent the unique challenges to DKA management in other settings. Although the baseline age was different in the preintervention and postintervention groups, the groups were otherwise well balanced in other demographic characteristics (Table 1). The BPA also triggers for patients not meeting criteria for DKA but who are being treated with the DKA protocol. These patients, however, were not included and the clinical outcomes for these patients were not measured. Additionally, the BPA could contribute to alarm fatigue and providers may simply choose to ignore it over time. A longer duration of the intervention phase of the project would be beneficial to assess long-term clinical trends. Furthermore, our study solely examined the timing of IV and SC insulin and did not assess other aspects of appropriate transitions in DKA management, such as the dosage of long-acting SC insulin used or whether DKA had resolved before the transition from IV to SC insulin therapy. Additional research is required to evaluate these outcomes.
After the culmination of the study in March 2023, the BPA has continued to be triggered in the EMR and is integrated into routine clinical practice. However, the method of identifying patients with DKA and extracting data manually was labor-intensive and not sustainable for long-term data monitoring. The measurement of outcomes stopped at the end of the intervention period to focus on data analysis and publication. Alternative and more automatic methods of data collection are needed to facilitate the evaluation of trends in DKA insulin management in the future.
Conclusion
This QI project reduced the rate of insufficient IV and SC insulin overlap during treatment of DKA by 15% through an EMR-based BPA. This also reduced the frequency of posttransition hypoglycemia.
Potential Competing Interests
The authors report no competing interests.
Acknowledgments
The Division of Endocrinology, Diabetes, and Metabolism from the Department of Medicine at Mayo Clinic Rochester did not play a role in the overall study, results, or the decision to submit the manuscript for publication. This project was initiated by the research group who had full autonomy throughout the process. Dr Welch served as project leader. Dr Brito served as methodological advisor. Dr Toro-Tobon performed the data analysis. Drs Welch, Toro-Tobon, Rachmasari, Sandooja, Rahimi, Mohan, Hewlett, Clark, Maheshwari, and Zhang performed data extraction. All authors contributed to the design of the study, critical appraisal of the results, and manuscript preparation. All authors reviewed and agreed on the final version of the article.
Footnotes
Supplemental material can be found online at http://www.mcpiqojournal.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Supplemental Online Material
References
- 1.Pasquel F.J., Messler J., Booth R., et al. Characteristics of and mortality associated with diabetic ketoacidosis among US patients hospitalized with or without COVID-19. JAMA Netw Open. 2021;4(3) doi: 10.1001/jamanetworkopen.2021.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eli Lilly . 2023. Humulin R Prescribing Insert.https://pi.lilly.com/us/humulin-r-pi.pdf [Google Scholar]
- 3.Porcellati F., Rossetti P., Ricci N.B., et al. Pharmacokinetics and pharmacodynamics of the long-acting insulin analog glargine after 1 week of use compared with its first administration in subjects with type 1 diabetes. Diabetes Care. 2007;30(5):1261–1263. doi: 10.2337/dc06-2208. [DOI] [PubMed] [Google Scholar]
- 4.ElSayed N.A., Aleppo G., Aroda V.R., et al. 16. Diabetes care in the hospital: standards of care in diabetes-2023. Diabetes Care. 2023;46(suppl 1):S267–S278. doi: 10.2337/dc23-S016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitabchi A.E., Umpierrez G.E., Miles J.M., Fisher J.N. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335–1343. doi: 10.2337/dc09-9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karajgikar N.D., Manroa P., Acharya R., et al. Addressing pitfalls in management of diabetic ketoacidosis with a standardized protocol. Endocr Pract. 2019;25(5):407–412. doi: 10.4158/EP-2018-0398. [DOI] [PubMed] [Google Scholar]
- 7.Joyner Blair A.M., Hamilton B.K., Spurlock A. Evaluating an order set for improvement of quality outcomes in diabetic ketoacidosis. Adv Emerg Nurs J. 2018;40(1):59–72. doi: 10.1097/TME.0000000000000178. [DOI] [PubMed] [Google Scholar]
- 8.Hsia E., Seggelke S., Gibbs J., et al. Subcutaneous administration of glargine to diabetic patients receiving insulin infusion prevents rebound hyperglycemia. J Clin Endocrinol Metab. 2012;97(9):3132–3137. doi: 10.1210/jc.2012-1244. [DOI] [PubMed] [Google Scholar]
- 9.Alawneh D., Younis M., Hamarshi M.S. The impact of a new best practice advisory on the management of diabetic ketoacidosis. Curr Diabetes Rev. 2021;17(5) doi: 10.2174/1573399816999201103141726. [DOI] [PubMed] [Google Scholar]
- 10.Sheikh-Ali M., Karon B.S., Basu A., et al. Can serum beta-hydroxybutyrate be used to diagnose diabetic ketoacidosis? Diabetes Care. 2008;31(4):643–647. doi: 10.2337/dc07-1683. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.